Abstract
One of the therapeutic approaches in treating diabetes is to reduce postprandial hyperglycemia by inhibiting major carbohydrate hydrolyzing enzymes. In the present study, crude extracts of marine seaweed, Turbinaria ornata, were tested for their antidiabetic potential using enzyme inhibitory assays (α-amylase, α-glucosidase, and dipeptidyl peptidase-IV). Among the tested extracts, methanol and acetone extracts showed significant inhibitory effects on α-amylase (IC50 250.9 μg/mL), α-glucosidase (535.6 μg/mL), and dipeptidyl peptidase-4 (55.2 μg/mL), respectively. Free radical scavenging activity of these extracts was analyzed using DPPH assay (65%). Extracts were tested for in vitro toxicity using DNA fragmentation assay, haemolytic assay, and MTT assay. None of the extracts showed toxicity in tested models. Furthermore, GC-MS analysis of lead extracts showed the presence of major compounds, hentriacontane, z, z-6, 28-heptatriactontadien-2-one, 8-heptadecene, and 1-heptacosanol. Our findings suggest that Turbinaria ornata can be used as a potential source for further in vivo studies in controlling hyperglycemia.
1. Introduction
Diabetes mellitus is a metabolic disorder which affects the endocrine system of the body, characterized by defects in carbohydrate, lipid, and protein metabolism [1]. Globally the frequency of disorder is rising gradually; patients suffering from this disorder are unable to turn out or respond properly to insulin produced in the body [2]. Control of postprandial hyperglycemia prevents chronic vascular complications, and it is a widely accepted goal for the management of type-2 diabetes [3]. α-Amylase and α-glucosidase are the two major enzymes which play a key role in the digestion of carbohydrates by hydrolysis of inner α-1, 4-glucosidic linkages in starch and several other polysaccharides and the uptake of glucose by intestinal α-glucosidases [4]. One of the effective strategies for the management of diabetes is by retarding the starch digestion rate by inhibiting these enzymes leading to the reduction of postprandial hyperglycemia in diabetic subjects [5]. A recent approach for the development of an antidiabetic drug is based on the development of incretin analogues and dipeptidyl peptidase-IV (DPP-IV) inhibitors which play a crucial role in the glucose homeostasis by promoting α and β cell function [6]. It also downregulates the gastric emptying and gastric acid secretion to reduce the postprandial glucose level [7, 8]. Currently, there are several commercially available DPP-IV inhibitors (sitagliptin, saxagliptin, and vildagliptin) to block DPP-IV action thereby prolonging the half-life and biological activity of incretin hormones [9]. On the other hand, some pharmacokinetics studies reveal that DPP-IV inhibitors, mainly vildagliptin, are not safe for the patients with severe liver problems [10, 11]. Similarly, other synthetic hypoglycemic agents (acarbose and voglibose) that inhibit α-amylase and α-glucosidase can cause hepatic and gastrointestinal disorders [12]. Experimental and clinical evidences show that hyperglycemia increases the production of free radicals and induces oxidative stress leading to various diseases like diabetes mellitus, cardiac disorders, inflammatory, and neurodegenerative diseases [13–15]. Hence, there is a need for new, safe, and efficacious antioxidant and antidiabetic agents.
Natural sources such as marine flora and fauna, bacteria, fungi, and higher plants are of new interest in the development of antidiabetic agents. Among them, marine seaweeds represent one of the richest sources of bioactive compounds which are widely used in pharmaceutical research [16–18]. Polyphenols and sulfated polysaccharides present in seaweeds have been proven for antiviral, antitumoral, anti-inflammatory, and anticoagulant activity [17–19]. Seaweeds contain prolific source of bioactive compounds which can be exploited for the treatment of major chronic diseases like diabetes through the inhibition of starch digesting enzymes and the regulation of glucose induced oxidative stress [20, 21].
In the present study, antidiabetic and antioxidant effect of five different solvent extracts of marine seaweed Turbinaria ornata has been evaluated. T. ornata, a wide spread species belonging to Phaeophyceae, is rich in fucoids and sulphated polysaccharides [22, 23]. It has been previously reported for its antioxidant, anti-inflammatory, and cytotoxicity activity on murine melanoma and colon cancer cells [23–25]. To the best of our knowledge, this is the first report on T. ornata elucidating three different antidiabetic mechanisms (α-amylase, α-glucosidase, and DPP-IV) in vitro and the extracts were also tested for their in vitro toxicity.
2. Materials and Methods
2.1. Chemicals
Porcine pancreatic α-amylase, dinitrosalicylic acid (DNSA), acarbose, p-nitrophenyl α-D-glucopyranoside, diprotin A (Ile-Pro-Ile), DPP-IV from porcine kidney, and Gly-pro-p-nitroanilide (GPPN) were purchased from Sigma, Bangalore, India. DPPH, butylated hydroxy toluene (BHT) was obtained from Himedia, Mumbai. α-Glucosidase and dimethyl sulphoxide (DMSO) were obtained from SRL, Mumbai.
2.2. Collection and Processing of Seaweed
Fresh brown seaweeds were collected from Mandapam coastal region (Latitude 9°17′′N; Longitude 79°11′′E), Gulf of Mannar, Tamilnadu, South India on low tide during October 2012. The voucher specimen was deposited in the Marine Biotechnology and Biomedicine Laboratory, VIT University. The seaweeds were cleaned and holdfasts were removed and stored at −20°C immediately. Before extraction, they were shade-dried, powdered, and stored in air-tight containers at −20°C.
2.3. Preparation of Seaweed Extracts
The dried seaweed samples (25 g) were milled and extracted using 250 mL of various solvents such as petroleum ether, benzene, ethyl acetate, acetone, and methanol for 24 h by using Soxhlet apparatus. Each filtrate was concentrated to dryness under reduced pressure using rotary evaporator (Super Fit, Rotavap, model, PBU-6, India). The samples were lyophilized by using freeze dryer (Lark, Penguin Classic Plus, India) and stored in a refrigerator at 2–8°C for use in subsequent experiments. Prior to experiments, various concentrations were prepared from 250 to 1000 μg/mL.
2.4. Preliminary Phytochemical Screening
Preliminary phytochemical screenings were carried out as per the standard protocols of Harborne [26].
2.5. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
Free radical scavenging activity was determined according to the method of Mensor et al. [27]. Briefly, 500 μL of 0.3 mM alcoholic solution of DPPH (Himedia, India) was added to 2.5 mL of test samples at varying concentrations (250–1000 μg/mL). After incubation of samples in the dark for 30 minutes, the optical density was measured at 518 nm in the UV-visible spectrophotometer (Systronics AU-2700, India) using methanol as blank and synthetic antioxidant butylated hydroxy toluene (BHT) as positive control. The experiments were performed in triplicates and scavenging activity was expressed as percentage inhibition, using the following formula:
| (1) |
2.6. In Vitro α-Amylase Inhibition Assay
The α-amylase inhibitory activity was determined as described in Jayasri et al. [28] with minor modifications. Briefly, 250 μL of algal extracts with varying concentrations (250–1000 μg/mL) and 250 μL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) containing α-amylase (porcine pancreatic α-amylase, Sigma, St. Louis, USA) solution (0.5 mg/mL) were incubated for 10 min at 25°C. After preincubation, 250 μL of 1% starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) was added to each tube at 5 sec intervals. The reaction mixtures were then incubated at 25°C for 10 min. The reaction was stopped with 500 μL dinitrosalicylic acid (Sigma, St. Louis, USA) colour reagent. The tubes were then incubated in a boiling water bath for 5 min and cooled to room temperature. The reaction mixture was then diluted by adding 5 mL of distilled water, and absorbance was measured at 540 nm in the UV-visible spectrophotometer (Systronics AU-2700, India). The experiments were performed in triplicate and the α-amylase inhibitory activity was calculated as percentage inhibition, using the formula
| (2) |
2.7. In Vitro α-Glucosidase Inhibition Assay
The α-glucosidase inhibitory activity was determined as described by Jayasri et al. [28] with slight modifications. Briefly, 50 μL of algal extracts with varying concentrations (250–1000 μg/mL) and 100 μL of 0.1 M phosphate buffer (pH-6.9) containing α-glucosidase (SRL, India) solution (1.0 U/mL) were incubated in 96-well plates at 25°C for 10 minutes. After preincubation, 50 μL of 5 mM p-nitrophenyl α-D-glucopyranoside (Sigma, St. Louis, USA) in 0.1 M phosphate buffer (pH-6.9) was added to each well at 5 sec intervals. The reaction mixture was then incubated at 25°C for 5 min. After incubation, absorbance readings were recorded at 405 nm using a plate reader (Bio-TEK, USA) and compared to the control which contained 50 μL of buffer solution in place of algal extract. The experiments were performed in triplicate and the α-glucosidase inhibitory activity was calculated as percentage inhibition. The percentage of enzyme inhibition was calculated as follows:
| (3) |
2.8. In Vitro Dipeptidyl Peptidase-IV (DPP-IV) Inhibition Assay
DPP-IV inhibitory activity was determined according to the method of Al-Masri et al. [29]. Standard diprotin A (Sigma, St. Louis, USA) was diluted to various concentrations (0.2, 0.4, 0.8, 1.6, 3.2, and 6.4 μg/mL) using Tris-HCl buffer (50 mM, pH 7.5) and the final volume was made to 35 μL and DPP-IV enzyme (15 μL) (Sigma, St. Louis, USA) was added to the above mixture. One unit of enzyme activity was defined as the amount of enzyme that catalyzes the release of 1 μmol para-nitroaniline (pNA) from the substrate/min under assay conditions. After adding the enzyme, the mixture was preincubated for 10 min at 37°C to enhance the binding capacity of the inhibitor. This was followed by the addition of 50 μL of (0.2 mM) Gly-pro-p-nitroanilide (Sigma, St. Louis, USA) in Tris-HCl as a substrate. Final incubation was done at 37°C for 30 min and the reaction was terminated by addition of 25 μL of 25% glacial acetic acid. The absorbance was measured at 405 nm using microtiter plate reader (Bio-TEK, USA). Experiments were done in triplicate and the results obtained were compared with negative control.
2.9. DPP-IV Inhibition Assay of Seaweed Extract
Seaweed extracts were dissolved in dimethyl sulphoxide to make a stock concentration of 1000 μg/mL. From the stock, the following concentrations were prepared (2.5, 10, 40, and 80 μg/mL) in Tris-HCl buffer (50 mM, pH 7.5) in a total volume of 100 μL/well. The assay was performed in triplicate according to standardized procedure of diprotin A. The percentage of DPP-IV inhibition was calculated as follows:
| (4) |
2.10. Maintenance of Cell Line
J774 cell line (mouse macrophage) was obtained from National Centre for Cell Science, Pune, India. The cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% (v/v) heat-inactivated foetal bovine serum (FBS), 2 mM l-glutamine, and 100 U/mL of penicillin/streptomycin with 5% CO2 at 37°C in a humidified incubator. Exponentially growing cells were used for the experiment.
2.11. Cytotoxicity Assay
Briefly, cells were seeded at a density of 5 × 105 cells/mL and allowed to attach for 24 h in 300 μL of medium incubated at 37°C and 5% CO2. After 24 h, seaweed extracts were added to the medium at various concentrations (1000–250 μg/mL) and incubated for 24 h. At the end of treatment, cells were incubated with MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] (5 mg/mL) for 4 h. The formazan crystals formed were dissolved in DMSO (dimethyl sulfoxide) after aspirating the medium. The extent of cytotoxicity was measured spectrophotometrically at 630 nm with a microplate reader (Bio-TEK, USA).
2.12. DNA Fragmentation Assay
Acridine orange-ethidium bromide staining was used to characterize the morphological changes and apoptotic body formation, assessed by fluorescent microscopy. Briefly, J774 cells (5 × 105) were seeded on sterile glass cover slips in six-well tissue culture plates and treated with test extracts (1000 μg/mL) for 24 h. The medium was removed and washed twice with PBS (phosphate-buffered saline). Cells grown on cover slips were loaded with acridine orange-ethidium bromide (4 μg/mL) of ratio (1 : 1) and incubated for 15 min at 37°C in the dark. Cells were viewed under Weswox LED fluorescent microscope (FM-3000) attached with a camera, and photographs were taken under 40 and 100x magnification at fluorescent conditions.
2.13. Haemolytic Assay
The seaweed extracts were evaluated for their haemolytic activity as described by Malagoli [30]. The blood sample was collected from a healthy volunteer (A+ve), using ethylene diamine tetra acetic acid (EDTA) as an anticoagulant. Whole blood was washed thrice in 9 volumes of sterile 0.9% NaCl saline solution; after each washing, the cells were centrifuged (150 ×g for 5 min) and the supernatant was discarded. The final pellet obtained was diluted, 1/9 (v/v) in sterile 0.9% NaCl saline solution and then 1/24 (v/v) in sterile Dulbecco's phosphate buffer saline (D-PBS), pH 7.0, containing 0.5 mM boric acid and 1 mM calcium chloride. The haemolytic activity of seaweed extracts was tested under in vitro conditions in 96-well plates. Briefly, each well received 100 μL of 0.85% NaCl solution containing 10 mM CaCl2. 100 μL of normal saline served as negative control, 0.1% Triton X-100 as a positive control, and test extracts of various concentrations (250–1000 μg/mL) were added to each well. Then each well received 100 μL of human erythrocytes diluted in D-PBS. After 30 min incubation at room temperature, the samples were centrifuged and supernatant was used to measure the absorbance of liberated haemoglobin at 630 nm [31].
2.14. GC/MS Analysis
GC/MS (Perkin Elmer, Clarus 680 GC coupled to a Clarus 600 MS) analysis was performed for the detection of major compounds present in various extracts of T. ornata. Column used in GC/MS was Elite-5MS (30.0 m, 0.25 mmID, and 250 μmdf). Carrier gas used was helium at a constant flow rate of 1 mL/min. EI mode with electron energy set at 70 eV was used. 1 μL of extract diluted with methanol was injected into GC/MS and the compounds were identified based on the molecular structure, molecular mass, and calculated fragment ratio of resolved spectra with that of mass spectra available from the library. Spectral data were interpreted using the database of National Institute Standard And Technology (NIST).
2.15. Statistical Analysis
All the data reported were expressed as mean ± S.E.M. Statistical analysis was performed using two-way ANOVA. The values were considered to be significantly different when the P value was <0.0001 compared to the baseline values. Software employed for statistical analysis was Graph-Pad Prism, Version 5.
3. Results
3.1. Identification of Collected Seaweeds
The samples were identified based on the morphological characteristics in response to environmental conditions: stiff erect stalks, hard thick leaves (blades) characterized by lateral ridges, and outer marginal blade with stiff row of spines (Figure 1). Based on the above characters, seaweed species was identified as T. ornata and authenticated by Dr. P. Kaladharan principle scientist and scientist in charge, Calicut Regional Centre of Central Marine Fisheries Research Institute.
Figure 1.
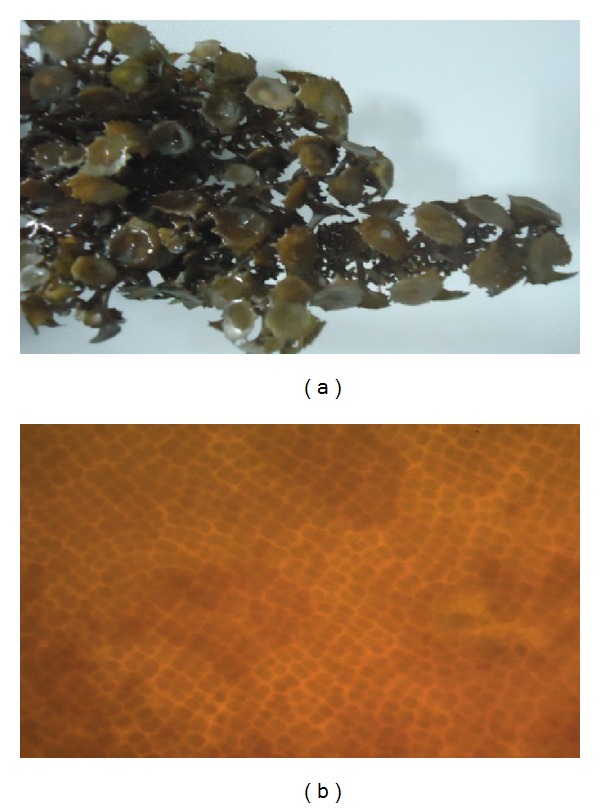
(a) Macroscopic view of Turbinaria ornata collected from Mandapam coastal region in Gulf of Mannar, Tamilnadu, India. Stiff erect stalks, hard thick leaves (blades) characterized by lateral ridges, and outer marginal blade with stiff row of spines are the unique characteristics of this species. (b) Microscopic image of Turbinaria ornata (magnification 40x).
3.2. Phytochemical Analysis of T. ornata
Qualitative analysis of the crude extracts of T. ornata showed the presence of major phytochemicals like alkaloids, phenols, flavanoids, proteins, lipids, carbohydrates, glycosides, tannins, and saponins in all the studied extracts (Table 1).
Table 1.
Qualitative phytochemical screening of various extracts of Turbinaria ornata (+: present, −: absent).
| Phytochemicals | Pet ether | Benzene | Ethyl acetate | Methanol | Acetone |
|---|---|---|---|---|---|
| Alkaloids | + | + | + | + | + |
| Phenols | + | + | + | + | + |
| Flavanoids | + | + | + | + | + |
| Proteins and amino acid | + | + | + | + | + |
| Lipids | + | + | + | + | + |
| Carbohydrates | + | + | + | + | + |
| Saponins | − | − | + | − | − |
| Glycosides | − | − | + | + | − |
| Tannins | + | + | + | − | − |
| Oils and fats | + | − | − | − | − |
3.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
The antioxidant potential of T. ornata was studied through its scavenging ability of the stable radical DPPH. Acetone extract showed significant scavenging ability on DPPH (65%) at a concentration of 1000 μg/mL when compared with that of a standard BHT (97%). However, none of the extracts exhibited higher activity than BHT at the same concentration (Figure 2).
Figure 2.
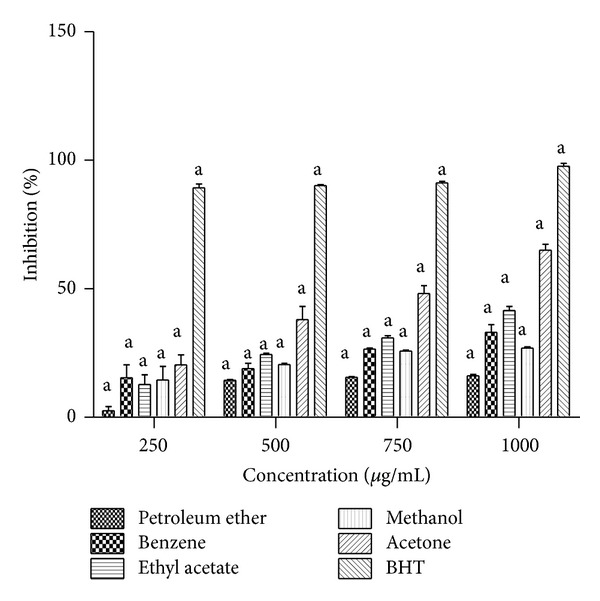
DPPH radical scavenging activity of petroleum ether, benzene, ethyl acetate, methanol, and benzene extracts of Turbinaria ornata. BHT is used as positive control and absorbance was measured at 517 nm. Values are means ± SD (n = 3) (a P < 0.0001 considered as significant).
3.4. In Vitro α-Amylase Inhibition Assay
Inhibitory effect of T. ornata on α-amylase is shown in Figure 3(a). Among the five extracts studied (petroleum ether, benzene, ethyl acetate, methanol, and acetone), three extracts showed (petroleum ether, methanol, and acetone) significant α-amylase inhibition at all the tested concentrations (250, 500, 750, and 1000 μg/mL). Methanol extract of T. ornata showed maximum inhibition of 96.5% with an IC50 of 250.9 μg/mL followed by acetone extract (87.6%) at a concentration of 1000 μg/mL.
Figure 3.
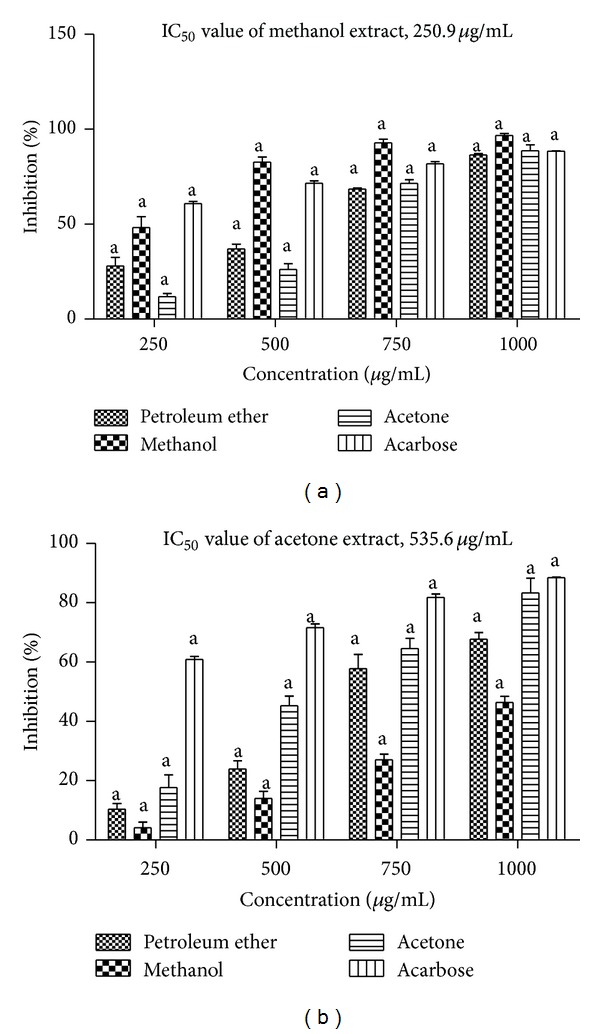
(a) In vitro α-amylase inhibitory activity of petroleum ether, methanol, and acetone extracts of Turbinaria ornata. Absorbance was measured at 540 nm. (b) In vitro α-glucosidase inhibitory activity of petroleum ether, methanol, and acetone extracts of Turbinaria ornata, acarbose is used as positive control and absorbance was measured at 405 nm. Values are means ± SD (n = 3) (a P < 0.0001 considered significant).
3.5. In Vitro α-Glucosidase Inhibition Assay
The α-glucosidase inhibitory activity of T. Ornata extracts was determined using p-nitrophenyl α-D-glucopyranoside as a substrate and these were compared with a standard acarbose (88%) (Figure 3(b)). At a concentration of 1000 μg/mL, acetone extract showed maximum inhibition (87.6%) with an IC50 value of 535.6 μg/mL. The inhibitory activity of acetone extracts was found to be more potent and similar when compared to the positive control acarbose.
3.6. In Vitro Dipeptidyl Peptidase-IV (DPP-IV) Inhibition Assay
T. ornata extracts were evaluated for their mode of action to stimulate insulin secretion through inhibition of DPP-IV enzyme. The effectiveness of various extracts was evaluated on the basis of percentage inhibition and IC50 values obtained. Among the five different solvent extracts (petroleum ether, benzene, ethyl acetate, methanol, and acetone), methanol extracts showed maximum percentage inhibition of 55.4% with an IC50 value of 55.2 μg/mL at a maximum concentration of 80 μg/mL. All the results were compared with a standard diprotin A (Figure 4).
Figure 4.
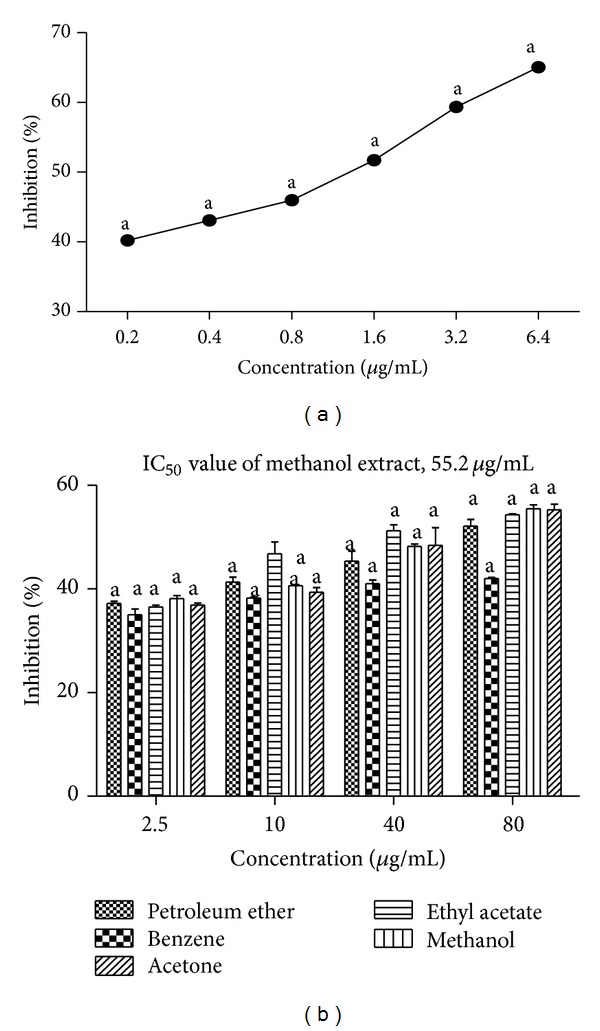
(a) DPP-IV inhibitory activity of diprotin A as positive control. (b) DPP-IV inhibitory activity of petroleum ether, benzene, ethyl acetate, methanol, and acetone extracts of Turbinaria ornata. Absorbance was measured at 405 nm. Values are means ± SD (n = 3) (a P < 0.0001 considered significant).
3.7. Cytotoxic Effect of T. ornata Extracts on J774 Cell Line
To evaluate the cytotoxic effect of the extracts, J774 cell line was incubated for 24 h with various concentrations of extracts. As shown in Figure 5(a), the decrease in viability correlates with the increase in concentration. MTT assay indicates that J774 cells treated with various extracts of T. ornata were safe and did not show any toxic effect against the cell treated at a lower concentration of 250 μg/mL. The observed cytotoxicity was found to be 2.57% when treated with methanol extract of T. ornata. 50% of cells were found to be viable when treated with a higher concentration of 1000 μg/mL.
Figure 5.
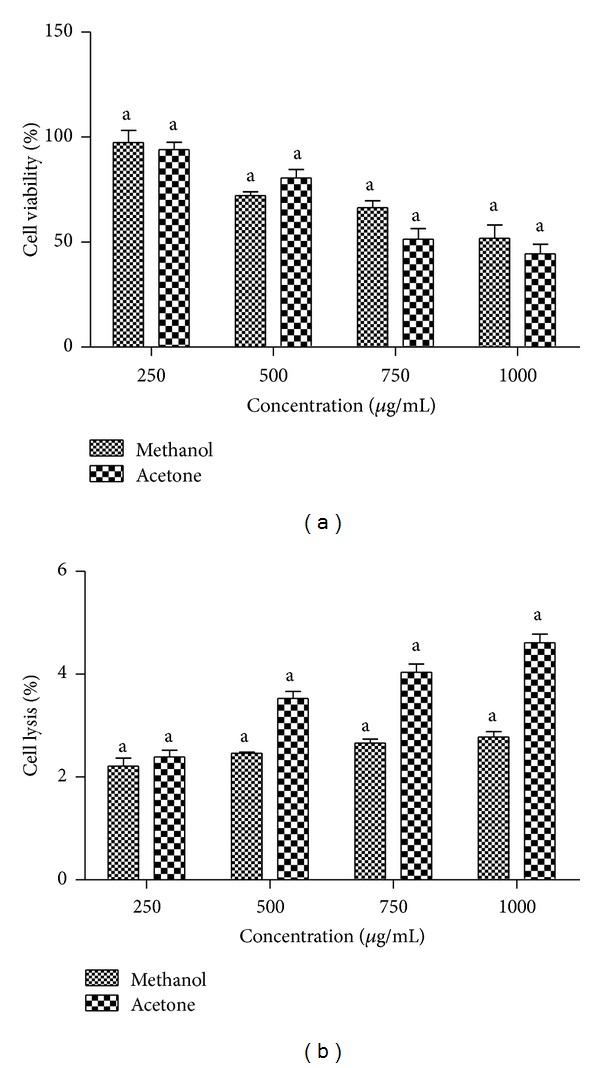
(a) Cell viability determined by MTT assay, J774 cells treated with methanol and acetone extracts of Turbinaria ornata. Absorbance was measured at 630 nm. (b) Haemolytic activity of methanol and acetone extracts of Turbinaria ornata. Absorbance was measured at 630 nm. Values are means ± SD (n = 3) (a P < 0.0001 considered significant).
3.8. DNA Fragmentation Assay
Staining cells with acridine orange-ethidium bromide is used to measure the proportion of viable, apoptotic, and necrotic cells. J774 cells treated with a maximum concentration of 1000 μg/mL of algal extracts were found to be viable and there was no change in cellular morphology including chromatin condensation, membrane blebbing, and fragmented nuclei (Figure 6).
Figure 6.
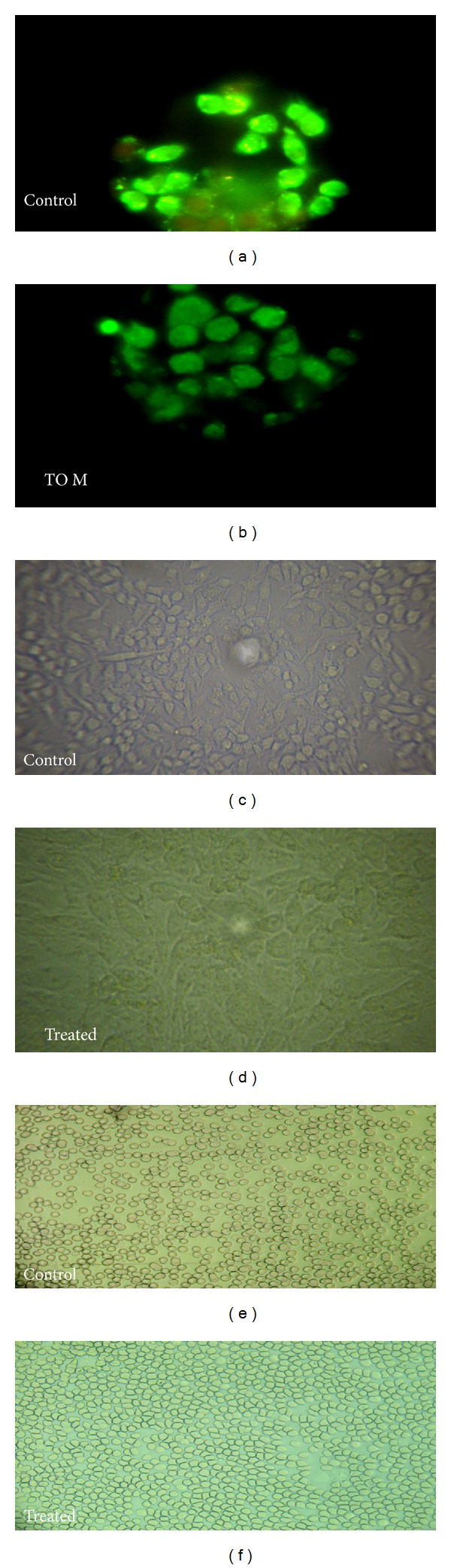
(a) Fluorescent photomicrographs (100x magnification) of J774 cells treated without seaweed extracts. (b) J774 cells treated with Turbinaria ornata methanol extract (TO M). (c) Phase contrast microscopic image of J774 cells treated with media alone (control). (d) Phase contrast microscopic image of J774 cells treated with test extracts. ((e)-(f)) Microscopic image (40x magnification) of in vitro haemolytic activity.
3.9. Haemolytic Assay
The lead extracts methanol (2.78%) and acetone (4.61%) were found to be nontoxic at a dosage of 1000 μg/mL (Figure 5(b)). Also, the extracts did not show any erythrocyte membrane damage at all the tested concentrations.
3.10. GC-MS Analysis of the Acetone Extract of T. ornata
The crude extracts were analyzed by GC-MS and compared with the retention time (RT) and mass spectra available in the data library of NIST. The GC-MS analysis reveals the presence of major bioactive compounds like hentriacontane, z, z-6, 28-heptatriactontadien-2-one, 8-heptadecene, and 1-heptacosanol. Chromatograms obtained were shown in Figure 7.
Figure 7.
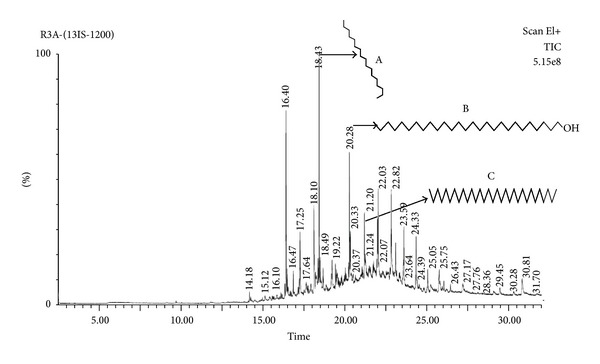
Gas chromatogram of acetone extract of Turbinaria ornata (A) 8-heptadecene, (B) 1-heptacosanol, and (C) hentriacontane.
4. Discussion
Marine seaweeds are good source of dietary fibres, polysaccharides, polyphenols, polyunsaturated fatty acids, minerals, and vitamins which possess various biological activities like antidiabetic, anti-inflammatory, and antioxidant activity [32]. Although this evidence seems to be valid, detailed research should be carried out to ensure the presence of various phytochemicals present in the seaweed extracts. In the current study, marine seaweed T. ornata was extracted and screened for both nonpolar and polar compounds using various solvents based on polarity. Qualitative analysis showed the presence of major bioactive compounds like alkaloids, phenols, flavanoids, glycosides, lipids, carbohydrates, proteins, tannins, oils, and fats. Presence of these bioactive molecules in various extracts of this seaweed may be responsible for various biological activities (antiviral, anti-inflammatory, and anticoagulant) [17–19].
The objective of this study was to investigate the antidiabetic and antioxidant potential of marine seaweed T. ornata and to enumerate major phytocompounds present in the extracts. One of the major causes for diabetes is due to the increased production of reactive oxygen species or impaired antioxidant defense system which leads to oxidative damage of beta cells [33]. In this study, acetone extract of T. ornata showed significant scavenging capability against DPPH (65%) at a maximum concentration of 1000 μg/mL compared with a positive control BHT (97%). As previously reported by Ananthi et al. and Chattopadhyay et al. [23, 25], the fucoidan fraction of Turbinaria conoides (61%) at a concentration of 1000 μg/mL and aqueous extract of T. ornata (80%) at a concentration of 500 μg/mL showed the similar scavenging capability on DPPH radicals. Our results were similar to these reports which confirm the antioxidant activity of T. ornata.
In the present study, the edible seaweed T. ornata was evaluated for in vitro α-amylase, α-glucosidase, and DPP-IV inhibitory property. Among the five extracts studied, petroleum ether, acetone, and methanol extracts showed significant percentage inhibition. Methanol extract of T. ornata showed maximum percentage inhibition on α-amylase (96.5%) with an IC50 value of 250.9 μg/mL with more inhibition than positive control acarbose (88%). Acetone extract of T. ornata showed more potent inhibition on α-glucosidase (84.3%) with an IC50 value of 535.6 μg/mL at a concentration of 1000 μg/mL, similar to the positive control acarbose (88%). The DPP-IV inhibitory potential of methanol extract of T. ornata showed (55.4%) inhibition with an IC50 value of 55.2 μg/mL which is comparable with that of positive control diprotin A (65%). Similar findings were shown by two brown seaweeds Ecklonia stolonifera Okumura and Eisenia bicyclis [34]. Several studies reported that the α-amylase and α-glucosidase inhibitory action of the class Phaeophyceae [35, 36] supports that these algal species are excellent source for antidiabetic agents. Apart from α-amylase and α-glucosidase, T. ornata extracts also showed significant DPP-IV inhibition. Based on these results, this seaweed can be used as alternative therapy for diabetes by raising the levels of endogenous incretin hormones (GLP-1 and GIP).
In this study, we also investigated the effects of crude extracts (methanol, acetone) of T. ornata in macrophage cell line (J774). The cytotoxic effects of the extracts were determined by using an MTT assay. After 24 h exposure, 250–1000 μg/mL of methanol and acetone extracts significantly decreased cell viability by increasing concentration. As previously reported, seaweed extracts which have higher phenol content may explain the level of cytotoxicity at higher concentrations (1000 μg/mL) [36, 37], whereby up to 500 μg/mL of seaweed extracts did not show any effect on J774 cell viability, and more than 90% of cells were found to be viable at a concentration of 250 μg/mL. Staining cells with fluorescent dyes is used to differentiate the nuclear morphology of live and apoptotic cells. J774 cells were analyzed in the presence of acridine orange and ethidium bromide to determine the nuclear morphology of viable and necrotic cells. Cells stained green represent viable cells and ethidium bromide selectively stains the cells that have lost membrane integrity and stains DNA orange. In this study acridine orange-ethidium bromide staining revealed that there was no change in cellular morphology, including chromatin condensation, membrane blebbing, and fragmented nuclei occurring due to the treatment of extracts even at higher concentration (1000 μg/mL). Our results indicate that the tested extracts did not induce haemolysis at a concentration of (1000 μg/mL). Based on the above results the methanol and acetone extracts of T. ornata were found to be nontoxic under in vitro conditions.
GC-MS analysis revealed the presence of major bioactive compounds like hentriacontane, z, z-6, 28-heptatriactontadien-2-one, 8-heptadecene, and 1-heptacosanol. Hentriacontane is the major component present in Oldenlandia diffusa, a natural source for the treatment of cancer in Asia [38], and also has reported activities like anti-inflammatory and antioxidant properties [38, 39]. Currently, there is no information regarding the antidiabetic effects of hentriacontane. Further, purification and characterization of active compounds along with mechanism studies will only give conclusive data on the antidiabetic property of tested algae.
5. Conclusions
The present study indicates that crude extracts of T. ornata possess in vitro α-amylase, α-glucosidase, and DPP-IV inhibitory activity. Due to their strong enzyme inhibitory activity, they are expected to suppress the glycemic response in diabetic conditions. Additionally, the extracts are capable of scavenging free radicals; in vitro toxicological parameters indicate that the IC50 of methanol and acetone extracts of T. ornata for α-amylase, α-glucosidase, and DPP-IV inhibition are greatly below cytotoxic levels. Future studies on in vivo antidiabetic activity of selected extracts and identification of major compounds responsible for antidiabetic action are in progress. These studies will reveal the possible antidiabetic mechanisms of T. ornata.
Acknowledgments
The authors wish to thank Department of Biotechnology, Government of India, for their financial support (Grant no. BT/Bio-CARe/03/347/2010-11), and VIT University for providing all necessary facilities.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a who consultation. Diabetic Medicine. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Gupta OP, Phatak S. Pandemic trends in prevalence of diabetes mellitus and associated coronary heart disease in India-their causes and prevention. International Journal of Diabetes in Developing Countries. 2003;23:37–50. [Google Scholar]
- 3.Ortiz-Andrade RR, García-Jiménez S, Castillo-España P, Ramírez-Ávila G, Villalobos-Molina R, Estrada-Soto S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: an anti-hyperglycemic agent. Journal of Ethnopharmacology. 2007;109(1):48–53. doi: 10.1016/j.jep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Gray GM. Carbohydrate digestion and absorption. Role of the small intestine. The New England Journal of Medicine. 1975;292(23):1225–1230. doi: 10.1056/NEJM197506052922308. [DOI] [PubMed] [Google Scholar]
- 5.Tarling CA, Woods K, Zhang R, et al. The search for novel human pancreatic α-amylase inhibitors: high-throughput screening of terrestrial and marine natural product extracts. ChemBioChem. 2008;9(3):433–438. doi: 10.1002/cbic.200700470. [DOI] [PubMed] [Google Scholar]
- 6.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. European Journal of Biochemistry. 1993;214(3):829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 7.Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50(3):609–613. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 8.Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. American Journal of Physiology—Endocrinology and Metabolism. 1997;273(5):E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 9.Lambeir A-M, Scharpé S, De Meester I. DPP4 inhibitors for diabetes—what next? Biochemical Pharmacology. 2008;76(12):1637–1643. doi: 10.1016/j.bcp.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 10.[EMEA] European Medicines Agency. European public assessment report (EPAR)—Januvia. 2007, http://www.ema.europa.eu/ema/
- 11.[EMEA] European Medicines Agency. European public assessment report (EPAR)—Galvus. 2007, http://www.ema.europa.eu/ema/
- 12.Murai A, Iwamura K, Takada M, Ogawa K, Usui T, Okumura J-I. Control of postprandial hyperglycaemia by galactosyl maltobionolactone and its novel anti-amylase effect in mice. Life Sciences. 2002;71(12):1405–1415. doi: 10.1016/s0024-3205(02)01844-1. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Letters. 1991;281(1-2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Hu N, Wu Y-L, Pan Y-J, Sun C-R. Preliminary studies on the chemical characterization and antioxidant properties of acidic polysaccharides from Sargassum fusiforme. Journal of Zhejiang University: Science B. 2008;9(9):721–727. doi: 10.1631/jzus.B0820025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butterfield DA, Castenga A, Pocernich CB, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s diseases. Journal of Nutritional Biochemistry. 2002;13:444–461. doi: 10.1016/s0955-2863(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 16.Mayer AMS, Lehmann VKB. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal and antiviral activities; with actions on the cardiovascular, endocrine, immune and nervous systems: and other miscellaneous mechanisms of action. Pharmacologist. 2000;42:62–69. [Google Scholar]
- 17.Cumashi A, Ushakova NA, Preobrazhenskaya ME, et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17(5):541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh T, Chattopadhyay K, Marschall M, Karmakar P, Mandal P, Ray B. Focus on antivirally active sulfated polysaccharides: from structure-activity analysis to clinical evaluation. Glycobiology. 2009;19(1):2–15. doi: 10.1093/glycob/cwn092. [DOI] [PubMed] [Google Scholar]
- 19.Pomin VH, Mourão PAS. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology. 2008;18(12):1016–1027. doi: 10.1093/glycob/cwn085. [DOI] [PubMed] [Google Scholar]
- 20.Lee S-H, Han J-S, Heo S-J, Hwang J-Y, Jeon Y-J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicology in Vitro. 2010;24(2):375–381. doi: 10.1016/j.tiv.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Lee S-H, Karadeniz F, Kim M-M, Kim S-K. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. Journal of the Science of Food and Agriculture. 2009;89(9):1552–1558. [Google Scholar]
- 22.Rohfritsch A, Payri C, Stiger V, Bonhomme F. Molecular and morphological relationships between two closely related species, Turbinaria ornata and T. conoides (Sargassaceae, Phaeophyceae) Biochemical Systematics and Ecology. 2007;35(2):91–98. [Google Scholar]
- 23.Ananthi S, Raghavendran HRB, Sunil AG, Gayathri V, Ramakrishnan G, Vasanthi HR. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga) Food and Chemical Toxicology. 2010;48(1):187–192. doi: 10.1016/j.fct.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Asari F, Kusumi T, Kakisawa H. Turbinaric acid, a cytotoxic secosqualene carboxylic acid from the brown alga Turbinaria ornata . Journal of Natural Products. 1989;52(5):1167–1169. doi: 10.1021/np50065a045. [DOI] [PubMed] [Google Scholar]
- 25.Chattopadhyay N, Ghosh T, Sinha S, Chattopadhyay K, Karmakar P, Ray B. Polysaccharides from Turbinaria conoides: structural features and antioxidant capacity. Food Chemistry. 2010;118(3):823–829. [Google Scholar]
- 26.Harborne JB. Phytochemical Methods. Vol. 3. Chapman & Hall; 1998. Methods of extraction and isolation; pp. 60–66. [Google Scholar]
- 27.Mensor LL, Menezes FS, Leitao GG, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 28.Jayasri MA, Radha A, Mathew TL. α-amylase and α-glucosidase inhibitory activity of Costus pictus D. Don in the management of diabetes. Journal of Herbal Medicine and Toxicology. 2009;3:91–94. [Google Scholar]
- 29.Al-Masri IM, Mohammad MK, Tahaa MO. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. Journal of Enzyme Inhibition and Medicinal Chemistry. 2009;24(5):1061–1066. doi: 10.1080/14756360802610761. [DOI] [PubMed] [Google Scholar]
- 30.Malagoli D. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invertebrate Survival Journal. 2007;4:92–94. [Google Scholar]
- 31.Muzykantov VR, Samokhin GP, Smirnov MD, Domogatsky SP. Hemolytic complement activity assay in microtitration plates. Journal of Applied Biochemistry. 1985;7(3):223–227. [PubMed] [Google Scholar]
- 32.Kim A-R, Shin T-S, Lee M-S, et al. Isolation and identification of phlorotannins from ecklonia stolonifera with antioxidant and anti-inflammatory properties. Journal of Agricultural and Food Chemistry. 2009;57(9):3483–3489. doi: 10.1021/jf900820x. [DOI] [PubMed] [Google Scholar]
- 33.Baynes JW. Chemical modification of proteins by lipids in diabetes. Clinical Chemistry and Laboratory Medicine. 2003;41(9):1159–1165. doi: 10.1515/CCLM.2003.179. [DOI] [PubMed] [Google Scholar]
- 34.Moon HE, Islam MN, Ahn BR, et al. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory phlorotannins from edible brown algae, Ecklonia stolonifera and Eisenia bicyclis. Bioscience, Biotechnology and Biochemistry. 2011;75(8):1472–1480. doi: 10.1271/bbb.110137. [DOI] [PubMed] [Google Scholar]
- 35.Apostolidis E, Karayannakidis PD, Kwon Y-I, Lee CM, Seeram NP. Seasonal variation of phenolic antioxidant-mediated a-glucosidase inhibition of Ascophyllum nodosum. Plant Foods for Human Nutrition. 2011;66(4):313–319. doi: 10.1007/s11130-011-0250-4. [DOI] [PubMed] [Google Scholar]
- 36.Nwosu F, Morris J, Lund VA, Stewart D, Ross HA, McDougall GJ. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chemistry. 2011;126(3):1006–1012. [Google Scholar]
- 37.Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radical Biology and Medicine. 2004;37(3):287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 38.Kim S-J, Chung W-S, Kim S-S, Ko S-G, Um J-Y. Antiinflammatory effect of Oldenlandia diffusa and its constituent, hentriacontane, through suppression of caspase-1 activation in mouse peritoneal macrophages. Phytotherapy Research. 2011;25(10):1537–1546. doi: 10.1002/ptr.3443. [DOI] [PubMed] [Google Scholar]
- 39.Agoramoorthy G, Chandrasekaran M, Venkatesalu V, Hsu MJ. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Brazilian Journal of Microbiology. 2007;38(4):739–742. [Google Scholar]


