Abstract
Non-alcoholic fatty liver disease (NAFLD) is currently the third most common indication for liver transplantation in the United States. With the growing incidence of obesity, NAFLD is expected to become the most common indication for liver transplantation over the next few decades. As the number of patients who have undergone transplantation for NAFLD increases, unique challenges have emerged in the management and long-term outcomes in patients. Risk factors such as obesity, hypertension, diabetes, and hyperlipidemia continue to play an important role in the pathogenesis of the disease and its recurrence. Patients who undergo liver transplantation for NAFLD have similar long-term survival as patients who undergo liver transplantation for other indications. Research shows that post-transplantation recurrence of NAFLD is commonplace with some patients progressing to recurrent non-alcoholic steatohepatitis and cirrhosis. While treatment of comorbidities is important, there is no consensus on the management of modifiable risk factors or the role of pharmacotherapy and immunosuppression in patients who develop recurrent or de novo NAFLD post-transplant. This review provides an outline of NAFLD as indication for liver transplantation with a focus on the epidemiology, pathophysiology and risk factors associated with this disease. It also provides a brief review on the pre-transplant considerations and post-transplant factors including patient characteristics, role of obesity and metabolic syndrome, recurrence and de novo NAFLD, outcomes post-liver transplantation, choice of medications, and options for immunosuppression.
Keywords: Liver transplantation, Non-alcoholic fatty liver disease, Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Nonalcoholic steatohepatitis, Cirrhosis, Obesity
Core tip: Non-alcoholic fatty liver disease (NAFLD) is a major cause of chronic liver disease and one of the leading indication for liver transplantation (LT) nowadays. Although, it remains the third most common indication for LT in the United States, it is projected to become the most common indication by 2025. It presents a unique challenge for the transplant community in terms of management and long-term outcomes. Many risk factors for NAFLD pre-transplant such as obesity, hypertension, hyperlipidemia, diabetes continue to play an important role in the pathogenesis of post-transplant NAFLD. In addition to therapy focused on prevention and management of coexisting medical conditions, physicians must weight the benefits and harms of both medical and surgical options in patients undergoing LT.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is increasingly recognized as a major etiology leading to chronic liver disease since its first description by Ludwig et al[1] in 1980. NAFLD has become an umbrella term to describe the pathologic picture of alcohol induced liver injury that occurs in the absence of alcohol abuse[2]. Histologically, NAFLD ranges from simple or bland steatosis to nonalcoholic steatohepatitis (NASH) and can progress to end-stage liver disease including fibrosis and cirrhosis. The pathologic definition of NASH is based on findings of macro vesicular steatosis, nuclear glycogenation, lobular and portal inflammation, and Mallory hyaline[1]. Progression of NASH to advanced fibrosis and cirrhosis is thought to be secondary to chronic inflammation and fibrosis[3]. Obesity has been strongly associated with NAFLD and NASH with some authors suggesting that NAFLD is the hepatic manifestation of metabolic syndrome[4]. With the global epidemic of obesity on the rise, there has been a consistent increase in NAFLD and NASH cases leading to increasing frequency of liver transplantation (LT) for this indication. According to the Scientific Registry of Transplant Recipients database (SRTR), NASH now represents the third most common indication for LT in the United States, surpassed only by hepatitis C and alcohol induced liver disease[5,6]. Furthermore, LT secondary to NASH is the only indication that has increased in frequency from 1.2% to 9.7% in less than a decade (from 2001-2009)[6]. Based on this data, end-stage liver failure secondary to NAFLD is estimated to become the most common indication for LT within the next two decades[5,6].
In this manuscript, we provide an overview of NAFLD in the context of LT. First, we review the epidemiology, pathophysiology and risk factors for NAFLD and how obesity and metabolic syndrome play a role in the development of the disease. We then explore the pre-transplant factors affecting this patient population such as patient characteristics and availability of livers available for transplantation. Finally, we discuss the post-transplant considerations such as recurrence and de-novo NAFLD, outcomes, pharmacotherapy and immunosuppression. The goal of this review is to educate and assist in the management of unique challenges for patients with NAFLD both pre- and post LT.
DEFINITION OF NAFLD AND NASH
An early diagnosis of NAFLD is often difficult as many patients remain asymptomatic until the disease has progressed to fibrosis and cirrhosis. Biochemically, there are no reliable serum biomarkers for NAFLD at the present time. Patients may have elevated serum transaminase levels; however, normal transaminases do not exclude the diagnosis. Per the United States Third National Health and Nutrition Examination Survey (NHANES III), the prevalence of NAFLD with and without elevated transaminases was found to be 3.1% and 16.4% respectively[7]. When elevated, aspartate aminotransferase and alanine aminotransferase are seldom greater than four times the upper limit of normal[8]. Therefore, the diagnosis of NAFLD remains a diagnosis of exclusion requiring elimination of other causes of abnormal liver function tests in presence of imaging or biopsy suggestive of steatosis. Liver biopsy remains the gold standard for its diagnosis. On biopsy, NAFLD must have histologic findings of macro vesicular steatosis in greater than 5% of hepatocytes[9]. For the diagnosis of NASH, most experts require additional findings suggestive of active inflammatory process including hepatocyte swelling, ballooning and degeneration with lobular inflammation[10]. The Nonalcoholic Steatohepatitis Clinical Research Network has designed and validated a histologic scoring system for NAFLD, called the NAFLD Activity Score that allows for evaluation of steatosis, inflammation and ballooning scores[11]. This scoring system assigns a score for steatosis (0-3), lobular inflammation (0-3) and hepatocyte ballooning (0-2) and sum of the scores if greater than or equal to five is defined as “definite NASH” and a score of less than or equal to three as “not NASH” (Table 1). In general, the diagnosis of both NAFLD and NASH requires the presence of hepatic steatosis, no significant alcohol consumption and no other etiology to explain liver disease[12,13]. Figure 1 illustrates the microscopic findings in biopsies of patients suspected of having NAFLD and depicts hepatocyte ballooning (Figure 1A), steatosis (Figure 1B) and lobular inflammation (Figure 1C).
Table 1.
Non-alcoholic fatty liver disease Activity Score
| Component | Score |
| Steatosis grade | |
| < 5% | 0 |
| 5%-33% | 1 |
| 33%-66% | 2 |
| > 66% | 3 |
| Lobular inflammation | |
| No foci | 0 |
| < 2 foci per 200 × field | 1 |
| 2-4 foci per 200 × field | 2 |
| > 4 foci per 200 × field | 3 |
| Ballooning | |
| None | 0 |
| Few balloon cells | 1 |
| Prominent/many cells | 2 |
Scoring system assigns a score for steatosis (0-3), lobular inflammation (0-3) and hepatocyte ballooning (0-2) and sum of the scores is correlated with a score of greater than or equal to five as “definite NASH” and a score of less than or equal to three as “not NASH”[66]. Adapted from Tanaka et al[66].
Figure 1.
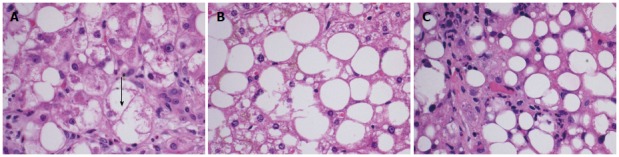
Microscopic findings in biopsies of patients’ suspected of having non-alcoholic fatty liver disease/nonalcoholic steatohepatitis. A: H and E stained liver tissue at × 40 showing ballooning degeneration of a hepatocyte (marked with black arrow); B: H and E stained liver tissue at × 40 showing steatosis without steatohepatitis. C: H and E stained liver tissue at × 40 showing inflammation (neutrophilic inflammation surrounding fatty hepatocytes).
EPIDEMIOLOGY AND RISK FACTORS IN NAFLD PATIENTS
Although the prevalence of NAFLD is unknown, its incidence is estimated to be on the rise with the concurrent obesity epidemic. According to the National Center for Health Statistics, the prevalence of obesity in the United States in 2009-2010 is estimated to be 35.5% of the male population and 35.8% of the female population[14]. A recent cross-sectional study in the setting of outpatient general internal medicine clinic in Texas shows the prevalence of NAFLD to be 46%, with findings of NASH in 12.2% of patients[15]. The projection from this study reports the anticipated prevalence of NASH in the US to be anywhere between three and eight million[15]. Despite these estimates, the frequency of progression from NAFLD to end-stage liver disease is unknown. In case series reports, transition from NASH to fibrosis are reported as high as a third of patients[16-18]. The rate of progression to decompensated cirrhosis and need for LT remains uncertain, however; this is the only indication for LT that has been steadily increasing[6]. Additionally, it is suggested that a high percentage of cases initially classified as cryptogenic cirrhosis may represent progression from NAFLD to cirrhosis[19]. As fibrosis distorts a fatty liver into a cirrhotic one, various histologic components such as steatosis and inflammatory changes become less evident and may even disappear[5]. Therefore, end-stage liver disease secondary to NAFLD is projected to become the most common indication for LT by 2025[6] given its increasing incidence and the steady decrease in frequency of hepatitis C infection and alcohol induced liver disease.
PATHOPHYSIOLOGY OF NAFLD AND NASH
NAFLD accounts for two types of fatty infiltration of the liver: simple steatosis and non-alcoholic steatohepatitis (NASH). Simple fatty liver infiltration, also called bland hepatic steatosis is a benign condition in which liver function tests are within normal limits or maybe slightly elevated. In this condition, liver biopsy shows liver tissue that is essentially normal except for fatty infiltration in hepatocytes. On the other hand, NASH is defined by the presence of inflammatory changes. The development of inflammation and subsequently NASH from hepatic steatosis is thought to be a complex mechanism involving insulin resistance, oxidative stress, and inflammatory cascade. Several models have been described in the literature to suggest the interplay between these processes and how simple steatosis is transformed into steatohepatitis, including the “two-hit hypothesis”. First described by Day et al[20], insulin resistance is the “first hit” that leads to steatosis in hepatocytes. During states of insulin resistance, both muscle and adipose tissues preferentially oxidize lipids, resulting in release of free-fatty acids. The liver incorporates these free fatty acids into triglycerides, and remaining free-fatty acids undergo oxidation in the mitochondria, peroxisomes or microsomes[21]. Then a “second hit” that occurs in the form of oxidative stress leads to inflammation and fibrosis[22]. Figure 2 summarizes the multiple factors that play a role in the development of NASH from steatosis. Others have also described a change in lipid metabolism through elevated peripheral fatty acids and de novo synthesis leading to an increase in fatty deposition in the liver. In patients with NAFLD, Donnelly et al[23] noted that the majority (60%) of the triacylglycerol in the liver arises from free fatty acids while 26% and 15% are attributable to de novo lipogenesis and diet, respectively[23,24]. Insulin resistance at the level of adipose tissue leads to an increased release of free fatty acids leading to an increased activation of macrophages and other immune cells. The entry of these free fatty acids in the liver also leads to the activation of intracellular inflammatory pathways causing hepatic inflammation and consequently fibrosis[25,26]. Furthermore, insulin resistance leads to hyperglycemia which in turn triggers stellate cell activation leading to fibrosis[27]. Genes also play an integral role in the development of NASH as evidenced by ethnic-specific allele frequencies and certain genotypes that purport a greater lipid content, more aggressive disease, and increase in serum aminotransferase levels[28].
Figure 2.
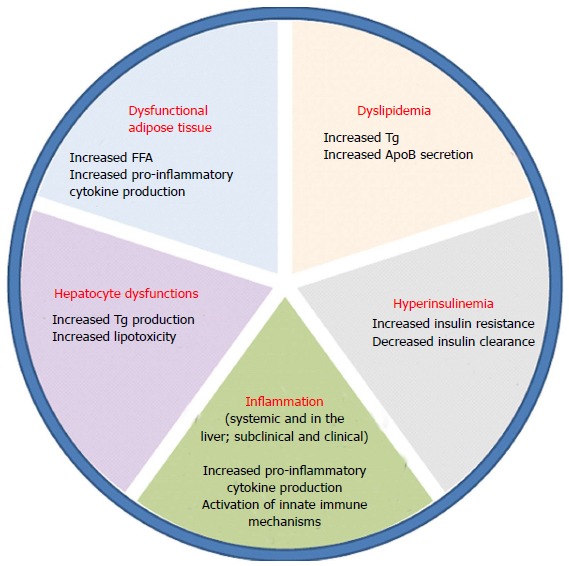
Multiple factors that play a role in the progression of steatosis to nonalcoholic steatohepatitis.
Several studies have shown an increased prevalence of risk factors in the form of hypertension, diabetes, obesity and hyperlipidemia - all components of metabolic syndrome in patients’ who have undergone LT[29]. In these patients, studies have also shown an increase in pro-steatotic cytokines such as leptin[30] and decrease in anti-steatotic cytokines such as adiponectin[31]. Additionally, the advanced age of the donors may exacerbate the effects of insulin resistance post-transplant due to accelerated fibrosis[32].
METABOLIC SYNDROME, OBESITY AND NAFLD
A large proportion of patients diagnosed with NAFLD have been identified to have the phenotype associated with metabolic syndrome. Although many organizations have defined the term “metabolic syndrome” differently, all definitions include risk factors for cardiovascular disease and type 2 diabetes such as hypertension, dyslipidemia (elevated triglycerides and lower high-density lipoprotein cholesterol), raised fasting glucose and central obesity[33]. Liver biopsies from patients who meet the strict definition of metabolic syndrome shows more advanced histologic changes and a high risk of severe fibrosis[34]. Additionally, obesity itself has been independently shown to be a predictor of advanced fibrosis in the liver. A study conducted by Dixon et al[35] showed that in 105 consecutive patients who underwent laparoscopic obesity surgery and had liver biopsies taken, there were findings of NASH in 25% with nearly half demonstrating findings of advanced fibrosis. Colicchio et al[36] also found severe steatosis to be uniformly present in non-diabetic patients with body mass index (BMI) greater than 39.9 kg/m2 (grade III obesity) when evaluated using liver ultrasound. It is however, the central or visceral obesity that is associated with the development of NAFLD independent of overall obesity[37,38]. Dyslipidemia and diabetes have also been shown to have an independent association with NAFLD. One study by Assy et al[38] showed that in patients with hypertriglyceridemia, there is a significantly higher risk of fatty infiltration than in patients’ with other forms of dyslipidemia, further supporting the association between metabolic syndrome and NAFLD.
PRE-TRANSPLANT CONSIDERATIONS
Patient characteristics
Obesity and insulin resistance have been implicated as the key pathogenic factors associated with NAFLD[39]. The risk factors associated with the histological severity of NASH in the non-transplant population include male sex, higher BMI, insulin resistance, hypertension, and presence of type II diabetes[18,40,41]. Analysis of the SRTR database by Charlton et al[6] showed that the people who underwent LT for NASH cirrhosis were older, had larger BMI, were more likely to be female, had a greater prevalence of diabetes and hypertension, and a lower incidence of hepatocellular carcinoma compared with other patients in the transplant cohort. Hence, prior to undergoing LT, optimization of modifiable factors in patients is essential for improved outcomes. In addition to medical optimization such as improved blood pressure and glycemic control, patients should strongly be encouraged to undergo supervised weight loss. A study by Nair et al[42] measured graft and patient survival in obese patients receiving LT in the United States. This study concluded that patients with morbid obesity (BMI > 40 kg/m2) had significantly higher rates of primary graft non-function and significantly increased immediate, one and two year mortality. Five year mortality rates were also significantly higher in severely obese (BMI between 35.1 and 40 kg/m2) and morbidly obese patients, secondary to increased cardiovascular mortality. Based on these findings, the American Association for the Study of Liver Disease (AASLD) considers morbid obesity a contraindication to LT[43], and recommends weight loss in all patients awaiting LT, especially if the patient’s BMI is greater than 35 kg/m2. Additionally, weight loss has been shown to help with improvement in the severity of steatosis and NASH prior to transplant. Meta-analysis by Mummadi et al[44] in the non-LT population who underwent bariatric surgery shows that a 19%-41% reduction in BMI was associated with improvement of steatosis in 91.6%, steatohepatitis in 81.3%, fibrosis in 65.5% and complete resolution of NASH in 69.5% of patient’s post-bariatric surgery.
Concurrent bariatric surgery and LT has also been evaluated in obese patients. A recent study analyzed thirty-seven patients referred for LT with BMI > 35 who had achieved weight loss prior to transplant and underwent LT alone and compared them with seven patients who underwent LT with sleeve gastrectomy[45]. This study reported that in patients with LT alone, there was a higher frequency of weight gain, steatosis, post-transplant diabetes, graft loss and death when compared with the sleeve gastrectomy group. This small study suggests that although bariatric surgery may play a promising role in patients undergoing transplant, more studies are needed to evaluate long-term survival in these patients and it may be appropriate for some patients who have persistent obesity and fail non-invasive management.
Availability of livers for transplant in the NAFLD population
The increasing prevalence of obesity has led to further increases in hepatic steatosis in potential donors, which has reduced the number of transplantable livers available for any indication. The use of steatotic livers for transplant depends on the level of fatty infiltration. Donor livers with greater than 60% steatosis are deemed non-transplantable whereas those with less than 30% are deemed useable with good function. Even though livers with 30%-60% steatosis are potentially used for patients, they have been associated with poor results due to decreased function, graft survival and decreased patient survival[46]. The biggest concern remains primary non-function of the graft which has been reported as high as 13% in donor livers with greater than 30% steatosis compared with < 3% in those with no steatosis on biopsy prior to transplant[47,48]. More recent studies show the rate of primary non-function of the graft to be less than 5% in those undergoing LT with steatosis of less than 30%[49-51]. Increased hepatic graft steatosis has also been associated with intrahepatic cholestasis and transient hyperbilirubinemia during regeneration after living donor transplant but the mechanism remains elusive[52].
The use of living donors for LT also has its challenges. Although the maximum percentage of steatosis in living donors is unknown for LT, most centers are reluctant to transplant grafts with greater than 30% steatosis given the increased risk of primary non-function of the graft[53]. With the growing incidence of obesity, finding grafts with less than 10% steatosis (preferred by most centers) is difficult[54]. Studies report that one third to one half of potential living donors have steatosis on liver biopsies and in these studies more than one-third of biopsies showed steatosis greater than 10%[55,56]. The need for liver biopsy in living transplant donors is also not without risk, given that the sensitivity of imaging modalities is low for small amounts of steatosis and improves with increasing steatosis[55].
POST-TRANSPLANT CONSIDERATIONS
Recurrence of NAFLD and NASH
The development of steatosis post-LT in patients is common with some observational studies reporting prevalence as high as 100%[57]. One study of post-liver transplant patients by Maor-Kendler et al[58], showed the incidence of grade 2 steatosis or higher in 38% of recipients with pre-transplant diagnosis of NASH/cryptogenic cirrhosis when compared to 6% in cholestasic disease, 16% in alcoholic disease and 9% in patients with HCV cirrhosis. Table 2 summarizes several studies that evaluated the incidence of NAFLD, NASH and cirrhosis post LT[57,59-66]. A recent study by Dureja et al[59] analyzed post-transplant data in eighty-eight patients who underwent transplant for NAFLD and report prevalence of recurrent NAFLD to be 39%, recurrent NASH to be 28.4% and fibrosis (stage 3 and 4) to be 3.4% respectively. Moreover, according to Contos et al[57] when comparing the cases of cryptogenic cirrhosis with those transplanted for alcoholic liver disease, primary biliary cirrhosis and primary sclerosing cholangitis, the rates of steatosis and subsequent NASH were significantly higher in the cryptogenic cirrhosis group. Similarly, Bhagat et al[61] reported the recurrence of NASH in 33% of the patients who were transplanted for cryptogenic cirrhosis with NASH phenotype compared with those transplanted for alcohol related cirrhosis at six months post-LT. Tanaka et al[66] recently reported recurrence of NASH in one patient who underwent living donor LT for NAFLD; however, this study is limited by small sample size and had only seven patients who were transplanted for this indication. Based on the studies (summarized in Table 2), the recurrence of steatosis, NASH and cirrhosis in patients transplanted for NAFLD is clearly possible and further studies are needed to determine the risk of recurrence in patients’ post-LT.
Table 2.
Various studies examining the incidence/recurrence of non-alcoholic fatty liver disease (de novo or recurrent), non-alcoholic steatohepatitis and Cirrhosis in the post-liver transplant population n (%)
| Ref. | Year of publication | Indication of transplant | Number of patients | Findings of NAFLD post-transplant | Findings of NASH post-transplant | Findings of cirrhosis post-transplant | Mean follow-up duration |
| Tanaka et al[66] | 2013 | Living donor transplant for NAFLD | 7 | 0 (0) | 1 (14) | None | 5.3 yr |
| Dureja et al[59] | 2011 | NAFLD | 88 | 34 (39) | 25 (28.4) | 3 (3.4) (reported as fibrosis grade 3/4) | 82 mo |
| Dumortier et al[60] | 2010 | Several indication | 599 | 131 (31.1) | 5 (3.8) | 3 (2.25) | 40 mo |
| Bhagat et al[61] | 2009 | Cryptogenic/NASH Cirrhosis vs alcoholic cirrhosis | 71 | N/A | 31 (33) | None | 1517 d |
| Lim et al[62] | 2007 | Non-NAFLD indication (18 HBV, 7 HCV, 5 others) | 30 | 12 (40) | 4 (13) | None | 44 mo |
| Seo et al[63] | 2007 | 68 various causes, 84% HCV | 68 | 121 (18) | 61 (9) | None | 28 mo |
| Ong et al[64] | 2001 | Cryptogenic cirrhosis | 51 | 13 (25.4) | 8 (15.7) | None | 26 mo |
| Contos et al[57] | 2001 | Cryptogenic/NASH cirrhosis | 30 | 30 (100) | 3 (10) | None | 3.5 yr |
| Charlton et al[65] | 2001 | NASH cirrhosis | 16 | 9 (60) | 5 (33) | 2 (12.5) | 28.1 mo |
De novo. HCV: Hepatitis C virus; HBV: Hepatitis B virus; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
De novo NAFLD/NASH
Little is known about the prevalence of de novo NAFLD and NASH in patients who undergo liver transplantation for non-NASH cirrhosis and have been transplanted a donor graft free of steatosis. Report by Seo et al[63] who evaluated sixty-eight liver transplant patients with various causes of liver cirrhosis using pre-transplant and post-transplant biopsies, noted the prevalence of de novo steatosis in twelve patients (18%) with prevalence of de novo NASH in six patients (9%). In another study that evaluated thirty patients with mostly infectious cirrhosis from HBV and HCV, incidence of steatosis and NASH were 40% and 13% respectively, although it is unclear how much of this was de novo[62]. In another case series in which patients underwent transplantation for HCV and alcohol cirrhosis, four patients developed de novo NAFLD post-transplant in the absence of graft steatosis[67]. Thus, the incidence, prevalence and the mechanism of de novo NAFLD or NASH remains unclear and there is an emerging need for studies in this area.
Influence of NAFLD/NASH on outcomes after liver transplantation
Data suggests that the outcome of LT in patients who undergo transplant for most common causes of cirrhosis in the United States, including cholestatic liver disease (primary biliary cirrhosis, primary sclerosing cholangitis), alcoholic liver disease, and HCV are excellent, with one year survival rates of 85%-90% and five year survival rates of 70%-80% respectively[6,68]. Review of literature for patients undergoing LT for NASH cirrhosis shows mortality after transplant to be similar at five years when compared with patients undergoing transplant for other indications, however the one and three year mortality in NASH cirrhosis patients were significantly higher[68]. Malik et al[68] reported a higher one year mortality in NASH patients with age ≥ 60 years and BMI ≥ 30 kg/m2 with diabetes and hypertension. A more recent review of transplant patients by Charlton et al[6] however reports survival at one year and three years after LT for NASH to be 84% and 78%, respectively and similar for other indications. They also report that patient and graft survival was similar to values for other indications when adjusted for age, sex, BMI and serum creatinine. There is, however, a higher incidence of cardiac events following LT in a subset of patients with higher BMI, elevated serum creatinine, diabetes, systolic blood pressure elevation, hypercholesterolemia, and these may represent to some extent the cause of poor outcomes in LT patients with NASH cirrhosis[69]. Malik et al[68] reported statistically significant differences in infection as the cause of death is NASH cirrhosis patients post-LT when compared with other indications and explain the likely cause to be elevated hyperglycemia and diabetes which may predispose these patients’ to increased risk of infection. With the growing number of NAFLD and NASH patients’ post-LT, it is expected that more studies would emerge in the upcoming years that would be high-powered to provide further details on these issues.
Management of NAFLD patients after liver transplant
Little data exists for the treatment of NAFLD patients’ post-LT. All recommendations for management of NAFLD post-transplant are a reflection of studies done on the non-LT population and can be divided into three broad categories: Lifestyle modifications, Pharmacotherapy and Bariatric Surgery.
Lifestyle modifications: The mainstay of medical management includes weight reduction through physical activity and diet modification and pharmacological management of medical co-morbidities such as hypertension, hypercholesterolemia and diabetes[4]. A low-carbohydrate (< 60 g of carbs/d) low caloric diet when compared with high carbohydrate (> 180 g of carbs/d) low caloric diet has been shown to lead to a more pronounced reduction in intrahepatic triglyceride content and improves insulin sensitivity[70]. Weight loss has also been shown to improve hepatic steatosis and inflammation with weight loss of 3%-5% showing improvement in steatosis and 7%-10% weight loss showing improvement in the level of steatohepatitis[13]. Physical activity has an important effect on the level of NAFLD and should be encouraged in patients. Moderate and vigorous activity was compared with controls that were generally inactive. This study showed that vigorous activity was beneficial in preventing progression to fibrosis in NAFLD patients over moderate activity[71] and thus should be encouraged. The role of caffeine in coffee has also been evaluated in patients with NAFLD. Molloy et al[72] showed that when comparing 4 different groups (controls, bland steatosis/not-NASH, NASH stage 0-1, and NASH stage 2-4), there was a significant reduction in the risk of fibrosis among patients with higher coffee consumption per day.
Pharmacotherapy: The use of insulin sensitizing medications including metformin and thiazolidinedione has been evaluated in patients with NAFLD and NASH. Although metformin use had been associated with normalization of aminotransferases and improvement in liver echographic findings in prior studies[73,74], pooled results from meta-analysis have found no significant improvement on steatosis, inflammation or fibrosis in metformin treated patients with NASH[75]. The study concluded that in patients without diabetes, targeted lifestyle interventions might be at least as beneficial as metformin and there is little evidence to suggest benefit of metformin in patients with NAFLD without pre-existing glucose intolerance regardless of the dose. Thiazolidinediones (TZDs), including rosiglitazone and pioglitazone, have been evaluated in multiple studies on its benefit in NASH patients. Rosiglitazone has however been shown to be associated with increased rate of myocardial infarction[76] and has been removed from European markets and highly restricted in the United States. Given the risk factors for NASH also mirror risk factors for coronary artery disease, rosiglitazone is likely not an optimal treatment option in patients. Pioglitazone was evaluated in a large multicenter study[77] for 96 wk at doses of 30 mg/d and compared with Vitamin E 800 IU/d or placebo in patients without diabetes with NASH. This study concluded that both treatment groups (Vitamin E and Pioglitazone) demonstrated improvement in hepatic steatosis, ballooning and inflammation, although only Vitamin E was associated with statistically significant improvements. Neither treatment had an effect on fibrosis but both Vitamin E and pioglitazone led to improvement in aminotransferase levels. Although Vitamin E may have a role in the treatment of NAFLD patients without diabetes, it is important to note that Vitamin E use has been associated with increased all-cause mortality and prostate cancer, especially at doses of 400 IU/d or higher[78,79]. Other small randomized control trials have also shown similar benefit of pioglitazone at 30-45 mg/d in NASH patients with or without diabetes demonstrating improvements in aminotransferase levels, hepatic steatosis, improved insulin sensitivity and inflammation[80,81] however no improvement in fibrosis were noted. Additionally, unlike rosiglitazone that has been associated with increased cardiovascular mortality[76], pioglitazone has only been associated with having a slightly positive or neutral effect on the cardiovascular system[82]. Based on this data, pioglitazone at doses of 30 mg/d and titrated up for glycemic control if necessary, may be recommended for patients with NAFLD, however should be used with caution in patients with history of heart failure and bladder cancer[82].
The use of statins has been investigated in small pilot studies for the treatment of NAFLD, although there have been mixed results. Rosuvastatin at dose of 10 mg/d given to NAFLD patients without diabetes, showed normalization of aminotransferase and cholesterol levels after follow-up for eight months[83] whereas another trial in NASH patients receiving simvastatin 40 mg/d demonstrated no significant differences in hepatocellular structure and aminotransferase levels when compared with placebo over a duration of one year[84]. Based on conflicting reports, AASLD has recommended against the use of statins in the treatment of NASH until more randomized clinical control trials can demonstrate its efficacy[13].
Ursodiol or ursodeoxycholic acid, approved for the treatment of primary biliary cirrhosis, has also been evaluated for NASH patients and trials thus far have not demonstrated significant differences in overall histology[85,86].
Pentoxifylline, a drug that inhibits the synthesis of TNF-α which is thought to be associated with possible progression to fibrosis[87] in NAFLD patients has also been studied for the treatment of NASH. A recent randomized control trial evaluated pentoxifylline 1200 mg/d compared to placebo in biopsy-confirmed NASH patients over a course of one year and found improvements in aminotransferase levels and histologic features from baseline but these were not significant when compared to placebo[88].
Use of pharmacological intervention to augment weight loss in NASH and NAFLD patients with orlistat has also shown improvement in steatosis and aminotransferase levels[89], however it is most likely the observed changes were associated with weight loss rather than the drug itself.
Role of bariatric surgery: As in the non-transplant population, weight loss has its own challenges in the post-LT population. In addition to obesity pre-transplant, many recipients experience rapid weight gain post-transplant that leads to recurrence and de novo steatosis in the graft liver[60]. Weight gain can partially be attributed to immunosuppressive medication such as steroids and calcineurin inhibitors taken to suppress the immune system post-LT. Few studies exist on the benefit of bariatric surgery post-OLT, mostly in the form of case reports and case series[90-93]. Duchini et al[92] reported Roux-en-Y bypass as a successful procedure in two NAFLD patients post-LT with morbid obesity demonstrating significant weight reduction, normalization of liver function and metabolic parameters, including lipid profile and hyperglycemia. A recent study from the University of Minnesota identified seven patients who underwent Roux-en-Y gastric bypass post-LT between 2001 and 2009[93], and reported therapeutic weight loss, improved glycemic control, and improved high-density lipoprotein in the presence of continued dyslipidemia. More studies however, are needed for consideration of bariatric surgery in post-LT patients before definite recommendations could be made.
Choice of Immunosuppression in NAFLD patients
Many immunosuppressive regimens used in the treatment of post-LT patients are associated with diabetes, hypertension, hyperlipidemia, obesity and increased risk of infection[94]. Patients who undergo LT for NASH often have metabolic syndrome and are at increased risk for the development of major vascular events[68]. Some studies have shown an increased risk of recurrence of hepatocellular carcinoma[95] in addition to other known adverse effects from steroids including diabetes, osteoporosis and obesity. Given that steroids have been linked to much adverse effects, they should be withdrawn from maintenance therapy within three months post-LT. Moving away from a steroid based immunosuppressive regimen in LT patients was evaluated by Segev et al[94] in their meta-analysis of thirty publications, including nineteen randomized control trials which showed there was no difference in death, graft loss and infection rates in patients who were on steroid-free regimens when compared with steroid-based immunosuppression. Additionally, the analysis showed a trend towards reduced hypertension and statistically significant decrease in CMV infection and cholesterol levels in steroid-free regimens. The authors also reported that if the steroids were replaced by another immunosuppression medication, there is a reduced risk of diabetes, rejection and severe rejection. This would advocate for the role of avoidance of steroids post-LT for immunosuppression, especially in patients with NASH cirrhosis.
Calcineurin inhibitors include tacrolimus (FK506) and cyclosporine and act by inhibiting T-cell activation. Although these drugs are commonly used, studies have shown acute and chronic nephrotoxicity as a major adverse effect of both tacrolimus and cyclosporine, occurring in up to 20% of patients depending on the organ transplanted[96]. Due to these outcomes, studies have advocated for conversion to sirolimus therapy in patients who develop renal insufficiency due to calcineurin inhibitors[97], however their complete avoidance has been associated with higher rejection rates[98]. Additionally, tacrolimus has been associated with neurotoxicity and development of de-novo diabetes, while cyclosporine has been associated with hypertension and hyperlipidemia[99,100].
Mycophenolic acid and Azathioprine are two other medications commonly used post-LT however require close monitoring due to the risk of bone marrow suppression[101] and their experience in NASH-related LT is limited. The decision on the type of immunosuppression regimen to be used should be based on maintaining a balance between drug toxicity and efficacy and dictated by patient factors such as age, ethnicity and etiology of their liver disease.
CONCLUSION
NAFLD is increasingly recognized as a major etiology leading to chronic liver disease and remains the only indication for LT that has steadily and steeply increased in frequency over the past decades. As the third most common indication for LT in the United States after HCV and alcoholic liver disease, NAFLD is projected to become the most common indication by 2025. The increasing prevalence of NAFLD both pre- and post-transplant presents unique challenges for the transplant community in terms of management and long-term outcomes. Many risk factors for NAFLD pre-transplant such as obesity, hypertension, hyperlipidemia, diabetes continue to play an important role in the pathogenesis of post-transplant NAFLD. In addition to prevention and management of coexisting medical conditions, physicians must weigh the benefits and harms of both medical and surgical therapies in patients undergoing LT. New research in pharmacotherapy such as insulin sensitizing drugs, statins, metformin and others continues to emerge, yet more research is needed to help identify methods to reduce and possibly reverse progression to fibrosis in these patients. The recommendation on avoidance of steroids and minimization of calcineurin inhibitors in this patient population would likely be beneficial in decreasing the risk factors associated with post-transplant NAFLD and should be considered. Further research is still needed to better understand the issues that affect this unique patient population.
ACKNOWLEDGMENTS
Pathology images and interpretation were provided by Dr. Lisa R. Dixon from the Department of Pathology at the University of Florida-College of Medicine.
Footnotes
P- Reviewers: Tapia G, Xian WP S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
References
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 2.Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis. 1986;8:283–298. [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 4.Saadeh S. Nonalcoholic Fatty liver disease and obesity. Nutr Clin Pract. 2007;22:1–10. doi: 10.1177/011542650702200101. [DOI] [PubMed] [Google Scholar]
- 5.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–1058. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 6.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 9.McClain CJ, Barve S, Deaciuc I. Good fat/bad fat. Hepatology. 2007;45:1343–1346. doi: 10.1002/hep.21788. [DOI] [PubMed] [Google Scholar]
- 10.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 11.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 12.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 14.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 15.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Evans CD, Oien KA, MacSween RN, Mills PR. Non-alcoholic steatohepatitis: a common cause of progressive chronic liver injury? J Clin Pathol. 2002;55:689–692. doi: 10.1136/jcp.55.9.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fassio E, Alvarez E, Domínguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 18.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 19.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 20.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 21.Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007;11:75–104; ix. doi: 10.1016/j.cld.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Pessayre D, Fromenty B, Mansouri A. Mitochondrial injury in steatohepatitis. Eur J Gastroenterol Hepatol. 2004;16:1095–1105. doi: 10.1097/00042737-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1139–1142. doi: 10.1172/JCI24930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725.e6. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 28.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 29.Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, Peltekian KM. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109–1114. doi: 10.1002/lt.21126. [DOI] [PubMed] [Google Scholar]
- 30.Modan-Moses D, Paret G. Leptin and transplantation: pieces are still missing in the puzzle. Isr Med Assoc J. 2002;4:207–208. [PubMed] [Google Scholar]
- 31.Man K, Zhao Y, Xu A, Lo CM, Lam KS, Ng KT, Ho JW, Sun CK, Lee TK, Li XL, et al. Fat-derived hormone adiponectin combined with FTY720 significantly improves small-for-size fatty liver graft survival. Am J Transplant. 2006;6:467–476. doi: 10.1111/j.1600-6143.2005.01201.x. [DOI] [PubMed] [Google Scholar]
- 32.Foxton MR, Quaglia A, Muiesan P, Heneghan MA, Portmann B, Norris S, Heaton ND, O’Grady JG. The impact of diabetes mellitus on fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2006;6:1922–1929. doi: 10.1111/j.1600-6143.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 33.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 34.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 35.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 36.Colicchio P, Tarantino G, del Genio F, Sorrentino P, Saldalamacchia G, Finelli C, Conca P, Contaldo F, Pasanisi F. Non-alcoholic fatty liver disease in young adult severely obese non-diabetic patients in South Italy. Ann Nutr Metab. 2005;49:289–295. doi: 10.1159/000087295. [DOI] [PubMed] [Google Scholar]
- 37.Angulo P. NAFLD, obesity, and bariatric surgery. Gastroenterology. 2006;130:1848–1852. doi: 10.1053/j.gastro.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 38.Assy N, Kaita K, Mymin D, Levy C, Rosser B, Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci. 2000;45:1929–1934. doi: 10.1023/a:1005661516165. [DOI] [PubMed] [Google Scholar]
- 39.Milić S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig Dis. 2012;30:158–162. doi: 10.1159/000336669. [DOI] [PubMed] [Google Scholar]
- 40.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 43.Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 44.Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–1402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, Hay JE, Wiesner RH, Sanchez W, Rosen CB, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13:363–368. doi: 10.1111/j.1600-6143.2012.04318.x. [DOI] [PubMed] [Google Scholar]
- 46.Perkins JD. Saying “Yes” to obese living liver donors: short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Liver Transpl. 2006;12:1012–1013. doi: 10.1002/lt.20807. [DOI] [PubMed] [Google Scholar]
- 47.D’Alessandro AM, Kalayoglu M, Sollinger HW, Hoffmann RM, Reed A, Knechtle SJ, Pirsch JD, Hafez GR, Lorentzen D, Belzer FO. The predictive value of donor liver biopsies on the development of primary nonfunction after orthotopic liver transplantation. Transplant Proc. 1991;23:1536–1537. [PubMed] [Google Scholar]
- 48.Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 49.Tevar AD, Clarke C, Wang J, Rudich SM, Woodle ES, Lentsch AB, Edwards ML. Clinical review of nonalcoholic steatohepatitis in liver surgery and transplantation. J Am Coll Surg. 2010;210:515–526. doi: 10.1016/j.jamcollsurg.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA. Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246:940–946; discussion 946-948. doi: 10.1097/SLA.0b013e31815c2a3f. [DOI] [PubMed] [Google Scholar]
- 51.Angele MK, Rentsch M, Hartl WH, Wittmann B, Graeb C, Jauch KW, Loehe F. Effect of graft steatosis on liver function and organ survival after liver transplantation. Am J Surg. 2008;195:214–220. doi: 10.1016/j.amjsurg.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 52.Cho JY, Suh KS, Lee HW, Cho EH, Yang SH, Cho YB, Yi NJ, Kim MA, Jang JJ, Lee KU. Hepatic steatosis is associated with intrahepatic cholestasis and transient hyperbilirubinemia during regeneration after living donor liver transplantation. Transpl Int. 2006;19:807–813. doi: 10.1111/j.1432-2277.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 53.Brandhagen D, Fidler J, Rosen C. Evaluation of the donor liver for living donor liver transplantation. Liver Transpl. 2003;9:S16–S28. doi: 10.1053/jlts.2003.50222. [DOI] [PubMed] [Google Scholar]
- 54.Trotter JF. Thin chance for fat people (to become living donors) Liver Transpl. 2001;7:415–417. doi: 10.1053/jlts.2001.24933. [DOI] [PubMed] [Google Scholar]
- 55.Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–1122. doi: 10.1053/jlts.2002.36740. [DOI] [PubMed] [Google Scholar]
- 56.AASLD abstracts (pp. 586a–686a) Hepatology. 2003;38:586A–686A. Available from: http://www.readcube.com/articles/10.1002/hep.1840380508?locale=en. [Google Scholar]
- 57.Contos MJ, Cales W, Sterling RK, Luketic VA, Shiffman ML, Mills AS, Fisher RA, Ham J, Sanyal AJ. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363–373. doi: 10.1053/jlts.2001.23011. [DOI] [PubMed] [Google Scholar]
- 58.Maor-Kendler Y, Batts KP, Burgart LJ, Wiesner RH, Krom RA, Rosen CB, Charlton MR. Comparative allograft histology after liver transplantation for cryptogenic cirrhosis, alcohol, hepatitis C, and cholestatic liver diseases. Transplantation. 2000;70:292–297. doi: 10.1097/00007890-200007270-00009. [DOI] [PubMed] [Google Scholar]
- 59.Dureja P, Mellinger J, Agni R, Chang F, Avey G, Lucey M, Said A. NAFLD recurrence in liver transplant recipients. Transplantation. 2011;91:684–689. doi: 10.1097/TP.0b013e31820b6b84. [DOI] [PubMed] [Google Scholar]
- 60.Dumortier J, Giostra E, Belbouab S, Morard I, Guillaud O, Spahr L, Boillot O, Rubbia-Brandt L, Scoazec JY, Hadengue A. Non-alcoholic fatty liver disease in liver transplant recipients: another story of “seed and soil”. Am J Gastroenterol. 2010;105:613–620. doi: 10.1038/ajg.2009.717. [DOI] [PubMed] [Google Scholar]
- 61.Bhagat V, Mindikoglu AL, Nudo CG, Schiff ER, Tzakis A, Regev A. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15:1814–1820. doi: 10.1002/lt.21927. [DOI] [PubMed] [Google Scholar]
- 62.Lim LG, Cheng CL, Wee A, Lim SG, Lee YM, Sutedja DS, Da Costa M, Prabhakaran K, Wai CT. Prevalence and clinical associations of posttransplant fatty liver disease. Liver Int. 2007;27:76–80. doi: 10.1111/j.1478-3231.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 63.Seo S, Maganti K, Khehra M, Ramsamooj R, Tsodikov A, Bowlus C, McVicar J, Zern M, Torok N. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007;13:844–847. doi: 10.1002/lt.20932. [DOI] [PubMed] [Google Scholar]
- 64.Ong J, Younossi ZM, Reddy V, Price LL, Gramlich T, Mayes J, Boparai N. Cryptogenic cirrhosis and posttransplantation nonalcoholic fatty liver disease. Liver Transpl. 2001;7:797–801. doi: 10.1053/jlts.2001.24644. [DOI] [PubMed] [Google Scholar]
- 65.Charlton M, Kasparova P, Weston S, Lindor K, Maor-Kendler Y, Wiesner RH, Rosen CB, Batts KP. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608–614. doi: 10.1053/jlts.2001.25453. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka T, Sugawara Y, Tamura S, Kaneko J, Takazawa Y, Aoki T, Hasegawa K, Sakamoto Y, Yamashiki N, Kokudo N. Living donor liver transplantation for non-alcoholic steatohepatitis: A single center experience. Hepatol Res. 2013:Jul 9; Epub ahead of print. doi: 10.1111/hepr.12200. [DOI] [PubMed] [Google Scholar]
- 67.Poordad F, Gish R, Wakil A, Garcia-Kennedy R, Martin P, Yao FY. De novo non-alcoholic fatty liver disease following orthotopic liver transplantation. Am J Transplant. 2003;3:1413–1417. doi: 10.1046/j.1600-6143.2003.00203.x. [DOI] [PubMed] [Google Scholar]
- 68.Malik SM, deVera ME, Fontes P, Shaikh O, Ahmad J. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9:782–793. doi: 10.1111/j.1600-6143.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 69.Guckelberger O, Mutzke F, Glanemann M, Neumann UP, Jonas S, Neuhaus R, Neuhaus P, Langrehr JM. Validation of cardiovascular risk scores in a liver transplant population. Liver Transpl. 2006;12:394–401. doi: 10.1002/lt.20722. [DOI] [PubMed] [Google Scholar]
- 70.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106:460–468; quiz 469. doi: 10.1038/ajg.2010.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molloy JW, Calcagno CJ, Williams CD, Jones FJ, Torres DM, Harrison SA. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology. 2012;55:429–436. doi: 10.1002/hep.24731. [DOI] [PubMed] [Google Scholar]
- 73.Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;(1):CD005166. doi: 10.1002/14651858.CD005166.pub2. [DOI] [PubMed] [Google Scholar]
- 74.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 75.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 76.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 77.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 80.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 81.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 82.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 83.Antonopoulos S, Mikros S, Mylonopoulou M, Kokkoris S, Giannoulis G. Rosuvastatin as a novel treatment of non-alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis. 2006;184:233–234. doi: 10.1016/j.atherosclerosis.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 84.Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990–994. doi: 10.1097/MCG.0b013e31819c392e. [DOI] [PubMed] [Google Scholar]
- 85.Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, Zeuzem S, Hein J, Berg T. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 86.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 87.Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol. 2011;17:2801–2811. doi: 10.3748/wjg.v17.i23.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, Rinella ME. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277–286. [PubMed] [Google Scholar]
- 89.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 90.Butte JM, Devaud N, Jarufe NP, Boza C, Pérez G, Torres J, Pérez-Ayuso RM, Arrese M, Martínez J. Sleeve gastrectomy as treatment for severe obesity after orthotopic liver transplantation. Obes Surg. 2007;17:1517–1519. doi: 10.1007/s11695-008-9432-z. [DOI] [PubMed] [Google Scholar]
- 91.Tichansky DS, Madan AK. Laparoscopic Roux-en-Y gastric bypass is safe and feasible after orthotopic liver transplantation. Obes Surg. 2005;15:1481–1486. doi: 10.1381/096089205774859164. [DOI] [PubMed] [Google Scholar]
- 92.Duchini A, Brunson ME. Roux-en-Y gastric bypass for recurrent nonalcoholic steatohepatitis in liver transplant recipients with morbid obesity. Transplantation. 2001;72:156–159. doi: 10.1097/00007890-200107150-00029. [DOI] [PubMed] [Google Scholar]
- 93.Al-Nowaylati AR, Al-Haddad BJ, Dorman RB, Alsaied OA, Lake JR, Chinnakotla S, Slusarek BM, Sampson BK, Ikramuddin S, Buchwald H, et al. Gastric bypass after liver transplantation. Liver Transpl. 2013;19:1324–1329. doi: 10.1002/lt.23734. [DOI] [PubMed] [Google Scholar]
- 94.Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, Montgomery RA, Cameron AM, Maley WR. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl. 2008;14:512–525. doi: 10.1002/lt.21396. [DOI] [PubMed] [Google Scholar]
- 95.Mazzaferro V, Rondinara GF, Rossi G, Regalia E, De Carlis L, Caccamo L, Doci R, Sansalone CV, Belli LS, Armiraglio E. Milan multicenter experience in liver transplantation for hepatocellular carcinoma. Transplant Proc. 1994;26:3557–3560. [PubMed] [Google Scholar]
- 96.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 97.Fairbanks KD, Eustace JA, Fine D, Thuluvath PJ. Renal function improves in liver transplant recipients when switched from a calcineurin inhibitor to sirolimus. Liver Transpl. 2003;9:1079–1085. doi: 10.1053/jlts.2003.50183. [DOI] [PubMed] [Google Scholar]
- 98.Farkas SA, Schnitzbauer AA, Kirchner G, Obed A, Banas B, Schlitt HJ. Calcineurin inhibitor minimization protocols in liver transplantation. Transpl Int. 2009;22:49–60. doi: 10.1111/j.1432-2277.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 99.Tueche SG. Diabetes mellitus after liver transplant new etiologic clues and cornerstones for understanding. Transplant Proc. 2003;35:1466–1468. doi: 10.1016/s0041-1345(03)00528-1. [DOI] [PubMed] [Google Scholar]
- 100.Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;(4):CD005161. doi: 10.1002/14651858.CD005161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]


