Abstract
Pelvic peritoneal adhesions constitute an important cause of concern which affects the life of millions of people worldwide due to complications like abdominal pain, bowel obstruction and infertility along with challenges in surgical exploration. Precise pre-operative diagnosis of the presence and extent of peritoneal adhesions is of great clinical and surgical importance. Diagnostic laparoscopy to detect peritoneal adhesions may itself lead to formation of adhesions. Routine CT and MRI studies are therefore useful non-invasive modalities to achieve this objective. This review article provides a brief background about the causation and patho-physiology of peritoneal adhesions. The article also addresses the range of clinical presentations in these patients, mainly from the gynecologic perspective. This article provides an illustrative review of CT and MRI findings with laparoscopic correlation. A new ‘imaging-based grading system’ for pre-operative quantification of the burden of peritoneal adhesions is also proposed. Despite practical challenges in accurate pre-operative diagnosis of peritoneal adhesions on imaging, detection of peritoneal adhesions is certainly feasible on routine CT and MRI scans and should be an integral part of image interpretation.
Keywords: Computed tomography, magnetic resonance imaging, pelvic peritoneal adhesions
Introduction
Peritoneal adhesions (2012 ICD-10-CM Diagnosis Code K66.0) are a distinct disease entity characterized by formation of bands of fibrous tissue that join intra-abdominal organs to each other or parietal peritoneal surfaces on either side. Adhesions forms as a result of body's healing process after an episode of traumatic, ischemic, infective, or irritative insult in a manner similar to scar formation. The term “adhesion” is applied when the scar tissue extends from the surface of organ to another organ, usually across a body cavity such as peritoneal cavity. Peritoneal adhesions had a significant impact over the quality of life of millions of people worldwide due to related small bowel obstruction (SBO), difficult surgical exploration, chronic abdomino-pelvic pain, and even female infertility.[1,2]
Patho-physiology of Peritoneal Adhesion Formation
The key site in peritoneal adhesion formation is the surface lining of the peritoneum. The delicacy of the peritoneal surface, its subsequent susceptibility to damage, and the rapid rate of re-mesothelialization are important factors.[3] Injury or inflammation of peritoneum triggers a coagulative state that releases multiple chemical messengers at injury site which lead to a series of events and consequent formation of peritoneal adhesions [Figure 1]. The adhesions may form quite rapidly after the triggering event, but the maturing process is usually slow and may last for months or years. Mature adhesions are the result of aberrant peritoneal healing and have been thought to consist of non-functional scar tissue. Recent studies have, however, established that the mature adhesions are highly cellular, vascularized, innervated, and suggest them to be dynamic, regenerating structures rather than inert non-functional fibrous tissue.[4]
Figure 1.
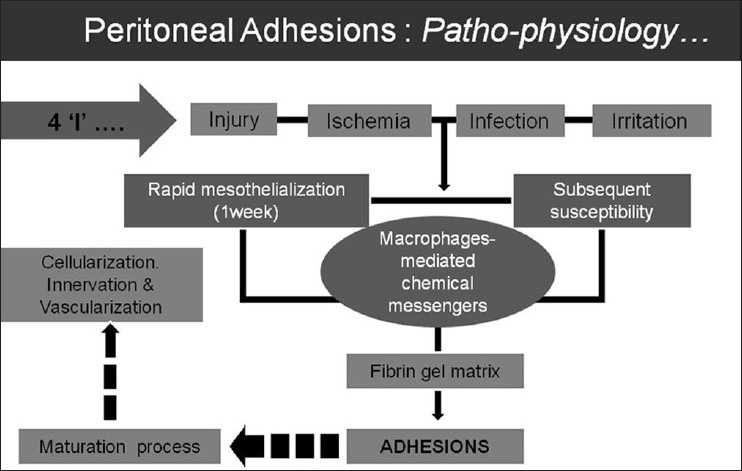
Flow chart to illustrate the causation and patho-physiology of adhesion formation in the peritoneal cavity
Peritoneal adhesions may form following one or more of the primary form of insult to the peritoneum, including injury, infection, ischemia, or irritation. The etiologic factors could be either congenital or acquired causes. The acquired causes include inflammatory (endometriosis, appendicitis, diverticulitis, etc.), infective (tuberculosis, pelvic inflammatory disease, etc.), post-surgical, or post-radiotherapy. Peritoneal adhesion related to the bowel loops may be either entero-enteric or entero-parietal, which determines the subsequent clinical impact [Figure 2].
Figure 2.
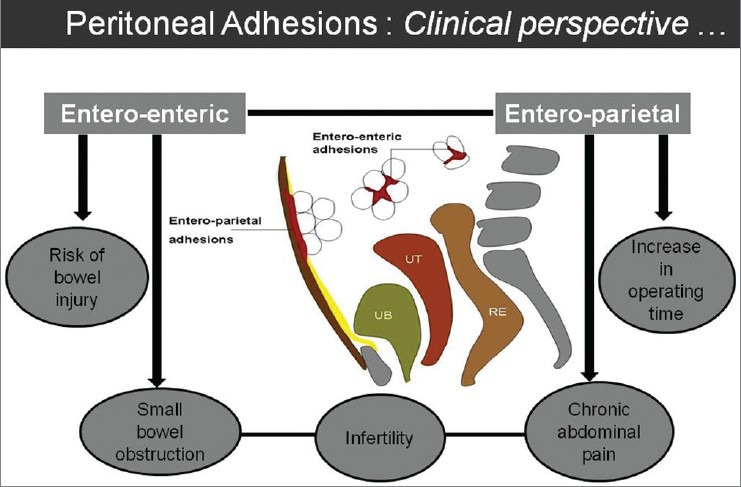
Line diagram to illustrate the types of peritoneal adhesions and their common clinical presentations
Post-surgical Peritoneal Adhesions
Operative procedures involving the peritoneal cavity are the commonest cause of peritoneal adhesions. This may lead to the formation of potentially obstructive peritoneal adhesions (matting or discrete bands) in almost 95% of patients.[5] In recent times, with increase in the incidence of abdominal surgeries, peritoneal adhesions have emerged as the most frequent cause of SBO. It has been reported that adhesive SBO occurs in 3% of all laparotomies and 1% occurs during the first postoperative year.[6,7] Postoperative soft adhesions form as early as from 72 h to 2 weeks. Twenty percent of these adhesions form within 1 month, while 40% form within 1 year.[8] Factors associated with the formation of post-surgical adhesions include trauma, thermal injury, infection, ischemia, and foreign bodies. The risk factors for post-surgical peritoneal adhesions include the patient's age, number of previous surgeries, and complexity of surgical procedures. Laparotomy is associated with higher risk of adhesion formation, as compared to laparoscopic approach.[9,10] Few procedures are associated with higher risk of adhesion formation, such as colo-rectal surgeries, abdominal hysterectomy, myomectomy, and non-elective appendicectomy.[3]
Gynecologic Considerations
Postoperative adhesions are an important concern for gynecologic surgeons, gynecologic oncologists, and infertility specialists. Several gynecologic and obstetric events have been a major source of peritoneal adhesions. Myomectomy is associated with a high degree of adnexal adhesions, mainly when the incision is performed on posterior uterine wall.[11] Surgical treatment of gynecologic malignancy may be associated with intestinal obstruction either by persistent tumor growth or by postoperative adhesions.[12] Peritoneal adhesions account for 15-20% cases of infertility, and the pregnancy rates are reported to increase from 38 to 52% with adhesiolysis.[13] Ectopic pregnancies have also been implicated as possible sequelae of peritubal adhesions.[14] The association between adhesions and chronic pelvic pain is well established in studies which have shown significant reduction of pain following adhesiolysis in 60-90% of patients.[15] Despite the measures to develop effective strategies to reduce or prevent adhesions, their formation remains a frequent occurrence after abdominal surgery. Emphasis on meticulous surgical technique is the key to reduce unnecessary morbidity from these untoward effects of surgery. Presence of peritoneal adhesions is an important concern in a patient who needs an abdominal surgery. Precise preoperative planning in terms of operative approach, surgical technique, and the need for an attending gastrointestinal surgeon to avoid any bowel injury is particularly relevant in these patients.
CT and MRI Evaluation of Peritoneal Adhesions
Evaluation of peritoneal adhesions may be feasible on routine computed tomography (CT) or magnetic resonance imaging (MRI) study done for assessment of the primary disease process. Peritoneal adhesions may be detected (apart from the primary disease process) in the same study without any additional cost, radiation, or side-effects to the patient. Peritoneal adhesions may be evaluated in terms of morphology, extent, and quantification. The effect of adhesions over abdominal wall and visceral structures can be better delineated, as compared to the direct visualization of the adhesion. The findings in these imaging modalities are likely to correlate with laparoscopy to a varying extent, depending upon the type and location of adhesions and also on the secondary effects on the adjoining structures. Preoperative CT and MRI studies have the potential to serve as the non-invasive surgical “road map” for the operative intervention and laparoscopic procedures. Imaging diagnosis of peritoneal adhesions is also clinically relevant to establish the cause of patient's symptoms, mainly in patients with chronic pelvic pain. CT and MRI diagnosis of peritoneal adhesions may help in deciding the operative approach (laparoscopic or open) and may facilitate precise preoperative planning. Preoperative CT or MRI for evaluation of peritoneal adhesions particularly makes sense in the present era of evidence-based clinical practice, as they provide objective evidence to justify the need for surgical adhesiolysis.
Multi-planar “sheet-like” nature of peritoneal adhesions and predominantly sparse vascularity constitutes the major challenge for delineation of peritoneal adhesions on CT and MRI. By virtue of excellent soft tissue resolution and recent advances in the multiplanar reconstruction capabilities of these imaging modalities, the evaluation of peritoneal adhesions is likely to be more feasible and reproducible than before. Comprehensive evaluation of the peritoneal cavity should become an integral component in this analysis. The peritoneum should be thoroughly traced and the course and thickness should be evaluated. The peritoneal recesses including the utero-vesical and recto-uterine pouch can be precisely evaluated [Figure 3]. The pro-peritoneal line of fat is an important radiologic parameter to detect anterior entero-parietal adhesions [Figure 4]. Focal obscuration of pro-peritoneal line of fat on CT or MRI may also be seen at the operative site without the presence of adhesions. This helps to localize the exact site of surgical scar. In patients with anterior entero-parietal adhesions at the operative site, the focal obscuration of the pro-peritoneal fat is likely to be accompanied by closely adherent omentum and small bowel loops [Figure 5]. The detection of peritoneal adhesion is often based on indirect signs on CT and MRI. Indirect signs are secondary to extrinsic indentation or kink over a bowel loop which may cause distortion of mucosal folds or luminal narrowing or even excessive peristalsis.[16] The serosal surface of the bowel loops should be surrounded by mesenteric-omental fat or may be abutting the wall of adjacent bowel wall. Linear or curvilinear soft tissue stands extending up to another loop of bowel or peritoneal surface may suggest peritoneal adhesion [Figure 6]. The external contours of pelvic organs should be carefully evaluated. Utero-cervical length and uterine surface may provide useful clues regarding the presence of adhesions [Figures 7 and 8]. Demonstration of peritoneal bands or sheet-like structures may constitute direct signs of adhesions. The adhesions may be thin “flimsy” or thick “band-like” adhesions which may or may not be deforming the visceral contours. Adhesions may be fibrous or vascularized and may show enhancement on post-contrast CT or MRI. Localizing features like “triangulation” feature of Picture archiving and communication system (PACS) should be used to ascertain the presence of thin adhesions. In patients with ascites, focal loculation of fluid is an important indicator of intraperitoneal adhesion. Well-defined linear area of stranding on CT or MRI is likely to correlate with the presence of peritoneal adhesion. CT and MRI may demonstrate deep pelvic adhesions, which may correlate with laparoscopy images [Figures 9 and 10]. Detailed preoperative information about the deep pelvic adhesions is particularly useful for effective adhesiolysis and clean surgical dissection.
Figure 3.
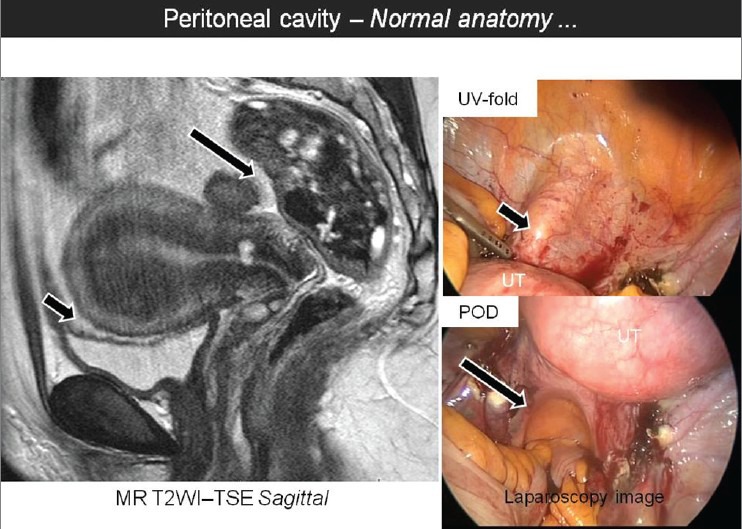
Sagittal T2W MR image showing the normal anatomy of the utero-vesical fold (UV-fold, short arrow) and the pouch of Douglas (POD, long arrow) with corresponding laparoscopic images
Figure 4.
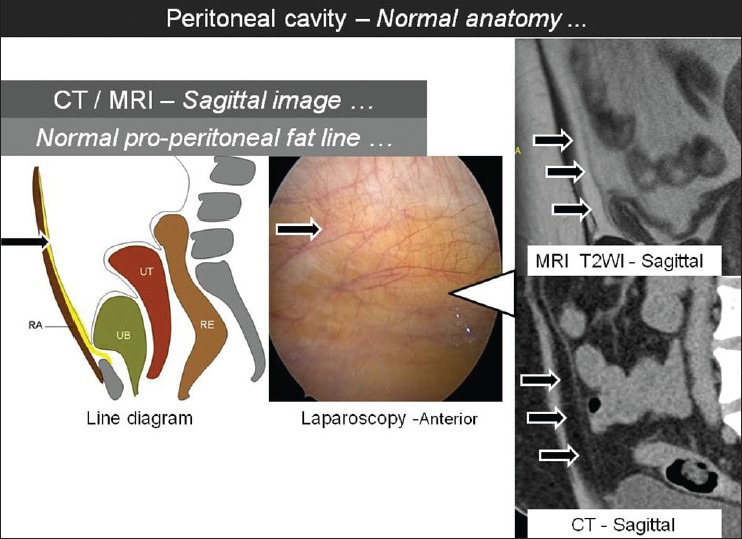
Sagittal T2W MR and sagittal CT images showing the normal appearance of pro-peritoneal fat line (arrows) with corresponding laparoscopic image and line diagram [RA: Rectus abdominis, UB: Urinary Bladder, UT: Uterus, RE: Rectum]
Figure 5.
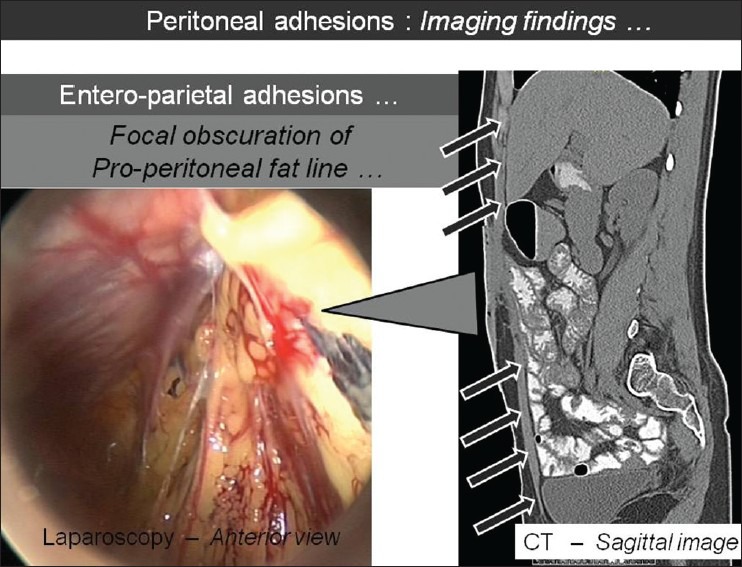
Sagittal CT image showing the focal obscuration of pro-peritoneal fat line (arrows) with corresponding laparoscopic image, which suggests entero-parietal adhesions
Figure 6.
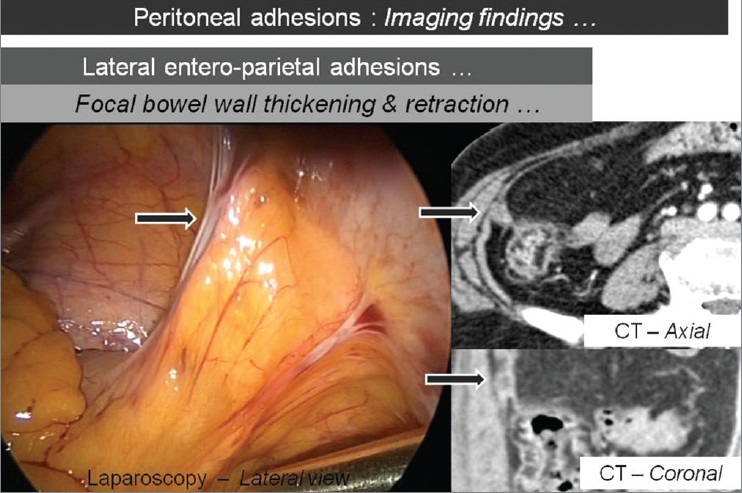
Axial and coronal CT images showing the focal thickening and retraction of bowel wall with corresponding laparoscopic image (arrows), which suggests lateral entero-parietal adhesions
Figure 7.
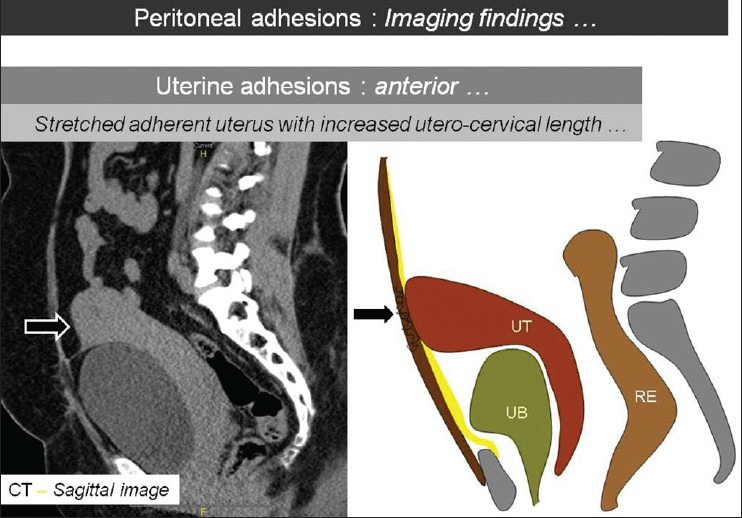
Sagittal CT image with corresponding line diagram showing the stretched adherent uterus (arrows) with increased utero-cervical length. [UB: Urinary Bladder, UT: Uterus, RE: Rectum]. This suggests anterior uterine adhesions
Figure 8.
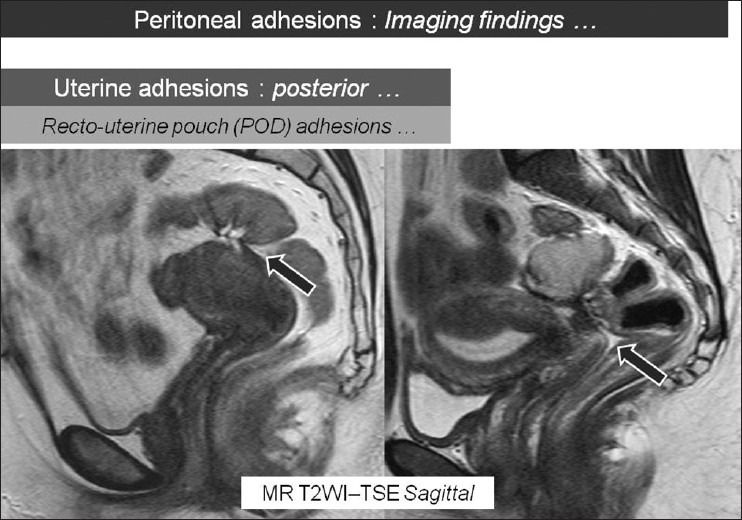
Sagittal T2W MR images showing recto-uterine pouch (Pouch of Douglas) adhesions (arrows). There is blunting of the POD due to these posterior uterine adhesions
Figure 9.
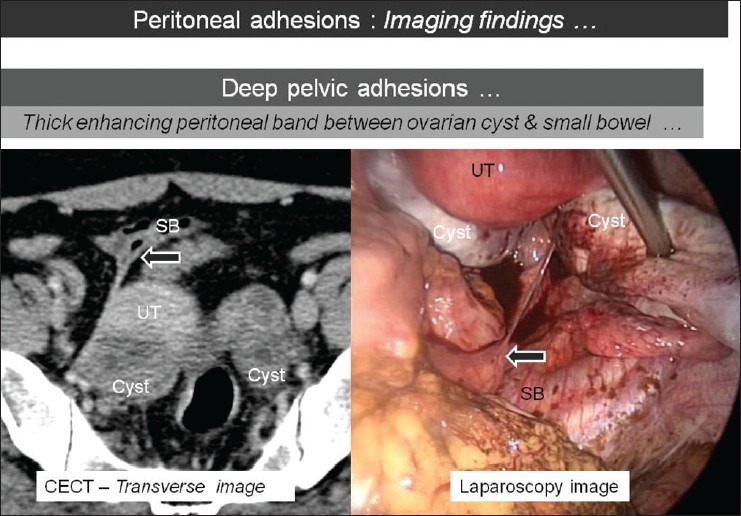
Axial CT image showing thick enhancing peritoneal band (arrow) extending between the ovarian cyst and the small bowel (SB) loop with corresponding laparoscopic image [UT: Uterus]. CT and MR images of these deep pelvic adhesions show excellent correlation with laparoscopy
Figure 10.
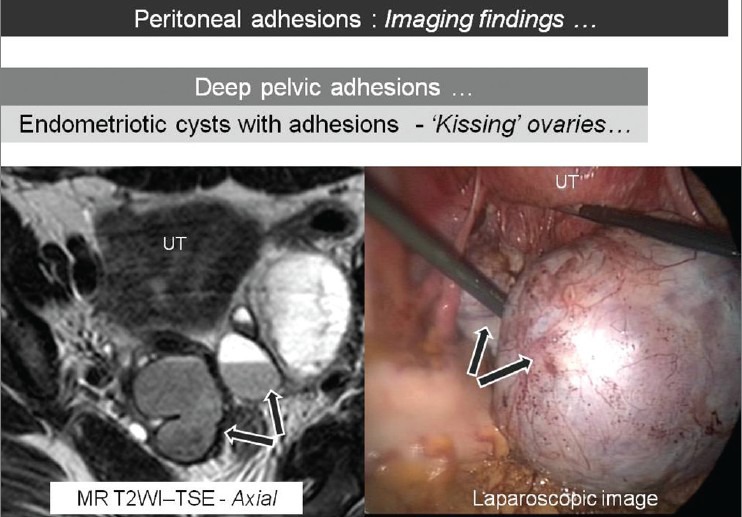
Axial T2W MR image showing bilateral ovarian cysts with shading phenomenon on T2WI (arrows) to suggest endometriomas. These cysts are seen posterior to uterus (UT) and are adherent to each other (‘kissing ovaries’). The corresponding laparoscopic image shows excellent correlation with MRI findings
Preoperative CT and MRI images may show excellent correlation with laparoscopy in a few cases and may not even demonstrate the adhesions in others. This primarily depends upon the type, thickness, and distribution of the peritoneal adhesions and the impact over the adjoining structures. In patients with thin “sheet-like” adhesions, the diagnosis may also significantly depend upon the technical factors including the type of CT or MRI equipment and the acquisition protocols. Use of multi-detector CT and evaluation of thin volume data, which is now a standard method of CT imaging, is expected to improve the detection of adhesions. Apart from the imaging technique, image interpretation is also an important component to ensure diagnosis of peritoneal adhesions. It is a common perception that the CT and MRI cannot at all diagnose the presence of peritoneal adhesions. It is however, important to look for several subtle clues on CT and MRI to suspect peritoneal adhesions.
There are instances when there were no definite demonstrable direct or indirect signs of peritoneal adhesions on CT and/or MRI and the patient was later on found to have adhesions. The diagnosis of peritoneal adhesions in these circumstances may be based on demonstration of adhesive SBO. CT is particularly useful in the diagnosis of SBO and in the identification of the underlying cause, which can be either extrinsic or intrinsic. The former includes peritoneal adhesions, closed loop obstruction, hernia, or extrinsic mass lesions. The intrinsic causes include adenocarcinoma, Crohn's disease, tuberculosis, or intussusception. Till recently, CT diagnosis of adhesive SBO was mainly a diagnosis of exclusion, when all other causes of obstruction were ruled out. This approach led to false diagnosis of adhesions when the other causes with subtle CT findings were the actual cause of obstruction.[17,18] It is important to differentiate obstruction due to adhesive bands from matted adhesions, as the former is more likely to be associated with high-grade obstruction with or without closed loop obstructions and the risk of strangulation, while the latter may be managed conservatively [Figure 11]. The presence of “beak sign” (abrupt luminal transition and luminal constriction) and “fat notch sign” (focal extraluminal compression due to a peritoneal band) at the site of luminal transition was reported to be associated with adhesive bands rather than matted adhesions.[19] “Small bowel feces sign” was reported to be more frequently associated with matted adhesions.[20] The matted adhesions are more commonly seen in the pelvis and are more likely to cause SBO in patients who had an underlying gynecologic inflammatory condition.[21]
Figure 11.
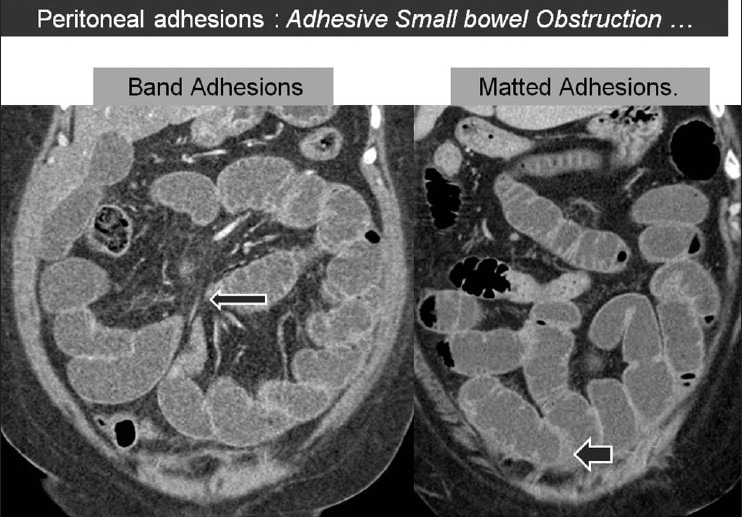
Coronal CT image (left panel) showing an enhancing peritoneal band (long arrow) causing focal compression over the small bowel loop with mild proximal obstruction. Another patient (right panel) showing presence of matted adhesions (short arrow) between the small bowel loops
Similarly, the diagnosis of peritoneal adhesions may be at times based on demonstration of peritoneal inclusion cysts (PIC) on CT or MRI. In patients with peritoneal adhesions, presence of active functioning ovary is an important predisposing cause for formation of PIC. They represent a non-neoplastic reactive mesothelial proliferation and occur almost exclusively in premenopausal women with history of previous surgery, trauma, pelvic inflammatory disease or endometriosis.[22] This is a relatively underdiagnosed entity which is commonly present and may mimic ovarian tumors. Preoperative diagnosis is crucial to avoid unnecessary interventions. Imaging diagnosis depends on the presence of normal ipsilateral ovary with the surrounding loculated fluid conforming to the shape of peritoneal space. Peritoneal adhesions extend to the ovarian surface and may distort the contour, but do not penetrate the parenchyma. Presence of centrally entrapped ovaries with peri-ovarian adhesions and loculated fluid often gives the appearance of “spider-in-web” on imaging studies [Figure 12]. Delineation of loculated fluid surrounding the ovary or elsewhere is an important clue to the presence of peritoneal adhesion. Imaging demonstration of extraovarian location of lesion and identification of entrapped normal ovary is the key to definitive diagnosis of PIC. Stable imaging appearance on follow-up imaging over a period of 6 months-1 year is also helpful.[23] CT and MRI offer accurate diagnosis of PIC and, therefore, provide important clue regarding the presence of pelvic peritoneal adhesions.
Figure 12.
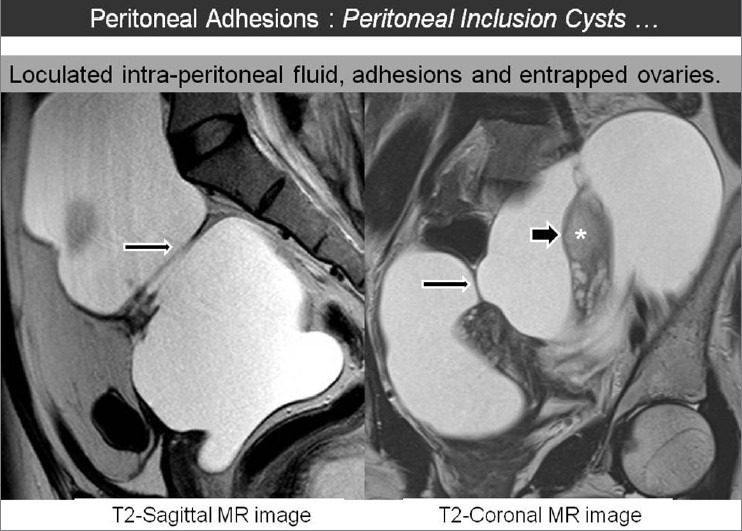
Sagittal and coronal T2W MR images showing a large hyperintense lesion which confirms to the shape of peritoneal cavity. Presence of several peritoneal adhesions are seen along the lesion extending up to the ovarian surface (marked as *). Entrapped ovaries in the centre of lesion constitute the ‘spider-in-web sign to diagnose peritoneal inclusion cyst
Quantification of peritoneal adhesion may also be feasible with CT and MRI, which may provide an estimate of adhesion burden in the peritoneal cavity. The proposed scoring system [Figure 13] is based on the morphological factors that facilitate diagnosis of peritoneal adhesions on imaging. The first part of the scoring system is based on eight parameters, three of which pertain to anterior abdominal wall, while the remaining five parameters pertain to peritoneal cavity. These eight parameters may have scores from 0 to 3, depending upon the presence or absence of any demonstrable peritoneal adhesions (normal: 0, thin flimsy adhesions: 1, thick adhesions: 2, and contour-deforming adhesions: 3). Thus, these eight parameters may have the maximum score of 24. As focal obscuration of pro-peritoneal fat alone is not a sign of adhesion and may only suggest the location of surgical scar, it is not included as a morphological parameter in this system. The status of mesenteric-omental fat and peritoneal fluid constitutes second part of this scoring system. Presence of focal linear area of fat stranding in the mesenteric-omental fat is likely to suggest a peritoneal band and should add 3 to the total score. Presence of loculated intraperitoneal fluid including the delineation of peritoneal inclusion cyst is also an important marker of peritoneal adhesion and should add 3 more to the total score. Thus, the maximum possible score with this adhesion scoring system is 30. This proposed scoring system would, however, need clinical validation in a prospective study to correlate the imaging and laparoscopy findings and the operative outcome. Such studies in future may also derive a cut-off adhesion score beyond which open surgery may be recommended, based on the prediction of total adhesion burden.
Figure 13.
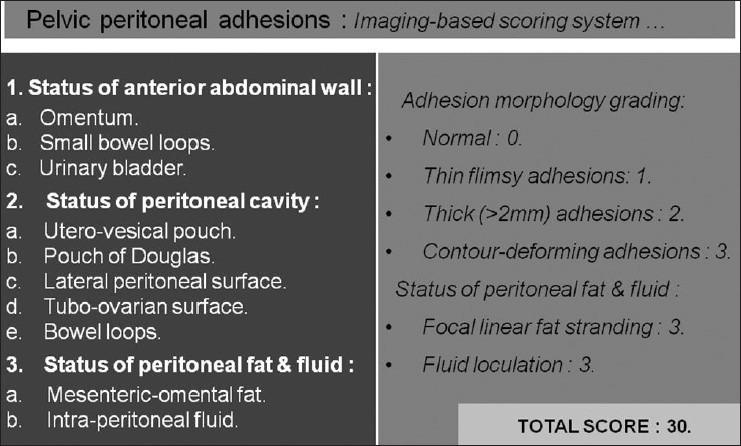
Proposed imaging-based scoring system to estimate the total adhesion burden in the peritoneal cavity. This system is based on the morphological parameters which help to diagnose the adhesions on routine CT and MRI
Precise preoperative diagnosis of the presence and extent of peritoneal adhesions is of great importance for a wide range of clinical presentations.[24] Diagnostic laparoscopy to detect peritoneal adhesions may itself lead to formation of adhesions.[25] CT and MRI are, therefore, likely to emerge as useful non-invasive modalities in future to achieve this objective. Apart from the routine CT and MR studies for the evaluation of peritoneal adhesions, functional cine-MR imaging is also reported to be an accurate method in the identification of intra-abdominal adhesions. Cine-MRI is based on the same principle as the “visceral-slide” technique initially reported with clinical pelvic examination and ultrasound.[26,27] Cine-MRI was found to have a sensitivity of 87.5% and a specificity of 92.5%.[28] The accuracy was particularly high for adhesions in the abdominal wall and the subperitoneal space, while entero-enteric adhesions were relatively overdiagnosed.
Routine CT and MR scans do contain useful information about peritoneal adhesions, which is relevant from a clinical perspective. It is, however, important for the radiologists to sensitize themselves to these subtle findings related to peritoneal adhesions on routine CT and MRI, which may have vital clinical and surgical relevance. The accuracy and performance of CT and MRI studies in the diagnosis of peritoneal adhesions and its impact on clinical outcome should be, however, further evaluated in a prospective clinical trial. The present article still emphasizes that radiologists should specifically look for peritoneal adhesions on routine preoperative CT and MR studies, which are performed as a part of a routine preoperative work-up. Apart from detection of the primary disease, the diagnosis of peritoneal adhesions in the same scans is likely to have important clinical and surgical implications.
Despite the practical challenges in accurate preoperative diagnosis of peritoneal adhesions on imaging, detection of peritoneal adhesions is possible on routine CT and MRI scans and should be an integral part of image interpretation.
Summary
This review article focuses on the pathophysiology, causation, clinical presentation, and diagnosis of peritoneal adhesions, mainly from a gynecologic perspective. The article provides an illustrative review of findings in patients with peritoneal adhesions on routine preoperative CT and MRI studies with laparoscopic correlation. The article emphasizes on several salient points that radiologists need to know for diagnosis of peritoneal adhesions.
Radiologists should specifically look for peritoneal adhesions on routine CT and MRI studies, as their preoperative diagnosis has important clinical and surgical implications. This may help to explain the cause of patient's symptoms and justify the need for surgical adhesiolysis. Preoperative diagnosis of peritoneal adhesions and their quantification is expected to ensure proper operative planning and may help to decide the surgical technique.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Eubanks S, Schauer PR. Laparoscopic surgery. In: Sabiston DC, Lyerly HK, editors. Textbook of surgery: The biological basis of modern surgical practice. 15th ed. Philadelphia: Saunders; 1997. pp. 801–7. [Google Scholar]
- 2.Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: Clinical significance, etiology and prevention. Am J Obstet Gynecol. 1994;170:1396–403. doi: 10.1016/s0002-9378(94)70170-9. [DOI] [PubMed] [Google Scholar]
- 3.Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: Etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260–73. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 4.Herrick SE, Mutsaers SE, Ozua P, Sulaiman H, Omer A, Boulos P, et al. Human peritoneal adhesions are highly cellular, innervated, and vascularized. J Pathol. 2000;192:67–72. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH678>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: A retrospective cohort study. Lancet. 1999;353:1476–80. doi: 10.1016/S0140-6736(98)09337-4. [DOI] [PubMed] [Google Scholar]
- 6.Miller G, Boman J, Shrier I, Gordon PH. Etiology of small bowel obstruction. Am J Surg. 2000;180:33–6. doi: 10.1016/s0002-9610(00)00407-4. [DOI] [PubMed] [Google Scholar]
- 7.Cox MR, Gunn IF, Eastman MC, Hunt RF, Heinz AW. The operative aetiology and types of adhesions causing small bowel obstruction. Aust N Z J Surg. 1993;63:848–52. doi: 10.1111/j.1445-2197.1993.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 8.Menzies D. Peritoneal adhesions: incidence, cause and prevention. Surg Annu. 1992;24:27–45. [PubMed] [Google Scholar]
- 9.Garrard CL, Clements RH, Nanney L, Davidson JM, Richards WO. Adhesion formation is reduced after laparoscopic surgery. Surg Endosc. 1999;13:10–3. doi: 10.1007/s004649900887. [DOI] [PubMed] [Google Scholar]
- 10.Kavic SM, Kavic SM. Adhesions and adhesiolysis: The role of laparoscopy. JSLS. 2002;6:99–109. [PMC free article] [PubMed] [Google Scholar]
- 11.Tulandi T, Murray C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213–5. [PubMed] [Google Scholar]
- 12.Krebs HB, Goplerud DR. Mechanical intestinal obstruction in patients with gynaecologic disease: A review of 368 patients. Am J Obstet Gynecol. 1987;157:577–83. doi: 10.1016/s0002-9378(87)80010-8. [DOI] [PubMed] [Google Scholar]
- 13.Luijendijk RW, de Lange DC, Wauters CC, Hop WC, Duron JJ, Pailler JL, et al. Foreign material in postoperative adhesions. Ann Surg. 1996;223:242–8. doi: 10.1097/00000658-199603000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil M. Assessing tubal damage. J Hum Reprod Sci. 2009;2:2–11. doi: 10.4103/0974-1208.51335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy DM, di Zerega GS. Adhesion controversies: Pelvic pain as a cause of adhesions, crystalloids in preventing them. J Reprod Med. 1996;41:19–26. [PubMed] [Google Scholar]
- 16.Bartram CI. Radiologic demonstration of adhesions following surgery for inflammatory bowel disease. Br J Radiol. 1980;53:650–3. doi: 10.1259/0007-1285-53-631-650. [DOI] [PubMed] [Google Scholar]
- 17.Boudiaf M, Soyer P, Terem C, Pelage JP, Maissiat E, Rymer R. CT Evaluation of small bowel obstruction. Radiographics. 2001;21:613–24. doi: 10.1148/radiographics.21.3.g01ma03613. [DOI] [PubMed] [Google Scholar]
- 18.Petrovic B, Nikalaidis P, Hammond NA, Grant TH, Miller FH. Identification of adhesions on CT in small bowel obstruction. Emerg Radiol. 2006;12:88–93. doi: 10.1007/s10140-005-0450-z. [DOI] [PubMed] [Google Scholar]
- 19.Delabrousse E, Lubrano J, Jehl J, Morati P, Rouget C, Mantion GA, et al. Small bowel obstruction from Adhesive bands and matted adhesions: CT differentiation. AJR Am J Roentgenol. 2009;192:693–7. doi: 10.2214/AJR.08.1550. [DOI] [PubMed] [Google Scholar]
- 20.Meissner K, Szecsi T, Jirikowski B. Intestinal Obstruction caused by the solitary bands: Aetiology, presentation, diagnosis, management, results. Acta Chr Hung. 1994;34:355–63. [PubMed] [Google Scholar]
- 21.Lazarus DE, Slywotsky C, Bennett GL, Megibow AJ, Macari M. Frequency and relevance of small bowel feces sign on CT in patients with small bowel obstruction. AJR Am J Roentgenol. 2004;183:1361–6. doi: 10.2214/ajr.183.5.1831361. [DOI] [PubMed] [Google Scholar]
- 22.Kim JS, Lee HJ, Woo SK, Lee TS. Peritoneal inclusion cysts and their relationship to ovaries: Evaluation with US. Radiology. 1997;204:481–4. doi: 10.1148/radiology.204.2.9240539. [DOI] [PubMed] [Google Scholar]
- 23.Jain KA. Imaging of Peritoneal Inclusion Cysts. AJR Am J Roentgenol. 2000;174:1559–63. doi: 10.2214/ajr.174.6.1741559. [DOI] [PubMed] [Google Scholar]
- 24.Freys SM, Fuchs KH, Heimbucher J, Thiede A. Laparoscopic adhesiolysis. Surg Endosc. 1994;8:1202–7. doi: 10.1007/BF00591051. [DOI] [PubMed] [Google Scholar]
- 25.Levrant SG, Bieber EJ, Barnes RB. Anterior abdominal wall adhesions after laparotomy or laparoscopy. J Am Assoc Gynecol Laparosc. 1997;4:353–6. doi: 10.1016/s1074-3804(05)80227-0. [DOI] [PubMed] [Google Scholar]
- 26.Stovall TG, Elder RF, Ling FW. Predictors of pelvic adhesions. J Reprod Med. 1989;34:345–8. [PubMed] [Google Scholar]
- 27.Sigel B, Golub RM, Loiacono LA, Parsons RE, Kodama I, Machi J, et al. Technique of ultrasonic detection and mapping of abdominal wall adhesions. Surg Endosc. 1991;5:161–5. doi: 10.1007/BF02653253. [DOI] [PubMed] [Google Scholar]
- 28.Lienemann A, Sprenger D, Steitz HO, Korell M, Reiser M. Detection and Mapping of Intra-abdominal Adhesions by Using Functional Cine MR Imaging: Preliminary Results. Radiology. 2000;217:421–5. doi: 10.1148/radiology.217.2.r00oc23421. [DOI] [PubMed] [Google Scholar]


