Abstract
Medical imaging is of crucial importance for diagnosis and initial staging as well as for differentiation of multiple myeloma (MM) from other monoclonal plasma cell diseases. Conventional radiography represents the reference standard for diagnosis of MM due to its wide availability and low costs despite its known limitations such as low sensitivity, limited specificity and its inability to detect extraosseous lesions. Besides conventional radiography, newer cross-sectional imaging modalities such as whole-body low-dose computed tomography (CT), whole-body magnetic resonance imaging (MRI) and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT are available for the diagnosis of osseous and extraosseous manifestations of MM. Whole-body low-dose CT is used increasingly, replacing conventional radiography at selected centers, due to its higher sensitivity for the detection of osseous lesions and its ability to diagnose extraosseous lesions. The highest sensitivity for both detection of bone marrow disease and extraosseous lesions can be achieved with whole-body MRI and 18F-FDG PET/CT. According to current evidence, MRI is the most sensitive method for initial staging while 18F-FDG PET/CT allows monitoring of treatment of MM. There is an evolving role for assessment of treatment response using newer MR imaging techniques. Future studies are needed to further define the exact role of the different imaging modalities for individual risk stratification and therapy monitoring.
Keywords: Multiple myeloma, Plasmocytoma, X-Ray, Magnetic resonance imaging, Diffusion-weighted imaging, Positron emission tomography-computed tomography, Imaging
Core tip: A comprehensive review about state-of-the-art imaging of multiple myeloma with a focus on whole-body imaging techniques including computed tomography (CT), magnetic resonance imaging and positron emission tomography/CT which are increasingly used for detection and visualization of both osseous and extraosseous myeloma manifestations.
INTRODUCTION
Multiple myeloma (MM) is the second most common (10%-15% of all) hematological malignancies and represents 1% of all malignant diseases[1,2]. It is responsible for 15%-20% of deaths from hematological malignancies and about 2% of all deaths from cancer[3-5]. The disease is characterized by clonal proliferation of plasma cells which may produce excessive amounts of monoclonal immunoglobulins that can be detected in serum and urine[2,3]. The proliferating plasma cells infiltrate the bone marrow leading to replacement of the normal myelopoiesis. Characteristic clinical symptoms include anemia and infections due to the progressive cytopenia, renal insufficiency due the excessive monoclonal light chains in the blood, and hypercalcaemia due to activation of osteoclasts with consecutive demineralization of the bones and pathologic fractures[6]. Moreover, there are extraosseous manifestations of MM, which may affect soft tissues and organs in 10%-16% of patients that can be detected using various imaging methods[7,8].
Treatment of MM consists of conventional chemotherapy or high dose chemotherapy and subsequent allogeneic or autologous stem cell transplantation[6]. The introduction of novel agents, such as immunomodulatory drugs thalidomide and lenalidomide and proteasome inhibitor bortezomib, combined with conventional chemotherapy has radically changed the treatment paradigm of elderly patients and improved outcome[9]. Due to these new and partly more aggressive treatments the progression free survival time has dramatically increased and the 10-year survival rate may reach up to 30%-40%[10].
Differentiation of MM from other monoclonal plasma cell diseases, such as the monoclonal gammopathy of undetermined significance (MGUS) and the so-called smoldering multiple myeloma (SMM) (Table 1), is of significant importance[11,12]. MGUS is also characterized by monoclonal plasma cells in the bone marrow and monoclonal immunoglobulins in serum/urine, but to a lower extent as compared to MM[11]. In addition, MGUS is characterized by an obligatory lack of end organ damage (no hypercalcaemia, no renal insufficiency, no anemia, and no bone lesions). SMM is regarded as a precursor and intermediate stage of MM and is also characterized by a lack of end organ damage[12]. MGUS and SMM have different risks for progression to MM: MGUS has a risk of 1% per year and SMM has a risk of 10% per year[11,13]. Currently, neither MGUS nor SMM represent an indication for therapy. The solitary plasmacytoma, which is a localized plasma cell tumor, has to be differentiated from these systemic plasma cell diseases. Plasmocytoma may be treated curatively in some cases with local treatments such as radiation therapy.
Table 1.
Monoclonal plasma cell disorders
| Plasma cell disorder | Diagnostic criteria |
| Monoclonal gammopathy of undetermined significance | Monoclonal serum paraprotein ≤ 3 g/dL and plasma cell infiltration of bone marrow ≤ 10% and no end organ damage1 |
| Asymptomatic smoldering multiple myeloma | Monoclonal serum paraprotein ≥ 3 g/dL and/or plasma cell infiltration of bone marrow ≥ 10% and no end organ damage1 |
| Symptomatic multiple myeloma | Monoclonal paraprotein in serum or urine and/or plasma cell infiltration of bone marrow ≥ 10% and end organ damage1 |
End organ damage: Anemia, hypercalcaemia, renal insufficiency, or bone lesions.
Role of imaging in multiple myeloma
Diagnosis of symptomatic and hence treatment requiring MM, as a differential diagnosis of MGUS and SMM, is based on the detection of osseous lesions as defined by osteolysis, a diffuse severe osteopenia or pathologic fractures[1,6]. The consensus statement of the International Myeloma Working Group (IMWG) still recommends conventional projection radiography for the majority of patients[1]. According to the Durie-Salmon-Staging system, the presence and number of osseous lesions contribute directly to the staging of the disease and thereby to the risk stratification of MM[14].
The use of more sophisticated imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) may help to better define osteolytic lesions allowing for earlier detection of the disease[9,15]. Whole body low-dose CT has replaced conventional radiography at many centers. Newer staging systems like the Durie-Salmon-PLUS staging system take into account results from more sensitive methods such as whole body MRI or 18F-fluorodeoxyglucose (FDG) PET/CT[16]. Two patterns of osseous involvement have to differentiated in MM[1]: On one hand, there are focal lesions with confirmed circumscriptive plasma cell infiltration of the bone marrow which may lead to destruction of the inner cortical bone (scalloping). On the other hand, there is diffuse bone marrow infiltration which leads to a mixture of monoclonal cells and physiologic hematopoietic cells while the spongiosa of the bone remains primarily intact.
These two involvement patterns of MM may occur isolated, synchronous or metachronous. Moreover, soft tissue and/or organ involvement can be observed, which may originate from primarily extraosseous lesions or arise secondarily from osseous lesions after destruction of the cortical bone. Therefore, the main role of imaging in MM is the reliable detection of osseous and extraosseous lesions, enabling exact staging and risk stratification of individual patients.
ROLE OF DIFFERENT IMAGING MODALITIES FOR MM
While the primary aim of this review is to provide a guideline-based overview of the currently recommended imaging modalities and their specific advantages and disadvantages (in the sometimes confusing context of numerous original studies, case reports and reviews), a number of other reviews with a different focus have recently been published including reviews addressing the specific role of imaging in the context of non-secretory myeloma[17] and the potential influence of newer imaging modalities on patient management[18-20]. In addition, other more pictorial reviews provide a good description of imaging features of both osseous and extraosseous myeloma[21-23].
Conventional projection radiography
Conventional projection radiography still represents the standard method for detection of bone lesions for initial staging and monitoring of MM. Lytic lesions in the plate bone of the skull and pelvis are typically characterized by stamped out lesions without a sclerotic rim (Figure 1). In the long bone various appearances may be detected: thinning of the inner cortical bone (scalloping), discrete small lytic lesions up to 1 cm, “moth-eaten” patterns deriving from multiple small lesions or large destructing osteolytic lesions[1]. All these lesions represent replacement of the physiological bone marrow by clonally expanding plasma cells with consecutive destruction of the bone[24]. According to the IMWG, a complete conventional radiographic status is recommended for each newly diagnosed patient with MM (Table 2)[1]. Nearly 80% of all newly diagnosed cases of MM reveal detectable changes using conventional radiography. The following sites are most commonly affected: vertebrae in 65% of patients, ribs in 45%, skull in 40%, shoulders in 40%, pelvis in 30% and long bones in 25%[1,25]. The detection of lytic bone lesions represents a criterion defining a symptomatic and treatment-requiring MM even in the absence of clinical symptoms[4,26]. The advantage of conventional radiography is its wide availability, low costs and coverage of almost the entire skeletal system.
Figure 1.
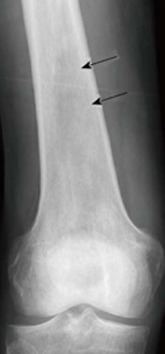
X-ray of an osseous myeloma lesion. Conventional X-ray of the right femoral bone showing an osteolytic lesion (arrows) representing an osseous myeloma manifestation.
Table 2.
Conventional radiographic status in multiple myeloma
| Region |
| Skull in 2 views |
| Spine (cervical/thoracic/lumbar) in 2 views |
| Chest AP |
| Pelvis AP |
| Long proximal bones AP |
AP: Anterior-posterior view.
The disadvantage of conventional radiography is its low sensitivity, which is explained by the fact that lytic lesions are only detectable if more than 30% of the trabecular bone is destroyed[27]. Hence, up to 20% of patients with normal skeletal status have non-detected osteolytic lesions[1,25]. In addition, conventional radiography can neither detect nor quantify a diffuse bone marrow infiltration nor extraosseous lesions. Another limitation of conventional radiography is the fact that it cannot be used for therapy monitoring, since lytic lesions rarely show radiographically detectable changes despite the presence of a therapy response[28]. Moreover, conventional radiography fails to differentiate benign reasons for focal lucent bone lesions, has a relatively high interobserver variability and certain regions can not be depicted free from superposition. Due to the aforementioned reasons, more sophisticated cross-sectional imaging methods are being established for diagnosis of MM[1].
CT
CT allows for detection of smaller osseous lesions that are not detectable by conventional radiography[3,5]. Early changes can be detected more reliably with CT. Another advantage of CT as compared to conventional radiography is its higher sensitivity, particularly in regions that are superimposed on conventional radiographs such as scapulae, ribs and sternum[3]. Importantly, potential instabilities and risk of fractures can be estimated better using cross-sectional CT (Figures 2 and 3)[2,29,30]. Another advantage of CT is short imaging times with modern multi-detector CT and complication free examinations of patients in the supine position without the need of repeated relocation, which might be of importance in anguished patients. Moreover, CT allows for detection of extraosseous manifestations of MM and the acquired 3D data sets can be used for radiation therapy if needed. In symptomatic patients with inconspicuous conventional radiographic imaging studies, a CT should be considered.
Figure 2.
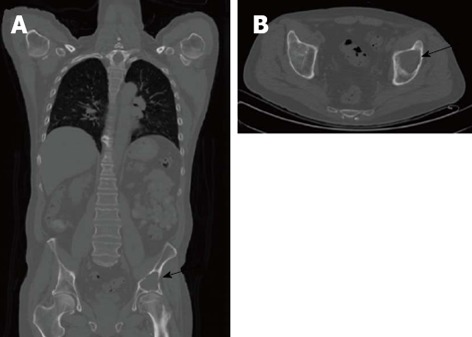
Computed tomography of an osseous myeloma lesion. Computed tomography in coronal (A) and transversal views (B) showing an osteolytic lesion in the left iliac bone (arrows) representing an osseous myeloma manifestation.
Figure 3.
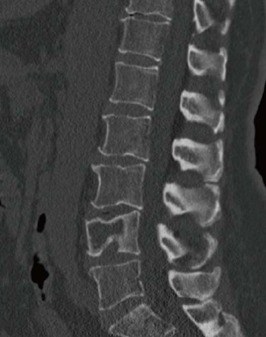
Computed tomography of an osseous myeloma lesion. Computed tomography in sagittal view showing an osteolytic lesion in L4 with associated pathologic fracture.
A known disadvantage of CT is its high radiation dose, which had led to the implementation of so called low-dose CT protocols, which are still highly specific for the detection of osteolytic bone lesions[4,5,31]. The dose of CT may be reduced even further in the future with newly developed iterative reconstruction techniques[2,32].
However, CT has limited sensitivity for detection of diffuse bone marrow infiltration, bone marrow lesions without lytic reaction and extraosseous lesions.
MRI
The use of MRI for imaging of MM has dramatically increased within the last decade[6,33]. MRI is clearly more sensitive than conventional radiography. Up to 50% of patients with inconspicuous conventional radiographic imaging reveal focal lesions detectable on a MRI (Figure 4)[7,8,33]. In particular, MRI offers improved detection of lesions in the spine, pelvis, sternum, skull and scapulae. Other advantages as compared to both conventional radiography and CT are the excellent depiction of the spinal cord and nerve roots, detection of soft tissue manifestations and the ability to differentiate between physiological and myeloma-infiltrated bone marrow[6,33-35]. The involvement of the bone marrow is classified in three different patterns[9,36-38]: focal lesions, homogenous diffuse bone marrow infiltration and mixed “salt-and-pepper” pattern with remaining islets of fatty bone marrow. An excellent review containing a large number of imaging examples for the different involvement patterns before and after treatment has recently been published[23].
Figure 4.
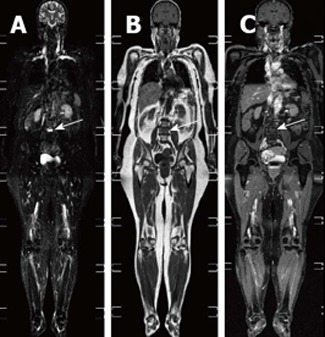
Whole-body magnetic resonance imaging of an osseous myeloma lesion. Whole-body magnetic resonance imaging: short-tau-inversion-recovery sequence (A), T1-weighted image (B) and T1-weighted image with fat suppression after contrast administration (C) showing an osseous lesion in L4 (arrows) representing an osseous myeloma manifestation.
An inconspicuous MRI indicates very low tumor burden, while diffuse involvement and contrast enhancement correspond to high tumor burden[10,39]. Several studies have shown that asymptomatic patients with detectable lesions on MRI have a higher probability to become symptomatic earlier than patients without such lesions[11,12,40,41]. Future studies are needed to evaluate whether detectable lesions on MRI have to be included in the definition of symptomatic MM. However, MRI of the spine and pelvis is indicated when there is suspicion of solitary plasmacytoma to rule out additional lesions[11,42]. Also in patients with suspicion of spinal chord or nerve root compression MRI is indicated, as well as in patients with painful myeloma manifestations for evaluation of the extent of potential soft tissue masses. Moreover there is an indication for MRI in patients with non-secretory myeloma for initial staging as well as for monitoring of treatment[12,34].
MRI has several disadvantages: relatively high costs, relatively long scanning time which may be difficult in ill patients, and the risk of development of nephrogenic systemic fibrosis after intravenous administration of gadolinium-based contrast agents, particularly in patients suffering from renal insufficiency.
Besides the morphological MR imaging, there are newer functional MR techniques such as diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MR imaging. However, published data particularly for DWI in the context of initial staging are very limited. A recent study has shown that diffusion-weighted imaging does not only allow for detection of myeloma manifestations, but also that the apparent diffusion coefficients significantly differ before and after initiation of therapy[43]. Concerning DCE imaging, data has been limited to several mainly small studies. In one such study on 24 patients with myeloma, DCI MRI reflected the degree of infiltration and vessel density in corresponding bone marrow biopsy specimens[44]. In another study, Hillengass et al[45] could demonstrate a prognostic significance of DCE-derived parameters for event-free survival (P = 0.02) in myeloma patients. DCE MRI may identify a subgroup of patients with asymptomatic monoclonal plasma cell disease and pathologic microcirculation. These patients show a significantly higher bone marrow plasmocytosis compared with patients with a low microcirculation pattern. However, the clinical significance of that finding is currently unclear[46]. Another study evaluating DCE MRI findings in patients with myeloma and metastases from non-haematological cancer has shown that characteristic DCE parameters, including the peak signal enhancement percentage (SE%), the steepest wash-in SE% during the ascending phase and the wash-out SE% may indicate if an unclear spinal lesion is of myelomatous origin or not[47]. In short, both DCE MRI and DWI are promising techniques particularly for response monitoring, but further prospective studies are needed to define their exact role.
The applied MR imaging protocols vary widely between different institutions, and may include standard non-enhanced T1- and T2-weighted imaging, STIR sequences and contrast-enhanced T1-weighted fat-saturated imaging[48-50]. The usefulness of contrast-enhanced MR imaging for the initial evaluation of multiple myeloma is debatable because it does not usually allow the identification of additional focal lesions compared to non-enhanced imaging protocols[49]. In addition, gadolinium-based contrast agents may cause nephrogenic systemic fibrosis, particularly in patients with impaired renal function.
Based on our experience, we recommend a whole-body MRI protocol containing a T1-weighted sequence without fat suppression, a STIR sequence and a contrast-enhanced T1-weighted sequence with fat suppression in all patients without contraindications to gadolinium-based contrast agents, particularly because contrast-enhanced imaging has been shown to predict diffuse bone marrow infiltration[51,52].
PET/CT
Combined PET/CT using 18F-FDG as radiotracer allows for the simultaneous acquisition of several morphological and function parameters relevant to MM. The excellent depiction of osseous structures and lesions by CT is supplemented with the high sensitivity of PET for detection of isolated focal medullary lesions without destruction of the osseous substance as well as for detection of extraosseous manifestations (Figures 5 and 6)[1,6,18,34,53]. Moreover, PET/CT allows for initial staging and treatment monitoring of non-secretory myeloma[1,54]. In contrast to MM, the MGUS is typically PET negative[1,14,54].
Figure 5.
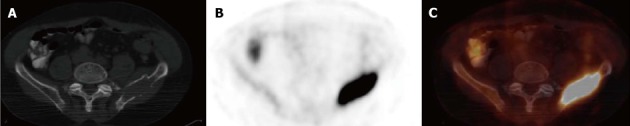
Positron emission tomography/computed tomography of an osseous myeloma lesion. Transversal computed tomography (CT) (A), 18F-fluorodeoxyglucose positron emission tomography (PET) (B) and fused PET/CT (C) showing an osteolytic lesion in the left iliac bone with cortical destruction representing an osseous myeloma manifestation.
Figure 6.

Positron emission tomography/computed tomography of extraosseous myeloma lesions. Transversal computed tomography (CT) (A), 18F-fluorodeoxyglucose positron emission tomography (PET) (B) and fused PET/CT (C) showing extraosseous myeloma manifestations (arrows).
The higher sensitivity of 18F-FDG PET/CT for detection of focal osseous lesions as compared to conventional radiography has been shown in several prospective studies. PET/CT detects more osseous myeloma manifestations in 40%-60% of cases as compared to conventional radiography and detects lesions in patients with false negative conventional radiography results[9,15,55,56]. Several studies have shown that in up to 40% of patients with initially solitary plasmacytoma, additional and so far unknown lesions may be detected by PET/CT leading to an upstaging and change of therapeutic management[1,16,57]. When compared to MRI, the sensitivity for detection of focal osseous lesions seems to be comparable. However, MRI has a higher sensitivity for detection of diffuse bone marrow infiltration, which may remain particularly undetected by PET/CT in cases of low degree plasma cell infiltration[1,56,58,59]. However, some newer studies have demonstrated a high sensitivity of PET also for detection of diffuse bone marrow infiltration. In a study by Sager et al[60], bone marrow involvement on FDG PET/CT of patients with MM was compared with bone marrow biopsy. In that study, the sensitivity of FDG PET in detecting bone marrow involvement at initial diagnosis was 90%. There was a significant correlation between SUVmax values, bone marrow biopsy cellularity and plasma cell ratios (r = 0.54 and r = 0.74, P < 0.01). Another study by Ak et al[61] also found a statistically significant positive correlation between the percentage of CD38/CD138 expressing plasma cells in bone marrow and both mean qualitative (r = 0.616) and semiquantitative (r = 0.755) FDG uptake.
PET imaging allows estimation of the standardized uptake value (SUV), which represents a quantitative measurement of 18F-fluorodeoxyglucose uptake and metabolic activity of a given lesion. Several studies have shown that a high SUV of lesions in MM patients correlates with faster disease progression and therefore with a worse prognosis[1,62,63]. A prospective study on 239 patients has shown that the presence of more than 3 PET-positive lesions represented the major independent parameter for predicting progression-free survival and overall survival[24,64]. In a study assessing the prognostic implications of serial FDG PET in 2 consecutive Total Therapy 3 trials for newly diagnosed myeloma, multivariate analysis showed that more than 3 focal lesions on day 7 of induction therapy imparted inferior overall survival and progression-free survival. Thus, the presence of > 3 focal lesions on day 7 PET follow-up may be exploited toward early therapy change[65]. In a study by Nanni et al[66], 107 patients had FDG PET 3 mo after therapy (autologous stem cell transplantation) and every 6 to 12 mo during the follow-up. In that series of patients, a negative posttherapy PET was predictive for nonrelapse or a long disease-free survival. In a study by Zamagni et al[62], 192 patients with newly diagnosed myeloma underwent FDG PET/CT at baseline and after autologous stem cell transplantation. In a multivariate analysis, both extramedullary disease detected by PET and SUV > 4.2 at baseline and persistence of FDG uptake after stem cell transplantation were independent variables adversely affecting progression-free survival. In addition to the parameters described above, the metabolic tumor volume, representing the metabolically active malignant tissue throughout the body has been shown to be useful for prediction of progression-free and overall survival in myeloma patients[67]. Future studies are required to further define the role of FDG PET/CT for individual risk stratification and therapy monitoring.
Apart from FDG, several other PET radiotracers have been evaluated for initial staging. In a study comparing FDG and 11C-acetate for initial staging of myeloma, 11C-acetate PET was able to detect diffuse bone marrow infiltration with a sensitivity of 100%, whereas FDG PET could establish a diagnosis of diffuse infiltration in only 40% of patients. In addition, the authors observed a positive correlation between bone marrow uptake values and percentages of plasma cell infiltrates (r = +0.63, P = 0.01) [68]. In a different study comparing the value of 11C-choline PET and FDG PET in assessing bone involvement in patients with multiple myeloma, 11C-Choline PET/CT scans detected 37 bone lesions, whereas 18F-FDG PET/CT scans detected 22 bone lesions. The authors concluded that 11C-Choline PET/CT appears to be more sensitive than 18F-FDG PET/CT for the detection of bony myelomatous lesions[69]. In a study by Nakamoto et al[70] assessing the clinical value of 11C-methionine (MET) as a radiolabelled amino acid tracer in plasma cell malignancies (which may also be useful because plasma cell malignancies are able to activate protein synthesis), MET PET revealed an equal or greater number of lesions than FDG (MET 156 lesions vs FDG 58 lesions) and tended to demonstrate higher uptake (maximum standardized uptake value 10.3 ± 5.6) than did FDG (3.4 ± 2.7, P < 0.001). The amino-acid tracer 18F-alpha-methyltyrosine (FAMT) was evaluated in a small study including eleven patients with MM. Although FAMT PET detected all lesions seen on FDG PET, uptake was significantly higher on FDG PET (P < 0.05)[71]. However, these new tracers are not widely available yet, usually require an on-site cyclotron for isotope production and an on-site radiochemistry for tracer synthesis.
IMAGING FOR MONITORING OF TREATMENT OF MM
According to the IMWG criteria, currently none of the presented imaging methods are mandatory for monitoring treatment of MM, as long as the response can be assessed by serum and urine analyses[1,6,34]. Repeated imaging is only indicated if ailment is likely induced by osseous lesions or in cases of relapse to exclude extraosseous lesions[1,6,25,34].
A characteristic feature of osseous manifestations of MM is the fact that the lesions regress only slowly or not at all, even in patients with complete remission[4,26,72]. Hence conventional radiography and CT cannot be adequately used for treatment monitoring. Typically, successfully treated inactive osteolytic lesions may show a sclerotic rim. A recent study has addressed the value of MRI for monitoring treatment of MM after stem cell transplantation, but found no additional benefit as compared to routinely performed hematological and immunological tests[27,38]. In contrast, 18F-FDG uptake represents a direct parameter of lesion activity (Figure 7), that enables detection of active myeloma lesions[1,16,25,54]. This allows 18F-FDG PET/CT to detect specific lesions after stem cell transplantation, albeit with lower sensitivity as compared to the initial staging[28,53]. In a study analyzing 197 whole-body 18F-FDG PET/CT scans performed in 99 patients with myeloma at different time points in the course of disease after autologous or allogeneic stem cell transplantation, PET/CT had a sensitivity of 54.6%, a specificity of 82.1%, a positive predictive value of 82.3%, a negative predictive value of 54.2% and an overall accuracy of 65.5%. The sensitivity of FDG PET/CT was shown to depend on the disease category according to the Uniform Response Criteria for myeloma. The authors concluded that FDG PET/CT may have a lower sensitivity for restaging after therapy compared to the pretreatment setting[53]. There are small PET studies on other tracers than FDG. In a recent prospective study, 13 patients underwent 11C-acetate PET/CT before and after treatment. After treatment, the diffuse bone marrow 11C-acetate uptake showed a mean SUVmax reduction of 66 % in patients with at least a very good partial response versus 34 % in those with at most a partial response only (P = 0.01), indicating a potential role of 11C-acetate PET for response assessment[68].
Figure 7.
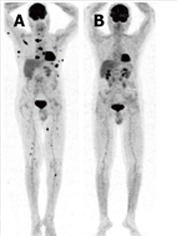
Positron emission tomography for therapy monitoring. Whole-body maximum-intensity-projection positron emission tomography (PET) images before and after stem cell transplantation showing extensive osseous and extraosseous myeloma manifestations before therapy (A) and complete resolution on PET after therapy (B).
There are several mainly small studies demonstrating changes on DWI or dynamic contrast-enhanced MRI after therapy, indicating a potential role of newer MRI techniques for response monitoring. In a study by Hillengass et al[43] on 56 patients with myeloma or monoclonal gammopathy of undetermined significance, the DWI-parameter apparent diffusion coefficient (ADC) correlated with bone marrow cellularity and micro-vessel density (P < 0.001). ADC was significantly different in 15 patients which underwent systemic treatment before and after that therapy (P < 0.001). In a study by Horger et al[73], twelve consecutive patients with myeloma underwent whole-body DWI both at baseline and 3 wk after onset of therapy. All involved lesions showed restricted diffusion at baseline, and ADC quantification yielded an increase of 63.9% in responders and a decrease of 7.8% in the sole non-responding patient during therapy, indicating that whole-body DWI with ADC analysis represents a feasible diagnostic tool for assessment of short-term treatment response. In a study by Lin et al[74], post-treatment bone marrow changes at whole-body dynamic contrast material-enhanced MR imaging were compared with clinical response in patients with multiple myeloma. Maximal percentages of bone marrow [BME(max)] and focal lesion [FLE(max)] enhancement were assessed. After induction chemotherapy, mean BME(max) differed between good and poor responders (94.3% vs 138.4%, respectively, P = 0.02). Mean timing [i.e., the number of post-contrast dynamic acquisitions where FLE(max) was observed] was significantly delayed in good responders compared with poor responders (4.7 vs 2.9, P < 0.0001). The authors concluded that whole-body dynamic contrast-enhanced MR imaging can be used to assess treatment response in patients with MM[74]. In another study comparing DWI and arterial spin labeling (ASL) perfusion in 10 patients, ASL showed a marked decrease in perfusion from baseline at 3 wk and at 8 wk (P = 0.01). In contrast, there was an increase in diffusion which was borderline significant (P = 0.0049). Both methods were able to correctly classify 9/10 patients as responder or non-responder. However, temporary changes in signal intensity between baseline and follow-up examinations were inconsistent on T1-weighted (w) and T2w images, indicating that standard MRI protocols may be of limited usefulness for response assessment[75]. This is in line with a whole-body MRI study on 66 patients after stem cell transplantation in which only moderate agreement was observed between MRI and routinely performed laboratory tests for the determination of remission[38]. Another study comparing 18F-FDG PET/CT and whole-body MRI for determination of remission status in patients with multiple myeloma after stem cell transplantation found that MRI may often be false positive because of persistent non-viable lesions in the post-treatment setting, indicating that PET/CT might be more suitable than MRI for determination of remission status[76].
As for the initial staging, future studies are needed to further define the exact value of the presented imaging techniques for monitoring treatment of MM.
CONCLUSION
Medical imaging is of crucial importance for diagnosis and initial staging as well as for differentiation of MM from other monoclonal plasma cell diseases. Despite the known limitations such as low sensitivity, limited specificity and inability to detect extraosseous lesions, conventional radiography still represents the reference standard for diagnosis of MM due to its wide availability and low costs. Besides conventional radiography, newer cross-sectional imaging modalities such as whole-body low-dose CT, whole-body MRI and 18F-FDG PET/CT are available for diagnosis of osseous and extraosseous manifestations of MM.
Among the cross-sectional imaging techniques, whole-body low-dose CT is currently replacing conventional radiography due to its high sensitivity for osseous lesions and the possibility to detect extraosseous lesions. Whole-body MRI and 18F-FDG PET/CT feature the highest sensitivity for osseous lesions, soft tissue lesions and organ manifestations. For that matter, MRI has the highest sensitivity for detection of diffuse bone marrow involvement and 18F-FDG PET/CT for detection of extraosseous lesions. Whole-body MRI should be considered in all patients with inconspicuous conventional radiography, all patients with apparently solitary plasmacytoma and patients with suspicion of spinal cord or nerve root compression.
Based on the results of recent studies and our experience, we recommend performing whole-body MRI for initial staging of MM due to its high sensitivity for detection of osseous and extraosseous lesions without the need for ionizing radiation. MRI allows for sensitive detection of both focal and diffuse bone marrow infiltration. A complementary CT may be indicated in case of conspicuous lesions to assess the presence of osteolytic lesions and to evaluate stability. For restaging of MM and detection of a possible relapse after initiation of treatment, we recommend performing 18F-FDG PET/CT due to its ability to differentiate between active and inactive lesions, enabling monitoring of MM treatment. There is an evolving role for assessment of treatment response using newer MR imaging techniques.
ACKNOWLEDGMENTS
The authors like to thank Curtis Wiens, PhD, Department of Radiology, University of Wisconsin, Madison, United States for his thorough revision and language editing of the manuscript.
Footnotes
P- Reviewers: Lichtor T, Ng SJ S- Editor: Gou SX L- Editor: A E- Editor: Lu YJ
References
- 1.Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, Siegel D, Lokhorst H, Kumar S, Rajkumar SV, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23:1545–1556. doi: 10.1038/leu.2009.89. [DOI] [PubMed] [Google Scholar]
- 2.Bataille R, Harousseau JL. Multiple myeloma. N Engl J Med. 1997;336:1657–1664. doi: 10.1056/NEJM199706053362307. [DOI] [PubMed] [Google Scholar]
- 3.Kröpil P, Fenk R, Fritz LB, Blondin D, Kobbe G, Mödder U, Cohnen M. Comparison of whole-body 64-slice multidetector computed tomography and conventional radiography in staging of multiple myeloma. Eur Radiol. 2008;18:51–58. doi: 10.1007/s00330-007-0738-3. [DOI] [PubMed] [Google Scholar]
- 4.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 5.Gleeson TG, Moriarty J, Shortt CP, Gleeson JP, Fitzpatrick P, Byrne B, McHugh J, O’Connell M, O’Gorman P, Eustace SJ. Accuracy of whole-body low-dose multidetector CT (WBLDCT) versus skeletal survey in the detection of myelomatous lesions, and correlation of disease distribution with whole-body MRI (WBMRI) Skeletal Radiol. 2009;38:225–236. doi: 10.1007/s00256-008-0607-4. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, Ludwig H, Joshua D, Mehta J, Gertz M, Avet-Loiseau H, Beksac M, Anderson KC, Moreau P, Singhal S, Goldschmidt H, Boccadoro M, Kumar S, Giralt S, Munshi NC, Jagannath S, on behalf of the International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701–4705. doi: 10.1182/blood-2010-10-299529. [DOI] [PubMed] [Google Scholar]
- 7.Wu P, Davies FE, Boyd K, Thomas K, Dines S, Saso RM, Potter MN, Ethell ME, Shaw BE, Morgan GJ. The impact of extramedullary disease at presentation on the outcome of myeloma. Leuk Lymphoma. 2009;50:230–235. doi: 10.1080/10428190802657751. [DOI] [PubMed] [Google Scholar]
- 8.Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325–330. doi: 10.1093/annonc/mdp329. [DOI] [PubMed] [Google Scholar]
- 9.Palumbo A, Mina R. Management of older adults with multiple myeloma. Blood Rev. 2013;27:133–142. doi: 10.1016/j.blre.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Barlogie B, Tricot GJ, van Rhee F, Angtuaco E, Walker R, Epstein J, Shaughnessy JD, Jagannath S, Bolejack V, Gurley J, et al. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. Br J Haematol. 2006;135:158–164. doi: 10.1111/j.1365-2141.2006.06271.x. [DOI] [PubMed] [Google Scholar]
- 11.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kröger N, Einsele H, Vesole DH, Dimopoulos M, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bladé J, Dimopoulos M, Rosiñol L, Rajkumar SV, Kyle RA. Smoldering (asymptomatic) multiple myeloma: current diagnostic criteria, new predictors of outcome, and follow-up recommendations. J Clin Oncol. 2010;28:690–697. doi: 10.1200/JCO.2009.22.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton LJ. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 14.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Bannas P, Kröger N, Adam G, Derlin T. [Modern imaging techniques in patients with multiple myeloma] Rofo. 2013;185:26–33. doi: 10.1055/s-0032-1325405. [DOI] [PubMed] [Google Scholar]
- 16.Durie BG. The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. Eur J Cancer. 2006;42:1539–1543. doi: 10.1016/j.ejca.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 17.Lonial S, Kaufman JL. Non-secretory myeloma: a clinician’s guide. Oncology (Williston Park) 2013;27:924–928, 930. [PubMed] [Google Scholar]
- 18.Agarwal A, Chirindel A, Shah BA, Subramaniam RM. Evolving role of FDG PET/CT in multiple myeloma imaging and management. AJR Am J Roentgenol. 2013;200:884–890. doi: 10.2214/AJR.12.9653. [DOI] [PubMed] [Google Scholar]
- 19.Zamagni E, Cavo M. The role of imaging techniques in the management of multiple myeloma. Br J Haematol. 2012;159:499–513. doi: 10.1111/bjh.12007. [DOI] [PubMed] [Google Scholar]
- 20.Terpos E, Moulopoulos LA, Dimopoulos MA. Advances in imaging and the management of myeloma bone disease. J Clin Oncol. 2011;29:1907–1915. doi: 10.1200/JCO.2010.32.5449. [DOI] [PubMed] [Google Scholar]
- 21.Hall MN, Jagannathan JP, Ramaiya NH, Shinagare AB, Van den Abbeele AD. Imaging of extraosseous myeloma: CT, PET/CT, and MRI features. AJR Am J Roentgenol. 2010;195:1057–1065. doi: 10.2214/AJR.10.4384. [DOI] [PubMed] [Google Scholar]
- 22.Philips S, Menias C, Vikram R, Sunnapwar A, Prasad SR. Abdominal manifestations of extraosseous myeloma: cross-sectional imaging spectrum. J Comput Assist Tomogr. 2012;36:207–212. doi: 10.1097/RCT.0b013e318245c261. [DOI] [PubMed] [Google Scholar]
- 23.Hanrahan CJ, Christensen CR, Crim JR. Current concepts in the evaluation of multiple myeloma with MR imaging and FDG PET/CT. Radiographics. 2010;30:127–142. doi: 10.1148/rg.301095066. [DOI] [PubMed] [Google Scholar]
- 24.Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood. 2006;108:3992–3996. doi: 10.1182/blood-2006-05-026112. [DOI] [PubMed] [Google Scholar]
- 25.Collins CD. Multiple myeloma. Cancer Imaging. 2010;10:20–31. doi: 10.1102/1470-7330.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelstyn GA, Gillespie PJ, Grebbell FS. The radiological demonstration of osseous metastases. Experimental observations. Clin Radiol. 1967;18:158–162. doi: 10.1016/s0009-9260(67)80010-2. [DOI] [PubMed] [Google Scholar]
- 28.Wahlin A, Holm J, Osterman G, Norberg B. Evaluation of serial bone X-ray examination in multiple myeloma. Acta Med Scand. 1982;212:385–387. doi: 10.1111/j.0954-6820.1982.tb03234.x. [DOI] [PubMed] [Google Scholar]
- 29.Horger M, Kanz L, Denecke B, Vonthein R, Pereira P, Claussen CD, Driessen C. The benefit of using whole-body, low-dose, nonenhanced, multidetector computed tomography for follow-up and therapy response monitoring in patients with multiple myeloma. Cancer. 2007;109:1617–1626. doi: 10.1002/cncr.22572. [DOI] [PubMed] [Google Scholar]
- 30.Horger M, Claussen CD, Bross-Bach U, Vonthein R, Trabold T, Heuschmid M, Pfannenberg C. Whole-body low-dose multidetector row-CT in the diagnosis of multiple myeloma: an alternative to conventional radiography. Eur J Radiol. 2005;54:289–297. doi: 10.1016/j.ejrad.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Chassang M, Grimaud A, Cucchi JM, Novellas S, Amoretti N, Chevallier P, Bruneton JN. Can low-dose computed tomographic scan of the spine replace conventional radiography? An evaluation based on imaging myelomas, bone metastases, and fractures from osteoporosis. Clin Imaging. 2007;31:225–227. doi: 10.1016/j.clinimag.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Laqmani A, Buhk JH, Henes FO, Klink T, Sehner S, von Schultzendorff HC, Hammerle D, Nagel HD, Adam G, Regier M. Impact of a 4th generation iterative reconstruction technique on image quality in low-dose computed tomography of the chest in immunocompromised patients. Rofo. 2013;185:749–757. doi: 10.1055/s-0033-1335577. [DOI] [PubMed] [Google Scholar]
- 33.Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD, Epstein J, van Hemert R, Erdem E, Hoering A, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007;25:1121–1128. doi: 10.1200/JCO.2006.08.5803. [DOI] [PubMed] [Google Scholar]
- 34.Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, Ludwig H, Joshua D, Mehta J, Gertz M, Avet-Loiseau H, Beksa M, Anderson KC, Moreau P, Singhal S, Goldschmidt H, Boccadoro M, Kumar S, Giralt S, Munshi NC, Jagannath S, International Myeloma Workshop Consensus Panel 3, et al. International Myeloma Workshop Consensus Panel 3; 2011. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3; pp. 4701–4705. [DOI] [PubMed] [Google Scholar]
- 35.Baur-Melnyk A, Buhmann S, Becker C, Schoenberg SO, Lang N, Bartl R, Reiser MF. Whole-body MRI versus whole-body MDCT for staging of multiple myeloma. AJR Am J Roentgenol. 2008;190:1097–1104. doi: 10.2214/AJR.07.2635. [DOI] [PubMed] [Google Scholar]
- 36.Baur-Melnyk A, Buhmann S, Dürr HR, Reiser M. Role of MRI for the diagnosis and prognosis of multiple myeloma. Eur J Radiol. 2005;55:56–63. doi: 10.1016/j.ejrad.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Moulopoulos LA, Dimopoulos MA, Alexanian R, Leeds NE, Libshitz HI. Multiple myeloma: MR patterns of response to treatment. Radiology. 1994;193:441–446. doi: 10.1148/radiology.193.2.7972760. [DOI] [PubMed] [Google Scholar]
- 38.Bannas P, Hentschel HB, Bley TA, Treszl A, Eulenburg C, Derlin T, Yamamura J, Adam G, Stübig T, Kröger N, et al. Diagnostic performance of whole-body MRI for the detection of persistent or relapsing disease in multiple myeloma after stem cell transplantation. Eur Radiol. 2012;22:2007–2012. doi: 10.1007/s00330-012-2445-y. [DOI] [PubMed] [Google Scholar]
- 39.Moulopoulos LA, Gika D, Anagnostopoulos A, Delasalle K, Weber D, Alexanian R, Dimopoulos MA. Prognostic significance of magnetic resonance imaging of bone marrow in previously untreated patients with multiple myeloma. Ann Oncol. 2005;16:1824–1828. doi: 10.1093/annonc/mdi362. [DOI] [PubMed] [Google Scholar]
- 40.Moulopoulos LA, Dimopoulos MA, Smith TL, Weber DM, Delasalle KB, Libshitz HI, Alexanian R. Prognostic significance of magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 1995;13:251–256. doi: 10.1200/JCO.1995.13.1.251. [DOI] [PubMed] [Google Scholar]
- 41.Hillengass J, Fechtner K, Weber MA, Bäuerle T, Ayyaz S, Heiss C, Hielscher T, Moehler TM, Egerer G, Neben K, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28:1606–1610. doi: 10.1200/JCO.2009.25.5356. [DOI] [PubMed] [Google Scholar]
- 42.Moulopoulos LA, Dimopoulos MA, Weber D, Fuller L, Libshitz HI, Alexanian R. Magnetic resonance imaging in the staging of solitary plasmacytoma of bone. J Clin Oncol. 1993;11:1311–1315. doi: 10.1200/JCO.1993.11.7.1311. [DOI] [PubMed] [Google Scholar]
- 43.Hillengass J, Bäuerle T, Bartl R, Andrulis M, McClanahan F, Laun FB, Zechmann CM, Shah R, Wagner-Gund B, Simon D, et al. Diffusion-weighted imaging for non-invasive and quantitative monitoring of bone marrow infiltration in patients with monoclonal plasma cell disease: a comparative study with histology. Br J Haematol. 2011;153:721–728. doi: 10.1111/j.1365-2141.2011.08658.x. [DOI] [PubMed] [Google Scholar]
- 44.Nosàs-Garcia S, Moehler T, Wasser K, Kiessling F, Bartl R, Zuna I, Hillengass J, Goldschmidt H, Kauczor HU, Delorme S. Dynamic contrast-enhanced MRI for assessing the disease activity of multiple myeloma: a comparative study with histology and clinical markers. J Magn Reson Imaging. 2005;22:154–162. doi: 10.1002/jmri.20349. [DOI] [PubMed] [Google Scholar]
- 45.Hillengass J, Wasser K, Delorme S, Kiessling F, Zechmann C, Benner A, Kauczor HU, Ho AD, Goldschmidt H, Moehler TM. Lumbar bone marrow microcirculation measurements from dynamic contrast-enhanced magnetic resonance imaging is a predictor of event-free survival in progressive multiple myeloma. Clin Cancer Res. 2007;13:475–481. doi: 10.1158/1078-0432.CCR-06-0061. [DOI] [PubMed] [Google Scholar]
- 46.Hillengass J, Zechmann C, Bäuerle T, Wagner-Gund B, Heiss C, Benner A, Ho A, Neben K, Hose D, Kauczor HU, et al. Dynamic contrast-enhanced magnetic resonance imaging identifies a subgroup of patients with asymptomatic monoclonal plasma cell disease and pathologic microcirculation. Clin Cancer Res. 2009;15:3118–3125. doi: 10.1158/1078-0432.CCR-08-2310. [DOI] [PubMed] [Google Scholar]
- 47.Lang N, Su MY, Yu HJ, Lin M, Hamamura MJ, Yuan H. Differentiation of myeloma and metastatic cancer in the spine using dynamic contrast-enhanced MRI. Magn Reson Imaging. 2013;31:1285–1291. doi: 10.1016/j.mri.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirowitz SA, Apicella P, Reinus WR, Hammerman AM. MR imaging of bone marrow lesions: relative conspicuousness on T1-weighted, fat-suppressed T2-weighted, and STIR images. AJR Am J Roentgenol. 1994;162:215–221. doi: 10.2214/ajr.162.1.8273669. [DOI] [PubMed] [Google Scholar]
- 49.Rahmouni A, Divine M, Mathieu D, Golli M, Dao TH, Jazaerli N, Anglade MC, Reyes F, Vasile N. Detection of multiple myeloma involving the spine: efficacy of fat-suppression and contrast-enhanced MR imaging. AJR Am J Roentgenol. 1993;160:1049–1052. doi: 10.2214/ajr.160.5.8470574. [DOI] [PubMed] [Google Scholar]
- 50.Shortt CP, Gleeson TG, Breen KA, McHugh J, O’Connell MJ, O’Gorman PJ, Eustace SJ. Whole-Body MRI versus PET in assessment of multiple myeloma disease activity. AJR Am J Roentgenol. 2009;192:980–986. doi: 10.2214/AJR.08.1633. [DOI] [PubMed] [Google Scholar]
- 51.Stäbler A, Baur A, Bartl R, Munker R, Lamerz R, Reiser MF. Contrast enhancement and quantitative signal analysis in MR imaging of multiple myeloma: assessment of focal and diffuse growth patterns in marrow correlated with biopsies and survival rates. AJR Am J Roentgenol. 1996;167:1029–1036. doi: 10.2214/ajr.167.4.8819407. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt GP, Baur A, Stäbler A, Schoenberg SO, Steinborn M, Baltin V, Reiser MF. [Estimation of diffuse bone marrow infiltration of the spine in multiple myeloma: correlation of MRT with histological results] Rofo. 2005;177:745–750. doi: 10.1055/s-2005-857869. [DOI] [PubMed] [Google Scholar]
- 53.Derlin T, Weber C, Habermann CR, Herrmann J, Wisotzki C, Ayuk F, Wolschke C, Klutmann S, Kröger N. 18F-FDG PET/CT for detection and localization of residual or recurrent disease in patients with multiple myeloma after stem cell transplantation. Eur J Nucl Med Mol Imaging. 2012;39:493–500. doi: 10.1007/s00259-011-1993-8. [DOI] [PubMed] [Google Scholar]
- 54.Durie BG, Waxman AD, D’Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med. 2002;43:1457–1463. [PubMed] [Google Scholar]
- 55.Nanni C, Zamagni E, Farsad M, Castellucci P, Tosi P, Cangini D, Salizzoni E, Canini R, Cavo M, Fanti S. Role of 18F-FDG PET/CT in the assessment of bone involvement in newly diagnosed multiple myeloma: preliminary results. Eur J Nucl Med Mol Imaging. 2006;33:525–531. doi: 10.1007/s00259-005-0004-3. [DOI] [PubMed] [Google Scholar]
- 56.Zamagni E, Nanni C, Patriarca F, Englaro E, Castellucci P, Geatti O, Tosi P, Tacchetti P, Cangini D, Perrone G, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92:50–55. doi: 10.3324/haematol.10554. [DOI] [PubMed] [Google Scholar]
- 57.Nanni C, Rubello D, Zamagni E, Castellucci P, Ambrosini V, Montini G, Cavo M, Lodi F, Pettinato C, Grassetto G, et al. 18F-FDG PET/CT in myeloma with presumed solitary plasmocytoma of bone. In Vivo. 2008;22:513–517. [PubMed] [Google Scholar]
- 58.Fonti R, Salvatore B, Quarantelli M, Sirignano C, Segreto S, Petruzziello F, Catalano L, Liuzzi R, Rotoli B, Del Vecchio S, et al. 18F-FDG PET/CT, 99mTc-MIBI, and MRI in Evaluation of Patients with Multiple Myeloma. J Nucl Med. 2008;49:195–200. doi: 10.2967/jnumed.107.045641. [DOI] [PubMed] [Google Scholar]
- 59.Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, Kumar S, Munshi NC, Dispenzieri A, Kyle R, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100) Leukemia. 2009;23:1904–1912. doi: 10.1038/leu.2009.127. [DOI] [PubMed] [Google Scholar]
- 60.Sager S, Ergül N, Ciftci H, Cetin G, Güner SI, Cermik TF. The value of FDG PET/CT in the initial staging and bone marrow involvement of patients with multiple myeloma. Skeletal Radiol. 2011;40:843–847. doi: 10.1007/s00256-010-1088-9. [DOI] [PubMed] [Google Scholar]
- 61.Ak I, Gulbas Z. F-18 FDG uptake of bone marrow on PET/CT scan: it’s correlation with CD38/CD138 expressing myeloma cells in bone marrow of patients with multiple myeloma. Ann Hematol. 2011;90:81–87. doi: 10.1007/s00277-010-1037-7. [DOI] [PubMed] [Google Scholar]
- 62.Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, Tacchetti P, Buttignol S, Perrone G, Brioli A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989–5995. doi: 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- 63.Haznedar R, Akı SZ, Akdemir ÖU, Özkurt ZN, Çeneli Ö, Yağcı M, Sucak GT, Ünlü M. Value of 18F-fluorodeoxyglucose uptake in positron emission tomography/computed tomography in predicting survival in multiple myeloma. Eur J Nucl Med Mol Imaging. 2011;38:1046–1053. doi: 10.1007/s00259-011-1738-8. [DOI] [PubMed] [Google Scholar]
- 64.Bartel TB, Haessler J, Brown TL, Shaughnessy JD, van Rhee F, Anaissie E, Alpe T, Angtuaco E, Walker R, Epstein J, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–2076. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Usmani SZ, Mitchell A, Waheed S, Crowley J, Hoering A, Petty N, Brown T, Bartel T, Anaissie E, van Rhee F, et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood. 2013;121:1819–1823. doi: 10.1182/blood-2012-08-451690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nanni C, Zamagni E, Celli M, Caroli P, Ambrosini V, Tacchetti P, Brioli A, Zannetti B, Pezzi A, Pantani L, et al. The value of 18F-FDG PET/CT after autologous stem cell transplantation (ASCT) in patients affected by multiple myeloma (MM): experience with 77 patients. Clin Nucl Med. 2013;38:e74–e79. doi: 10.1097/RLU.0b013e318266cee2. [DOI] [PubMed] [Google Scholar]
- 67.Fonti R, Larobina M, Del Vecchio S, De Luca S, Fabbricini R, Catalano L, Pane F, Salvatore M, Pace L. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med. 2012;53:1829–1835. doi: 10.2967/jnumed.112.106500. [DOI] [PubMed] [Google Scholar]
- 68.Lin C, Ho CL, Ng SH, Wang PN, Huang Y, Lin YC, Tang TC, Tsai SF, Rahmouni A, Yen TC. (11)C-acetate as a new biomarker for PET/CT in patients with multiple myeloma: initial staging and postinduction response assessment. Eur J Nucl Med Mol Imaging. 2014;41:41–49. doi: 10.1007/s00259-013-2520-x. [DOI] [PubMed] [Google Scholar]
- 69.Nanni C, Zamagni E, Cavo M, Rubello D, Tacchetti P, Pettinato C, Farsad M, Castellucci P, Ambrosini V, Montini GC, et al. 11C-choline vs. 18F-FDG PET/CT in assessing bone involvement in patients with multiple myeloma. World J Surg Oncol. 2007;5:68. doi: 10.1186/1477-7819-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamoto Y, Kurihara K, Nishizawa M, Yamashita K, Nakatani K, Kondo T, Takaori-Kondo A, Togashi K. Clinical value of ¹¹C-methionine PET/CT in patients with plasma cell malignancy: comparison with ¹⁸F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:708–715. doi: 10.1007/s00259-012-2333-3. [DOI] [PubMed] [Google Scholar]
- 71.Isoda A, Higuchi T, Nakano S, Arisaka Y, Kaira K, Kamio T, Mawatari M, Matsumoto M, Sawamura M, Tsushima Y. ¹⁸F-FAMT in patients with multiple myeloma: clinical utility compared to ¹⁸F-FDG. Ann Nucl Med. 2012;26:811–816. doi: 10.1007/s12149-012-0645-9. [DOI] [PubMed] [Google Scholar]
- 72.Callander NS, Roodman GD. Myeloma bone disease. Semin Hematol. 2001;38:276–285. doi: 10.1016/s0037-1963(01)90020-4. [DOI] [PubMed] [Google Scholar]
- 73.Horger M, Weisel K, Horger W, Mroue A, Fenchel M, Lichy M. Whole-body diffusion-weighted MRI with apparent diffusion coefficient mapping for early response monitoring in multiple myeloma: preliminary results. AJR Am J Roentgenol. 2011;196:W790–W795. doi: 10.2214/AJR.10.5979. [DOI] [PubMed] [Google Scholar]
- 74.Lin C, Luciani A, Belhadj K, Deux JF, Kuhnowski F, Maatouk M, Beaussart P, Cuenod CA, Haioun C, Rahmouni A. Multiple myeloma treatment response assessment with whole-body dynamic contrast-enhanced MR imaging. Radiology. 2010;254:521–531. doi: 10.1148/radiol.09090629. [DOI] [PubMed] [Google Scholar]
- 75.Fenchel M, Konaktchieva M, Weisel K, Kraus S, Claussen CD, Horger M. Response assessment in patients with multiple myeloma during antiangiogenic therapy using arterial spin labeling and diffusion-weighted imaging: a feasibility study. Acad Radiol. 2010;17:1326–1333. doi: 10.1016/j.acra.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Derlin T, Peldschus K, Münster S, Bannas P, Herrmann J, Stübig T, Habermann CR, Adam G, Kröger N, Weber C. Comparative diagnostic performance of ¹⁸F-FDG PET/CT versus whole-body MRI for determination of remission status in multiple myeloma after stem cell transplantation. Eur Radiol. 2013;23:570–578. doi: 10.1007/s00330-012-2600-5. [DOI] [PubMed] [Google Scholar]


