Abstract
Melorheostosis is an uncommon mesenchymal dysplasia that rarely affects the axial skeleton. We describe two atypical cases of melorheostosis with classical imaging findings – the first one involving the cervico-dorsal spine with encroachment of left vertebral artery canal causing attenuation of the left vertebral artery and the second one of mixed sclerosing bony dysplasia (monomelic involvement coexisting with osteopoikilosis).
Keywords: Hyperostosis, melorheostosis, osteopoikilosis, sclerosis
Introduction
Melorheostosis, also known as Leri's disease and first reported by Leri and Joanny in 1922,[1] is a rare, nonhereditary, and benign sclerosing mesenchymal dysplasia. The age of presentation is variable; however, in most of the cases, it manifests by 20 years of age. This bony dysplasia has a classical imaging appearance and is characterized by irregular and wavy hyperostosis also referred to as “flowing candle wax” appearance. Appendicular skeleton is more commonly involved as compared to axial skeleton. The majority of cases described in the literature suggest that involvement of the lower extremity is more common. We highlight two rare cases of melorheostosis- the first one involving the cervico-dorsal spine and ribs compressing the left vertebral artery and the second one of mixed sclerosing bone dystrophy, i.e. melorheostosis coexisting with osteopoikilosis.
Case Reports
Case 1
An 18-year-old male presented with the complaint of giddiness on and off since 2 years. He also complained of having pain in the left side of neck and over the left shoulder since 2 years, which was of insidious onset and was progressively increasing. On physical examination, there was a midline swelling at the back of the neck. No neurological deficit was noted. No other deformity was seen. Laboratory investigations revealed serum calcium, phosphate, and alkaline phosphatase to be within normal limits. Patient underwent radiograph of the cervical spine which showed flowing hyperostosis involving contiguous vertebral segments limited to left side of the skeleton [Figure 1]. Computed tomography (CT) of cervico-dorsal spine showed narrowing of the spinal canal, neural foraminae, and foramen transversarium by hyperostotic bone [Figure 2]. Magnetic resonance imaging (MRI) done subsequently revealed the spinal cord to be displaced to the contralateral side without any signal change, neural foraminal narrowing, and vertebral artery attenuation on the ipsilateral side [Figure 3]. Patient also underwent technetium-99m-MDP bone scan which showed asymmetrical hyperactivity involving contiguous vertebrae and ribs [Figure 4].
Figure 1(A-C).
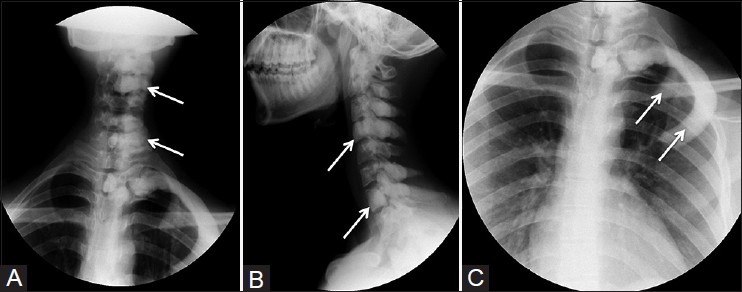
Radiographs of cervical spine AP and lateral views (A and B) showing flowing hyperostosis involving cervical and upper thoracic vertebrae (arrows).(C) Focused radiograph showing hyperostosis involving upper two ribs (arrows) on the left side
Figure 2(A-C).
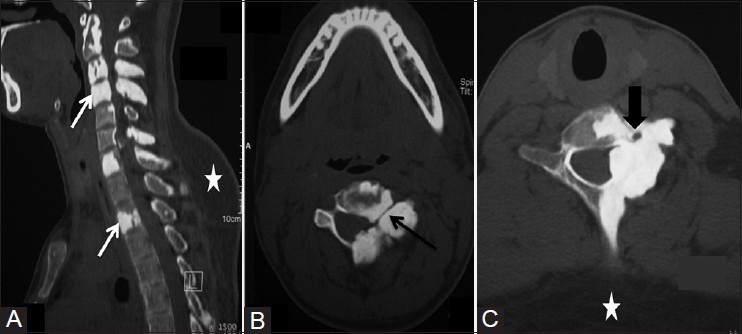
(A) Sagittal reformatted CT scan image showing hyperostosis involving cervical and dorsal vertebrae. (B and C) Axial CT scan images showing narrowing of spinal canal, neural foraminae (black arrow), and left foramen transversarium (arrowhead). Incidental note is made of lipoma (asterisk) at back
Figure 3(A-C).
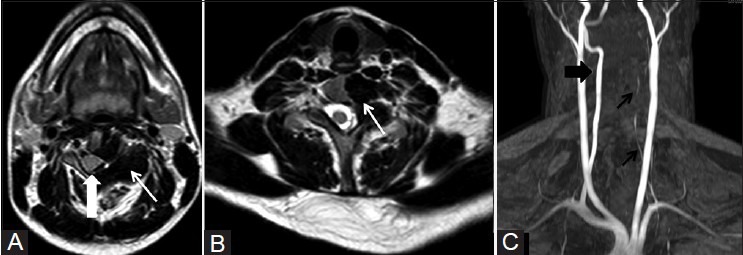
(A and B) Axial T2W MR images showing cortical hyperostosis as hypointense signal (arrow) involving left-sided hemivertebrae causing displacement of the spinal cord to the right (arrowhead) and narrowing ofleft neural foramina (arrow). (C) MR angiogram time-of-flight (TOF) image showing attenuated left vertebral artery (black arrows) and normal right vertebral artery (arrowhead)
Figure 4.
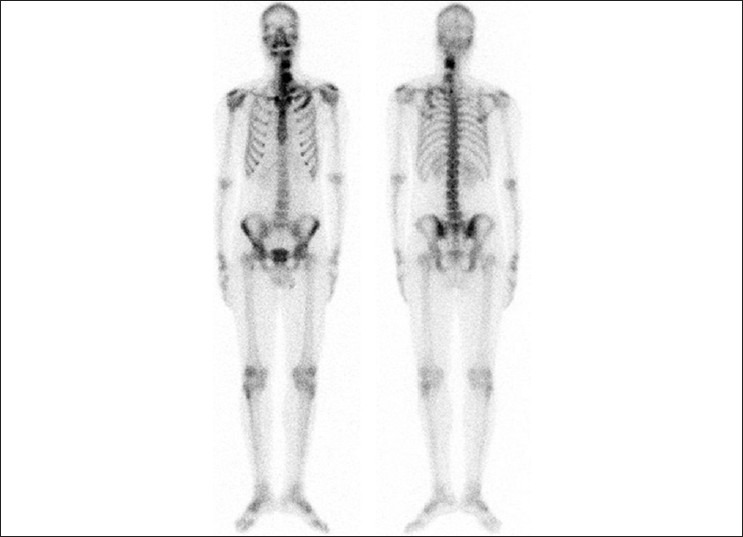
Technetium-99m-MDP bone scan image showing increased uptake in the contiguous vertebrae and ribs
Case 2
A 30-year-old female presented with pain in left hip, limping, and stiffness of left lower limb since 8 months. Radiographs of the pelvis and left leg showed cortical hyperostosis involving the left iliac bone extending and involving left femur, knee crossing the intervening joint, and also involving the left tibia and tarsal bones. Gross osteoarthritic changes were seen in left hip joint with downward tilting of left hemi-pelvis. In addition, multiple bone islands were seen in left femur, tibia, and patella on the left side suggestive of coexisting osteopoikilosis [Figure 5]. CT was also done which confirmed the findings [Figure 6].
Figure 5(A-D).
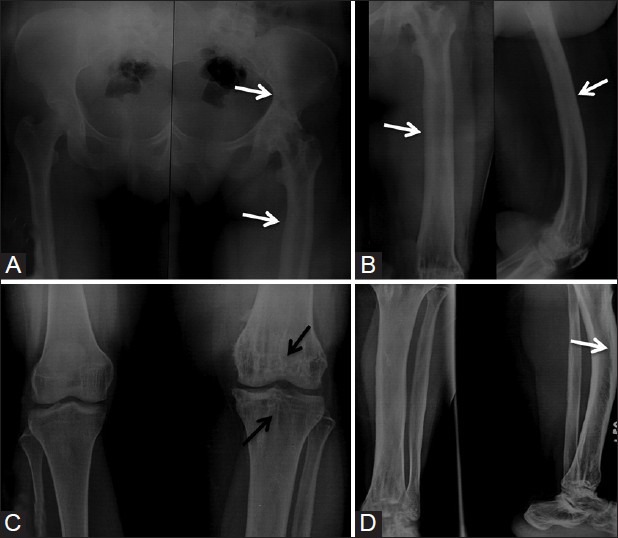
Radiographs of pelvis, femur, knee joint, and leg showing cortical hyperostosis (white arrows) extending from left iliac bone, left femur, and left knee, crossing the knee joint, and involving left tibia and tarsal bones (white arrows). Multiple bone islands (black arrows) are seen in left femur and tibia suggestive of osteopoikilosis
Figure 6(A-C).
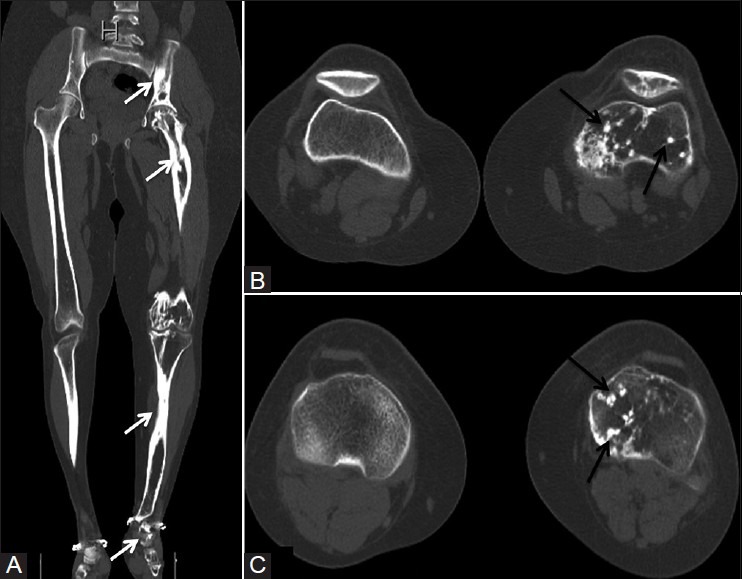
(A) Coronal reformatted CT scan image showing flowing cortical hyperostosis (white arrows) involving the left limb. (B and C) Axial CT scan images depicting multiple bone islands (black arrows) seen in femur, tibia, and patella
Discussion
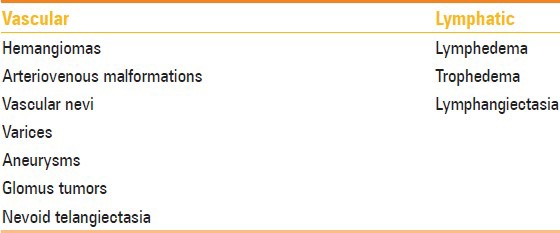
The word melorheostosis is derived from the Greek words, “melos” which means limb and “rhein” which means flow.[2] It is a mixed sclerosing dysplasia with disturbance of both intramembranous (predominantly) and endochondral ossification. It has an incidence of 0.9 per million with no sex predilection. This rare sclerosing dysplasia has a variable age of presentation, ranging from 2 to 64 years. However, majority of the patients present by 20 years of age.[3] Clinically, patients may present with painless asymmetric joint contracture, painful limb swelling, or restricted range of movements. The genetic basis of this entity is the loss of function mutation in LEMD3 gene, which encodes an inner nuclear membrane protein.[4] This disorder tends to be segmental and has a sclerotomal distribution. One side of the skeleton may only be involved and may affect only one bone (monostotic), multiple bones (polyostotic) on same side of skeleton, and rarely one limb (monomelic). There may be also generalized skeletal involvement. Involvement of the axial skeleton has been reported sporadically. Involvement of axial skeleton (skull, vertebral column, ribs, and facial bones) is very rare and is described in the form of case reports. Usually, spinal involvement is asymptomatic. Review of literature reveals symptomatic cases may present with scoliosis, stiffness, back pain, progressive myelopathy, radiculopathy, giddiness, and symptoms of vertebrobasilar insufficiency.[5,6,7,8,9,10] Soft tissue abnormalities consisting of osseous, chondroid, vascular, and fibrocartilaginous tissue have been reported in 76% of cases of melorheostosis. Vascular and lymphatic malformations associated with melorheostosis have been enlisted in Table 1.[11,12] Other associations of melorheostosis are neurofibromatosis, tuberous sclerosis, scleroderma, tricho-dento-osseous syndrome, rheumatoid arthritis, and hypophosphatemic rickets. Rarely, overlap syndromes with osteopoikilosis and osteopathia striata are also seen. Recently, Hellemans et al.[4] have demonstrated mutation on chromosome 12q that is responsible for osteopoikilosis, melorheostosis, and Buschke–Ollendorff syndrome.
Table 1.
Vascular and lymphatic lesions associated with melorheostosis
On imaging, five patterns have been described – classical, osteoma-like, myositis ossificans-like, osteopathia striata-like, and mixed type. Radiographs show asymmetric, irregular, linear bands of increased sclerosis, often described as molten wax dripping down from one side of a candle, involving the outer bony cortex. There is usually a distinct demarcation between the affected bone and the normal bone. Diaphyseal involvement is more common. CT scan reveals its clear demarcation from the normal bone more effectively. MRI shows decreased signal intensity localized to the affected bone on all pulse sequences. CT and MRI are more useful for assessment of lesions involving the axial skeleton. MRI also allows assessment of mineralized and nonmineralized soft tissue masses associated with this condition. Radionuclide bone scanning is helpful to distinguish a focus of melorheostosis from other lesions. In melorheostosis, focal increased uptake is seen due to increased osteoblastic activity, local hyperemia, immature collagen, and changes in capillary permeability.[13,14,15]
Histologic analysis shows variable degrees of cortical thickening consisting of chondroid islands surrounded by mature lamellar and woven bone, as well adjacent zones of fibrocartilage with irregular surface fibrillation.[11,16] Biochemical investigations reveal normal serum calcium, phosphorus, and alkaline phosphatase levels. Laboratory abnormalities affected in melorheostosis include osteoblastic specific factor-2 (osf-2), osteonectin, fibronectin, transforming growth factor-β (TGF-β), and fibroblast growth factor-23 (FGF-23).[11]
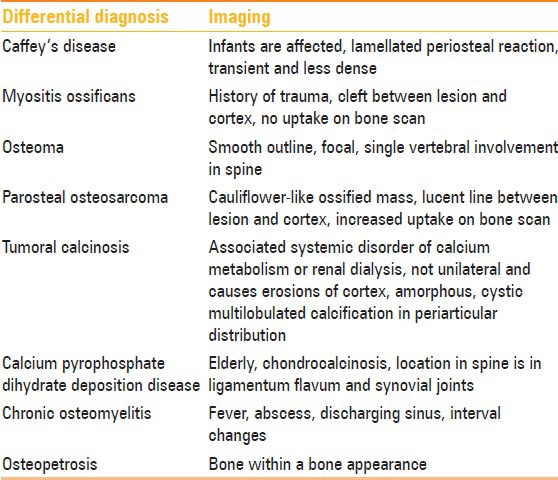
On imaging, differential diagnoses of melorheostosis include myositis ossificans, osteopetrosis, chronic osteomyelitis, parosteal and periosteal osteosarcoma, calcium pyrophosphate dihydrate (CPPD), deposition disease, osteoma, and Caffey's disease. Rare differentials include calcified synovial sarcoma, extraskeletal osteosarcoma, or tumoral calcinosis in view of para-articular calcified masses [Table 2].
Table 2.
Radiological differential diagnosis of melorheostosis
Management and prognosis of a patient with melorheostosis varies depending on age, extent, anatomic location, and associated soft tissue changes. The condition is not fatal, but morbidity is considerable. Treatment is mainly symptomatic. Recurrence after operative excision is seen. Primary aims of treatment are pain relief and restoration of full range of motion. Medical treatment is recommended to control bone pain. Conservative measures include analgesia, manipulation, braces, serial casting, physiotherapy, nerve blocks, and sympathectomy. Surgical treatment for melorheostosis is considered when symptoms are disabling. Literature shows very few cases being managed surgically. According to Saxena et al.,[10] no surgical treatment is required in osteopoikilosis. Management is considered only when there are complications like fracture.
In our first case, the patient's symptoms were attributed to neurovascular compression and the patient was advised surgical decompression, but the patient refused to undergo surgery. Conservative management with cervical bracing and physiotherapy was given and the patient is on follow-up. Our second case is on regular follow-up with physical medicine and rehabilitation department and is being managed symptomatically.
Conclusion
Melorheostosis is a rare, benign, and disabling condition. Involvement of axial skeleton is rare and coexistence of two bony dysplasias in one patient is even rarer. Knowledge of classical radiological findings and its pattern of involvement can prevent an unwarranted biopsy in these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Leri A, Joanny J. Une affection non de×crite des oshyperostose “encoule×e” surtoute la longeur d’un member ou “melorhe×ostose”. Bull Memoires Soc Med Hopitaux Paris. 1992;46:1141–5. [Google Scholar]
- 2.Bansal A. The dripping candle wax sign. Radiology. 2008;246:638–40. doi: 10.1148/radiol.2462050537. [DOI] [PubMed] [Google Scholar]
- 3.Resnick D. 3rd ed. Vol. 6. Philadelphia, PA: WB Saunders; 1995. Diagnosis of bone and joint disorders; pp. 4410–3. [Google Scholar]
- 4.Hellemans J, Preobrazhenska O, Willaert A, Debeer P, Verdonk PC, Costa T, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat Genet. 2004;36:1213–8. doi: 10.1038/ng1453. [DOI] [PubMed] [Google Scholar]
- 5.Kalbermatten NT, Vock P, Rüfenacht D, Anderson SE. Progressive melorheostosis in the peripheral and axial skeleton with associated vascular malformations: Imaging findings over three decades. Skeletal Radiol. 2001;30:48–52. doi: 10.1007/s002560000283. [DOI] [PubMed] [Google Scholar]
- 6.Suresh S, Muthukumar T, Saifuddin A. Classical and unusual imaging appearances of melorheostosis. Clin Radiol. 2010;65:593–600. doi: 10.1016/j.crad.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Zeiller SC, Vaccaro AR, Wimberley DW, Albert TJ, Harrop JS, Hilibrand AS. Severe myelopathy resulting from melorheostosis of the cervicothoracic spine. A case report. J Bone Joint Surg Am. 2005;87:2759–62. doi: 10.2106/JBJS.D.02653. [DOI] [PubMed] [Google Scholar]
- 8.Yoon J, Al Shafai L, Nahal A, Turcotte RE, Martin MH. Melorheostosis of the sacrum causing acute-onset neurological symptoms. Skeletal Radiol. 2011;40:1369–73. doi: 10.1007/s00256-011-1216-1. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy M, Mehdian H, Fairbairn KJ, Stevens A. Melorheostosis of the tenth and eleventh thoracic vertebrae crossing the facet joint: A rare cause of back pain. Skeletal Radiol. 2004;33:283–6. doi: 10.1007/s00256-004-0749-y. [DOI] [PubMed] [Google Scholar]
- 10.Saxena A, Neelakantan A, Jampana R, Sangra M. Melorheostosis causing lumbar radiculopathy: A case report and a review of the literature. Spine J. 2013;13:e27–9. doi: 10.1016/j.spinee.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 11.Jain VK, Arya RK, Bharadwaj M, Kumar S. Melorheostosis: Clinicopathological Features, Diagnosis, and Management. orthopedics. 2009;32:512. doi: 10.3928/01477447-20090527-20. [DOI] [PubMed] [Google Scholar]
- 12.Arbuckle HA, Morelli JG. Pigmentary disorders: Update on neurofibromatosis1 and tuberous sclerosis. Curr Opin Pediatr. 2000;12:354–8. doi: 10.1097/00008480-200008000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Choi IH, Kim JI, Yoo WJ, Chung CY, Cho TJ. Ilizarov treatment for equinoplanovalgus foot deformity caused by melorheostosis. Clin Orthop Relat Res. 2003;414:238–41. doi: 10.1097/01.blo.0000076801.53006.80. [DOI] [PubMed] [Google Scholar]
- 14.Brown RR, Steiner GC, Lehman WB. Melorheostosis: Case report with radiologic-pathologic correlation. Skeletal Radiol. 2000;29:548–52. doi: 10.1007/s002560000255. [DOI] [PubMed] [Google Scholar]
- 15.Motimaya AM, Meyers SP. Melorheostosis involving the cervical and upper thoracic spine: Radiographic, CT, and MR imaging findings. AJNR Am J Neuroradiol. 2006;27:1198–200. [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy M, Mehdian H, Fairbairn KJ, Stevens A. Melorheostosis of the tenth and eleventh thoracic vertebrae crossing the facet joint: A rare cause of back pain. Skeletal Radiol. 2004;33:283–6. doi: 10.1007/s00256-004-0749-y. [DOI] [PubMed] [Google Scholar]


