Graphical abstract
Keywords: Trichuris trichiura, Trichuris suis, Benzimidazole, Drug-uptake, Treatment efficacy
Highlights
-
•
The low uptake of FBZ by Trichuris suis in vitro was confirmed in vivo in pigs.
-
•
High correlation between plasma and worm concentrations of OXF and FBZSO2.
-
•
No correlation between plasma and worm concentrations of FBZ.
-
•
The blood–enterocyte pathway seems important for FBZ-metabolites to reach T. suis.
-
•
FBZ may enter T. suis from the blood–enterocyte pathway and the intestinal content.
Abstract
It is recognized that the clinical efficacy of single dose benzimidazoles (BZs) against the nematode, Trichuris suis of pigs and the closely related Trichuris trichiura in humans is only poor to moderate. Recent in vitro studies have indicated that a low uptake of fenbendazole (FBZ) in T. suis may be responsible for its poor efficacy. The aim of this study was to investigate this hypothesis by measuring the concentrations of FBZ and its metabolites, oxfendazole (OXF) and FBZ sulphone (FBZSO2), in T. suis isolated from FBZ treated pigs and in plasma of the pigs. The highest concentration of FBZ measured in T. suis was 66.6 pmol/mg dry worm tissue which was approximately half of what was measured in a previous in vitro study. The correlation between drug concentrations in plasma and in T. suis worms was highly positive for OXF (r = 0.93, P = 0.0007) and FBZSO2 (r = 0.85, P = 0.007), but no correlation was found for FBZ. This study shows that the low uptake of FBZ observed for T. suis in vitro, also takes place in vivo. The high and significant correlations between OXF and FBZSO2 concentrations in plasma of the pigs and T. suis (and the lack of this correlation for FBZ) suggests that the metabolites reach the worms via the blood–enterocyte interface while FBZ primarily reaches the worms via the intestinal lumen of the host.
1. Introduction
The whipworm Trichuris trichiura has been estimated to infect 464.6 million people worldwide resulting in an estimated 0.64 million disability adjusted life-years lost globally (Pullan et al., 2014). The anthelmintic drug regimens recommended by the World Health Organization (WHO) against human trichuriasis are a single-dose treatment with albendazole (ALB) or mebendazole (MBD) (WHO, 2013), which have shown only low to moderate cure rates (Keiser and Utzinger, 2008; Olsen et al., 2009; Knopp et al., 2010; Steinmann et al., 2011; Namwanje et al., 2011). Fenbendazole (FBZ), another BZ is widely used against gastrointestinal nematodes of veterinary importance, amongst these the whipworm of the pig, Trichuris suis. The current treatment recommendation of T. suis infection with FBZ is a single dose of 25 mg/kg, according to the Danish Health and Medicine Authority (2013), which is 5 times higher than recommended for other common gastrointestinal nematodes of the pig, e.g., Ascaris suum and Oesophagostomum spp. The dose of 5 mg/kg has resulted in a worm count reduction (WCR) of 93.1%, whereas a dose of 25 mg/kg reduced worm counts by 97.4% (Batte, 1978). A high efficacy has been reported when 5 mg/kg was used for 3 consecutive days resulting in a WCR of 99.7% and 99.9% four and five days after the last treatment day (Batte, 1978; Stewart et al., 1981). Treatment recommendations for another BZ, flubendazole, is a standard dose (5 mg/kg) divided over 7 days, which indicate that long term treatments of T. suis infections is a premise for achieving adequate drug efficacy (Taylor, 1999).
Efficacy of an anthelmintic is dependent on the ability of the active compound to reach the location of the parasite and to enter and bind to specific receptors within the parasite in sufficient and sustained concentrations (Alvarez et al., 2007). For BZs, the receptor is the β-tubulin in nematodes (Lacey, 1988). The fate of the drug in the host therefore plays a crucial role for in drug concentration and duration of exposure at the target site. FBZ is oxidised to the pharmacological active oxfendazole (OXF) and the less active fenbendazole sulphone (FBZSO2) in a range of mammals, fish and birds (Short et al., 1988).
Previous in vitro studies on motility and drug uptake of T. suis and Oesophagostomum dentatum (the nodular worm of the pig) have shown a lower pharmacological effect on T. suis than O. dentatum when exposed to FBZ (Hansen et al., 2014). We hypothesized that a low drug-uptake of FBZ in T. suis in vitro was responsible for this effect. The main objective of this study was therefore to confirm this hypothesis by measuring the concentrations of FBZ and metabolites within T. suis worms isolated from FBZ treated pigs.
2. Materials and methods
2.1. Experimental animals
Fourteen castrated male pigs (∼25 kg) were purchased from a Specific Pathogen Free (SPF) farm (Frenderupgård, Sorø, DK) with no history of helminth infections for 10 years. Pigs were acclimatized 1 week prior to experimental infection. The pigs were identified by ear tags and tested for current gastrointestinal parasite infections on the day of arrival and on the day of infection by means of a modified McMaster method (Roepstorff and Nansen, 1998). The animals had free access to water and were fed restrictively with a diet consisting of 75% barley and 25% supplementary feed (NAG, Helsinge, DK). Due to risk of coprophagia the two control pigs (no drug treatment) were kept separately.
2.2. Ethic statement
The current study was approved by the Experimental Animal Unit, University of Copenhagen, (Denmark) based on national regulations from the Danish Animal Experiments Inspectorate (permission No. 2010/561-1914, C5).
2.3. Experimental design
All animals were inoculated with 5000 embryonated T. suis eggs (kindly provided by Parasite Technologies A/S, DK) using a stomach tube. Infection was confirmed in all pigs by a modified McMaster method at day 48 post inoculation (p.i.), and on the first treatment day (day 49 p.i.). After stratification for faecal egg count (FEC) (49 day p.i.), pigs were randomly allocated into 4 treatment groups with 3 animals in each (Table 1). The remaining two pigs served as control group. On day 49 p.i. the pigs in group 1 and 2 were given FBZ (Panacur® 4% oral powder as an aqueous suspension) through a stomach tube at a dose rate of 5 mg/kg, whereas the pigs in group 3 and 4 were given 5 mg/kg daily for 3 days. The pigs in group 1 and 2 were euthanized 12 and 24 h after the first treatment, respectively, whereas the pigs in group 3 and 4 were euthanized 12 and 24 h after the third treatment, respectively. These time points were chosen match the in vitro study where worms were incubated for 24 and 72 h, respectively, and to obtain information about the dynamics of the uptake after a single and triple treatments. Two heparinized blood samples were collected from venae jugularis from all pigs just prior to euthanasia. Blood samples were centrifuged at 2000g for 15 min and recovered plasma stored at −20 °C until HPLC analysis. The two control animals were euthanized at the same time as the animals in group 4. All pigs were euthanized by captive bolt pistol followed by exsanguination.
Table 1.
Mean (±SD) faecal egg counts (FEC) in the treated groups and control group on day 49 post inoculated (p.i.) with a single dose of 5000 embryonated Trichuris suis eggs.
| Groups | Mean FEC 49 days p.i. (egg per gram of faeces) |
|---|---|
| Control (n = 2) | 1890 ± 834 |
| Group 1 (n = 3) | 4553 ± 3855 |
| Group 2 (n = 3) | 2880 ± 3248 |
| Group 3 (n = 3) | 4527 ± 3012 |
| Group 4 (n = 3) | 3853 ± 3707 |
No significant difference was found between group means (P = 0.87).
2.4. Worm recovery
The pigs were immediately eviscerated and the caecum and large intestines separated from the stomach and the small intestine. The caecum and the large intestine were divided into 4 sections starting at the caecum. Section 1: caecum; section 2: 0–25% of the total length of the colon, section 3: 26–50% of the total length of the colon; section 4: 51–100% of the total length of the colon including rectum. All sections were opened and gently liberated from intestinal content, which was kept for later worm count, and rinsed briefly in 39 °C saline. The adult worms were gently collected from the intestinal wall by hand, counted and washed in 50 mL 39 °C saline for a maximum of 30 s by carefully inverting the 50 mL centrifuge tubes. From each pig, 3 times 30 worms were collected from caecum; if the number of worms was insufficient more worms were included distally in colon, i.e., from sections 2, 3 or 4 in the mentioned order. If no worms were observed attached to the mucosa, worms were isolated from the intestinal content. After washing the worms were poured into petri dishes in approximately 0.5 mL of saline from where the worms were gently transferred to Eppendorf tubes with a curved forceps. Excess water in the Eppendorf tubes was removed using a pipette and the worms frozen in liquid nitrogen. All samples were kept at −20 °C until HPLC analysis.
2.5. High-performance liquid chromatography analysis (HPLC)
FBZ and it metabolites in worms and plasma were measured using the HPLC method described for plasma by Petersen and Friis (2000) with minor modifications. Vials with worms were thawed and then dried under phosphorous pentoxide until constant weight. Each vial with dried worm (10–50 mg, equivalent to 18–30 worms) was mixed with 200 μl 0.05 M phosphate buffer (pH 7.4). After gentle homogenization with plastic pestle another 200 μl buffer was added and the homogenization repeated before addition of 400 μl 6 M guanidine HCl. The sample was mixed on a vortex mixer for 1 min and placed at 20 °C for 15 min before centrifugation at 8000×g for 10 min. The supernatant was transferred to a clean tube and an additional 400 μl of 6 M guanidine HCl was added to the sample residue. The procedure was repeated and the two supernatants were combined and loaded on an activated cartridge (Oasis HLB, 60 mg, 3 mL). The cartridge was activated with 2 mL methanol followed by 2 mL of water. The loaded cartridge was washed with 2 mL 5% methanol and allowed to dry under vacuum for 1 min, before eluting the analyte with 2 mL methanol. The eluate was dried at 37 °C and the residuum was dissolved in 100 μL 50% methanol. This solution was centrifuged at 8000×g before 50 μL were injected into the HPLC-system. A blank worm sample and standards in phosphate buffer and guanidine HCl were run in parallel. Standards of FBZ, OXF and FBZSO2 were prepared from stock solutions in DMSO. Concentration of analyte in worms was expressed as pmol/mg dry worm tissue. Plasma samples were prepared for HPLC analyses by using Oasis HLB cartridge as described above. Instead of guanidine HCl solution 1 mL of plasma was loaded on the activated cartridge. The rest of the cleaning up procedure was as described above. The HPLC system (Waters) was equipped with an auto sampler, 2 HPLC pumps, and a UV detector set at 294 nm. Separation of analytes was accomplished at 30 °C on a Novapak C18 (5 μ, 15 cm). The mobile phase consisted of a gradient mixed from acetonitrile and 0.025 M ammonium acetate (pH 7.2) at a flow rate of 1 mL/min. The proportion of acetonitrile was 30% acetonitrile for the first 3 min, progressing linearly to 40% at 3.5 min, held constant at 40% until 11 min and finally reduced to 30% at 11.5 min for the remaining run time of 17 min. Retention times for FBZ, OXF and FBZSO2 were 13 min, 2.5 min and 4.5 min, respectively. Peak area of an analyte was used to calculate concentration of the analyte. The method for detecting FBZ, OXF and FBZSO2 in worms (buffer-guanidine suspension) was validated in-house by evaluating the following parameters: linear range, recovery, matrix effect, precision, selectivity and limit of quantification. As described above the dry worms were suspended in buffer-guanidine and the validation was conducted based on the amount of drug loaded on the SPE cartridge. The calibration curves for FBZ were linear between 0.1 and 1 μg loaded on the SPE and for OXF and FBZSO2 between 0.05 and 0.5 μg loaded on SPE. The recovery of FBZ, OXF and FBZSO2 was determined by comparing the responses of matrix standards in the range from 0.05 to 1 μ loaded on SPE with water standards in the same concentrations. The recovery was 76%, 101% and 75% for FBZ, OXF and FBZSO2, respectively. No matrix effect could be demonstrated. The intra-assay and inter-assay variability were 10% and 12%, respectively. The limit of quantification (LOQ) was 1.5, 0.5 and 0.8 μg/mg dry worm for FBZ, OXF and FBZSO2, respectively corresponding to 5.3, 1.7 and 2.4 pmol/mg dry worm tissue.
2.6. Worm count reduction (WCR)
For each pig any remaining worms attached to the mucosa were collected and mixed with the intestinal contents from caecum and the large intestine. Each sample was then mixed thoroughly and a 10% subsample was washed on a 212 μM sieve and the collected material stained with iodine and stored for later counting. Due to low numbers of pigs in each treatment group and the early time of treatment evaluation, worm counts were only used to get indicative information on the treatment efficiency. Worm count reduction was calculated as follows:
where g is the geometric mean number of parasites (Hennessy et al., 2006).
2.7. Statistical analysis
One-way analysis of Variance (ANOVA) (JMP version 8) was used to test for similar FEC distribution between treatment groups. For each group, ANOVA and Student’s t-test (JMP version 8) was used to compare log-transformed drug concentrations (log_conc) of FBZ, OXF and FBZSO2 measured within worms. Student’s t-test was used to compare log-concentrations of each drug compound in worms 12 and 24 h after a single and triple dose treatment, respectively. Pearson correlation coefficient (r) was calculated using GraphPad Prism (version 6) to examine the relationship between drug concentrations measured in the worms and in the plasma of the pig.
3. Results
3.1. Establishment of infections and worm counts
Patent infections were observed in all pigs day 49 p.i. and no significant differences were found between group mean FEC (P = 0.87) (Table 1). Individual worm counts and worm count reductions are shown in Table 2. Based on the two control animals the establishment rate was 39%. Fenbendazole showed an immediate effect on worm counts with reductions of respectively, 46% and 51.5% as soon as 12 and 24 h after the first treatment (Table 2). Fenbendazole given for 3 consecutive days resulted in WCR of 86.6% and 98.5% 12 and 24 h after the last treatment, respectively.
Table 2.
Number of Trichuris suis in 14 pigs and worm count reductions 12 and 24 h after 1 or 3 times treatment with FBZ (5 mg/kg), respectively.
| Control | Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|
| 12 h after 1 × 5 mg/kg | 24 h after 1 × 5 mg/kg | 12 h after 3 × 5 mg/kg | 24 h after 3 × 5 mg/kg | ||
| (n = 2) | (n = 3) | (n = 3) | (n = 3) | (n = 3) | |
| Worm count | 1092 | 78 | 839 | 161 | 1 |
| 3393 | 3615 | 386 | 202 | 200 | |
| 3982 | 2512 | 523 | 122 | ||
| Geometric mean | 1925 | 1039 | 934 | 257 | 29 |
| Worm count reduction (%) | n.a. | 46.0 | 51.5 | 86.6 | 98.5 |
Group 1: 12 h after 1 dose of FBZ; group 2: 24 h after 1 dose of FBZ; group 3: 12 h after 3 doses of FBZ; group 4: 24 h after 3 doses of FBZ.
3.2. Drug concentrations in plasma and worms
After a single oral administration total drug concentration in plasma (FBZ plus metabolites) was 3851 pmol/mL at 12 h and 4511 pmol/mL at 24 h (Table 3). OXF was the major component accounting 65% and 58% of total drug at 12 and 24 h, respectively, followed by FBZSO2 (28%; 39%) and FBZ (7%; 3%) (Table 3). Repeated administration of FBZ for 3 days resulted in total drug concentrations of 9654 pmol/mL at 12 h and 8242 pmol/mL at 24 h after the last dose, indicating an accumulation of drug after repeated dosing. OXF still remained the major compound in plasma (60% at 12 h and 49% at 24 h), followed by FBZSO2 (36%; 48%) and FBZ (5%; 3%).
Table 3.
Mean concentrations (±SD) of FBZ, OXF and FBZSO2 in plasma and in Trichuris suis 12 and 24 h after 1 or 3 times treatment with FBZ (5 mg/kg). Group 1: 12 h after 1 dose of FBZ; groups 2: 24 h after 1 dose of FBZ; group 3: 12 h after 3 doses of FBZ; group 4: 24 h after 3 doses of FBZ.
| n = number of animals | Mean concentration in plasma (pmol/mL) |
n = number of worm samples | Mean concentration in worms (pmol/mg dry worm tissue) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FBZ | OXF | FBZSO2 | Total | FBZ | OXF | FBZSO2 | Total | ||
| Control (n = 2) | 0 | 0 | 0 | 0 | Control (n = 2) | 0 | 0 | 0 | 0 |
| Group 1 (n = 3) 12 h |
269.31 (±97.20) (7.0) |
2491.16 (±690.77) (64.7) |
1090.15 (±421.54) (28.3) |
3850.62 (100) |
Group 1 (n = 2) |
6.47a,A (±1.1) (14.5) |
20.72b,A (±6.53) (46.3) |
17.53b,A (±3.59) (39.2) |
44.71 (100) |
| Group 2 (n = 3) 24 h |
125.51 (±89.94) (2.8) |
2610.68 (±720.86) (57.9) |
1774.78 (±470.84) (39.3) |
4510.97 (100) |
Group 2 (n = 2) |
14.57a,C (±1.4) (22.7) |
16.02a,C (±10.87) (25.0) |
33.57b,C (± 5.66) (52.3) |
64.16 (100) |
| Group 3 (n = 3) 12 h |
434.64 (±210.43) (4.5) |
5743.10 (±1707.80) (59.5) |
3476.55 (±533.06) (36.0) |
9654.29 (100) |
Group 3 (n = 2) |
29.3a,B (±19.27) (15.8) |
56.73a,B (±1.20) (30.6) |
99.18a,B (±72.48) (53.6) |
185.21 (100) |
| Group 4 (n = 3) 24 h |
237.25 (±53.76) (2.9) |
4030.95 (±342.70) (48.9) |
3974.11 (±1416.90) (48.2) |
8242.31 (100) |
Group 4 (n = 2) |
66.63a,D (±1.6) (39.7) |
26.73b,C (±13.43) (15.9) |
74.67a,C (±75.09) (44.4) |
168.03 (100) |
| Total mean | 235.0 (3.8) |
3432 (55.1) |
2558.1 (41.1) |
6225 (100) |
Total mean | 31.7 (27.7) |
33.0 (28.8) |
49.9 (43.5) |
114.4 (100) |
Each worm sample consisted of triplets except one where only a single subsample was obtained. Significant differences (P < 0.05) within groups (horizontally) are indicated by different superscripts (a and b). Significant differences between groups (vertically) are indicated by different superscripts (A, B for groups 1 and 3 and C, D for groups 2 and 4). Numbers in italic and brackets are relative concentrations (%) in plasma of the pig and T. suis, respectively, measured at each time-point and of the total mean.
In T. suis total concentration of drug was 44.7 pmol/mg dry worm tissue at 12 h and 64.2 pmol/mg dry worm tissue at 24 h after single administration. Repeated administration of FBZ for 3 consecutive days resulted in an accumulation of drug and metabolites of 185.2 and 168.0 pmol/mg 12 and 24 h after the last dose. The distribution pattern of FBZ and its metabolites differ to some extent between the worms and the host plasma. In T. suis OXF accounted for the major fraction 12 h after a single dose but 24 h post administration and after repeated doses, FBZSO2 was dominating Correlations between plasma and worm concentrations of each drug-metabolite, including all pigs, are shown in Fig. 1. The correlation analysis revealed that OXF and FBZSO2 concentrations in the host plasma were highly correlated with the concentrations in T. suis (r = 0.93, P = 0.0007; r = 0.85, P = 0.007, respectively, Fig. 1b and c) whereas no correlation was found for FBZ (r = 0.37, P = 0.36). The concentration of FBZ remained relatively higher in the worms than in the plasma of the host (Table 3).
Fig. 1.
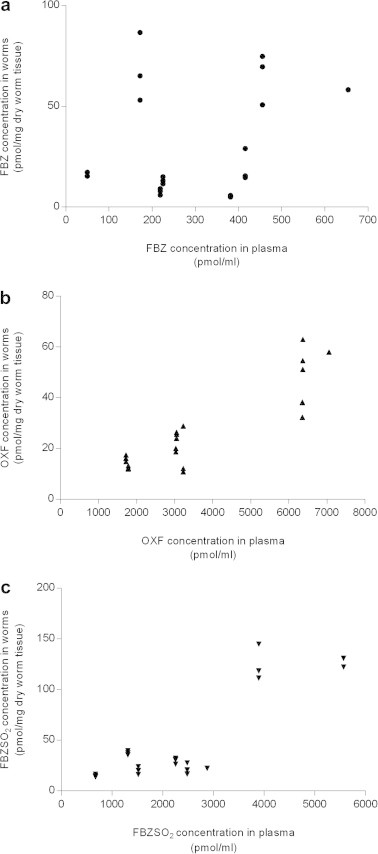
Correlation between concentration of (a) -●- FBZ (r = 0.37, P = 0.36), (b) -▴- OXF (r = 0.93, P = 0.0007), and (c) -▾- FBZSO2 (r = 0.85, P = 0.007) in plasma of pigs and in Trichuris suis isolated from these pigs.
4. Discussion
The main objective of this study was to measure the concentration of FBZ and metabolites within T. suis in order to investigate whether the low drug uptake observed in vitro (Hansen et al., 2014) could be confirmed in vivo and thereby explain the low pharmacological effect against this species. Data confirmed that the low drug uptake of FBZ measured in T. suis in vitro also take place in vivo. Indeed, the highest concentrations of FBZ measured in T. suis after single and repeated administration of FBZ for 3 days were 14.6 and 66.6 pmol/mg dry worm tissue, respectively (Table 3). These concentrations were approximately six and two times lower than those measured in the worms in vitro after 24 and 72 h exposure to FBZ at a range of 0.01–30 μM (2–88 and 3–120 pmol/mg dry worm tissue, respectively) (Hansen et al., 2014). It can be argued that the drug concentrations the worms were exposed to in vivo are not comparable with the FBZ concentrations used in vitro. However, in a study by Petersen (1998) single-dose FBZ (5 mg/kg) was administered to 12 pigs through a stomach tube, as in this study. The concentration of FBZ in caecum content varied between 1 and 2.6 μg/g and between 1.5 and 7.1 μg/g in mucosa of the caecum during the 24 h observation period. The lowest and the highest values, 1 and 7.1 μg/g wet tissue are equivalents to 3 and 24 μM. It is therefore likely that within 24 h of the first treatment, worms were exposed to concentrations between 0.01 and 30 μM as used in vitro (Hansen et al., 2014). However, when worms were exposed to 1 μM FBZ for 24 and 72 h in vitro, FBZ concentrations in the worms were almost as high (83 and 93 pmol/mg dry worm tissue, respectively) as when exposed to 30 μM (Hansen et al., 2014). In the present study, pigs were dosed FBZ (5 mg/kg) at an interval of 24 h for three days, hence, drug concentrations at the predilection site were expected not to fall below 3 μM during the last treatment day, but highly likely to increase due to accumulation. Therefore we conclude that the exposure in vitro and in vivo has been within the same range and that the uptake of FBZ by T. suis is even lower in vivo than in vitro.
Adult stages of T. suis inhabit the lower part of the intestine, primarily the caecum and proximal colon (Pedersen and Saeed, 2000; Kringel and Roepstorff, 2006). The anterior oesophageal part of the worm is embedded in the mucosa in a tunnel like-construction of epithelial cells, while the posterior thick part is protruding freely into the lumen (Jenkins, 1970). Consequently, Trichuris spp. may be exposed to anthelmintic drugs present in both the mucosa and the digesta of the intestinal lumen which is quite unique for adults of a nematode species. High correlations between drug concentrations of OXF and FBZSO2 in host plasma and worms suggest that these metabolites enter the worms via the blood–enterocyte pathway whereas FBZ, showing no correlation between concentrations in plasma and worms, may enter the worms both by the blood–enterocyte pathway and directly from the digesta. As this study was designed to measure the drug uptake of T. suis in vivo, no attempts were made to measure the concentrations of FBZ, OXF and FBZSO2 at the predilection sites of the worms (i.e. mucosa and digesta of caecum). However, by comparing our results with those reported by Petersen (1998) transport pathways can be considered. Comparison is possible since the plasma concentrations in this study are only slightly higher than reported by Petersen and the fractions of FBZ, OXF and FBZSO2 were found to be similar (Table 4). In this study the major drug compounds found in worms after a single dose treatment were OXF and FBZSO2. From the in vitro studies we know that only small fraction of FBZ is metabolised by T. suis and therefore, the high concentration of OXF and FBZSO2 in the worms may originate from the host. According to Petersen (1998) the major drug fraction in the content of caecum is FBZ (75.7% and 85.7%) whereas OXF is the major fraction in plasma (75.8% and 54.6%) (Table 4). Supported by the high correlation between concentrations of OXF in plasma and worms (Fig. 1), we suggest that OXF reaches the worms via the blood–enterocyte pathway. FBZSO2 showed a similar distribution pattern as OXF, as FBZSO2 amounted 19.2% in plasma and only 2.7% in the content of the caecum, respectively. The high correlation between concentrations of FBZSO2 in worms and plasma (Fig. 1) further indicate that these drug metabolites might reach the worm from the systemic circulation. After a single dose treatment the concentration of FBZ in worms was higher 24 h in comparison to 12 h p.t. Only a minor proportion of FBZ was found in plasma (5.0%) whereas high proportions were present at the predilection site (mucosa: 58.1%, content: 75.7%). Furthermore, no correlation was found between FBZ concentrations in worms and plasma (Fig. 1). We therefore suggest that the main fraction of FBZ, accumulating in the worms originate from the intestinal digesta of the host although some minor part may enter from the systemic circulation.
Table 4.
Relative (%) and actual concentrations of FBZ, OXF and FBZSO2 in pigs and worms 12 and 24 h after one treatment with FBZ (5 mg/kg).
| 12 h |
Total | 24 h |
Total | |||||
|---|---|---|---|---|---|---|---|---|
| FBZ | OXF | FBZSO2 | FBZ | OXF | FBZSO2 | |||
| Petersen (1998): Plasma (%) (pmol/ml) |
5.0 67 |
75.8 (1268) |
19.2 (362) |
100 (1883) |
8.2 (167) |
54.6 (1111) |
37.1 (754) |
100 (2031) |
| Caecal (%) mucosa (pmol/g) |
58.1 (13028) |
36.8 (8245) |
5.1 (1132) |
100 (22405) |
53.0 (4176) |
20.1 (1586) |
26.8 (2113) |
100 (7874) |
| Caecal (%) content (pmol/g) |
75.7 (8351) |
21.6 (2379) |
2.7 (302) |
100 (11032) |
85.7 (3341) |
8.1 (317) |
6.2 (241) |
100 (3899) |
| Present study (%) | ||||||||
| Plasma | 7 | 64.7 | 28.3 | 100 | 2.8 | 57.9 | 39.3 | 100 |
| Worms (in vivo) | 14.5 | 46.3 | 39.2 | 100 | 22.7 | 25 | 52.3 | 100 |
|
Hansen et al. (2014) Worms (in vitro) |
– | – | – | – | 96 | 4.0 | 0 | 100 |
Relative concentrations in italics are from the present study. Relative concentrations in bold are from the pharmacokinetic study of FBZ in pigs (Petersen, 1998). Concentrations of FBZ, OXF and FBZSO2 in brackets are given in pmol/mL (plasma) and pmol/g (Caecal mucosa and Caecum content). Underlined relative concentrations from a previous in vitro study where Trichuris suis was exposed to 30 μM FBZ (Hansen et al., 2014).
The World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anthelmintic in swine recommend a period of 5–7 days after treatment before worm count is performed (Hennessy et al., 2006). The present work was not directed towards estimating the efficacy of a triple dose regime with FBZ, but to obtain indicative information on the treatment efficacy which showed an immediate and very high worm count reduction (98.5%) as early as 24 h after the last treatment. The worms isolated from pigs in group 4 (24 h after repeated FBZ treatments) were expected to be undergoing expulsion as they were all isolated from the intestinal content of the colon, suggesting an even higher reduction in worm count if post mortem was conducted according to guidelines. This finding is in accordance with previous studies where WCR reported after 5 mg/kg FBZ given for 3 consecutive days were 99.7% and 99.9% four to five days after the last treatment day (Batte, 1978; Stewart et al., 1981). With the relatively high concentration of OXF observed in the worms, the high WCR and the low effect of FBZ observed in vitro (Hansen et al., 2014) we speculate that OXF, as compared to FBZ, is the drug compound with the highest availability, thus a higher efficacy against Trichuris spp. infections. Future in vitro studies comparing FBZ and OXF are needed.
Acknowledgements
The authors gratefully acknowledge Associate Professor Christian Ritz (from Institute of Nutrition, Exercise and Sport, University of Copenhagen) for providing statistical analytic assistance, Business Unit Lead Kirsten Volmer Larsen (from MSD, Denmark) for kindly providing fenbendazole (Panacur® Vet., oral powder 4%), Helena Mejer, Lise-Lotte Christiansen, Anna Sofie Eckhoff, Rikke Jess, Fredrik Samuelson at Section for Parasitology and Aquatic Disease, University of Copenhagen for assisting in practical work.
References
- Alvarez L.I., Mottier M.L., Lanusse C.E. Drug transfer into target helminth parasites. Trends Parasitol. 2007;23(9):97–104. doi: 10.1016/j.pt.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Batte E.G. Evaluation of fenbendazole as a swine anthelmintic. Vet. Med. Small Anim. Clin. 1978;73(9):1183–1186. [PubMed] [Google Scholar]
- Danish Health and Medicine Authority, 2013. Panacur 4% oral powder, online, (cited the 17th of March 2014). Available at: http://www.produktresume.dk/docushare/dsweb/View/Collection-87.
- Hansen T.V.A., Nejsum P., Friis C., Olsen A., Thamsborg S.M. Trichuris suis and Oesophagostomum dentatum show different sensitivity and accumulation of fenbendazole, albendazole and levamisole in vitro. PLoS Negl. Trop. Dis. 2014;8(4):e2752. doi: 10.1371/journal.pntd.0002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy D.R., Bauer C., Boray J.C., Conder G.A., Daugschies A., Johansen M.V., Maddox-Hyttel C., Roepstorff A. World association for the advancement of veterinary parasitology (WAAVP): second edition of guidelines for evaluating the efficacy of anthelmintics in swine. Vet. Parasitol. 2006;141(1–2):138–149. doi: 10.1016/j.vetpar.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Jenkins T. A morphological and histochemical study of Trichuris suis (Schrank, 1788) with special reference to the host–parasite relationship. Parasitology. 1970;61(3):357–374. doi: 10.1017/s0031182000041202. [DOI] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299(16):1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- Knopp S., Mohammed K.A., Speich B., Hattendorf J., Khamis I.S., Khamis A.N., Stothard J.R., Rollinson D., Marti H., Utzinger J. Albendazole and mebendazole administered alone or in combination with ivermectin against Trichuris trichiura: a randomized controlled trial. Clin. Infect. Dis. 2010;51(12):1420–1428. doi: 10.1086/657310. [DOI] [PubMed] [Google Scholar]
- Kringel H., Roepstorff A. Trichuris suis population dynamics following a primary experimental infection. Vet. Parasitol. 2006;139(1–3):132–139. doi: 10.1016/j.vetpar.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lacey E. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int. J. Parasitol. 1988;18(7):885–936. doi: 10.1016/0020-7519(88)90175-0. [DOI] [PubMed] [Google Scholar]
- Namwanje H., Kabatereine N.B., Olsen A. Efficacy of single and double doses of albendazole and mebendazole alone and in combination in the treatment of Trichuris trichiura in school-age children in Uganda. Trans. R. Soc. Trop. Med. Hyg. 2011;105(10):586–590. doi: 10.1016/j.trstmh.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Olsen A., Namwanje H., Nejsum P., Roepstorff A., Thamsborg S.M. Albendazole and mebendazole have low efficacy against Trichuris trichiura in school-age children in Kabale district, Uganda. Trans. R. Soc. Trop. Med. Hyg. 2009;103(5):443–446. doi: 10.1016/j.trstmh.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Saeed I. Experimental infection of pigs with three dose levels of Trichuris suis. Parasite. 2000;7(4):275–281. doi: 10.1051/parasite/2000074275. [DOI] [PubMed] [Google Scholar]
- Petersen, M.B., 1998. Pharmacodynamic and pharmacokinetic parameters of benzimidazoles in the pig using Oesophagostomum dentatum as model parasite. Ph.D Thesis, Royal Veterinary and Agricultural University, Denmark.
- Petersen M.B., Friis C. Pharmacokinetics of fenbendazole following intravenous and oral administration to pigs. Am. J. Vet. Res. 2000;61(5):573–576. doi: 10.2460/ajvr.2000.61.573. [DOI] [PubMed] [Google Scholar]
- Pullan R.L., Smith J.L., Jasrasaria R., Brooker S.J. Global numbers of infection and disease burden of soil transmitted helminth in 2010. Parasit. Vectors. 2014;(7):37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff A., Nansen P. Food and Agriculture Organization of the United Nations; Rome: 1998. Epidemiology, diagnosis and control of helminth parasites of swine. FAO Animal Health Manual. [Google Scholar]
- Short C.R., Flory W., Hsieh L.C., Barker S.A. The oxidative metabolism of fenbendazole: a comparative study. J. Vet. Pharmacol. Ther. 1988;11(1):50–55. doi: 10.1111/j.1365-2885.1988.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Steinmann P., Utzinger J., Du Z., Jiang J., Chen J., Hattendorf J., Zhou H., Zhou X. Efficacy of single-dose and triple-dose albendazole and mebendazole against soil-transmitted helminths and Taenia spp.: a randomized controlled trial. PLoS ONE. 2011;6(9):e25003. doi: 10.1371/journal.pone.0025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart T.B., Marti O.G., Hale O.M. Efficacy of fenbendazole against five genera of swine parasites. Am. J. Vet. Res. 1981;42(7):1160–1162. [PubMed] [Google Scholar]
- Taylor D.J. In: Pig Diseases. seventh ed. Taylor D.J., editor. St. Edmundsburry Press Ltd; Glasgow: 1999. [Google Scholar]
- WHO . World Health Organ. WHO Press; Geneva: 2013. Assessing the efficacy of anthelminthic drugs against schistosomiasis and soil-transmitted helminthiases. [Google Scholar]


