Abstract
Poly(ADP-ribose) polymerase-1 (PARP1) catalyzes the poly(ADP-ribosyl)ation of protein acceptors using NAD+ as the substrate is now considered as an important target for development of anticancer therapy. PARP1 is known to be post-translationally modified in various ways including phosphorylation and ubiquitination, but the physiological role of PARP1 methylation is not well understood. Herein we demonstrated that the histone methyltransferase SMYD2, which plays critical roles in human carcinogenesis, mono-methylated PARP1. We confirmed lysine 528 to be a target of SMYD2-dependent PARP1 methylation by LC-MS/MS and Edman Degradation analyses. Importantly, methylated PARP1 revealed enhanced poly(ADP-ribose) formation after oxidative stress, and positively regulated the poly(ADP-ribosyl)ation activity of PARP1. Hence, our study unveils a novel mechanism of PARP1 in human cancer through its methylation by SMYD2.
Introduction
Poly(ADP-ribose) polymerase-1 (PARP1) is one of the most abundant nuclear proteins and catalyzes the transfer of the ADP-ribose unit from its substrate, NAD+, to some protein acceptors such as histones and PARP1 itself. Poly(ADP-ribosyl)ation (PARylation) is one type of post-translational modification, characterized by addition of ADP-ribose units to glutamic acid, aspartic acid and/or lysine residues in target proteins by members of the PARP family, and alters the properties and functions of the proteins. Through its interaction with partner proteins or/and the poly(ADP-ribosyl)ation of the proteins, PARP1 is involved in DNA repair, chromatin modification, transcriptional regulation and genomic stability [1,2]. This PAR-dependent response network is crucial for both physiological and pathological responses. As a molecular nick-sensor of DNA breaks, PARP1 has a crucial role in the organization of the DNA repair machinery [3]. PARP1 has been shown to play multiple critical roles in the repair of DNA single-strand breaks (SSBs) and double strand breaks (DSBs) [4-9]. The activation of PARP1 after DNA damage recruits enzymes including XRCC1, DNA ligase III and DNA polymerase β, which are required for DNA repair, to the DNA damage sites. The involvement of PARP1 in the DNA repair pathway prompted researchers to investigate the effect of PARP1 inhibition on DNA-damaging anticancer therapies [10,11]. Inhibition of PARP1 was proven to enhance the cytotoxicity of DNA-damaging agents to cancer cells [12,13]. Thus, several PARP1 inhibitors have been taken into the clinical trials [14,15].
Mammalian PARP1 is a 116-kDa protein, comprising an N-terminal DNA-binding domain including three zinc-binding domains, a central auto-modification domain, and a C-terminal catalytic domain. The central auto-modification domain contains several glutamate, aspartate and lysine residues as acceptors for its auto(ADP-ribosyl)ation. PARP1 has been reported to be modified through multiple post-translational modifications, including phosphorylation, acetylation, sumoylation, ubiquitination and also ADP-ribosylation [16-20]. Meanwhile, the detailed mechanism how PARP1 activity is controlled by these post-translational modifications still remains to be elucidated.
The accumulated evidence implicates deregulation of histone methylation appears to play crucial roles in human carcinogenesis [21]; for example, many lysine methyltransferases have been shown to function as oncogenes [22-26]. SET and MYND domain containing 2 (SMYD2) is one of the SMYD methyltransferase family proteins, containing the SET domain and the MYND domain. SMYD2 methylates histone H3K36 and H3K4, and functions as a transcriptional regulator in cooperation with the Sin3A and HDAC1 histone deacetylase complex [27,28]. SMYD2 is also known to methylate non-histone protein substrates, including p53 and RB1, and methylated p53 was reported to lose its tumor suppressive function [29,30]. Overexpression of SMYD2 was observed in various types of cancer [29,31]. Given the importance of SMYD2 in cancer cell proliferation, SMYD2 become an attractive drug target that is actively pursued by the pharmaceutical industry [32].
In this study, we found that SMYD2 methylates PARP1 and enhances its poly(ADP-ribosyl)ation activity. Our study implicates a novel mechanism of PARP1 in human carcinogenesis through the methylation by SMYD2.
Materials and Methods
Cell Culture
293T and HeLa cells were from American Type Culture Collection (ATCC) in 2001 and 2003, and tested and authenticated by DNA profiling for polymorphic short tandem repeat (STR) markers (Table W1). Both cell lines were grown in monolayers in appropriate media: Dulbecco's modified Eagle's medium (D-MEM) for 293T cells; Eagle's Minimum Essential Medium (E-MEM) for HeLa cells supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic solution (Sigma-Aldrich, St. Louis, MO). We also generated stable HeLa cell lines constitutively expressing SMYD2. The pCAGGS-SMYD2-HA or empty pCAGGS-HA mock vector was transfected into HeLa cells by FuGENE6 (Roche Applied Science, Penzberg, Germany) according to the manufacturer's protocol [22,33], and the antibiotics-resistant clones were selected with the culture media containing 0.5 mg/ml Geneticin®. SMYD2 stably expressing HeLa cells were transfected with SMYD2-specific siRNA duplex (5′- GAAUGACCGGUUAAGAGA-3′), or siEGFP siRNA duplex (5′- GCAGCACGACUUCUUCAAG-3′) as a negative control, respectively, by using Lipofectamin RNAiMAX (Life Technologies, Carlsbad, CA) according to the manufacturer's recommendations.
In Vitro Methyltransferase Assay
In vitro methyltransferase assays were performed as described previously [34]. Briefly, 1 μg of His-PARP1 protein was incubated with 1 μg of His-SMYD2 in 50 mM Tris–HCl (pH 8.8), 1.0 μCi/ml S-adenosyl-L-[methyl-3H]-methionine (Perkin Elmer, Waltham, MA) and Milli-Q water for 1 hour at 30°C. After boiling in sample buffer, the samples were subjected to SDS-PAGE, and visualized by fluorography [34].
Immunoprecipitation
293T cells were seeded at a density of 40% on a 100-mm dish. After cell attachment, the cells were transfected with expression vector constructs using FuGENE6, and after 48 h, transfected 293T cells were washed with PBS and lysed in CelLyticTM M Cell Lysis Reagent (Sigma-Aldrich) containing complete protease inhibitor cocktail (Roche Applied Science). Five hundred micrograms of whole-cell extract were incubated with anti-FLAG M2 agarose (Sigma-Aldrich) for 1 h at 4°C. After the beads were washed 3 times with 1 ml of TBS buffer (pH 7.6), the FLAG-tagged proteins bound to the beads were eluted by boiling in Lane Marker Sample Buffer (Thermo Fisher Scientific, Waltham, MA). Samples were then subjected to SDS-PAGE, and detected by western blot.
Immunocytochemistry
Cells fixed by 4% paraformaldehyde were incubated with a rabbit anti-HA antibody (Y-11, Santa Cruz Biotechnology) at a 1:1000 dilution ratio and a mouse anti-poly(ADP-ribose) (PAR) monoclonal antibody (Trevigen, Gaithersburg, MD) at a 1:1000 dilution ratio. After washing with PBS (−), cells were stained by an Alexa Fluor® 488-conjugated anti-mouse secondary antibody (Life Technologies) and an Alexa Fluor® 594-conjugated anti-rabbit secondary antibody (Life Technologies) at a 1:1000 dilution ratio. Stained preparations were mounted with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA).
Mass Spectrometry
The reaction mixture of in vitro methyltransferase assay was analyzed by nano liquid chromatography–tandem mass spectrometry (LC-MS/MS) using LCQ Deca XP plus (Thermo Fisher Scientific, San Jose, CA). The peptides were separated using nano ESI spray column (100 μm [ID] × 50 mm [L]) packed with a reversed-phase material (Inertsil ODS-3,3 μm; GL Sciences, Tokyo, Japan) at a flow rate 200 nl/min. The mass spectrometer was operated in the positive-ion mode, and the spectra were acquired in a data-dependent MS/MS mode. The MS/MS spectra were searched against the in-house database using local MASCOT server (version 2.2.1; Matrix Sciences, London, United Kingdom). The reaction mixture was desalted and applied to MALDI-TOF-MS using an Ultraflex (Bruker Daltonik GmbH, Bremen, Germany).
Edman Degradation
The in vitro methylation product was subjected to Edman degradation using a Procise HT protein sequencing system (Applied Biosystems, Foster City, CA).
Amino Acid Analysis
The excised protein bands blotted on the polyvinylidene fluoride membrane were individually inserted in clean 6 × 32-mmglass tubes containing 50 pmol of norvaline as internal standard and hydrolyzed in 6N HCl vapor at 110°C for 20 hours. The hydrolyzed samples were derivatized in situ by 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate for fluorophore detection. The 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate amino acids were separated by ion-pair chromatography on a C18 reversed-phase column (InertSustain C18HP, 3.0 mm [ID] × 250 mm, 3 μm; GL Sciences, Tokyo, Japan). Both a laser-induced fluorescence detector (LIF726; GL Sciences) and a fluorescence detector with Xe flush lamp (G1321A; Agilent Technologies, Santa Clara, CA) were used to reveal the existence of monomethylated Lys [35,36].
PARP1 Enzymatic Activity Assay
PARP1 activities were assayed using the universal colorimetric PARP assay kit (Trevigen, Gaithersburg, MD) as described previously [37]. One μg of PARP1 (Alexis, San Diego, CA) was incubated with 1 μg SMYD2 in 50 mM Tris–HCl (pH 8.8), with or without 160 nM S-adenosyl-L-methionine (SAM, New England Biolabs, Ipswich, MA) at 30°C for 4 h respectively. Methylated PARP1 (with SAM) or unmethylated PARP1 (without SAM) were loaded into a 96-well plate coated with histone H1, and incubated with biotinylated poly(ADP-ribose) and nicked DNA (Trevigen) for 1 hour. All the steps were performed strictly according to the manufacturer's instructions. Finally, the absorbance was measured at 450 nm in a spectrometrophotometer.
Results
Lysine 528 on PARP1 Is Methylated by SMYD2
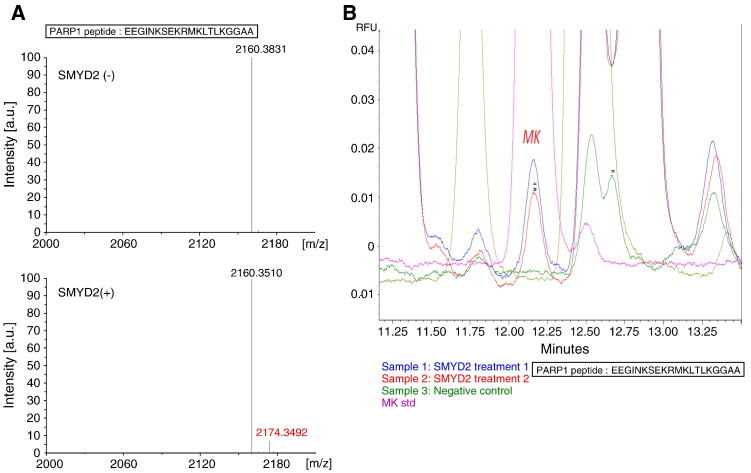
Since the physiological function of PARP1 methylation is unknown, we conducted an in vitro methyltransferase assay of recombinant PARP1 protein using a variety of recombinant histone methyltransferases to identify an enzyme(s) that possibly methylates PARP1 and found that the histone methyltransferase SMYD2 could methylate PARP1 in a dose-dependent manner (Figure 1A and B). Subsequently we attempted to identify a methylation site(s) of PARP1 using in vitro-methylated full-length of PARP1 by liquid chromatography-tandem mass spectrometry (LC-MS/MS), but failed to identify it probably due to the low coverage of the peptide fragments of PARP1. Therefore we focused on amino-acid residues 513–532, which correspond to a critical portion of PARP1 auto-modification [20] with three lysine residues, Lys 521, Lys 524 and Lys 528. We synthesized the peptide (513–532) and then performed an in vitro methyltransferase assay with S-adenosyl-L-[3H-methyl]-methionine, and found methylation of this peptide (data not shown). Subsequent matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis showed 14 Da-shift when it was mixed with SMYD2, implying that the PARP1 peptide is likely to be mono-methylated (Figure 2A). The mono-methylation on PARP1 after SMYD2 treatment was also confirmed by amino acid analysis (Figure 2B).
Figure 1.
SMYD2 methylates PARP1 in vitro. (A) Recombinant PARP1 protein was methylated by SMYD2 in a dose-dependent manner. An in vitro methyltransferase assay was performed by using purified His-tagged PARP1 and SMYD2 recombinant proteins. Methylated PARP1 was detected by fluorography. (B) Signals from methylated PARP1 gradually increased with the increase of SMYD2. Amounts of loading proteins were confirmed by staining the MemCodeTM Reversible Protein Stain (Thermo Fisher Scientific).
Figure 2.
Confirmation of PARP1 methylation by MALDI-TOF mass spectrometry and amino acid analyses. (A) MALDI-TOF MS spectra of the specific peptides (amino acid residues 513–532; EEGINKSEKRMKLTLKGGAA) before (upper panel) and after (lower panel) in vitro methyltransferase assay. (B) The chromatograms of amino acid analysis of the specific peptides before (sample3, green) and after (sample 1 and 2, blue and red, respectively) in vitro methyltransferase assay. MK indicates the retention time of mono-methyl lysine standard (MK, purple).
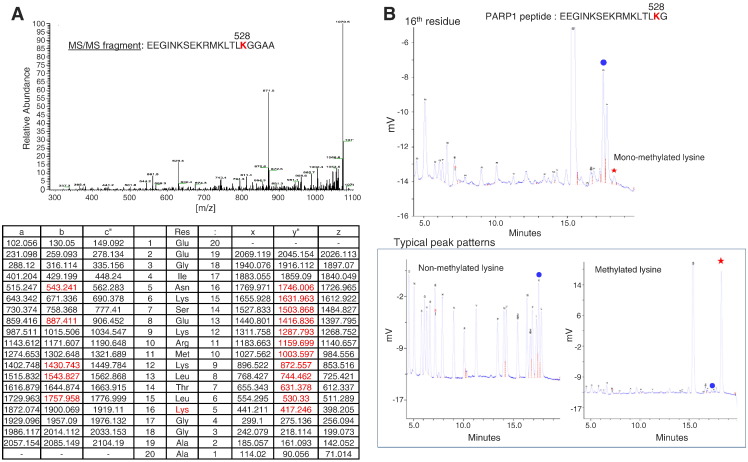
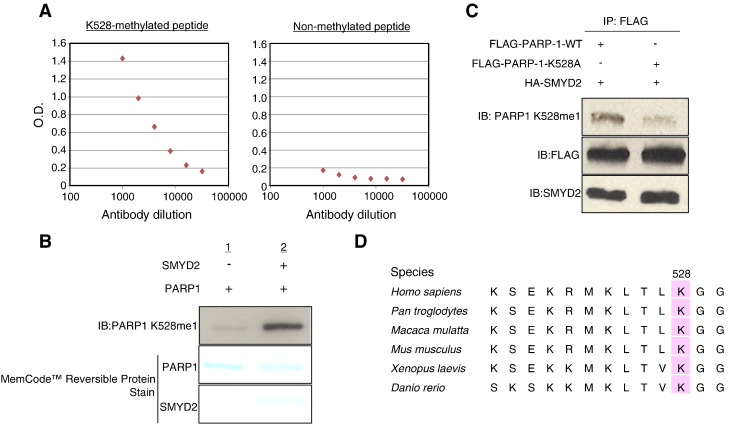
To further identify a methylation site(s) of PARP1 by SMYD2, we performed LC-MS/MS analysis and found that lysine 528 to be the methylation site by SMYD2 (Figure 3A). To validate this result, we also conducted Edman degradation analysis and confirmed the methylation of lysine 528 (Figure 3B). We subsequently generated an antibody against a synthetic peptide with mono-methylation at K528 and confirmed high affinity and specificity of the antibody by ELISA (Figure 4A). Using this specific antibody, we examined an in vitro methyltransferase assay using full-length recombinant PARP1 protein followed by western blot analysis and confirmed the increase of the methylation specific signal by treatment with SMYD2 (Figure 4B). To further verify the K528-methylation of PARP1, we prepared and transfected an expression vector for FLAG-tagged wild-type PARP1 (FLAG-PARP1-WT) or that for FLAG-tagged K528-substituted PARP1 (FLAG-PARP1-K528A) with an HA-SMYD2 expression vector into 293T cells. We then precipitated FLAG-proteins by immunoprecipitation method, and western blot analysis using the PARP1 K528-methylation antibody detected the signal in the wild-type PARP1 protein, but not in the K528-substituted PARP1 protein (Figure 4C). Taken together, SMYD2 can methylate lysine 528 on PARP1 and this methylation is observed both in vitro and in vivo. Additionally, since the lysine 528 is highly conserved from Danio rerio to Homo sapiens, it is likely that this lysine methylation might have a critical role in the function of PARP1 (Figure 4D).
Figure 3.
Lysine 528 on PARP1 is methylated by SMYD2. (A) The nano-LC-MS/MS spectrum of the specific peptide after in vitro methyltransferase assay (upper panel) and the theoretical value table of MS fragments (lower panel). The values observed were indicated in red bold. (B) Chromatograms of Edman degradation. The typical chromatograms of PTH-amino acids standard mixture (left) and α,ε−di PTH-ε-mono-methyl lysine (right) were shown in lower panel. The Edman degradation chromatogram of 16th residue of the specific peptides (amino acid residues 513–532) after in vitro methyltransferase assay was shown in upper panel. Asterisk indicated the retention time of mono-methyl lysine.
Figure 4.
Confirmation of Lys 528 methylation on PARP1 by specific antibody. (A) Determination of the titer and specificity of the anti-mono-methylated K528 PARP1 (Sigma-Aldrich) antibody analyzed by ELISA. (B) Purified His-PARP1 proteins were reacted in the presence or absence of His-SMYD2 protein with SAM. The membrane was immunoblotted with a rabbit anti-PARP1 K528me1 antibody. Amounts of loading proteins on the membrane were visualized by MemCodeTM Reversible Protein Stain (Thermo Fisher Scientific). (C) The pCAGGS-PARP1-FLAG or pCAGGS-PARP1 K528A-FLAG vector was co-transfected with the pCAGGS-SMYD2-HA vector into 293T cells. The whole cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads. Immunoprecipitants were immunoblotted with anti-PARP1 K528me1, anti-FLAG (Sigma-Aldrich) and anti-SMYD2 (Santa Cruz Biotechnology) antibodies. (D) Amino acid sequence alignment of human (Homo sapiens), common chimpanzee (Pan troglodytes), Nazuri monkey (Macaca mulatta), mouse (Mus musculus), African clawed frog (Xenopus laevis) and zebrafish (Danio renio) PARP1. Lysine 528 is conserved across these species.
SMYD2-dependent Methylation Enhances PARP1 Activity
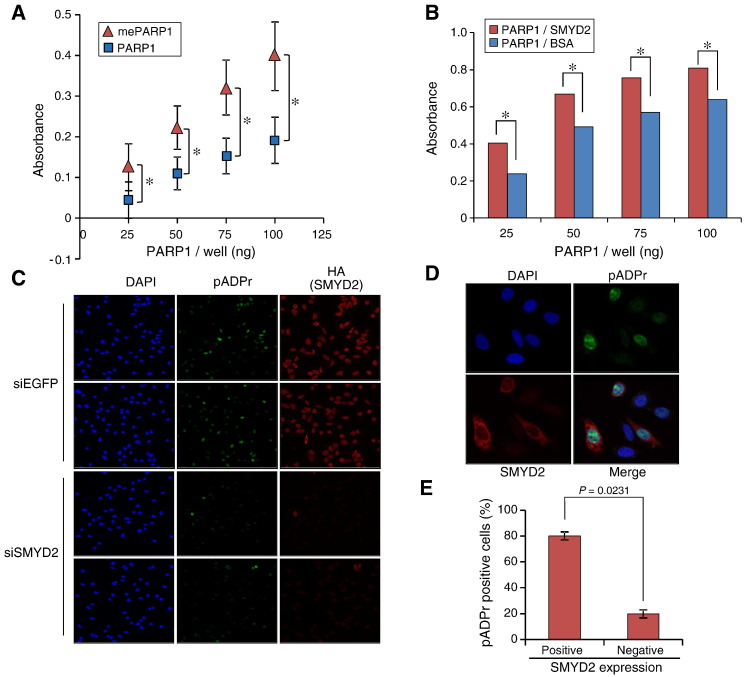
PARP1 poly(ADP-ribosyl)ation activity is well-known to be involved in multiple cellular processes. To investigate the effect of methylated PARP1 on its enzymatic activity, recombinant PARP1 was incubated with SMYD2 enzyme in the presence or absence of the methyl donor S-adenosyl-L-methionine (SAM) at 30°C for 4 h, and PARP1 activities were measured by the universal colorimetric PARP assay kit. Consequently, we observed that methylated PARP1 (with SAM) showed much higher enzymatic activities than unmethylated PARP1 (without SAM) (Figure 5A). In addition, we incubated PARP1 with SMYD2 or BSA in the presence of SAM, and analyzed PARP1 PARylation activities as described in Materials and Methods. Consistently, PARP1 protein incubated with SMYD2 exhibited the higher activities (Figure 5B), suggesting that SMYD2 mediated PARP1 methylation enhanced PARP1 enzymatic activity.
Figure 5.
SMYD2 mediated PARP1 methylation positively regulates PARP1 enzymatic activity both in vitro and in vivo. (A) PARP1 protein was incubated with SMYD2 in the presence or absence of SAM at 30°C for 4 h. The universal colorimetric PARP assay kit was used to measure PARP1 activities. Results are the mean ± SD of three independent experiments, and each experiment was performed in duplicate. X-axis represents the amount of PARP1 protein per well, Y-axis represents the absorbance of each well read at 450 nm. P-values were calculated using Student's t-test (*, P < .05). (B) PARP1 was reacted with same amount of SMYD2 or BSA in the presence of SAM, and the relative activities of PARP1 were examined by the colorimetric PARP assay kit. Results are the mean of three independent experiments. P-values were calculated using Student's t-test (*, P < .05). (C) HA-SMYD2 expressing or SMYD2 depleted HeLa cells were treated with 1 mM H2O2 for 10 min. Cells were then fixed with 4% paraformaldehyde and immunostained with an anti-poly(ADP-ribose) monoclonal antibody and an anti-HA monoclonal antibody for analysis by immunofluorescence. Immunocytochemical analysis showed much higher poly(ADP-ribose) positivity (green) in HA-SMYD2 expressing HeLa cells than SMYD2 depleted HeLa cells. (D) Each 1 × 105 HA-SMYD2 stably expressing HeLa cells and 1 × 105 SMYD2-depleted HeLa cells were mixed well and seeded into the chamber well, follow by treatment of 1 mM H2O2 for 10 min. SMYD2 overexpressing cells (red-positive) harbored much stronger signals of poly(ADP-ribose) (green) than SMYD2 depleted cells. (E) Among 250 poly(ADP-ribose) positive cells counted, nearly 80% cells showed the positive expression of SMYD2. Only 20% cells were characterized as poly(ADP-ribose)-positive/SMYD2-negative. Data show the averaged value of two independent experiments. P-values were calculated using Student's t-test.
PARP1 Methylation Enhances Cellular Response to Oxidative DNA Damage
PARP1 is activated in response to DNA damage leading to auto(ADP-ribosyl)ation and poly(ADP-ribosyl)ation by transferring the ADP-ribose from NAD+ to other receptor proteins and PARP1 itself, and plays an important role in DNA repair [38]. To elucidate whether SMYD2-dependent PARP1 methylation influences PARP1 activity in vivo, we examined poly(ADP-ribose) (PAR) synthesis in cancer cells exposed to H2O2. The HeLa cell lines constitutively overexpressing SMYD2 were transfected with siSMYD2 or siEGFP, and cultured for 72 hrs, followed by treatment of cells with 1 mM H2O2. After 10 minutes, cells were fixed by 4% paraformaldehyde immediately, and immunostained with a rabbit anti-HA antibody and a mouse anti-poly(ADP-ribose) (PAR) monoclonal antibody (Trevigen). SMYD2-depleted cells exhibited the significant reduction of poly(ADP-ribose) signals compared with those treated with siEGFP (Figure 5C), implying that poly(ADP-ribosyl)ation activity was affected by knockdown of SMYD2. Concordantly, SMYD2-overexpressing cells showed significantly higher poly(ADP-ribosyl)ation activity than control cells (Figure W1). Additionally, we mixed and seeded the equal numbers of siSMYD2- or siEGFP-treated HeLa cells, followed by treatment with H2O2. As expected, we confirmed that SMYD2-positive HeLa cells showed much higher poly(ADP-ribose) signals than SMYD2-negative HeLa cells, further supporting that PARP1 methylation is likely to enhance cellular response to H2O2 induced DNA damage (Figure 5D and E).
Discussion
The poly (ADP-ribose) polymerase (PARP) family of enzymes plays a critical role in the maintenance of DNA integrity as part of the base excision pathway of DNA repair. PARP1 is overexpressed in a variety of cancers, and its expression level has been associated with overall survival rate of cancer patients, especially in the case of breast cancer [39,40]. As a novel class of anticancer drugs, more than 40 preclinical studies or clinical trials of PARP inhibitors are ongoing [39,41-44]. In this study, we demonstrated that the oncogenic methyltransferase SMYD2 methylates lysine 528 on PARP1 and enhance PARP1 poly(ADP-ribosyl)ation enzymatic activity in cancer cells.
Increased PARP activity is known as one of the mechanisms by which cancer cells escape from the apoptosis signals induced by DNA-damaging agents [45]. To maintain genome integrity, cells possess multiple mechanisms to efficiently repair the different kinds of DNA damage, including mismatch repair (MMR), base excision repair (BER), nucleotide excision repair (NER), single-strand break repair (SSBR), double-strand break repair (DSBR), and so on [46]. Compelling the data from previous studies indicate that mutation of genes responding to DNA damage often promotes human carcinogenesis [46]. Additionally, inhibiting proteins involved in the DNA damage response can lead to increase the sensitivity of cancer cells to chemotherapy. Therefore, studies of the DNA damage response can elucidate the fundamental mechanisms triggering human carcinogenesis and provide novel strategies for cancer therapy. In this regard, since PARP plays an important role in the repair of DNA strand breaks known to be generated by radiation and chemotherapeutic drugs, inhibition of elevated PARP enzyme activity in cancer cells have potential to improve the outcome of cancer chemotherapy or radiotherapy [47,48].
According to our data, SMYD2 depletion resulted in the reduction of PARP1 enzymatic activity, suggesting that SMYD2 inhibition might have also potential to improve cancer chemotherapy. As we previously reported that SMYD2 was highly overexpressed in multiple cancer cells and its expression in normal tissues was significantly low [29], targeting this enzyme may be an ideal strategy for cancer therapy. In fact, histone methyltransferases have recently been recognized as good targets for development of cancer therapy, and inhibitors targeting some histone methyltransferases including SMYD2 have already been reported as candidates of anticancer drugs [32,49,50]. Although further functional analyses are required, a SMYD2 inhibitor has great potential to be applied to treat various types of human cancer.
Acknowledgments
We thank Dr. Hyun-Soo Cho for helpful discussion and also thank the member of Nakamura laboratory for kind support.
Footnotes
This article refers to supplementary materials, which is designated by Table W1 and Figure W1 is available online at www.neoplasia.com.
Disclosure of Potential Conflicts of Interest: L. Piao, Y. Nakamura and R. Hamamoto have research grants from Oncotherapy Science, Inc. Y. Nakamura is a stock holder and a scientific advisor of Oncotherapy Science, Inc. No potential conflicts of interest were disclosed by the other authors.
Supplementary Materials.
Table W1.
Information of certificated cell lines.
| Name | Origin | Certification institution | Tested method | DNA profile or characteristics |
|---|---|---|---|---|
| 293T | human embryonic kidney fibroblast | ATCC | STR | Amelogenin: X CSF1PO: 11, 12 D13S317: 12, 14 D16S539: 9, 13 D5S818: 8, 9 D7S820: 11 THO1: 7, 9.3 TPOX: 11 vWA: 16, 18, 19 |
| HeLa | human cervix carcinoma | ATCC | STR | Amelogenin: X,Y CSF1PO: 11,12 D13S317: 11,14 D16S539: 9,11 D5S818: 11,12 D7S820: 10,11 THO1: 8 TPOX: 8 vWA: 15 |
ATCC; American Type Culture Collection.
Figure W1.
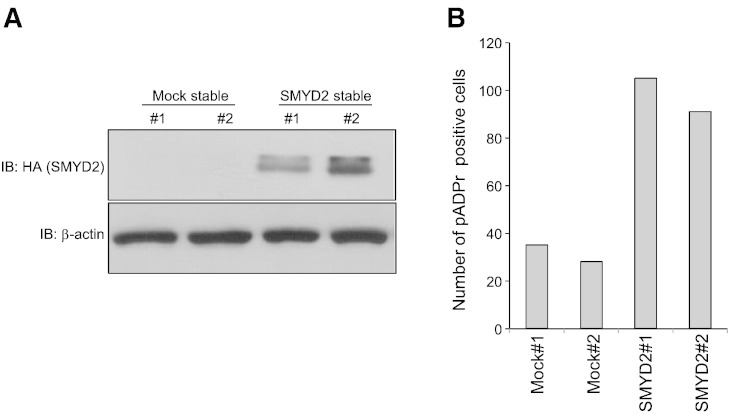
SMYD2 mediated PARP1 methylation positively regulates PARP1 enzymatic activity. (A) Two lots of mock-transfected HeLa cells (Mock stable#1 and #2)and SMYD2-transfected HeLa cells (SMYD2 stable #1 and #2) were lysed and fractionated by SDS-PAGE. Samples were immunoblotted with anti-HA (Y-11; Santa Cruz)and anti-β-actin (AC-15; SIGMA)antibodies.(B)Number of pADPr positive cells in Mock stable cells (#1 and #2) and SMYD2 stable cells. Each 1 x 105 Mock stables cells and 1 x 105 SMYD2 stable cells were seeded into the chamber well, followedby treatment of cells with1 mM H2O2 for 15min.Immunocytochemical analysis showed much higher poly(ADP-ribose) positivity in SMYD2 stable cells than Mock stable cells.
References
- 1.Kameshita I., Matsuda Z., Taniguchi T., Shizuta Y. Poly (ADP-Ribose) synthetase. Separation and identification of three proteolytic fragments as the substrate-binding domain, the DNA-binding domain, and the automodification domain. J Biol Chem. 1984;259:4770–4776. [PubMed] [Google Scholar]
- 2.Ogata N., Ueda K., Kawaichi M., Hayaishi O. Poly(ADP-ribose) synthetase, a main acceptor of poly(ADP-ribose) in isolated nuclei. J Biol Chem. 1981;256:4135–4137. [PubMed] [Google Scholar]
- 3.de Murcia G., Menissier de Murcia J. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci. 1994;19:172–176. doi: 10.1016/0968-0004(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 4.Audebert M., Salles B., Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279:55117–55126. doi: 10.1074/jbc.M404524200. [DOI] [PubMed] [Google Scholar]
- 5.Audebert M., Salles B., Weinfeld M., Calsou P. Involvement of polynucleotide kinase in a poly(ADP-ribose) polymerase-1-dependent DNA double-strand breaks rejoining pathway. J Mol Biol. 2006;356:257–265. doi: 10.1016/j.jmb.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Dantzer F., de La Rubia G., Menissier-De Murcia J., Hostomsky Z., de Murcia G., Schreiber V. Base excision repair is impaired in mammalian cells lacking Poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39:7559–7569. doi: 10.1021/bi0003442. [DOI] [PubMed] [Google Scholar]
- 7.D'Silva I., Pelletier J.D., Lagueux J., D'Amours D., Chaudhry M.A., Weinfeld M., Lees-Miller S.P., Poirier G.G. Relative affinities of poly(ADP-ribose) polymerase and DNA-dependent protein kinase for DNA strand interruptions. Biochim Biophys Acta. 1999;1430:119–126. doi: 10.1016/s0167-4838(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 8.Durkacz B.W., Omidiji O., Gray D.A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980;283:593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- 9.Wang M., Wu W., Wu W., Rosidi B., Zhang L., Wang H., Iliakis G. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006;34:6170–6182. doi: 10.1093/nar/gkl840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng C.L., Johnson S.P., Keir S.T., Quinn J.A., Ali-Osman F., Szabo C., Li H., Salzman A.L., Dolan M.E., Modrich P. Poly(ADP-ribose) polymerase-1 inhibition reverses temozolomide resistance in a DNA mismatch repair-deficient malignant glioma xenograft. Mol Cancer Ther. 2005;4:1364–1368. doi: 10.1158/1535-7163.MCT-05-0128. [DOI] [PubMed] [Google Scholar]
- 11.Tentori L., Leonetti C., Scarsella M., D'Amati G., Vergati M., Portarena I., Xu W., Kalish V., Zupi G., Zhang J. Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin Cancer Res. 2003;9:5370–5379. [PubMed] [Google Scholar]
- 12.Hoeijmakers J.H. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 13.Longley D.B., Johnston P.G. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 14.Calvert A.H., Plummer R. The development of phase I cancer trial methodologies: the use of pharmacokinetic and pharmacodynamic end points sets the scene for phase 0 cancer clinical trials. Clin Cancer Res. 2008;14:3664–3669. doi: 10.1158/1078-0432.CCR-07-4559. [DOI] [PubMed] [Google Scholar]
- 15.Plummer R., Jones C., Middleton M., Wilson R., Evans J., Olsen A., Curtin N., Boddy A., McHugh P., Newell D. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2008;14:7917–7923. doi: 10.1158/1078-0432.CCR-08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagne J.P., Moreel X., Gagne P., Labelle Y., Droit A., Chevalier-Pare M., Bourassa S., McDonald D., Hendzel M.J., Prigent C. Proteomic investigation of phosphorylation sites in poly(ADP-ribose) polymerase-1 and poly(ADP-ribose) glycohydrolase. J Proteome Res. 2009;8:1014–1029. doi: 10.1021/pr800810n. [DOI] [PubMed] [Google Scholar]
- 17.Hassa P.O., Haenni S.S., Buerki C., Meier N.I., Lane W.S., Owen H., Gersbach M., Imhof R., Hottiger M.O. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005;280:40450–40464. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- 18.Martin N., Schwamborn K., Schreiber V., Werner A., Guillier C., Zhang X.D., Bischof O., Seeler J.S., Dejean A. PARP-1 transcriptional activity is regulated by sumoylation upon heat shock. EMBO J. 2009;28:3534–3548. doi: 10.1038/emboj.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T., Simbulan-Rosenthal C.M., Smulson M.E., Chock P.B., Yang D.C. Polyubiquitylation of PARP-1 through ubiquitin K48 is modulated by activated DNA, NAD +, and dipeptides. J Cell Biochem. 2008;104:318–328. doi: 10.1002/jcb.21624. [DOI] [PubMed] [Google Scholar]
- 20.Altmeyer M., Messner S., Hassa P.O., Fey M., Hottiger M.O. Molecular mechanism of poly(ADP-ribosyl)ation by PARP1 and identification of lysine residues as ADP-ribose acceptor sites. Nucleic Acids Res. 2009;37:3723–3738. doi: 10.1093/nar/gkp229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai H.C., Baylin S.B. Cancer epigenetics: linking basic biology to clinical medicine. Cell Res. 2011;21:502–517. doi: 10.1038/cr.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamamoto R., Furukawa Y., Morita M., Iimura Y., Silva F.P., Li M., Yagyu R., Nakamura Y. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat Cell Biol. 2004;6:731–740. doi: 10.1038/ncb1151. [DOI] [PubMed] [Google Scholar]
- 23.Hamamoto R., Silva F.P., Tsuge M., Nishidate T., Katagiri T., Nakamura Y., Furukawa Y. Enhanced SMYD3 expression is essential for the growth of breast cancer cells. Cancer Sci. 2006;97:113–118. doi: 10.1111/j.1349-7006.2006.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takawa M., Cho H.S., Hayami S., Toyokawa G., Kogure M., Yamane Y., Iwai Y., Maejima K., Ueda K., Masuda A. Histone Lysine Methyltransferase SETD8 Promotes Carcinogenesis by Deregulating PCNA Expression. Cancer Res. 2012;72:3217–3227. doi: 10.1158/0008-5472.CAN-11-3701. [DOI] [PubMed] [Google Scholar]
- 25.Takawa M., Masuda K., Kunizaki M., Daigo Y., Takagi K., Iwai Y., Cho H.S., Toyokawa G., Yamane Y., Maejima K. Validation of the histone methyltransferase EZH2 as a therapeutic target for various types of human cancer and as a prognostic marker. Cancer Sci. 2011;102:1298–1305. doi: 10.1111/j.1349-7006.2011.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyokawa G., Cho H.S., Masuda K., Yamane Y., Yoshimatsu M., Hayami S., Takawa M., Iwai Y., Daigo Y., Tsuchiya E. Histone Lysine Methyltransferase Wolf-Hirschhorn Syndrome Candidate 1 Is Involved in Human Carcinogenesis through Regulation of the Wnt Pathway. Neoplasia. 2011;13:887–898. doi: 10.1593/neo.11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abu-Farha M., Lambert J.P., Al-Madhoun A.S., Elisma F., Skerjanc I.S., Figeys D. The tale of two domains: proteomics and genomics analysis of SMYD2, a new histone methyltransferase. Mol Cell Proteomics. 2008;7:560–572. doi: 10.1074/mcp.M700271-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Brown M.A., Sims R.J., 3rd, Gottlieb P.D., Tucker P.W. Identification and characterization of Smyd2: a split SET/MYND domain-containing histone H3 lysine 36-specific methyltransferase that interacts with the Sin3 histone deacetylase complex. Mol Cancer. 2006;5:26. doi: 10.1186/1476-4598-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho H.S., Hayami S., Toyokawa G., Maejima K., Yamane Y., Suzuki T., Dohmae N., Kogure M., Kang D., Neal D.E. RB1 Methylation by SMYD2 Enhances Cell Cycle Progression through an Increase of RB1 Phosphorylation. Neoplasia. 2012;14:476–486. doi: 10.1593/neo.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J., Perez-Burgos L., Placek B.J., Sengupta R., Richter M., Dorsey J.A., Kubicek S., Opravil S., Jenuwein T., Berger S.L. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006;444:629–632. doi: 10.1038/nature05287. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu S., Imoto I., Tsuda H., Kozaki K.I., Muramatsu T., Shimada Y., Aiko S., Yoshizumi Y., Ichikawa D., Otsuji E. Overexpression of SMYD2 relates to tumor cell proliferation and malignant outcome of esophageal squamous cell carcinoma. Carcinogenesis. 2009;30:1139–1146. doi: 10.1093/carcin/bgp116. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson A.D., Larsen N.A., Howard T., Pollard H., Green I., Grande C., Cheung T., Garcia-Arenas R., Cowen S., Wu J. Structural basis of substrate methylation and inhibition of SMYD2. Structure (London, England: 1993) 2011;19:1262–1273. doi: 10.1016/j.str.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Hayami S., Kelly J.D., Cho H.S., Yoshimatsu M., Unoki M., Tsunoda T., Field H.I., Neal D.E., Yamaue H., Ponder B.A. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int J Cancer. 2011;128:574–586. doi: 10.1002/ijc.25349. [DOI] [PubMed] [Google Scholar]
- 34.Cho H.S., Shimazu T., Toyokawa G., Daigo Y., Maehara Y., Hayami S., Ito A., Masuda K., Ikawa N., Field H.I. Enhanced HSP70 lysine methylation promotes proliferation of cancer cells through activation of Aurora kinase B. Nat Commun. 2012;3:1072. doi: 10.1038/ncomms2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda A., Dohmae N. Amino acid analysis of sub-picomolar amounts of proteins by precolumn fluorescence derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. Biosci Trends. 2011;5:231–238. doi: 10.5582/bst.2011.v5.6.231. [DOI] [PubMed] [Google Scholar]
- 36.Masuda A., Dohmae N. Examination of an absolute quantity of less than a hundred nanograms of proteins by amino acid analysis. Anal Bioanal Chem. 2013;405:8073–8081. doi: 10.1007/s00216-013-7056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piao L., Nakagawa H., Ueda K., Chung S., Kashiwaya K., Eguchi H., Ohigashi H., Ishikawa O., Daigo Y., Matsuda K. C12orf48, termed PARP-1 binding protein, enhances poly(ADP-ribose) polymerase-1 (PARP-1) activity and protects pancreatic cancer cells from DNA damage. Genes Chromosomes Cancer. 2011;50:13–24. doi: 10.1002/gcc.20828. [DOI] [PubMed] [Google Scholar]
- 38.Maynard S., Schurman S.H., Harboe C., de Souza-Pinto N.C., Bohr V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kummar S., Chen A., Parchment R.E., Kinders R.J., Ji J., Tomaszewski J.E., Doroshow J.H. Advances in using PARP inhibitors to treat cancer. BMC Med. 2012;10:25. doi: 10.1186/1741-7015-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojo F., Garcia-Parra J., Zazo S., Tusquets I., Ferrer-Lozano J., Menendez S., Eroles P., Chamizo C., Servitja S., Ramirez-Merino N. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann Oncol. 2012;23:1156–1164. doi: 10.1093/annonc/mdr361. [DOI] [PubMed] [Google Scholar]
- 41.Khan O.A., Gore M., Lorigan P., Stone J., Greystoke A., Burke W., Carmichael J., Watson A.J., McGown G., Thorncroft M. A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours. Br J Cancer. 2011;104:750–755. doi: 10.1038/bjc.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Audeh M.W., Carmichael J., Penson R.T., Friedlander M., Powell B., Bell-McGuinn K.M., Scott C., Weitzel J.N., Oaknin A., Loman N. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 43.Kummar S., Chen A., Ji J., Zhang Y., Reid J.M., Ames M., Jia L., Weil M., Speranza G., Murgo A.J. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 2011;71:5626–5634. doi: 10.1158/0008-5472.CAN-11-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heitz F., Harter P., Ewald-Riegler N., Papsdorf M., Kommoss S., du Bois A. Poly(ADP-ribosyl)ation polymerases: mechanism and new target of anticancer therapy. Expert Rev Anticancer Ther. 2010;10:1125–1136. doi: 10.1586/era.10.53. [DOI] [PubMed] [Google Scholar]
- 45.Peralta-Leal A., Rodriguez M.I., Oliver F.J. Poly(ADP-ribose)polymerase-1 (PARP-1) in carcinogenesis: potential role of PARP inhibitors in cancer treatment. Clin Transl Oncol. 2008;10:318–323. doi: 10.1007/s12094-008-0207-8. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Wang F., Tang T., Guo C. The role of PARP1 in the DNA damage response and its application in tumor therapy. Front Med. 2012;6:156–164. doi: 10.1007/s11684-012-0197-3. [DOI] [PubMed] [Google Scholar]
- 47.Southan G.J., Szabo C. Poly(ADP-ribose) polymerase inhibitors. Curr Med Chem. 2003;10:321–340. doi: 10.2174/0929867033368376. [DOI] [PubMed] [Google Scholar]
- 48.Beneke S., Diefenbach J., Burkle A. Poly(ADP-ribosyl)ation inhibitors: promising drug candidates for a wide variety of pathophysiologic conditions. Int J Cancer. 2004;111:813–818. doi: 10.1002/ijc.20342. [DOI] [PubMed] [Google Scholar]
- 49.Knutson S.K., Wigle T.J., Warholic N.M., Sneeringer C.J., Allain C.J., Klaus C.R., Sacks J.D., Raimondi A., Majer C.R., Song J. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol. 2012;8:890–896. doi: 10.1038/nchembio.1084. [DOI] [PubMed] [Google Scholar]
- 50.McCabe M.T., Ott H.M., Ganji G., Korenchuk S., Thompson C., Van Aller G.S., Liu Y., Graves A.P., Della Pietra A., III, Diaz E. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]