Abstract
Cordycepin is one of the most important bioactive compounds produced by species of Cordyceps sensu lato, but it is hard to produce large amounts of this substance in industrial production. In this work, single factor design, Plackett-Burman design, and central composite design were employed to establish the key factors and identify optimal culture conditions which improved cordycepin production. Using these culture conditions, a maximum production of cordycepin was 2008.48 mg/L for 700 mL working volume in the 1000 mL glass jars and total content of cordycepin reached 1405.94 mg/bottle. This method provides an effective way for increasing the cordycepin production at a large scale. The strategies used in this study could have a wide application in other fermentation processes.
1. Introduction
Cordyceps militaris is an entomopathogenic fungus belonging to Ascomycota, Sordariomycetidae, Hypocreales, and Cordycipitaceae [1] and is one of the most important traditional Chinese medicinal mushrooms. Cordyceps militaris is the type species of Cordyceps, which internally parasitizes larva or pupa of lepidopteran insects and forms fruiting bodies on their insect hosts. Cordyceps militaris has long been recognized as a desirable alternative for natural Ophiocordyceps sinensis [2] as it has been given Chinese Licence number Z20030034/35. This is because the gathering of Ophiocordyceps sinensis is causing substantial reductions in populations [3]. Cordyceps militaris produces many bioactive compounds, including polysaccharides, cordycepin, adenosine, amino acid, organic selenium, ergosterol, sterols, cordycepic acid, superoxide dismutase (SOD), and multivitamins [4, 5].
Cordycepin (3′-deoxyadenosine), a nucleoside analog, was first isolated from C. militaris [6] and is one of the species most important biologically active metabolites. It has been regarded as a medicinal agent responsible for immunological regulation [7], anticancer [8], antifungus [9], antivirus [10], antileukemia [11, 12], and antihyperlipidemia [13] activities. Cordycepin is also a Phase I/II clinical stage drug candidate for treatment of refractory acute lymphoblastic leukemia (ALL) patients who express the enzyme terminal deoxynucleotidyl transferase (TdT) (http://www.ClinicalTrials.Gov verified by OncoVista, Inc., 2009).
In previous work, cordycepin has been synthesized by chemical [14, 15] and microbial fermentation using C. militaris [6] or Aspergillus nidulans [16, 17]. Solid-state fermentation [18, 19], submerged culture [4, 20–24], and surface liquid culture [25–27] have been used in microbial fermentation of cordycepin. Cordycepin obtained through chemistry pathways is hard to purify, and the cost is much higher than through biology fermentation. Thus a major need is to improve the biology methodology [28]. Fermentation time is too long and is difficult to achieve large scale production via solid-state fermentation [18, 19]. Productivity is generally low, the costs are high, and fermentation processes are easily contaminated in submerged culture in large fermenters [4, 20, 21, 29]. Productivity in surface culture techniques is higher as compared to other methods [29, 30] and the cost is lower [23]. New technologies, such as space mutation treatment and high-energy ion beam irradiation, have been used to obtain better Cordycepin producing, novel mutants of C. militaris. The resulting mutants were higher cordycepin produces, than the wild strain [30, 31]. Bu et al. [20] reported that the cordycepin in C. militaris was substantially increased by the elicitor of Phytophthora sp. Research result showed that glucose and yeast extract were effective media components for improved cordycepin production by C. militaris [32, 33]. There have been other studies using different culture conditions [21, 24, 25, 32], culture medium, and additives [4, 22–24, 26, 27] for the production of cordycepin via liquid culture. However, as far as we know, these reports studied cordycepin production in 250 mL or 500 mL Erlenmeyer flasks, and there have been no reports to improved cordycepin production using static liquid culture in 1000 mL glass jars. The latter process is a good way to scale up large scale cordycepin production from the laboratory to industry.
In this study, the effects of working volume, carbon sources, nitrogen sources, inorganic salts, growth factor, nucleoside analogue, and amino acid additions were studied in order to improve the cordycepin production by static liquid culture of C. militaris (strain CGMCC2459) in 1000 mL glass jars. The results suggested that the optimization medium conditions were helpful for improved large scale cordycepin production.
2. Materials and Methods
2.1. Microorganism and Seed Culture
The isolate of C. militaris (strain CGMCC2459) used in the present study was collected from Mt. Qingcheng in Sichuan Province, China. The microorganism was maintained on potato dextrose agar (PDA) slants. Slants were incubated at 25°C for 7 days and then stored at 4°C. The seed culture was grown in a 250 mL flask containing 70 mL of basal medium (sucrose 20 g/L; peptone 20 g/L; KH2PO4 1 g/L; and MgSO4 ·7H2O 0.5 g/L) at 25°C on a rotary shaker incubator at 150 rev/min for 5 days [24].
2.2. Basal Medium and Static Culture of Glass Jars
The basal medium composition for the fermentation was as follows: sucrose 20 g/L; peptone 20 g/L; KH2PO4 1 g/L; and MgSO4 ·7H2O 0.5 g/L. The pH was not adjusted, followed by autoclaving for 30 min on the 121°C. The static culture experiments were performed in 1000 mL glass jars (inner diameter 110 mm, height 150 mm) containing basal medium after inoculating with 10% (v/v; the biomass dry weight of seed culture is 54 mg/mL) of the seed culture. The culture was incubated at 25°C without moving for 35 days, and samples were collected at the end of the fermentation from the glass jars for analyzing biomass dry weight and cordycepin production.
2.3. Static Culture Conditions
The effects of factors affecting cell growth and the production of cordycepin by C. militaris were studied using a one-factor-at-a-time method for static culture. The effects of carbon sources on cordycepinproduction were studied by substituting carbon sources such as sucrose, lactose, soluble starch, and dextrin for glucose at 25°C for 35 days. Effects of nitrogen sources (yeast extract, beef extract, NH4NO3, NaNO3, NH4Cl, casein, and carbamide) and inorganic salts (MgCl2 ·6H2O, MgSO4 ·7H2O, KCl, ZnSO4, CaCl2 ·2H2O, CaSO4 ·2H2O, FeSO4 ·7H2O, and K2HPO4 ·3H2O) were also studied using static culture. Growth factors (Vitamin B1 (VB1), Vitamin B6 (VB6), Vitamin B7 (VB7), Vitamin B11 (VB11), α-naphthylacetic acid (NAA), 3-Indoleacetlc acid (IAA), and 2,4-dichlorophenoxyacetic acid (2,4-D)) were supplemented for 10 mg/L in basal media. Nucleoside analogues (1 g/L) and amino acids (8 g/L) established in our previous study [23] as an initial concentration were separately added to the optimal concentration of carbon and nitrogen source, inorganic salts, and growth factors and cultivated at 25°C for 35 days. All experiments were carried out at triplicate, and mean of results is presented.
2.4. Analytical Methods
Samples collected at 35 days from the glass jars were centrifuged at 2810 ×g for 20 min. The mycelium at the bottom of tubes was washed sufficiently with a large amount of distilled water and dried to a constant dry weight at 55°C.
For analysis of extracellular cordycepin, the resulting culture filtrate was obtained by centrifugation at 2810 ×g for 20 min. The supernatant was filtered through a 0.45 μm membrane and the filtrate was analyzed by HPLC (1100 series, Agilent Technology, USA). Accurate quantities of cordycepin (Sigma, USA) were dissolved in distilled water, to give various concentrations for calibration. The mobile phase was 10 mmol/L KH2PO4, which was dissolved in methanol/distilled water (6 : 94). Elution was performed at a flow rate of 1.0 mL/min with column temperature at 45°C and UV wavelength of 259 nm. Mean values were computed from triplicate samples.
2.5. Plackett-Burman Design
The Plackett-Burman design, an effective technique for medium-component optimization [35, 36], was used to select factors that significantly influenced hydrogen production. Sucrose (X 1), peptone (X 2), K2HPO4 ·3H2O (X 4), MgSO4 ·7H2O (X 5), and VB1 (X 6) were investigated as key ingredients affecting cordycepin production. Based on the Plackett-Burman design, a 15-run was applied to evaluate eleven factors (including two virtual variables). Each factor was prepared in two levels: −1 for low level and +1 for high level. Table 1 illustrates the variables and their corresponding levels used in the experimental design. The values of two levels were set according to our preliminary experimental results. The Plackett-Burman design and the response value of cordycepin production are shown in Table 2.
Table 1.
Range of different factors investigated with Plackett-Burman design.
| Symbol | Variables | Experimental value | |
|---|---|---|---|
| Low (−1) | High (+1) | ||
| X 1 | Sucrose (g/L) | 20 | 25 |
| X 2 | Peptone (g/L) | 20 | 25 |
| X 3 | Virtual 1 | −1 | 1 |
| X 4 | K2HPO4 ·3H2O (g/L) | 1 | 1.25 |
| X 5 | VB1 (g/L) | 10 | 12.5 |
| X 6 | MgSO4 ·7H2O (g/L) | 1 | 1.25 |
| X 7 | Virtual 2 | −1 | 1 |
Table 2.
Plackett-Burman design and response values.
| Runs | Experimental value | Y (mg/L) Cordycepin production | ||||||
|---|---|---|---|---|---|---|---|---|
| X 1 | X 2 | X 3 | X 4 | X 5 | X 6 | X 7 | ||
| 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 812.36 ± 26.83 |
| 2 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1395.18 ± 8.4 |
| 3 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | 900.25 ± 10.29 |
| 4 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | 802.45 ± 45.43 |
| 5 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | 1097.66 ± 25.57 |
| 6 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 845.87 ± 24.94 |
| 7 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | 786.35 ± 7.61 |
| 8 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 805.08 ± 29.1 |
| 9 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 920.48 ± 16.21 |
| 10 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | 694.01 ± 79.51 |
| 11 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 497.28 ± 4.44 |
| 12 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 592.83 ± 16.13 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1134.14 ± 2.59 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1100.21 ± 0.08 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1133.56 ± 1.85 |
2.6. Response Surface Methodology
Response surface methodology using a central composite design was applied to batch cultures of C. militaris, for identifying the effects of process variables [35, 36]. In this study, the basic nutrient (carbon sources, nitrogen sources, inorganic salts, and growth factors) and additives (amino acid, nucleoside analogue) were studied for cordycepin production using static liquid culture. In the first test, a three-factor, five-level central composite design with 20 runs was employed. Tested variables (sucrose, K2HPO4 ·3H2O, and MgSO4 ·7H2O) were denoted as X 1, X 4, and X 6, respectively, and each of them was assessed at five different levels, combining factorial points (−1, −1), axial points (−1.6818, +1.6818), and central point (0), as shown in Table 3. Based on the above results, another test, a three-factor, five-level central composite design with 20 runs was employed. Tested variables (amino acid, nucleoside analogue, and culture time) were denoted as A, B, and C, respectively, and each of them was assessed at five different levels, combining factorial points (−1, +1), axial points (−1.6818, +1.6818), and central point (0), as shown in Table 4.
Table 3.
Factors and levels of central composite design for carbon sources and inorganic salts.
| Symbol | Variables | Code level | ||||
|---|---|---|---|---|---|---|
| −1.6818 | −1 | 0 | 1 | 1.6818 | ||
| X 1 | Sucrose (g/L) | 3.1821 | 10 | 20 | 30 | 36.8179 |
| X 4 | K2HPO4 ·3H2O (g/L) | 0.1591 | 0.5 | 1 | 1.5 | 1.8409 |
| X 6 | MgSO4 ·7H2O (g/L) | 0.1591 | 0.5 | 1 | 1.5 | 1.8409 |
Table 4.
Factors and levels of central composite design for amino acid, nucleoside analogue, and time.
| Symbol | Variables | Code level | ||||
|---|---|---|---|---|---|---|
| −1.6818 | −1 | 0 | 1 | 1.6818 | ||
| A | Hypoxanthine (g/L) | 0.53 | 1 | 5 | 9 | 10.53 |
| B | L-alanine (g/L) | 5.27 | 8 | 12 | 16 | 18.72 |
| C | Culture time (days) | −0.09 | 4 | 10 | 16 | 20.09 |
2.7. Statistical Analysis
Dry weight and cordycepin production are expressed as means ± SD. An analysis of variance (ANOVA) followed by Tukey's test was applied for multiple comparisons of significant analyses at P < 0.05. Statistical data analyses were performed in SPSS version 17.0 software packet. Design-Expert Version 8.0.5b software package (Stat-Ease Inc., Minneapolis, USA) was used for designing experiments as well as for regression and graphical analysis of the experimental data obtained.
3. Results and Discussion
3.1. Effects of Working Volume on the Biomass and Cordycepin Production
Dissolved oxygen concentration is the key factor in the medium for cell growth and metabolite biosynthesis [21]. Dissolved oxygen does not only have an important function in the respiratory chain, but also in metabolite composition [37, 38]. A previous study showed that the highest cordycepin production and productivity were obtained at lower dissolved oxygen levels [21]. Masuda et al. [25] also reported that a lower medium depth was most efficient for cordycepin production in C. militaris by surface culture.
In this study, we tried to establish the most efficient working volume of medium for improved cordycepin production. Cultures of C. militaris were prepared at the working volumes of 100 to 900 mL (corresponding to a medium depth of 1.26 to 11.31 cm). As shown in Figure 1, cordycepin production reduced gradually with increasing working volume of the medium, from 100 to 700 mL. However, there was no significant difference in cordycepin production in different working volumes. Obviously, the highest working volume of 900 mL did not help in cordycepin production. The result indicates that there is an upper dissolved oxygen limit in the medium for cordycepin production [21]. Lower working volumes result in higher cordycepin productivity, with the highest peak (463.33 ± 56.72 mg of cordycepin) produced at using 700 mL of media. Changes in biomass values were small (between 300 and 700 mL) because of the restricted area and thickness of the mycelial mat. In order to obtain higher cordycepin production, the most effective medium amount was 700 mL (corresponding to an 8.8 cm medium depth) and used as the media volume for next experiment.
Figure 1.
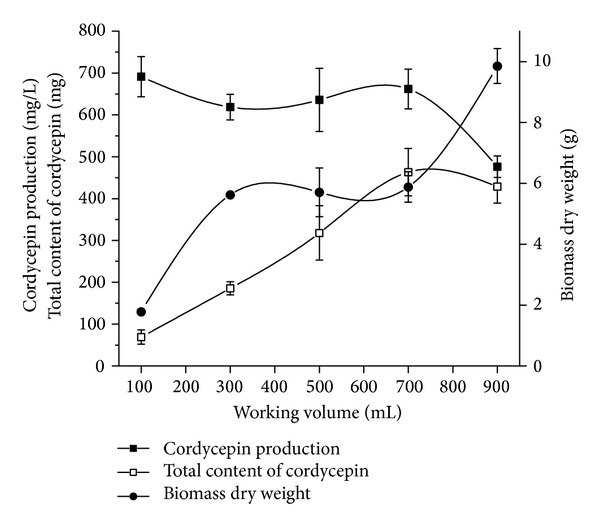
Effects of working volume on the production of cordycepin, total production of cordycepin, and biomass dry weight (total content ofcordycepin (mg) = cordycepin production (mg/L) × working volume (mL)).
3.2. Effects of Carbon and Nitrogen Sources on Cordycepin Production
To find a suitable carbon source for C. militaris cordycepin production we added various carbon sources at a concentration of 20 g/L to the sugar-free basal medium. Glucose was previously found to be an excellent precursor of cordycepin production [39]. However, as shown in Figure 2(a), sucrose and lactose proved to be better carbon sources for cordycepin production than glucose in this study. Cordycepin production reached 843.63 ± 66.70 mg/L of sucrose and 823.72 ± 85.64 mg/L of lactose, respectively. Therefore, sucrose was selected as the main carbon source in the remaining experiment.
Figure 2.
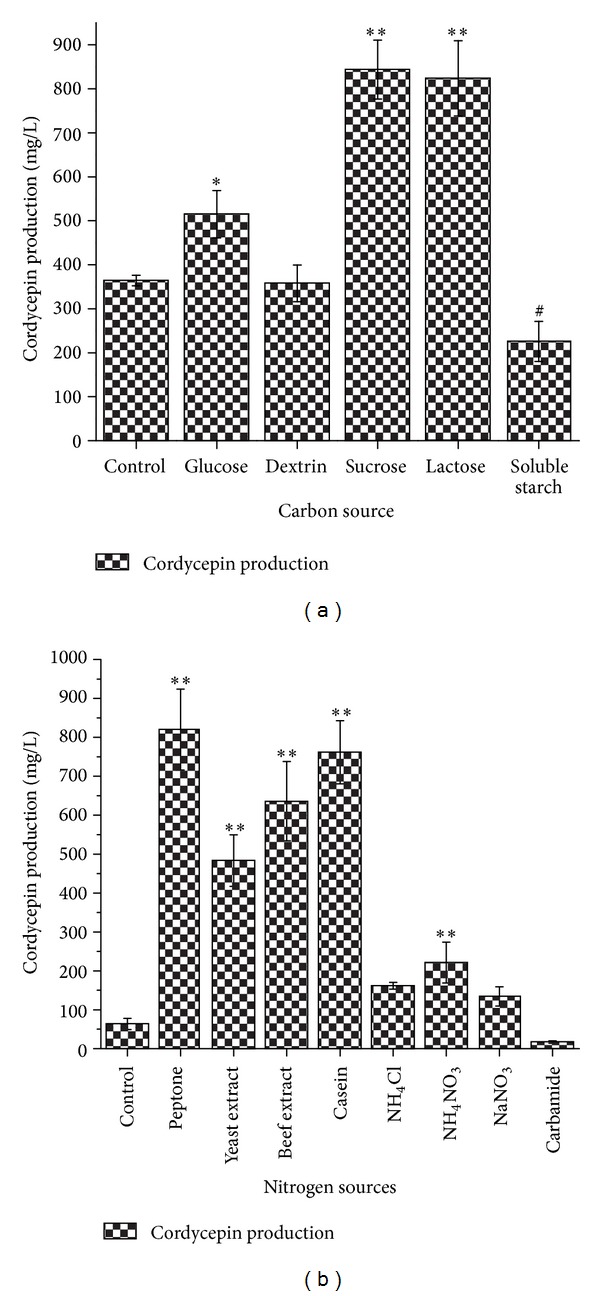
Effects of carbon sources and nitrogen sources on the production of cordycepin: carbon sources (a); nitrogen sources (b); *5% significance level (test group versus control group); **1% significance level (test group versus control group); #5% significance level (control group versus test group).
In previous work, nitrogen showed a regulating role important in cordycepin production and had two effects [40]. One effect was negative since, in excess, N promoted a faster mycelial growth and consequently diverted the source of carbon toward energy and biomass production. The other effect was positive because a moderate input contributed to the maintenance of citric acid productive biomass [40]. To investigate the effect of nitrogen sources on cordycepin production in C. militaris, various compounds containing nitrogen (inorganic and organic nitrogen) were added individually to nitrogen free basal medium at a concentration of 20 g/L. Among the 8 nitrogen sources tested, peptone, yeast extract, beef extract, casein, and NH4NO3 were favorable to the cordycepin production (Figure 2(b)). Organic nitrogen was advantageous to both growth and biosynthesis of metabolites. The result is consistent with the experimental data reported [18] and showed that maximum cordycepin production resulted when the peptone was used as a nitrogen source.
3.3. Effects of Inorganic Salt and Growth Factor on the Cordycepin Production
Inorganic ion was one of the most important nutrition components of medium for the mycelial growth [41]. In order to investigate the effects of inorganic salt for the cordycepin production in C. militaris, we tested nine types (at 1 g/L) of inorganic salts (Figure 3(a)). Media with only 20 g/L glucose and 20 g/L peptone were used as the control. The highest cordycepin production (1120.30 ± 105.28 mg/L) by C. militaris was observed in medium, when K2HPO4 ·3H2O was used as an inorganic salt. KH2PO4, MgSO4 ·7H2O, KCl, and MgCl2 ·6H2O were also useful inorganic salts. At last, MgSO4 ·7H2O and K2HPO4 ·3H2O were recognized as favorable bioelements for production of cordycepin.
Figure 3.
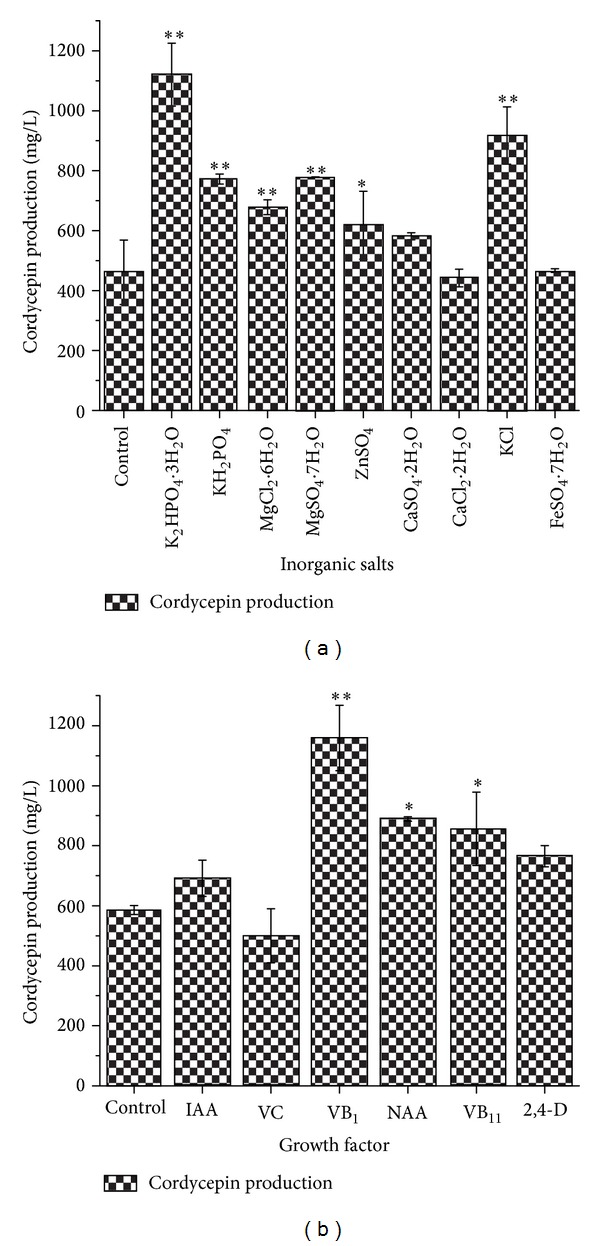
Effects of inorganic salt and growth factors on the production of cordycepin: inorganic salt (a); growth factors (b); *5% significance level (test group versus control group); **1% significance level (test group versus control group).
Growth factor is essential for growth response and metabolite production [42]. In order to find the optimal growth factor for cordycepin production, C. militaris was cultured in a basal medium with different vitamins and plant growth hormones in static liquid culture. Cordycepin production increased in media with added 10 mg/L of VB1, NAA, and VB11 (Figure 3(b)). Maximum cordycepin production (1159.34 ± 109.01 mg/L) occurred when VB1 was used as the growth factor.
3.4. Screening of Important Variables Using Plackett-Burman Design
The data (Table 2) indicated wide variation in cordycepin production in the 15 tests. The data suggested that process optimization is important for improving the efficiency of cordycepin production. Analysis of the regression coefficients and t values of 7 factors (Table 5) showed that X 1, X 2, X 4, and X 5 had positive effects on cordycepin production. X 6 had negative effects. The variable affects with a confidence level above 95% are considered as significant factors. Based on these results, three factors (X 1, sucrose; X 4, K2HPO4 ·3H2O; and X 6, MgSO4 ·7H2O) were considered as significant for cordycepin production by static liquid culture methodology.
Table 5.
Results of regression analysis of Plackett-Burman design.
| Symbol | Regression analysis | ||||
|---|---|---|---|---|---|
| Effect | Coefficient | Standard error | T | P | |
| 845.82 | 32.86 | 25.74 | 0.000** | ||
| X 1 | 190.88 | 95.44 | 32.86 | 2.90 | 0.027* |
| X 2 | 149.23 | 74.62 | 32.86 | 2.27 | 0.064 |
| X 3 | −40.85 | −20.42 | 32.86 | −0.62 | 0.557 |
| X 4 | 244.10 | 122.05 | 32.86 | 3.71 | 0.010* |
| X 5 | 62.81 | 31.41 | 32.86 | 0.96 | 0.376 |
| X 6 | −176.15 | −88.08 | 32.86 | −2.68 | 0.037* |
| X 7 | −127.39 | −63.69 | 32.86 | −1.94 | 0.101 |
| Ct Pt | 276.82 | 73.48 | 3.77 | 0.009** | |
*5% significance level; **1% significance level; X 1–X 7 are symbols shown in Table 1.
3.5. Optimization by Response Surface Methodology for Carbon Sources and Inorganic Salts
In order to evaluate the influence of medium component on cordycepin production, sucrose, K2HPO4 ·3H2O, and MgSO4 ·7H2O should be examined. The levels of variables for central composite design experiments were selected according to the above results of Plackett-Burman design. Table 6 shows the detailed experimental design and results. Regression analysis was performed to fit the response function (cordycepin production) with the experimental data. From the variables obtained (Table 6), the model is expressed by (1), which represents cordycepin production (Y 1) as a function of sucrose (X 1), K2HPO4 ·3H2O (X 4), and MgSO4 ·7H2O (X 6) concentrations:
| (1) |
Table 6.
Experimental design and responses of the central composite design for carbon sources and inorganic salts.
| Run | Variables Code |
Y (mg/L) Cordycepin production |
Run | Variables Code |
Y (mg/L) Cordycepin production |
||||
|---|---|---|---|---|---|---|---|---|---|
| X 1 | X 4 | X 6 | X 1 | X 4 | X 6 | ||||
| 1 | −1 | −1 | −1 | 1080.55 ± 109.69 | 11 | 0 | 0 | 0 | 1399.43 ± 124.44 |
| 2 | 1 | −1 | −1 | 1359.48 ± 12.61 | 12 | 0 | 0 | 0 | 1415.43 ± 42.13 |
| 3 | −1 | 1 | −1 | 1158.59 ± 12.15 | 13 | 0 | 0 | 0 | 1487.42 ± 16.38 |
| 4 | 1 | 1 | −1 | 1289.90 ± 47.00 | 14 | 0 | 0 | 0 | 1409.43 ± 155.22 |
| 5 | −1 | −1 | 1 | 980.55 ± 34.72 | 15 | −1.6818 | 0 | 0 | 876.91 ± 16.69 |
| 6 | 1 | −1 | 1 | 1097.48 ± 52.42 | 16 | 1.6818 | 0 | 0 | 1290.00 ± 14.71 |
| 7 | −1 | 1 | 1 | 987.95 ± 2.89 | 17 | 0 | −1.6818 | 0 | 1110.57 ± 157.63 |
| 8 | 1 | 1 | 1 | 1117.38 ± 116.84 | 18 | 0 | 1.6818 | 0 | 1344.66 ± 65.54 |
| 9 | 0 | 0 | 0 | 1485.38 ± 12.19 | 19 | 0 | 0 | −1.6818 | 958.39 ± 224.14 |
| 10 | 0 | 0 | 0 | 1333.48 ± 94.22 | 20 | 0 | 0 | 1.6818 | 958.00 ± 75.82 |
Results of F-test analysis of variance (ANOVA) showed that the regression was statistically significant at 95% and 99% confidence levels (Table 7). The “F value” of the model was 9.21, and the value of “Prob > F” < 0.01 indicated that the model was significant. In this case, linear terms of X 1 and quadratic terms of X 1 2, X 4 2, X 6 2 were significant of model terms for cordycepin production. The “Lack of Fit F value” of 0.0903 implied that the “Lack of Fit” was not significant relative to the pure error (P > 0.05). The Pred-R 2 of 0.3183 was not as close to the Adj-R 2 of 0.7954 as one might normally expect. The result suggested that some factors were not considered in the model. However, the “Adeq Precision” of 8.173 indicated that the model was adequate for prediction production of cordycepin.
Table 7.
ANOVA for response surface quadratic polynomial model for carbon sources and inorganic salts.
| Source | Sum of quares | df | Mean Square | F-value | P-value Prob > F |
|---|---|---|---|---|---|
| Model | 6.507E + 005 | 9 | 72300.77 | 9.21 | 0.0009** |
| X 1-X 1 | 1.337E + 005 | 1 | 1.337E + 005 | 17.03 | 0.0021** |
| X 4-X 4 | 13504.30 | 1 | 13504.30 | 1.72 | 0.2190 |
| X 6-X 6 | 36477.48 | 1 | 36477.48 | 4.65 | 0.0565 |
| X 1 X 4 | 2282.55 | 1 | 2282.55 | 0.29 | 0.6016 |
| X 1 X 6 | 3356.89 | 1 | 3356.89 | 0.43 | 0.5279 |
| X 4 X 6 | 44.38 | 1 | 44.38 | 5.653E − 003 | 0.9416 |
| X 1 2 | 1.620E + 005 | 1 | 1.620E + 005 | 20.63 | 0.0011** |
| X 4 2 | 43685.18 | 1 | 43685.18 | 5.56 | 0.0400** |
| X 6 2 | 3.256E + 005 | 1 | 3.256E + 005 | 41.47 | <0.0001∗∗ |
| Residual | 78513.73 | 10 | 7851.37 | ||
| Lack of Fit | 61670.32 | 5 | 12334.06 | 3.66 | 0.0903 |
| Pure Error | 16843.41 | 5 | 3368.68 | ||
| Cor Total | 7.292E + 005 | 19 |
R 2 = 0.8923; CV = 7.34%; Pred-R 2 = 0.3183; Adj-R 2 = 0.7954; Adeq Precision = 8.173; ∗5% significance level; ∗∗1% significance level.
The response surface plot obtained from (1) is shown in Figure 4. It is evident that cordycepin production reached its maximum at a combination of coded level (X 1, sucrose, level 0.47; X 4, K2HPO4 ·3H2O, level 0.21; X 6, MgSO4 ·7H2O, level −0.20) when using canonical analysis of the Design-Expert Version 8.0.5b software package. The model predicted a maximum response of 1451.43 mg/L cordycepin production at levels of sucrose 24.7 g/L, K2HPO4 ·3H2O 1.11 g/L, and MgSO4 ·7H2O 0.90 g/L as optimized medium components.
Figure 4.
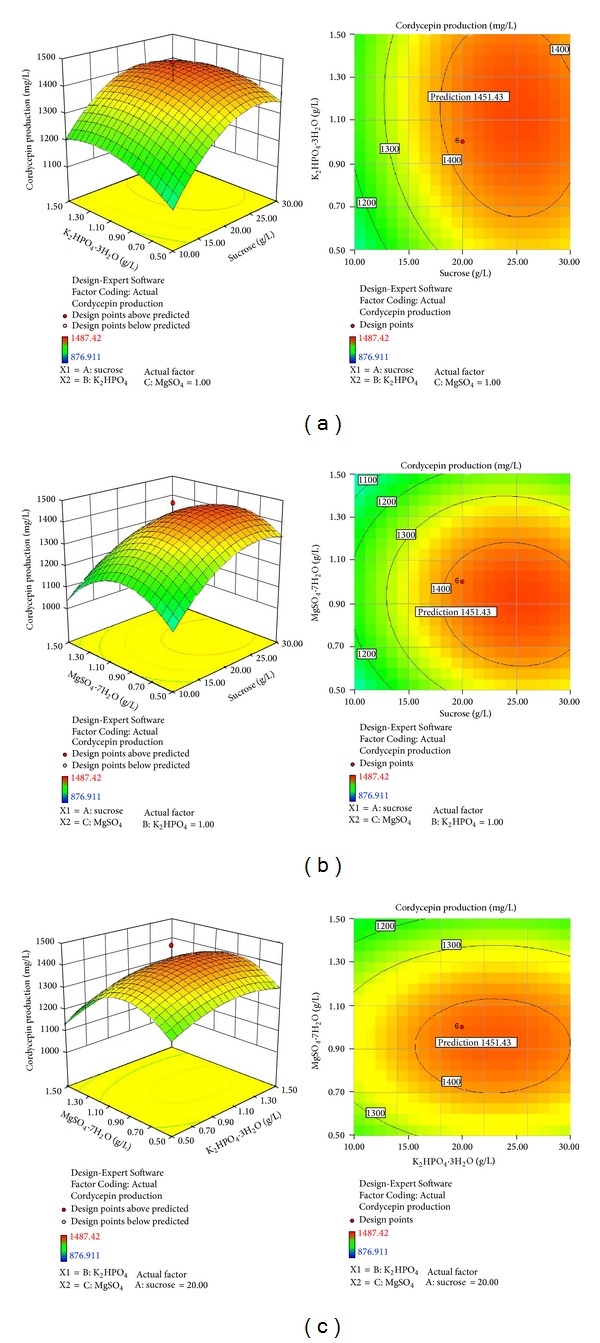
Three-dimensional response surface plots and two-dimensional contour plots for cordycepin production by C. militaris (strain CGMCC2459) showing variable interactions of (a) sucrose and K2HPO4 ·3H2O; (b) sucrose and MgSO4 ·7H2O; (c) K2HPO4 ·3H2O and MgSO4 ·7H2O.
3.6. Effects of Nucleoside Analogue and Amino Acid on the Production of Cordycepin
Chassy and Suhadolnik [43] reported that adenine and adenosine were precursors for cordycepin synthesis. Amino acids were regarded as the best substance for improved cordycepin production [4, 23]. Based on these results, among 10 different kinds of nucleoside analogue were supplemented for 1 g/L in this study. As shown in Figure 5(a), cordycepin production increased obviously in the medium with hypoxanthine, thymine, and thymidine additives. The highest production of cordycepin was achieved, when hypoxanthine was used as the nucleoside analogue. Hypoxanthine's molecular structure is similar to purine bases found in cordycepin. Substituent on purine bases structure is –OH on hypoxanthine rather than –NH2. The –OH should be replaced in metabolic pathways. In addition, among 14 different amino acids were tested for 8 g/L. As shown in Figure 5(b), L-alanine can improve cordycepin production. Previous research showed that adenine, adenosine, and glycine were good additives for increased cordycepin production [4, 23, 26, 27]. L-alanine may be an important nutritional element for C. militaris or component of cordycepin production. Hypoxanthine and L-alanine were the best additives to promote cordycepin production in this study.
Figure 5.
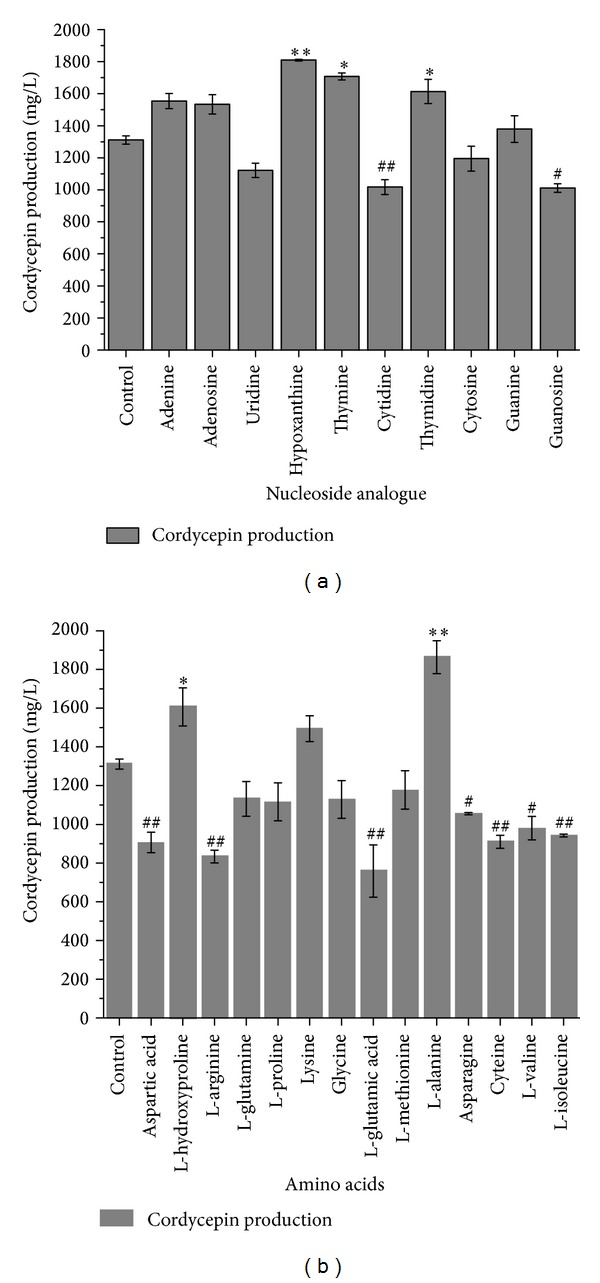
Effects of nucleoside analogue and amino acid on the production of cordycepin: nucleoside analogue (a); amino acid (b); *5% significance level (test group versus control group); **1% significance level (test group versus control group); #5% significance level (control group versus test group); ##1% significance level (control group versus test group).
3.7. Optimization by Response Surface Methodology for Amino Acid, Nucleoside Analogue, and Fermentation Time
Similarly, central composite design was also applied to study the significant factors (hypoxanthine, L-alanine, and culture time) and their optimal levels. Figure 6 shows the morphological characteristics of C. militaris in 1000 mL glass jars after fermentation by static liquid fermentation. Table 8 shows the detailed experimental design and results. Regression analysis was performed to fit the response function (cordycepin production) with the experimental data. From the variables obtained (Table 9), the model was expressed by (2), which represented cordycepin production (Y 2) as a function of hypoxanthine (A), L-alanine (B), and culture time (C, time), concentrations:
| (2) |
Figure 6.

Morphology of C. militaris (strain CGMCC2459) in 700/1000 mL glass jars at the end of the fermentation process by response surface methodology: symbols in photos indicated 20 runs.
Table 8.
Experimental design and responses of the central composite design for amino acid, nucleoside analogue, and time.
| Run | Variables Code | Y (mg/L) Cordycepin production | Run | Variables Code | Y (mg/L) Cordycepin production | ||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | ||||
| 1 | −1 | −1 | −1 | 1383.01 ± 41.53 | 11 | 0 | 0 | 0 | 2041.25 ± 54.70 |
| 2 | 1 | −1 | −1 | 1422.52 ± 39.41 | 12 | 0 | 0 | 0 | 2020.97 ± 73.70 |
| 3 | −1 | 1 | −1 | 1216.88 ± 8.69 | 13 | 0 | 0 | 0 | 1998.18 ± 49.48 |
| 4 | 1 | 1 | −1 | 1857.51 ± 164.86 | 14 | 0 | 0 | 0 | 2068.60 ± 72.79 |
| 5 | −1 | −1 | 1 | 1216.87 ± 253.38 | 15 | −1.6818 | 0 | 0 | 1590.14 ± 222.14 |
| 6 | 1 | −1 | 1 | 1111.18 ± 170.50 | 16 | 1.6818 | 0 | 0 | 1573.90 ± 776.16 |
| 7 | −1 | 1 | 1 | 1536.05 ± 75.17 | 17 | 0 | −1.6818 | 0 | 1636.44 ± 65.23 |
| 8 | 1 | 1 | 1 | 851.70 ± 17.01 | 18 | 0 | 1.6818 | 0 | 1527.98 ± 177.46 |
| 9 | 0 | 0 | 0 | 2073.27 ± 65.85 | 19 | 0 | 0 | −1.6818 | 1211.14 ± 82.58 |
| 10 | 0 | 0 | 0 | 1743.09 ± 14.81 | 20 | 0 | 0 | 1.6818 | 676.97 ± 142.74 |
Table 9.
ANOVA for response surface quadratic polynomial model for amino acid, nucleoside analogue, and time.
| Source | Sum of quares | df | Mean Square | F-value | P-value Prob > F |
|---|---|---|---|---|---|
| Model | 2.899E + 006 | 9 | 3.221E + 005 | 11.91 | 0.0003∗∗ |
| A-A | 1378.39 | 1 | 1378.39 | 0.051 | 0.8260 |
| B-B | 1564.06 | 1 | 1564.06 | 0.058 | 0.8148 |
| C-C | 3.115E + 005 | 1 | 3.115E + 005 | 11.51 | 0.0068∗ |
| AB | 63.06 | 1 | 63.06 | 2.331E − 003 | 0.9624 |
| AC | 2.702E + 005 | 1 | 2.702E + 005 | 9.99 | 0.0102∗ |
| BC | 5468.49 | 1 | 5468.49 | 0.20 | 0.6626 |
| A 2 | 3.076E + 005 | 1 | 3.076E + 005 | 11.37 | 0.0071∗∗ |
| B 2 | 3.073E + 005 | 1 | 3.073E + 005 | 11.36 | 0.0071∗∗ |
| C 2 | 1.990E + 006 | 1 | 1.990E + 006 | 73.58 | <0.0001∗∗ |
| Residual | 2.705E + 005 | 10 | 27053.63 | ||
| Lack of Fit | 1.928E + 005 | 5 | 38561.98 | 2.48 | 0.1707 |
| Pure Error | 77726.41 | 5 | 15545.28 | ||
| Cor Total | 3.169E + 006 | 19 |
R 2 = 0.9146; CV = 10.70%; Pred-R 2 = 0.4162; Adj-R 2 = 0.8378; Adeq Precision = 11.222; ∗5% significance level; ∗∗1% significance level.
Results of F-test analysis of variance (ANOVA) showed that the regression was statistically significant at 95% and 99% confidence level (Table 9). The “F value” of the model was 11.91, and the value of “Prob > F” < 0.01 indicated that the model was significant. In this case, linear terms of C; interactive terms of AC; and quadratic terms of A 2, B 2, and C 2 were significant in model terms for cordycepin production. The “Lack of Fit F value” of 0.1707 implied that the “Lack of Fit” was not significant relative to the pure error (P > 0.05). The Pred-R 2 of 0.4162 was not as close to the Adj-R 2 of 0.8378 as one might normally expect. The result suggested that some factors were not also considered in the model. However, the “Adeq Precision” of 11.222 indicated that the model was adequate for prediction production of cordycepin.
The response surface plot obtained from (2) is shown in Figure 7. It is evident that cordycepin production reached its maximum with a combination of coded level (A, hypoxanthine, level 0.11; B, L-alanine, level 0.06; C, time, level −0.23) by canonical analysis of the Design-Expert Version 8.0.5b software package. The model predicted a maximum response of 2008.48 mg/L cordycepin production at levels of hypoxanthine 5.45 g/L, L-alanine 12.23 g/L, and time 8.6 days (in the practical test 8 days) as optimized medium components.
Figure 7.
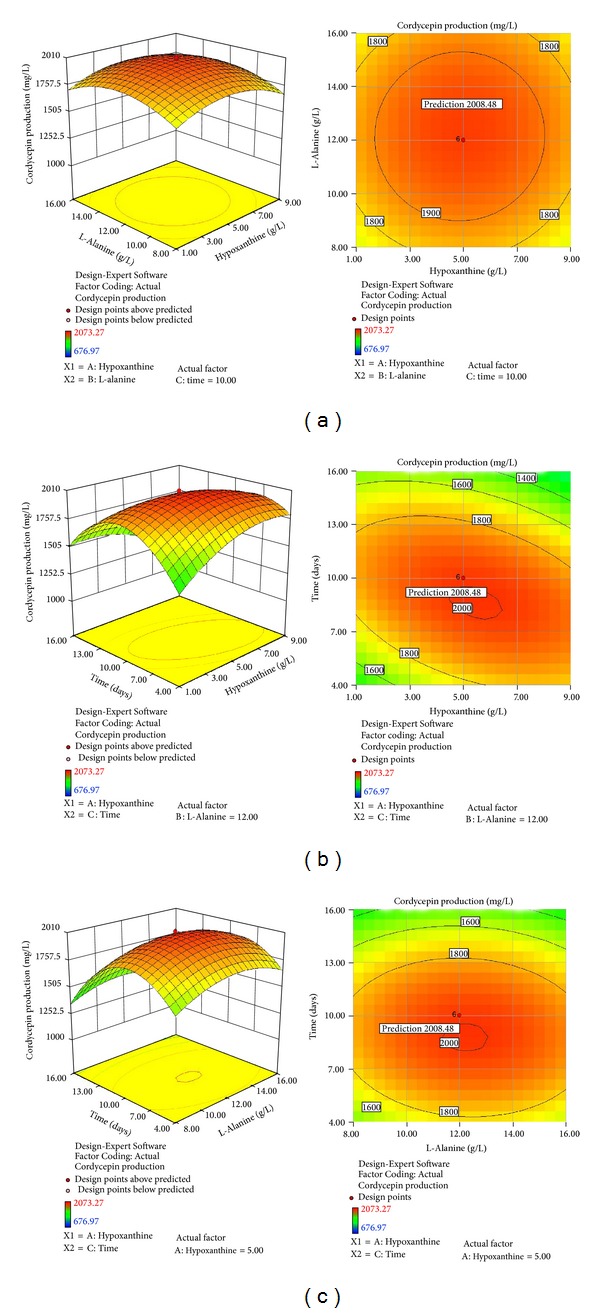
Three-dimensional response surface plots and two-dimensional contour plots for cordycepin production by C. militaris (strain CGMCC2459) showing variable interactions of (a) hypoxanthine and L-alanine; (b) hypoxanthine and time; (c) L-alanine and time.
In previous work, the orthogonal design method [44–46], Box-Behnken design [34, 47], and central composite design [31] were used to optimize culture conditions for cordycepin production by Cordyceps sp. These experimental designs have been successfully used to optimize medium for the mycelial growth and microbial metabolite production in liquid culture processes. In this study, static liquid culture conditions are optimized for the cordycepin production using response surface methodology and are an effective way to enhance the productivity of cordycepin and biomass in C. militaris.
3.8. Verification Experiments and Batch Culture
Based on the results of response surface methodology, the optimized medium was prepared as follows: peptone 20 g/L; sucrose 24.7 g/L; K2HPO4 ·3H2O 1.11 g/L; MgSO4 ·7H2O 0.90 g/L; VB1 10 mg/L; hypoxanthine 5.45 g/L; and L-alanine 12.23 g/L. Five experiments were performed to confirm the above optimal culture requirements. The data were 2011.15 mg/L, 2000.69 mg/L, 1989.22 mg/L, 1969.6 mg/L, and 2061.37 mg/L, respectively. The average cordycepin production was 2006.41 ± 34.37 mg/L. The experimental values were particularly close to the predicted values (2008.48 mg/L). The result confirmed the model suited the predictive of hyperproduction of cordycepin by C. militaris in static liquid culture. Batch culture was carried for cordycepin production under optimized culture conditions (Figure 8).
Figure 8.

Batch culture for cordycepin production under optimized culture conditions by static liquid culture using C. militaris (strain CGMCC2459).
3.9. In Vitro Cordycepin Production Using Liquid Culture in Other Studies
The highest report for cordycepin production was 14300 mg/L by Masuda et al. [29] (Table 10). In our experiment, cordycepin production at 2008.48 mg/L was lower. However, a maximum total content of cordycepin (1405.94 mg) was achieved in our study. This is a second higher report of cordycepin production in one single fermenter. The results showed that the culture conditions will provide an effective way for increasing cordycepin production.
Table 10.
Cordycepin production in the medium by liquid culture in different studies.
| No. | Methodology | Working volume of the medium v/v (mL/mL) |
Cordycepin production (mg/L) | Total content of cordycepin in one bottle (mg) | References |
|---|---|---|---|---|---|
| 1 | Submerged culture | 50/250 | 245.7 | 12.5 | Mao et al., [32] |
| 2 | Submerged culture | 50/250 | 420.5 | 21.03 | Mao and Zhong [21] |
| 3 | Surface liquid culture | 100/500 | 640 | 64 | Masuda et al., [25] |
| 4 | Shaking + Static | 100/250 | 2214.5 | 221.45 | Shih et al., [34] |
| 5 | Surface liquid culture | 100/500 | 2500 | 250 | Masuda et al., [26] |
| 6 | Surface liquid culture | 100/500 | 3100 | 310 | Das et al., [30] |
| 7 | Surface liquid culture | 100/500 | 8570 | 857 | Das et al., [27] |
| 8 | Submerged culture | 100/500 | 1644.21 | 164.42 | Wen et al., [23] |
| 9 | Dark + Shaking | 100/500 | 1015 | 101.5 | Kang et al., [24] |
| 10 | Surface liquid culture | 150/500 | 14300 | 2145 | Masuda et al., [29] |
| 11 | Static liquid culture | 700/1000 | 2008.48 | 1405.94 | In this study |
4. Conclusion
In this work, single factor design, Plackett-Burman design, and central composite design were employed to establish the key factors and identify optimal culture conditions which improved cordycepin production by C. militaris CGMCC2459. Optimal media contained peptone 20 g/L; sucrose 24.7 g/L; K2HPO4 ·3H2O 1.11 g/L; MgSO4 ·7H2O 0.90 g/L; VB1 10 mg/L; hypoxanthine 5.45 g/L; and L-alanine 12.23 g/L. Hypoxanthine and L-alanine were added to the optimal medium at 8.6 days. Optimal incubation conditions were 25°C at an unaltered pH of 35 days. Using these culture conditions, a maximum production of cordycepin was 2008.48 mg/L for 700 mL working volume in the 1000 mL glass jars, and total content of cordycepin reached 1405.94 mg/bottle (700 mL/1000 mL). This method provides an effective way for increasing the cordycepin production at a large scale. The strategies used in this study could have a wide application in other fermentation process.
Acknowledgments
This work was supported by the Agricultural Science and Technology Foundation of Guizhou Province (no. (2011)3054), the National Natural Science Foundation of China (no. 31200016), and the Modernization of Traditional Chinese Medicine Program of Guizhou Province (no. (2012)5008).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Chao Kang and Ting-Chi Wen contributed equally to this work.
References
- 1.Sung G-H, Hywel-Jones NL, Sung J-M, Luangsa-ard JJ, Shrestha B, Spatafora JW. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu JT. Medicinal Fungus in China. Beijing, China: Beijing Medical University, Peking Union Medical College Joint Press; 1997 (Chinese) [Google Scholar]
- 3.Mortimer PE, Karunarathna SC, Li Q, et al. Prized edible Asian mushrooms: ecology, conservation and sustainability. Fungal Diversity. 2012;56(1):31–47. [Google Scholar]
- 4.Wen TC, Lei BX, Kang JC, Li GR, He J. Enhanced production of mycelial culture using additives and cordycepin by submerged in Cordyceps militaris . Food and Fermentation Industries. 2009;35(8):49–53. [Google Scholar]
- 5.Li HC, Sun P, Feng CQ. The research of cordycepin as an active component in Cordyceps . Journal of Jinggangshan University (Natural Science) 2010;31(2):93–96. [Google Scholar]
- 6.Cunningham KG, Manson W, Spring FS, Hutchinson SA. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) link. Nature. 1950;166(4231):p. 949. doi: 10.1038/166949a0. [DOI] [PubMed] [Google Scholar]
- 7.de Silva DD, Rapior S, Fons F, Bahkali AH, Hyde KD. Medicinal mushrooms in supportive cancer therapies: an approach to anti-cancer effects and putative mechanisms of action. Fungal Diversity. 2012;55:1–35. [Google Scholar]
- 8.Yoshikawa N, Yamada S, Takeuchi C, et al. Cordycepin (3′-deoxyadenosine) inhibits the growth of B16-BL6 mouse melanoma cells through the stimulation of adenosine A3 receptor followed by glycogen synthase kinase-3β activation and cyclin D1 suppression. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2008;377(4–6):591–595. doi: 10.1007/s00210-007-0218-y. [DOI] [PubMed] [Google Scholar]
- 9.Sugar AM, McCaffrey RP. Antifungal activity of 3’-deoxyadenosine (cordycepin) Antimicrobial Agents and Chemotherapy. 1998;42(6):1424–1427. doi: 10.1128/aac.42.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto K, Simizu B. Effect of cordycepin on the replication of western equine encephalitis virus. Archives of Virology. 1976;52(4):341–345. doi: 10.1007/BF01315623. [DOI] [PubMed] [Google Scholar]
- 11.Kodama EN, McCaffrey RP, Yusa K, Mitsuya H. Antileukemic activity and mechanism of action of cordycepin against terminal deoxynucleotidyl transferase-positive (TdT+) leukemic cells. Biochemical Pharmacology. 2000;59(3):273–281. doi: 10.1016/s0006-2952(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 12.de Silva DD, Rapior S, Sudarman E, et al. Bioactive metabolites from macrofungi: ethnopharmacology, biological activities and chemistry. Fungal Diversity. 2013;62:1–40. [Google Scholar]
- 13.de Silva DD, Rapior S, Hyde KD, Bahkali AH. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Diversity. 2012;56(1):1–29. [Google Scholar]
- 14.Todd A, Ulbricht TLV. Deoxynucleosides and related compounds—part IX: a synthesis of 3′-deoxyadenosine. Journal of the Chemical Society. 1960:3275–3277. [Google Scholar]
- 15.Kwon DW, Jeon J-H, Kang C, Kim YH. New synthesis of 3′-deoxypurine nucleosides using samarium(III) iodide complex. Tetrahedron Letters. 2003;44(43):7941–7943. [Google Scholar]
- 16.Kaczka EA, Dulaney EL, Gitterman CO, Woodruff HB, Folkers K. Isolation and inhibitory effects on KB cell cultures of 3′-deoxyadenosine from Aspergillus nidulans (Eidam) wint. Biochemical and Biophysical Research Communications. 1964;14(5):452–455. doi: 10.1016/0006-291x(64)90085-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang HX, Wu W, Chen W, Gao XH, Tang LS. Comparative analysis of cordycepin and adenosine contents in fermentation supernatants between Aspergillus nidulans and Cordyceps militaris . Acta Agriculturae Shanghai. 2006;22(2):28–31. [Google Scholar]
- 18.Wen TC, Kang JC, Lei BX, Li GR, He J. Effects of different solid culture condition on fruit body and cordycepin output of Cordyceps militaris . Guizhou Agricultural Sciences. 2008;36(4):92–94. [Google Scholar]
- 19.Chen ZH, Yu H, Zeng WB, Yang JY, Yuan J, Chen YJ. Solid fermentation technique for cordycepin production by Cordyceps nigrella strain cnig-56. Acta Edulis Fungi. 2010;17(1):80–82. [Google Scholar]
- 20.Bu L, Zhu ZY, Liang ZQ, Liu AY. Utilization of fungal elicitor in increasing cordycepin of Cordyceps militaris . Mycosystema. 2002;21(2):252–256. [Google Scholar]
- 21.Mao X-B, Zhong J-J. Hyperproduction of cordycepin by two-stage dissolved oxygen control in submerged cultivation of medicinal mushroom Cordyceps militaris in bioreactors. Biotechnology Progress. 2004;20(5):1408–1413. doi: 10.1021/bp049765r. [DOI] [PubMed] [Google Scholar]
- 22.Mao XB, Tu YQ. Enhanced production of cordyceipn by fed batch cultivation using amino acid in Cordyceps militaris . Chongqing Journal of Research on Chinese Drugs and Herbs. 2005;(1):18–22. [Google Scholar]
- 23.Wen TC, Kang JC, Lei BX, Li GR, He J. Enhanced production of cordycepin by submerged culture using additives in Cordyceps militaris . Food Science. 2010;31(5):175–179. [Google Scholar]
- 24.Kang C, Wen TC, Kang JC, Qian YX, Lei BX. Effects of additives and different culture conditions on cordycepin production by the medicinal fungus Cordyceps militaris . Mycosystem. 2012;31(3):389–397. [Google Scholar]
- 25.Masuda M, Urabe E, Sakurai A, Sakakibara M. Production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris . Enzyme and Microbial Technology. 2006;39(4):641–646. [Google Scholar]
- 26.Masuda M, Urabe E, Honda H, Sakurai A, Sakakibara M. Enhanced production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris . Enzyme and Microbial Technology. 2007;40(5):1199–1205. [Google Scholar]
- 27.Das SK, Masuda M, Sakurai A, Sakakibara M. Effects of additives on cordycepin production using a Cordyceps militaris mutant induced by ion beam irradiation. African Journal of Biotechnology. 2009;8(13):3041–3047. [Google Scholar]
- 28.Aman S, Anderson DJ, Connolly TJ, Crittall AJ, Ji G. From adenosine to 3′-deoxyadenosine: development and scale up. Organic Process Research and Development. 2000;4(6):601–605. [Google Scholar]
- 29.Masuda M, Das SK, Hatashita M, Fujihara S, Sakurai A. Efficient production of cordycepin by the Cordyceps militaris mutant G81-3 for practical use. Process Biochemistry. 2014;49:181–187. [Google Scholar]
- 30.Das SK, Masuda M, Hatashita M, Sakurai A, Sakakibara M. A new approach for improving cordycepin productivity in surface liquid culture of Cordyceps militaris using high-energy ion beam irradiation. Letters in Applied Microbiology. 2008;47(6):534–538. doi: 10.1111/j.1472-765X.2008.02456.x. [DOI] [PubMed] [Google Scholar]
- 31.Wen L, Zhang C, Xia M, Weng L. Effects of Cordyceps militaris space mutation treatment on active constituent content. Food Science. 2008;29(5):382–384. [Google Scholar]
- 32.Mao X-B, Eksriwong T, Chauvatcharin S, Zhong J-J. Optimization of carbon source and carbon/nitrogen ratio for cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris . Process Biochemistry. 2005;40(5):1667–1672. [Google Scholar]
- 33.Das SK, Masuda M, Hatashita M, Sakurai A, Sakakibara M. Optimization of culture medium for cordycepin production using Cordyceps militaris mutant obtained by ion beam irradiation. Process Biochemistry. 2010;45(1):129–132. [Google Scholar]
- 34.Shih I-L, Tsai K-L, Hsieh C. Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of Cordyceps militaris . Biochemical Engineering Journal. 2007;33(3):193–201. [Google Scholar]
- 35.Guo WQ, Ren NQ, Wang XJ, et al. Optimization of submerged culture conditions for exo-biopolymer production by Paecilomyces japonica . Bioresource Technology. 2009;100:1192–1196. doi: 10.1016/j.biortech.2008.07.070. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Yu X, Ding C, et al. Optimization of phenol degradation by Candida tropicalis Z-04 using Plackett-Burman design and response surface methodology. Journal of Environmental Sciences. 2011;23(1):22–30. doi: 10.1016/s1001-0742(10)60369-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhao DF, Liang CY, Ren X, Sun SM. Effect of dissolved oxygen on the biotransformation of tylosin to its acyl derivative. Chinese Journal of Pharmaceutical. 2006;37(3):162–164. [Google Scholar]
- 38.Xie T, Fang HY, Zhu GB, Zhu GJ. Effect of dissolved oxygen on glycerol production by Candida glycerinogenes . China Biotechnology. 2008;28(5):65–70. [Google Scholar]
- 39.Kredich NM, Guarino AJ. Studies on the biosynthesis of cordycepin. Biochimica et Biophysica Acta. 1961;47(3):529–534. doi: 10.1016/0006-3002(61)90546-7. [DOI] [PubMed] [Google Scholar]
- 40.Pintado J, Torrado A, González MP, Murado MA. Optimization of nutrient concentration for citric acid production by solid-state culture of Aspergillus niger on polyurethane foams. Enzyme and Microbial Technology. 1998;23(1-2):149–156. [Google Scholar]
- 41.Bae J-T, Sinha J, Park J-P, Song C-H, Yun J-W. Optimization of submerged culture conditions for exo-biopolymer production by Paecilomyces japonica . Journal of Microbiology and Biotechnology. 2000;10(4):482–487. [Google Scholar]
- 42.Lei K, Ke Y, Mao N. The optimization of the culture media for high production of cordycepin by Cordyceps militaris Fjnu-01. Pharmaceutical Biotechnology. 2011;18(6):504–508. [Google Scholar]
- 43.Chassy BM, Suhadolnik RJ. Nucleoside antibiotics IV. Metabolic fate of adenosine and cordycepin by Cordyceps militaris during cordycepin biosynthesis. Biochimica et Biophysica Acta. 1969;182(2):307–315. doi: 10.1016/0005-2787(69)90181-6. [DOI] [PubMed] [Google Scholar]
- 44.Xiao JH, Chen DX, Liu JW, et al. Optimization of submerged culture requirements for the production of mycelial growth and exopolysaccharide by Cordyceps jiangxiensis JXPJ 0109. Journal of Applied Microbiology. 2004;96(5):1105–1116. doi: 10.1111/j.1365-2672.2004.02235.x. [DOI] [PubMed] [Google Scholar]
- 45.Xiao JH, Chen DX, Xiao Y, et al. Optimization of submerged culture conditions for mycelial polysaccharide production in Cordyceps pruinosa . Process Biochemistry. 2004;39(12):2241–2247. [Google Scholar]
- 46.Dong C-H, Yao Y-J. Nutritional requirements of mycelial growth of Cordyceps sinensis in submerged culture. Journal of Applied Microbiology. 2005;99(3):483–492. doi: 10.1111/j.1365-2672.2005.02640.x. [DOI] [PubMed] [Google Scholar]
- 47.Xie C, Liu G, Gu Z, Fan G, Zhang L, Gu Y. Effects of culture conditions on mycelium biomass and intracellular cordycepin production of Cordyceps militaris in natural medium. Annals of Microbiology. 2009;59(2):293–299. [Google Scholar]


