Abstract
The diagnosis of acute right lower quadrant pain in a pediatric population is challenging. Acute appendicitis is the most common cause of an acute surgical abdomen. The common mimics of acute appendicitis are acute gastrointestinal and gynecologic diseases. This article reviews the sonographic findings of the spectrum of common acute abdominal emergencies in children with a focus on imaging clues to a specific diagnosis. This awareness can impact on diagnostic accuracy and impact patient management.
Keywords: Appendicitis, pediatric, sonography
Introduction
Acute abdominal pain is a common complaint in infancy and childhood and is associated with a variety of surgical and nonsurgical conditions.[1,2,3,4] Most children presenting with acute abdominal pain will have nonsurgical disease. The most frequent condition requiring acute surgical intervention is acute appendicitis.[1,2] With the continued improvement in gray-scale and color Doppler techniques and introduction of graded-compression sonography, the use of ultrasonography has increased in the evaluation of children with acute abdominal pain. This review addresses the sonographic evaluation of suspected appendicitis in children, along with the imaging features of other causes of acute pain mimicking appendicitis.
Imaging Modalities
Ultrasonography is the most frequently used imaging modality in the evaluation of children with suspected appendicitis. Other tests include abdominal radiography, which is simple and readily available, but is rarely diagnostic of appendicitis. Its primary role is to assess for potential complications associated with acute inflammation, including small bowel obstruction and perforation. Computed tomography (CT) has a role in patients with large body habitus and in those with marked abdominal pain who cannot tolerate the graded-compression sonographic technique. CT is also useful when perforation with periappendiceal abscess is suspected, as it helps to establish the diagnosis and determine whether percutaneous abscess drainage can be safely performed.
Graded-compression sonography
The graded compression technique described by Puylaert et al. is used in the initial imaging test in the evaluation of suspected appendicitis.[5] A linear or curved array transducer (5 or 7.5 MHz) is used to gradually compress the right lower quadrant. Gentle gradual pressure is applied to the anterior abdominal wall, resulting in displacement and compression of normal bowel loops. At the start of the examination, the patient is asked to point with a single finger to the site of maximal tenderness or pain.[6] Self-localization expedites the search for an inflamed appendix, especially an aberrantly located appendix, and reduces the time of examination.
Scanning begins in the transverse plane with identification of the ascending colon, which appears as a non-peristaltic bowel segment containing gas and fluid. The transducer is then moved inferiorly to identify the cecal tip, where the appendix should arise. Pressure is gradually applied to displace gas and fecal material from the cecum, thus improving visualization of the appendix and distal small bowel. Normal bowel is easily compressible and displays active peristalsis. The psoas muscle and external iliac vessels should be identified, since the appendix lies anteriorly to these structures. Visualization of iliac vessels is also a sign of technical adequacy. Gray-scale and color Doppler images are acquired.
The ultrasound examination should include a survey of the entire abdomen to assess potential complications such as ascites, abscess, peritonitis, and hydronephrosis that can complicate appendicitis.
Pelvic sonography
In adolescent females, pelvic pathology can mimic appendicitis. If the examination for appendicitis is negative, sonography of pelvic organs should be performed. Sonographic assessment can be performed with a transabdominal approach using the distended urinary bladder as the acoustic window or with an endovaginal approach. The distended bladder displaces gas-filled bowel loops out of the pelvis, allowing transmission of sound into deeper structures of the pelvis and easier visualization of the ovaries and uterus. Bladder filling can be achieved with oral intake of fluid or via catheterization with instillation of sterile fluid. Pelvic sonography is performed with a sector or curved array transducer, and scans are obtained in both sagittal and transverse planes. Gray-scale and color Doppler images are acquired.[7]
Endovaginal sonography is an alternative technique in pubertal females who use tampons or who are sexually active. A major advantage of endovaginal over transabdominal scanning is the obviation of bladder filling. The major limitation of this technique is a limited field of view. Because of this problem, transabdominal sonography with full bladder is routinely carried out before a transvaginal sonography. An endovaginal study can be done if the transabdominal findings are indeterminate or confusing or to better characterize ovarian and tubal pathology.[6]
Acute Appendicitis
In the pediatric population, appendicitis is most common in the the 2nd decade and is rare in children under the age of 2 years.[8,9] The classic clinical presentation is acute onset of abdominal pain that may originate in the periumbilical region and migrate to the right lower quadrant. Diffuse abdominal distension and tenderness may be present if there is bowel obstruction or perforation. Approximately one-third of children with appendicitis have atypical clinical signs and symptoms.[10,11] In adolescent females, in particular, gynecologic disorders, as discussed below, can produce acute lower abdominal pain that mimics appendicitis.
Sonographic criteria
The normal appendix may be visible with graded-compression sonography and needs to be distinguished from the pathologic appendix.[3,8,9,12] The normal appendix is compressible, blind-ending, and measures 6 mm or less in maximum diameter. It has a tubular appearance on long axis scans and a target appearance in the axial plane [Figure 1]. The thin echogenic inner layer of mucosa/submucosa and the hypoechoic outer zone representing the muscularis propria are usually identifiable. A small amount of fluid or gas may be noted within the lumen.[13] On color flow Doppler imaging, there is no appreciable flow.[14,15]
Figure 1(A, B).
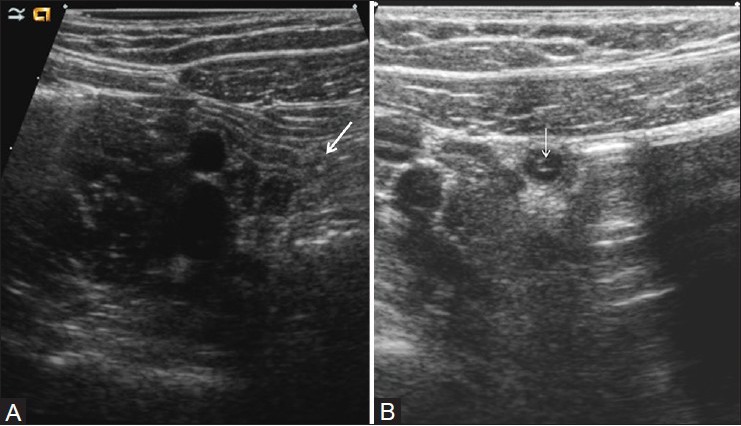
Normal appendix. Long axis (A) and short axis views (B) demonstrate a small appendix, measuring 5.4-mm in diameter. Note the central echogenic stripe representing the mucosa and submucosa and the peripheral hypoechoic muscularis. Arrow=blind ending appendiceal tip
On long axis views, the inflamed appendix appears as a fluid-filled, noncompressible tubular structure with one blind end and a diameter from outside wall to outside wall of at least 6 mm [Figure 2a]. In the axial plane, it has a target appearance, reflecting the fluid-filled center, the adjacent echogenic mucosa and submucosa, and the outer hypoechoic muscular wall [Figure 2b]. Demonstration of an appendicolith, which appears as an echogenic focus with posterior acoustic shadowing, is supportive evidence of appendicitis [Figure 3].[16] Of note, intraluminal air can mimic an appendicolith. The posterior acoustic shadowing from air tends to be ill-defined and associated with ring-down artifacts, whereas an appendicolith has clean posterior shadowing. Ancillary findings of appendicitis include enlarged mesenteric lymph nodes, a heterogeneous mass representing periappendiceal phlegmon, and a walled-off fluid collection representing abscess. As discussed below, the latter two findings are often complications of perforation. The inflamed appendix is hyperemic on color Doppler sonography [Figure 4].[15]
Figure 2(A, B).
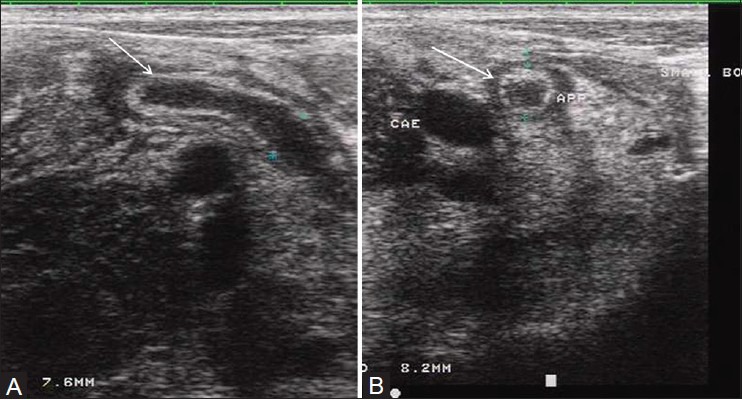
Acute appendicitis. (A) Long axis view through the right lower quadrant demonstrates a noncompressible, enlarged, fluid-filled blind-ending appendix (arrow). (B) Short axis scan shows a target appearance characterized by a fluid-filled center, subadjacent layer of hyperechoic mucosa, and outer layer of hypoechoic muscle (arrow)
Figure 3(A, B).
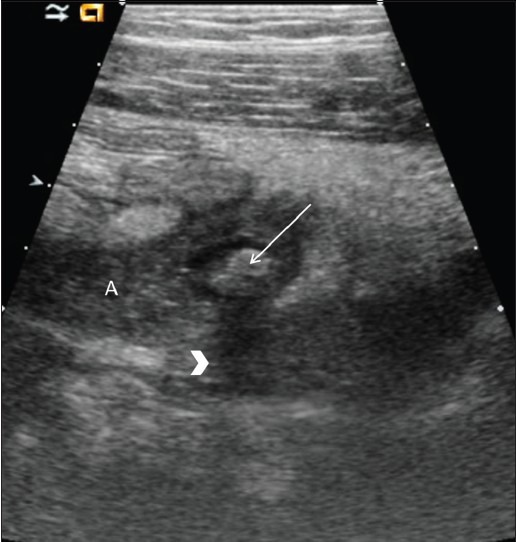
Acute appendicitis with an appendicolith. Long axis view shows an enlarged, fluid-filled appendix (A) with an echogenic appendicolith (arrow). Note the acoustic shadowing (arrowhead) caused by the appendicolith
Figure 4.
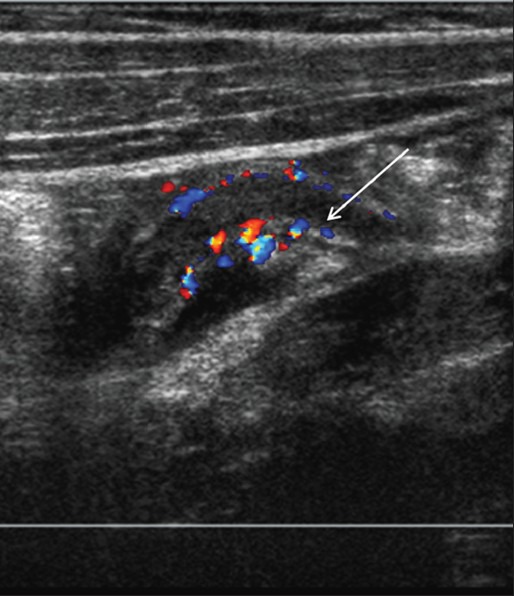
Acute appendicitis, color Doppler imaging. Longitudinal color flow Doppler images show a dilated appendix with peripheral hyperemia (arrow)
Complications of appendicitis
Perforation occurs in 20-30% of children with appendicitis.[17] The diagnosis of perforation can be difficult as the appendiceal lumen decompresses, and hence becomes non-identifiable at sonography (40-60% of cases). In this scenario, the diagnosis is based on identification of secondary signs such as loss of echogenic mucosal lining, increased periappendiceal echogenicity (phlegmon), abscess formation, thickening of adjacent aperistaltic bowel loops, and interloop fluid.[17]
Appendiceal abscess appears as a hypoechoic or complex mass with internal echoes and/or septations [Figure 5]. Occasionally, the appendiceal stump is displayed as an echogenic tubular structure projecting into the hypoechoic fluid-filled abscess. Color flow Doppler sonography usually demonstrates flow in the wall of the abscess and adjacent soft tissues.[15,18] Peritonitis is characterized by dilated bowel loops with thick hyperechoic walls and ascites. Color Doppler imaging shows increased blood flow in the bowel wall and adjacent mesentery.
Figure 5(A, B).
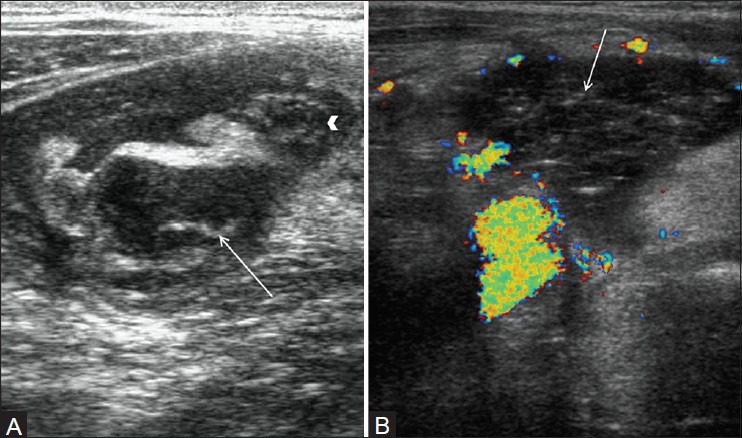
Perforated appendicitis. (A) Short axis view through right lower quadrant demonstrates enlarged appendix (arrowhead) with surrounding collection in right iliac fossa and appendicolith (arrow) in the fluid collection consistent with perforated appendix. (B) Color Doppler image in another patient shows complex collection (arrow) in right iliac fossa without internal vascularity consistent with abscess secondary to appendicular perforation
Diagnostic efficacy of sonography
The sensitivity of sonography for diagnosing appendicitis is between 80% and 95%, with a specificity of 89-100% and an accuracy of 90-96%[8,9,10,18,19,20,21] The sensitivity and specificity of color Doppler sonography are approximately 90% and 95%, respectively.[15] Although color Doppler sonography does not increase the sensitivity of the examination, it enhances interpretation of the gray-scale findings and increases observer confidence in the diagnosis of appendicitis.
False-positive diagnoses
Causes of false-positive diagnoses include mistaking the normal appendix for the abnormal appendix and mistaking other inflammatory processes, such as bowel disease and Meckel's diverticulum, for appendicitis.
False-negative diagnoses
The causes of false-negative diagnoses include focal (tip) appendicitis, an aberrant location of the appendix, such as a retrocecal position, and perforation. In focal appendicitis, the inflammation is localized to the distal end of the appendix; the proximal appendix being normal [Figure 6].[3,8] This pitfall can be minimized by imaging the entire length of the appendix. A retrocecal appendix can be difficult to visualize, particularly if the ascending colon and distal small bowel contain large amounts of air that cannot be easily compressed. The problem of a retrocecal appendix can be minimized by having the patient identify the site of maximal tenderness and by scanning in a coronal plane with the transducer paralleling the iliac wing. As noted above, the diagnosis of appendicitis can be challenging if the appendix peforates and decompresses.[17] The identification of secondary findings help to establish the correct diagnosis.
Figure 6(A, B).
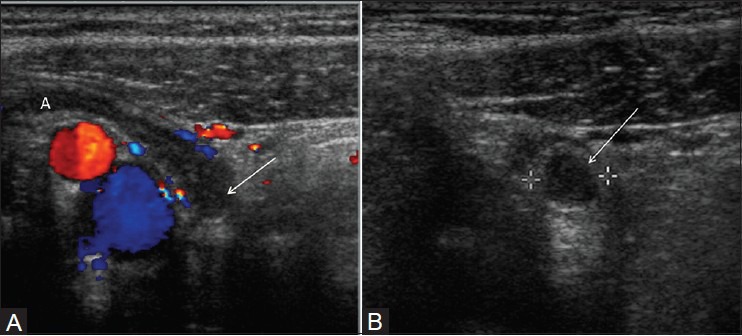
Focal appendicitis. (A) Longitudinal color Doppler image of right lower quadrant demonstrates enlarged and hyperemic appendiceal tip (arrow) with normal appearing proximal appendix. (B) Transverse view of right lower quadrant image shows enlarged appendiceal tip (arrow)
Alternative diagnoses
Another important contribution of sonography to the management of children with suspected appendicitis is in the diagnosis of other abdominal and pelvic conditions that can mimic appendicitis.[8,21] Approximately one-fourth to one-third of children referred for sonographic evaluation of suspected appendicitis will have that condition and another one-fourth to one-third of children will have specific alternative diagnoses, usually gastrointestinal and gynecologic diseases, established by sonography.[21] Many of these children, almost one-third to one-half, will have abdominal pain relief without a specific diagnosis being established.[8,9,21] The common mimics of acute appendicitis are acute gastroinestinal and gynecologic diseases.[21,23] Thus, the entire abdomen and pelvis should be scanned in children who have a normal graded-compression examination of the right lower quadrant and no sonographic evidence of appendicitis.
Mesenteric Adenitis/Mesenteric Lymphadenopathy
Mesenteric lymph node enlargement or adenitis can be associated with infectious, inflammatory, and neoplastic processes, and it can be a cause of acute right lower quadrant pain.[24,25] Histologic assessment of the affected lymph nodes demonstrates nonspecific, reactive lymphoid hyperplasia.[24]
Mesenteric lymph nodes are most commonly identified at sonography in the right lower quadrant, but they also can be seen in the mesenteric root along the course of the ileal and jejunal arteries and the superior mesenteric vein. They are considered enlarged when the maximum anteroposterior diameter is at least 4mm. The lymph nodes are iso- or hypoechoic relative to surrounding tissues and muscles and have an oval shape with a central echogenic hilum, which demonstrates color flow [Figure 7].[4,24,25]
Figure 7(A, B).
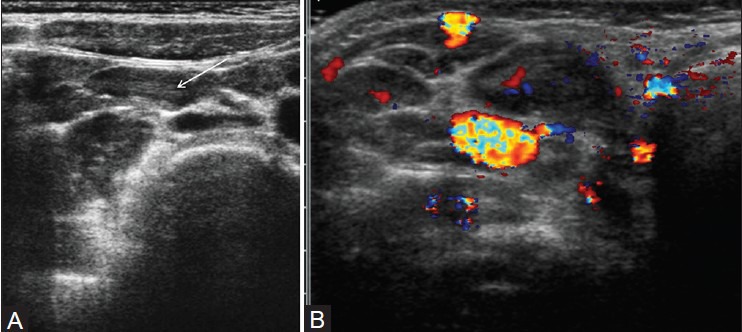
Mesenteric lymphadenopathy. (A) Longitudinal view through the right lower quadrant using graded-compression sonography shows several enlarged, oval-shaped, mesenteric lymph node with echogenic central hilum (arrow). (B) Color Doppler scan in another patient shows an enlarged node (arrow) with central flow
Acute Gastrointestinal Diseases
Inflammatory bowel disease, infectious terminal ileitis, Henoch-Schönlein purpura, Meckel's diverticulum, and intussusception are the gastrointestinal causes of acute abdominal pain that can clinically mimic appendicitis.
Infectious and inflammatory bowel diseases
Yersinia enterocolitica, Campylobacterjejuni, Salmonella typhosa, and Yersinia pseudotuberculosis are the causes of acute infectious terminal ileitis, while Crohn's disease or regional enteritis is the most frequent inflammatory bowel disease in children. The sonographic findings of Crohn's disease and acute terminal ileitis are similar and include partially compressible, concentrically thickened bowel wall. Wall thickness ranges between 5 and 14 mm (normal is < 3 mm) [Figure 8].[26,27,28,29] On short axis views, it has a target or bull's-eye appearance, representing the central echogenic mucosa and surrounding hypoechoic wall. Peristalsis is usually present, but it is often diminished. Color Doppler imaging shows increased color signal in the mucosa, submucosa, and/or the bowel wall. Associated findings include hyperechoic mesenteric fat and enlarged mesenteric lymph nodes.
Figure 8(A-C).
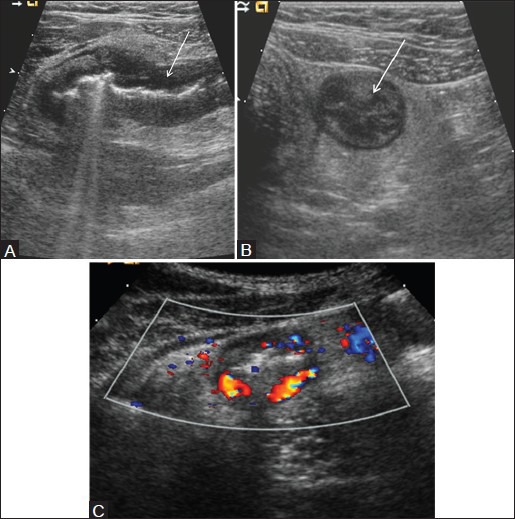
Yersinia enterocolitis. (A) Longitudinal and (B) axial scan of the right lower quadrant show abnormal ileum with thickened hypoechoic bowel wall (arrows) surrounding central echogenic mucosa. The bowel lumen is collapsed. (C) Color Doppler image shows hyperemic bowel wall
Vasculitis
Henoch–Schönlein purpura is an idiopathic systemic vasculitis characterized by purpura, abdominal pain, arthralgia, and sometimes nephritis. The common sites of bowel involvement are the proximal jejunum and distal ileum. Abdominal pain can precede the onset of the skin lesions and mimic acute appendicitis. Sonography shows circumferential bowel wall thickening, usually ranging between 5 and 8 mm in diameter [Figure 9].[29,30,31] Color flow imaging shows hypervascular bowel wall. Intussusception is an occasional complication[32]
Figure 9.
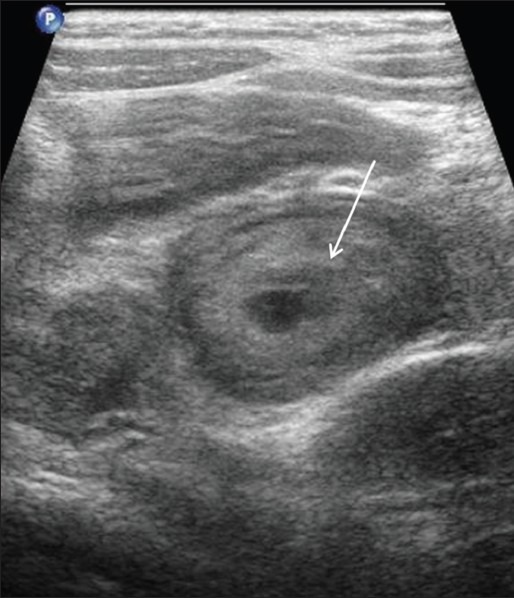
Henoch-Schönlein purpura: Long axis of the terminal ileum show thickened echogenic small bowel wall (arrow)
Meckel's diverticulum
The omphalomesenteric duct is an in utero structure connecting the midgut at the level of the ileum to the yolk sac.[33] It usually involutes in the first trimester. Meckel's diverticulum develops when the ileal end of the duct remains open. Patients may present with abdominal pain due to inflammation or intussusception with the diverticulum as the lead point (see discussion below). At sonography, the inflamed Meckel's diverticulum appears as a hypoechoic tubular or cyst-like structure on the antimesenteric border of the distal ileum [Figure 10].[34] Doppler sonography reveals hyperemia of the wall.[35] This appearance can mimic that of appendicitis, but an origin from small bowel rather than cecum should suggest the diagnosis of Meckel's diverticulum.
Figure 10(A, B).
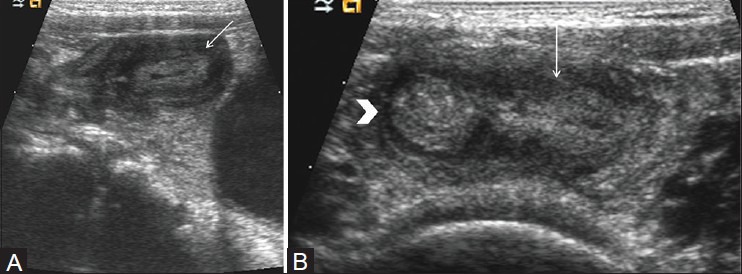
Meckel's diverticulum. (A) Longitudinal view of right lower quadrant shows oval structure (arrow) with central echogenic mucosa and peripheral hypoechoic muscle. (B) Longitudinal view of right lower quadrant shows connection (arrow) of this oval hypoechoic structure with distal ileum (arrow head) Normal appendix was seen separate from the lesion. On surgery, inflamed Meckel's diverticulum was identified
Intussusception
Intussusception is one of the most common causes of acute abdominal pain in early childhood, typically affecting children between 3 months and 2 years of age. Approximately 90% of intussusceptions are ileocolic; the rest are ileoileocolic, colocolic, or ileoileal. More than 90% of intussusceptions have no pathologic lead point and are believed to be due to inflammation of Peyer patches (lymphoid tissue in the ileum). Meckel's diverticulum, intestinal polyps, enteric duplication cysts, intramural hematoma, and lymphoma may also cause intussusception.
The graded-compression sonographic technique is utilized for investigation of intussusception. When viewed in cross-section, the intussusception has a bull's-eye or target appearance.[36,37] In the transverse plane, the intussusception appears as a complex mass with an echogenic center surrounded by a variable number of alternating, concentric, hypoechoic, and hyperechoic rings (target or doughnut sign). On long axis images, the intussusception often has a reniform shape (the pseudokidney sign) [Figure 11]. Pathologic lead points appear as intraluminal cystic or soft tissue masses. The presence of blood flow suggests viable bowel, while the absence of blood flow suggests that gangrenous changes have occurred.[38]
Figure 11(A-C).
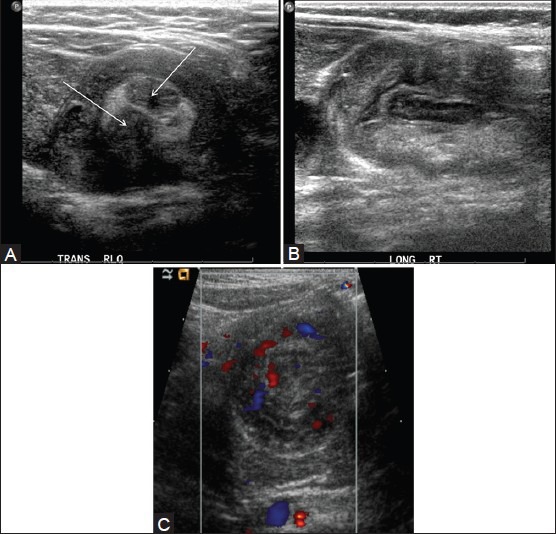
Intussusception. (A) Transverse view through the right mid-abdomen demonstrates a soft-tissue mass. Note the hypoechoic outer rim representing the receiver loop, or intussuscipiens. The central region, or intussusceptum, has loops of bowel and echogenic mesenteric fat and two lymph nodes (arrows). (B) Longitudinal view shows a reniform shape of the intussuscipiens. (C) Transverse color doppler image shows normal vascularity in the intestinal wall in intussusceptum and intussuscipiens
Acute Gynecologic Conditions
The common gynecologic causes of acute right lower quadrant pain mimicking appendicitis are ovarian cysts and adnexal torsion.[7,39]
Functional ovarian cysts
Functional ovarian cysts are found in pubertal girls and result when a Graafian follicle continues to grow after failed ovulation or when a corpus luteum fails to involute after ovulation. The distinction between a functional or pathologic cyst and a normal physiologic follicle is based on size, with cysts larger than 3cm considered pathologic. Functional cysts are often discovered incidentally on pelvic sonograms performed for other reasons, but large ones may cause pain related to compression of adjacent structures. Acute pain can follow cyst hemorrhage, rupture, or torsion.[7,39]
Uncomplicated non-hemorrhagic cysts are anechoic, thin-walled, unilocular masses (>3 cm in diameter) exhibiting posterior acoustic enhancement [Figure 12]. Hemorrhagic ovarian cysts appear as complex masses containing low-level echogenic internal debris and septations, which can produce a fishnet weave or reticular appearance [Figure 13].[40] Other findings include a fluid-debris level, clotted blood products, a thick wall, and a small amount of cul-de-sac fluid. Larger amounts of echogenic peritoneal fluid may be seen if there has been cyst rupture. Hemorrhagic and non-hemorrhagic cysts are both avascular on color Doppler sonography.[41]
Figure 12.
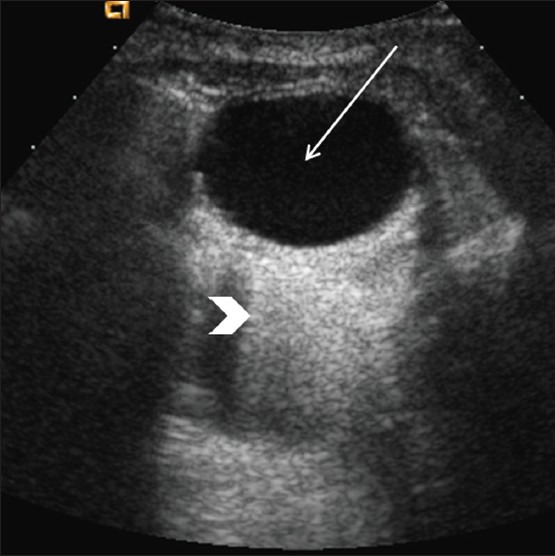
Simple ovarian cyst. Longitudinal image through the pelvis show a well-defined, unilocular, anechoic lesion (arrow) with posterior acoustic enhancement (arrowhead)
Figure 13.
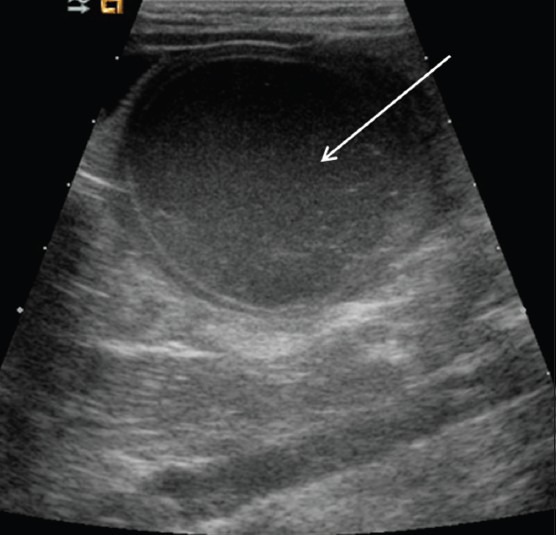
Hemorrhagic ovarian cyst. Longitudinal view of the pelvis demonstrates a large cystic mass with internal debris (arrow)
Most ovarian cysts undergo spontaneous resolution within one to two menstrual cycles. The likelihood of resolution is higher if the cyst diameter is < 5-6 cm. Hence, cysts over 5cm are generally followed until resolution with sonography. The arbitrary time period between studies is 1-2 weeks after the start of the next menstrual cycle. Cysts that persist for several cycles may need to be investigated further with CT, magnetic resonance imaging (MRI), or percutaneous or surgical aspiration to exclude a cystic neoplasm.[42]
Adnexal torsion
Adnexal torsion involves both the ovary and fallopian tube and is the result of partial or complete rotation of the ovary on its vascular pedicle.[43] Torsion is a surgical emergency as prolonged compromise of the arterial blood supply to the ovary will lead to hemorrhagic infarction. Although torsion can occur in all pediatric age groups, it is more common in adolescents. Torsion can occur in the normal adnexa or in association with an underlying mass, which acts as a fulcrum. Neonates and infants are more likely than adolescent girls to have underlying lesions. The explanation for torsion of a normal adnexum is excessive mobility secondary to lax supporting ligaments. Adnexal torsion is nearly always unilateral. Bilateral torsion is asynchronous.[44]
The sonographic findings of torsion of the normal adnexum are an enlarged hypoechoic ovary with multiple small peripheral cysts (follicles) and good sound transmission [Figure 14].[43,45,46,47,48] The positive predictive value of multifollicular enlargement is 87.5% and the specificity is 93.3%.[48] The torsed ovary often assumes a midline position, either behind the bladder or cephalad to the uterus. Associated masses are usually large (>4 cm) and may be cystic or solid.[43] Other features of adnexal torsion are a twisted vascular pedicle, ipsilateral deviation of the uterus to the side of the torsion, and cul-de-sac fluid.[7,43,47,49]
Figure 14(A and B).
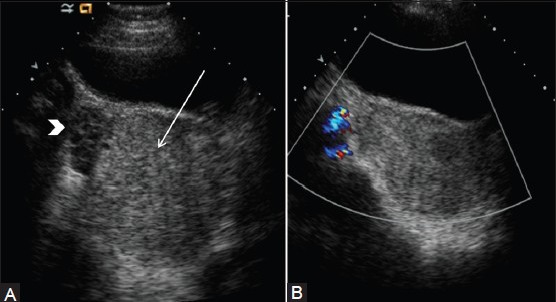
Adnexal torsion. (A) Transverse sonogram in a 13-year-old girl shows an enlarged right ovary (arrow) with acoustic enhancement. Notice peripheral follicles in enlarged ovary (arrowhead) (B) Color flow Doppler image shows the enlarged right ovary with no demonstrable flow. An infarcted hemorrhagic adnexum with 360° torsion of the fallopian tube was noted at surgery
Color Doppler sonography can be helpful in diagnosis by demonstrating absence of ovarian blood flow.[41] Occasionally, however, arterial flow can be noted in the torsed ovary or the twisted vascular pedicle,[41,50,51,52] reflecting the dual blood supply from uterine and ovarian arteries. Gray-scale sonographic features always need to be considered in combination with color Doppler findings. If the gray-scale images are typical of torsion, regardless of the color flow pattern, the diagnosis of torsion should be made. The diagnosis of early ovarian torsion may be difficult by any imaging modality.
Conclusion
Sonography plays an important role in the evaluation of children with acute abdominal pain. The diagnosis of acute right lower quandrant pain in a pediatric population is challenging and requires awareness of the sonographic findings of acute appendicitis and its imaging mimics. A negative sonogram in patients with appendicits does not exclude the possiblity of other diseases, and a complete examination of the pelvis and upper abdomen should be performed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Carty HM. Paediatric emergencies: Non-traumatic abdominal emergencies. Eur Radiol. 2002;12:2835–48. doi: 10.1007/s00330-002-1499-7. [DOI] [PubMed] [Google Scholar]
- 2.John SD. Imaging of acute abdominal emergencies in infants and children. Curr Probl Diagn Radiol. 2000;29:141–79. doi: 10.1016/s0363-0188(00)90011-2. [DOI] [PubMed] [Google Scholar]
- 3.Siegel M. Gastrointestinal tract. In: Siegel MJ, editor. Pediatric Sonography. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2010. pp. 339–83. [Google Scholar]
- 4.Vasavada P. Ultrasound evaluation of acute abdominal emergencies in infants and children. Radiol Clin North Am. 2004;42:445–56. doi: 10.1016/j.rcl.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Puylaert JB. Acute appendicitis: US evaluation using graded compression. Radiology. 1986;158:355–60. doi: 10.1148/radiology.158.2.2934762. [DOI] [PubMed] [Google Scholar]
- 6.Soda K, Nemoto K, Yoshizawa S, Hibiki T, Shizuya K, Konishi F. Detection of pinpoint tenderness on the appendix under ultrasonography is useful to confirm acute appendicitis. Arch Surg. 2002;136:1136–40. doi: 10.1001/archsurg.136.10.1136. [DOI] [PubMed] [Google Scholar]
- 7.Siegel M. Female pelvis. In: Siegel MJ, editor. Pediatric Sonography. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2010. pp. 509–53. [Google Scholar]
- 8.Sivit CJ, Siegel MJ, Applegate KE, Newman KD. When appendicitis is suspected in children. Radiographics. 2001;21:247–62. doi: 10.1148/radiographics.21.1.g01ja17247. [DOI] [PubMed] [Google Scholar]
- 9.Sivit CJ. Imaging the child with right lower quadrant pain and suspected appendicitis: current concepts. Pediatr Radiol. 2004;34:447–53. doi: 10.1007/s00247-004-1179-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko K, Tsudo M. Ultrasound-based decision making in the treatment of acute appendicitis in children. J Pediatr Surg. 2004;39:1316–20. doi: 10.1016/j.jpedsurg.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Smink DS, Finkelstein JA, Garcia Peña BM, Shannon MW, Taylor GA, Fishman SJ. Diagnosis of acute appendicitis in children using a clinical practice guideline. J Pediatr Surg. 2004;39:458–63. doi: 10.1016/j.jpedsurg.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Wiersma F, Sramek A, Hoscher HC. US features of the normal appendix and surrounding area in children. Radiology. 1995;235:1018–22. doi: 10.1148/radiol.2353040086. [DOI] [PubMed] [Google Scholar]
- 13.Rettenbacher T, Hollerweger A, Macheiner P, Rettenbacher L, Frass R, Schneider B, et al. Presence of absence of gas in the appendix: Additional criteria to rule out or confirm acute appendicitis – evaluation with US. Radiology. 2000;214:183–7. doi: 10.1148/radiology.214.1.r00ja20183. [DOI] [PubMed] [Google Scholar]
- 14.Baldisserotto M, Peletti AB. Is colour Doppler sonography a good method to differentiate normal and abnormal appendices in children? Clin Radiol. 2007;62:365–9. doi: 10.1016/j.crad.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Quillin SP, Siegel MJ. Appendicitis in children: Color doppler sonography. Radiology. 1992;184:745–7. doi: 10.1148/radiology.184.3.1509060. [DOI] [PubMed] [Google Scholar]
- 16.Lowe LH, Penney MW, Scheker LE, Perez R, Jr, Stein SM, Heller RM, et al. Appendicolith revealed on CT in children with suspected appendicitis: How specific is it in the diagnosis ofappendicitis? AJR Am J Roentgenol. 2000t;175:981–4. doi: 10.2214/ajr.175.4.1750981. [DOI] [PubMed] [Google Scholar]
- 17.Quillin SP, Siegel MJ, Coffin CM. Acute appendicitis in children: Value of sonography in detecting perforation. AJR Am J Roentgenol. 1992;159:1265–8. doi: 10.2214/ajr.159.6.1442398. [DOI] [PubMed] [Google Scholar]
- 18.Quillin SP, Siegel MJ. Appendicitis: Efficacy of color Doppler sonography. Radiology. 1994;191:557–60. doi: 10.1148/radiology.191.2.8153340. [DOI] [PubMed] [Google Scholar]
- 19.Sivit CJ, Applegate KE, Stallion A, Dudgeon DL, Salvator A, Schluchter M, et al. Imaging evaluation of suspected appendicitis in a pediatric population: Effectiveness of sonography versus CT. AJR Am J Roentgenol. 2000;175:977–80. doi: 10.2214/ajr.175.4.1750977. [DOI] [PubMed] [Google Scholar]
- 20.Applegate KE, Sivit CJ, Salvator AE, Borisa VJ, Dudgeon DL, Stallion AE, et al. Effect of cross-sectional imaging on negative appendectomy and perforation rates in children. Radiology. 2001;220:103–7. doi: 10.1148/radiology.220.1.r01jl17103. [DOI] [PubMed] [Google Scholar]
- 21.Siegel MJ, Carel C, Surratt S. Ultrasonography of acute abdominal pain in children. JAMA. 1991;266:1987–9. [PubMed] [Google Scholar]
- 22.Hernandez JA, Swischuk LE, Angel CA, Chung D, Chandler R, Lee S. Imaging of acute appendicitis: US as the primary imaging modality. Pediatr Radiol. 2005;35:392–5. doi: 10.1007/s00247-004-1372-8. [DOI] [PubMed] [Google Scholar]
- 23.Sung TC, Allahan MJ, Taylor GA. Clinical and imaging mimickers of acute appendicitis in the pediatric population. AJR Am J Roentgenol. 2006;186:67–74. doi: 10.2214/AJR.04.1380. [DOI] [PubMed] [Google Scholar]
- 24.Sivit CJ, Newman KD, Chandra RS. Visualization of enlarged mesenteric lymph nodes at US examination: Clinical significance. Pediatr Radiol. 1993;23:471–5. doi: 10.1007/BF02012457. [DOI] [PubMed] [Google Scholar]
- 25.Puylaert JB, van der Zant FM. Mesenteric lymphadenitis or appendicitis? AJR Am J Roentgenol. 1995;165:490. doi: 10.2214/ajr.165.2.7618596. [DOI] [PubMed] [Google Scholar]
- 26.Alison M, Kheniche A, Azoulay R, Roche S, Sebag G, Belarbi N. Ultrasonography of Crohn disease in children. Pediatr Radiol. 2007;37:1071–82. doi: 10.1007/s00247-007-0559-1. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto T, Iida M, Sakai T, Kimura Y, Fujishima M. Yersinia terminal ileitis: Sonographic findings in eight patients. AJR Am J Roentgenol. 1991;156:965–7. doi: 10.2214/ajr.156.5.2017961. [DOI] [PubMed] [Google Scholar]
- 28.Ruess L, Blask AR, Bulas DI, Mohan P, Bader A, Latimer JS, et al. Inflammatory bowel disease in children and young adults: Correlation of sonographic and clinical parameters during treatment. AJR Am J Roentgenol. 2000;175:79–84. doi: 10.2214/ajr.175.1.1750079. [DOI] [PubMed] [Google Scholar]
- 29.Siegel MJ, Friedland JA, Hildebolt CF. Bowel wall thickening in children: Differentiation with US. Radiology. 1997;203:631–5. doi: 10.1148/radiology.203.3.9169680. [DOI] [PubMed] [Google Scholar]
- 30.Bömelburg T, Claasen U, vonLengerke HJ. Intestinal ultrasonographic findings in Schönlein-Henoch syndrome. Eur J Pediatr. 1991;150:158–60. doi: 10.1007/BF01963556. [DOI] [PubMed] [Google Scholar]
- 31.Couture A, Veyrac C, Baud C, Galifer RB, Armelin I. Evaluation of abdominal pain in Henoch-Schonlein syndrome by high frequency ultrasound. Pediatr Radiol. 1992;22:12–7. doi: 10.1007/BF02011602. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy HJ, Tizard EJ. Clinical practice: Diagnosis and management of Henoch-Schönlein purpura. Eur J Pediatr. 2010;169:643–50. doi: 10.1007/s00431-009-1101-2. [DOI] [PubMed] [Google Scholar]
- 33.Levy AD, Hobbs CM. Meckel's diverticulum: Radiologic features with pathologic correlation. Radiographics. 2004;24:565–87. doi: 10.1148/rg.242035187. [DOI] [PubMed] [Google Scholar]
- 34.Baldisserotto M, Mffazzoni DR, Dora MD. Sonographic findings of Meckel's diverticulitis in children. AJR Am J Roentgenol. 2003;180:425–8. doi: 10.2214/ajr.180.2.1800425. [DOI] [PubMed] [Google Scholar]
- 35.Baldisserotto M. Color Doppler sonographic findings of inflamed and perforated Meckel's diverticulum. J Ultrasound Med. 2004;23:843–8. doi: 10.7863/jum.2004.23.6.843. [DOI] [PubMed] [Google Scholar]
- 36.Beasley S. Intussusception. Pediatr Radiol. 2004;34:302–4. doi: 10.1007/s00247-003-1074-7. [DOI] [PubMed] [Google Scholar]
- 37.Daneman A, Navarro O. Intussusception. Part I: A review of diagnostic approaches. Pediatr Radiol. 2003;33:79–85. doi: 10.1007/s00247-002-0832-2. [DOI] [PubMed] [Google Scholar]
- 38.Lim HK, Bae SH, Lee KH, Seo GS, Yoon GS. Assessment of reducibility of ileocolic intussusception in children: Usefulness of color Doppler sonography. Radiology. 1994;191:781–5. doi: 10.1148/radiology.191.3.8184064. [DOI] [PubMed] [Google Scholar]
- 39.Garel L, Dubois J, Grignon A, Filiatrault D, Van Vliet G. US of the pediatric female pelvis: A clinical perspective. Radiographics. 2001;21:1393–407. doi: 10.1148/radiographics.21.6.g01nv041393. [DOI] [PubMed] [Google Scholar]
- 40.Jain KA. Sonographic spectrum of hemorrhagic ovarian cysts. J Ultrasound Med. 2002;21:879–86. doi: 10.7863/jum.2002.21.8.879. [DOI] [PubMed] [Google Scholar]
- 41.Quillan SP, Siegel MJ. Transabdominal color Doppler ultrasonography of the painful adolescent ovary. J Ultrasound Med. 1994;13:549–55. doi: 10.7863/jum.1994.13.7.549. [DOI] [PubMed] [Google Scholar]
- 42.Helmrath MA, Shin CE, Warner BW. Ovarian cysts in the pediatric population. Semin Pediatr Surg. 1998;7:19–28. doi: 10.1016/s1055-8586(98)70002-2. [DOI] [PubMed] [Google Scholar]
- 43.Chang HC, Bhatt S, Dogra VS. Pearls and pitfalls in diagnosis of ovarian torsion. Radiographics. 2008;28:1355–68. doi: 10.1148/rg.285075130. [DOI] [PubMed] [Google Scholar]
- 44.Kurtoglu E, Kokcu A, Danaci M. Asynchronous Bilateral Ovarian Torsion. A Case Report and Mini Review. J Pediatr Adolesc Gynecol. 2013;26:S1083–3188. doi: 10.1016/j.jpag.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Linam LE, Darolia R, Naffaa LN, Breech LL, O’hara SM, Hillard PJ. US finding of adnexal torsion in children and adolescents: Size really does matter. Pediatr Radiol. 2007;37:1013–9. doi: 10.1007/s00247-007-0599-6. [DOI] [PubMed] [Google Scholar]
- 46.Stark JE, Siegel MJ. Ovarian torsion in prepubertal and pubertal girls: Sonographic findings. AJR Am J Roentgenol. 1994;163:1479–82. doi: 10.2214/ajr.163.6.7992751. [DOI] [PubMed] [Google Scholar]
- 47.Servaes S, Zurakowski D, Laufer MR, Feins N, Chow JS. Sonographic findings of ovarian torsion in children. Pediatr Radiol. 2007;37:446–51. doi: 10.1007/s00247-007-0429-x. [DOI] [PubMed] [Google Scholar]
- 48.Graif M, Itzhak Y. Sonographic evaluation of ovarian torsion in childhood and adolescence. AJR Am J Roentgenol. 1988;150:647–9. doi: 10.2214/ajr.150.3.647. [DOI] [PubMed] [Google Scholar]
- 49.Harmon JC, Binkovitz LA, Stephens J. Uterine position in adnexal torsion: specificity and sensitivity of ipsilateral deviation of the uterus. Pediatr Radiol. 2009;39:354–8. doi: 10.1007/s00247-009-1172-2. [DOI] [PubMed] [Google Scholar]
- 50.Hurh PJ, Meyer JS, Shaaban A. Ultrasound of a torsed ovary: Characteristic gray-scale appearance despite normal arterial and venous flow on Doppler. Pediatr Radiol. 2002;32:586–8. doi: 10.1007/s00247-002-0739-y. [DOI] [PubMed] [Google Scholar]
- 51.Fleischer AC, Stein SM, Cullinan JA, Warner MA. Color Doppler sonography of adnexal torsion. J Ultrasound Med. 1995;14:523–8. doi: 10.7863/jum.1995.14.7.523. [DOI] [PubMed] [Google Scholar]
- 52.Vijayaraghavan SB. Sonographic whirlpool sign in ovarian torsion. J Ultrasound Med. 2004;23:1643–9. doi: 10.7863/jum.2004.23.12.1643. [DOI] [PubMed] [Google Scholar]


