Abstract
Na+/H+ exchanger 3 regulating factor 1/ezrin-radixin-moesin (ERM)–binding phosphoprotein 50 (NHERF1/EBP50), an adaptor molecule that interacts with the ERM–neurofibromatosis type 2 family of cytoskeletal proteins through its ERM-binding region and with phosphatase and tensin homolog (PTEN) and β-catenin through its PDZ domains, has been recently implicated in the progression of various human malignancies, including colorectal cancer (CRC). We report here that NHERF1 controls gland morphogenesis, as demonstrated in three-dimensional (3D) human intestinal glands developing from a single nonpolarized cell. Starting from the early two-cell developmental stage, NHERF1 concentrates at the cellular interface in a central membrane disc that marks the apical pole delimiting the forming lumen. NHERF1 depletion leads to severe disruption of the apical-basal polarity, with formation of enlarged and distorted cell spheroids devoid of a central lumen. This characteristic and the increased number of mitoses in NHERF1-depleted spheroids, including multipolar ones, mimic high-grade dysplasia lesions observed in CRC progression. NHERF1 ERM-binding or PDZ-domain mutants fail to localize apically and impair gland formation most likely by outcompeting endogenous ligands, with the latter mutant completely aborting gland development. Examination of NHERF1 ligands showed that even if both ezrin and moesin colocalized with NHERF1 at the apical membrane, moesin but not ezrin depletion disrupted morphogenesis similarly to NHERF1. NHERF1 depletion resulted also in membrane displacement of PTEN and nuclear translocation of β-catenin, events contributing to polarity loss and increased proliferation. These findings reveal an essential role of NHERF1 in epithelial morphogenesis and polarity and validate this 3D system for modeling the molecular changes observed in CRC.
Abbreviations: 3D, three-dimensional; CIP, calf intestinal phosphatase; IF, immunofluorescence; CRC, colorectal cancer; ERM, ezrin-radixin-moesin; NF2, neurofibromatosis type 2; NHERF1/EBP50, Na+/H+ exchanger 3 regulating factor 1/ERM-binding phosphoprotein 50; pHH3, phospho-histone H3; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PM, plasma membrane
Introduction
Epithelial gland morphogenesis is the process resulting in the establishment of polarized tubular or acinar structures formed by tightly connected cells arranged on a basement membrane that delimit a lumen by their apical surface. The polarized architecture of glandular epithelia allows unidirectional fluid and solute transport, which is the basis for the absorptive and secretory functions. Its alteration leads to epithelial sheet disorganization and development of initially benign glandular tumors, or adenomas, that, on further accumulation of mutations, may become malignant, or adenocarcinomas. Epithelial morphogenesis is initiated by cell adhesion molecules, such as cadherins, followed by organization of the cytoskeleton and differential sorting of proteins to the cortical apical and basolateral compartments [1]. Recently, the compartmentalization of lipids, especially phosphoinosides, was shown to maintain epithelial cell polarity, with phosphatidylinositol 4,5-bisphosphate (PIP2) enriched at the apical membrane and phosphatidylinositol 3,4,5-trisphosphate (PIP3), at the basolateral membrane [2]. PTEN tumor suppressor, a phosphoinositide phosphatase that dephosphorylates PIP3 to PIP2 [3] and opposes the activity of the phosphoinositol 3-OH kinase, has been implicated in maintaining this balance [4].
Na+/H+ exchanger 3 regulating factor 1/ezrin-radixin-moesin (ERM)–binding phosphoprotein 50 (NHERF1/EBP50) is an adaptor protein that interacts directly with PTEN [5] and is localized mainly at the apical plasma membrane (PM) in human epithelial tissues [6]. NHERF1 knockout mice have ultrastructural defects of the apical intestinal brush border membrane [7,8] and also show mammary alveolar membrane polarity disruption with lactation deficit [9]. Our mechanistic studies in cultured cells showed that NHERF1 behaves as a tumor and epithelial-to-mesenchymal transition suppressor through its effects on PTEN and β-catenin [5,10,11]. We and others have also shown that NHERF1 is involved in a series of human cancers, including adenocarcinomas of colon and breast [6,10,12]. We have now examined the involvement of NHERF1 in three-dimensional (3D) intestinal gland formation in which glands develop from single stem cells. We found that NHERF1 is required for epithelial morphogenesis by interacting with moesin and stabilizing PTEN at the apical membrane. This morphogenetic study provides mechanistic support to our previous observations of NHERF1 loss in human colorectal cancer (CRC) that occurs as an early step in adenoma progression [10].
Materials and Methods
Plasmids and Retroviral Infections
The retroviral constructs encoding Myc-tagged NHERF1 wild type and ΔSNL mutant in the pCXb vector (blasticidin selection) and the NHERF1 short hairpin RNA (shRNA) Nos 1 and 4, ezrin short hairpin RNA (shRNA) No. 8, and moesin shRNA No. 4 used for knockdown in pSIREN-RetroQ vector (puromycin selection) have been described [11,13–15]. The Myc-tagged PDZ-domain double mutant (PDZ1-2-DM) construct in pCXb retroviral vector, which has both PDZ-domain binding pockets disrupted by GF to AA substitutions at positions 25 to 26 (PDZ1) and 165 to 166 (PDZ2), was obtained by polymerase chain reaction mutagenesis. The retroviral constructs encoding Akt, PTEN, and PTEN-ΔPDZ (former PTEN-401) [16] were obtained by inserting in the pCXn vector (G418 selection) the cDNAs in frame with HA or Myc tags, respectively. Transfections and retroviral infections were performed as previously described [16].
Epithelial Morphogenesis Assay
Caco-2 human CRC cells were grown in a 50/50 mixture of Dulbecco's modified Eagle's medium /Ham's F-12 nutrients supplemented with 10% FBS. Caco-2 cells were embedded in 40% vol/vol Growth Factor–Reduced Matrigel (BD Biosciences, La Jolla, CA)/growth medium to produce 3D glands as previously described [17]. Immunofluorescence (IF) staining of glands was performed 2 to 8 days post-embedding. The following primary antibodies were used at 1 mg/ml overnight at 4˚C: E-cadherin, ezrin, moesin, GM130 (BD Biosciences), NHERF1 (Affinity BioReagents/Thermo, Rockford, IL), laminin, β-catenin, (Sigma-Aldrich, St Louis, MO), ZO-1 (Zymed/Invitrogen, Carlsbad, CA), atypical protein kinase C (aPKC) (C-20), PTEN (A2B1), Myc (9E10) (Santa Cruz Biotechnology, Santa Cruz, CA), HA (Boehringer Mannheim/ Roche, Indianapolis, IN), and phospho-histone H3 (pHH3)–S10 (Upstate Biotechnology/Millipore, Billerica, MA). Actin cytoskeleton was stained with rhodamine-labeled phalloidin (Molecular Probes/Invitrogen, Carlsbad, CA) at 1:200. Both the secondary antibodies Alexa Fluor 568 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG (Molecular Probes/Invitrogen) were used at 1:200, with TO-PRO-3 iodide (Molecular Probes/Invitrogen) at 1:2000. The slides were mounted with the SlowFade Gold antifade reagent (Invitrogen). The Carl Zeiss MicroImaging, Thornwood, NY 510 confocal microscope was used to acquire images, with × 63/1.40 objective with oil immersion.
CRC Resection Specimens and Histology
Resection specimens from patients with CRC were obtained from the Gastrointestinal Tumor Bank at MD Anderson Cancer Center (Houston, TX) and contained areas of adenoma, carcinoma, and adjacent normal mucosa on the same slide [10]. The CRC specimens were deparaffinated and hydrated as previously described [11], followed by hematoxylin and eosin staining or immunohistochemistry with NHERF1 antibody (Affinity BioReagents/Thermo), at 1:200.
Protein Analysis
Cell lysis and immunoblot analysis were performed as previously described [5]. Antibodies for Western blot analysis were NHERF1, ezrin (BD Biosciences), glyceraldehyde 3-phosphate dehydrogenase; Erk, extracellular signal-regulated kinase (GAPDH) (0411), Myc (9E10), PTEN (A2B1), moesin (C15), Erk1 (C16) and Erk2 (C-14) (Santa Cruz Biotechnology), Akt (Cell Signaling Technology, Danvers, MA), and HA (Boehringer Mannheim/Roche). Calf intestinal phosphatase (CIP) assay was performed as per manufacturer's instructions (New England Biolabs, Ipswich, MA). Briefly, cells were lysed in lysis buffer (40 mM Hepes (pH 7.5), 120 mM NaCl, and 1% Triton X-100). Lysates were incubated with 10 U of CIP or no enzyme control at 37°C for 1 hour and then analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. ImageJ program (National Institutes of Health, Bethesda, MD) was used for densitometric analysis.
Results
NHERF1 Controls the Morphogenesis of Intestinal Cell Glands
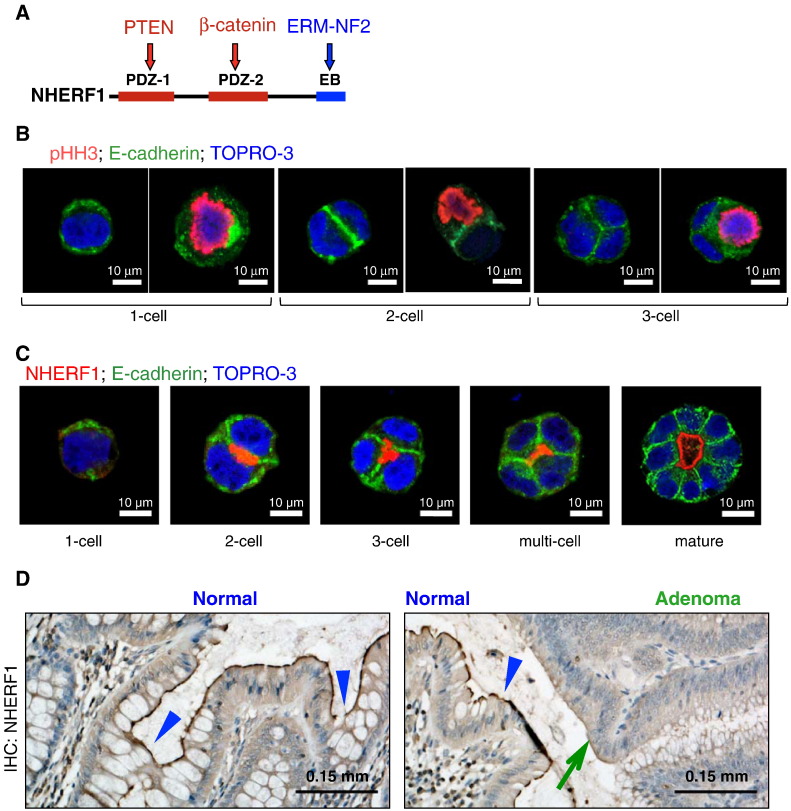
NHERF1 is a 50-kDa adaptor protein comprising two tandem PDZ domains and a carboxyl (C)-terminal ERM-binding region [18,19]. It associates with the PTEN tumor suppressor protein through its PDZ1 domain [5] and with β-catenin through its PDZ2 domain [20] (Figure 1A).
Figure 1.
NHERF1 is localized at the apical PM in intestinal 3D glands. (A) Schematic NHERF1 structure shows N-terminal PDZ domains (1 and 2) and C-terminal ERM-binding (EB) region with selected ligands. (B) Caco-2 cell gland development by successive divisions from a nonpolarized single cell is shown by confocal IF with pHH3 antibody labeling mitoses. (C) Confocal IF with indicated antibodies of Caco-2 cells fixed 2 to 8 days post-plating in Matrigel shows progressive stages of gland development. (D) Immunohistochemistry with NHERF1 of human normal colonic mucosa and adenoma (green arrow) shows apical positivity of normal surface epithelium and glands (purple arrowheads) and loss in adenoma.
To model the development of adenocarcinoma, we examined the effect of NHERF1, a molecule lost early in CRC progression [10], on the development of 3D glands. We have previously shown that NHERF1 exhibits apical PM localization in Caco-2 CRC cells similarly to normal colon epithelial cells [10]. Caco-2 cells have been used as a model of normal intestinal cells because they form polarized cell monolayers with well-developed apical microvilli in confluent 2D, two-dimensional cultures [21] and 3D cysts with a central lumen when grown in Matrigel [17]. These 3D cysts or glands develop from nonpolarized single cells through successive symmetric cell divisions, labeled by pHH3 (Figure 1B), and, progressing through two-cell, three-cell, and multicell stages, reach the mature gland stage by 8 days in culture (Figure 1C). As early as from the two-cell stage, polarity is apparent (see also polarity markers in Figure 2B), and NHERF1 strongly concentrates at the membrane interface in a central disc that marks the apical membrane (Figure 1C). It then remains tightly associated with the apical PM at later stages until the lumen forms. In mature glands, NHERF1 localizes at the apical PM, strongly supporting the notion that this 3D model closely resembles the normal colonic gland (Figure 1D).
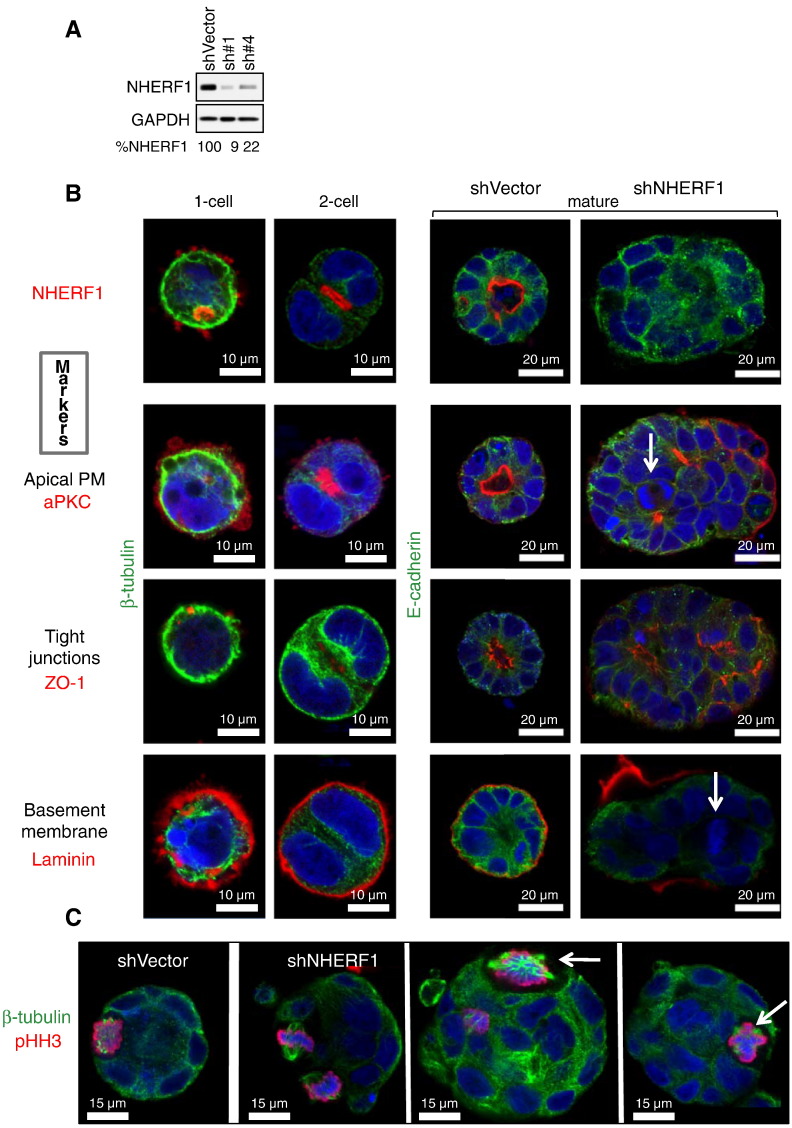
Figure 2.
NHERF1 controls 3D gland morphogenesis. (A) NHERF1 shRNA knockdown in Caco-2 cells. NHERF1 knockdown efficiency (%) is shown as GAPDH-normalized NHERF1 levels. (B) Confocal IF with indicated markers of developing glands at various stages. Mature glands formed by control cells (shVector) and NHERF1 knockdown cells (shNHERF1) highlight severe central lumen disruption, polarity loss, and numerous mitoses (arrows) in NHERF1-depleted spheroids. (C) Unipolar and multipolar (arrows) mitoses in NHERF1-depleted spheroids are shown.
In CRC, NHERF1 loss from the apical PM occurs from early adenoma stages (Figure 1D) [10]. We depleted NHERF1 in Caco-2 cells by using two different shRNAs (Figure 2A) and further used for 3D experiments shNHERF1 No. 1 cells showing greater than 90% NHERF1 depletion. A panel of markers for the apical PM (aPKC), tight junctions (ZO-1), adherent junctions (E-cadherin), basement membrane (laminin) (Figure 2B), and Golgi complex (GM130) (Figure W1) were used to characterize the consequences of NHERF1 depletion on cell polarity. The localization of these polarity markers at one-cell and two-cell normal development stages is also shown (Figure 2B, left columns). Control (shVector) glands contain a spherical monolayer of polarized cells joined by adherens (lateral PM) and tight (apico-lateral PM) junctions facing a central lumen through their apical surface and sitting on a tightly apposed basement membrane. In contrast, NHERF1 depletion severely disrupted this morphology and induced the formation of compact spheroids of larger size, lacking a central lumen or having multiple aborted lumina and often showing distorted shapes (Figure 2B, right column). Individual cells preserved their lateral contacts through E-cadherin but lost apical polarity, as indicated by the latero-basal dispersion or even absence of aPKC and ZO-1. Laminin was either absent (not shown) or unevenly distributed and detached from the base of NHERF1-depleted spheroids. NHERF1 depletion also induced random distribution of the Golgi apparatus around a centrally displaced nucleus, contrasting with the normal basal polarization of the nucleus and supranuclear Golgi complex localization in NHERF1-expressing cells (Figure W1). Careful examination of the NHERF1-depleted spheroids also revealed frequent mitotic figures (Figure 2B, arrows). Labeling with β-tubulin, a marker for the mitotic spindle, and pHH3 showed rare typical bipolar mitoses in control glands and frequent mitoses in spheroids, some of which were multipolar (Figure 2C, arrows). Overall, NHERF1 depletion induced massive cellular disorganization with inversion or loss of apical-basal polarity, heterogeneous cell shapes and sizes, and increased mitotic activity. These changes are reminiscent of the transition from normal epithelium to adenoma and carcinoma seen in CRC progression.
NHERF1 PDZ Domains and ERM-Binding Region Are Required for Gland Morphogenesis
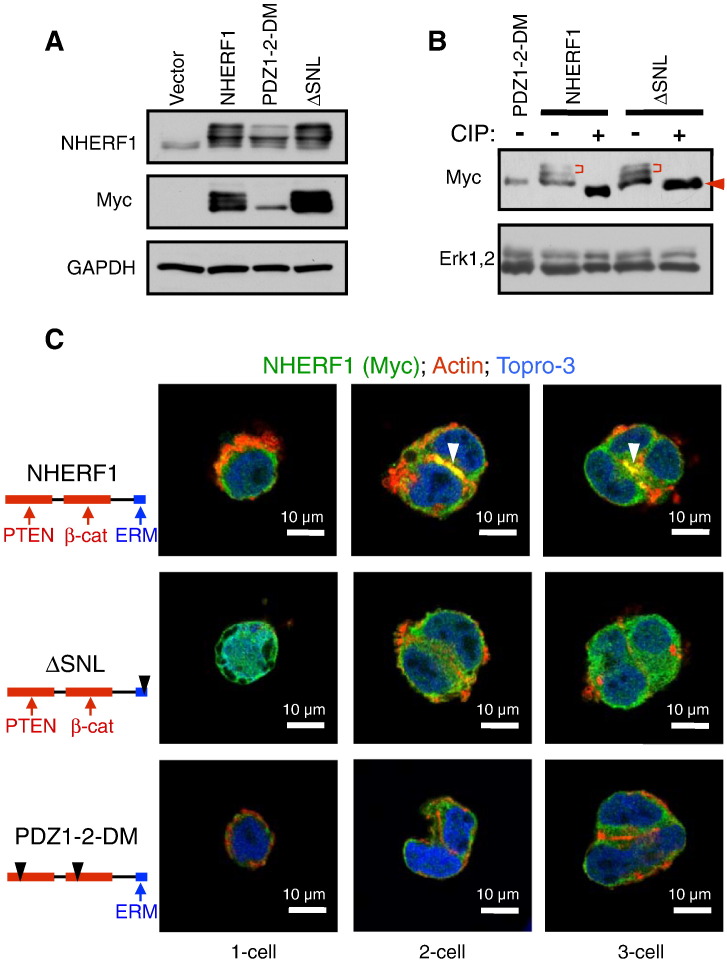
NHERF1 is an adaptor protein that interacts with multiple ligands through its domains [22]. To determine the molecular requirements for gland morphogenesis, Myc-tagged NHERF1 wild type and two complementary mutants in the ERM-binding region (ΔSNL) or the PDZ domains (PDZ1-2-DM) were overexpressed in cells (Figure 3, A and B). At the one-cell development stage, NHERF1 wild type was expressed in the cytoplasm, but starting from the two-cell stage, a notable fraction concentrated at the apical PM (Figure 3C, arrowheads), allowing cell polarity, as illustrated here by actin labeling. In contrast, both mutants failed to localize at the apical PM and prevented gland formation, implying that proper NHERF1 localization at the apical PM is required for gland morphogenesis. However, important distinctions were observed between these mutants (Figure 3C). The ΔSNL mutant that abolishes ERM binding but enhances PDZ-domain ligand binding [14] showed cytoplasmic and nuclear localization and allowed the formation of small compact spheroids. We have previously shown loss of apical PM localization in 2D polarized epithelia for this mutant [14], most likely due to disrupted interaction with ERM proteins. The PDZ1-2 double mutant, which exhibits reverse binding capabilities, had drastic consequences on morphogenesis, aborting it at the transition to the two-cell stage, in which only few distorted forms were still viable. Interestingly, this mutant was also hypophosphorylated compared to NHERF1 wild type or the ΔSNL mutant (Figure 3B). Although PDZ1-2-DM appears to have cytoplasmic expression in the few two-cell stage forms, we found PM localization in 2D cultures of glioblastoma cells (not shown), indicating that this mutant could localize at the PM, but most likely, it prevented organoid growth before polarity could be established. Overall, these experiments suggested that NHERF1 apical PM localization, most likely by interaction with ERM proteins, is necessary for gland morphogenesis and that NHERF1 itself organizes PDZ ligands essential for this process.
Figure 3.
NHERF1 apical PM localization is required for 3D gland development. (A) Immunoblot with indicated antibodies of whole lysates (30-μg proteins) from cells expressing Myc-tagged NHERF1 wild type, double PDZ domain mutant (PDZ1-2-DM), and ERM-binding–deficient ΔSNL mutant is shown. (B) Immunoblot as in A shows collapse of phosphorylated forms (bracket) to a single form (arrowhead) following CIP treatment. (C) IF analysis of early gland development from Caco-2 cells expressing the diagrammed NHERF1 and mutants. Arrowheads indicate NHERF1 expression at the apical PM.
Both Ezrin and Moesin Colocalize with NHERF1 at the Apical PM, but Only Moesin Controls Gland Morphogenesis Similarly to NHERF1
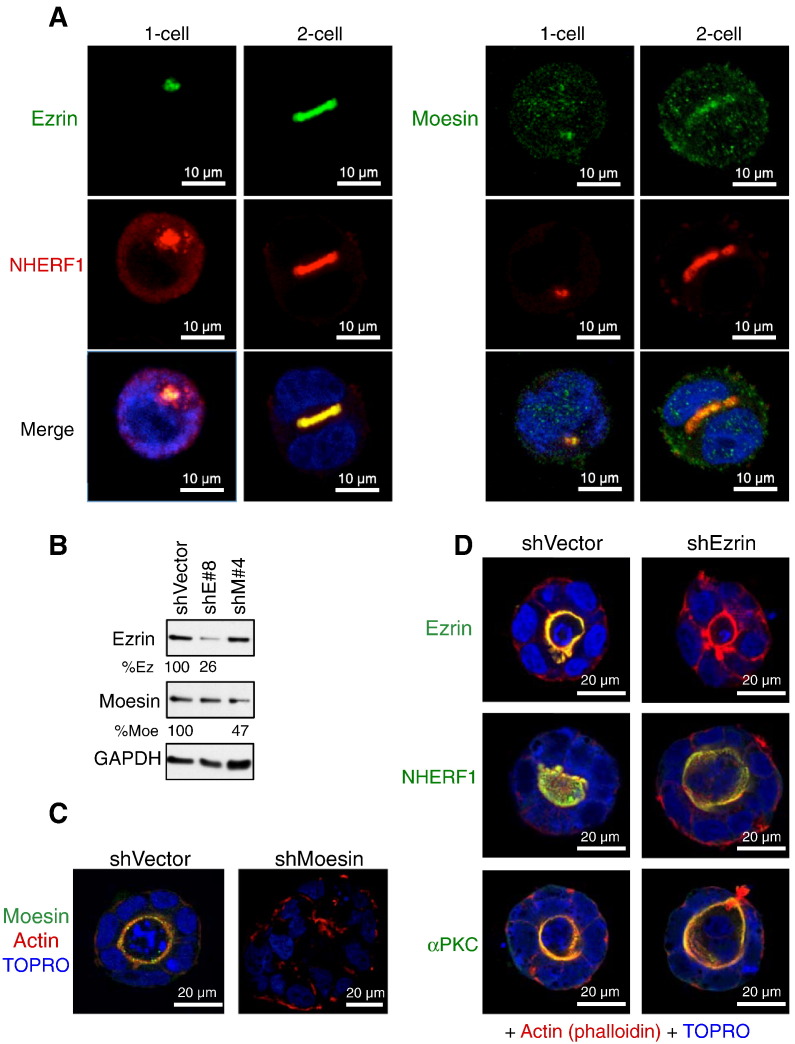
To identify the specific ERM protein controlling morphogenesis in association with NHERF1, we double-labeled one-cell and two-cell stage 3D cultures with NHERF1 and either ezrin or moesin (Figure 4A). We could not perform similar studies for radixin, because a radixin-specific antibody for IF could not be identified. Both ezrin and moesin colocalized with NHERF1 at the inner disc of the intercellular membranes at the two-cell stage. Interestingly, at one-cell stage, they also colocalized with NHERF1 in a juxtanuclear punctate structure (see also Figure 2B), possibly an endosome-derived precursor form of the apical membrane.
Figure 4.
Differential roles of ezrin and moesin in gland morphogenesis. (A) IF analysis with NHERF1 and ezrin (left) or moesin (right) antibodies of early gland formation shows colocalization of both ERMs with NHERF1 at the apical PM. (B) Western blot of proteins from Caco-2 cell lysates shows shRNA knockdown of ezrin (shE No. 8) and moesin (shM No. 4) and corresponding GAPDH-normalized levels (%). (C and D) Confocal IF of moesin-depleted (shMoesin) (C) and ezrin-depleted (shEzrin) (D) mature structures shows disrupted gland morphogenesis only by moesin depletion.
We have previously developed a battery of shRNAs for knockdown of individual ERM proteins [15]. In Caco-2 cells, ezrin shE No. 8 and moesin shM No. 4 had relatively efficient knockdown (Figure 4B). Moesin depletion resulted in loss of polarity and disrupted gland morphogenesis with development of large deformed spheroids (Figure 4C), exhibiting a phenotype similar to NHERF1. Surprisingly, ezrin depletion did not impair morphogenesis, and normally formed glands developed in the absence of ezrin (Figure 4D, upper panels). We confirmed that these ezrin-depleted glands express NHERF1 apically (Figure 4D, middle panels), implying that the localization of NHERF1 is not dependent on ezrin in this model. The localization of the apical polarity marker aPKC was also correct, confirming the formation of structurally normal glands in the absence of ezrin. Conversely, NHERF1 depletion had the expected effect on ezrin, inducing basal mislocalization in compact spheroids (Figure W2). These results indicate that, in this 3D colonic gland formation model, ezrin is dispensable, whereas NHERF1 and moesin are both required for gland morphogenesis.
NHERF1 Loss Displaces PTEN from the Apical PM and Induces Nuclear Shift of β-Catenin
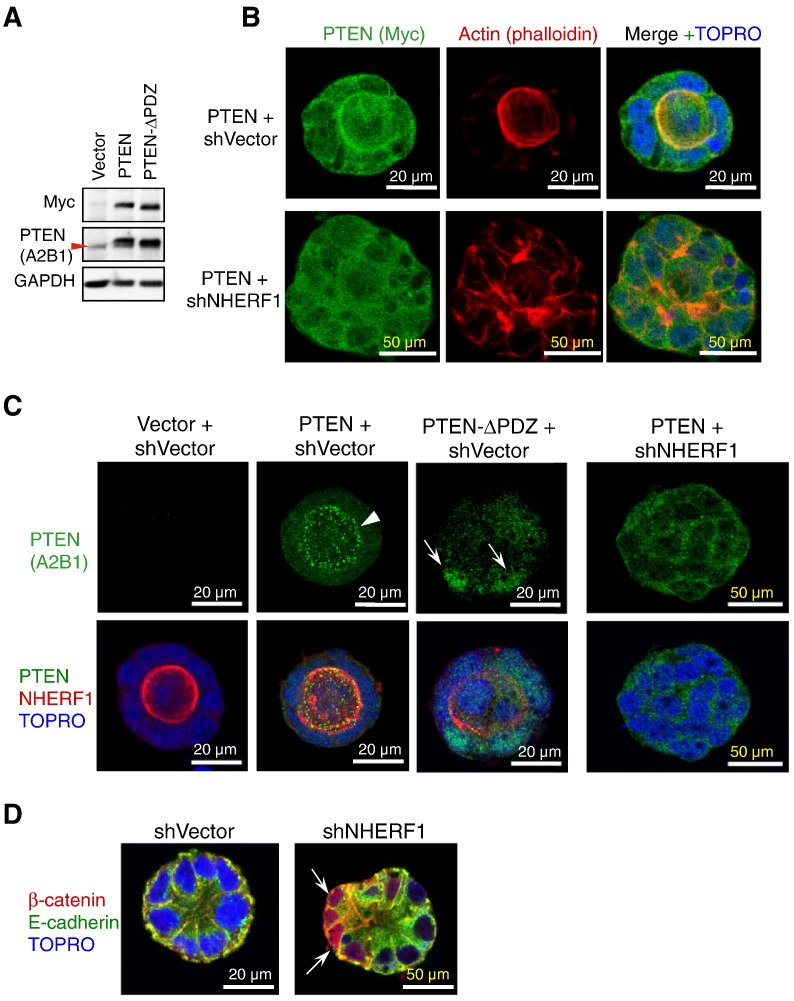
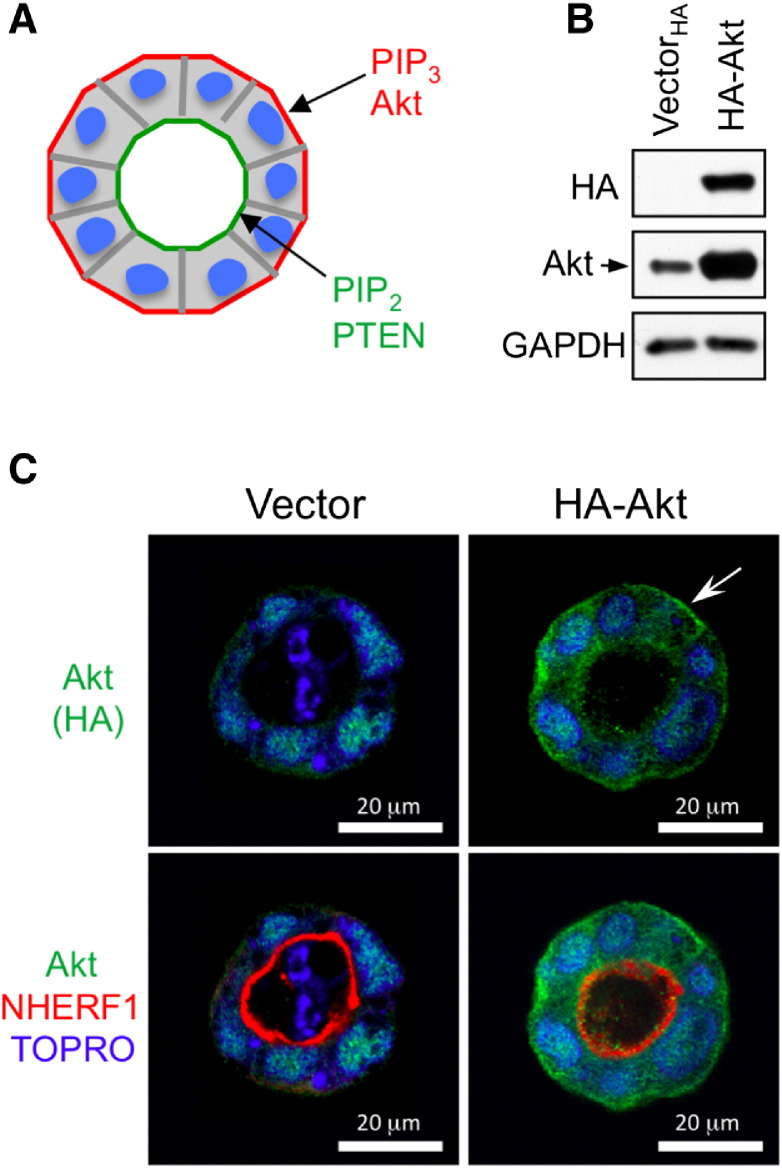
NHERF1 interacts with many ligands through its PDZ domains. Of these, PTEN has been linked to the establishment of epithelial polarity in MDCK cell cysts, resulting in the apical compartmentalization of PIP2 and baso-lateral distribution of PIP3 [2] (Figure W3A). We confirmed the distribution of PIP3 to the basal PM in Caco-2 glands by expressing HA-tagged Akt that contains a PH domain with high affinity for PIP3 (Figure W3, B and C). Akt displayed a polarized distribution to the basal PM, sparing the apical PM where NHERF1 was localized. To analyze the localization of PTEN in Caco-2 glands, we stably expressed Myc-tagged PTEN, as endogenous PTEN could not be detected by IF analysis (Figure 5A). PTEN localized to the cytoplasm and nucleus of cells, as reported for other cell types [23], and a fraction localized at the apical PM (Figure 5B, upper panels), confirming the reported findings in MDCK cells [4]. By inducing polarity loss, NHERF1 depletion led to dispersion of PTEN in the cytoplasmic and nuclear compartments of the cells forming the spheroids (Figure 5, B, lower panels, and C, right panels).
Figure 5.
NHERF1 depletion induces displacement of apical PTEN and nuclear translocation of β-catenin in 3D spheroids. (A) Western blot of proteins (30 μg) from whole cell lysates with either Myc or PTEN A2B1 antibodies shows expression of Myc-tagged PTEN and PTEN-ΔPDZ mutant in Caco-2 cells. Endogenous PTEN is indicated by arrowhead. (B) IF of 3D structures developing from control and NHERF1 shRNA-depleted (shNHERF1) cells expressing Myc-tagged PTEN is shown. (C) IF analysis of 3D glands expressing Myc-tagged PTEN and PTEN-ΔPDZ shows apical PM localization of wild-type PTEN (arrowhead) as in B but not of PTEN-ΔPDZ that is partially localized to the nucleus (arrows). NHERF1 depletion delocalizes PTEN to the cytoplasm (right panels). (D) Confocal IF shows strong nuclear β-catenin (arrows) in some cells of NHERF1-depleted spheroids.
We have previously shown that NHERF1 interacts with PTEN through the PDZ1 domain of NHERF1 and the PDZ-binding motif of PTEN [5,14]. Costaining with PTEN and NHERF1 antibodies showed that apical PTEN colocalized with NHERF1 in 3D glands (Figure 5C). Moreover, deletion of the last three amino acids that form the PDZ binding motif in PTEN (PTEN-ΔPDZ) (Figure 5A) prevented PTEN's apical PM localization and resulted in its concentration in cell nuclei (Figure 5C). These findings imply that the PDZ binding motif of PTEN is required for PTEN apical PM localization, most likely through interaction with NHERF1.
Although Caco-2 cells resemble normal intestinal cells, they are derived from CRC and have mutated Apc [24] that however does not seem to interfere with the degradation of β-catenin. Accordingly, β-catenin colocalized predominantly with E-cadherin at the baso-lateral membranes and was not observed in the nucleus of cells from control glands (Figure 5D). In NHERF1-depleted spheroids, a significant number of cells demonstrated nuclear β-catenin (Figure 5D, arrows), confirming previous data in 2D tissue culture [10,11].
Discussion
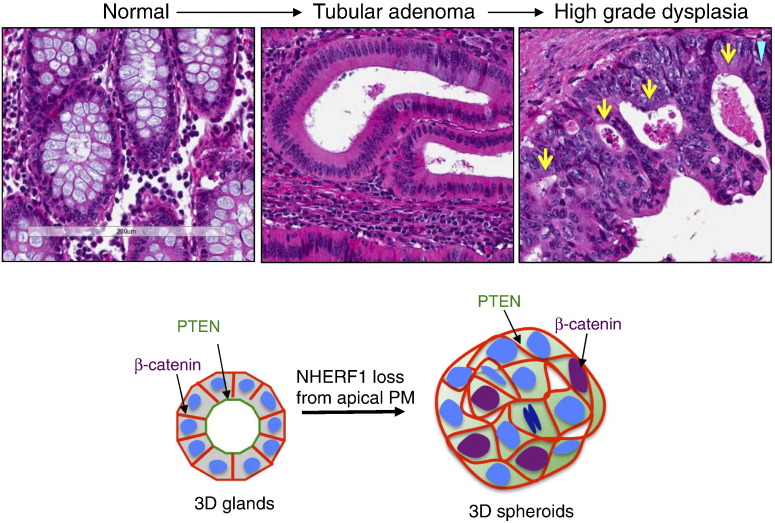
Accurate experimental modeling of pathologic changes observed in human conditions is a powerful tool for understanding the mechanism of disease and for therapy design. In this study, we modeled the initial NHERF1 molecular changes that we have reported in CRC [10] in a previously described 3D model of human intestinal gland formation [17], which was also tested by the introduction of K-Ras and B-Raf mutations occurring in a subset of human CRCs [25]. We noted strong correspondence between the pathologic changes occurring in the transformation sequence of CRC from normal-> adenoma -> carcinoma and the alteration by NHERF1 loss of the normal gland morphology toward tumorlike 3D structures characterized by enhanced and disorganized growth (Figure 6). These 3D spheroids share similar morphologic changes with colonic-epithelium adenomatous growth, characterized by crowded cells showing enlarged nuclei and increased number of mitoses, some of which are atypical. High-grade dysplasia, a feature of progression from adenoma to carcinoma characterized by cribriform architecture and total loss of polarity has correspondent changes in the formation of aborted lumina and polarity loss in NHERF1-depleted spheroids. A break of the basement membrane with invasion of neoplastic cells through muscularis mucosae characterizes adenocarcinoma. A detached or absent basement membrane in NHERF1-depleted spheroids, sometimes with cells budding off the main structure, may mimic initial phases of invasion (see Figure 2). The 3D changes induced by NHERF1 loss are best consistent with high-grade dysplasia, also called carcinoma in situ, and functionally implicate NHERF1 in CRC progression.
Figure 6.
Parallelism between morphologic changes occurring in the CRC transformation paradigm and in 3D glands by NHERF1 apical PM loss. Hematoxylin and eosin staining of a CRC resection specimen (original magnification, × 200) shows areas of normal mucosa, tubular adenoma, and high-grade dysplasia, the latter showing cribriform architecture (arrows), mitoses (arrowhead), nuclear enlargement and pleomorphism, and complete loss of cell polarity. The development of 3D spheroids by NHERF1 depletion reproduces high-grade dysplasia and validates the 3D intestinal gland system.
The mechanism by which NHERF1 controls cell polarity is related to its correct apical PM localization. NHERF1 is localized at the apical PM in both normal intestine surface epithelium and glands. As previously observed in polarized opossum kidney cells, disruption of the ERM-binding region redirects NHERF1 to the cytoplasm [14,26]. Expression of this ERM-binding deficient mutant with altered subcellular compartmentalization is sufficient to prevent Caco-2 gland morphogenesis with central lumen formation. These observations suggest that apically localized NHERF1 is required to maintain cell polarity, including the apical localization of ERM proteins, and that it is the interaction with ERM proteins that properly localizes NHERF1 at the apical PM. In contrast to knockout mice, where ezrin is the ERM protein involved in proper organization of the apical intestinal brush border membrane similarly to NHERF1 [8,27], specific ezrin knockdown in the human Caco-2 gland model had no effect on gland morphogenesis. This may be explained by a compensatory interaction of NHERF1 with moesin, which also colocalized apically with NHERF1 in 3D glands. Surprisingly, moesin knockdown disrupted gland morphogenesis similarly to NHERF1. Depletion of another ERM family member, neurofibromatosis type 2 (NF2), was shown to promote formation of Caco-2 3D glands with multiple lumens [28]. Interestingly, NF2 or Drosophila moesin knockdowns were also shown to alter spindle morphogenesis during mitosis, leading to chromosomal misalignment and multipolar mitoses [28–30]. Most likely, the membrane destabilization of ERM-NF2 proteins in NHERF1-depleted spheroids leads to defective cortical rigidity and spindle positioning during mitosis, with multipolar spindle formation. ERM proteins have high sequence similarity and functional redundancy, but nonredundant functions are being investigated [31]. They have been found differentially overexpressed or sometimes downregulated in various cancers [15,32]. Interestingly, moesin was found overexpressed in CRC compared to normal mucosa, and ezrin was found overexpressed in metastatic CRC compared to primary tumors [33,34]. More studies are therefore warranted to characterize the individual ERM protein expression and function in the CRC transformation sequence, as well as in relationship to NHERF1.
An important step in gland morphogenesis also involves the PDZ domain interactions of NHERF1. Of NHERF1's PDZ-domain ligands, PTEN has been shown to maintain high PIP2 levels at the apical PM, which are critical for apical-basal polarity in Drosophila photoreceptor epithelial cells and MDCK cell cysts [2,4,35]. Recently, PTEN knockdown has also been shown to disrupt Caco-2 3D gland morphogenesis [36]. However, how PTEN localizes at the apical PM remained unknown. A polarized distribution of PTEN was initially observed in migratory single cells of the slime mold Dictyostelium discoideum where PTEN and phosphoinositol 3-OH kinase were shown to occupy distinct PM compartments during chemotaxis [37,38]. Similar to MDCK cells, PTEN was required to maintain phosphoinositide partition in Dictyostelium during chemotaxis, and the PIP2-binding motif from PTEN N terminus was required for its PM localization [39]. Mutations in this motif were shown not only to disrupt PM binding but also to inactivate PTEN's tumor suppressor function in nonpolarized tumor cells [40–42]. In Drosphila epithelial cells, PTEN interaction and colocalization with Bazooka/Par-3 at the developing apically situated cell-cell junctions were described [35,43]. We show here that the C-terminal PDZ-binding motif, which is dispensable for PTEN's growth-suppressive function in nonpolarized tumor cells [16], provides the specialized signal for apical PM localization in polarized epithelial cells. NHERF1 stabilizes PTEN apically through PDZ-domain interactions [5], and NHERF1 loss leads to PTEN cytosolic redistribution. This change will, in turn, alter the distribution of PIP2 and contribute to the more severe apical-basal polarity disruption. Of note is that ERM proteins interact with PIP2 [44] and they are required for relaxing the “closed” conformation of NHERF1, allowing interaction with PTEN [14]. We conclude that the disruption of epithelial morphogenesis seen after NHERF1 depletion or apical displacement is a consequence of dissociation of NHERF1 complexes with both PTEN and the ERM proteins from the apical PM, leading to polarity loss.
Acknowledgments
The authors thank deeply Alan Hall and Noriko Kaji for their kindness in sharing the 3D intestinal gland system.
Footnotes
This work was supported by NCI-CA107201, American Recovery and Reinvestment Act ARRA supplement, (ARRA) supplement and GS Hogan Gastrointestinal Research Fund to M.-M.G. The authors declare no conflict of interest.
This article refers to supplementary materials, which are designated by Figures W1 to W3 and are available online at www.neoplasia.com.
Appendix A. Supplementary data
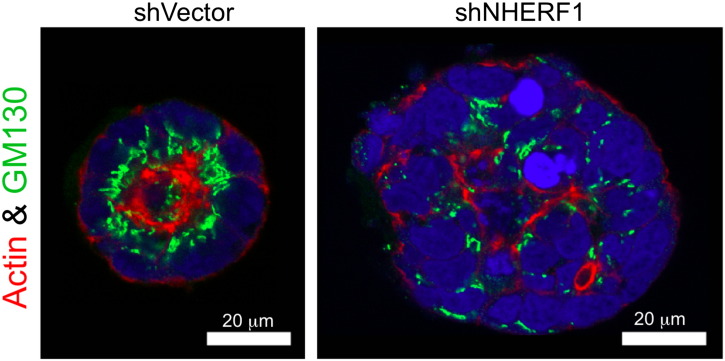
Figure W1.
Loss of Golgi complex polarized distribution by NHERF1 depletion. Confocal IF analysis of Golgi complex distribution with GM130 antibody, phalloidin labeling for actin cytoskeleton and TOPRO-3 iodide (blue) for the nuclei in the Caco-2 3D structures. Note polarized supranuclear Golgi localization in glands formed by control cells (shVector) and its complete disorganization in NHERF1 shRNA-depleted (shNHERF1) spheroids.
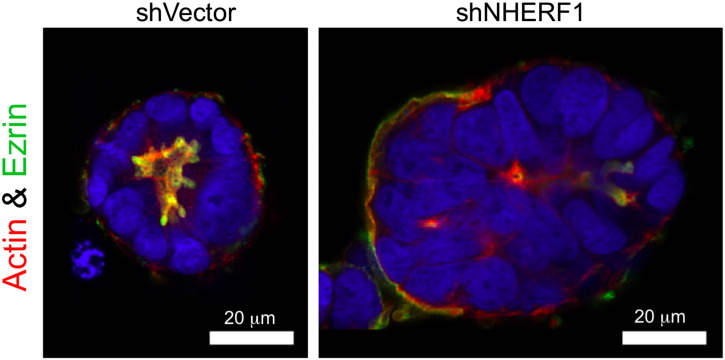
Figure W2.
Loss of ezrin polarized distribution by NHERF1 depletion. Confocal IF analysis of ezrin distribution with ezrin antibody, phalloidin labeling for actin cytoskeleton and TOPRO-3 iodide (blue) for the nuclei in the Caco-2 3D structures. Note apical PM ezrin localization in glands formed by control cells (shVector) and its complete displacement to the basal membrane or to minute aborted lumens in NHERF1 shRNA-depleted (shNHERF1) spheroids.
Figure W3.
Akt localizes at the basal cell membrane in 3D Caco-2 glands. A. Schematic drawing of a 3D gland with the polarized distribution of phosphoinositides. B. Western blot of whole cell lysates (30 μg proteins) showing expression of HA-tagged Akt and vector control in Caco-2 cells. Endogenous Akt is indicated. C. Confocal IF analysis of Caco-2 3D glands with HA and NHERF1 antibodies. Note basal membrane distribution of HA-Akt(arrow).
References
- 1.Mullin J.M. Epithelial barriers, compartmentation, and cancer. Sci STKE. 2004;126:pe2. doi: 10.1126/stke.2162004pe2. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Belmonte F., Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20:227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Maehama T., Dixon J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi Y., Morales F.C., Kreimann E.L., Georgescu M.M. PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J. 2006;25:910–920. doi: 10.1038/sj.emboj.7600979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stemmer-Rachamimov A.O., Wiederhold T., Nielsen G.P., James M., Pinney-Michalowski D., Roy J.E., Cohen W.A., Ramesh V., Louis D.N. NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am J Pathol. 2001;158:57–62. doi: 10.1016/S0002-9440(10)63944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broere N., Chen M., Cinar A., Singh A.K., Hillesheim J., Riederer B., Lünnemann M., Rottinghaus I., Krabbenhöft A., Engelhardt R. Defective jejunal and colonic salt absorption and altered Na+/H + exchanger 3 (NHE3) activity in NHE regulatory factor 1 (NHERF1) adaptor protein-deficient mice. Pflugers Arch. 2009;457:1079–1091. doi: 10.1007/s00424-008-0579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales F.C., Takahashi Y., Kreimann E.L., Georgescu M.M. Ezrin–radixin–moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci U S A. 2004;101:17705–17710. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales F.C., Hayashi Y., van Pelt C.S., Georgescu M.M. NHERF1/EBP50 controls lactation by establishing basal membrane polarity complexes with prolactin receptor. Cell Death Dis. 2012;3:e391. doi: 10.1038/cddis.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi Y., Molina J.R., Hamilton S.R., Georgescu M.M. NHERF1/EBP50 is a new marker in colorectal cancer. Neoplasia. 2010;12:1013–1022. doi: 10.1593/neo.10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreimann E.L., Morales F.C., de Orbeta-Cruz J., Takahashi Y., Adams H., Liu T.J., McCrea P.D., Georgescu M.M. Cortical stabilization of β-catenin contributes to NHERF1/EBP50 tumor suppressor function. Oncogene. 2007;26:5290–5299. doi: 10.1038/sj.onc.1210336. [DOI] [PubMed] [Google Scholar]
- 12.Mangia A., Chiriatti A., Bellizzi A., Malfettone A., Stea B., Zito F.A., Reshkin S.J., Simone G., Paradiso A. Biological role of NHERF1 protein expression in breast cancer. Histopathology. 2009;55:600–608. doi: 10.1111/j.1365-2559.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 13.Morales F.C., Molina J.R., Hayashi Y., Georgescu M.M. Overexpresssion of ezrin inactivates NF2 tumor suppressor in glioblastoma. Neuro Oncol. 2010;12:528–539. doi: 10.1093/neuonc/nop060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morales F.C., Takahashi Y., Momin S., Adams H., Chen X., Georgescu M.M. NHERF1/EBP50 head-to-tail intramolecular interaction masks association with PDZ domain ligands. Mol Cell Biol. 2007;27:2527–2537. doi: 10.1128/MCB.01372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X., Morales F.C., Agarwal N.K., Dogruluk T., Gagea M., Georgescu M.M. Moesin is a glioma progression marker that induces proliferation and Wnt/β-catenin pathway activation via interaction with CD44. Cancer Res. 2013;73:1142–1155. doi: 10.1158/0008-5472.CAN-12-1040. [DOI] [PubMed] [Google Scholar]
- 16.Georgescu M.M., Kirsch K.H., Akagi T., Shishido T., Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci U S A. 1999;96:10182–10187. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffe A.B., Kaji N., Durgan J., Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–633. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reczek D., Berryman M., Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinman E.J., Steplock D., Tate K., Hall R.A., Spurney R.F., Shenolikar S. Structure–function of recombinant Na/H exchanger regulatory factor (NHE-RF) J Clin Invest. 1998;101:2199–2206. doi: 10.1172/JCI204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata T., Chuma M., Kokubu A., Sakamoto M., Hirohashi S. EBP50, a β–catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology. 2003;38:178–186. doi: 10.1053/jhep.2003.50270. [DOI] [PubMed] [Google Scholar]
- 21.Simon-Assmann P., Turck N., Sidhoum-Jenny M., Gradwohl G., Kedinger M. In vitro models of intestinal epithelial cell differentiation. Cell Biol Toxicol. 2007;23:241–256. doi: 10.1007/s10565-006-0175-0. [DOI] [PubMed] [Google Scholar]
- 22.Georgescu M.M., Morales F.C., Molina J.R., Hayashi Y. Roles of NHERF1/EBP50 in cancer. Curr Mol Med. 2008;8:459–468. doi: 10.2174/156652408785748031. [DOI] [PubMed] [Google Scholar]
- 23.Sulis M.L., Parsons R. PTEN: from pathology to biology. Trends Cell Biol. 2003;13:478–483. doi: 10.1016/s0962-8924(03)00175-2. [DOI] [PubMed] [Google Scholar]
- 24.Gayet J., Zhou X.P., Duval A., Rolland S., Hoang J.M., Cottu P., Hamelin R. Extensive characterization of genetic alterations in a series of human colorectal cancer cell lines. Oncogene. 2001;20:5025–5032. doi: 10.1038/sj.onc.1204611. [DOI] [PubMed] [Google Scholar]
- 25.Magudia K., Lahoz A., Hall A. K-Ras and B-Raf oncogenes inhibit colon epithelial polarity establishment through up-regulation of c-myc. J Cell Biol. 2012;198:185–194. doi: 10.1083/jcb.201202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernando N., Déliot N., Gisler S.M., Lederer E., Weinman E.J., Biber J., Murer H. PDZ-domain interactions and apical expression of type IIa Na/Pi cotransporters. Proc Natl Acad Sci U S A. 2002;99:11957–11962. doi: 10.1073/pnas.182412699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saotome I., Curto M., McClatchey A.I. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Hebert A.M., DuBoff B., Casaletto J.B., Gladden A.B., McClatchey A.I. Merlin/ERM proteins establish cortical asymmetry and centrosome position. Genes Dev. 2012;26:2709–2723. doi: 10.1101/gad.194027.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carreno S., Kouranti I., Glusman E.S., Fuller M.T., Echard A., Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J Cell Biol. 2008;180:739–746. doi: 10.1083/jcb.200709161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunda P., Pelling A.E., Liu T., Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 31.Hirata T., Nomachi A., Tohya K., Miyasaka M., Tsukita S., Watanabe T., Narumiya S. Moesin-deficient mice reveal a non-redundant role for moesin in lymphocyte homeostasis. Int Immunol. 2012;24:705–717. doi: 10.1093/intimm/dxs077. [DOI] [PubMed] [Google Scholar]
- 32.Clucas J., Valderrama F. ERM proteins in cancer progression. J Cell Sci. 2014;127:267–275. doi: 10.1242/jcs.133108. [DOI] [PubMed] [Google Scholar]
- 33.Kim C.Y., Jung W.Y., Lee H.J., Kim H.K., Kim A., Shin B.K. Proteomic analysis reveals overexpression of moesin and cytokeratin 17 proteins in colorectal carcinoma. Oncol Rep. 2012;27:608–620. doi: 10.3892/or.2011.1545. [DOI] [PubMed] [Google Scholar]
- 34.Leiphrakpam P.D., Rajput A., Mathiesen M., Agarwal E., Lazenby A.J., Are C., Brattain M.G., Chowdhury S. Ezrin expression and cell survival regulation in colorectal cancer. Cell Signal. 2014;26:868–879. doi: 10.1016/j.cellsig.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinal N., Goberdhan D.C., Collinson L., Fujita Y., Cox I.M., Wilson C., Pichaud F. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol. 2006;16:140–149. doi: 10.1016/j.cub.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 36.Jagan I., Fatehullah A., Deevi R.K., Bingham V., Campbell F.C. Rescue of glandular dysmorphogenesis in PTEN-deficient colorectal cancer epithelium by PPARγ-targeted therapy. Oncogene. 2013;32:1305–1315. doi: 10.1038/onc.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funamoto S., Meili R., Lee S., Parry L., Firtel R.A. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 38.Iijima M., Huang Y.E., Devreotes P. Temporal and spatial regulation of chemotaxis. Dev Cell. 2002;3:469–478. doi: 10.1016/s1534-5807(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 39.Iijima M., Huang Y.E., Luo H.R., Vazquez F., Devreotes P.N. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem. 2004;279:16606–16613. doi: 10.1074/jbc.M312098200. [DOI] [PubMed] [Google Scholar]
- 40.Denning G., Jean-Joseph B., Prince C., Durden D.L., Vogt P.K. A short N-terminal sequence of PTEN controls cytoplasmic localization and is required for suppression of cell growth. Oncogene. 2007;26:3930–3940. doi: 10.1038/sj.onc.1210175. [DOI] [PubMed] [Google Scholar]
- 41.Rahdar M., Inoue T., Meyer T., Zhang J., Vazquez F., Devreotes P.N. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci U S A. 2009;106:480–485. doi: 10.1073/pnas.0811212106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker S.M., Leslie N.R., Perera N.M., Batty I.H., Downes C.P. The tumour-suppressor function of PTEN requires an N-terminal lipid-binding motif. Biochem J. 2004;379:301–307. doi: 10.1042/BJ20031839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Stein W., Ramrath A., Grimm A., Müller-Borg M., Wodarz A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development. 2005;132:1675–1686. doi: 10.1242/dev.01720. [DOI] [PubMed] [Google Scholar]
- 44.Hirao M., Sato N., Kondo T., Yonemura S., Monden M., Sasaki T., Takai Y., Tsukita S., Tsukita S. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]