Abstract
The amniotic membrane (AM) is the inner layer of the fetal membranes and consist of 3 different layers: the epithelium, basement membrane and stroma which further consists of three contiguous but distinct layers: the inner compact layer, middle fibroblast layer and the outermost spongy layer. The AM has been shown to have anti-inflammatory, anti-fibrotic, anti-angiogenic as well as anti-microbial properties. Also because of its transparent structure, lack of immunogenicity and the ability to provide an excellent substrate for growth, migration and adhesion of epithelial corneal and conjunctival cells, it is being used increasingly for ocular surface reconstruction in a variety of ocular pathologies including corneal disorders associated with limbal stem cell deficiency, surgeries for conjunctival reconstruction, as a carrier for ex vivo expansion of limbal epithelial cells, glaucoma surgeries and sceral melts and perforations. However indiscriminate use of human AM needs to be discouraged as complications though infrequent can occur. These include risk of transmission of bacterial, viral or fungal infections to the recipient if the donors are not adequately screened for communicable diseases, if the membrane is not processed under sterile conditions or if storage is improper. Optimal outcomes can be achieved only with meticulous case selection. This review explores the ever expanding ophthalmological indications for the use of human AM.
Keywords: Human amniotic membrane, Limbus, Stem cells, Ocular surface, Cornea
Core tip: Amniotic membrane transplantation is a very useful armamentarium in the hands of the ophthalmic surgeons for treating a variety of ocular surface disorders. Because of its transparent structure, anti- inflammatory, anti-fibrotic and anti-angiogenic properties and ability to provide a substrate for growth of corneal and conjunctival epithelial cells, it forms an ideal material for ocular surface reconstruction.
INTRODUCTION
The ocular surface is an extremely sensitive and dynamic structure, the health of which is crucial for the optimal functioning of the eye. Any mechanical or chemical insult to it either from exogenous sources, i.e., chemical injuries by substances like acids and alkalis, or from endogenous factors, i.e., change in the amount and composition of the tear film due to severe dry eye states associated with conditions like Stevens Johnson syndrome (SJS), rheumatoid arthritis and other collagen vascular diseases ,can result in anatomic, physiologic and optical dysfunction of the eye as a whole.
Various biological tissues have been attempted to be used as donor tissue to repair and reconstruct the ocular surface or to decrease the inflammation in instances where the conjunctiva and cornea get significantly damaged. These include among others oral, labial and vaginal mucous membranes and rabbit peritoneum. Amniotic membrane (AM) was first used therapeutically by Davis for skin transplantation in 1910[1]. De Roth however is the first person credited with having used fetal membranes in ophthalmic surgery in an attempt to reconstruct the ocular surface in patients with symblepharon[2]. The initial enthusiasm for use of this tissue however disappeared from documented ophthalmic literature, till the early nineties, when Batlle et al[3] used it to repair conjunctival defects and reconstruct the fornices.
STRUCTURE OF THE FETAL MEMBRANES
The fetal membranes consist of two layers: the outer chorion which is vascular and in contact with the uterine wall, and the amnion which is avascular, lies inner to the chorion and is in contact with amniotic fluid. The AM is 0.02-0.05 mm thick and is classically considered to be composed of three layers (Figure 1).
Figure 1.
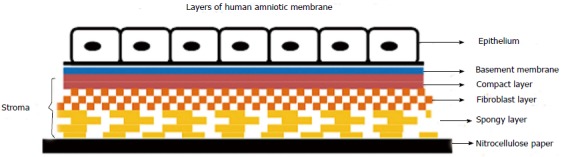
Line diagram showing layers of cryopreserved human amniotic membrane, oriented with the stromal side in contact with the nitrocellulose filter paper and epithelial side facing up.
Epithelium
Which is a monolayer of metabolically active cuboidal cells with microvilli present on its apical surface.
Basement membrane
Made up of type IV, V and VII collagen(also found in conjunctival and corneal basement membranes) in addition to fibronectin and laminin[4], It is one of the thickest membranes in the human body and can withstand current cryopreservation techniques.
Stroma
This is further divided into three contiguous but distinct layers: the inner compact layer which is in contact with the basement membrane and contributes to the tensile strength of the membrane, middle fibroblast layer which is thick and made up of a loose fibroblast network and the outermost spongy layer.
MECHANISM OF ACTION
Several mechanisms of action are attributed to the AM’s ability to help in healing and reconstruction of the ocular surface.
Mechanical
The AM acts as a biological bandage and shields the regenerating epithelium from the frictional forces generated by the blinking movements of the eyelids[5]. This is especially of significance in cases where entropion, trichiasis, keratinization of lid margin/palpebral conjunctiva or other such lid pathology exists which can damage the fragile epithelium, e.g., trachoma, SJS, ocular cicatricial pemphigoid (OCP), etc. Use of the AM in addition to tilting the balance of the ocular surface towards healing, also dramatically decreases the subjective symptoms of pain and discomfort experienced by these patients, especially when implanted on deepithelized areas of the cornea. This has been attributed to a purely mechanical effect and not because of the biological mediators present in the membrane, as elegantly demonstrated by Lee et al[6] in experimental studies on rabbits where application of amniotic fluid to denuded corneas (created by subjecting the animals to excimer laser photo keratectomy) increased the corneal sensitivity and upregulated regeneration of nerves.
Promotion of epithelialization
The basement membrane of the AM closely resembles that of the conjunctiva and cornea especially with regards to its collagen composition. It thus serves as a substrate on which epithelial cells can grow easily. Four main effects on the regenerating corneal epithelium have been described: (1) facilitation of epithelial cell migration[7,8]; (2) reinforcement of basal epithelial cell adhesion[9-11]; (3) promotion of epithelial cell differentiation[12-14]; and (4) Prevention of apoptosis[15,16]. These properties render it suitable for use in cases of nonhealing or persistent epithelial defects of the ocular surface, especially that of the cornea.
Anti-fibrotic and anti-inflammatory properties
Fetal hyaluronic acid is an important constituent of the stromal matrix of the AM. This helps to suppress TGF β signaling with reduced expression of TGF β-1, β-2, and β-3 isoforms in addition to reduced expression of TGF-Receptor II. This inhibits proliferation of corneal, limbal and conjunctival fibroblasts. Differentiation of fibroblasts into myofibroblasts is also inhibited, thus reducing scarring after pterygium surgery and ocular surface reconstruction[17]. Anti-inflammatory effect of AM is driven by inhibition of expression of pro inflammatory cytokines from the damaged ocular surface, e.g., interleukin (IL) 1a, IL-2, IL-8, interferon-γ, tumor necrosis factor-β, basic fibroblast growth factor and platelet derived growth factor[18]. In addition to the chemically mediated anti- inflammatory effect, Shimmura et al[19] also demonstrated a more mechanical effect by showing that inflammatory cells get trapped and undergo apoptosis in the matrix of the AM.
Anti-angiogenic properties
In addition to the anti-inflammatory properties which retard new vessel proliferation, a specific anti-angiogenic effect has also been ascribed to the AM. This has been demonstrated to be due to the production of several potent anti angiogenic chemicals including thrombospondin -1, endostatin and all four tissue inhibitors of metalloproteases (TIMP-1, 2, 3 and 4)[20]. Though beneficial in most situations the anti-angiogenic effect of AM needs to be kept in mind and balanced against its other potential benefits when using it in limbal stem cell deficiency associated with limbal ischaemia, i.e., in chemical injuries of the ocular surface.
Anti- microbial properties
A literature review reveals conflicting reports about the anti-microbial properties of AM. Burn patients treated with AM have been shown to have decreased bacterial counts and control of infections[21,22]. Antibacterial effects effects have been demonstrated against both gram positive cocci including streptococci and Staphylococcus aureus as well as gram negative bacilli including Escherichia coli and Pseudomonas aeruginosa[23,24]. These antibacterial effects have been attributed to the presence of several anti-microbial factors in the amniotic fluid including bactricidin, beta-lysin , lysozyme, transferrin and 7S immunoglobulin[25,26]. Other investigators however believe that the AM does not per se contain any chemical antimicrobial substances, but rather just constitutes an effective physical barrier against infection because of its ability to adhere closely to the underlying surface[24,27].
In addition to the above properties another important characteristic of the human AM is a lack of expression of the major histocompatibility antigens HLA-A, B, or DR antigens[28,29]. Hence immunological rejection after its transplantation does not occur and obviates the need for any immune suppression. This feature along with the transparent structure and ability to be preserved for prolonged periods make the AM an ideal substrate for ocular surface transplantation.
PROCURING, PROCESSING AND PRESERVING THE AM
AM is retrieved under strict aseptic conditions from donors undergoing elective cesarean section and who have been previously screened serologically for potentially communicable diseases including human immunodeficiency virus, hepatitis B and C viruses and syphilis. Placenta obtained after vaginal delivery are not used for this purpose because of the potential for contamination with bacteria from the vagina. It is recommended that the maternal donor should undergo repeat serological screening after 6 mo (to cover the window period for transmission of communicable diseases) before the AM is released for use[30]. Tissue is used for transplantation only when both the samples are negative.
Antibiotics covering both gram positive and gram negative bacteria as well as fungi (50 μg/mL penicillin, 50 μg/mL streptomycin, 100 μg/mL of neomycin, 2.5 μg/mL of amphotericin B) are used to wash the placenta under sterile conditions. Blunt dissection is then used to separate the amnion from the chorion. The AM may be preserved by means of cryopreservation (cryopreserved human amniotic membrane, CHAM) or in a dry deepithelialized form (dry human amniotic membrane, DHAM). To prepare CHAM Kim et al[31,32] and Lee et al[33] recommended using 50% glycerol in Dulbecco’s modified Eagle Medium (DMEM) in a ratio of 1:1 to store the membrane. The membrane is cut into multiple pieces and placed on nitrocellulose paper strips with epithelial side up. It is then placed in vials containing the glycerol/DMEM storage medium and cryopreserved at -80 °C. Just prior to use the membrane should be taken out and warmed to room temperature for 10 min. CHAM stored in glycerol may be safely and effectively used for over a year with the added advantage of antiviral and antibacterial properties of glycerol[34]. One drawback with using CHAM is the need of a -80 °C refrigerator. This precludes its use outside big institutions.
DHAM does not require to be attached to nitrocellulose paper and is free standing. DHAM is prepared by subjecting the amniotic membrane to sterilization using low energy electron beam radiation and then preserving it using low heat and air vaccum .DHAM can be stored at room temperature for upto 2-5 years and is rehydrated prior to use. It is usually 35-40 microns thick but a third generation, 110-μm-thick, freeze dried, and freestanding human AM allograft (Ambio 5; IOP Inc, Costa Mesa, California) is commercially available. This has an additional thick layer of retained collagen from the chorionic membrane, which makes it thicker and confers greater tectonic function.
Both fresh and preserved AM have been found to be equally effective when transplanted onto the ocular surface[35]. Use of freshly acquired AM however is associated with certain disadvantages including the risk of transmission of communicable diseases as the donor cannot undergo repeat serological testing, and wastage of unused tissue ( with preserved AM, up to 30 grafts can be prepared with one placenta). Preservation of the AM by any means has been shown to adversely affect the viability and proliferative capacity of its epithelial cells[36,37]. Kruse et al[36] proposed that AM grafts function primarily as a matrix and not by virtue of transplanted functional cells. Other literature on the subject also supports the view that viability of cellular components of the AM is not essential for its biological effectiveness[38].
SURGICAL PRINCIPLES AND METHODS OF IMPLANTATION
Rationale for determining orientation of AM on ocular surface
This is important as the indication for which the AM is being used and the endpoint desired determines the preferred orientation with which it is used on the ocular surface. Histopathological analysis has revealed that after application of AM the re-epithelialization of the ocular surface by the host epithelium (i.e., by the host corneal or conjunctival epithelium) occurs preferentially on the basement membrane side of the epithelium[39], though Seitz et al[40] have also demonstrated that corneal epithelial cells do possess the ability to grow on the stromal side of the membrane. Hence where the membrane is used with the aim of providing conjunctival or corneal cells a substrate to grow on, the AM is used epithelial/basement side up. The stromal matrix of the AM on the other hand has the ability to trap inflammatory cells and induce their apoptosis, thus down regulating the inflammatory response[38]. Thus in the presence of acute inflammation, the membrane may be used to protect the ocular surface from the deleterious effects of the pro inflammatory cells and mediators- here it is used epithelial side down, so that the stromal side faces the palpebral aperture.
Determining the orientation of the AM
The AM supplied on the nitrocellulose filter paper is usually oriented epithelial side up, with the stromal side in direct contact with the paper. The stromal surface can be identified by the presence of vitreous-like strands that can be raised with a sponge or a fine forceps. This may need to be performed at a few points for confirmation.
Depending on the indication for which it is used there are three surgical techniques by which the AM can be used over the ocular surface.
Graft or inlay technique: In this technique the AM is intended to act as a substrate or scaffold for epithelial cells to grow. The AM is placed epithelial/basement membrane side up and is trimmed to fit the size of the underlying epithelial or stromal defect. It is usually sutured to the cornea using non absorbale 10-0 nylon sutures and to the episclera and conjunctiva using 9-0 or 10-0 vicryl (Figure 2). It is preferred to keep the epithelial/basement membrane side up in this technique because the basement membrane of the amnion acts as an excellent substrate for growth of the progenitor epithelial cells by prolonging their lifespan, maintaining clonigenecity and preventing apoptosis[41]. The surrounding 1-2 mm of the host corneal epithelium is debrided. This ensures that the regenerating epithelium grows over the basement membrane of the AM, and consequently the AM stroma gets incorporated into the host tissue (“graft”). Depending on the depth of the underlying defect this technique may be used as a single layer graft inlay where a single layer of AM is used, or multilayer graft inlay where multiple layers of the AM are placed into the ground of the ulcer, which is filled without sutures before a superficial graft is sutured to the periphery of the ulcer, again after depithelialization of a ring-shaped area around the cornea ulcer. The epithelium is expected to grow over the uppermost layer of this multilayer graft[40]. This is also referred to as the layered or fill in technique. The layering may be done either by cutting the AM into multiple pieces and placing them one on top of one another or by using a larger piece of AM which is repeatedly folded (blanket fold) upon itself.
Figure 2.
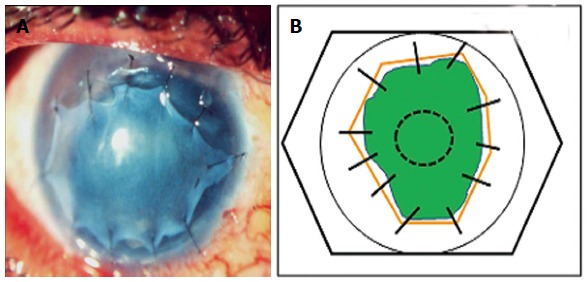
(A) Amniotic membrane used as an inlay graft (B) line diagram showing amniotic membrane (solid orange lines) used as an Inlay graft for a non-healing epithelial defect (green). The membrane is trimmed to fit the size of the underlying defect and sutured to the cornea using interrupted 10-0 nylon monofilament sutures.
Patch or overlay technique: Here the AM is sutured to the ocular surface keeping it larger than the underlying defect so that host epithelium is present below the membrane. The membrane is sutured to the surrounding conjunctiva or episclera using 9-0 vicryl suture. An additional 10-0 nylon suture may be applied to the peripheral cornea in a purse string manner to ensure prolonged retention (Figure 3). The AM may be used epithelial side up or stromal side up as the host epithelium is expected to grow under the membrane which basically acts only as a “biological bandage contact lens” to protect the fragile new epithelium from the frictional forces generated due to eyelid movements. In this situation the AM is expected to fall off or be removed after a certain time.
Figure 3.
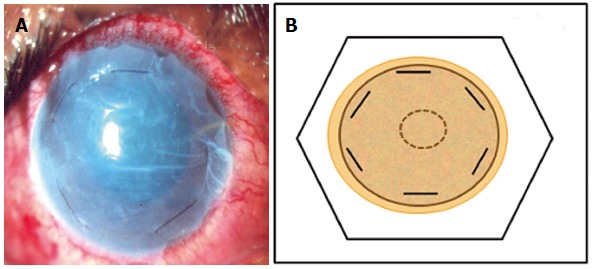
Amniotic membrane used as an overlay patch. A: Clinical photograph; B: Line diagram. The amniotic membrane is trimmed to cover the whole of the corneal surface and fixed by 10-0 nylon monofilamemt sutures at the corneal periphery parallel to the limbus.
Combined (inlay and overlay) technique: Two or more layers of AM are used in this technique, with the inner smaller layer/layers acting as a graft and the outer larger layer acting as a patch. Also known as the “sandwich technique” a single-layer( Figure 4A) or multilayer inlay (Figure 4B) is combined with an onlay[40]. The epithelium is expected to grow under the patch but over the uppermost inlay graft.
Figure 4.
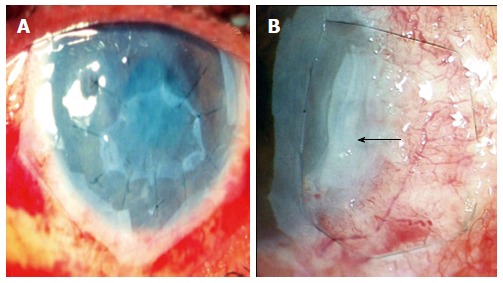
Single layer inlay covered by a larger “patch” or “onlay” (A), multi-layer inlay ( amniotic membrane folded upon itself in form of a blanket fold- black arrow) covered by a larger “patch” which is fixed to underlying episclera by a purse string suture in a case of deep, non-resolving peripheral ulcerative keratitis (B).
The availability of fibrin glue for ophthalmic use has in many cases supplanted the use of sutures, and the AM may be adhered to the ocular surface using the recombitant fibrin glue. This reduces the surgical time and also increases patient comfort.
OPHTHALMOLOGICAL INDICATIONS FOR USE OF AM
The list of indications for which AM is used in ophthalmology is expanding with each passing year. Broadly its use can be classified into (1) corneal surface disorders, without limbal stem cell deficiency (LSCD); (2) corneal surface disorders with associated LSCD; (3) conjunctival surface reconstruction, e.g., pterygium removal, after removal of large lesions other than pterygium, after symblepharon lysis; (4) as a carrier for ex vivo expansion of corneal epithelial cells; (5) glaucoma; (6) treatment of sclera melts and perforations; and (7) other miscellaneous indications.
Persitent epithelial defects and Non Healing corneal ulcers
Persitent epithelial defects (PED’s) may occur due to a variety of mechanisms including innervations deficits of the cornea (e.g., neurotrophic keratopathy following Herpes Zoster keratitis, after penetrating keratoplasty), chronic inflammation or mechanical factors. These factors may act individually or in synergy, and lead to epithelial defects which are unresponsive to conventional management strategies, e.g., lubrication, bandage contact lenses, tarsorrahaphy, etc. Untreated these PED’s can progress to stromal collagenolysis, ulceration, perforation or scarring. In these situations AM may be used as a single layer or multilayer graft (inlay) depending on the depth of the lesion providing a substrate for the epithelial cells to migrate and adhere to the basement membrane. The inlay may also be combined with an epithelial side down onlay patch graft especially if significant ocular surface inflammation co-exists as stromal surface of the onlay graft will help mop up the inflammatory cells and mediators on the palpebral surface. Success rates of using AM for PED’s have been reported as varying from 64% to 91%[33,42]. Early detachment of the membrane however remains a major problem despite the use of multiple sutures or a protective bandage contact lens (BCL)[42].
AM has also been used successfully in nonhealing infective ulcers due to bacteria, fungi, viruses and protozoa. The nonhealing of the ulcer inspite of adequate antimicrobial therapy in these situations may be because of release of proinflammatory mediators, proteolytic enzymes and collagenazes by the microorganisms, stromal keratocytes and polymorphonuclear cells[43,44]. Single or multilayer AM has an inhibitory effect on these proteolytic enzymes and also provides a healthy basement membrane, thus tilting the ocular surface milieu in favour of rapid healing.
Corneal perforations and descemetoceles
Corneal perforations and descemetoceles are globe and sight threatening complications associated with loss of tectonic strength of corneal stroma as well as associated underlying inflammation. A majority of the methods used to treat these conditions including tissue adhesives, lamellar keratoplasty, penetrating keratoplasty, bandage contact lenses and conjunctival flaps provide tectonic support, but do not directly address the inflammatory component. Multilayer AM has been used to treat non traumatic microperforations and descemetoceles with upto 72.7% to 82.3% success rate being reported[45,46]. AM in this situation provides tectonic support, collagen substitution for corneal stroma and anti- inflammatory and anti-fibrotic actions which halt progressive tissue degradation. Depending on the underlying severity and extent of the disease process it may be used as a permanent surgical therapy or as a temporizing measure till a more definitive surgical procedure can be performed. One of the authors of this review (AKJ) has reported successful management of a case of perforated peripheral corneal ulcer in a patient of acne rosacea with amniotic membrane after three applications of cyanoacrylate glue with bandage contact lens failed to seal the perforation successfully[47]. A final best corrected visual acuity of 6/6 was achieved in this patient. Though used most commonly for small perforations, Kim et al[48] have reported successful outcomes even in patients with perforations > 2 mm in size using fibrin glue to augment the thickness of the AM-a procedure they termed “fibrin glue assisted augmented AMT”.
Symptomatic bullous keratopathy
In symptomatic patients with good visual potential and intolerant to a BCL, AM may be used as a temporizing measure till a definitive treatment for the bullous keratopathy, i.e., endothelial or penetrating keratoplasty is undertaken. It may also be used as an alternative to anterior stromal puncture in patients with a poor visual potential with the objective of providing longer pain free periods. Espana et al[49] have reported a mean follow up of 25 mo and noted that 88 % patients were able to obtain a pain free status.
Band keratopathy
Band keratopathy due to abnormal deposition of calcium on the corneal surface results in ocular irritation and epithelial surface breakdown. Primary treatment involves removal of calcium deposits by ethylene diamine tetra acetic acid chelation or superficial keratectomy. AM transplantation has been used as an adjunct after primary surgical treatment in band keratopathy with pain relief being reported in 93% cases and visual acuity improvement in 44% of sighted eyes[50].
Corneal disorders with associated LSCD: Any acute or chronic insult to the the limbal epithelial stem cells can lead to a state of partial or total LSCD which may manifest as conjunctivalization of the cornea, neovascularization, PED’s and chronic inflammation. This may be seen after thermal or chemical burns, cicatrizing disorders like SJS and OCP, aniridia, chronic contact lens wear, untreated vernal keratoconjunctivitis (VKC) and multiple surgeries involving the limbal area. Successful long term outcome in these eyes after lamellar or penetrating keratoplasty requires prior optimization of the ocular surface and restoration of the stem cell population. Success of AM in these scenarios depends on the severity of the LSCD. In cases of partial LSCD amniotic membrane alone can be an effective therapy to restore the ocular surface as by promoting epithelialization and reducing inflammation it restores a normal stroma which maximizes functioning of the remaining limbal stem cells[51,52]. In cases of total LSCD however AM transplantation has only an adjunct role to limbal stem cell transplantation which is the definitive modality of treatment, as AM can only optimize functioning of existent limbal stem cells (LSC’s) as is seen in partial LSCD, but it cannot cause repopulation of the affected eye with LSC’s in cases of advanced total LSCD. In these situations use of de-epithelized AM as a carrier for ex vivo expansion of limbal autologus or allolimbal stem cells is another good option as it combines the advantages of both techniques, i.e., simultaneous optimization of the ocular surface by the AM and replacement of the stem cells.
Conjunctival reconstruction: AM can be used for reconstruction of the conjunctival surface as a substitute for conjunctival grafts in situations where availability of autologous conjunctival tissue is limited, i.e., after removal of large conjunctival lesions, patients having undergone repeated conjunctival surgery leading to a scarred conjunctiva, or where the conjunctiva needs to be preserved, i.e., patients with glaucoma who may require filtering surgery in the future. Use of AM in these situations is helpful as in addition to providing a healthy basement membrane for growth of conjunctival epithelial cells it also helps in maintaining the normal goblet cell containing phenotype of these cells[53]. However as the AM is only a temporary substitute, to provide long term reepithelialization of the conjunctival surface, the surrounding conjunctival tissue must be healthy with an intact vascular bed.
Pterygium: Use of AM as an alternative to autologous conjunctival grafts was described by Prabhasawat et al[54]. They reported higher recurrence rates (10.9%) for primary pterygia with the use of AM as compared to recurrence with use of autologous conjunctival autografts (2.6%). However later studies which emphasized extensive removal of fibrovascular tissue adjacent to the pterygium have reported that recurrence rates with use of AM transplantation (3%-3.8% in primary pterygia and 9.5% in recurrent pterygia) were similar to those reported after conjunctival autografting and intra operative mitimycin C use[55,56]. Jain et al[57] have described the use of AM transplantation using fibrin glue in primary pterygia using a ‘tuck in technique’ where the edges of the AM graft were tucked underneath the adjacent free margin of conjunctiva on 3 sides and reported no recurrences in 11 out of 12 patients after a follow up of one year (Figure 5).
Figure 5.
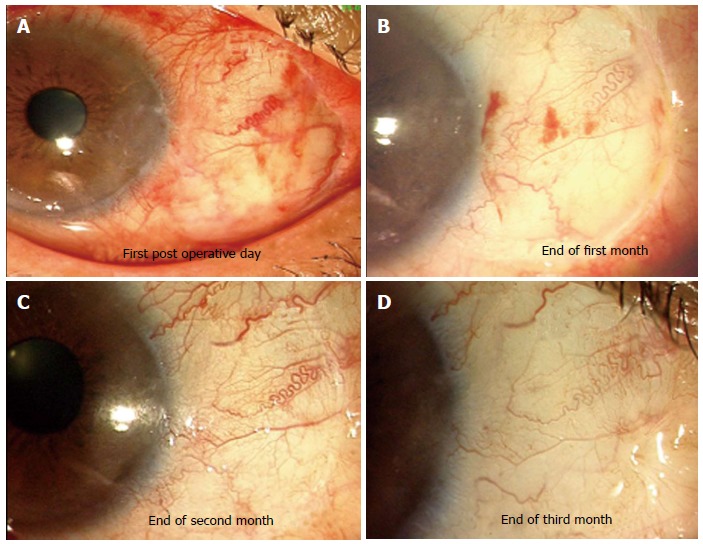
Amniotic membrane used to cover bare sclera after excision of primary pterygium. A-D: Serial photographs showing appearance of amniotic membrane graft. At end of 3 mo excellent integration and cosmetic appearance was achieved.
However conjunctival autograft is still considered to be the gold standard for treatment of primary pterygia and AM may be a reserved as a reasonable option in cases with diffuse conjunctival involvement, i.e., primary extensive biheaded pterygia, in previous multiple failed surgeries and in patients in whom the bulbar conjunctiva must be preserved for a prospective glaucoma filtering procedure[58].
Conjunctival tumours and ocular surface squamous neoplasias: AM has been used for conjunctival reconstruction after excision of both benign and malignant tumours including ocular surface squamous neoplasias(OSSN), melanomas, lymphomas and complex choristomas. The AM provides a substrate for migration of the conjunctival epithelial cells. Advantages of using amniotic membrane as compared to conjunctival autografts in these situations include a lack of donor site morbidity and the ability to clinically monitor local tumour recurrence beneath the transparent AM graft[59].
After symblepharon lysis: AM can be used both in the prevention as well as treatment of symblepharon. In the acute phases of chemical injury the AM can be used as a patch to cover the entire ocular surface and sutured to the fornices through the eyelids to prevent symblepharon formation as well as simultaneously reduce ocular surface inflammation. The AM should be a continuous sheet devoid of buttonholes. A large sheet is placed on the ocular surface and it is first anchored to the inner surface of the everted lower lid close to the lid margin using multiple interrupted 10-0 vicryl sutures. To anchor the sheet to the fornices two sets of double armed 4-0 chromic gut sutures on a cutting needle are used and the needles are passed from the AM surface through the inferior fornix, via the full-thickness of the eyelid and are made to exit through the eyelid skin. The two needles of each of the two sets of sutures are passed through two segments of an encircling band and then tied[60]. A sutureless amniotic patch (ProKeras; Bio-Tissue Inc., Miami,Florida) is also available for this purpose. Another modification suggested by Rahman et al[61] is the use of a conformer on which the AM is sutured and placed on the ocular surface, with the AM acting as a patch and the conformer maintaining the fornices because of its rigidity. Though the AM has also been used for symblephera associated with SJS and OCP the outcomes are usually not as successful as compared to stable non progressive cicatrization because of the chronic ongoing inflammation associated with these diseases[62].
As a carrier for ex vivo expansion of epithelial cells: Progenitor stem cells for the conjunctiva and cornea have been established to reside in the conjunctival fornices and limbal area respectively. In the eye, i.e., under in- vivo conditions these migrate onto the ocular surface and differentiate into daughter cells to continuously regenerate the conjunctival and corneal surface epithelia[63]. Expansion of these cell populations on basement membrane side of AM (with amniotic epithelium intact or de-epithelized AM) as well as on the stromal side of AM have been demonstrated previously. The corneal epithelial cells have been shown to migrate rapidly when limbal explants are placed on AM denuded of amniotic epithelial cells but with the basement membrane intact, relatively slowly when the amniotic epithelium is left behind and slowest when the membrane has been flipped over and the cells are grown on the stromal surface. Culturing the explants on an intact AM with devitalized epithelium favors expansion of an epithelial phenotype that closely resembles limbal stem cells[38].
Clinically in cases of LSCD, limbal biopsies can be used to harvest corneal epithelial stem cells for ex vivo expansion on AM, which can then be transplanted onto the eye after appropriate preparation of the host bed by resecting the vascularized pannus or any other procedure which may be required. The main advantage of this approach of expanding corneal epithelial cells ex vivo on AM is that only a small amount of limbal tissue is required to be harvested from the contralateral eye as compared to conventional limbal allografts which require up to 12-clock hours of limbal tissue and have the potential risk for limbal deficiency developing in the donor eye. Another advantage is that the AM is a natural substrate and when transplanted onto the corneal surface gets integrated into it. Excellent outcomes have been reported after transplantation of cultivated limbal stem cell on denuded AM for LSCD[64-66].
Glaucoma: AM has been used in glaucoma to reduce scarring at the time of filtering surgery, to repair early or late leaks, and act as a cover for valve procedures. Fujishima et al[67] attempted to reduce scarring in filtering surgery by incorporating a layer of amnion between the scleral flap and bed to prevent an adhesion between the two layers, but achieved only limited success. Use of AM to repair bleb leaks is controversial with some authors reporting good results[68] while others have reported it as being ineffective[69].
Treatment of corneo scleral melts and perforations: Small sclera perforations or melts can be treated by multilayerd AM alone while for larger scleral defects AM has been used over the sclera patch, basement side up so as to facilitate epithelialization of the scleral patch graft as well as to reduce the inflammation[45,70]. It has been used with success for both infectious scleral ulcerations after appropriate antimicrobial therapy[71]. as well as after noninfectious scleral melts. Tay et al[72] have reported using a double layer of freeze dried AM (Ambio 5; IOP Inc, Costa Mesa, California) in a crescentric manner to manage a case of carrier graft melt in a patient with Boston Keratoprosthesis Type 1.
Miscellaneous indications: Severe shield ulcers due to VKC which do not heal with conservative management respond well to surgical debridement of the mucous plaque and debri followed by using the AM as a patch to promote epithelialization. Using this technique Sridhar et al[73] achieved a success rate of 94.7% with shield ulcers. AM has been occasionally in oculoplasty for lid reconstruction, for treatment of punctual occlusion by applying it as a patch over the denuded punctual orifice[74] as a cover for ocular prosthesis at the time of insertion and to cover the tarsal plate in lid split procedure for correction of cicatricial entropion[75].
Complications and limitations of am use: Though the AM is finding ever expanding uses in ophthalmology, it must not be used indiscriminately as complications though infrequent can occur. Risk of transmission of bacterial, viral or fungal infections to the recipient exists if the donors are not adequately screened for communicable diseases, if the membrane is not processed under sterile conditions or if storage is improper. Incidence rates of 1.6%-8.0% have been reported post AM transplantation with gram positive isolates being reported most frequently[76-78]. Premature degradation of the membrane and cheese wiring may need frequent repeat transplantations. Occasionally, a residual subepithelial membrane may persist in some cases and inadvertently opacify the visual axis[38].
CONCLUSION
The AM is proving to be a very versatile tool in the hands of the ophthalmologist, and the indications for its use are rapidly expanding as there is a better understanding of its properties. However a judicious use and appropriate patient selection is important for achieving optimal outcomes.
Footnotes
P- Reviewers: Fisher RA, Kita K, Mathis AS S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307. [Google Scholar]
- 2.deRoth A. Plastic repair of conjunctival defects with fetal membrane. Arch Ophthalmol. 1940;23:522–525. [Google Scholar]
- 3.Batlle JF, Perdomo FJ. Placental membranes as a conjunctival substitute. Ophthalmol. 1993:100: A107. [Google Scholar]
- 4.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of sub-chains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea and conunctiva. Cornea. 1999;18:73–79. [PubMed] [Google Scholar]
- 5.Baum J. Thygeson lecture. Amniotic membrane transplantation: why is it effective? Cornea. 2002;21:339–341. doi: 10.1097/00003226-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Lee HS, Kim JC. Effect of amniotic fluid in corneal sensitivity and nerve regeneration after eximer laser ablation. Cornea. 1996;15:517–524. [PubMed] [Google Scholar]
- 7.Meller D, Pires RT, Tseng SC. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–471. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40:878–886. [PubMed] [Google Scholar]
- 9.Keene DR, Sakai LY, Lunstrum GP. Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol. 1987;104:611–621. doi: 10.1083/jcb.104.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LM, Falcioni R, Kennel SJ, Aplin JD, Baker J, Loizidou M. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terranova VP, Lyall RM. Chemotaxis of human gingival epithelial cells to laminin. A mechanism for epithelial cell apical migration. J Periodonto. 1986;57:311–317. doi: 10.1902/jop.1986.57.5.311. [DOI] [PubMed] [Google Scholar]
- 12.Guo M, Grinnell F. Basement membrane and human epidermal differentiation in vitro. J Invest Dermatol. 1989;93:372–378. [PubMed] [Google Scholar]
- 13.Kurpakus MA, Stock EL, Jones JC. The role of the basement membrane in differential expression of keratin proteins in epithelial cells. Dev Biol. 1992;150:243–255. doi: 10.1016/0012-1606(92)90239-d. [DOI] [PubMed] [Google Scholar]
- 14.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–334. [PubMed] [Google Scholar]
- 18.Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of Interleukin 1 alpha and Interleukin 1 beta in the human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85:444–449. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20:408–413. doi: 10.1097/00003226-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Rao TV, Chandrasekharam V. Use of dry human and bovine amnion as a biological dressing. Arch Surg. 1981;116:891–896. doi: 10.1001/archsurg.1981.01380190029007. [DOI] [PubMed] [Google Scholar]
- 22.Robson MC, Krizek TJ. The effect of human amniotic membranes on the bacteria population of infected rat burns. Ann Surg. 1973;177:144–149. doi: 10.1097/00000658-197302000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schønheyder HC, Uldbjerg N, Madsen H. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–229. doi: 10.1016/s0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 24.Kjaergaard N, Helmig RB, Schønheyder HC, Uldbjerg N, Hansen ES, Madsen H. Chorioamniotic membranes constitute a competent barrier to group b streptococcus in vitro. Eur J Obstet Gynecol Reprod Biol. 1999;83:165–169. doi: 10.1016/s0301-2115(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 25.Gudson J. A Bactericidin for bacillus subtilis in pregnancy. J Immunol. 1962;88:494–499. [PubMed] [Google Scholar]
- 26.Galask RP, Snyder IS. Antimicrobial factors in amniotic fluid. Am J Obstet Gynecol. 1970;106:59–65. doi: 10.1016/0002-9378(70)90126-2. [DOI] [PubMed] [Google Scholar]
- 27.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–288. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- 28.Adinolfi M, Akle CA, McColl I, Fensom AH, Tansley L, Connolly P, Hsi BL, Faulk WP, Travers P, Bodmer WF. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982;295:325–327. doi: 10.1038/295325a0. [DOI] [PubMed] [Google Scholar]
- 29.Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 30.Dua HS, Azuara-Blanco A. Amniotic membrane transplantation. Br J Ophthalmol. 1999;83:748–752. doi: 10.1136/bjo.83.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–484. [PubMed] [Google Scholar]
- 32.Kim JC, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Ophthalmol. 1995;9:32–46. doi: 10.3341/kjo.1995.9.1.32. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123:303–312. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]
- 34.Maral T, Borman H, Arslan H, Demirhan B, Akinbingol G, Haberal M. Effectiveness of human amnion preserved long-term in glycerol as a temporary biological dressing. Burns. 1999;25:625–635. doi: 10.1016/s0305-4179(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 35.Adds PJ, Hunt CJ, Dart JK. Amniotic membrane grafts, “fresh” or frozen? A clinical and in vitro comparison. Br J Ophthalmol. 2001;85:905–907. doi: 10.1136/bjo.85.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kruse FE, Joussen AM, Rohrschneider K, You L, Sinn B, Baumann J, Völcker HE. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch Clin Exp Ophthalmol. 2000;238:68–75. doi: 10.1007/s004170050012. [DOI] [PubMed] [Google Scholar]
- 37.Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539–1546. [PubMed] [Google Scholar]
- 38.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Hu DJ, Basti S, Wen A, Bryar PJ. Prospective comparison of corneal re-epithelialization over the stromal and basement membrane surfaces of preserved human amniotic membrane. ARVO. 2003:3151. [Google Scholar]
- 40.Seitz B, Resch MD, Schlötzer-Schrehardt U, Hofmann-Rummelt C, Sauer R, Kruse FE. Histopathology and ultrastructure of human corneas after amniotic membrane transplantation. Arch Ophthalmol. 2006;124:1487–1490. doi: 10.1001/archopht.124.10.1487. [DOI] [PubMed] [Google Scholar]
- 41.Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch Ophthalmol. 2002;120:783–790. doi: 10.1001/archopht.120.6.783. [DOI] [PubMed] [Google Scholar]
- 42.Letko E, Stechschulte SU, Kenyon KR, Sadeq N, Romero TR, Samson CM, Nguyen QD, Harper SL, Primack JD, Azar DT, et al. Amniotic membrane inlay and overlay grafting for corneal epithelial defects and stromal ulcers. Arch Ophthalmol. 2001;119:659–663. doi: 10.1001/archopht.119.5.659. [DOI] [PubMed] [Google Scholar]
- 43.Brown SI, Bloomfield SE, Tam W. The cornea-destroying enzyme of Pseudomonas aeruginosa. Invest Ophthalmol. 1974;13:174–180. [PubMed] [Google Scholar]
- 44.Buxton JN, Fox ML. Conjunctival flaps in the treatment of refractory pseudomonas corneal abscess. Ann Ophthalmol. 1986;18:315–318. [PubMed] [Google Scholar]
- 45.Hanada K, Shimazaki J, Shimmura S, Tsubota K. Multilayered amniotic membrane transplantation for severe ulceration of the cornea and sclera. Am J Ophthalmol. 2001;131:324–331. doi: 10.1016/s0002-9394(00)00825-4. [DOI] [PubMed] [Google Scholar]
- 46.Solomon A, Meller D, Prabhasawat P, John T, Espana EM, Steuhl KP, Tseng SC. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002;109:694–703. doi: 10.1016/s0161-6420(01)01032-6. [DOI] [PubMed] [Google Scholar]
- 47.Jain AK, Sukhija J. Amniotic membrane transplantation in ocular rosacea. Ann Ophthalmol (Skokie) 2007;39:71–73. doi: 10.1007/BF02697331. [DOI] [PubMed] [Google Scholar]
- 48.Kim HK, Park HS. Fibrin glue-assisted augmented amniotic membrane transplantation for the treatment of large noninfectious corneal perforations. Cornea. 2009;28:170–176. doi: 10.1097/ICO.0b013e3181861c54. [DOI] [PubMed] [Google Scholar]
- 49.Espana EM, Grueterich M, Sandoval H, Solomon A, Alfonso E, Karp CL, Fantes F, Tseng SC. Amniotic membrane transplantation for bullous keratopathy in eyes with poor visual potential. J Cataract Refract Surg. 2003;29:279–284. doi: 10.1016/s0886-3350(02)01525-0. [DOI] [PubMed] [Google Scholar]
- 50.Anderson DF, Prabhasawat P, Alfonso E, Tseng SC. Amniotic membrane transplantation after the primary surgical management of band keratopathy. Cornea. 2001;20:354–361. doi: 10.1097/00003226-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 52.Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85:567–575. doi: 10.1136/bjo.85.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prabhasawat P, Tseng SC. Impression cytology study of epithelial phenotype of ocular surface reconstructed by preserved human amniotic membrane. Arch Ophthalmol. 1997;115:1360–1367. doi: 10.1001/archopht.1997.01100160530001. [DOI] [PubMed] [Google Scholar]
- 54.Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104:974–985. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- 55.Solomon A, Pires RTF, Tseng SCG: Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmol. 2001;108:449–460. doi: 10.1016/s0161-6420(00)00567-4. [DOI] [PubMed] [Google Scholar]
- 56.Ma DH, See LC, Liau SB, Tsai RJ. Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol. 2000;84:973–978. doi: 10.1136/bjo.84.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain AK, Bansal R, Sukhija J. Human amniotic membrane transplantation with fibrin glue in management of primary pterygia: a new tuck-in technique. Cornea. 2008;27:94–99. doi: 10.1097/ICO.0b013e318158b47f. [DOI] [PubMed] [Google Scholar]
- 58.Sangwan VS, Murthy SI, Bansal AK, Rao GN. Surgical treatment of chronically recurring pterygium. Cornea. 2003;22:63–65. doi: 10.1097/00003226-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Paridaens D, Beekhuis H, van Den Bosch W, Remeyer L, Melles G. Amniotic membrane transplantation in the management of conjunctival malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol. 2001;85:658–661. doi: 10.1136/bjo.85.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am. 2003;16:43–65; vi. doi: 10.1016/s0896-1549(02)00110-4. [DOI] [PubMed] [Google Scholar]
- 61.Rahman I, Said DG, Maharajan VS, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye (Lond) 2009;23:1954–1961. doi: 10.1038/eye.2008.410. [DOI] [PubMed] [Google Scholar]
- 62.Maharajan VS, Shanmuganathan V, Currie A, Hopkinson A, Powell-Richards A, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction: indications and outcomes. Clin Experiment Ophthalmol. 2007;35:140–147. doi: 10.1111/j.1442-9071.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 63.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–426. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 66.Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108:1569–1574. doi: 10.1016/s0161-6420(01)00694-7. [DOI] [PubMed] [Google Scholar]
- 67.Fujishima H, Shimazaki J, Shinozaki N, Tsubota K. Trabeculectomy with the use of amniotic membrane for uncontrollable glaucoma. Ophthalmic Surg Lasers. 1998;29:428–431. [PubMed] [Google Scholar]
- 68.Nagai-Kusuhara A, Nakamura M, Fujioka M, Negi A. Long-term results of amniotic membrane transplantation-assisted bleb revision for leaking blebs. Graefes Arch Clin Exp Ophthalmol. 2008;246:567–571. doi: 10.1007/s00417-007-0727-x. [DOI] [PubMed] [Google Scholar]
- 69.Budenz DL, Barton K, Tseng SC. Amniotic membrane transplantation for repair of leaking glaucoma filtering blebs. Am J Ophthalmol. 2000;130:580–588. doi: 10.1016/s0002-9394(00)00600-0. [DOI] [PubMed] [Google Scholar]
- 70.Oh JH, Kim JC. Repair of scleromalacia using preserved scleral graft with amniotic membrane transplantation. Cornea. 2003;22:288–293. doi: 10.1097/00003226-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 71.Ma DH, Wang SF, Su WY, Tsai RJ. Amniotic membrane graft for the management of scleral melting and corneal perforation in recalcitrant infectious scleral and corneoscleral ulcers. Cornea. 2002;21:275–283. doi: 10.1097/00003226-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Tay E, Utine CA, Akpek EK. Crescenteric amniotic membrane grafting in keratoprosthesis-associated corneal melt. Arch Ophthalmol. 2010;128:779–782. doi: 10.1001/archophthalmol.2010.95. [DOI] [PubMed] [Google Scholar]
- 73.Sridhar MS, Sangwan VS, Bansal AK, Rao GN. Amniotic membrane transplantation in the management of shield ulcers of vernal keratoconjunctivitis. Ophthalmology. 2001;108:1218–1222. doi: 10.1016/s0161-6420(01)00622-4. [DOI] [PubMed] [Google Scholar]
- 74.Murube J, Olivares C, Murube E: Treatment of dry eye by punctum patch. Orbit. 1995;14:1–7. [Google Scholar]
- 75.Ti SE, Tow SL, Chee SP. Amniotic membrane transplantation in entropion surgery. Ophthalmology. 2001;108:1209–1217. doi: 10.1016/s0161-6420(01)00599-1. [DOI] [PubMed] [Google Scholar]
- 76.Marangon FB, Alfonso EC, Miller D, Remonda NM, Muallem MS, Tseng SC. Incidence of microbial infection after amniotic membrane transplantation. Cornea. 2004;23:264–269. doi: 10.1097/00003226-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Khokhar S, Sharma N, Kumar H, Soni A. Infection after use of nonpreserved human amniotic membrane for the reconstruction of the ocular surface. Cornea. 2001;20:773–774. doi: 10.1097/00003226-200110000-00023. [DOI] [PubMed] [Google Scholar]
- 78.Messmer EM. Hypopyon after amniotic membrane transplantation. Ophthalmology. 2001;108:1714–1715. doi: 10.1016/s0161-6420(01)00734-5. [DOI] [PubMed] [Google Scholar]


