Abstract
Detection of Helicobacter pylori after triple therapy is usually carried out by either rapid urease test (RUT), urea breath test (UBT), histology, bacterial isolation, and single round PCR or serological tests. In this study, antral biopsy specimens from 25 patients were tested for H. pylori by RUT, culture, histology, and nested PCR in their antral biopsy specimens before and after treatment. Three genes, namely, heat shock protein (hsp60), phosphoglucosamine mutase (ureC), and flagellar export ATP synthase (fliI) of H. pylori were targeted. Of the 25 antral biopsy specimens, the RUT, culture, histology, and nested PCR positivity dropped from 81.8% to 12%, 31% to 0%, 100 to 84%, and 100% to 92%, respectively, before and after therapy. Further, hsp60 specific amplicons from 23 out of 25 patients gave identical restriction pattern, while 6 fliI and 1 ureC specific amplicon produced different restriction pattern. Furthermore, variations in fliI gene sequences in H. pylori after treatment were also confirmed by sequencing and compared in silico. Nested PCR based detection of H. pylori is more sensitive method to detect H. pylori after therapy than culture, RUT, and histology. Further, this study suggests that H. pylori is not eradicated completely after triple therapy.
1. Introduction
Association of Helicobacter pylori (H. pylori) with acid peptic diseases including duodenal ulcer is well established [1]. Further, H. pylori has been designated as class I carcinogen by WHO [2]. Although, prevalence of H. pylori ranges between 20 and 80% in different geographical areas depending on different socioeconomic factors, eradication has been advised only in symptomatic cases [3–6]. Triple therapy constituting omeprazole, amoxicillin, and clarithromycin has been found to be quite effective to eliminate H. pylori from stomach [7, 8]. However, occasional recurrences have been reported with isolation of strain similar to that of pretreatment [9–13]. It is not clear whether the infection persisted after triple therapy or reinfection occurred from the other niches, for example, oral cavity, or acquired from other close family members. For primary diagnosis and posttherapy evaluation of H. pylori, most of the studies have used rapid urease test (RUT)/CLO test, histology, C13/14-urea breath test (UBT), and culture and stool antigen detection test. Because of poor sensitivity of isolation of H. pylori, histology and UBT are usually considered as gold standard for the assessment of eradication therapy. But these tests are found sensitive enough only when density of H. pylori remains high. Contrary to this, PCR based technique can detect even a few bacteria. There are scant reports using single round PCR based detection of H. pylori after therapy. It has already been established that the sensitivity of nested PCR based detection is very high in comparison to single round [14]. Interestingly, there is no report till date by using nested PCR based detection of the bacterium after eradication therapy in H. pylori associated diseases. Therefore, present study was planned to see whether, H. pylori is really eradicated by using nested PCR protocol and if not, whether the persistent strains are the same or different.
2. Methods
2.1. Patient Selection
This study was conducted at Sir Sunder Lal Hospital, Banaras Hindu University, Varanasi, during June, 2009 to March, 2010. Ethical committee clearance was obtained before commencement of the study and well informed written consent was obtained from each of the participants. The patients who had severe acid peptic diseases on endoscopy with positive test for H. pylori were given clarithromycin 500 mg, amoxicillin 1 gm, and pantoprazole 40 mg; all twice daily for 14 days. Patients were initially asked to visit again for follow up at 4 weeks after the completion of anti-H. pylori therapy. A total of 93 patients (63 male and 30 female; mean age 42.4 y; age range 20 to 85 y) were enrolled in the present study. Biopsies were collected in the endoscopy units of the Department of Gastroenterology. Patients taking proton pump inhibitors and/or antibiotics having bleeding ulcers or an acute hemorrhage from other sites in the upper gastrointestinal tract and patients with stomach surgery were excluded. The endoscope was mechanically washed and then disinfected using activated 2% glutaraldehyde. The 4-5 biopsy specimens were collected from each patient of the 93 enrolled patients. A total of 25 patients who had antral gastritis (n = 12) and peptic ulcer (n = 13) diagnosed previously could be followed up and upper gastrointestinal endoscopy was performed on both the occasions, that is, pre- and posttherapy, and biopsy samples were collected from gastric antrum. Since 68 patients did not report for the follow up after treatment, they were excluded and only 25 patient's antral biopsies could be analyzed in this study.
2.2. Rapid Urease Test (RUT)
For RUT, biopsy was inoculated into 1 mL of 10% urea dissolved in deionized water (pH 6.8), to which two drops of 1% phenol red solution were added and incubated at 37°C for 24 h. A positive result was recorded when the color changed from yellow to pink within 30 min [15].
2.3. Histology
Antral biopsy specimens, collected during pre- and posttreatment were fixed in 10% buffered formalin, embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin to examine the presence/absence of curved rod shaped H. pylori on the mucosal surface.
2.4. Culture of H. pylori from Gastric Biopsy Specimens
The biopsy piece was homogenized into phosphate buffer saline (PBS) in an all glass disposable homogenizer. This tissue homogenate was plated onto the media containing brain heart infusion (BHI) agar (Difco, Becton Dickinson, Sparks, MD, USA), supplemented with 7% sheep blood, 0.4% IsoVitaleX, and Skirrow selective supplement (vancomycin 10 μg/mL; polymixin B sulfate 2.5 IU/mL; trimethoprim lactate 5 μg/mL) (Difco, Becton Dickinson, Sparks, MD, USA). Plates were incubated at 37°C in an atmosphere of 5% O2, 10% CO2, and 85% N2 for 3–7 days. Plates were opened after 72 h and every 24 h afterwards, if no growth was obtained. Plates were discarded only after 7 days of incubation. Organisms were identified as H. pylori based on typical colony morphology, Gram staining, and positive oxidase, catalase, and rapid urease tests [15].
2.5. Preparation of Genomic DNA for PCR Assay
Genomic DNA from tissue homogenate was extracted using a standard proteinase K and phenol-chloroform method [16]. One set of double distilled water was included in each batch of DNA extraction to check cross-contamination of DNA during DNA extraction.
2.6. PCR Amplification
PCR was carried out in a 25 μL volume using 10 ng of DNA, 1 U of Taq polymerase (Bangalore Genie, India), and 10 pmol of each primer (SBS Genetech), 0.25 mM (each) deoxynucleotide triphosphate, and 1.5 mM MgCl2 in standard PCR buffer. For the internal amplification, the PCR product from the primary cycle was diluted 1/50 and 1 μL was used as the template in the nested PCR [15]. All the amplifications were carried out in a thermal cycler (Biometra, Goettingen, Germany). Details of primers and their protocol are given in Table 1. Amplification of all the three conserved genes were carried out by nested protocol. Universal eubacterial primers were used for all the samples to exclude PCR inhibition. DNA from H. pylori reference BHUHPSKP3 (KC525436) and a tube containing water in place of DNA were assayed in each PCR run as positive and negative controls, respectively. The PCR products were analyzed by electrophoresis on 1.4% agarose gels (Bangalore Genie, India) containing 0.5 μg of ethidium bromide per mL. The gel was run at 70 V with TBE (Tris Boric acid EDTA) buffer and was examined by transilluminator and photographed.
Table 1.
Primers used for detection of H. pylori before and after treatment targeting specific genes.
| S. number | Targeted gene | Primer sequence (5′-3′) | Product size | PCR condition, (annealing temperature and cycles) MgCl2 conc. | |
|---|---|---|---|---|---|
| 1 | Heat shock protein (hsp60)14, conserved | Primary | AAGGCATGCAAGATAGAGGCT CTTTTTTCTCTTTCATTTCCACTT |
590 bp | 94°C, 30 seconds, 56°C, 30 seconds; 72°C, 30 s (35 cycles) (60 mol/lit) |
| Nested | TTGATAGAGGCTACCTCTCC TGTCATAATCGCTTGTCGTGC |
501 bp | 94°C, 30 seconds, 56°C, 30 seconds; 72°C, 30 s (35 cycles) (60 mol/lit) |
||
| 2 | Phosphoglucosamine mutase (ureC/glmM), conserved | Primary | TTGGGGGTATAATTCAAGGG TTAGTGAGCGCTCTAACTTCC |
945 bp | 94°C, 1 min, 59°C, 1 min; 72°C, 1 min (35 cycles) (60 mol/lit) |
| Nested | GCAACAGAGCTTACCTGCTTG GATTCAAATAGGGCCTATGC |
882 bp | 94°C, 1 min, 59°C, 1 min; 72°C, 1 min (35 cycles) (60 mol/lit) |
||
| 3 | Flagellum-specific ATP synthase (fliI)∗, conserved | Primary | CCCGATGCGAATGAGCATTTC GCTTAACCCTTTAGGGCAAGTC |
858 bp | 94°C, 1 min, 56°C, 1 min; 72°C, 1 min (35 cycles) (60 mol/lit) |
| Nested | GATGTCTTTAGCCACCCTTGATGT GAGCATTGATGGGCTTTTGACTTGC |
640 bp | 94°C, 1 min, 56°C, 1 min; 72°C, 1 min (35 cycles) (60 mol/lit) |
||
*In-house designed primers for this study; PCR-polymerase chain reaction.
2.7. PCR Based Restriction Enzyme Analysis (PCR-REA)
After amplification, the PCR products [hsp60, ureC or glmM, and fliI gene] were precipitated with 2.5 volumes of ethanol. The pellets were washed twice with 75% ethanol and dissolved in Tris-EDTA buffer (pH 8.0). A 10 μL precipitated amplified DNA was then digested with the 10 U of restriction enzyme in appropriate buffered solution recommended by the manufacturer (Genie, Bangalore, India) and incubated for 3 h at 37°C. Hind III restriction enzyme was used for hsp60 and ureC, and Mnl I was used for fliI gene. The digested DNA fragments were analyzed by electrophoresis on 2% agarose gels (Genie, Bangalore, India) containing 0.5 μg of ethidium bromide per mL. The gel was run at 70 V with TBE (Tris Boric acid EDTA) buffer for 3 h and was examined by transilluminator and photographed. The sizes of digested DNA fragments were estimated from migration distances of a 100-bp DNA ladder molecular weight standard (Genie, Bangalore, India) and compared with in silico restriction digestion specified with concerned restriction enzyme.
2.8. Sequencing
The amplified fliI gene segment, which had different restriction pattern from previous strain were purified from salts and primers using HiPura silica kit for DNA isolation (HiMedia). A total of 6 (5 mutated and 1 wild type) purified amplicons generated targeting fliI were outsourced for partial sequencing to Genei, Bangalore, India. Sequences were analyzed using BLASTN (http://www.ncbi.nlm.nih.gov/BLAST/) to verify mutations/changes in the sequences of fliI gene specific for H. pylori.
3. Results
3.1. Bacteriological Study
Of the 25 antral biopsy specimens collected from patients, 81.8% (18/25) were found positive by RUT and 31% (7/25) by culture for H. pylori, before triple therapy. Four weeks after anti-H. pylori triple therapy, 3 (12%) patients (2 PUD and 1 gastritis) were positive by RUT and none of them were positive for H. pylori isolation after triple therapy.
3.2. Histology
Of the 25 patients with gastroduodenal diseases that completed eradication treatment, 16% (4/25) were still H. pylori positive by histology.
3.3. Nested PCR
Genomic DNA extracted from biopsy specimens were subjected to amplification by primers specific for hsp60, ureC, and fliI genes of H. pylori. All the 25 patients were positive for the 501 bp, 840 bp, and 640 bp of amplicon for hsp60, ureC, and fliI genes, respectively, before therapy. However, 92% (23/25) antral biopsies were positive for H. pylori gDNA by nested PCR after 4 weeks, while 2 patients were found negative (Figures 1, 2, and 3).
Figure 1.
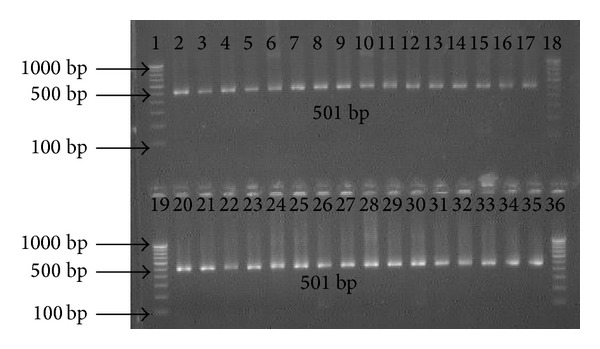
Amplification of partial 501 bp hsp60 gene with specific nested primer for H. pylori in antral biopsies. Lanes 1, 18, 19, and 36: molecular marker (100 bp); lanes 2 and 20: positive control; lanes 3 to 17: gDNA from antral biopsies before treatment; and lanes 21 to 35: gDNA from antral biopsies after treatment.
Figure 2.
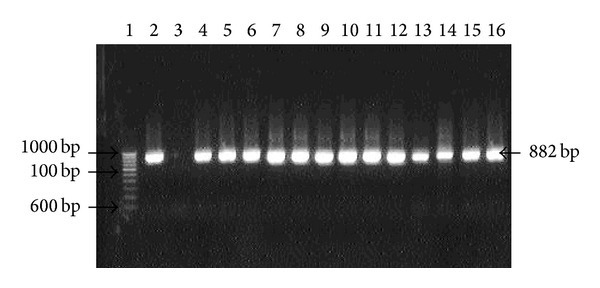
Amplification of ureC (glmM) gene with internal nested primer specific for H. pylori in antral biopsies. Lane 1: molecular marker (100 bp); lane 2: positive control; lane 3: negative control; lanes 4 to 16: gDNA from antral biopsies collected after treatment.
Figure 3.
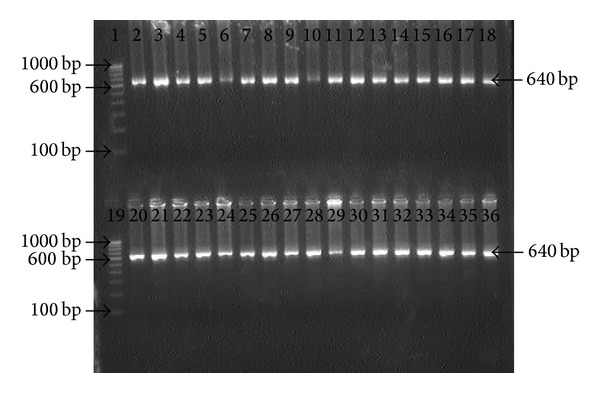
Electrophotograph showing amplification of fliI gene with specific internal primer for H. pylori in antral biopsies. Lanes 1 and 19: molecular marker (100 bp); lanes 2 and 20: positive control; Lanes 3 to 18: gDNA from antral biopsies before treatment; and Lanes 21 to 35: gDNA from antral biopsies after treatment.
3.4. PCR-REA
The amplified PCR products of hsp60 gene were digested by Hind III enzyme. All nested amplicons of 501 bp were restricted into two fragments of 310 and 191 bp by the Hind III restriction enzyme. There was no difference in restriction pattern of amplified partial hsp60 gene of strains of H. pylori before and after treatment (Table 2; Figure 4).
Table 2.
Representation of strains of H. pylori after nested PCR restriction analysis.
| Biopsy specimens | Restriction pattern of partial amplified genes of H. pylori by different restriction enzymes | |||||
|---|---|---|---|---|---|---|
| hsp60 by Hind III | ureC (glmM) by Hind III | fliI by Mnl I | ||||
| Before therapy | After therapy | Before therapy | After therapy | Before therapy | After therapy | |
| NUDG11 | A | A | A | A | A | A |
| NUDG13 | A | A | A | A | A | A |
| PUDG18 | A | A | A | A | A | AA |
| NUDG35 | A | A | A | A | A | A |
| NUDG37 | A | A | A | A | A | A |
| PUDG43 | A | A | A | A | A | A |
| NUDG46 | A | A | A | A | A | AB1 |
| PUDG47 | A | — | A | — | A | — |
| PUDG57 | A | A | A | A | A | A |
| NUDG61 | A | A | A | A | A | A |
| PUDG63 | A | A | A | A | A | A |
| NUDG64 | A | A | A | A | A | A |
| NUDG67 | A | A | A | AX | A | A |
| PUDG73 | A | A | A | A | A | AB2 |
| NUDG80 | A | A | A | A | A | AA |
| PUDG81 | A | A | A | A | A | A |
| PUDG85 | A | — | A | — | A | — |
| PUDG88 | A | A | A | A | A | AC |
| NUDG95 | A | A | A | A | A | A |
| NUDG96 | A | A | A | A | A | A |
| PUDG101 | A | A | A | A | A | A |
| PUDG104 | A | A | A | A | A | A |
| NUDG109 | A | A | A | A | A | AA |
| PUDG115 | A | A | A | A | A | A |
| PUDG118 | A | A | A | A | A | A |
Fragment length of AX-303 and 579 bp; AA-486 and 154 bp; AB1-386, 154, 55, and 45 bp; AB2-386, 134, 55, 45, and 22 bp; and AC-431, 300, and 154 bp.
Figure 4.
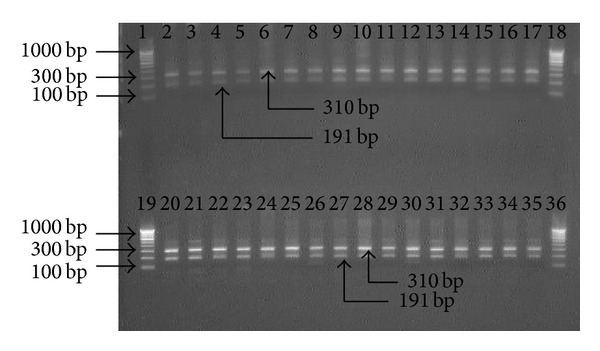
Electrophotograph of restriction digestion of hsp60 gene of H. pylori strains with Hind III, restricted 501 bp hsp60 gene amplicon into two fragments (310 and 191 bp). Lanes 1, 18, 19, and 36: 100 bp molecular marker; lanes 2 to 17: restriction pattern of PCR product specific to hsp60 before treatment; lanes 20–35: restriction pattern of amplicons specific to hsp60 gene after treatment.
The amplified PCR products of ureC gene were restricted by Hind III enzyme. All amplified PCR products were restricted into 3 segments of 435, 303, and 144 bp. Strains amplified from all the patients were same except one patient with antral gastritis. The amplicon from this tissue restricted only into fragments of 303 and 579 bp (Table 2; Figure 5).
Figure 5.
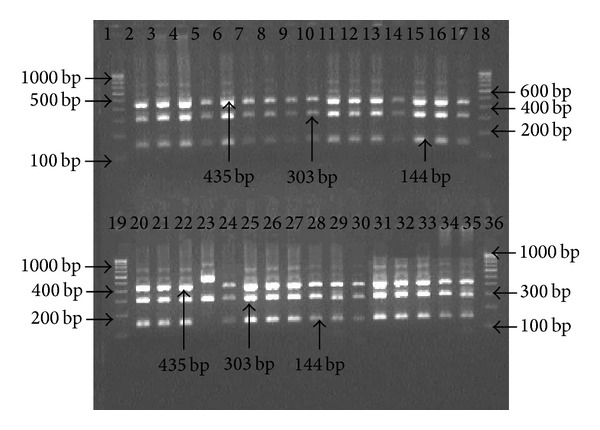
Electrophotograph of restriction analysis of ureC (glmM) gene of H. pylori strains with Hind III, restricted 882 bp ureC gene amplicon into 3 fragments (435, 303, and 144 bp). Lanes 1, 18, 19, and 36: 100 bp molecular marker; lanes 2 to 17: restriction pattern of PCR product specific to ureC before treatment; lanes 20–35: restriction pattern of amplicons specific to ureC gene after treatment.
The amplified PCR products of fliI gene were digested using Mnl I enzyme. Digestions of PCR product with Mnl I resulted into 3 fragments of 431, 154, and 55 bp similar to in silico. The DNA amplified from the 17 patients were identical, while 6 patients exhibited different restricted pattern than what were obtained before therapy (4 with peptic ulcer and 2 had antral gastritis). Restriction pattern of amplified fliI gene of H. pylori from 6 patients exhibited four different types. Three patients were type A, while remaining 3 strains belonged to each of the B1, B2 and C types. Type A strain showed two fragments of 486 and 154 bp. Type B was fragmented into three fragments 386, 154, and 55 bp, but on the ground of sequencing type B was further subdivided into two subgroups B1 and B2. Subtype B1 was restricted into 386, 154, 55, and 45 bp and subtype B2 was digested into 386, 134, 55, 45, and 22 bp. However, the smaller fragments could not be visualized on 1.8% agarose gel. Type C amplicon could be digested into 3 fragments, that is, 431, 300, and 154 bp (Table 2; Figure 6). However, this type could not be sequenced.
Figure 6.
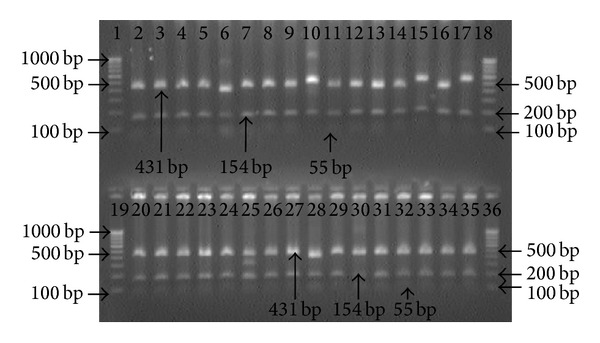
Electrophotograph of restriction digestion of fliI gene of H. pylori strains with Mnl I, restricted 640 bp fliI gene amplicon into three fragments (431, 154, and 55 bp). Lanes 1, 18, 19, and 36: 100 bp molecular marker; lanes 2 to 17: restriction pattern of PCR product specific to fliI after treatment; lanes 6, 10, 15, and 17 exhibited different band pattern comparison to their previous strains; and lanes 20–35: restriction pattern of amplicons specific to fliI gene before treatment.
3.5. DNA Sequence of fliI Gene before and after Treatment and In Silico Restriction
DNA sequences of fliI gene have been submitted to NCBI gene data Bank (GenBank accession number KC525439, KC525440, KC525441, KC525442, and KC525443). Comparison of the nucleotide sequences with the NCBI database showed 99% similarity with H. pylori flagellum-specific ATP synthase (fliI). The partial nucleotide sequence of fliI of 5 strains were flanked with nucleotide sequences from J99 as reference up to 640 bp similar to internal amplicon with assurance that no additional site could be generated during flanking. All the five sequenced nucleotides were restricted by Mnl I in silico (Figure 7). In silico restriction pattern was similar to experimental observation.
Figure 7.
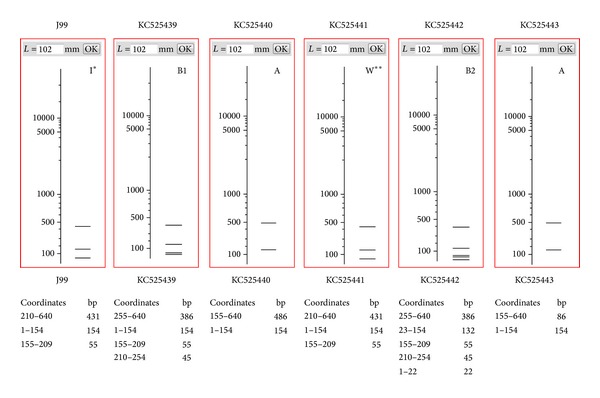
In silico restriction pattern of fliI gene (640 bp) specific to H. pylori digested with Mnl I. The virtual gel was generated by interpolating experimental data using NEB cutter V2.0. KC525441 (GenBank accession number) represents in silico restriction pattern like J99; KC525439, KC525440, KC525442, and KC525443 produced varying restriction pattern. I*: restriction pattern of J99; W**: wild type restriction pattern; A, B1, and B2: mutated/changed restriction pattern of Mnl I.
4. Discussion
The positivity for H. pylori in 23 of 25 patients (92%) who came for follow up after 4 weeks of completion of anti-H. pylori therapy is really surprising in the light of previous reports, where eradication rates ranged between 70 and 100%. This remarkable difference may be explained on the basis of relatively poor sensitivity of H. pylori detection by serological, UBT, fecal antigen, RUT, histopathology, culture, and single round PCR methods than by nested PCR. Anti-H. pylori antibody based method could demonstrate eradication rate of 85%, but it should be taken in the light of the fact that antibody fall may take time and also presence of H. pylori in body sites other than stomach cannot be excluded. UBT has been found to show eradication rates ranging between 75 and 100%. However, for positivity by UBT, urease producing bacterial density in stomach should be sufficient enough which is naturally reduced significantly by anti-H. pylori regimen. The same logic of low H. pylori density in stomach very well explains the quite high eradication rate assessed by fecal antigen detection, RUT, histology, single round PCR, and bacterial isolation methods. Further RUT, UBT and bacterial isolation primarily depends on viable and metabolically active form of H. pylori. But this is already established that the antimicrobial therapy not only causes reduction in bacterial load but also transforms active spiral bacteria to coccoid (viable but not culturable: VBNC) form [17, 18]. Further, single round PCR may give positive amplification only when more than 70 bacterial cells are present in a given biopsy sample [19], while nested PCR is capable of detecting the bacterium as low as 3 cells only [14]. In the present study, we targeted 3 genes (hsp60, ureC, and fliI) to rule out possibility of PCR contamination and all the targets gave specific amplification in each antral biopsies collected from 23 of the 25 patients. Further, we have taken full precaution to avoid cross-contamination through endoscopes by proper sterilization and performing PCR in 3 completely separated rooms. These observations suggest that extremely sensitive methods of H. pylori should be employed specifically for evaluation of therapeutic efficacy.
Further, we tried to see whether the H. pylori strains detected pre- and posttherapy are similar or they are the cases of reinfection by new strains. All amplicon originating from 3 different targets from each of 23 patients subjected to restriction analysis showed that pre- and poststrains were identical. However, one amplicon of ureC origin and 6 of fliI were found to give different banding pattern than the initial amplification experiment on antral biopsy in the same patient. Although possibility of rising mutations during therapy cannot be ruled out, majority of patients were found to harbor the same strain after 4 weeks posttherapy which suggests that complete H. pylori eradication has not occurred in these patients. Our observation goes in the same line as reported previously, where the authors have shown that infection of H. pylori persists after therapy [20–26]. However, reinfection by the bacterium having identical restriction pattern may occur a result of recolonisation of stomach originating from the oral cavity of the same patients or contacting infection from a family member harboring the same strain [27]. It may also be quite likely, however, that H. pylori may survive in the gastric pits where sufficient concentration of antibiotics may not be achieved or bacteria transformed to coccoid (VBNC) that makes antibiotic ineffective. In an animal model, Cellini et al. (1994) demonstrated that up to 3 months after inoculation viable but not culturable forms of H. pylori could still be detected in the mouse stomach [28]. A few studies [29, 30] were carried out to evaluate triple therapy comparing PCR with culture. However no report has included nested PCR. Interestingly, Hammar et al. (1992) described gastric biopsy samples that were H. pylori positive by PCR but negative by culture [19]. Similar findings of persistence of H. pylori antigens, detected both with single round PCR and enzyme immunoassay (EIA), in the stool of successfully eradicated patients have been reported [31].
Therefore, it may be proposed that H. pylori causes chronic infection and usually eradication does not occur by anti-H. pylori regimens. The symptomatic relief occurring in the patients might be due to overall reduction in the bacterial density which might have aggravated the problem. Further, the possibility of presence of other bacteria than H. pylori causing acid peptic disease which are taken care off by the same antimicrobial agents may be also considered.
In conclusion, conventional methods to detect H. pylori especially posttherapy could not detect the pathogens as can be done by nested PCR protocol. Therefore, nested PCR may be proposed as the gold standard. Moreover, RUT, UBT, and histopathology are unable to discriminate the reinfection or recrudescence, while PCR based method (restriction analysis or sequencing) has capability to indicate either of the two possibilities.
Acknowledgments
This study was funded by the Department of Biotechnology, Government of India, Grant no. and sanction order no. 102/IFD/SAN/PR1310/2006-07. The authors gratefully acknowledged the Council of Scientific and Industrial Research (CSIR) New Delhi, India, in the form of Senior Research Fellowship awarded to Saurabh Kumar Patel.
Conflict of Interests
The authors have no conflict of interests.
References
- 1.van der Hulst RWM, Keller JJ, Rauws EAJ, Tytgat GNJ. Treatment of Helicobacter pylori infection: a review of the world literature. Helicobacter. 1996;1(1):6–19. doi: 10.1111/j.1523-5378.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Schistosomes, Liver Flukes and Helicobacter pylori. Vol. 61. Lyon, France: International Agency for Research on Cancer; 1994. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans). [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura K, Ido K, Saifuku K, et al. A 1-h topical therapy for the treatment of Helicobacter pylori infection. American Journal of Gastroenterology. 1995;90(1):60–63. [PubMed] [Google Scholar]
- 4.Sung JJY, Chung SCS, Ling TKW, et al. Antibacterial treatment of gastric ulcers associated with Helicobacter pylori . The New England Journal of Medicine. 1995;332:139–142. doi: 10.1056/NEJM199501193320302. [DOI] [PubMed] [Google Scholar]
- 5.Bayerdorffer E, Mannes GA, Sommer A, et al. Long-term follow-up after eradication of Helicobacter pylori with a combination of omeprazole and amoxycillin. Scandinavian Journal of Gastroenterology. 1993;28, supplement 196:19–25. doi: 10.3109/00365529309098337. [DOI] [PubMed] [Google Scholar]
- 6.Graham DY, Opekun AR, Klein PD. Clarithromycin for the eradication of Helicobacter pylori . Journal of Clinical Gastroenterology. 1993;16(4):292–294. doi: 10.1097/00004836-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Logan RPH, Gummett PA, Schaufelberger HD, et al. Eradication of Helicobacter pylori with clarithromycin and omeprazole. Gut. 1994;35(3):323–326. doi: 10.1136/gut.35.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia H, Daw MA, Sant S, Beattie S, Keane CT, O’Morain CA. Clinical efficacy of triple therapy in Helicobacter pylori-associated duodenal ulcer. European Journal of Gastroenterology & Hepatology. 1993;5(3):141–144. [Google Scholar]
- 9.Wheeldon TU, Hoang TT, Phung DC, Björkman A, Granström M, Sörberg M. Long-term follow-up of Helicobacter pylori eradication therapy in Vietnam: reinfection and clinical outcome. Alimentary Pharmacology & Therapeutics. 2005;21(8):1047–1053. doi: 10.1111/j.1365-2036.2005.02408.x. [DOI] [PubMed] [Google Scholar]
- 10.Okimoto T, Murakami K, Sato R, et al. Is the recurrence of Helicobacter pylori infection after eradication therapy resultant from recrudescence or reinfection, in Japan. Helicobacter. 2003;8(3):186–191. doi: 10.1046/j.1523-5378.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 11.Gisbert JP, Arata IG, Boixeda D, et al. Role of partner's infection in reinfection after Helicobacter pylori eradication. European Journal of Gastroenterology and Hepatology. 2002;14(8):865–871. doi: 10.1097/00042737-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrand P, Bardhan P, Rossi L, et al. Recrudescence and reinfection with Helicobacter pylori after eradication therapy in Bangladeshi adults. Gastroenterology. 2001;121(4):792–798. doi: 10.1053/gast.2001.28018. [DOI] [PubMed] [Google Scholar]
- 13.Xia HX, Windle HJ, Marshall DG, Smyth CJ, Keane CT, O'Morain CA. Recrudescence of Helicobacter pylori after apparently successful eradication: novel application of randomly amplified polymorphic DNA fingerprinting. Gut. 1995;37(1):30–34. doi: 10.1136/gut.37.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh V, Mishra S, Rao GRK, et al. Evaluation of nested PCR in detection of Helicobacter pylori targeting a highly conserved gene: HSP60. Helicobacter. 2008;13(1):30–34. doi: 10.1111/j.1523-5378.2008.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel SK, Pratap CB, Verma AK, Jain AK, Dixit VK, Nath G. Pseudomonas fluorescens-like bacteria from the stomach: a microbiological and molecular study. World Journal of Gastroenterology. 2013;19(7):1056–1067. doi: 10.3748/wjg.v19.i7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho S-A, Hoyle JA, Lewis FA, et al. Direct polymerase chain reaction test for detection of Helicobacter pylori in humans and animals. Journal of Clinical Microbiology. 1991;29(11):2543–2549. doi: 10.1128/jcm.29.11.2543-2549.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clinical Microbiology Reviews. 1997;10(4):720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole SP, Cirillo D, Kagnoff MF, Guiney DG, Eckmann L. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infection and Immunity. 1997;65(2):843–846. doi: 10.1128/iai.65.2.843-846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammar M, Tyszkiewicz T, Wadström T, O'Toole PW. Rapid detection of Helicobacter pylori in gastric biopsy material by polymerase chain reaction. Journal of Clinical Microbiology. 1992;30(1):54–58. doi: 10.1128/jcm.30.1.54-58.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor NS, Fox JG, Akopyants NS, et al. Long-term colonization with single and multiple strains of Helicobacter pylori assessed by DNA fingerprinting. Journal of Clinical Microbiology. 1995;33(4):918–923. doi: 10.1128/jcm.33.4.918-923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salama SM, Jiang Q, Chang N, Sherbaniuk RW, Taylor DE. Characterization of chromosomal DNA profiles from Helicobacter pylori strains isolated from sequential gastric biopsy specimens. Journal of Clinical Microbiology. 1995;33(9):1496–1497. doi: 10.1128/jcm.33.9.2496-2497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tee W, Lambert J, Smallwood R, Schembri M, Ross BC, Dwyer B. Ribotyping of Helicobacter pylori from clinical specimens. Journal of Clinical Microbiology. 1992;30(6):1562–1567. doi: 10.1128/jcm.30.6.1562-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costas M, Owen RJ, Bickley J, Morgan DR. Molecular techniques for studying the epidemiology of infection by Helicobacter pylori . Scandinavian Journal of Gastroenterology. 1991;26(181):20–32. doi: 10.3109/00365529109093204. [DOI] [PubMed] [Google Scholar]
- 24.Owen RJ, Bickley J, Costas M, Morgan DR. Genomic variation in Helicobacter pylori: application to identification of strains. Scandinavian Journal of Gastroenterology. 1991;26(181):43–50. doi: 10.3109/00365529109093207. [DOI] [PubMed] [Google Scholar]
- 25.Oudbier JH, Langenberg W, Rauws EAJ, Bruin-Mosch C. Genotypical variation of Campylobacter pylori from gastric mucosa. Journal of Clinical Microbiology. 1990;28(3):559–565. doi: 10.1128/jcm.28.3.559-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langenberg W, Rauws EAJ, Houthoff HJ, et al. Follow-up study of individuals with untreated Campylobacter pylori-associated gastritis and of noninfected persons with non-ulcer dyspepsia. The Journal of Infectious Diseases. 1988;157(6):1245–1249. doi: 10.1093/infdis/157.6.1245. [DOI] [PubMed] [Google Scholar]
- 27.Schutze K, Hentschel E, Dragosics B, Hirschl AM. Helicobacter pylori reinfection with identical organisms: transmission by the patients' spouses. Gut. 1995;36(6):831–833. doi: 10.1136/gut.36.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cellini L, Allocati N, Angelucci D, et al. Coccoid Helicobacter pylori not culturable in vitro reverts in mice. Microbiology and Immunology. 1994;38(11):843–850. doi: 10.1111/j.1348-0421.1994.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 29.Perkins SE, Yan LL, Shen Z, Hayward A, Murphy JC, Fox JG. Use of PCR and culture to detect Helicobacter pylori in naturally infected cats following triple antimicrobial therapy. Antimicrobial Agents and Chemotherapy. 1996;40(6):1486–1490. doi: 10.1128/aac.40.6.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rollan A, Giancaspero R, Fuster F, et al. The long-term reinfection rate and the course of duodenal ulcer disease after eradication of Helicobacter pylori in a developing country. American Journal of Gastroenterology. 2000;95(1):50–56. doi: 10.1111/j.1572-0241.2000.01700.x. [DOI] [PubMed] [Google Scholar]
- 31.Makristathis A, Barousch W, Pasching E, et al. Two enzyme immunoassays and PCR for detection of Helicobacter pylori in stool specimens from pediatric patients before and after eradication therapy. Journal of Clinical Microbiology. 2000;38(10):3710–3714. doi: 10.1128/jcm.38.10.3710-3714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


