Abstract
Rheumatoid arthritis is a chronic systemic inflammatory disease that often affects the cervical spine. While it was initially thought that cervical involvement was innocuous, natural history studies have substantiated the progressive nature of untreated disease. Over the past 50 years, there has been further elucidation in the pathophysiology of the disease, as well as significant advancements in medical and surgical therapy. The introduction of disease modifying drugs and biologic agents has reduced the amount of patients with advanced stages of the disease needing surgery. Advancement in instrumentation techniques has improved patient outcomes and fusion rates. The introduction of endoscopic approaches for ventral decompression may further lower surgical morbidity. In this review, we give a brief overview of the pertinent positives of the disease. A discussion of historical techniques and the evolution of surgical therapy into the modern era is provided. With improved medical therapies and less invasive approaches, we will likely continue to see less advanced cases of disease and less surgical morbidity. Nonetheless, a thorough understanding of the disease is crucial, as its systemic involvement and need for continued medical therapy have tremendous impact on overall complications and outcomes even in patients being seen for standard degenerative disease with comorbid rheumatoid.
Keywords: Atlantoaxial instability, Cranial settling, Subaxial subluxation, Cervical, Surgery, Morbidity, Rheumatoid arthritis
Core tip: This review summarizes the pertinent features of cervical rheumatoid arthritis. A discussion of important preoperative considerations and surgical approaches in a modern era with advancing medical therapy is provided. The evolution of surgical techniques and outcomes are also highlighted.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic relapsing inflammatory disorder that primarily affects synovial joints with varying degrees of systemic involvement[1,2]. Although an awareness of the disease and its propensity to involve the cervical spine were appreciated as early as the 18th and 19th century[3,4], an observational approach was generally advocated until Matthew and colleagues’ seminal natural history study demonstrated clear radiographic and neurologic progression[5]. Several studies have since confirmed the progressive and grim natural history of untreated disease of the cervical spine[6-11]. Thus, a role for surgery was established, and it is now widely accepted that operative intervention should ideally occur before neurologic deterioration, particularly given poor outcomes with advanced disease[6]. With advancement in surgical instrumentation and newer medical therapies, however, there have been recent trends towards less surgery and less invasive approaches, along with a decrease in surgical volume as biologic agents have been shown to reduce the amount of de novo cervical lesions[12,13]. In this review, we provide an overview of the pertinent disease features of rheumatoid and important preoperative considerations for surgical planning. Finally, we briefly discuss the evolution of surgical therapies and modern techniques and emerging techniques and their impact on overall morbidity and surgical outcomes.
PRESENTATION
Rheumatoid arthritis typically presents in the 4th and 5th decades of life, and more often afflicts females than males (2-4 fold)[1]. The prevalence in the United States among whites is estimated to be 0.5%-1% (roughly 1.3 million adults)[14,15]. RA accounts for significant disability and loss of work force worldwide[16]. Half of those affected are unable to work within 10 years of disease onset and life time costs rival that of coronary artery disease and stroke[1].
In general, rheumatoid can manifest in any joint. However, the metacarpophalangeal and proximal interphalangeal joints of the hand, the metatarsophalangeal joints of the feet, and the wrists and knee are most often affected[17]. Given the systemic nature of the disease, patients can also present with constitutional symptoms or a myriad of extra-articular manifestations. Extra-articular manifestations have a higher incidence in those with an accompanying vasculitis and may range from subcutaneous nodules and nail bed thrombi to pleurisy, pulmonary fibrosis, and/or pericarditis[18-23]. Other peripheral complications include cutaneous ulcers and neuropathy[18,24]. The cervical region is the most frequently involved area of the spine and can show radiographic changes in up to 88%[25], though both the thoracic and lumbar spine can also be involved[26,27].
PATHOPHYSIOLOGY AND CERVICAL MANIFESTATIONS
A general understanding of RA pathophysiology can help explain its presentation in patients whose cervical spine is affected by the disease. While the precise etiology has yet to be fully elucidated, the prevailing hypothesis is that RA results from a humoral autoimmune response arising from exposure to an environmental agent (i.e., Epstein-Barr Virus) in genetically predisposed individuals[28,29]. Following exposure to an environmental trigger, antigen presentation by macrophages (particularly in those with variants in HLA-DR4 and DR-1) initiates an inflammatory cascade and release of cytokines[30]. This is believed to result in both the formation of autoantibodies such as rheumatoid factor (present in 80% of individuals of the disease[18]) and an inflammatory infiltrate of synovial joints, otherwise known as a pannus. The synovial infiltrate is comprised of T cells (predominantly Th1 cells), B cells, plasma cells, natural killer cells, dendritic cells, and mast cells[31]. The autoantibodies lead to further activation of the complement system and neutrophils. Synovial fibroblasts, macrophages, and T cells secrete cytokines (interleukin-1, interleukin-17, tumor necrosis factor etc) and digestive enzymes (e.g., matrix metalloproteinases, collagenases) that result in osteoclast activation and ultimately destruction of adjacent cartilage, tendons, and bone[32-35]. The ongoing inflammatory response leads to either progressive spinal instability from ligamentous laxity and facet involvement, direct neural compression (i.e., pannus), or compromises in blood supply to the spinal cord in cases with cervical disease[36].
Classically, rheumatoid can manifest in the cervical spine as atlantoaxial subluxation, cranial settling (also termed “basilar invagination”), and/or subaxial subluxation (Figure 1). Other manifestations include a C1-2 pannus (present in up to 81% on MRI)[25], odontoid erosions or fracture[37], or rarely an inflammatory discitis[26]. In a moderate sized cohort (N = 106), Kawaguchi et al[27] found the overall rate of cervical spine involvement to be 65%, with atlantoaxial subluxation occurring most commonly (47%), followed by odontoid erosion (35%) and subaxial subluxation (20%). More subtle signs of early involvement include a neurocentral synovitis of superficial joints and erosion of the lateral disk margins, with little osteophyte formation[9,38,39]. Varying degrees of fibrosis and ankylosis are also not uncommon[40]. Histologic analysis has also confirmed the presence of fibrinoid changes in the apical and interspinous ligaments[41]. Finally, osteoporosis can also often accompany rheumatoid[41,42].
Figure 1.
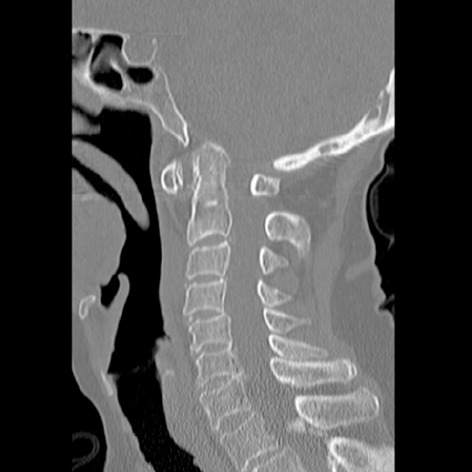
Sagittal computed tomography of the cervical spine of an 82-year-old female with rheumatoid arthritis and neck pain with cranial settling.
SIGNS AND SYMPTOMS OF CERVICAL SPINE MANIFESTATIONS
The aforementioned changes can result in a variety of symptoms in rheumatoid patients including pain (occipital headache and neck pain), myelopathy and cranial nerve palsies, or signs of vascular insufficiency (i.e., Wallenberg syndrome or syncope)[43-45]. While pain is the most common symptom of cervical involvement and may suggest instability, it is nonspecific; Neva and colleagues found that 65% of patients in a rheumatoid cohort reported pain and had no evidence of radiographic subluxation[43]. Occipital headaches may indicate impingement of the greater or lesser occipital nerves and is present in 60% of patients with atlantoaxial subluxation and 90% with cranial settling[46]. Pain can be somewhat regionalized to the area involved. In other words, suboccipital pain can indicate atlantoaxial pathology, while mid to lower cervical pain can correlate with subaxial instability. Patients with C1-2 instability may report a “clunking” with movement, also termed the Sharp-Purser test[47]. Signs and symptoms of myelopathy include limb paresthesias, numbness, weakness, and bladder or bowel disturbances. Hand deformities and peripheral neuropathy can mask myelopathy in RA. Bell’s cruciate paralysis has been described with cervicomedullary compression with cranial settling and describes upper motor neuron weakness greater in the arms than legs due to a more caudal decussation of the lateral corticospinal tracts supplying the legs[48]. Cranial nerve involvement (usually glossopharyngeal, vagus, and hypoglossal) can also result from cranial settling. Dysfunction of one or more cranial nerves has been reported in up to 20% of individuals[46]. Other signs of bulbar compression that can result include internuclear ophthalmoplegia, facial diplegia, nystagmus, loss of sensation in the trigeminal distribution, quadriparesis, sleep apnea, and locked-in syndrome[49]. Lastly, sudden death can result from direct brainstem compression or vascular insufficiency[50,51].
NATURAL HISTORY
The majority of the natural history studies for cervical rheumatoid were conducted in the 1980’s, before the development of biological therapies such as anti-tumor necrosis factor agents. As previously mentioned, these agents have been shown to impede de novo involvement of cervical spine. However, they have not been shown to prevent further progression of instability once it has occurred[12,13]. Nonetheless, improved medical therapy has reduced the overall need for surgical intervention. Cervical disease usually develops within 2-10 years of disease onset[52]. Generally, it is felt that atlantoaxial subluxation precedes cranial settling, and that subluxation can falsely appear to reduce once this occurs[53]. The degree of progression has been shown to correlate with peripheral disease of the hands and feet[54]. Fujiwara et al[7] followed a moderate size cohort (N = 173), 29% of which had atlantoaxial subluxation. At 5 years of follow up, they found that 63% with atlantoaxial subluxation progressed and that 39% without prior evidence of disease developed de novo subluxation. Ten patients became myelopathic[7]. Similarly, Pellici noted worsening in subluxation in 80%, de novo subluxation in 27%, and an overall 5-year mortality of 17%[8]. Mikulowski et al[50] reported postmortem findings in 104 rheumatoid patients and found that 11 deaths were associated with cervicomedullary compression from atlantoaxial dislocation[50].
More advanced stages of the disease can have an even worse natural history. Out of 31 total patients, Marks and Sharp noted 15 deaths within 6 mo of presentation[9]. All patients who did not undergo treatment and 50% treated with a soft collar alone died. Casey and colleagues reported on patients with cranial setting (classified as Ranawat 3B or with the inability to walk or feed oneself)[6]. Three out 58 patients refused surgery and died at 1 wk, 2 mo, and 6 mo respectively. The 30-d mortality rate in those who underwent surgery was 13%, and 60% died within 4 years. Only 25% had a favorable outcome. Because of the poor natural history, a general consensus has arisen to intervene before cervical myelopathy or cranial settling occurs[6,10,11,46]. Approximately, 10% with cervical spine involvement will require surgery[8].
While a detailed description of specific medical therapies is beyond the scope of this review, we briefly mention the various medical agents as their cessation becomes important when considering surgical intervention. Commonly employed therapies include non-steroidal anti-inflammatory drugs (NSAIDs), oral steroids, disease-modifying anti-rheumatic drugs (DMARDs) such as methotrexate, sulfasalazine, hydroxychloroquine, and newer biologic agents such as tumor necrosis factor or interleukin-1 antagonists[55]. Other potential adjuncts include osteoporosis agents to improve bone density[56]. In an older series by Sunahara, 76% of patients showed further progression at six years despite medical therapy[51]. Although newer agents show less de novo disease, they are less effective at halting further cervical progression unlike with peripheral disease[57]. Mutilating disease, corticosteroid use, high seropositivity, vasculitis, rheumatoid nodules, and male gender are established risk factors for progression[58,59].
PREOPERATIVE EVALUATION
Radiographic evaluation and criteria for instability
Plain cervical X-rays are recommended for screening RA patients for cervical spine disease. Useful views for evaluation are upright AP and lateral, open-mouth (odontoid view), and flexion-extension for detection of instability (Figure 2). Plain radiographs can be limited, however, by bony erosion and inability to visualize soft tissue compression. CT is useful for determining overall bone quality and for surgical planning especially with C2 fixation techniques[60]. MRI has the highest sensitivity for detecting disease of all three modalities[61]. General recommendations for obtaining MRI are the presence of neurologic deficits, a predental space of 7 to 8 mm, and abnormal radiographic pathology (i.e., cranial settling, odontoid erosion, or subaxial subluxation). Additionally, MRI can be useful for detecting pannus. While it was initially though that T2 hyperintensity correlates with regression after fusion, it has been since been shown that pannus regression is independent of MR signal and can resolve after posterior fusion despite its MR intensity (Figure 3)[62-64]. Lastly, preoperative CT and MRI are useful for preoperative planning and can be incorporated into the operative theater with intraoperative navigation to improve safety during transoral decompression[65].
Figure 2.
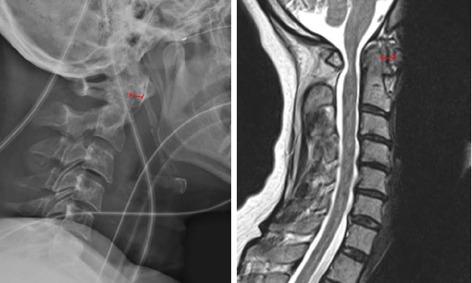
Lateral X-ray (left) and sagittal magnetic resonance imaging (right) of a 52-year-old with atlantoaxial subluxation. Left: Lateral X-ray; Right: Sagittal Magnetic resonance imaging. The anterior atlantodental interval is shown (red line).
Figure 3.
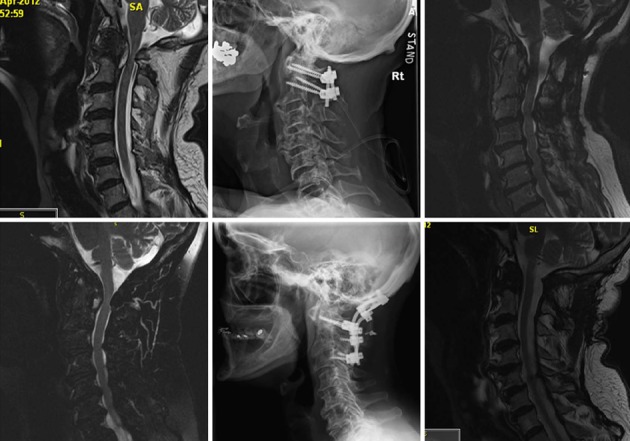
Resolving rheumatoid pannus after occipital cervical (top) and C1-2 fusion (bottom). Left: Preoperative magnetic resonance imagings; Middle: Postoperative lateral X-ray; Right: Postoperative magnetic resonance imagings.
Evaluating for atlantoaxial instability
Although the atlanto-dental interval (ADI) has been used as a measure for atlantoaxial instability, the posterior atlanto-dental interval (PADI) has been shown to be a more reliable indicator and correlates with neurologic improvement after surgery and the development of myelopathy. Normal ADI is defined as 0-3 mm, whereas values between 6-10 mm have been cited as cutoffs for instability and indications for surgery[37,66-69]. Cut-off values on lateral radiographs of 13 and 14 mm have been suggested for PADI[70-73]. These values correlate with anatomical studies at C1 showing the width of the cord, dura (anterior and posterior) and cerebrospinal fluid space of 10 mm, 1 mm, and 2 mm, respectively[71,74]. More recently, it has been shown that preoperative neurologic function is directly related to increased intramedullary T2 signal, which also corresponds to ADI and PADI on lateral radiographs[75]. Open mouth odontoid views are useful for detecting lateral subluxation, with a distance of greater than 2 mm being shown to correlate with spinal cord compression[37,76].
Evaluation for cranial settling
A variety of radiographic parameters have been used to identify cranial settling (Table 1). Classic indicators evaluate the location of the odontoid process with respect to the foramen magnum (McRae’s line)[77] or with respect to the hard palate and the base (Chamberlain’s line)[78] and midpoint of the opisthion (McGregor’s line)[79]. Newer classifications describe the relation of C1 relative to C2 (e.g., Ranawat’s Criteria and Clark’s station of the atlas) or the base of C2 relative the palatal-occipital line (Redlund-Johnell)[66,80,81]. While all can potentially be used, none have a sensitivity, specificity, or negative or positive predictive value of greater than 90%[79]. Riew and colleagues conducted a meta-analysis which found that combining Clark station, Redlund-Johnell criteria, and Ranawat criteria yielded a sensitivity and negative predictive values of 94% and 91%, and thus recommend the use of all three when evaluating for cranial settling[79].
Table 1.
Criteria for cranial settling in rheumatoid
| Measurement/Criteria | Description | Definition of abnormal |
| McRae’s line | Tip of the basion to opisthion | If any portion of the odontoid extends superior to this line |
| McGregor’s line | Hard palate to caudal aspect of the opisthion | > 4.5 mm of the dens is superior to this line |
| Chamberlain’s line | Hard palate to the midpoint of the opisthion | > 3 mm of the dens extends superior to this line |
| Ranawat’s1 | Distance from the C2 pedicle to a line bisecting the ring of C1 | < 15 mm in males, < 13 mm in females |
| Redlund-Johnell and Peterson1 | Distance from the inferior end plate of C2 to McGregor’s palato-occipital line | < 34 mm in males, < 29 mm in females |
| Clark’s station of the atlas1 | Position of C1 with relation to the body of C2 (divided into thirds) | If C1 extends below the rostral third of C2 |
Recommended criteria by Riew et al[79].
Evaluation of the subaxial spine
Boden and colleagues define a cut off for critical stenosis as less than 14 mm in subaxial spine[71]. Other commonly used criteria on plain radiographs include White and Panjabi’s, which uses a value of greater than 3.5 mm of vertebral translation or greater than 11 degrees between adjacent motion segments as markers for subaxial instability[82].
Cervicomedullary angle
The cervicomedullary angle measured on MRI or myelography is predictive of neurologic compression[83]. The cervicomedullary angle corresponds with the angle formed by the intersection of vertical lines drawn along the anterior surface of the brainstem and the spinal cord on sagittal MRI. The range in normal individuals is 135-175 compared to less than 135 degrees in those with myelopathy.
EFFECT OF RHEUMATOID MEDICATION ON SURGERY
The cessation or continuation of various rheumatoid medications is an important perioperative consideration (summary provided in Table 2). Because NSAIDs inhibit platelet function and thus increase the risk for intraoperative blood loss and postoperative hemorrhage, they should be discontinued 3 to 5 half-lives before surgery[84]. Additionally, NSAIDs have been shown to inhibit bone formation and should be withheld after surgery if possible. Corticosteroids impair bone and wound healing and can cause adrenal suppression in patients on an equivalent of 20 mg/d of prednisone or more. Perioperative stress doses should be given to these individuals[84,85]. While methotrexate has not been shown to increase in infection rates[86], it may affect bone healing[87] and should be discontinued for 6 to 8 wk if possible. Finally, biologic agents (tumor necrosis factor-α and interleukin-1 antagonists) increase the risk of opportunistic infections (11% reported by Giles et al[88]) and should be stopped preoperatively and held until 10 to 14 d after surgery[84,85].
Table 2.
Common rheumatoid arthritis medications and perioperative considerations
| RA medication | Preoperative action |
| NSAIDs | Discontinue 3-5 half-lives before surgery |
| Corticosteroids | Administer perioperative stress doses |
| Methotrexate | Discontinue for 6-8 wk if possible |
| Biologic agents (TNF-α and interleukin-1 antagonists) | Discontinue preoperatively and hold until 10-14 d post-surgery |
RA: Rheumatoid arthritis; NSAIDs: Non-steroidal anti-inflammatory drugs; TNF-α: Tumor necrosis factor α.
OVERALL IMPACT OF RA ON SURGICAL COMPICATIONS AND OUTCOMES
There are several general considerations that should be noted about rheumatoid patients before considering any surgery. Because of the overall systemic effects of the disease, patients will have higher complication rates than would be expected for other indications that involve the same surgery. For example, patients with comorbid rheumatoid and lumbar pathology have been shown to have higher wound and implant related complications[89-91]. Similar findings have also been reported in other orthopedic procedures such as total hip arthroplasty[92,93]. Nonunion and instrumentation failures are impart related to baseline osteopenia or osteoporosis and also due to anti-rheumatoid agents that impair fusion[88,89,91]. Rheumatoid patients are also more prone to develop infections[94-96]. In a large matched cohort with over 10 years of follow up, Doran et al[95] found that rheumatoid patients had higher overall incidence of infections with particular predilection for bone, joints, skin, soft tissues, and the respiratory tract. The higher infection rates were felt to be due to alterations in immunity from rheumatoid and immunosuppression from rheumatoid medication. Pulmonary involvement especially has an impact on overall morbidity with surgery and also increases the risk of premature mortality[93,97,98]. Increased morbidity and ongoing systemic disease result in higher resource utilization and worse outcome in rheumatoid patients[93,99,100]. Thus, it is imperative that these patients undergo thorough medical evaluation and preoperative optimization to mitigate these effects as much as possible. Lastly, outcomes are largely determined by preoperative neurologic function. Wolfs and colleagues performed a meta-analysis on 752 rheumatoid patients (25 studies) and found that those who were Ranawat class I and II rarely had deterioration in neurologic function. Whereas, patients with Ranawat class IIIB function had significantly worse outcomes with 43% and 70% mortality rates at 5 and 10 years, respectively[101].
SURGICAL INDICATIONS
Indications for surgery include medically refractory pain, neurologic deficits (myelopathy or cranial nerve/bulbar dysfunction), and radiographic instability as defined previously[41,64,71,102-104]. The ultimate goals of surgery are to relieve neurologic compression and eradicate instability, thereby preventing further neurologic decline[44].
PREOPERATIVE TRACTION AND SURGICAL APPROACH
Prior to surgery both the reducibility of the lesion and vector of compression need to be considered (Figure 4). Atlantoaxial instability can often be reduced with positioning intraoperatively, and thus, preoperative traction is mostly used for cases of cranial settling. Traction is contraindicated only in cases of complex rotary subluxations and posterior occipito-atlantoaxial dislocations due to risk of distracting the vertebral artery[40]. Otherwise, traction can be initiated with 5-7 lbs. and gradually increased to a maximum of 10-11 lbs. by 36 h[40]. Periodic radiographs and serial neurologic examinations should be performed. Instrumented fusion can be performed in cases of cranial settling which reduce with traction. If no reduction occurs by 4 to 5 d, however, decompression in the vector of the offending pathology should be performed followed by instrumented fusion. Roughly 80% of cases will reduce[44]. Negative predictors for reduction include odontoid penetration beyond 15 mm through the foramen magnum, large pannus, odontoid fractures, and cranial settling complicated by lateral or rotatory subluxation[40].
Figure 4.
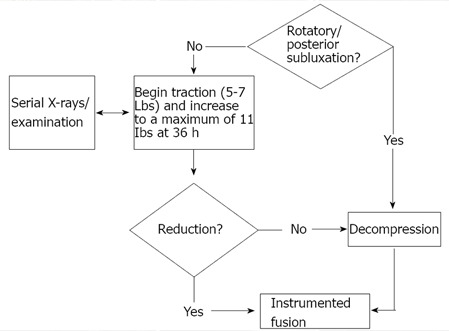
Preoperative traction and surgical approach to cervical rheumatoid.
FUSION AND DECOMPRESSION TECHNIQUES
Occipitocervical fusion is indicated for cranial settling or for fixed atlantoaxial subluxations with posterior cord impingement by C1 in which case a C1 laminectomy is also performed[105-107]. The technique was originally described by Foerster in 1927 and then modified by Hamblen with the addition of iliac crest grafts in 1967[108,109]. Ransford and Flint later popularized the loop-rod technique[110,111], which eventually became supplanted by occipitocervical plating as described by Grob[112,113] and Smith[114]. Occipitocervical plating is more rigid and commonly used today[112,115,116]. Typically occipitocervical fusions extend down to at least C2 with or without a C1 laminectomy (which is preferred by some even in reducible lesions). The fusion may need to extend further into the subaxial spine depending on the bone quality and screw fixation.
A C1-2 fusion is considered the surgery of choice for atlantoaxial subluxation (Figure 5). Historically, Gallie wiring and grafting techniques were used[117], which were further modified by Brooks and Jenkins[118], Wertheim and Bohlman[119], and Clark and colleagues[66]. Halo immobilization was often used to supplement wiring techniques to improve arthrodesis rates, but still could have failure rates of 20%[69,120,121]. As instrumentation methods improved, however, these techniques have been replaced or combined with screw and rod instrumentation. Magerl originally described the use of C1-2 articular screws[122,123]. Goel would later describe plate and screw fixation for atlanto-axial subluxation which was further modified by Harms and Melcher to posterior C1 lateral mass and C2 pedicle or pars screws[124,125]. When using C2 fixation techniques it is paramount to consider the course of the vertebral artery which can be defined with preoperative CT or CT angiography[126]. A high riding vertebral artery or narrow C2 isthmus can be prohibitive to C2 transarticular screw fixation. Other contraindications include collapsed lateral masses or significant cranial settling, irreducible subluxations, poor bone quality, or loss of osseous integrity of C1 or C2. The C2 isthmus should be wide enough to accommodate a 3.5 mm screw. The starting point is 3 mm above the C2-3 facet articulation, 2-3 mm lateral to the medial border of the C2 facet, and the trajectory is 0-10 degrees medially aimed at the anterior arch of C1. Screw size is typically a width of 3.5 to 4.5 mm and 40 to 44 mm long. Optimally placed transarticular screws have a fusion rate of roughly 95%[127], though it can be difficult to capture both the C1 and C2 vertebrae. The Harm’s technique involves placing polyaxial screws (3.5 mm) directly into the lateral mass of C1 and into the pars or pedicle of C2 bilaterally. C2 pedicle fixation can be performed provided the pedicle width is wide enough to accommodate a screw (at least 6 mm per Alosh et al[60]). Overall the literature suggests that incidence of vertebral artery injury is low with either transarticular or pedicle screw fixation techniques, and both have greater than 90% fusion rates[128-131]. Although no prospective comparisons have been conducted, pooled meta-analysis suggests that pedicle screws may have a lower risk of misplacement and vertebral artery injury[132]. In the authors’ opinion, either technique is acceptable provided the surgeon has sufficient experience and a thorough knowledge of the patient’s anatomy. Other C2 fixation techniques have been described to lower the risk for vertebral artery injury or for cases with unfavorable anatomy. Tokuhashi and colleagues describe an alternative technique that involves the use of Halifax interlaminar clamps to achieve intraoperative reduction and placement of an interference screw that is secured to a corticocancellous graft[133]. Intralaminar screws can also be used as an alternative method of C2 fixation when anatomy for pedicle or transarticular screws is unfavorable[134]. From a biomechanical perspective, C2 pedicle screws provide greatest overall stability[135]. Lapiswala et al[136] demonstrated superior lateral bending moments with pedicle and transarticular screws compared to intralaminar screws, though with wire supplementation, all have equivalent moments in flexion, extension, and axial rotation. The senior author prefers pedicle screw fixation in patients with favorable vertebral artery anatomy due to their increased biomechanical strength.
Figure 5.
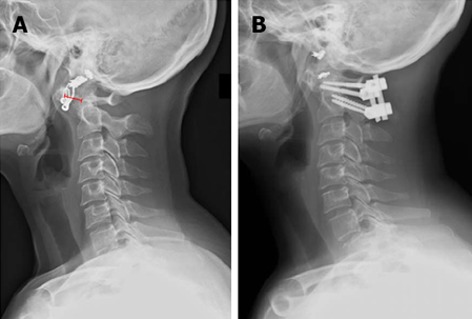
Lateral X-ray images of the spine before (A) and after (B) surgical intervention. The anterior atlantodental interval is shown (red line).
Subaxial fusion techniques have also evolved from wiring (Bohwman’s triple wire technique) to plating and screws, to polyaxial lateral screws and rods[123,137,138]. Three variants of lateral mass screws have been described by An, Magerl, and Roy-Camille. While pedicle screws have been shown to have the highest pullout strength, we do not recommend their routine use due to a higher risk of vertebral artery injury[139,140]. Our personal preference is to use a modified An technique due to a lower rate of nerve root violation compared to other techniques[141], and to reserve other techniques as potential rescue methods.
For cases that are irreducible or in cases in which a pannus fails to regress, decompression is indicated. A standard C1 laminectomy is indicated for cases of posterior impingement of the cord. Ventral decompression has traditionally been performed through a transoral approach. These are often complicated by swallowing dysfunction and postoperative airway swelling, necessitating tracheostomy and percutaneous gastrostomy placement[142]. Because of a high failure rate of successful postoperative extubation, we often place a tracheostomy prior to transoral approach. We have also found it resourceful to use intraoperative navigation as an adjunct[65]. More recently, endoscopic approaches via a transnasal or transoral route have been advocated[143-145]. In addition to reducing swallowing dysfunction and the need for tracheostomy, these approaches may also allow for preservation of the anterior arch of C1 and perhaps obviate the need for posterior fixation in select cases[143-151]. A transcervical endoscopic approach is also feasible and may mitigate morbidity as demonstrated in a small cohort (N = 15) by Dasenbrock et al[152] in which all were able to avoid the need for postoperative tracheostomy. While these approaches potentially offer less invasive techniques for ventral decompression, further prospective and comparative studies will be necessary to determine their role in the management of rheumatoid patients.
OUTCOMES
Surgical outcomes are generally better in patients with less preoperative impairment. In a series of 28 patients, Schmitt-Sody et al[153] found that 7 out of 10 patients that were Ranawat class II improved to class I, whereas 1 out of 11 class IIIA improved to class II, and 2 patients deteriorated to Class IIIB. Ranawat et al[154] noted that outcomes were particularly poor in non-ambulatory patients (Ranawat Class IIIB). Other poor prognosticators include a spinal cord area of less than 44 mm2 and PADI of less than 10 mm[154]. Boden et al[71] noted significant motor improvement in patients with had preoperative PADI of 14 mm or more. Advanced age, atlantoaxial instability, and postoperative complications have all been found to be predictors of mortality[39,155]. Lastly, intervening early and before cranial settling occurs has been shown to decrease the risk for future instability. Agarwal and colleagues found that 5.5% of patients undergoing early intervention for atlantoaxial subluxation developed recurrent instability (mean 9 years) compared to 36% who underwent occipitocervical fusion cranial settling[156]. Clarke et al[157] found that 39% of rheumatoid patients undergoing surgery for atlantoaxial subluxation subsequently developed subaxial subluxation, 54% of which required further fusion.
CONCLUSION
The treatment of cervical rheumatoid has significantly evolved over the past 50 years. A disease with potentially grim outcomes has been improved with surgery. Additionally, the advanced stages of the disease are less commonly seen due to improved medical therapies. Significant advances in surgical instrumentation no longer require internal and external fixation, and fusion rates have improved. Finally, the use of endoscopic approaches may potentially lower the morbidity with ventral decompression, though further prospective study will be necessary to elucidate their role and whether they can obviate the need for fusion.
Footnotes
P- Reviewers: Iizuka H, Sumi M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Koopman WJ. Prospects for autoimmune disease: Research advances in rheumatoid arthritis. JAMA. 2001;285:648–650. doi: 10.1001/jama.285.5.648. [DOI] [PubMed] [Google Scholar]
- 2.Silman AJ. Rheumatoid arthritis and infection: a population approach. Ann Rheum Dis. 1989;48:707–710. doi: 10.1136/ard.48.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen SE. The recognition of rheumatoid arthritis in the eighteenth century. The contribution of Linné and Boissier de la Croix de Sauvages. Scand J Rheumatol. 1993;22:178–182. doi: 10.3109/03009749309099267. [DOI] [PubMed] [Google Scholar]
- 4.Garrod AE. A Treatise on Rheumatism and Rheumatoid Arthritis. London: Griffin; 1890. [Google Scholar]
- 5.Mathews JA. Atlanto-axial subluxation in rheumatoid arthritis. A 5-year follow-up study. Ann Rheum Dis. 1974;33:526–531. doi: 10.1136/ard.33.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey AT, Crockard HA, Bland JM, Stevens J, Moskovich R, Ransford A. Predictors of outcome in the quadriparetic nonambulatory myelopathic patient with rheumatoid arthritis: a prospective study of 55 surgically treated Ranawat class IIIb patients. J Neurosurg. 1996;85:574–581. doi: 10.3171/jns.1996.85.4.0574. [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara K, Owaki H, Fujimoto M, Yonenobu K, Ochi T. A long-term follow-up study of cervical lesions in rheumatoid arthritis. J Spinal Disord. 2000;13:519–526. doi: 10.1097/00002517-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Pellicci PM, Ranawat CS, Tsairis P, Bryan WJ. A prospective study of the progression of rheumatoid arthritis of the cervical spine. J Bone Joint Surg Am. 1981;63:342–350. [PubMed] [Google Scholar]
- 9.Marks JS, Sharp J. Rheumatoid cervical myelopathy. Q J Med. 1981;50:307–319. [PubMed] [Google Scholar]
- 10.Crockard HA, Essigman WK, Stevens JM, Pozo JL, Ransford AO, Kendall BE. Surgical treatment of cervical cord compression in rheumatoid arthritis. Ann Rheum Dis. 1985;44:809–816. doi: 10.1136/ard.44.12.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden SD. Rheumatoid arthritis of the cervical spine. Surgical decision making based on predictors of paralysis and recovery. Spine (Phila Pa 1976) 1994;19:2275–2280. doi: 10.1097/00007632-199410150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Kaito T, Hosono N, Ohshima S, Ohwaki H, Takenaka S, Fujiwara H, Makino T, Yonenobu K. Effect of biological agents on cervical spine lesions in rheumatoid arthritis. Spine (Phila Pa 1976) 2012;37:1742–1746. doi: 10.1097/BRS.0b013e318256b584. [DOI] [PubMed] [Google Scholar]
- 13.Kaito T, Ohshima S, Fujiwara H, Makino T, Yonenobu K. Predictors for the progression of cervical lesion in rheumatoid arthritis under the treatment of biological agents. Spine (Phila Pa 1976) 2013;38:2258–2263. doi: 10.1097/BRS.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 14.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 15.Cathcart ES, O’Sullivan JB. Rheumatoid arthritis in a New England town. A prevalence study in Sudbury, Massachusetts. N Engl J Med. 1970;282:421–424. doi: 10.1056/NEJM197002192820804. [DOI] [PubMed] [Google Scholar]
- 16.Sokka T, Kautiainen H, Pincus T, Verstappen SM, Aggarwal A, Alten R, Andersone D, Badsha H, Baecklund E, Belmonte M, et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther. 2010;12:R42. doi: 10.1186/ar2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grassi W, De Angelis R, Lamanna G, Cervini C. The clinical features of rheumatoid arthritis. Eur J Radiol. 1998;27 Suppl 1:S18–S24. doi: 10.1016/s0720-048x(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 18.Condemi JJ. The autoimmune diseases. JAMA. 1992;268:2882–2892. [PubMed] [Google Scholar]
- 19.Helmers R, Galvin J, Hunninghake GW. Pulmonary manifestations associated with rheumatoid arthritis. Chest. 1991;100:235–238. doi: 10.1378/chest.100.1.235. [DOI] [PubMed] [Google Scholar]
- 20.Hurd ER. Extraarticular manifestations of rheumatoid arthritis. Semin Arthritis Rheum. 1979;8:151–176. doi: 10.1016/s0049-0172(79)80005-0. [DOI] [PubMed] [Google Scholar]
- 21.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 37-1992. A 68-year-old woman with rheumatoid arthritis and pulmonary hypertension. N Engl J Med. 1992;327:873–880. doi: 10.1056/NEJM199209173271209. [DOI] [PubMed] [Google Scholar]
- 22.Young ID, Ford SE, Ford PM. The association of pulmonary hypertension with rheumatoid arthritis. J Rheumatol. 1989;16:1266–1269. [PubMed] [Google Scholar]
- 23.Wiedemann HP, Matthay RA. Pulmonary manifestations of the collagen vascular diseases. Clin Chest Med. 1989;10:677–722. [PubMed] [Google Scholar]
- 24.Brito-Zerón P, Akasbi M, Bosch X, Bové A, Pérez-De-Lis M, Diaz-Lagares C, Retamozo S, Gandía M, Pérez-Alvarez R, Soto-Cárdenas MJ, et al. Classification and characterisation of peripheral neuropathies in 102 patients with primary Sjögren’s syndrome. Clin Exp Rheumatol. 2013;31:103–110. [PubMed] [Google Scholar]
- 25.Zikou AK, Alamanos Y, Argyropoulou MI, Tsifetaki N, Tsampoulas C, Voulgari PV, Efremidis SC, Drosos AA. Radiological cervical spine involvement in patients with rheumatoid arthritis: a cross sectional study. J Rheumatol. 2005;32:801–806. [PubMed] [Google Scholar]
- 26.Heywood AW, Meyers OL. Rheumatoid arthritis of the thoracic and lumbar spine. J Bone Joint Surg Br. 1986;68:362–368. doi: 10.1302/0301-620X.68B3.3733796. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi Y, Matsuno H, Kanamori M, Ishihara H, Ohmori K, Kimura T. Radiologic findings of the lumbar spine in patients with rheumatoid arthritis, and a review of pathologic mechanisms. J Spinal Disord Tech. 2003;16:38–43. doi: 10.1097/00024720-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Balandraud N, Roudier J, Roudier C. Epstein-Barr virus and rheumatoid arthritis. Autoimmun Rev. 2004;3:362–367. doi: 10.1016/j.autrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Toussirot E, Roudier J. Epstein-Barr virus in autoimmune diseases. Best Pract Res Clin Rheumatol. 2008;22:883–896. doi: 10.1016/j.berh.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Ollier W, Thomson W. Population genetics of rheumatoid arthritis. Rheum Dis Clin North Am. 1992;18:741–759. [PubMed] [Google Scholar]
- 31.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 32.Koch AE. The pathogenesis of rheumatoid arthritis. Am J Orthop (Belle Mead NJ) 2007;36:5–8. [PubMed] [Google Scholar]
- 33.Firestein GS. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005;11:S39–S44. doi: 10.1097/01.rhu.0000166673.34461.33. [DOI] [PubMed] [Google Scholar]
- 34.Müller-Ladner U, Pap T, Gay RE, Neidhart M, Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005;1:102–110. doi: 10.1038/ncprheum0047. [DOI] [PubMed] [Google Scholar]
- 35.Ainola MM, Mandelin JA, Liljeström MP, Li TF, Hukkanen MV, Konttinen YT. Pannus invasion and cartilage degradation in rheumatoid arthritis: involvement of MMP-3 and interleukin-1beta. Clin Exp Rheumatol. 2005;23:644–650. [PubMed] [Google Scholar]
- 36.Delamarter RB, Bohlman HH. Postmortem osseous and neuropathologic analysis of the rheumatoid cervical spine. Spine (Phila Pa 1976) 1994;19:2267–2274. doi: 10.1097/00007632-199410150-00004. [DOI] [PubMed] [Google Scholar]
- 37.Weissman BN, Aliabadi P, Weinfeld MS, Thomas WH, Sosman JL. Prognostic features of atlantoaxial subluxation in rheumatoid arthritis patients. Radiology. 1982;144:745–751. doi: 10.1148/radiology.144.4.7111719. [DOI] [PubMed] [Google Scholar]
- 38.Ball J, Sharp J. Rheumatoid arthritis of the cervical spine. Mod Trends Rheumatol. 1971;2:117–138. [PubMed] [Google Scholar]
- 39.Sharp J, Purser DW, Lawrence JS. Rheumatoid arthritis of the cervical spine in the adult. Ann Rheum Dis. 1958;17:303–313. doi: 10.1136/ard.17.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menezes AH AG. Acquired Abnormalities of the Craniocervical Junction. In: Youmans Neurological Surgery, Vol 3., editors. 6th ed. Online Version. Philadelphia: WB Saunders; 2011. p. 289. [Google Scholar]
- 41.Lorber A Pearson CM, Rene RM. Osteolytic vertebral lesions as a manifestation of rheumatoid arthritis and related disorders. Arthritis Rheum. 1961;4:514–532. doi: 10.1002/art.1780040508. [DOI] [PubMed] [Google Scholar]
- 42.Mohammad A, Lohan D, Bergin D, Mooney S, Newell J, O’Donnell M, Coughlan RJ, Carey JJ. The prevalence of vertebral fracture on vertebral fracture assessment imaging in a large cohort of patients with rheumatoid arthritis. Rheumatology (Oxford) 2014;53:821–827. doi: 10.1093/rheumatology/ket353. [DOI] [PubMed] [Google Scholar]
- 43.Neva MH, Häkkinen A, Mäkinen H, Hannonen P, Kauppi M, Sokka T. High prevalence of asymptomatic cervical spine subluxation in patients with rheumatoid arthritis waiting for orthopaedic surgery. Ann Rheum Dis. 2006;65:884–888. doi: 10.1136/ard.2005.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menezes AH, VanGilder JC, Clark CR, el-Khoury G. Odontoid upward migration in rheumatoid arthritis. An analysis of 45 patients with “cranial settling”. J Neurosurg. 1985;63:500–509. doi: 10.3171/jns.1985.63.4.0500. [DOI] [PubMed] [Google Scholar]
- 45.Gurley JP, Bell GR. The surgical management of patients with rheumatoid cervical spine disease. Rheum Dis Clin North Am. 1997;23:317–332. doi: 10.1016/s0889-857x(05)70332-x. [DOI] [PubMed] [Google Scholar]
- 46.Menezes AH. Rheumatological disorders. In: Menezes AH, Sonntag VKH, editors. Principles of Spinal Surgery, Vol 1. New York: McGraw-Hill; 1996. pp. 705–722. [Google Scholar]
- 47.Sharp J, Purser DW. Spontaneous Atlanto-Axial Dislocation in Ankylosing Spondylitis and Rheumatoid Arthritis. Ann Rheum Dis. 1961;20:47–77. doi: 10.1136/ard.20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell HS. Paralysis of both arms from injury of the upper portion of the pyramidal decussation: “cruciate paralysis”. J Neurosurg. 1970;33:376–380. doi: 10.3171/jns.1970.33.4.0376. [DOI] [PubMed] [Google Scholar]
- 49.Rana NA, Hancock DO, Taylor AR, Hill AG. Upward translocation of the dens in rheumatoid arthritis. J Bone Joint Surg Br. 1973;55:471–477. [PubMed] [Google Scholar]
- 50.Mikulowski P, Wollheim FA, Rotmil P, Olsen I. Sudden death in rheumatoid arthritis with atlanto-axial dislocation. Acta Med Scand. 1975;198:445–451. doi: 10.1111/j.0954-6820.1975.tb19573.x. [DOI] [PubMed] [Google Scholar]
- 51.Sunahara N, Matsunaga S, Mori T, Ijiri K, Sakou T. Clinical course of conservatively managed rheumatoid arthritis patients with myelopathy. Spine (Phila Pa 1976) 1997;22:2603–2607; discussion 2608. doi: 10.1097/00007632-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 52.Winfield J, Cooke D, Brook AS, Corbett M. A prospective study of the radiological changes in the cervical spine in early rheumatoid disease. Ann Rheum Dis. 1981;40:109–114. doi: 10.1136/ard.40.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oda T, Fujiwara K, Yonenobu K, Azuma B, Ochi T. Natural course of cervical spine lesions in rheumatoid arthritis. Spine (Phila Pa 1976) 1995;20:1128–1135. doi: 10.1097/00007632-199505150-00004. [DOI] [PubMed] [Google Scholar]
- 54.Winfield J, Young A, Williams P, Corbett M. Prospective study of the radiological changes in hands, feet, and cervical spine in adult rheumatoid disease. Ann Rheum Dis. 1983;42:613–618. doi: 10.1136/ard.42.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis JM, Matteson EL. My treatment approach to rheumatoid arthritis. Mayo Clin Proc. 2012;87:659–673. doi: 10.1016/j.mayocp.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mawatari T, Miura H, Hamai S, Shuto T, Nakashima Y, Okazaki K, Kinukawa N, Sakai S, Hoffmann PF, Iwamoto Y, et al. Vertebral strength changes in rheumatoid arthritis patients treated with alendronate, as assessed by finite element analysis of clinical computed tomography scans: a prospective randomized clinical trial. Arthritis Rheum. 2008;58:3340–3349. doi: 10.1002/art.23988. [DOI] [PubMed] [Google Scholar]
- 57.Cha TD, An HS. Cervical spine manifestations in patients with inflammatory arthritides. Nat Rev Rheumatol. 2013;9:423–432. doi: 10.1038/nrrheum.2013.40. [DOI] [PubMed] [Google Scholar]
- 58.Paimela L, Laasonen L, Kankaanpää E, Leirisalo-Repo M. Progression of cervical spine changes in patients with early rheumatoid arthritis. J Rheumatol. 1997;24:1280–1284. [PubMed] [Google Scholar]
- 59.Young A, Corbett M, Winfield J, Jaqueremada D, Williams P, Papasavvas G, Hay F, Roitt I. A prognostic index for erosive changes in the hands, feet, and cervical spines in early rheumatoid arthritis. Br J Rheumatol. 1988;27:94–101. doi: 10.1093/rheumatology/27.2.94. [DOI] [PubMed] [Google Scholar]
- 60.Alosh H, Parker SL, McGirt MJ, Gokaslan ZL, Witham TF, Bydon A, Wolinsky JP, Sciubba DM. Preoperative radiographic factors and surgeon experience are associated with cortical breach of C2 pedicle screws. J Spinal Disord Tech. 2010;23:9–14. doi: 10.1097/BSD.0b013e318194e746. [DOI] [PubMed] [Google Scholar]
- 61.Zoli A, Priolo F, Galossi A, Altomonte L, Di Gregorio F, Cerase A, Mirone L, Magarò M. Craniocervical junction involvement in rheumatoid arthritis: a clinical and radiological study. J Rheumatol. 2000;27:1178–1182. [PubMed] [Google Scholar]
- 62.Stiskal MA, Neuhold A, Szolar DH, Saeed M, Czerny C, Leeb B, Smolen J, Czembirek H. Rheumatoid arthritis of the craniocervical region by MR imaging: detection and characterization. AJR Am J Roentgenol. 1995;165:585–592. doi: 10.2214/ajr.165.3.7645475. [DOI] [PubMed] [Google Scholar]
- 63.Yonezawa I, Okuda T, Won J, Sakoda J, Nakahara D, Nojiri H, Muto O, Momomura R, Kaneko K. Retrodental mass in rheumatoid arthritis. J Spinal Disord Tech. 2013;26:E65–E69. doi: 10.1097/BSD.0b013e3182621a05. [DOI] [PubMed] [Google Scholar]
- 64.Grob D, Würsch R, Grauer W, Sturzenegger J, Dvorak J. Atlantoaxial fusion and retrodental pannus in rheumatoid arthritis. Spine (Phila Pa 1976) 1997;22:1580–1583; discussion 1584. doi: 10.1097/00007632-199707150-00010. [DOI] [PubMed] [Google Scholar]
- 65.Krauss WE, Bledsoe JM, Clarke MJ, Nottmeier EW, Pichelmann MA. Rheumatoid arthritis of the craniovertebral junction. Neurosurgery. 2010;66:83–95. doi: 10.1227/01.NEU.0000365854.13997.B0. [DOI] [PubMed] [Google Scholar]
- 66.Clark CR, Goetz DD, Menezes AH. Arthrodesis of the cervical spine in rheumatoid arthritis. J Bone Joint Surg Am. 1989;71:381–392. [PubMed] [Google Scholar]
- 67.Heywood AW, Learmonth ID, Thomas M. Cervical spine instability in rheumatoid arthritis. J Bone Joint Surg Br. 1988;70:702–707. doi: 10.1302/0301-620X.70B5.3192564. [DOI] [PubMed] [Google Scholar]
- 68.Conaty JP, Mongan ES. Cervical fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1981;63:1218–1227. [PubMed] [Google Scholar]
- 69.Papadopoulos SM, Dickman CA, Sonntag VK. Atlantoaxial stabilization in rheumatoid arthritis. J Neurosurg. 1991;74:1–7. doi: 10.3171/jns.1991.74.1.0001. [DOI] [PubMed] [Google Scholar]
- 70.Oda T, Yonenobu K, Fujimura Y, Ishii Y, Nakahara S, Matsunaga S, Shimizu T, Matsumoto M. Diagnostic validity of space available for the spinal cord at C1 level for cervical myelopathy in patients with rheumatoid arthritis. Spine (Phila Pa 1976) 2009;34:1395–1398. doi: 10.1097/BRS.0b013e3181a2b486. [DOI] [PubMed] [Google Scholar]
- 71.Boden SD, Dodge LD, Bohlman HH, Rechtine GR. Rheumatoid arthritis of the cervical spine. A long-term analysis with predictors of paralysis and recovery. J Bone Joint Surg Am. 1993;75:1282–1297. doi: 10.2106/00004623-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Kawaida H, Sakou T, Morizono Y, Yoshikuni N. Magnetic resonance imaging of upper cervical disorders in rheumatoid arthritis. Spine (Phila Pa 1976) 1989;14:1144–1148. doi: 10.1097/00007632-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Greenberg AD. Atlanto-axial dislocations. Brain. 1968;91:655–684. doi: 10.1093/brain/91.4.655. [DOI] [PubMed] [Google Scholar]
- 74.Koehler PR, Haughton VM, Daniels DL, Williams AL, Yetkin Z, Charles HC, Shutts D. MR measurement of normal and pathologic brainstem diameters. AJNR Am J Neuroradiol. 1985;6:425–427. [PMC free article] [PubMed] [Google Scholar]
- 75.Iizuka H, Iizuka Y, Kobayashi R, Nishinome M, Sorimachi Y, Takagishi K. The relationship between an intramedullary high signal intensity and the clinical outcome in atlanto-axial subluxation owing to rheumatoid arthritis. Spine J. 2014;14:938–943. doi: 10.1016/j.spinee.2013.07.448. [DOI] [PubMed] [Google Scholar]
- 76.Burry HC, Tweed JM, Robinson RG, Howes R. Lateral subluxation of the atlanto-axial joint in rheumatoid arthritis. Ann Rheum Dis. 1978;37:525–528. doi: 10.1136/ard.37.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MCRAE DL, BARNUM AS. Occipitalization of the atlas. Am J Roentgenol Radium Ther Nucl Med. 1953;70:23–46. [PubMed] [Google Scholar]
- 78.Chamberlain WE. Basilar Impression (Platybasia): A Bizarre Developmental Anomaly of the Occipital Bone and Upper Cervical Spine with Striking and Misleading Neurologic Manifestations. Yale J Biol Med. 1939;11:487–496. [PMC free article] [PubMed] [Google Scholar]
- 79.Riew KD, Hilibrand AS, Palumbo MA, Sethi N, Bohlman HH. Diagnosing basilar invagination in the rheumatoid patient. The reliability of radiographic criteria. J Bone Joint Surg Am. 2001;83-A:194–200. doi: 10.2106/00004623-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 80.Kawaida H, Sakou T, Morizono Y. Vertical settling in rheumatoid arthritis. Diagnostic value of the Ranawat and Redlund-Johnell methods. Clin Orthop Relat Res. 1989;(239):128–135. [PubMed] [Google Scholar]
- 81.Redlund-Johnell I, Pettersson H. Radiographic measurements of the cranio-vertebral region. Designed for evaluation of abnormalities in rheumatoid arthritis. Acta Radiol Diagn (Stockh) 1984;25:23–28. doi: 10.1177/028418518402500105. [DOI] [PubMed] [Google Scholar]
- 82.White AA PM. Clinical Biomechanics of the Spine. 2nd ed. Philadelphia: JB Lippincott; 1990. [Google Scholar]
- 83.Bundschuh C, Modic MT, Kearney F, Morris R, Deal C. Rheumatoid arthritis of the cervical spine: surface-coil MR imaging. AJR Am J Roentgenol. 1988;151:181–187. doi: 10.2214/ajr.151.1.181. [DOI] [PubMed] [Google Scholar]
- 84.Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14:544–551. doi: 10.5435/00124635-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Scanzello CR, Figgie MP, Nestor BJ, Goodman SM. Perioperative management of medications used in the treatment of rheumatoid arthritis. HSS J. 2006;2:141–147. doi: 10.1007/s11420-006-9012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grennan DM, Gray J, Loudon J, Fear S. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60:214–217. doi: 10.1136/ard.60.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerster JC, Bossy R, Dudler J. Bone non-union after osteotomy in patients treated with methotrexate. J Rheumatol. 1999;26:2695–2697. [PubMed] [Google Scholar]
- 88.Giles JT, Bartlett SJ, Gelber AC, Nanda S, Fontaine K, Ruffing V, Bathon JM. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum. 2006;55:333–337. doi: 10.1002/art.21841. [DOI] [PubMed] [Google Scholar]
- 89.Crawford CH, Carreon LY, Djurasovic M, Glassman SD. Lumbar fusion outcomes in patients with rheumatoid arthritis. Eur Spine J. 2008;17:822–825. doi: 10.1007/s00586-008-0610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inaoka M, Tada K, Yonenobu K. Problems of posterior lumbar interbody fusion (PLIF) for the rheumatoid spondylitis of the lumbar spine. Arch Orthop Trauma Surg. 2002;122:73–79. doi: 10.1007/s004020100321. [DOI] [PubMed] [Google Scholar]
- 91.Mitsuyama T, Kubota M, Yuzurihara M, Mizuno M, Hashimoto R, Ando R, Okada Y. The pitfalls in surgical management of lumbar canal stenosis associated with rheumatoid arthritis. Neurol Med Chir (Tokyo) 2013;53:853–860. doi: 10.2176/nmc.oa2012-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, Hawker GA. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:254–263. doi: 10.1002/art.38231. [DOI] [PubMed] [Google Scholar]
- 93.Stundner O, Chiu YL, Sun X, Goodman SM, Russell LA, Calloway JJ, MacKenzie CR, Mazumdar M, Memtsoudis SG. Perioperative outcomes in patients with rheumatoid versus osteoarthritis for total hip arthroplasty: a population-based study. Clin Exp Rheumatol. 2013;31:889–895. [PubMed] [Google Scholar]
- 94.Baum J. Infection in rheumatoid arthritis. Arthritis Rheum. 1971;14:135–137. doi: 10.1002/art.1780140119. [DOI] [PubMed] [Google Scholar]
- 95.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 96.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46:2294–2300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- 97.Nannini C, Ryu JH, Matteson EL. Lung disease in rheumatoid arthritis. Curr Opin Rheumatol. 2008;20:340–346. doi: 10.1097/BOR.0b013e3282f798ed. [DOI] [PubMed] [Google Scholar]
- 98.Amital A, Shitrit D, Adir Y. The lung in rheumatoid arthritis. Presse Med. 2011;40:e31–e48. doi: 10.1016/j.lpm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 99.Rittmeister M, Patzel U, Kerschbaumer F. [The influence of rheumatoid and degenerative disease on hospital resources in the operative treatment of cervical spine instability] Orthopade. 2002;31:1123–1131. doi: 10.1007/s00132-002-0400-3. [DOI] [PubMed] [Google Scholar]
- 100.Miyamoto H, Sumi M, Uno K. Outcome of surgery for rheumatoid cervical spine at one institute over three decades. Spine J. 2013;13:1477–1484. doi: 10.1016/j.spinee.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 101.Wolfs JF, Kloppenburg M, Fehlings MG, van Tulder MW, Boers M, Peul WC. Neurologic outcome of surgical and conservative treatment of rheumatoid cervical spine subluxation: a systematic review. Arthritis Rheum. 2009;61:1743–1752. doi: 10.1002/art.25011. [DOI] [PubMed] [Google Scholar]
- 102.Nakano KK, Schoene WC, Baker RA, Dawson DM. The cervical myelopathy associated with rheumatoid arthritis: analysis of patients, with 2 postmortem cases. Ann Neurol. 1978;3:144–151. doi: 10.1002/ana.410030210. [DOI] [PubMed] [Google Scholar]
- 103.Santavirta S, Slätis P, Kankaanpää U, Sandelin J, Laasonen E. Treatment of the cervical spine in rheumatoid arthritis. J Bone Joint Surg Am. 1988;70:658–667. [PubMed] [Google Scholar]
- 104.Crockard HA. Surgical management of cervical rheumatoid problems. Spine (Phila Pa 1976) 1995;20:2584–2590. doi: 10.1097/00007632-199512000-00022. [DOI] [PubMed] [Google Scholar]
- 105.Lipson SJ. Cervical myelopathy and posterior atlanto-axial subluxation in patients with rheumatoid arthritis. J Bone Joint Surg Am. 1985;67:593–597. [PubMed] [Google Scholar]
- 106.Peppelman WC, Kraus DR, Donaldson WF, Agarwal A. Cervical spine surgery in rheumatoid arthritis: improvement of neurologic deficit after cervical spine fusion. Spine (Phila Pa 1976) 1993;18:2375–2379. doi: 10.1097/00007632-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 107.McAfee PC, Cassidy JR, Davis RF, North RB, Ducker TB. Fusion of the occiput to the upper cervical spine. A review of 37 cases. Spine (Phila Pa 1976) 1991;16:S490–S494. doi: 10.1097/00007632-199110001-00007. [DOI] [PubMed] [Google Scholar]
- 108.Foerster O. Die Leitungsbahnen des Schmerzgefuehls. Berlin: Urban und Schwarzenburg; 1927. p. 266. [Google Scholar]
- 109.Hamblen DL. Occipito-cervical fusion. Indications, technique and results. J Bone Joint Surg Br. 1967;49:33–45. [PubMed] [Google Scholar]
- 110.Ransford AO, Crockard HA, Pozo JL, Thomas NP, Nelson IW. Craniocervical instability treated by contoured loop fixation. J Bone Joint Surg Br. 1986;68:173–177. doi: 10.1302/0301-620X.68B2.3514627. [DOI] [PubMed] [Google Scholar]
- 111.Flint GA, Hockley AD, McMillan JJ, Thompson AG. A new method of occipitocervical fusion using internal fixation. Neurosurgery. 1987;21:947–950. doi: 10.1227/00006123-198712000-00032. [DOI] [PubMed] [Google Scholar]
- 112.Grob D, Dvorak J, Panjabi MM, Antinnes JA. The role of plate and screw fixation in occipitocervical fusion in rheumatoid arthritis. Spine (Phila Pa 1976) 1994;19:2545–2551. doi: 10.1097/00007632-199411001-00009. [DOI] [PubMed] [Google Scholar]
- 113.Grob D, Dvorak J, Panjabi M, Froehlich M, Hayek J. Posterior occipitocervical fusion. A preliminary report of a new technique. Spine (Phila Pa 1976) 1991;16:S17–S24. [PubMed] [Google Scholar]
- 114.Smith MD, Anderson P, Grady MS. Occipitocervical arthrodesis using contoured plate fixation. An early report on a versatile fixation technique. Spine (Phila Pa 1976) 1993;18:1984–1990. doi: 10.1097/00007632-199310001-00010. [DOI] [PubMed] [Google Scholar]
- 115.Sasso RC, Jeanneret B, Fischer K, Magerl F. Occipitocervical fusion with posterior plate and screw instrumentation. A long-term follow-up study. Spine (Phila Pa 1976) 1994;19:2364–2368. doi: 10.1097/00007632-199410150-00021. [DOI] [PubMed] [Google Scholar]
- 116.Huckell CB, Buchowski JM, Richardson WJ, Williams D, Kostuik JP. Functional outcome of plate fusions for disorders of the occipitocervical junction. Clin Orthop Relat Res. 1999;(359):136–145. doi: 10.1097/00003086-199902000-00014. [DOI] [PubMed] [Google Scholar]
- 117.Gallie WE. Fractures and dislocations of the cervical spine. Am J Surg. 1939;46:495–499. [Google Scholar]
- 118.Brooks AL, Jenkins EB. Atlanto-axial arthrodesis by the wedge compression method. J Bone Joint Surg Am. 1978;60:279–284. [PubMed] [Google Scholar]
- 119.Wertheim SB, Bohlman HH. Occipitocervical fusion. Indications, technique, and long-term results in thirteen patients. J Bone Joint Surg Am. 1987;69:833–836. [PubMed] [Google Scholar]
- 120.Coyne TJ, Fehlings MG, Wallace MC, Bernstein M, Tator CH. C1-C2 posterior cervical fusion: long-term evaluation of results and efficacy. Neurosurgery. 1995;37:688–692; discussion 692-693. doi: 10.1227/00006123-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 121.Chan DP, Ngian KS, Cohen L. Posterior upper cervical fusion in rheumatoid arthritis. Spine (Phila Pa 1976) 1992;17:268–272. doi: 10.1097/00007632-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 122.Magerl F, Seeman P. Stable posterior fusion of the atlas and axis by transarticular screw fixation. In: Kehr P, Weidner A, editors. Cervical Spine. Berlin: Springer-Verlag; 1986. pp. 322–327. [Google Scholar]
- 123.Grob D, Magerl F. [Surgical stabilization of C1 and C2 fractures] Orthopade. 1987;16:46–54. [PubMed] [Google Scholar]
- 124.Harms J, Melcher RP. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine (Phila Pa 1976) 2001;26:2467–2471. doi: 10.1097/00007632-200111150-00014. [DOI] [PubMed] [Google Scholar]
- 125.Goel A, Laheri V. Plate and screw fixation for atlanto-axial subluxation. Acta Neurochir (Wien) 1994;129:47–53. doi: 10.1007/BF01400872. [DOI] [PubMed] [Google Scholar]
- 126.Yamazaki M, Okawa A, Furuya T, Sakuma T, Takahashi H, Kato K, Fujiyoshi T, Mannoji C, Takahashi K, Koda M. Anomalous vertebral arteries in the extra- and intraosseous regions of the craniovertebral junction visualized by 3-dimensional computed tomographic angiography: analysis of 100 consecutive surgical cases and review of the literature. Spine (Phila Pa 1976) 2012;37:E1389–E1397. doi: 10.1097/BRS.0b013e31826a0c9f. [DOI] [PubMed] [Google Scholar]
- 127.Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255–265. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- 128.Elliott RE, Tanweer O, Boah A, Morsi A, Ma T, Frempong-Boadu A, Smith ML. Outcome comparison of atlantoaxial fusion with transarticular screws and screw-rod constructs: meta-analysis and review of literature. J Spinal Disord Tech. 2014;27:11–28. doi: 10.1097/BSD.0b013e318277da19. [DOI] [PubMed] [Google Scholar]
- 129.Meyer B, Kuhlen D. Atlantoaxial fusion: transarticular screws versus screw-rod constructs. World Neurosurg. 2013;80:516–517. doi: 10.1016/j.wneu.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 130.Elliott RE, Tanweer O, Boah A, Morsi A, Ma T, Smith ML, Frempong-Boadu A. Atlantoaxial fusion with screw-rod constructs: meta-analysis and review of literature. World Neurosurg. 2014;81:411–421. doi: 10.1016/j.wneu.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 131.Elliott RE, Tanweer O, Boah A, Morsi A, Ma T, Frempong-Boadu A, Smith ML. Atlantoaxial fusion with transarticular screws: meta-analysis and review of the literature. World Neurosurg. 2013;80:627–641. doi: 10.1016/j.wneu.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 132.Elliott RE, Tanweer O, Boah A, Smith ML, Frempong-Boadu A. Comparison of safety and stability of C-2 pars and pedicle screws for atlantoaxial fusion: meta-analysis and review of the literature. J Neurosurg Spine. 2012;17:577–593. doi: 10.3171/2012.9.SPINE111021. [DOI] [PubMed] [Google Scholar]
- 133.Tokuhashi Y, Ajiro Y, Oshima M, Umezawa N. C1-C2 intra-articular screw fixation for atlantoaxial subluxation due to rheumatoid arthritis. Orthopedics. 2009;32:172. [PubMed] [Google Scholar]
- 134.Gorek J, Acaroglu E, Berven S, Yousef A, Puttlitz CM. Constructs incorporating intralaminar C2 screws provide rigid stability for atlantoaxial fixation. Spine (Phila Pa 1976) 2005;30:1513–1518. doi: 10.1097/01.brs.0000167827.84020.49. [DOI] [PubMed] [Google Scholar]
- 135.Dmitriev AE, Lehman RA, Helgeson MD, Sasso RC, Kuhns C, Riew DK. Acute and long-term stability of atlantoaxial fixation methods: a biomechanical comparison of pars, pedicle, and intralaminar fixation in an intact and odontoid fracture model. Spine (Phila Pa 1976) 2009;34:365–370. doi: 10.1097/BRS.0b013e3181976aa9. [DOI] [PubMed] [Google Scholar]
- 136.Lapsiwala SB, Anderson PA, Oza A, Resnick DK. Biomechanical comparison of four C1 to C2 rigid fixative techniques: anterior transarticular, posterior transarticular, C1 to C2 pedicle, and C1 to C2 intralaminar screws. Neurosurgery. 2006;58:516–521; discussion 516-521. doi: 10.1227/01.NEU.0000197222.05299.31. [DOI] [PubMed] [Google Scholar]
- 137.An HS, Gordin R, Renner K. Anatomic considerations for plate-screw fixation of the cervical spine. Spine (Phila Pa 1976) 1991;16:S548–S551. doi: 10.1097/00007632-199110001-00019. [DOI] [PubMed] [Google Scholar]
- 138.Heller JG, Carlson GD, Abitbol JJ, Garfin SR. Anatomic comparison of the Roy-Camille and Magerl techniques for screw placement in the lower cervical spine. Spine (Phila Pa 1976) 1991;16:S552–S557. doi: 10.1097/00007632-199110001-00020. [DOI] [PubMed] [Google Scholar]
- 139.Hostin RA, Wu C, Perra JH, Polly DW, Akesen B, Wroblewski JM. A biomechanical evaluation of three revision screw strategies for failed lateral mass fixation. Spine (Phila Pa 1976) 2008;33:2415–2421. doi: 10.1097/BRS.0b013e31818916e3. [DOI] [PubMed] [Google Scholar]
- 140.44th Annual Meeting. Council on Arteriosclerosis. Dallas, Texas, November 1990. Abstracts. Arteriosclerosis. 1990;10:751a–871a. [PubMed] [Google Scholar]
- 141.Xu R, Haman SP, Ebraheim NA, Yeasting RA. The anatomic relation of lateral mass screws to the spinal nerves. A comparison of the Magerl, Anderson, and An techniques. Spine (Phila Pa 1976) 1999;24:2057–2061. doi: 10.1097/00007632-199910010-00016. [DOI] [PubMed] [Google Scholar]
- 142.Van Gompel JJ, Morris JM, Kasperbauer JL, Graner DE, Krauss WE. Cystic deterioration of the C1-2 articulation: clinical implications and treatment outcomes. J Neurosurg Spine. 2011;14:437–443. doi: 10.3171/2010.12.SPINE10302. [DOI] [PubMed] [Google Scholar]
- 143.Gladi M, Iacoangeli M, Specchia N, Re M, Dobran M, Alvaro L, Moriconi E, Scerrati M. Endoscopic transnasal odontoid resection to decompress the bulbo-medullary junction: a reliable anterior minimally invasive technique without posterior fusion. Eur Spine J. 2012;21 Suppl 1:S55–S60. doi: 10.1007/s00586-012-2220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu JC, Huang WC, Cheng H, Liang ML, Ho CY, Wong TT, Shih YH, Yen YS. Endoscopic transnasal transclival odontoidectomy: a new approach to decompression: technical case report. Neurosurgery. 2008;63:ONSE92–ONSE4; discussion ONSE94. doi: 10.1227/01.neu.0000335020.06488.c8. [DOI] [PubMed] [Google Scholar]
- 145.Frempong-Boadu AK, Faunce WA, Fessler RG. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery. 2002;51:S60–S66. [PubMed] [Google Scholar]
- 146.Iacoangeli M, Gladi M, Alvaro L, Di Rienzo A, Specchia N, Scerrati M. Endoscopic endonasal odontoidectomy with anterior C1 arch preservation in elderly patients affected by rheumatoid arthritis. Spine J. 2013;13:542–548. doi: 10.1016/j.spinee.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 147.Gempt J, Lehmberg J, Grams AE, Berends L, Meyer B, Stoffel M. Endoscopic transnasal resection of the odontoid: case series and clinical course. Eur Spine J. 2011;20:661–666. doi: 10.1007/s00586-010-1629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Laufer I, Greenfield JP, Anand VK, Härtl R, Schwartz TH. Endonasal endoscopic resection of the odontoid process in a nonachondroplastic dwarf with juvenile rheumatoid arthritis: feasibility of the approach and utility of the intraoperative Iso-C three-dimensional navigation. Case report. J Neurosurg Spine. 2008;8:376–380. doi: 10.3171/SPI/2008/8/4/376. [DOI] [PubMed] [Google Scholar]
- 149.Nayak JV, Gardner PA, Vescan AD, Carrau RL, Kassam AB, Snyderman CH. Experience with the expanded endonasal approach for resection of the odontoid process in rheumatoid disease. Am J Rhinol. 2007;21:601–606. doi: 10.2500/ajr.2007.21.3089. [DOI] [PubMed] [Google Scholar]
- 150.Kassam AB, Snyderman C, Gardner P, Carrau R, Spiro R. The expanded endonasal approach: a fully endoscopic transnasal approach and resection of the odontoid process: technical case report. Neurosurgery. 2005;57:E213; discussion E213. doi: 10.1227/01.neu.0000163687.64774.e4. [DOI] [PubMed] [Google Scholar]
- 151.Gande A, Tormenti MJ, Koutourousiou M, Paluzzi A, Fernendez-Miranda JC, Snydermnan CH, Gardner PA. Intraoperative computed tomography guidance to confirm decompression following endoscopic endonasal approach for cervicomedullary compression. J Neurol Surg B Skull Base. 2013;74:44–49. doi: 10.1055/s-0032-1329627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dasenbrock HH, Clarke MJ, Bydon A, Sciubba DM, Witham TF, Gokaslan ZL, Wolinsky JP. Endoscopic image-guided transcervical odontoidectomy: outcomes of 15 patients with basilar invagination. Neurosurgery. 2012;70:351–359; discussion 351-359. doi: 10.1227/NEU.0b013e318230e59a. [DOI] [PubMed] [Google Scholar]
- 153.Schmitt-Sody M, Kirchhoff C, Buhmann S, Metz P, Birkenmaier C, Troullier H, Jansson V, Veihelmann A. Timing of cervical spine stabilisation and outcome in patients with rheumatoid arthritis. Int Orthop. 2008;32:511–516. doi: 10.1007/s00264-007-0349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ranawat CS, O’Leary P, Pellicci P, Tsairis P, Marchisello P, Dorr L. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1979;61:1003–1010. [PubMed] [Google Scholar]
- 155.Ronkainen A, Niskanen M, Auvinen A, Aalto J, Luosujärvi R. Cervical spine surgery in patients with rheumatoid arthritis: longterm mortality and its determinants. J Rheumatol. 2006;33:517–522. [PubMed] [Google Scholar]
- 156.Agarwal AK, Peppelman WC, Kraus DR, Pollock BH, Stolzer BL, Eisenbeis CH, Donaldson WF. Recurrence of cervical spine instability in rheumatoid arthritis following previous fusion: can disease progression be prevented by early surgery? J Rheumatol. 1992;19:1364–1370. [PubMed] [Google Scholar]
- 157.Clarke MJ, Cohen-Gadol AA, Ebersold MJ, Cabanela ME. Long-term incidence of subaxial cervical spine instability following cervical arthrodesis surgery in patients with rheumatoid arthritis. Surg Neurol. 2006;66:136–140; discussion 140. doi: 10.1016/j.surneu.2005.12.037. [DOI] [PubMed] [Google Scholar]


