Abstract
Inhibitors of the mammalian target of rapamycin (mTOR) have improved the treatment of renal cell carcinoma (RCC). However, chronic drug exposure may trigger resistance, limiting the utility of these agents. The metastatic behavior of RCC cells, susceptible (RCCpar) or resistant (RCCres) to the mTOR inhibitor temsirolimus, was investigated. Adhesion to vascular endothelium or immobilized collagen and fibronectin was quantified. Chemotactic motility was evaluated with a modified Boyden chamber assay. Integrin α and β subtype receptors were analyzed by flow cytometry and Western blot analysis. The physiological relevance of the integrins was then determined by blocking studies and small interfering RNA knockdown. Adhesion to endothelial cells and to fibronectin (not to collagen) and chemotaxis were enhanced in RCCres compared to RCCpar. RCCres detached from fibronectin and motile activity further increased under retreatment with low-dosed temsirolimus. α5 integrin was diminished inside the cell and at the cell surface, whereas the β3 subtype was reduced intracellularly but elevated at the plasma membrane. In RCCpar, blocking α5 surface receptors enhanced RCC-collagen but reduced RCC-fibronectin interaction, whereas the opposite was true for RCCres. Chemotaxis of RCCpar but not of RCCres was strongly diminished by the α5 antibody. Blocking β3 significantly lowered chemotaxis with stronger effects on RCCres, compared to RCCpar. Importantly, β3 knockdown reduced chemotaxis of RCCpar but upregulated the motile behavior of RCCres. Temsirolimus resistance is characterized by quantitative alterations of integrin α5 and β3 expression, coupled to functional changes of the integrin molecules, and forces a switch from RCC adhesion to RCC migration.
Introduction
Renal cell carcinoma (RCC) is one of the most aggressive tumor types. Approximately one third of patients have already developed metastases at diagnosis, and up to 40% of patients undergoing surgical resection will have disease recurrence. Once metastasized, the 5-year survival rate is less than 5% [1].
Increasing knowledge about the molecular alterations driving a cell to become malignant has led to the development of novel compounds targeting those pathways, which are aberrantly activated in cancer. This is particularly true for the phosphatidyl inositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling, which is dysregulated in RCC [2], and activation of this pathway has been suggested to correlate with aggressive behavior and poor prognosis in RCC tumors [3].
In the targeted treatment of RCC, mTOR inhibition plays a principal role. Temsirolimus has been approved for the first-line treatment of patients with RCC with poor prognosis, whereas the oral mTOR inhibitor everolimus has been recommended for patients with advanced progressive RCC or for patients with failed vascular endothelial growth factor–targeted therapy [1,4].
Though mTOR targeting offers significantly enhanced response rates, it is rarely curative [5]. The reason for the insufficient therapeutic response has not been fully elucidated. It is argued that chronic drug exposure may activate an undesired escape mechanism, leading to resistance development. It has recently been demonstrated that long-term mTOR blockade triggers undesired feedback loops in RCC cells [6], associated with drug nonresponsiveness and accelerated tumor growth [7]. Similar effects have been observed with resistant prostate cancer cells, evidenced by elevated cell cycle progression compared to those from drug-sensitive sublines [8].
When discussing the pros and cons of mTOR inhibitors, it must be kept in mind that invasion and metastasis are critical for malignant tumor progression. They are the main causes of treatment failure. How circulating RCC cells are transferred from blood vessels into the target tissue when resistance toward mTOR inhibitors develops is unclear. The object of the present study was to drive RCC cells into temsirolimus resistance and investigate altered adhesion and invasion dynamics. Because adhesion molecules of the integrin family are critically involved in the process of tumor transmigration and metastasis [9,10], modification of integrin α and β subtype expression was analyzed and correlated to the invasive behavior of the tumor cells.
Materials and Methods
Cell Culture
Kidney carcinoma Caki-1, KTCTL-26, and A498 cells were purchased from LGC Promochem (Wesel, Germany). The tumor cells were grown and subcultured in RPMI 1640 medium (Gibco/Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal calf serum (FCS), 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified 5% CO2 incubator. The temsirolimus-resistant subline was cultivated for 12 months by exposing the parental cells to temsirolimus (Torisel; LC Laboratories, Woburn, MA), starting at 1 nM/ml and increasing stepwise to 1 μM/ml. The resistant variants were termed Cakires, KTCres, and A498res. The parental control cells were designated Cakipar, KTCpar, and A498par.
Human umbilical vein endothelial cells (HUVEC) were isolated from human umbilical veins and harvested by enzymatic treatment with dispase (Gibco/Invitrogen). Human endothelial cells were grown in Medium 199 (M199; Biozol, Munich, Germany), supplemented with 10% FCS, 10% pooled human serum, 20 mg/ml endothelial cell growth factor (Boehringer, Mannheim, Germany), 0.1% heparin, 100 ng/ml gentamycin, and 20 mM Hepes buffer (pH 7.4). Subcultures from passages 2 to 5 were employed.
Drug Treatment
Temsirolimus was dissolved in DMSO as a 10 mM stock solution and stored as aliquots at − 20°C. Before experiments, temsirolimus was diluted in cell culture medium to the final concentration. Control cell cultures received cell culture medium alone. To exclude toxic effects of the compounds, cell viability was determined by trypan blue (Gibco/Invitrogen).
To analyze the influence of temsirolimus on adhesion and chemotactic movement of resistant compared to sensitive tumor cells, cell culture medium of Cakires, KTCres, or A498res cells containing 1 μM temsirolimus was replaced by temsirolimus-free medium to avoid unspecific effects. A medium change was also carried out in the drug-sensitive cell culture system. After 3 days, 10 nM/ml temsirolimus was added to both resistant and sensitive cells (controls received fresh medium without temsirolimus), and adhesion and chemotactic movement were analyzed.
Tumor Cell Adhesion
To analyze tumor cell adhesion, HUVECs were transferred to six-well multiplates (Falcon Primaria; BD Biosciences, Heidelberg, Germany) in complete HUVEC medium. When confluency was reached, RCC cells (resistant and sensitive) were detached from their culture flasks by Accutase treatment (PAA Laboratories, Cölbe, Germany). Cells (0.5 × 106) were then added to the HUVEC monolayer for 30, 60, or 120 minutes. Subsequently, nonadherent tumor cells were washed off using warmed (37°C) M199. The remaining cells were fixed with 1% glutaraldehyde. Adherent tumor cells were counted in five different fields of a defined size (5 × 0.25 mm2) using a phase-contrast microscope, and the mean cellular adhesion rate was calculated.
Attachment to Extracellular Matrix Components
Six-well plates (Falcon Primaria) were coated with collagen G [extracted from calfskin, consisting of 90% collagen type I and 10% collagen type III; diluted to 400 μg/ml in phosphate-buffered saline (PBS); Seromed, Berlin, Germany] or fibronectin (derived from human plasma, diluted to 50 μg/ml in PBS; BD Biosciences) overnight. Unspecific cell binding was evaluated using culture plates treated with Poly-D-Lysine (Nunc, Wiesbaden, Germany). Plastic dishes served as the background control. Plates were washed with 1% BSA in PBS to block nonspecific cell adhesion. Tumor cells (0.5 x 106) were then added to each well for 30 minutes. Subsequently, nonadherent tumor cells were washed off, and the remaining adherent cells were fixed with 2% glutaraldehyde and counted under a microscope. The mean cellular adhesion rate, defined by adherent cellscoated well − adherent cellsbackground, was calculated from five different observation fields (5 × 0.25 mm2).
Tumor Cell Motility (Chemotaxis)
Serum-induced chemotactic movement was investigated using six-well Transwell chambers (Greiner Bio-One, Frickenhausen, Germany) with 8-μm pores. RCC cells (0.5 × 106) per milliliter were placed in the upper chamber in serum-free medium. The lower chamber contained 10% serum. After 20-hour incubation, the upper surface of the Transwell membrane was gently wiped with a cotton swab to remove nonmigrating cells. Cells, which had moved to the lower surface of the membrane, were stained using hematoxylin and counted under a microscope. The mean chemotaxis rate was calculated from five different observation fields (5 × 0.25 mm2).
Integrin Surface Expression
RCC cells were detached from their culture flasks by Accutase (PAA Laboratories GmbH, Pasching, Austria) and washed in blocking solution (PBS, 0.5% BSA). The cells were then incubated for 60 minutes at 4°C with phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) directed against the following integrin subtypes: anti-a1 (mouse IgG1, clone SR84), anti-a2 (mouse IgG2a, clone 12 F1-H6), anti-a3 (mouse IgG1, clone C3 II.1), anti-a4 (mouse IgG1, clone 9 F10), anti-a5 (mouse IgG1, clone IIA1), anti-a6 (rat IgG2a, clone GoH3), anti-b1 (mouse IgG1, clone MAR4), anti-b3 (mouse IgG1, clone VI-PL2), or anti-b4 (rat IgG2a; clone 439–9B; all: BD Biosciences). Tumor cell integrin expression was then measured using a FACScan (BD Biosciences; FL-2H (log) channel histogram analysis; 1 × 104 cells per scan) and expressed as mean fluorescence units. A mouse IgG1-PE (MOPC-21) or IgG2a-PE (G155–178; all: BD Biosciences) was used as an isotype control.
Western Blot Analysis
To investigate the integrin protein level in Cakires and Cakipar cells, tumor cell lysates were applied to a 7% to 12% polyacrylamide gel and electrophoresed for 90 minutes at 100 V. The protein was then transferred to nitrocellulose membranes (1 hour, 100 V). After blocking with nonfat dry milk for 1 hour, the membranes were incubated overnight with mAbs directed against integrin α3 (rabbit, polyclonal, 1:1000; Chemicon/Millipore, Schwalbach, Germany), integrin α5 (mouse IgG2a, 1:5000, clone 1; BD Biosciences), and integrin β3 (mouse IgG1, 1:2500, clone 1; BD Biosciences).
HRP-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG (both: 1:5.000; Upstate Biotechnology, Lake Placid, NY) served as the secondary antibody. The membranes were briefly incubated with ECL detection reagent (ECL; Amersham/GE Healthcare, München, Germany) to visualize the proteins and then analyzed by the Fusion FX7 system (Peqlab, Erlangen, Germany). β-Actin (1:1.000; clone AC-15; Sigma-Aldrich, Taufenkirchen, Germany) served as the internal control.
Blocking and Knockdown Studies
RCC cells were incubated for 60 minutes with 10 μg/ml function-blocking anti–integrin β3 (clone B3A) or anti–integrin α5 (clone P1D6) mouse mAb (both: Millipore). Control cells were incubated with cell culture medium alone.
Additionally, tumor cells (3 × 105 per well) were transfected with small interfering RNA (siRNA) directed against integrin β3 (2 μM, HS_ITGB3_5 FlexiTube siRNA: NM_000212; Qiagen, Hilden, Germany) or integrin α5 (2 μM, Hs_ITGA5_5 FlexiTube siRNA: NM_002205; Qiagen) with a siRNA/transfection reagent (HiPerFect Transfection Reagent; Qiagen) ratio of 1:6. Nontreated cells and cells treated with 5 nM control siRNA (AllStars Negative Control siRNA; Qiagen) served as controls. Subsequently, tumor cell adhesion to HUVEC, immobilized collagen, or fibronectin as well as RCC chemotaxis were analyzed as indicated above.
Statistics
All experiments were performed three to six times. Statistical significance was calculated with the Wilcoxon–Mann-Whitney U test. Differences were considered statistically significant at a P value less than .05. Inhibitory concentration of 50% (IC50) values were calculated by CalcuSyn software (Biosoft, Cambridge, UK).
Results
Tumor Cell Adhesion and Chemotaxis
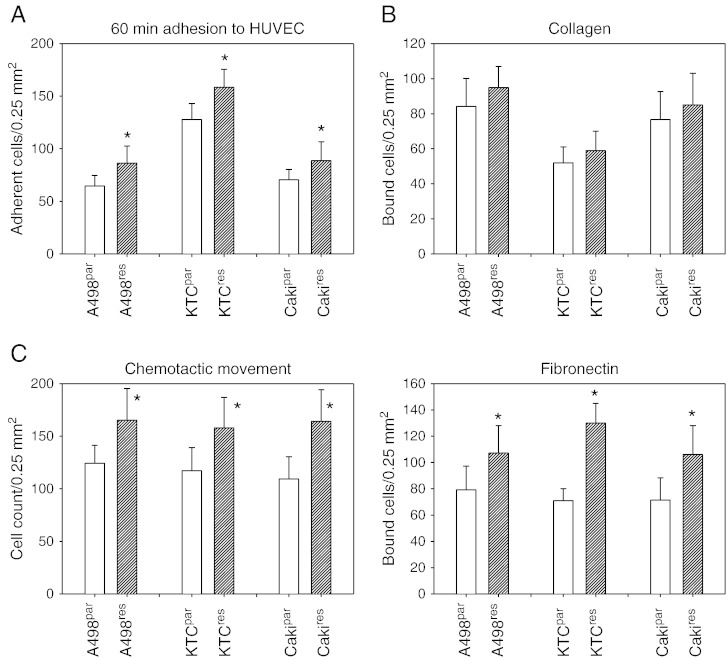
IC50 values were given as follows: A498par = 3.32 ± 0.82; A498res = 17.01 ± 0.32; KTCpar = 0.49 ± 0.18; KTCres = 29.73 ± 8,76; Cakipar = 7.41 ± 3.24; and Cakires = 160.53 ± 46.71 (each: nM/ml). Evaluation of tumor cell endothelial cell interaction revealed that more A498res, KTCres, or Cakires cells adhered to HUVEC than did the respective parental cell lines (Figure 1A). Similar behavior was apparent for the matrix binding assay. Significantly more A498res, KTCres, or Cakires cells bound to immobilized fibronectin (but not to collagen) compared to A498par, KTCpar, or Cakipar (Figure 1B). Regarding chemotaxis, more A498res, KTCres, or Cakires cells penetrated the Transwell membrane, compared to the parental cell lines (Figure 1C).
Figure 1.
Adhesion and chemotactic behavior of temsirolimus-resistant (res) versus temsirolimus sensitive (par) RCC cells. (A) RCC adhesion to HUVEC after 60 minutes. (B) Adhesion to the extracellular matrix proteins collagen and fibronectin. Resistant (res) or sensitive (par) RCC cells were added to immobilized collagen or fibronectin for 60 minutes, and binding was measured. (C) Chemotactic movement was assessed in a Transwell chamber assay. Tumor cells were seeded in the upper chamber in serum-free medium, and 10% FCS, as the chemoattractant, was placed in the lower well. A to C show means calculated from five counts. Each diagram represents one of six experiments. * indicates significant difference between the resistant and the sensitive tumor cell subline.
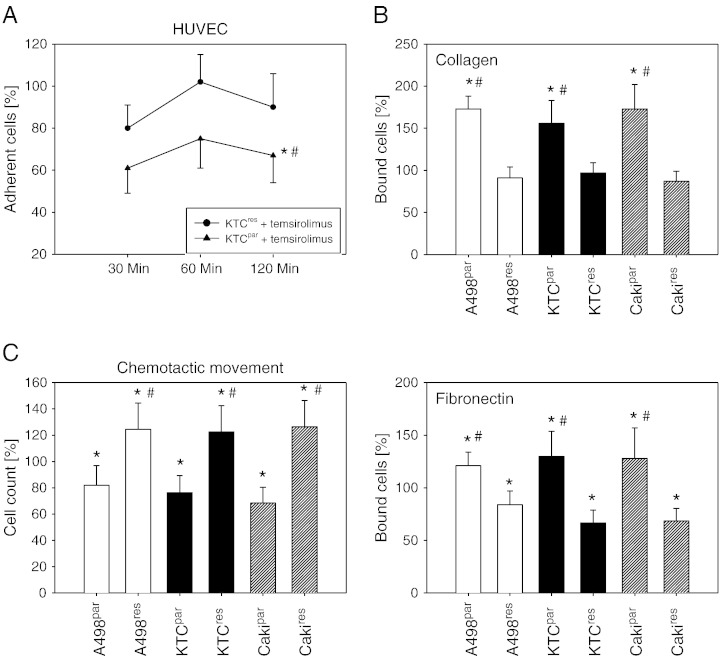
Tumor cells were retreated with a therapeutically relevant temsirolimus concentration (10 nM), and the response was analyzed. Drug treatment caused a significant reduction in the number of drug-sensitive cells adhering to HUVEC. This effect was not found in the resistant cell lines (Figure 2A, representative for KTCTL-26). Adhesion of A498par, KTCpar, or Cakipar to collagen or fibronectin increased with 10 nM temsirolimus. However, temsirolimus did not elevate A498res, KTCres, or Cakires cell binding to collagen, and the number of bound cells was even diminished in the fibronectin-coated plates, compared to nontreated cells (Figure 2B). Inversely, chemotactic movement of A498par, KTCpar, or Cakipar was diminished, whereas this was not true with respect to A498res, KTCres, or Cakires cells. Chemotaxis of the resistant cell lines was increased, compared to the controls (Figure 2C).
Figure 2.
Short-term treatment with low-dosed temsirolimus differentially alters adhesion and migration of resistant and nonresistant RCC cells. The resistant tumor cells were treated with fresh medium (without temsirolimus) for 3 days and then exposed to 10 nM temsirolimus. Medium change followed by 10 nM temsirolimus treatment was also carried out with the drug-sensitive cell lines. A shows time-dependent RCC adhesion to HUVEC (representative for KTCpar and KTCres), B shows the collagen and fibronectin binding assay, and C demonstrates chemotactic behavior of the tumor cell sublines evaluated by the Transwell chamber assay (60-minute values). Percentage is related to controls not treated with 10 nM temsirolimus, set to 100%. Each diagram represents one of six experiments. * indicates significant difference to the temsirolimus-free control. # indicates significant difference between the resistant and the sensitive RCC cell line.
Because all cell lines responded similarly to temsirolimus, subsequent experiments were limited to the cell line KTCTL-26.
Integrins Are Modified in KTCres Cells
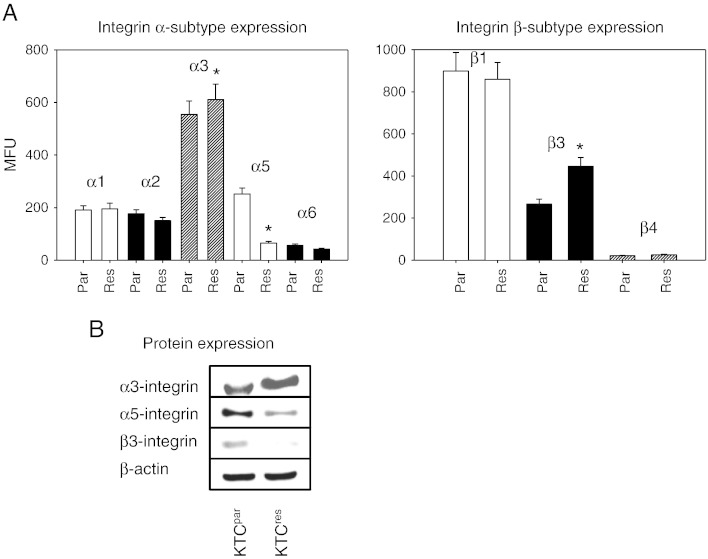
Surface levels of integrin α and β adhesion receptors were analyzed by a FACScan. The integrin subtypes α3 and β1 were strongly expressed, and α1, α2, α5, and β3 were moderately expressed, whereas α6 and β4 were only minimally detectable on KTCpar cells (Figure 3A). Comparative analysis between KTCpar and KTCres cells revealed distinct differences of the integrin expression pattern. The α3 integrin subtype was slightly elevated, and the β3 subtype member was strongly enhanced, whereas integrin α5 was dramatically downregulated on the KTCres cell membrane, compared to KTCpar (Figure 3A). No significant differences were seen with respect to α1, α2, α6, β1, and β4 integrins.
Figure 3.
Integrin α and β expression in KTCpar and KTCres cells. A depicts the FACS results given as mean fluorescence units. Tumor cells were washed in blocking solution and then stained with specific mAbs as listed in Materials and Methods section. To evaluate background staining of PE-conjugated antibodies, goat anti-mouse IgG1-PE or IgG2a-PE was used. Fluorescence was measured using a FACScan flow cytometer. * indicates significant difference between the resistant and the sensitive tumor subline. (B) Modification of intracellular integrin protein level. Tumor cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted on the membrane incubated with appropriate mAbs. β-Actin served as the internal control. The figure shows one representative from three separate experiments.
According to the flow cytometry data, integrin α3 protein was elevated, and α5 protein diminished in KTCres compared to KTCpar cells. The β3 integrin protein content was lowered in the drug-resistant tumor cells, compared to the drug-sensitive cells (Figure 3B). This finding contrasts with the FACS data demonstrating β3 up-regulation under resistance.
Blocking Studies
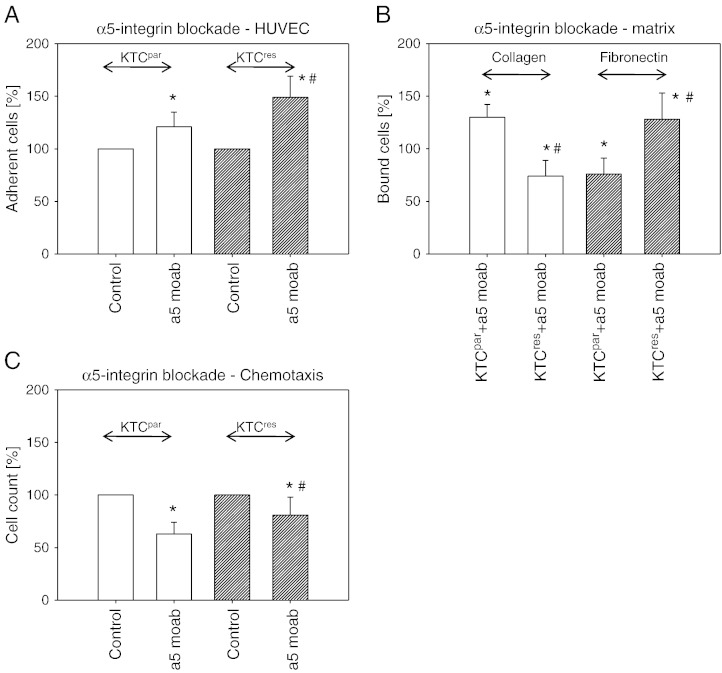
Blocking studies were carried out to investigate the functionality of α5 and β3 integrins, which were most strongly altered in KTCres compared to KTCpar cells. Blockade of α5 on the cell surface by a mAb led to significant enhancement of tumor cell adhesion to HUVEC (Figure 4A). The effect was more pronounced in KTCres compared to KTCpar cells. Integrin α5 blockade correlated with an increased binding of KTCpar but with a decreased binding of KTCres cells to collagen. Inversely, α5 blocking reduced KTCpar binding but elevated KTCres binding to fibronectin (Figure 4B). The motile behavior of KTCpar and KTCres cells was influenced by α5, in as much as receptor blockade triggered a distinct (KTCpar) or moderate (KTCres) loss of chemotactic activity (Figure 4C).
Figure 4.
Influence of integrin α5 blockade on tumor cell adhesion to HUVEC (A), binding to immobilized collagen or fibronectin (B) and on chemotaxis (C). KTCpar or KTCres cells were preincubated for 60 minutes with a function-blocking anti–integrin α5 mAb. Controls were untreated. Values are percentage difference to the 100% control. * indicates significant difference between the RCC control subline and the RCC subline treated with the function-blocking antibody. # indicates significant difference between KTCpar and KTCres cells whose integrin subtype was blocked.
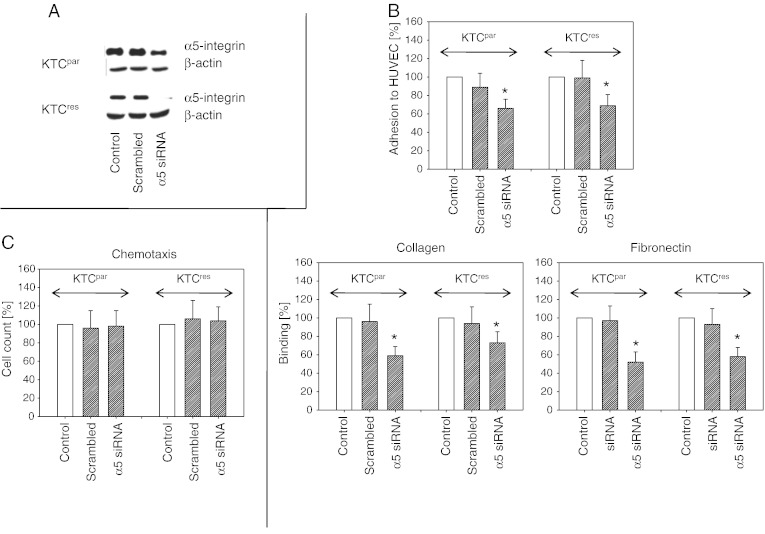
Knocking down the intracellular integrin α5 content by siRNA (Western blot controls are shown in Figure 5A) diminished the interaction of both KTCpar and KTCres cells with endothelium as well as with the matrix proteins collagen and fibronectin, compared to untreated controls (Figure 5B). Chemotaxis was not influenced (Figure 5C) with no differences between KTCpar and KTCres cells.
Figure 5.
Influence of integrin α5 knockdown on tumor cell adhesion and motility. KTCpar and KTCres cells were transfected with α5 siRNA or scrambled siRNA, and knockdown was controlled by Western blot analysis (A). Cells were then subjected to the HUVEC, collagen, and fibronectin adhesion assay (B) or to the chemotaxis assay (C). Values are shown as percentage difference to controls, set to 100%. Each diagram represents one of six experiments. * indicates significant difference to the siRNA-free control.
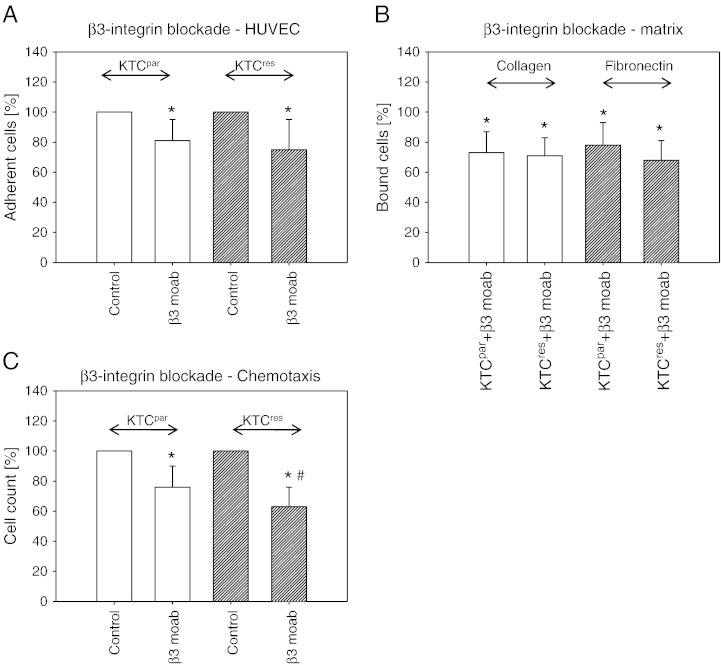
Experiments were repeated using a β3 integrin–blocking antibody. In doing so, tumor cell adhesion to HUVEC was significantly lowered, compared to the untreated controls (Figure 6A), whereby no quantitative differences were seen between KTCpar compared to KTCres cells. A similar phenomenon was induced in the collagen and fibronectin binding assay (Figure 6B). Integrin β3 blockade also prevented integrin migration through the transmembrane pores. However, the number of migrating KTCres cells was reduced to a higher extent than for KTCpar cells (Figure 6C).
Figure 6.
Integrin β3 blocking studies. KTCpar or KTCres cells were preincubated for 60 minutes with a function-blocking anti–integrin β3 mAb, and tumor cell adhesion to HUVEC (A), tumor cell binding to immobilized collagen or fibronectin (B), and chemotactic motility (C) were evaluated. Controls remained untreated. Each experiment was repeated five times. Mean values from one representative test are shown as percentage difference to the 100% control. * indicates significant difference between the control RCC and RCC cells treated with the function-blocking antibody. # indicates significant difference between KTCpar and KTCres cells whose integrin subtype was blocked.
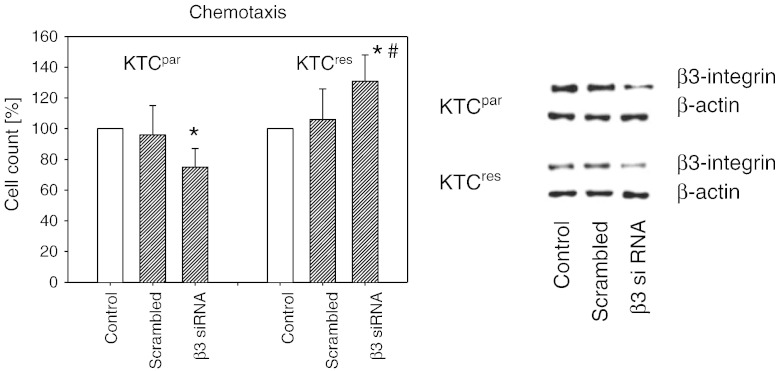
Chemotaxis of the KTCTL-26 tumor cells whose β3 integrin had been knocked down was additionally investigated. This led to a significant reduction of KTCpar migration, whereas motility of KTCres cells was upregulated (Figure 7).
Figure 7.
Knockdown of integrin β3 differentially alters chemotaxis of KTCpar and KTCres cells. Tumor cells were transfected with β3 siRNA or scrambled siRNA. Controls remained untreated. Efficacy of receptor knockdown was evaluated by Western blot analysis (right panel). Subsequently, cells were subjected to the chemotaxis assay. Values are shown as percentage difference to the 100% control. * indicates significant difference to the untreated control. # indicates significant difference between KTCpar and KTCres cells whose integrin subtype was knocked down.
Discussion
Evidence is presented here showing that temsirolimus resistance is coupled to enhanced RCC cell adhesion to vascular endothelium and to extracellular matrix components, accompanied by increased chemotactic activity. Transendothelial migration and motile spreading are critical steps in tumor dissemination and progression [11]. With this in mind, it is concluded that long-term exposure to temsirolimus may alter the invasive behavior, creating highly aggressive RCC cells. Interaction of the drug-resistant tumor cells with fibronectin, but not with collagen, was distinctly escalated. This is clinically important because Knowles et al. recently demonstrated that fibronectin is the dominant factor promoting lung metastasis of RCC [9]. In good accordance, comparative analysis of primary and metastatic RCC cells displayed an increased capacity of the metastatic subtype to strongly attach to fibronectin, whereas cross talk with collagen was only of minor relevance [12]. Therefore, long-term use of temsirolimus may change the RCC phenotype, driving the fibronectin-dependent invasion process forward. This hypothesis is supported by the present investigation, whereby the tumor cells exposed to a therapeutically relevant temsirolimus dosage exhibit altered binding of the resistant RCC cells only to fibronectin. In contrast, both collagen and fibronectin binding to temsirolimus-sensitive RCC cells was altered, with collagen-dependent adhesion being more modified than fibronectin-dependent adhesion.
In drug-resistant prostate cancer cells, an inverse correlation between adhesion and migration properties has been reported [10]. Although the complex scenario of metastatic colonization is not fully understood, there is no doubt that loosening tumor-matrix contact is a necessary prerequisite to allow motile crawling into the surrounding tissue [11,13]. It is therefore not surprising that the basal attachment rate of the drug-resistant RCC to fibronectin was higher than the one of the drug-sensitive cells but was then diminished under short-term retreatment with low-dosed temsirolimus. At the same time, the resistant tumor increased its motile activity, indicating a behavioral switch from being adhesive to becoming invasive. Such a two-step process could play a role during resistance acquisition. The first step may involve facilitating fibronectin instead of collagen-dependent tumor-matrix interaction, and the second step may involve a conversion from an adhesive to an invasive phenotype. Isogai et al. have defined a critical role of fibronectin in providing a cellular switch between stationary and migratory cell phases [14], which would support this hypothesis.
The mechanism responsible for increased motile behavior indicates modification of the integrin expression pattern. The α5 integrin subtype was drastically downregulated on the surface membrane as well as within the cytoplasm of drug-resistant RCC cells. Detailed information on the role of integrin α5 is sparse. Studies on A498 cells have revealed that α5 regulates tumor binding to fibronectin [15] and controls chemotaxis [16]. This corroborates the present data demonstrating diminished contact of KTCpar cells to fibronectin and reduced migratory potential once α5 surface expression has been blocked. Nevertheless, the situation appears more complex than initially thought because KTCres behaved differently under α5 blockade, compared to the KTCpar cells. The pronounced effect of α5 on KTCpar chemotaxis was not seen with KTCres. Most notably, attachment of KTCres to collagen was inhibited, and attachment to fibronectin was enhanced, whereas KTCpar responded to α5 blockade in the opposite way. Obviously, the relevance of the α5 receptor for KTCpar is not transferable to the KTCres cells. On the basis of the present investigation, a functional switch of the α5 integrin during resistance development is proposed, in as much as this integrin subtype may no longer control the tumor cell's motility but rather shifts the tumor cell's binding affinity from collagen to fibronectin.
Change of the integrin function seems also to be reflected in the endothelial cell binding assay, because blocking α5 distinctly enhanced KTCres but only slightly elevated KTCpar adhesion to HUVEC. Apart from hypothesizing differences in linking α5 to a (still unknown) endothelial cell receptor, HUVECs are predestined to deposit collagen and fibronectin on their surface [17]. Given that matrix proteins serve as the specific integrin ligands [18], α5 may promote KTCres accumulation along the endothelial fibronectin fibers. However, involvement of α5 in KTCpar adhesion includes both collagen and fibronectin with a reciprocal relationship. Consequently, only mild alterations of KTCpar binding to HUVEC in the presence of the α5 antibody can be expected.
The different effects of α5 on temsirolimus-responsive compared to temsirolimus-nonresponsive RCC cells were not inducible by knocking down the α5 protein content. Therefore, it seems likely that the α5 surface receptor is the relevant factor responsible for modifying tumor cell adhesion. Loss of α5 together with a functional switch has recently been observed in everolimus-resistant prostate cancer [10]. Presumably, the role of α5 seen in drug-resistant RCC is not restricted to this tumor entity. Nevertheless, further experiments on different tumor types are required to investigate whether the role of the α5 integrin in mTOR inhibitor–based regimen can be generalized.
Blocking the β3 integrin surface molecule diminished RCC chemotaxis with KTCres being more influenced than KTCpar cells. Considering the strong elevation of this receptor on the KTCres membrane, it seems likely that membranous β3 is, at least partially, responsible for the enhanced migratory activity seen in the resistant RCC tumor cells. Because the β3 level inversely correlates with the KTCres-binding activity, receptor enhancement might also be responsible for fibronectin detachment occurring during temsirolimus retreatment. Although no data from others are available regarding this issue, β3 integrin expression correlated well with the invasive potential of lung [19], breast [20], and colorectal [21] carcinomas as well as of melanoma cells [22]. Classification of 45 human tumor cell lines derived from various tissues has revealed cell surface localization of β3 integrin receptors exclusively in cell lines crossing an endothelial cell barrier [23]. Hence, upregulating β3 along the RCC cell surface under chronic temsirolimus treatment might entail the severe risk of accelerating metastatic tumor spreading. The development of undesired countermechanisms caused by an mTOR inhibitor regimen should therefore be carefully controlled. Whether the evaluation of the β3 expression level in patients with cancer might be an innovative tool to monitor drug response is the subject of ongoing studies.
Diminishing the cytoplasmic integrin β3 pool by siRNA knockdown differentially altered the chemotactic activity of KTCpar, compared to KTCres cells. Indeed, loss of this protein significantly lowered KTCpar but increased KTCres chemotaxis. Reduction of the intracellular β3 content, becoming overt during resistance acquisition, is therefore a signal that RCC cells undergo conversion toward a highly motile phenotype. Because loss of cytoplasmic β3 is paralleled by enrichment of this receptor on the cell membrane, it may be assumed that β3 is translocated from the intracellular space to the outer cell surface. The same reciprocal distribution of β3 has been observed in Cakires and A498res, indicating a common mechanism of redistribution (data not shown). Indeed, trafficking integrins has been documented to play an important role in regulating invasive migration [24]. Because both reduced intracellular β3 as well as enhanced β3 surface expressions separately promote RCC migration, dynamic receptor trafficking may further encourage metastatic dissemination.
The different chemotactic response of sensitive and resistant tumor cells in the presence of β3 siRNA points to a functional switch of the β3 integrin, as has already been postulated with the α5 molecule. The α5 subtype forces fibronectin-RCC interaction, possibly as a prerequisite for initiating invasion, whereas β3 drives the invasion process forward. The molecular background underlying the functional switch of α5 and β3 is still a matter of debate. Flevaris et al. indicate that the β3 integrin may inhibit the RhoA signaling pathway, subsequently inducing the conversion from adhesion to migration [25]. This is important because everolimus has recently been demonstrated to prevent migration of drug-sensitive cells by RhoA activation [26] and, consequently, activates the motile machinery by diminishing RhoA. Although how RhoA contributes to the conflicting processes of stable adhesion and motile spreading is not well understood, it is plausible to assume modification of the β3-RhoA cross-communication in RCC cells during resistance development. Whether this speculation is transferable to the α5 integrin is not yet clear. However, a link from α5 to RhoA has recently been observed in melanoma cells [27], making the existence of an α5-RhoA axis in RCC cells likely.
This study shows that temsirolimus resistance drives RCC cancer cells to become highly motile. The process is accompanied by two different processes: 1) quantitative alteration of the integrin α5 and β3 expression and 2) functional change of the integrin molecules, forcing the switch from adhesion to migration. Analysis of the integrin-driven alterations of the intracellular signaling machine is the subject of ongoing experiments.
Acknowledgment
We would like to thank Karen Nelson for critically reading the manuscript.
Footnotes
This work was supported by the “Alfons und Gertrud Kassel-Stiftung.”
Contributed equally as senior authors.
References
- 1.Najjar Y.G., Rini B.I. Novel agents in renal carcinoma: a reality check. Ther Adv Med Oncol. 2012;4:183–194. doi: 10.1177/1758834012443725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho D.C., Hutson T.E., Samlowski W., Sportelli P., Somer B., Richards P., Sosman J.A., Puzanov I., Michaelson M.D., Flaherty K.T. Two phase 2 trials of the novel Akt inhibitor perifosine in patients with advanced renal cell carcinoma after progression on vascular endothelial growth factor-targeted therapy. Cancer. 2012;118:6055–6062. doi: 10.1002/cncr.27668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantuck A.J., Seligson D.B., Klatte T., Yu H., Leppert J.T., Moore L., O'Toole T., Gibbons J., Belldegrun A.S., Figlin R.A. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 4.Hutson T.E. Targeted therapies for the treatment of metastatic renal cell carcinoma: clinical evidence. Oncologist. 2011;16(Suppl 2):14–22. doi: 10.1634/theoncologist.2011-S2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen N., Rathmell W.K. Looking beyond inhibition of VEGF/mTOR: emerging targets for renal cell carcinoma drug development. Curr Clin Pharmacol. 2011;6:199–206. doi: 10.2174/157488411797189389. [DOI] [PubMed] [Google Scholar]
- 6.Harada K., Miyake H., Kumano M., Fujisawa M. Acquired resistance to temsirolimus in human renal cell carcinoma cells is mediated by the constitutive activation of signal transduction pathways through mTORC2. Br J Cancer. 2013;109:2389–2395. doi: 10.1038/bjc.2013.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juengel E., Dauselt A., Makarević J., Wiesner C., Tsaur I., Bartsch G., Haferkamp A., Blaheta R.A. Acetylation of histone H3 prevents resistance development caused by chronic mTOR inhibition in renal cell carcinoma cells. Cancer Lett. 2012;324:83–90. doi: 10.1016/j.canlet.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Tsaur I., Makarević J., Hudak L., Juengel E., Kurosch M., Wiesner C., Bartsch G., Harder S., Haferkamp A., Blaheta R.A. The cdk1-cyclin B complex is involved in everolimus triggered resistance in the PC3 prostate cancer cell line. Cancer Lett. 2011;313:84–90. doi: 10.1016/j.canlet.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Knowles L.M., Gurski L.A., Engel C., Gnarra J.R., Maranchie J.K., Pilch J. Integrin αvβ3 and fibronectin upregulate Slug in cancer cells to promote clot invasion and metastasis. Cancer Res. 2013;73:6175–6184. doi: 10.1158/0008-5472.CAN-13-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsaur I., Makarević J., Juengel E., Gasser M., Waaga-Gasser A.M., Kurosch M., Reiter M., Wedel S., Bartsch G., Haferkamp A. Resistance to the mTOR-inhibitor RAD001 elevates integrin α2- and β1-triggered motility, migration and invasion of prostate cancer cells. Br J Cancer. 2012;107:847–855. doi: 10.1038/bjc.2012.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Zijl F., Krupitza G., Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messai Y., Noman M.Z., Derouiche A., Kourda N., Akalay I., Hasmim M., Stasik I., Ben Jilani S., Chebil M., Caignard A. Cytokeratin 18 expression pattern correlates with renal cell carcinoma progression: relationship with Snail. Int J Oncol. 2010;36:1145–1154. doi: 10.3892/ijo_00000597. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz M., Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 2010;8:629–642. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- 14.Isogai C., Laug W.E., Shimada H., Declerck P.J., Stins M.F., Durden D.L., Erdreich-Epstein A., DeClerck Y.A. Plasminogen activator inhibitor-1 promotes angiogenesis by stimulating endothelial cell migration toward fibronectin. Cancer Res. 2001;61:5587–5594. [PubMed] [Google Scholar]
- 15.Jones J., Berkhoff S., Weich E., Engl T., Wedel S., Relja B., Jonas D., Blaheta R.A. Transient down-regulation of beta1 integrin subtypes on kidney carcinoma cells is induced by mechanical contact with endothelial cell membranes. J Cell Mol Med. 2007;11:826–838. doi: 10.1111/j.1582-4934.2007.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones J., Marian D., Weich E., Engl T., Wedel S., Relja B., Jonas D., Blaheta R.A. CXCR4 chemokine receptor engagement modifies integrin dependent adhesion of renal carcinoma cells. Exp Cell Res. 2007;313:4051–4065. doi: 10.1016/j.yexcr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Leroy-Dudal J., Demeilliers C., Gallet O., Pauthe E., Dutoit S., Agniel R., Gauduchon P., Carreiras F. Transmigration of human ovarian adenocarcinoma cells through endothelial extracellular matrix involves αv integrins and the participation of MMP2. Int J Cancer. 2005;114:531–543. doi: 10.1002/ijc.20778. [DOI] [PubMed] [Google Scholar]
- 18.Heino J., Käpylä J. Cellular receptors of extracellular matrix molecules. Curr Pharm Des. 2009;15:1309–1317. doi: 10.2174/138161209787846720. [DOI] [PubMed] [Google Scholar]
- 19.Li N., Zhang J.P., Guo S., Min J., Liu L.L., Su H.C., Feng Y.M., Zhang H.L. Down-regulation of β3-integrin inhibits bone metastasis of small cell lung cancer. Mol Biol Rep. 2012;39:3029–3035. doi: 10.1007/s11033-011-1065-y. [DOI] [PubMed] [Google Scholar]
- 20.Liu H., Radisky D.C., Yang D., Xu R., Radisky E.S., Bissell M.J., Bishop J.M. MYC suppresses cancer metastasis by direct transcriptional silencing of αv and β3 integrin subunits. Nat Cell Biol. 2012;14:567–574. doi: 10.1038/ncb2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei Y., Huang K., Gao C., Lau Q.C., Pan H., Xie K., Li J., Liu R., Zhang T., Xie N. Proteomics identification of ITGB3 as a key regulator in reactive oxygen species-induced migration and invasion of colorectal cancer cells. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.005397. M110.005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn J., Sanz-Moreno V., Marshall C.J. The metastasis gene NEDD9 product acts through integrin β3 and Src to promote mesenchymal motility and inhibit amoeboid motility. J Cell Sci. 2012;125:1814–1826. doi: 10.1242/jcs.101444. [DOI] [PubMed] [Google Scholar]
- 23.Bauer K., Mierke C., Behrens J. Expression profiling reveals genes associated with transendothelial migration of tumor cells: a functional role for αvβ3 integrin. Int J Cancer. 2007;121:1910–1918. doi: 10.1002/ijc.22879. [DOI] [PubMed] [Google Scholar]
- 24.Jacquemet G., Humphries M.J., Caswell P.T. Role of adhesion receptor trafficking in 3D cell migration. Curr Opin Cell Biol. 2013;25:627–632. doi: 10.1016/j.ceb.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flevaris P., Stojanovic A., Gong H., Chishti A., Welch E., Du X. A molecular switch that controls cell spreading and retraction. J Cell Biol. 2007;179:553–565. doi: 10.1083/jcb.200703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeruschke S., Büscher A.K., Oh J., Saleem M.A., Hoyer P.F., Weber S., Nalbant P. Protective effects of the mTOR inhibitor everolimus on cytoskeletal injury in human podocytes are mediated by RhoA signaling. PLoS One. 2013;8:e55980. doi: 10.1371/journal.pone.0055980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goundiam O., Nagel M.D., Vayssade M. Akt and RhoA inhibition promotes anoikis of aggregated B16F10 melanoma cells. Cell Biol Int. 2012;36:311–319. doi: 10.1042/CBI20110069. [DOI] [PubMed] [Google Scholar]