Abstract
Purpose
We retrospectively studied the efficacy and safety of different surgical approaches to treating pediatric limbal dermoids with regard to intra and postoperative complications.
Methods
The data of 12 consecutive patients (14 eyes), who underwent monocentric surgery of a limbal demoid in the past 9 years, were retrospectively analyzed for intra and postoperative complications. Group one consists of eleven eyes: seven eyes with a bare-sclera deep lamellar excision of the dermoid and four eyes with an additional amniotic membrane transplantation subsequent to excision. Group two consists of three eyes: two eyes treated with Mitomycin C 0.02% over 2 min following the excision, and one eye treated with Mitomycin C and amniotic membrane transplantation after removal of the dermoid. Follow-up ranged from 2 to 53 months (median 17 months).
Results
Intraoperative complications did not occur in any of the patients. Postoperatively, all patients showed corneal reepithelialization within a week. Limbal stem cell deficiency with a pseudopterygium developed in four eyes, one treated with bare-sclera excision and the others with amniotic membrane transplantation. One pseudopterygium had to be removed surgically because of visual acuity deterioration. Not a single eye treated with Mitomycin C developed a pseudopterygium.
Conclusions
The transplantation of amniotic membrane following removal of a limbal dermoid cannot prevent the occurrence of a pseudopterygium. However, the use of Mitomycin C seems to have a protective effect.
Introduction
Limbal dermoids rank among the most common tumors of the corneal limbus.1 They are ocular choristomas and can occur in a variety of sizes ranging from only small lesions to larger masses in the epibulbar region.2 Epibulbar dermoids are classified into three grades. Grade I limbal or epibulbar dermoid are lesions with a superficial tumor measuring <5 mm. Grade II limbal dermoids are of larger size and extend into the corneal stroma down to Descemet's membrane. Grade III limbal dermoids involve the whole cornea and stuctures of the anterior chamber. The most common location for epibulbar dermoids is the temporal inferior quadrant of the limbus.3 Though being a benign tumor, the removal of a limbal dermoid is not only performed to improve the cosmetic appearance of the eye but more importantly to prevent loss of visual acuity. Vision is often impaired from astigmatic refractive errors caused by corneal astigmatism. This may lead to irreversible anisometropic amblyopia in children.4, 5 Large dermoids can also lead to surface irritation and discomfort or even central corneal opacification.
The appropriate time of intervention and the best surgical technique for removal of the pediatric limbal dermoids are subject to discussion. In the past, several different surgical techniques for the removal of dermoids have been described.6 These techniques include bare excision, amniotic membrane transplantation, and even lamellar and penetrating keratoplasty. The adequate choice depends on the location and size of the lesion.
Major risks of the excision of the limbal dermoid are intraoperative perforation, postoperative epithelial defects and peripheral vascularization of the cornea.7 Lamellar keratoplasty is reported to result in the improvement of visual acuity, but may also lead to an increased corneal astigmatism.8, 9 In order to avoid scarring and conjunctivalization of the cornea, two different possibilities are described.
(1) Covering of the defect: several reports describe the possibility to combine the excision of the dermoid with placement of different kinds of tissue on the ocular surface. Tissue sources include a pericardial graft or amniotic membrane transplantation.10, 11, 12 The fibrin-glued multilayered amniotic membrane transplantation was discussed to improve postoperative reepithelialization, prevent post-operative scarring, and protect the limbal stem cells.11, 12
(2) The use of Mitomycin C: Mitomycin C as an antitumor antibiotic was first isolated from Streptomyces caespitosus in 1958.13 It is used in pterygium surgery since 1963 in order to reduce the recurrence rates.14 This seems to be achieved by the inhibition of fibroplast proliferation at the level of the episclera.15, 16 Therefore, in our opinion the use of Mitomycin C can be beneficial in the treatment of limbal dermoids in matters of postoperative complications.
The goal of our study was to compare the efficacy of Mitomycin C in surgical treatment of limbal dermoids.
Patients and methods
The data with respect to efficacy and intra and postoperative safety of the removal of limbal dermoids were assessed in a monocentric, retrospective trial. All patients had been treated consecutively in the past 9 years. We included a total of 14 eyes of 12 consecutive patients who had undergone a removal of a limbal dermoid.
Seven patients were male and five were female (Table 1). Three patients had a diagnosis of Goldenhar syndrome. Thirteen limbal dermoids were located in the temporal inferior quadrant of the eye (Figures 1a and 2a), one was in the upper quadrant. All limbal dermoids were classified as grade one.
Table 1. Patients and surgical techniques.
| Surgical technique | BCVA preOP | BCVA postOP | Complications | Follow-up postOP | Miscellaneous |
|---|---|---|---|---|---|
| Group one: plain removal | |||||
| Patient 1 | 0.3 | 0.5 | None | 20 months | |
| Patient 2 | 0.9 | 0.8 | Pseudopterygium | 4 months | Goldenhar syndrome |
| Patient 3 | 1 | 0.8 | Corneal opacification | 3 months | |
| Patient 4 | 0.05 | 0.05 | None | 3 days | |
| Patient 5 | 1.25 | 0.9 | None | 1 day | |
| Patient 6 | 0.3 | 0.1 | None | 1 day | |
| Patient 7 | 0.8 | k.A. | None | 1 day | |
| Removal and amniotic membrane | |||||
| Patient 8 OD | 0.6 | 0.5 | Pseudopterygium | 27 months | Removal of pseudopterygium with MMC 17 months after first surgery |
| Patient 8 OS | 0.8 | 0.63 | Pseudopterygium | 26 months | Goldenhar syndrome |
| Patient 9 | 0.4 | 0.6 | Pseudopterygium | 2 months | |
| Patient 10 | NA | NA | None | 28 days | |
| Group two: removal and mitomycon C | |||||
| Patient 11 OD | 0.5 | 0.4 | None | 53 months | Goldenhar syndrome |
| Patinet 11 OS | 0.8 | 1 | None | 45 months | Goldenhar syndrome |
| Patient 12 | 1.25 | 1 | None | 17 months | Additional amniotic membrane transplantation |
The table contains all patients; only patients with a follow-up of >2 months were included in the analysis of postoperative complications.
Figure 1.
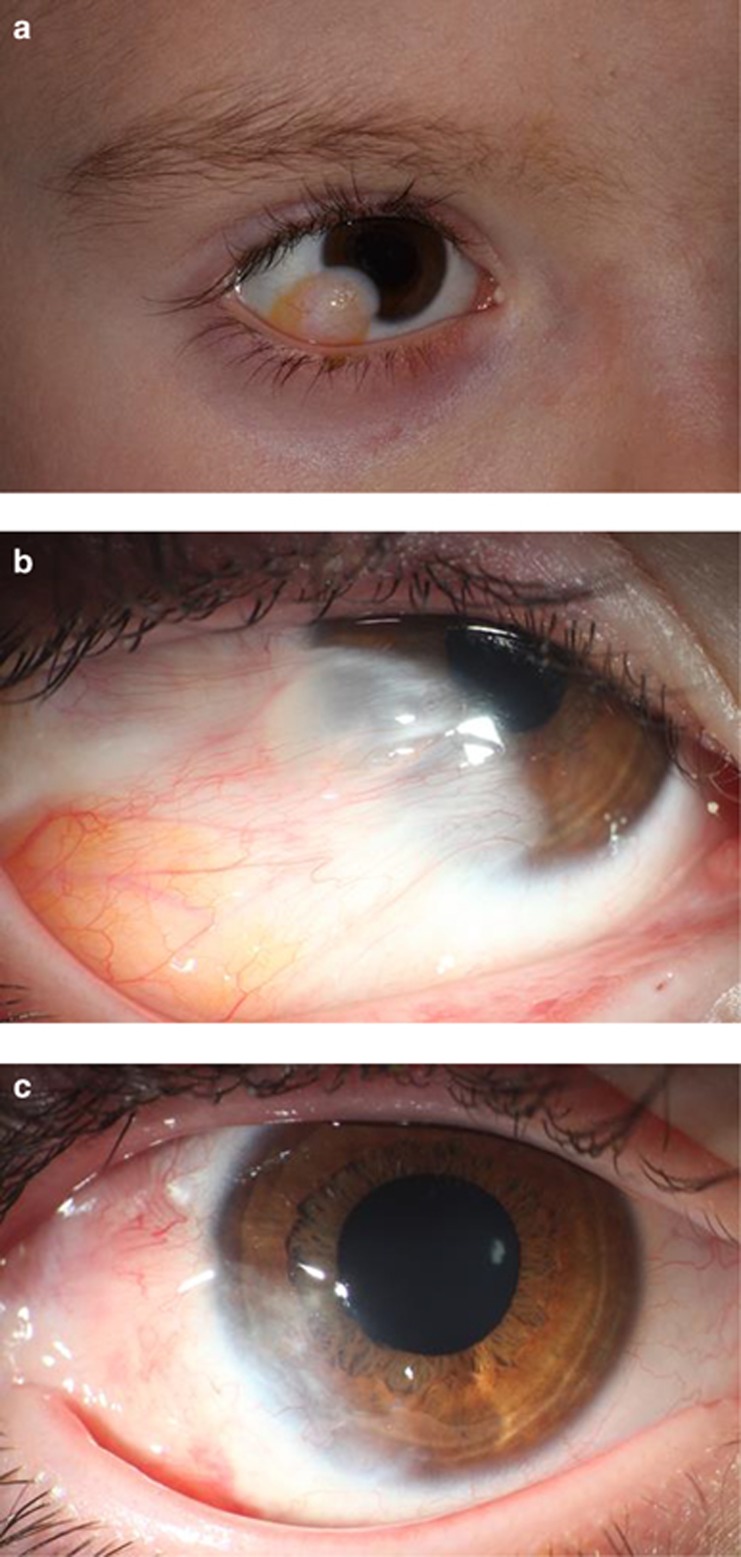
(a) Limbal dermoid before surgery (Patient 8). (b) Pseudopterygium 4 months after initial surgery with amniotic membrane (Patient 8). (c) After removal of the pseudopterygium (Patient 8).
Figure 2.
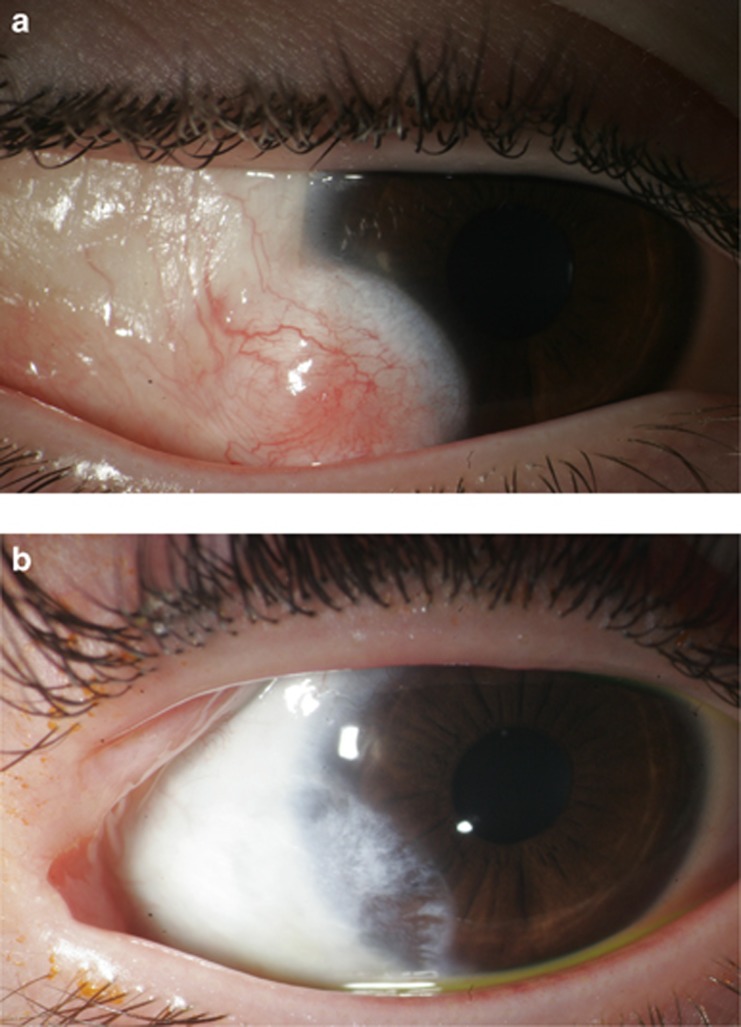
(a) Limbal dermoid before surgery (Patient 11). (b) After removal of the limbal dermoid with use of Mitomycin C (Patient 11).
The indication for the surgery was an increase in the size of the dermoid in three patients. In two patients, the indication for excision was an increasing astigmatism and visual deterioration. Seven patients were afflicted with discomfort of the affected eye. Three patients suffered from strabism associated with amblyopia. One of these patients also had a central opacification of the cornea.
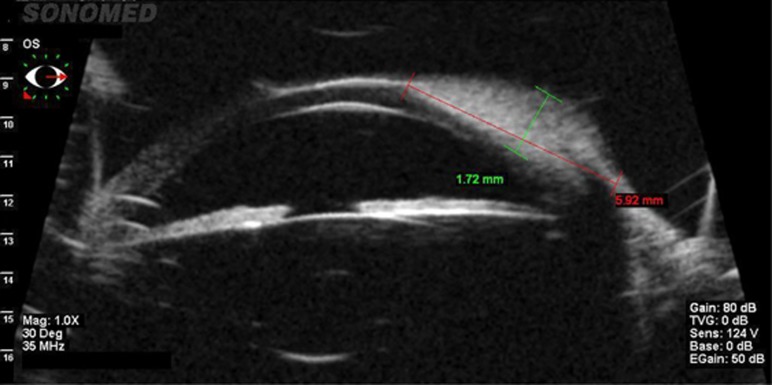
Preoperative evaluation of the limbal dermoid included measurement of best corrected visual acuity, slit-lamp examination and ophthalmoscopy. Ultrasound biomicroscopy (SonoMed VuMax 35 mHz; SonoMed Escalon, Lake Success, NY, USA) (Figure 3) could be obtained in six of the patients (seven eyes). However, the depth of the dermoid could not be definitely measured with this technique. All surgeries were performed by the same surgeon.
Figure 3.
Ultrasound biomicroscopy (Frequency 35 mHz) of a limbal dermoid: Difficult evaluation of the depth (Patient 8).
The surgical techniques were divided in two groups:
Group one included eleven eyes. The mean age at the time of the surgery was 4.4 years (lower quartile: 3.8; upper quartile: 8.9). Seven eyes were treated by removal of the limbal dermoid by lamellar excision without further procedures. At first, the border of the limbal dermoid was marked using a trepan or a diamond blade. Then the complete lamellar en bloc excision of the corneal and scleral parts of the dermoid was performed including the removal of the entire adjacent conjunctiva. Finally a smoothing of the corneal surface was performed if necessary.
Amniotic membrane transplantation was carried out in four eyes. The amniotic membrane was prepared at our hospital, stored at −80 °C on a Sartolon Polyamid Filter by Sartorius Stedim biotech (Sartorius AG, Göttingen, Germany) in a culture medium containing Gycerlol, Streptomycin, penicillin, L-Glutamin, Amphotericin B, HEPES Buffer, NaHCO3, and MEM-Earles's. The amniotic membrane was grafted as single layer and fixed with multiple sutures of Vicryl 7.0 after the removal of the dermoid. Amniotic membrane only covered the sclera and the cornea at area of the excision. All sutures were removed within 2 weeks after surgery.
Group two consisted of three eyes that had been additionally treated with Mitomycin C 0.02% (Medac GmbH, Wedel, Germany). The mean age at the time of the surgery was 4.4 years (lower quartile: 3.8; upper quartile: 8.9). We exposed the bare sclera for 2 min after the complete removal of the dermoid. One of these eyes with treatment with Mitomycin C 0.02% over 2 min had supplemental amniotic membrane transplantation.
Postoperative treatment included antibiotic eye drops and artificial tear eye drops until reepithelialization occurred. Sutures were removed within 2 weeks. Pre and postoperative photographical data were available for all patients (Figures 1a and b, and 2a and b). When applicable, we assessed the best corrected visual acuity at each follow-up. All excised limbal dermoids underwent histological examination.
For the evaluation of postoperative complications, only the patients with a follow-up of >2 months were included. We estimated the rate of pseudopterygium formation with the Kaplan–Meier method. We also tested the difference in pseudopterygium formation between the Mitomycin C-treated and the conventional group for statistical significance by means of the log-rank test. This test was intended to be interpreted in a descriptive sense owing to the lack of an a priori statistical power calculation.
Results
There was no statistical difference between the groups concerning age (P=0.344). We observed no intraoperative complications. A complete removal of the dermoid was achieved in all but one of the operated eyes.
Reepithelialization was complete after 1 week the latest in all eyes.
The histopathological examination confirmed the diagnosis of a solid limbal dermoid in all cases. Squamous epithelium, hair follicles, and sebaceous glands were unanimously present in the specimen.
For the evaluation of postoperative complications, the assessed patients had a postoperative follow-up from 2 to 53 months. Median follow-up was 17 months.
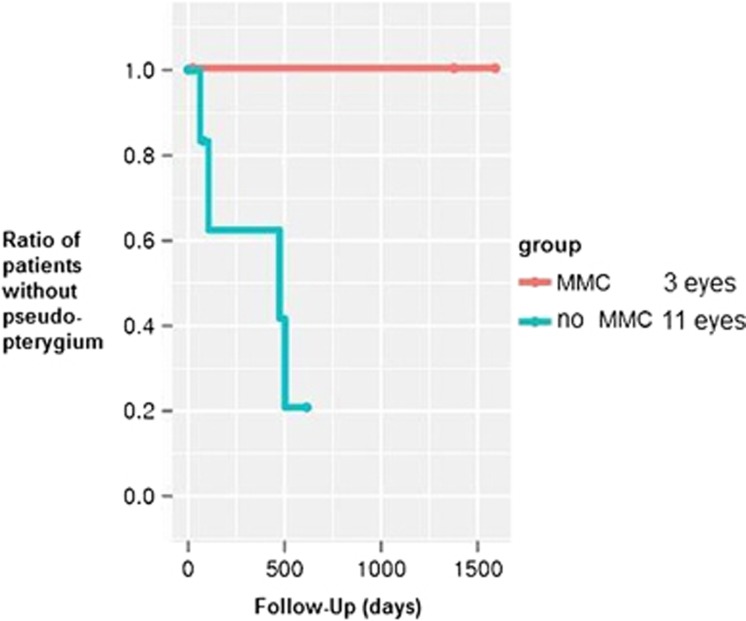
A total of four eyes from group one developed a limbal stem cell deficiency within 2–16 months, resulting in a pseudopterygium formation (Figure 1b). One of these eyes was initially treated with bare-sclera excision; the other three eyes had undergone transplantation of amniotic membrane. One eye had to undergo surgery of the pseudopterygium because of visual loss due to an increasing astigmatism. A removal of the pseudopterygium with the intraoperative use of Mitomycin C 0.02% for 2 min and the transplantation of amniotic membrane were performed (Figure 1c). No eye treated with Mitomycin C developed a postoperative pseudopterygium (Figure 4). The difference between the groups was not statistically different in the log-rank test (P=0.053). Temporary signs of ischemia after treatment with Mitomycin C (Figure 2b) resolved completely and did not pose any further complications.
Figure 4.
Ratio of patients without pseudopterygium in the groups with or without MMC.
Mean visual acuity was 0.21 logMAR in group one and 0.0 logMAR in group two. There was no statistical difference (P=0.18). Postoperative astigmatism showed −0.25 D in group one and −4.25 D in group two. This also did not show statistical significance (P=0.56).
Discussion
The method of choice to treat a limbal dermoid is surgical excision.1, 2, 3, 4, 5, 6, 7
The use of amniotic membrane transplantation in the removal of a limbal dermoid has recently been described by others.10, 12, 13 The main reasons for using amniotic membrane transplantation on conjunctival defects are the positive effects on reepithelialization and inhibition of postoperative inflammation, neovascularisation, and fibrosis.17
Pirouzian et al11 reported that the purpose of amniotic membrane transplantation was primarily to achieve a volumetric filling of the stromal and epithelial defect after the removal of a limbal dermoid. They also anticipated reduced scarring due to faster reepithelialization. Reduced inflammation with consecutive protection of the limbal stem cells is another commonly assumed mechanism of action.11, 12 A removal of a limbal dermoid alone can lead to a limbal stem cell deficiency and pseudopterygium formation.18 Therefore, stem cell transplantation may be a more beneficial approach.19, 20 However, we decided to choose a less invasive technique, namely intraoperative application of Mitomycin C with or without amniotic membrane transplantation. Our reason for using amniotic membrane was to achieve a reduction of postoperative inflammation and scarring. The use of Mitomycin C in our patients was based on the positive results from limbokeratoplasty in limbal stem cell deficiency.19 We hypothesize that Mitomycin C inhibits fibroblast growth and consequently the occurrence of a pseudopterygium or corneal neovascularization. The inhibition of fibroblast growth by Mitomycin C is also reported in the treatment of a primary pterygium, a recurrent pterygium, and in filtering bleb surgery.14, 15, 16 Regarding our data, three out of four limbal stem cell deficiencies occurred in eyes, which had amniotic membrane transplantation after removal of the limbal dermoid. Therefore, we did not observe a protective effect of amniotic membrane transplantation regarding the limbal stem cells or the growth of a postoperative pseudopterygium. The poor outcome of amniotic membrane transplantation in our study stands in contrast to the previous reports.10, 12, 13 All eyes treated with Mitomycin C, however, showed no development of a pseudopterygium during the follow-up. Therefore, a beneficial effect of Mitomycin C on postoperative pseudopterygium formation has to be considered likely.
The main limitation of our trial is the small number of cases that forecloses adequate statistical reasoning from our results. In addition, the patients need a longer postoperative follow-up until a final evaluation is possible. This is especially true for our five patients with short follow-up, which could either not be contacted owing to unknown address or were not available for another examination. Therefore, the use of Mitomycin C in the removal of limbal dermoids and the transplantation of amniotic membrane should be evaluated in a larger, prospective and controlled clinical trial. Nevertheless, our preliminary results are promising, and we believe that Mitomycin C is a viable option in limbal dermoid surgery.
The authors declare no conflict of interest.
References
- Sunderraj PP, Viswanathan RK, Balachander R. Neoplasms of the limbus. Indian J Ophthalmol. 1991;39:168–169. [PubMed] [Google Scholar]
- Mansour AM, Barber JC, Reinecke RD, Wang FM. Ocular choristomas. Review Surv Ophthalmol. 1989;33:339–358. doi: 10.1016/0039-6257(89)90011-8. [DOI] [PubMed] [Google Scholar]
- Sommer F, Pillunat LE. Epibulbäre Dermoide. Klin Monatsbl Augenheilkd. 2004;221:872–877. doi: 10.1055/s-2004-813599. [DOI] [PubMed] [Google Scholar]
- Burillon C, Durand L. Solid dermoids of the limbus and the cornea. Ophthalmologica. 1997;211:367–372. doi: 10.1159/000310832. [DOI] [PubMed] [Google Scholar]
- Robb RM. Astigmatic refractive errors associated with limbal dermoids. J Pediatr Ophthalmol Strabismus. 1996;33:241–243. doi: 10.3928/0191-3913-19960701-08. [DOI] [PubMed] [Google Scholar]
- Stergiopoulos P, Link B, Naumann GO, Seitz B. Solid corneal dermoids and subconjunctival lipodermoids: impact of differentiated surgical therapy on the functional long-term outcome. Cornea. 2009;28 (6:644–651. doi: 10.1097/ICO.0b013e3181914305. [DOI] [PubMed] [Google Scholar]
- Panton RW, Sugar J. Excision of limbal dermoids. Ophthalmic Surg. 1991;22:85–89. [PubMed] [Google Scholar]
- Scott JA, Tan DT. Therapeutic lamellar keratoplasty for limbal dermoids. Ophthalmology. 2001;108:185818–185867. doi: 10.1016/s0161-6420(01)00705-9. [DOI] [PubMed] [Google Scholar]
- Watts P, Michaeli-Cohen A, Abdolell M, Rootman D. Outcome of lamellar keratoplasty for limbal dermoids in children. J AAPOS. 2002;6:209–215. doi: 10.1067/mpa.2002.124651. [DOI] [PubMed] [Google Scholar]
- Lazzaro DR, Coe R. Repair of limbal dermoid with excision and placement of a circumlimbal pericardial graft. Eye Contact Lens. 2010;36:228–229. doi: 10.1097/icl.0b013e3181e465bf. [DOI] [PubMed] [Google Scholar]
- Pirouzian A, Holz H, Merrill K, Sudesh R, Karlen K. Surgical management of pediatric limbal dermoids with sutureless amniotic membrane transplantation and augmentation. J Pediatr Ophthalmol Strabismus. 2011;30:1–6. doi: 10.3928/01913913-20110823-01. [DOI] [PubMed] [Google Scholar]
- Pirouzian A, Ly H, Holz H, Sudesh R, Chuck R. Fibrin-glue assisted multilayered amniotic membrane transplantation in surgical management of pediatric corneal limbal dermoid: a novel approach. Graefes Arch Clin Exp Ophthalmol. 2011;249:261–265. doi: 10.1007/s00417-010-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakaki S, Marumo H, Tomioka K. Isolation of new fractions of antitumor mitomycins. Antibiot Chemother. 1958;8:228–240. [PubMed] [Google Scholar]
- Cheng HC, Tseng SH, Kao PL, Chen FK. Low-dose intraoperative mitomycin C as chemoadjuvant for pterygium surgery. Cornea. 2001;20:24–29. doi: 10.1097/00003226-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Hutchinson AmyK, Grossniklaus HansE, Brown ReayH, McManus PaulE, Bradley CharlesK. Clinicopathologic features of excised mitomycin filtering blebs. Arch Ophthalmol. 1994;112:74–79. doi: 10.1001/archopht.1994.01090130084023. [DOI] [PubMed] [Google Scholar]
- Fakhry MA. The use of mitomycin C with autologous limbal-conjunctival autograft transplantation for management of recurrent pterygium. Clin Ophthalmol. 2011;26:123–127. doi: 10.2147/OPTH.S16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005;16:233–240. doi: 10.1097/01.icu.0000172827.31985.3a. [DOI] [PubMed] [Google Scholar]
- Mohan M, Mukherjee G, Panda A. Clinical evaluation and surgical intervention of limbal dermoid. Indian J Ophthalmol. 1981;29:69–73. [PubMed] [Google Scholar]
- Eberwein P, Böhringer D, Schwartzkopff J, Birnbaum F, Reinhard T. Allogenic limbo-keratoplasty with conjunctivoplasty, mitmycin c, and amniotic membrane for bilateral limbal stemm cell deficiency. Ophthalmology. 2012;119 (5:930–937. doi: 10.1016/j.ophtha.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Reinhard T, Spelsberg H, Henke L, Kontopoulos T, Enczmann J, Wernet P, et al. Long-term results of allogenic penetrating limbo-keratoplasty in total limbal stem cell deficiency. Ophthalmology. 2004;111 (4:775–782. doi: 10.1016/j.ophtha.2003.07.013. [DOI] [PubMed] [Google Scholar]