Abstract
Total Knee Arthroplasty has been shown to be a successful procedure for treating patients with osteoarthritis, and yet approximately 5%-10% of patients experience residual pain, especially in the anterior part of the knee. Many theories have been proposed to explain the etiology of this anterior knee pain (AKP) but, despite improvements having been made, AKP remains a problem. AKP can be described as retropatellar or peripatellar pain, which limits patients in their everyday lives. Patients suffering from AKP experience difficulty in standing up from a chair, walking up and down stairs and riding a bicycle. The question asked was: “How can a ‘perfectly’ placed total knee arthroplasty (TKA) still be painful: what can cause this pain?”. To prevent AKP after TKA it is important to first identify the different anatomical structures that can cause this pain. Greater attention to and understanding of AKP should lead to significant pain relief and greater overall patient satisfaction after TKA. This article is a review of what pain is, how nerve signalling works and what is thought to cause Anterior Knee Pain after a Total Knee Arthroplasty.
Keywords: Anterior knee pain, Patellofemoral pain, Total knee arthroplasty, Malrotation, Homeostasis
Core tip: Total knee arthroplasty (TKA) has been shown to be a successful procedure for treating patients with osteoarthritis, and yet approximately 5%-10% of patients experience anterior knee pain (AKP). To prevent AKP after TKA it is important to first identify the different anatomical structures that can cause this pain. Greater attention to and understanding of AKP should lead to significant pain relief and greater overall patient satisfaction after TKA. This article is a review of what pain is, how nerve signalling works and what is thought to cause AKP after a TKA.
INTRODUCTION
One of the remaining challenges in the field of total knee arthroplasty (TKA) is the elimination of anterior knee pain (AKP). TKA itself has been shown to be a successful procedure for treating patients with osteoarthritis[1], and yet a significant proportion of the patients still experience AKP after surgery. The symptoms of AKP after TKA can be described as retropatellar or peripatellar pain limiting patients in their everyday lives[2]. Patients suffering from AKP experience difficulty in standing up from a chair, walking up and down stairs, and riding a bicycle.
In the early TKA designs the primary goal was resurfacing the medial and lateral compartments and little attention was paid to the patellofemoral joint (PFJ)[3]. In these designs a 40% to 58% rate of patellofemoral pain was identified[4]. Insall et al[5] in 1976 hypothesized that the residual pain was most frequently attributed to the patellar compartment. During the early years of TKA residual AKP was often treated with either a patellectomy and/or soft tissue realignment[5]. It would therefore, seem more appropriate to refer to AKP rather than patellofemoral pain as the general term for pain in the anterior part of knee. The continuing high incidence of postoperative AKP led to the changes in the shape of the patellofemoral part of the TKA, such as modifying the femoral trochlea and placing a patella component[3,5].
The first step in understanding AKP after TKA is to look at a healthy functioning knee. According to the theory of Dye, “the healthy knee can be viewed as a biologic transmission with a complex assemblage of living asymmetrical moving parts whose purpose is to accept, transfer, and ultimately dissipate often high loads generated at the ends of the long mechanical lever arms of the femur, tibia, patella, and fibula”[6]. Furthermore, Dye’s refers to each knee having a unique “envelope of function”; a potential range of activity in which it maintains a homeostasis of all surrounding tissues[6]. The potential range of activity is different for individual knees, whether healthy, arthritic or with a knee arthroplasty. An arthritic knee can be viewed as a transmission with worn bearings, and thus with a limited capacity to accept and transfer loads. In other words, arthritis causes the potential range of activity to become limited, causing pain and restrictions in daily activities such as walking and cycling, and sometimes even pain when at rest.
The fundamental aim of knee arthroplasty or joint replacement surgery is to restore, as far as possible, a normal functioning, pain free knee. Applying Dye’s theory to a TKA patient gives an interesting perspective. A TKA knee can then be viewed as a knee functioning with a combined biologic and artificial transmission with a limited potential range of activity. The limitation is in part due to the use of artificial products containing metals and polyethylene, which are harder and less flexible than the original cartilage and therefore make it unlikely that the knee will return fully to its pre-injury/pre-arthritis state. A pain free knee with a good function can be described in Dye’s terms as functioning in a zone of homeostasis of all knee tissues. The knee that is structurally overloaded, and thus functioning in a zone of abnormal loading (supraphysiological) of knee tissues is clearly no longer functioning in the zone of homeostasis[6]. If this abnormal loading of the knee continues for long periods of time, a TKA can ultimately fail (i.e., it enters the zone of structural failure)[6].
Although numerous design improvements have reduced the incidence of AKP, nevertheless approximately 5%-10% of patients still experience Anterior Knee Pain (AKP)(ranging from 0.4%-49.0%)[1-4,7-24]. Many theories have been proposed to explain the etiology of AKP[1-4,7-25]. In sports medicine it has been observed that some patients can have severe patellofemoral pain while their articular cartilage appears normal, and that patients with severe patellofemoral cartilage damage can be pain free[26]. Even though significant knowledge exists about AKP after TKA, its etiology and pathogenesis are still not fully understood. In this review the most important factors related to the development of AKP after TKA are therefore analysed, starting with how this pain signalling works and what anatomical structures can cause this pain to occur after TKA.
PATHOPHYSIOLOGY AND ETIOLOGY OF PAIN
Pain is a normal manifestation of everyday life and serves as a protective mechanism for the body, causing the individual to react to try to eliminate the pain stimulus[2,27]. However, excessive pain after a TKA can diminish or hinder quality of life[12,21,22,27]. This form of pain typically originates in the peripheral nervous system (PNS)[27].
The PNS consists of all the nerves outside of the brain and the spinal cord[27,28]. It is made up of bundles of axons which are enclosed by connective tissue to maintain the continuity, nourish and protect the axon[28]. Each axon in the PNS can be surrounded by a myelin sheath, called a Schwann cell sheath, and these two together form a nerve fibre[28]. An axon can be myelinated once it reaches a thickness of one or two micro metre[28]. The difference between a myelinated and non-myelinated nerve fibre is the conduction velocity (myelinated is faster)[28]. Nerve fibres can therefore be classified into different types depending on their diameter and conducting velocity[28].
When nerve fibres transmit information from sensory receptors to the central nervous system (CNS) they are called afferent nerves[28]. Afferent nerves are triggered by specialized neural structures called sensory receptors[28]. These receptors are sensitive to a specific form of physical energy[28]. In the joint these are called joint receptors or mechanoreceptors and can be divided into four basic categories: Ruffini endings (stretch), Pacinian corpuscles (pressure and pain), Golgi tendon organ-like endings and Free Nerve Endings (FNE)[2,28]. Pain occurs when tissues are being compressed, damaged or irritated[2]. The perception of pain appears to be activated by pain-specific sensory receptors called nociceptors in a process called nociception[29]. FNE, especially type IV FNEs detect touch, pressure, pain, heat and cold[2].
These FNEs primarily form the basis of the nociceptive system by sending signals to the spinal cord and CNS on pain and inflammation[2,29]. Under normal circumstances FNEs remain inactive, but they become active when subjected to abnormal mechanical deformation, thermal stimuli or special chemical agents[2,29]. Pain signals are transmitted to the CNS by a fast or a slow pathway[2]. Fast pain can be triggered by mechanical and thermal stimuli, but slow pain can be caused by mechanical and thermal stimuli and by chemical agents[2]. Fast pain is transmitted through A fibres (also called A delta fibres, they are myelinated) at velocities estimated to be between 6 m and 100 m per second[2,28]. This is often experienced as a sharp, acute or electric pain[2]. The slow pain is transmitted through C fibres (unmyelinated) with velocities between 0.5 m and 2 m per second and is often described as burning, aching or chronic pain[2,28]. This type of pain is normally present with tissue destruction[2]. Slow pain can occur in the skin and deep tissues whereas fast pain is not felt in the deeper structures[2].
Chemical substances, as mentioned, play an important role in stimulating the slow type of pain[2]. Chemicals that excite pain include histamine (also causes itching), serotonin and bradykinin[2,29]. Bradykinin acts G-protein-linked receptors to produce a range of proinflammatory effects including vasodilatation and edema[2,29]. Prostaglandins and Substance P (SP) enhance the sensitivity of nociceptors but do not directly excite them[2,29]. SP can function as a vasodilator and therefore can produce inflammation[2]. SP is a neuropeptide that can function as a neurotransmitter and is detected and isolated in the lateral retinaculum, the infrapatellar fat pad, synovial membrane, periosteum and subchondral bone of patellae affected with degenerative disease[2,30]. Nerve fibres immunoreactive for SP are not observed in the intact articular cartilage and are present in degenerative erosion of the patella[2]. Examination of subchondral bone, however, shows the presence of SP positive nerve fibres in a degenerative joint.
The problem with pain receptors is that, unlike smell or taste for example, they adapt very little and sometimes not at all[2]. The continuous excitation of nociceptors, therefore, tends to lead to a chronic aching pain[2]. This increase in sensitivity of the nociceptors is called hyperalgesia[2].
Having looked at how nerves transmit pain, the next step is to focus on which nerves are responsible for different parts of the knee joint. The innervation of the knee joint follows Hilton’s law, meaning that all of the motor efferent nerves carry afferent branches from the knee capsule[31,32]. The innervation of the knee joint can be divided into two groups; a posterior and an anterior group[31,32]. The posterior group is made up of branches of the tibial nerve and a terminal branch of the obturator nerve. If necessary, signals from the posterior capsule and cruciate ligaments are transmitted to the CNS[31,32]. The anterior group consists of branches of the femoral, common peroneal and saphenous nerves[31,32]. The femoral nerve divides into the vastus muscles and the anterior medial joint capsule. The saphenous nerve innervates the anterior medial capsule and some sensory branches to the patellar tendon[31,32]. The medial side of the patella is innervated by the nerves of the vastus medialus muscle[2]. The lateral part of the knee is innervated by the nerves of the biceps femoris muscle and the vastus lateralis muscle[2].
The knee is a hinge type of joint and in the Total Knee Arthroplasty (TKA) is made up of the articulations between the femoral and tibial components and between the patella and the patellar surface of the femur, the patellofemoral joint (PFJ). The femur component (metal alloy) articulates with a Polyethylene surface either on a tibial base plate (metal alloy) or a total Polyethylene tibial component. A hinge joint mainly allows for motion in one plane and only slight side to side motion is possible. The femur and tibia are connected together by strong collateral ligaments and depending on the arthroplasty used, sometimes a posterior cruciate ligament.
The complexity of patellofemoral joint and the high number of pain transmitters are of relevance to the issue of whether or not to resurface the patella, which is an area of ongoing controversy in the field of TKA[1,3,4,7,14,17,24,33,34]. If the patella is resurfaced, the articular cartilage is removed and substituted with a polyethylene component. Alternatively, if needed, the patella can be reshaped (denervation, a partial resection of the lateral facet, removal of osteophytes or a combination of the above mentioned). The complexity of the PFJ means it is not only the articulation of the patella on the femoral groove of the femur component (metal alloy). Several muscle as well as ligament forces act on the patella to provide stability and a proper patellar tracking. The patella allows for multidirectional movement, especially cranial and caudal, but it also tilts and even rotates. There are, therefore, numerous points of contact between the undersurface of the patella and the femur component. The PFJ also depends on the synovial plica, infrapatellar fat pad, tendons, retinacula and the capsule[2,26]. The PFJ appears to be very sensitive to pain due to the high number of FNEs in different structures[2]. The highest numbers are found in the quadriceps muscles, with significant numbers also in the retinacula, patellar tendon and synovium[2,26]. Intact hyaline cartilage is completely free of nerve fibres[2,30]. Therefore intact aneural cartilage cannot be a source of pain[2,26]. Dye had an arthroscopic palpation of his patellar cartilage lesion done on his own knee. The operation was performed without intraarticular analgesia. Dye reported experiencing no pain when the patellar cartilage lesion was palpated[26]. However, he did experience severe pain when the synovial plica, infrapatellar fat pad, tendons, retinacula and the capsule were palpated[26].
REGENERATION OF NERVES
After looking at how pain signalling works, the interesting next question for clinicians is: how can these findings be translated into practical daily patient care? With the skin incision in a TKA, nerves are surgically cut, and axons are injured or damaged[35]. The neurons can regenerate a new axon in a process called chromatolysis[35]. The process starts with exudation, cell proliferation and then collagen synthesis[28]. First the gap is filled with blood corpuscles and macrophages and a fibrin clot is formed[28]. This results into an ingrowth of capillaries and fibroblasts. This ingrowth only develops in the proximal stump of the damaged axon by forming sprouts, as the distal part of the axon dies[27,35]. These sprouts grow along the path of the original nerve, if this route is still available[35]. In the distal stump the Schwann cells of the nerves not only survive the wallerian degeneration, but also proliferate and form rows along the course previously taken by the axons[35]. These Schwann cells form nerve growth factor, which attracts the nerve fibres to grow back across the surgical cut[27]. The rate of regeneration is slow and is approximately 1 mm per day in the knee[28,35]. When peripheral nerve regeneration gets blocked in the scar tissue, a neuroma can be formed[27,28].
Another way pain might be transmitted is when nerves around the knee are compressed. The nerve, a soft structure, can be compressed between bone, ligaments or due to swelling (hematoma)[27], but also by a knee arthroplasty or the scar tissue that is formed after a TKA. Dellon describes how, when a nerve is compressed, blood flow to the nerve is reduced[27]. When a nerve does not get enough oxygen, it stops conducting normal signals to the CNS[27]. This can be felt as a “buzzing” or “tingling”feeling[27].
CLINICAL IMPLICATIONS
Assuming that the incapacitating AKP is caused by the activation of FNE, the main interest of this study is to know how they become active and whether this is due to an abnormal mechanical deformation, thermal stimuli or special chemical agent[2,29]. It is known that the structures in and around the PFJ are very sensitive to pain, being full of nociceptors[2,29]. The synovium, lateral retinaculum, infrapatellar fat pad, periosteum and subchondral bone of patellae affected with degenerative disease are all richly supplied with type IVa FNEs and fibres containing Substance P[2].
A key aim of this study was to identify literature evaluating the different determinants of AKP after a TKA was found[2,8,9,12,13,15,18,22,23,25-27,30,34,36-41]. The articles reviewed confirm this study’s hypothesis that anything that alters the patellofemoral joint (PFJ) mechanics can activate these FNEs and thus induce AKP after TKA[22]. It can further be hypothesised that the nociceptive system can be activated by several factors, either alone or in combination: Hoffa impingement, peripatellar synovitis, increased osseous pressure and mechanical changes that alter the PFJ in an abnormal way.
The influence of preoperative AKP does not seem to be predictive in relation to postoperative AKP[8,12,22,24,42]. However, the influence of preoperative AKP does seem to be predictive in relation to postoperative AKP[8,12,22,24,42]. Some researchers investigated the influence of walking on AKP after TKA. A higher knee extension moment in the early midstance phase of walking causes higher forces on the PFJ and consequently a higher frequency and severity of AKP after TKA[42]. Interestingly, the patients that modify and decrease the PFJ loading had less or no AKP[42]. Muscle balance also has an influence on AKP, for instance the preoperative weakness of the vastus medialis muscle was seen to lead to increased activation of the vastus lateralis, and the ensuing lateral maltracking of the patella can lead to AKP after TKA[25,43]. Weakness of the hip adductors was also seen to lead to a dynamic valgus and thus to lateral patella maltracking, which therefore makes it a contributor to AKP after TKA[25].
Moving on to focus on the influence of the knee design, Mahoney et al[44] suggested that the design of the specific TKA can have an influence on PFJ loading. They studied which designs were able to change the posterior flexion extension axis, thereby lengthening the extensor mechanism moment arm, which could decrease the quadriceps’ muscle force and reduce the PFJ forces[44]. The importance of trochlear design was emphasised by Popovic, who found a high number of patients experiencing pain due to an inappropriate trochlear design[40]. Some other researchers have suggested that the posterior stabilized knees have less AKP compared to the cruciate retaining designs, but a recent meta-analysis could not prove this difference (6% AKP in both groups)[45]. Breugem et al[12,13] performed two studies that specifically evaluated whether the mobile bearing knee could reduce AKP compared to the fixed bearing knee. In the short-term follow-up study less AKP in the mobile bearing knees compared to the fixed knees[12] was observed, which seemed to suggest that the mobile bearing knee did have an influence on AKP[12]. Kim et al[46] reported less pain in the mobile bearing group compared to a fixed bearing group at a mean follow-up of 2.6 years. Wohlrab et al[47] found a difference in pain scores at 3 mo favouring the mobile bearing, but found no difference after three and five years. In the 6 to 10 year (median 7.9 years) follow-up study the mobile bearing knees did not sustain the difference in AKP that had been found in the short-term study[12,13]. These results are comparable to the findings of other systematic reviews and/or meta-analysis, most of which have not shown an advantage of using a mobile bearing[22,36,48-51]. Based on these results combined with a recent meta-analysis showing less AKP in the mobile bearing TKA, it seems that the debate can be reopened as to whether the mobile bearing TKA is part of the solution to AKP[52]. Aglietti et al[36] suggested that the performance of a mobile bearing knee might decline over time. Yet, even if this difference is only relevant in the short term, it would still seem advantageous to use a mobile bearing TKA for the benefit of patients that experience less pain in this period. The reason why the mobile bearing TKA seems to make a difference in AKP, could be due to the femur component being highly congruent to the PE bearing this in combination with the self alignment ability of the PE bearing and the fixed tibial base plate, in a knee that is well balanced in flexion and extension thus unloading the PFJ.
The influence of placement of the TKA is another issue. The PFJ loading is influenced both by changes in the joint line height and by selecting a femoral component that is too large[39,53]. Tibia-femoral instability, due to inappropriate placement or other causes, leads to increased pressure on the PFJ, especially in flexion[54]. However, perhaps the most important aspect of placement is the influence of malrotation after TKA[8,9,18,34,55-57]. Berger et al[9] reported that combined component internal rotation is associated with lateral tracking followed by patellar tilting and potential patellar subluxation. Dislocation and component failure were reported when severe malrotation was present[9]. Barrack et al[8] studied the influence of malrotation on AKP and found a combined internal rotation had a relative risk of AKP which was five times higher than cases without combined component internal rotation. It is thought that a femoral component placed in internal rotation shifts and tilts the patella medially and this can have a negative influence on the PFJ[8,9,18,55]. This could be an important reason to explain why secondary resurfacing of the patella does not always solve AKP after TKA[34,58].
Without doubt the most studied aspect of AKP has been the influence of the patella, especially in relation to resurfacing[3,7,14,24,33,34,58-61]. Many studies have focused on patella resurfacing, some demonstrating no difference[4,14,22], some showing better results after resurfacing[1,3,17,24,33], and others advising against resurfacing[7,58,62]. It is known that simply resurfacing the patella is not a universal solution for AKP, although it may solve the problem in selected cases[3,4,7,14,16,58]. A meta analysis of 7 high quality studies showed no advantage for resurfacing the patella with regard to AKP[61]. Interesting publications from the Oxford group show that full thickness cartilage damage of the PFJ is not a contraindication for the placement of the medial Oxford mobile bearing knee arthroplasty, since this does not seem to influence the outcome or the revision rate[63,64]. It is still not clear why in a mobile bearing uni compartmental knee, PFJ arthritis does not seem to play an important role, whereas in a TKA it is a recurring debate. Maybe the influence of the intact anterior cruciate ligament (propriocepsis) is part of the AKP solution. Future research will hopefully provide new insights to these questions.
Finally, although soft tissue irritation is often related to malalignment, the mechanical cause cannot always be defined. Therefore, it may be that soft tissue problems play a significant role in AKP after TKA. Synovial impingement after TKA can play an important role in postoperative pain[65]. Two studies evaluated the influence of denervation of the patella and Van Jonbergen found a difference in favour of circumpatellar electrocautery denervation[23]. One study found a lower prevalence of AKP due to the resection of Hoffa’s fat pad[66]. Other factors contributing to AKP have been proposed; wear[21], referred pain[21,22], patella height[24,39], patellar thickness[67,68] and patella baja (pseudonaja), but these studies could not demonstrate a correlation with AKP after TKA. This is often more a lack of power than proof.
Applying Dye’s theory to patients with AKP after TKA provides useful insights (Figure 1). The zone of homeostasis can then be seen as a zone without AKP. The zone of supraphysiological “overload” can be seen as the zone for abnormal loading of the PFJ and therefore a cause of AKP after TKA[6]. A good example of this is the relationship between malrotation and the severity of experienced AKP[8]. If the components of the TKA are malrotated, the knee tissues are overloaded and thus clearly functioning in a zone of abnormal loading rather than in the zone of homeostasis[6]. If this abnormal loading of the knee continues for long periods of time, a TKA can ultimately fail (i.e., it enters the zone of structural failure)[6]. As mentioned by Berger et al[9], slightly combined component internal rotation is associated with lateral tracking of the patella followed by tilting and potential patellar subluxation and the knee is thus functioning in the zone of abnormal loading, which could lead to AKP after TKA. Dislocation and component failure were reported when severe malrotation of the components was present and thus the knee was functioning in the zone of structural failure[9].
Figure 1.
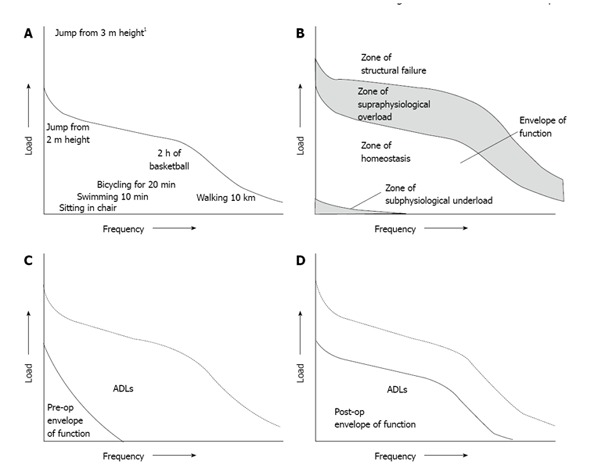
Applying Dye’s theory to patients with Anterior Knee Pain after total knee arthroplasty provides useful insights. A: The potential range of activity or envelope of function for a specific joint: all activities fall within the zone of homeostasis except the jump from 3 m height1; B: The different zones of loading across the knee joint. The first zone is the zone of subphysiological underloading, the second is the preferred zone of tissue homeostasis, the third is the zone of supraphysiological overloading and the fourth is the zone of structural failure; C: Arthritis causes the potential range of activity (envelope of function) to become limited, causing pain and restrictions in daily activities such as walking and cycling. The dotted line is the original zone of homeostasis for this specific knee; D: The postoperative situation where total knee arthroplasty placement increases the potential range of activity but does not return it to the original range of activity. (Reprinted with permission from Dye et al[6]).
If AKP is present after TKA placement, it is interesting to find out if it is possible to bring the knee back into a zone of homeostasis, and if this can influence the AKP. This can sometimes be achieved by conservative measures, for example in the study by Smith et al[42], where patients that were able to learn how to walk in a different way could reduce or even had no AKP, compared to patients who could not learn how. Other corrective measures include muscle-training (the quadriceps/hamstrings but also the hip and trunk muscles, therefore avoiding a dynamic valgus[25,43]), medication (painkillers), taping of the patella, using inlays or avoiding certain activities. If structural mechanical causes are present, revision surgery may be indicated, but caution is advised within the first 12 mo. This review will not focus on operative treatment options, as these are areas for separate review.
CONCLUSION
It can be concluded that anterior knee pain after TKA can be seen as the presenting symptom of a multifactorial problem. It is known that the nociceptive system is being activated by abnormal mechanical deformation, thermal stimuli or special chemical agents. The pain is caused by soft tissues (i.e., retinaculum, infrapatellar fat pad and the synovial membrane) being overloaded and/or due to impingement; for example due to malrotation of the components, overstuffing of PFJ, instability of the PFJ or a combination. Simply changing to a mobile bearing, releasing the retinacula or resurfacing the patella does not seem to be a universal solution. A perfect placement of a well-designed TKA can minimize AKP, however it cannot be concluded that it will prevent AKP, due to the large number of different factors playing a role in the origin of AKP. It is suggested that clinicians determine the specific envelope of function or zone of tissue homeostasis for TKA patients. Future research should focus on making further progress towards understanding the complex interaction of factors causing AKP in order to find a solution to prevent and eliminate it. The recommendation of this study is that the main focus should be on determining the correct placement and maybe incorporating the ACL (if present) in TKA placement.
Footnotes
P- Reviewers: AkronJE, Chiroiu V, Moilanen T, Willis-Owen CA S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
References
- 1.Bourne RB, Burnett RS. The consequences of not resurfacing the patella. Clin Orthop Relat Res. 2004;(428):166–169. doi: 10.1097/01.blo.0000147137.05927.bf. [DOI] [PubMed] [Google Scholar]
- 2.Biedert RM, Sanchis-Alfonso V. Sources of anterior knee pain. Clin Sports Med. 2002;21:335–347, vii. doi: 10.1016/s0278-5919(02)00026-1. [DOI] [PubMed] [Google Scholar]
- 3.Rand JA. Patellar resurfacing in total knee arthroplasty. Clin Orthop Relat Res. 1990;(260):110–117. [PubMed] [Google Scholar]
- 4.Burnett RS, Bourne RB. Indications for patellar resurfacing in total knee arthroplasty. Instr Course Lect. 2004;53:167–186. [PubMed] [Google Scholar]
- 5.Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee-replacement prostheses. 1976. Clin Orthop Relat Res. 1999;(367):3–17; discussion 2. doi: 10.1097/00003086-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10–18. doi: 10.1097/00003086-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Barrack RL, Bertot AJ, Wolfe MW, Waldman DA, Milicic M, Myers L. Patellar resurfacing in total knee arthroplasty. A prospective, randomized, double-blind study with five to seven years of follow-up. J Bone Joint Surg Am. 2001;83-A:1376–1381. [PubMed] [Google Scholar]
- 8.Barrack RL, Schrader T, Bertot AJ, Wolfe MW, Myers L. Component rotation and anterior knee pain after total knee arthroplasty. Clin Orthop Relat Res. 2001;(392):46–55. doi: 10.1097/00003086-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Berger RA, Crossett LS, Jacobs JJ, Rubash HE. Malrotation causing patellofemoral complications after total knee arthroplasty. Clin Orthop Relat Res. 1998;(356):144–153. doi: 10.1097/00003086-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Berger RA, Rubash HE, Seel MJ, Thompson WH, Crossett LS. Determining the rotational alignment of the femoral component in total knee arthroplasty using the epicondylar axis. Clin Orthop Relat Res. 1993;(286):40–47. [PubMed] [Google Scholar]
- 11.Brander VA, Stulberg SD, Adams AD, Harden RN, Bruehl S, Stanos SP, Houle T. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res. 2003;(416):27–36. doi: 10.1097/01.blo.0000092983.12414.e9. [DOI] [PubMed] [Google Scholar]
- 12.Breugem SJ, Sierevelt IN, Schafroth MU, Blankevoort L, Schaap GR, van Dijk CN. Less anterior knee pain with a mobile-bearing prosthesis compared with a fixed-bearing prosthesis. Clin Orthop Relat Res. 2008;466:1959–1965. doi: 10.1007/s11999-008-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breugem SJ, van Ooij B, Haverkamp D, Sierevelt IN, van Dijk CN. No difference in anterior knee pain between a fixed and a mobile posterior stabilized total knee arthroplasty after 7.9 years. Knee Surg Sports Traumatol Arthrosc. 2014;22:509–516. doi: 10.1007/s00167-012-2281-2. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DG, Duncan WW, Ashworth M, Mintz A, Stirling J, Wakefield L, Stevenson TM. Patellar resurfacing in total knee replacement: a ten-year randomised prospective trial. J Bone Joint Surg Br. 2006;88:734–739. doi: 10.1302/0301-620X.88B6.16822. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhuth SA, Saleh KJ, Cui Q, Clark CR, Brown TE. Patellofemoral instability after total knee arthroplasty. Clin Orthop Relat Res. 2006;446:149–160. doi: 10.1097/01.blo.0000214415.83593.db. [DOI] [PubMed] [Google Scholar]
- 16.Healy WL, Wasilewski SA, Takei R, Oberlander M. Patellofemoral complications following total knee arthroplasty. Correlation with implant design and patient risk factors. J Arthroplasty. 1995;10:197–201. doi: 10.1016/s0883-5403(05)80127-5. [DOI] [PubMed] [Google Scholar]
- 17.Helmy N, Anglin C, Greidanus NV, Masri BA. To resurface or not to resurface the patella in total knee arthroplasty. Clin Orthop Relat Res. 2008;466:2775–2783. doi: 10.1007/s11999-008-0420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann S, Romero J, Roth-Schiffl E, Albrecht T. [Rotational malalignment of the components may cause chronic pain or early failure in total knee arthroplasty] Orthopade. 2003;32:469–476. doi: 10.1007/s00132-003-0503-5. [DOI] [PubMed] [Google Scholar]
- 19.Mont MA, Yoon TR, Krackow KA, Hungerford DS. Eliminating patellofemoral complications in total knee arthroplasty: clinical and radiographic results of 121 consecutive cases using the Duracon system. J Arthroplasty. 1999;14:446–455. doi: 10.1016/s0883-5403(99)90100-6. [DOI] [PubMed] [Google Scholar]
- 20.Motsis EK, Paschos N, Pakos EE, Georgoulis AD. Review article: Patellar instability after total knee arthroplasty. J Orthop Surg (Hong Kong) 2009;17:351–357. doi: 10.1177/230949900901700322. [DOI] [PubMed] [Google Scholar]
- 21.Toms AD, Mandalia V, Haigh R, Hopwood B. The management of patients with painful total knee replacement. J Bone Joint Surg Br. 2009;91:143–150. doi: 10.1302/0301-620X.91B2.20995. [DOI] [PubMed] [Google Scholar]
- 22.van Jonbergen HP, Reuver JM, Mutsaerts EL, Poolman RW. Determinants of anterior knee pain following total knee replacement: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22:478–499. doi: 10.1007/s00167-012-2294-x. [DOI] [PubMed] [Google Scholar]
- 23.van Jonbergen HP, Scholtes VA, van Kampen A, Poolman RW. A randomised, controlled trial of circumpatellar electrocautery in total knee replacement without patellar resurfacing. J Bone Joint Surg Br. 2011;93:1054–1059. doi: 10.1302/0301-620X.93B8.26560. [DOI] [PubMed] [Google Scholar]
- 24.Wood DJ, Smith AJ, Collopy D, White B, Brankov B, Bulsara MK. Patellar resurfacing in total knee arthroplasty: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A:187–193. doi: 10.2106/00004623-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Petersen W, Rembitzki IV, Brüggemann GP, Ellermann A, Best R, Koppenburg AG, Liebau C. Anterior knee pain after total knee arthroplasty: a narrative review. Int Orthop. 2014;38:319–328. doi: 10.1007/s00264-013-2081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26:773–777. doi: 10.1177/03635465980260060601. [DOI] [PubMed] [Google Scholar]
- 27.Dellon AL. Pain solutions 2013. La Vergne, USA: Lightning Source Inc; 2013. [Google Scholar]
- 28.Bodine SCLR. Peripheral Nerve, Physiology, Anatomy and Pathology. In: Orthopaedic Basic Science., editor. ET Buckwaters JA, Simon SR. 2 nd ed. Rosemont, Illinois, USA: American Academy of orthopaedic Surgeons; 2010. pp. 617–682. [Google Scholar]
- 29.Page CP. Integrated Pharmocology. London: Mosby; 1997. [Google Scholar]
- 30.Witoński D, Wagrowska-Danielewicz M. Distribution of substance-P nerve fibers in the knee joint in patients with anterior knee pain syndrome. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1999;7:177–183. doi: 10.1007/s001670050144. [DOI] [PubMed] [Google Scholar]
- 31.Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221–226. [PubMed] [Google Scholar]
- 32.Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10:329–335. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- 33.Waters TS, Bentley G. Patellar resurfacing in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Am. 2003;85-A:212–217. doi: 10.2106/00004623-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Bhattee G, Moonot P, Govindaswamy R, Pope A, Fiddian N, Harvey A. Does malrotation of components correlate with patient dissatisfaction following secondary patellar resurfacing? Knee. 2014;21:247–251. doi: 10.1016/j.knee.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Berny R, Levy MN. Physiology. St. Louis, Missouri, USA: Mosby; 1993. [Google Scholar]
- 36.Aglietti P, Baldini A, Buzzi R, Lup D, De Luca L. Comparison of mobile-bearing and fixed-bearing total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20:145–153. doi: 10.1016/j.arth.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100–110. doi: 10.1097/01.blo.0000172303.74414.7d. [DOI] [PubMed] [Google Scholar]
- 38.Kessler O, Patil S, Colwell CW, D’Lima DD. The effect of femoral component malrotation on patellar biomechanics. J Biomech. 2008;41:3332–3339. doi: 10.1016/j.jbiomech.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 39.Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta?: influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91:344–350. doi: 10.1302/0301-620X.91B3.21592. [DOI] [PubMed] [Google Scholar]
- 40.Popovic N, Lemaire R. Anterior knee pain with a posterior-stabilized mobile-bearing knee prosthesis: the effect of femoral component design. J Arthroplasty. 2003;18:396–400. doi: 10.1016/s0883-5403(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 41.Scuderi GR, Insall JN, Scott NW. Patellofemoral Pain After Total Knee Arthroplasty. J Am Acad Orthop Surg. 1994;2:239–246. doi: 10.5435/00124635-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Smith AJ, Lloyd DG, Wood DJ. Pre-surgery knee joint loading patterns during walking predict the presence and severity of anterior knee pain after total knee arthroplasty. J Orthop Res. 2004;22:260–266. doi: 10.1016/S0736-0266(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 43.Chester R, Smith TO, Sweeting D, Dixon J, Wood S, Song F. The relative timing of VMO and VL in the aetiology of anterior knee pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2008;9:64. doi: 10.1186/1471-2474-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahoney OM, McClung CD, dela Rosa MA, Schmalzried TP. The effect of total knee arthroplasty design on extensor mechanism function. J Arthroplasty. 2002;17:416–421. doi: 10.1054/arth.2002.32168. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Tan Y, Deng Y, Chen L. Posterior cruciate-retaining versus posterior stabilized total knee arthroplasty: a meta-analysis of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc. 2014;22:556–564. doi: 10.1007/s00167-012-2275-0. [DOI] [PubMed] [Google Scholar]
- 46.Kim YH, Yoon SH, Kim JS. Early outcome of TKA with a medial pivot fixed-bearing prosthesis is worse than with a PFC mobile-bearing prosthesis. Clin Orthop Relat Res. 2009;467:493–503. doi: 10.1007/s11999-008-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wohlrab D, Hube R, Zeh A, Hein W. Clinical and radiological results of high flex total knee arthroplasty: a 5 year follow-up. Arch Orthop Trauma Surg. 2009;129:21–24. doi: 10.1007/s00402-008-0665-z. [DOI] [PubMed] [Google Scholar]
- 48.Beard DJ, Pandit H, Price AJ, Butler-Manuel PA, Dodd CA, Murray DW, Goodfellow JW. Introduction of a new mobile-bearing total knee prosthesis: minimum three year follow-up of an RCT comparing it with a fixed-bearing device. Knee. 2007;14:448–451. doi: 10.1016/j.knee.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Smith H, Jan M, Mahomed NN, Davey JR, Gandhi R. Meta-analysis and systematic review of clinical outcomes comparing mobile bearing and fixed bearing total knee arthroplasty. J Arthroplasty. 2011;26:1205–1213. doi: 10.1016/j.arth.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 50.van der Voort P, Pijls BG, Nouta KA, Valstar ER, Jacobs WC, Nelissen RG. A systematic review and meta-regression of mobile-bearing versus fixed-bearing total knee replacement in 41 studies. Bone Joint J. 2013;95-B:1209–1216. doi: 10.1302/0301-620X.95B9.30386. [DOI] [PubMed] [Google Scholar]
- 51.Van der Bracht H, Van Maele G, Verdonk P, Almqvist KF, Verdonk R, Freeman M. Is there any superiority in the clinical outcome of mobile-bearing knee prosthesis designs compared to fixed-bearing total knee prosthesis designs in the treatment of osteoarthritis of the knee joint? A review of the literature. Knee Surg Sports Traumatol Arthrosc. 2010;18:367–374. doi: 10.1007/s00167-009-0973-z. [DOI] [PubMed] [Google Scholar]
- 52.Li YL, Wu Q, Ning GZ, Feng SQ, Wu QL, Li Y, Hao Y. No difference in clinical outcome between fixed- and mobile-bearing TKA: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2014;22:565–575. doi: 10.1007/s00167-012-2313-y. [DOI] [PubMed] [Google Scholar]
- 53.Kawahara S, Matsuda S, Fukagawa S, Mitsuyasu H, Nakahara H, Higaki H, Shimoto T, Iwamoto Y. Upsizing the femoral component increases patellofemoral contact force in total knee replacement. J Bone Joint Surg Br. 2012;94:56–61. doi: 10.1302/0301-620X.94B1.27514. [DOI] [PubMed] [Google Scholar]
- 54.Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;(356):39–46. doi: 10.1097/00003086-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Incavo SJ, Wild JJ, Coughlin KM, Beynnon BD. Early revision for component malrotation in total knee arthroplasty. Clin Orthop Relat Res. 2007;458:131–136. doi: 10.1097/BLO.0b013e3180332d97. [DOI] [PubMed] [Google Scholar]
- 56.Nicoll D, Rowley DI. Internal rotational error of the tibial component is a major cause of pain after total knee replacement. J Bone Joint Surg Br. 2010;92:1238–1244. doi: 10.1302/0301-620X.92B9.23516. [DOI] [PubMed] [Google Scholar]
- 57.Romero J, Stähelin T, Wyss T, Hofmann S. [Significance of axial rotation alignment of components of knee prostheses] Orthopade. 2003;32:461–468. doi: 10.1007/s00132-003-0475-5. [DOI] [PubMed] [Google Scholar]
- 58.Mockford BJ, Beverland DE. Secondary resurfacing of the patella in mobile-bearing total knee arthroplasty. J Arthroplasty. 2005;20:898–902. doi: 10.1016/j.arth.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Barrack RL, Burak C. Patella in total knee arthroplasty. Clin Orthop Relat Res. 2001;(389):62–73. doi: 10.1097/00003086-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Pakos EE, Ntzani EE, Trikalinos TA. Patellar resurfacing in total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2005;87:1438–1445. doi: 10.2106/JBJS.D.02422. [DOI] [PubMed] [Google Scholar]
- 61.He JY, Jiang LS, Dai LY. Is patellar resurfacing superior than nonresurfacing in total knee arthroplasty? A meta-analysis of randomized trials. Knee. 2011;18:137–144. doi: 10.1016/j.knee.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Wyatt MC, Frampton C, Horne JG, Devane P. Mobile- versus fixed-bearing modern total knee replacements- which is the more patella-friendly design?: The 11-year New Zealand Joint Registry study. Bone Joint Res. 2013;2:129–131. doi: 10.1302/2046-3758.27.2000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beard DJ, Pandit H, Gill HS, Hollinghurst D, Dodd CA, Murray DW. The influence of the presence and severity of pre-existing patellofemoral degenerative changes on the outcome of the Oxford medial unicompartmental knee replacement. J Bone Joint Surg Br. 2007;89:1597–1601. doi: 10.1302/0301-620X.89B12.19259. [DOI] [PubMed] [Google Scholar]
- 64.Beard DJ, Pandit H, Ostlere S, Jenkins C, Dodd CA, Murray DW. Pre-operative clinical and radiological assessment of the patellofemoral joint in unicompartmental knee replacement and its influence on outcome. J Bone Joint Surg Br. 2007;89:1602–1607. doi: 10.1302/0301-620X.89B12.19260. [DOI] [PubMed] [Google Scholar]
- 65.Dajani KA, Stuart MJ, Dahm DL, Levy BA. Arthroscopic treatment of patellar clunk and synovial hyperplasia after total knee arthroplasty. J Arthroplasty. 2010;25:97–103. doi: 10.1016/j.arth.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Maculé F, Sastre S, Lasurt S, Sala P, Segur JM, Mallofré C. Hoffa’s fat pad resection in total knee arthroplasty. Acta Orthop Belg. 2005;71:714–717. [PubMed] [Google Scholar]
- 67.Erak S, Rajgopal V, Macdonald SJ, McCalden RW, Bourne RB. Ten-year results of an inset biconvex patella prosthesis in primary knee arthroplasty. Clin Orthop Relat Res. 2009;467:1781–1792. doi: 10.1007/s11999-009-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koh JS, Yeo SJ, Lee BP, Lo NN, Seow KH, Tan SK. Influence of patellar thickness on results of total knee arthroplasty: does a residual bony patellar thickness of & lt; or=12 mm lead to poorer clinical outcome and increased complication rates? J Arthroplasty. 2002;17:56–61. doi: 10.1054/arth.2002.29320. [DOI] [PubMed] [Google Scholar]


