Graphical abstract

Keywords: Nematode, Cuticle, Collagen, Moulting, Ecdysis, Protease, C. elegans
Highlights
-
•
The nematode cuticle and its enzymology represent a novel target in nematode control.
-
•
Cuticle collagens are the major structural components of this vital structure.
-
•
Prolyl 4-hydroxylase, disulphide bonding and proline isomerisation are key modifications that occur within the endoplasmic reticulum (ER).
-
•
Collagen N- and C-terminal processing and tyrosine cross-linking are critical and exploitable post-ER events.
-
•
Moulting and collagenase enzymes are prime targets for inhibitor design in parasitic nematode control.
Abstract
All nematodes possess an external structure known as the cuticle, which is crucial for their development and survival. This structure is composed primarily of collagen, which is secreted from the underlying hypodermal cells. Extensive studies using the free-living nematode Caenorhabditis elegans demonstrate that formation of the cuticle requires the activity of an extensive range of enzymes. Enzymes are required both pre-secretion, for synthesis of component proteins such as collagen, and post-secretion, for removal of the previous developmental stage cuticle, in a process known as moulting or exsheathment. The excretion/secretion products of numerous parasitic nematodes contain metallo-, serine and cysteine proteases, and these proteases are conserved across the nematode phylum and many are involved in the moulting/exsheathment process. This review highlights the enzymes required for cuticle formation, with a focus on the post-secretion moulting events. Where orthologues of the C. elegans enzymes have been identified in parasitic nematodes these may represent novel candidate targets for future drug/vaccine development.
1. The cuticle
All nematodes are encased in an exoskeleton (known as the cuticle), a structure key to the success and diversity of nematode species. This complex extracellular matrix covers the outermost layer of cells and is required for body shape, movement, and functions as the primary interface with the environment (Fig. 1A). The cuticle structure and its biogenesis have been most extensively studied in the free-living model nematode Caenorhabditis elegans (Singh and Sulston, 1978; Page and Winter, 2003; Page and Johnstone, 2007). As a new cuticle is generated for each developmental stage, it is synthesised five times during the nematode lifecycle, with synthesis of the first cuticle beginning during late embryogenesis. Progression through development requires that the cuticle from the previous stage is shed and replaced with the new cuticle in a process known as moulting. The importance of the cuticle in maintaining body shape has been illustrated by genetic analysis in C. elegans. Strains carrying mutations in either the structural components of the cuticle, or the enzymes required for cuticle formation, result in drastically altered body morphology or lethality (Kramer, 1997; Page and Winter, 2003; Page and Johnstone, 2007).
Fig. 1A.
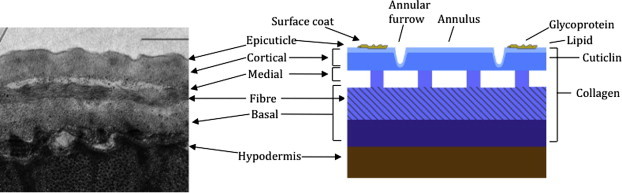
The organization and structure of the C. elegans cuticle. Left image is a transmission electron micrograph (TEM) depicting a longitudinal cross-section of the adult cuticle. Right panel is a cartoon depiction highlighting the distinct structural layers and their composition. With the exception of the epicuticle and surface coat, collagens are present in all major layers. Cuticlins are restricted to the cortical layer. The epicuticle contains lipids and is covered by a glycoprotein-rich coat (scale bar 1 μm).
Collagen and collagen-like proteins, at 80% of total protein, constitute the vast majority of the cuticular structural components. Compared with vertebrate collagen monomers, which are large and consist of long uninterrupted runs of the defining Gly-X-Y motif (where Gly is glycine, and X and Y are most often proline and hydroxyproline, respectively), nematode collagen monomers are generally smaller, around 35 kDa, and contain multiple interruptions within the Gly-X-Y repeat regions. The C. elegans cuticle collagen family consists of 167 members, 22 of which result in informative body morphology defects when mutated; these include phenotypes known in C. elegans nomenclature as dumpy (or Dpy, which are short and fat), roller (or Rol, helically twisted), long (or Lon), squat (or Sqt, short and twisted) and blistered (or Bli, fluid-filled blistering of the cuticle) (Page and Johnstone, 2007). Similar morphological phenotypes are found in mutants where the enzymes required for collagen and cuticle synthesis are defective (Page and Winter, 2003; Page and Johnstone, 2007). In addition to collagen, an unusual highly cross-linked class of insoluble protein called cuticlin is present in the nematode cuticle (Sapio et al., 2005), with C. elegans cuticlin mutants displaying dumpy morphological defects in specific developmental stages (Muriel et al., 2003; Sapio et al., 2005).
Collagen biogenesis is a complex, multi-step process with modifications that occur both intra- and extra-cellularly and requires the function of numerous enzymes (Fig. 1B). Some of the key enzymes involved in this pathway in C. elegans will be discussed in detail and their relevance to important human and animal parasitic nematodes will be highlighted.
Fig. 1B.

The cuticle collagen biogenesis pathway in C. elegans. Steps within the endoplasmic reticulum (ER) (blue) include, proline hydroxylation by DPY-18, disulphide bond formation by PDI, and proline isomerisation, by proline isomerases (PPIases). Export from the ER requires a functional COPII-pathway (sec-23). All cuticle collagens are predicted to be N-terminally processed by the subtilisin-like protease BLI-4, whereas a subset of collagens are C-terminally processed by the BMP-like protease DPY-31. The final step in collagen maturation involves tyrosine cross-link formation and is catalysed by peroxidase enzymes including BLI-3 and MLT-7. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. Enzymatic modifications occurring within the endoplasmic reticulum
2.1. Hydroxylation of proline residues
The first step in collagen synthesis involves the co-translational hydroxylation of Y position proline residues to 4-hydroxyproline in the Gly-X-Y domain, a modification that is required to stabilise the final assembled collagen triple helix. This reaction is catalysed by collagen prolyl 4-hydroxylase (C-P4H), which in multi-cellular animals are oligomeric enzymes consisting of α subunits, which contain the active site residues, and β subunits, formed by protein disulphide isomerase (PDI). Our lab has studied C-P4H extensively in the free-living nematode C. elegans where they are developmentally essential. C. elegans lacking a single C-P4H α subunit (DPY-18) are viable but show abnormal body morphology, cuticle structure, collagen localisation, and reduced levels of cuticular 4-hydroxyproline (Winter and Page, 2000), while combined loss of both α subunits (DPY-18 and PHY-2) results in embryonic lethality (Winter and Page, 2000; Winter et al., 2007b). Oligomeric C-P4Hs in all species examined contain only one type of β subunit PDI, which is present in all forms of the complex. Therefore, in C. elegans single loss of the β subunit (PDI-2) results in phenotypes equivalent to combined disruption of both α subunits (Winter and Page, 2000; Winter et al., 2007b) (Fig. 2). Recombinant C. elegans C-P4H are effectively inhibited using co-substrate analogues and in vivo these compounds replicate the phenotypes found by genetic disruption in C. elegans (Myllyharju et al., 2002). In vitro and in vivo work demonstrated that the C. elegans C-P4H complexes were formed from combinations of subunits that are unique to nematodes (Myllyharju et al., 2002). Similar analysis of a close relative of C. elegans, Caenorhabditis briggsae, revealed further nematode-specific C-P4H complexes that differ significantly from their vertebrate counterparts (Winter et al., 2007b).
Fig. 2.
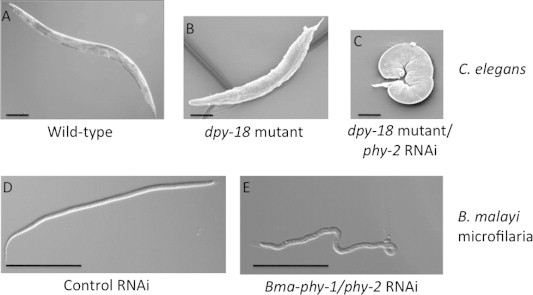
Collagen prolyl 4-hydroxylase (C-P4H) is essential in C. elegans and B. malayi. (A) Wild type (N2) adult C. elegans showing normal body morphology. (B) Mutation in C. elegans dpy-18 C-P4H α subunit resulting in a dumpy (short fat) phenotype. (C) C. elegans phy-2 RNAi in a dpy-18 mutant background causing larval lethality from loss of both C-P4H α subunits. (D) Wild type morphology of control (C. elegans phy-3) RNAi on B. malayi microfilaria following treatment of cultured adult females (Winter et al., 2013). (E) Mutant microfilaria body morphology phenotype following double C-P4H α subunit RNAi treatment of cultured adult females (Winter et al., 2013). Scale bars 100 μm.
Using a novel RNAi approach, our lab demonstrated that C-P4H is also developmentally essential in the human infective filarial nematode Brugia malayi (Winter et al., 2013). Analysis of the B. malayi genome (Ghedin et al., 2007) aided identification of all potential C-P4H subunits. Simultaneous RNAi of both α subunits (Bma-PHY-1 and -2) in cultured B. malayi adult females resulted in a highly penetrant body morphology defect in the released microfilariae (L1s) (Winter et al., 2013) (Fig. 2). This effect was replicated following RNAi of the single β subunit (Bma-PDI-2) (Winter et al., 2013). These results are supported by previous studies which showed that 4-hydroxyproline levels in B. malayi cuticle collagens were similar to those of C. elegans (Cox et al., 1981; Petralanda and Piessens, 1991) and that moulting of cultured B. malayi L3s was dependent on addition of ascorbate, a co-factor essential for C-P4H activity (Rajan et al., 2003). While the B. malayi RNAi results clearly mirrored the C. elegans genetic analysis, biochemical approaches revealed unusual aspects of the parasitic nematode proteins. In contrast to α subunits from every other multi-cellular species examined, recombinant Bma-PHY-1 and -2 proteins did not require PDI for solubility (Winter et al., 2003). Also, co-expression of all three B. malayi C-P4H components (Bma-PHY-1, -2 and Bma-PDI-2) in all combinations failed to produce highly active enzyme. In addition, the B. malayi proteins did not complement a C. elegans C-P4H mutant. Importantly, lack of complementation using B. malayi proteins is not a general phenomenon, as Bma-PDI-2 can complement a C. elegans pdi-2 mutant. This also confirmed that Bma-PDI-2 was a bona fide C-P4H β subunit. Further investigation into the B. malayi proteins revealed that parasite-derived, but not recombinant, B. malayi material contained a non-reducible covalent bond linking the subunits. This indicated that B. malayi C-P4H activity was dependent on this modification and its absence in recombinant proteins accounted for lack of activity/function in the assays employed. Enzymes from additional parasitic nematode species will need to be examined to determine if the B. malayi enzymes are unique in this respect. However, a covalent crosslink is not found between C-P4H subunits from any other species examined to date, including the extensively studied vertebrate enzymes. It is possible that the major differences in enzyme assembly between nematodes and vertebrate enzymes may be exploitable in the development of nematode-specific C-P4H inhibitors.
2.2. Procollagen registration: disulphide bond formation
The next important step in collagen biogenesis is the registration and trimerisation of the collagen chains. Registration involves the correct association of the monomers and is catalysed by PDI through disulphide bond formation of highly conserved C-terminal cysteine clusters in the cuticle collagens. This event may be key to the selection and assembly of the correct partners to form homo- or indeed hetero-trimers. In addition to the role in proline hydroxylation, it has been established that PDI-2 is also involved in this oxidative registration step in C. elegans (Winter et al., 2007b). Mutations in pdi-2 cause severe cuticle defects and adult sterility, whereas the combined mutations of pdi-1, pdi-2 and pdi-3 cause embryonic lethality and it was concluded that PDI-2 performs two essential functions during morphogenesis: one that is C-P4H dependent and the other contributing to disulphide bond formation. The importance of disulphide bonds and the oxidative/reductive state of the cuticle has also been found to be critical during the moulting process (Stenvall et al., 2011) and will be addressed further under the moulting section.
2.3. Procollagen trimerisation and proline isomerisation
In vitro analysis of vertebrate type III collagen demonstrates that trimer folding is rate-limited by the slow cis–trans isomerisation of proline and hydroxyproline residues and requires the assistance of peptidyl prolyl cis–trans isomerase (PPIase) enzymes (Bachinger, 1987). The PPIase enzyme superfamily contains the cyclophilin (CYN) and FK506-binding protein (FKB) families, members of which are defined as receptors for the structurally unrelated immunosuppressive drugs cyclosporin A (CsA) and FK506, respectively.
CsA (Page et al., 1995; Bell et al., 1996) and novel PPIase inhibitors (Yang et al., 2007; Dunsmore et al., 2011) cause severe gut and cuticle related phenotypes in C. elegans suggesting that PPIase enzymes are required to fold the structural components, such as the proline and hydroxyproline-rich collagens. To date, the exact combination of CYN enzymes, from a pool of 18, that are involved in this process have not been defined, as genetic analysis of cyns in C. elegans revealed widespread redundancy (Bell et al., 1996). However, combined loss of two ER-localised C. elegans FKBs, fkb-4 and -5, caused a cold-sensitive lethal phenotype with associated collagen and cuticle matrix defects (Winter et al., 2007a). It is therefore envisaged that this rate-limiting folding event is thermally catalysed under normal conditions and that only under adverse cold stress conditions is there an absolute requirement for these PPIase enzymes.
3. Modifications that occur outside the endoplasmic reticulum
The transit of collagen from the ER to through the secretory pathway coincides with the N- and C-terminal processing events. The removal of the globular non-Gly-X-Y domain results in protein insolubility and precedes the final multimerisation and cross-linking events. A number of key enzymes play important roles in the post-ER maturation of collagens, and may therefore represent novel drug targets.
3.1. Collagen N-terminal processing
All C. elegans cuticle collagens possess a highly conserved N-terminal Kex2, furin-type cleavage site (RX(K/R)R), which has been experimentally defined as a requirement for processing the cuticle collagens ROL-6, SQT-1 (Yang and Kramer, 1994, 1999) and DPY-5 (Thacker et al., 2006). The serine protease BLI-4 is required for post-embryonic viability in C. elegans (Peters et al., 1991; Thacker et al., 1995) and was originally defined by a viable partial loss-of-function mutant with a blistered adult cuticle. Similarly, RNAi depletion of BLI-4 reveals another cuticle-related phenotype as moulting defects were observed (Kamath et al., 2003). These findings suggested that BLI-4 is the enzyme that processes the N-terminal pro-region of the cuticle collagens and this is supported by its hypodermal expression pattern (Thacker et al., 1995) and by the genetic suppression of the bli-4 phenotype by mutations in the cuticle collagen gene dpy-5 (Thacker et al., 2006). Orthologues of BLI-4 are present in many parasitic nematode genomes including Onchocerca volvulus (Poole et al., 2003). Thus, BLI-4 is an essential enzyme during embryogenesis, and is required for formation of the cuticle in C. elegans, most likely due to processing the N-terminal pro-region of cuticle collagens, and is likely to perform this role in a range of parasitic nematode species.
A targeted RNAi screen for disruption of cuticle collagen localisation performed in our lab, identified BLI-5 (Page et al., 2006). Mutation of bli-5 resulted in a blistered cuticle, while overexpression produced a severe moulting phenotype (Page et al., 2006). Protein annotation suggested BLI-5 was a protease inhibitor; however biochemical characterisation of C. elegans BLI-5 revealed that it activated, rather than inhibited, serine protease activity (Stepek et al., 2010b). BLI-5 orthologues from the parasitic nematodes Haemonchus contortus and B. malayi were both able to complement the C. elegans bli-5 mutant and both also activated serine protease activity in vitro (Stepek et al., 2010b). The biochemical characterisation and phenotypic similarity between bli-5 and -4 mutants suggests BLI-5 might regulate BLI-4 (Stepek et al., 2010b). In support of this, both bli-5 and bli-4 were found to share common temporal and spatial expression patterns and combined bli-4/bli-5 RNAi produced a variable but synergistic effect (Stepek et al., 2010b).
3.2. Collagen C-terminal processing
The C-terminal processing of vertebrate fibrillar collagens is an essential step that is carried out by zinc metalloproteases of the astacin BMP class (bone morphogenic protein) (Canty and Kadler, 2005). The nematode astacins (NAS) represent a large gene family of 39 members in C. elegans that includes the subgroup V forms that, in addition to a metalloprotease domain, have a unique nematode-specific domain arrangement that includes an epidermal growth factor (EGF) domain, a complement subcomponent C1r/C1s (CUB) domain and a thrombospondin (TSP) type 1 domain (Mohrlen et al., 2003; Stepek et al., 2010a). Mutations in the C. elegans astacin V-encoding gene nas-35 (a.k.a. dpy-31) resulted in a temperature sensitive severe dumpy phenotype as well as embryonic and post-embryonic lethality (Novelli et al., 2004). This enzyme is expressed in the hypodermal tissue and is required for normal collagen secretion. Screens for genetic suppressors of dpy-31 identified the cuticle collagen SQT-3 as a major substrate for this enzyme, and highlighted its cleavage site adjacent to the C-terminal tyrosine cross-linking site. dpy-31 therefore encodes an essential procollagen C-peptidase (Novelli et al., 2004, 2006); (Fig. 1B). Orthologues of this essential enzyme are found in the parasitic nematodes B. malayi and H. contortus (Stepek et al., 2010a). Heterologous expression of the Brugia dpy-31 promoter in C. elegans showed it directed strong expression of a reporter gene to the secretory gland cells and the gut. Complementation experiments revealed that the H. contortus gene could rescue the C. elegans dpy-31 mutant whereas the B. malayi gene did not. The functional significance of the nematode enzymes was confirmed biochemically by performing metalloprotease assays and by the in vitro cleavage of recombinant C. elegans SQT-3 cuticle collagen at the specific C-terminal site (Stepek et al., 2010a). These assays substantiated the key role that this enzyme plays in the processing of nematode collagens and highlighted the conservation of function between the C. elegans and parasitic nematode enzymes.
3.3. Collagen cross-linking
The final steps in collagen maturation and cuticle synthesis are the structural cross-linking events. The cuticle collagens and cuticlins are covalently cross-linked via di- and tri-tryrosine cross-links. These non-reducible cross-links impart the characteristic strength and integrity to the cuticle (Page, 2001) and are distinct from the hydroxylysine-derived cross-links commonly found in the vertebrate collagens (Myllyharju and Kivirikko, 2004). The major cuticle cross-linking enzyme is a NADPH dual oxidase enzyme called BLI-3 (Edens et al., 2001; Simmer et al., 2003) (Fig. 1B). This large enzyme has a membrane-bound peroxide generating domain and a pseudo-peroxidase domain (Edens et al., 2001). RNAi (Edens et al., 2001) or mutation (Simmer et al., 2003) of BLI-3 results in weakened cuticles that lack tyrosine cross-links, producing adult worms that are devoid of cuticle struts and exhibit dumpy and blister phenotypes. BLI-3 does however lack a highly conserved haem-binding motif that is essential for peroxidase activity, and through RNAi screens an additional Haem peroxidase called MLT-7 was subsequently identified (Thein et al., 2003, 2009). MLT-7 protein has haem-binding and metridin ShK toxin domains, with mutants arresting as larvae with moulting defects. Like bli-3 mutants, mlt-7 mutants have fragile, permeable cuticles that are devoid of tyrosine cross-links and combined bli-3/mlt-7 mutants arrest prematurely as severely misshapen L1s. BLI-3 and MLT-7 are therefore the key enzymes that cooperate to promote the oxidative cross-linking of the collagens with a model suggesting that BLI-3 produces the H2O2 that is used by MLT-7 to generate the tyrosine linkages (Thein et al., 2009). Within their peroxidase domains, the MLT-7 orthologues from H. contortus and B. malayi show 82% and 50% identity, respectively, with C. elegans MLT-7 (Thein et al., 2009). The plant nematode, Meloidogyne incognita, has a dual oxidase gene (Miduox) that encodes a multifunctional enzyme involved in tyrosine cross-linking of the developing cuticle during normal cuticle biosynthesis (Charlton et al., 2010). RNAi knockdown of this gene resulted in a slower developmental rate and a reduction in plant infectivity (Bakhetia et al., 2005). This Duox enzyme has significant identity to BLI-3 in C. elegans.
4. The enzymology of the moulting process
All nematodes are encased in a protective cuticle but this resilient structure restricts growth and therefore must be successively shed and re-synthesised. All nematodes undergo four cuticle moults from the L1 hatchling to the mature adult. The moulting process replaces the cuticle from the previous stage with a new cuticle, and occurs in three general steps: (1) lethargus, in which the activity of the worm decreases; (2) apolysis, in which the old cuticle separates from the hypodermis; and (3) ecdysis, in which the old cuticle is completely shed and the worm emerges as the next stage with a new cuticle. In C. elegans, this moulting process is repeated every 10 h and lasts for approximately 2 h, with the final ecdysis stage occurring rapidly within a matter of minutes (Singh and Sulston, 1978).
The process of moulting is unique to the Ecdysozoa clade, which in addition to nematodes, also includes arthropods, and may therefore represent a more general target for a wider group of pest species. The endocrine trigger for the moulting process in nematodes remains to be identified but in arthropods, such as Drosophila, this pathway is well characterised and centres on the hormone ecdysone, the nuclear ecdysone receptor (ECR) and ultraspiracle (USP) (Tzertzinis et al., 2010). In C. elegans, an ecdysone hormone has not been identified but a functional ECR homologue has been characterised in the filarial nematode B. malayi (Tzertzinis et al., 2010). The moulting process in C. elegans does require cholesterol, an essential low-density lipoprotein receptor (LRP-1) (Yochem et al., 1999) and an essential sterol synthesising enzyme LET-767 that is localised to the nematode gut (Kuervers et al., 2003). The genome of C. elegans does, however, encode a large family of orphan nuclear hormone receptors, two of which, NHR-23 and NHR-25, are essential for proper moulting (Kostrouchova et al., 1998, 2001).
Characterisation of the components of the moulting pathway in C. elegans is helping to uncover many nematode-specific features that may prove fruitful in future nematode control strategies. A global RNAi screen in C. elegans uncovered 159 genes that, when disrupted, affect the moulting process to some degree (Frand et al., 2005); this list includes proteases, protease inhibitors, peroxidases, matrix components, sterol sensing proteins, nucleic acid binding/interacting proteins, signalling proteins and a range of other novel proteins, many of which are exclusively found in members of the nematode phylum. The importance of a key moulting component, MLT-10, identified in this screen (Frand et al., 2005) has been confirmed following a cholesterol-sensitivity mutagenic screen (Meli et al., 2010). MLT-10 is a member of a large family of novel nematode-specific proline-rich proteins and is essential for both the synthesis of the new and removal of the old cuticle (Meli et al., 2010).
The oxidative state of disulphide linkages in the nematode cuticle has recently been shown to be a key feature in the apolysis process. Characterisation of the sole selenocysteine containing protein in C. elegans, thioredoxin TRXR-1, revealed that in combination with the glutathione reductase (GSR-1), both proteins are essential for the normal moulting process (Stenvall et al., 2011). It was noted that the cuticular disulphide linkages are actively reduced during apolysis, an effect that could be induced prematurely by incubating worms in strong reducing agents or glutathione (GSH). TRXR-1 is expressed in the hypodermis and has an absolute requirement for selenocysteine. This study revealed that the oxidative state of the disulphide groups in the cuticle is tightly regulated during the moulting cycle, and that, when the combined functions of trxr-1 and gsr-1 are perturbed, these groups remain in an oxidised state resulting in moulting defects (Stenvall et al., 2011). This process may be linked to the PDI-catalysed collagen chain registration steps (Fig. 1A) (Winter et al., 2007c) and/or the higher order PDI-catalysed matrix formation events (Eschenlauer and Page, 2003).
4.1. Ecdysis enzymes
In addition to the collagen biogenesis and cuticle assembly pathways, numerous enzymes play key roles in the subsequent cuticle shedding and moulting processes. The astacin metalloproteases, as well as being involved in procollagen C-processing, are also involved in the ecdysis process in C. elegans, and recombinant forms of these C. elegans enzymes can specifically digest the cuticle and permit exsheathment of the parasitic nematode H. contortus (Davis et al., 2004; Stepek et al., 2011). NAS-36 and -37 represent the subgroup V astacins that play key roles in cuticle ecdysis in C. elegans (Novelli et al., 2004).
Mutations in nas-36 and nas-37 result in moulting defects where worms are incarcerated in the cuticle from the previous stage, which remains unshed due to a tight anterior ring of undigested cuticle (Davis et al., 2004; Suzuki et al., 2004) (Fig. 3). NAS-37 is expressed in the C. elegans hypodermis just prior to ecdysis. The protein accumulates in the anterior cuticle and aids the proper digestion of the anterior cap, an event that concludes the moulting process and allows the worms to escape from the old cuticle (Davis et al., 2004). This specific enzymatic function is analogous to exsheathment in H. contortus, where an anterior refractile ring forms in the L2 cuticle sheath prior to infection of the host by the L3 stage (Gamble et al., 1989, 1996). A specific function was recently established when recombinantly-expressed C. elegans NAS-37 and NAS-36 were shown to induce refractile ring formation in the H. contortus L2 cuticle sheath (Davis et al., 2004; Stepek et al., 2011). Both H. contortus and B. malayi have a NAS-36, but lack a NAS-37 orthologue. The B. malayi gene can complement C. elegans strains mutant in either nas-36 or nas-37, and a combined mutant of nas-36/nas-37, indicating that NAS-36 is the dominant molecule involved in cuticle ecdysis in parasitic nematodes. This result also confirmed that NAS-36 metalloprotease plays a functionally conserved role in phylogenetically divergent nematode species (Stepek et al., 2011).
Fig. 3.

Moulting defects due to mutations in C. elegans metalloprotease NAS-37. (A) Mid-body constriction due to unshed cuticle and incomplete ecdysis due to mutation in nas-37. (B) SEM image of mid-body cuticle constriction due to nas-37 mutation. Scale bars 100 μm.
4.2. Additional cuticle-associated zinc metalloproteases
Zinc metalloproteases, with a similar crucial involvement in the L2 to L3 moult and exsheathment of L3, are present in the excretion/secretion (ES) of the infective L3 of many additional parasitic nematodes, including Brugia pahangi (Hong et al., 1993), Dirofilaria immitis (Richer et al., 1992), Ancylostoma caninum and Ancylostoma duodenale (Hotez et al., 1990; Hawdon et al., 1995), and the bovine filarial nematode, Setaria cervi (similar to Wuchereria bancrofti) (Pokharel et al., 2006). A similar zinc metalloprotease is also involved in the formation and degradation of the old and new cuticles during the moulting of the L3 to L4 of Ascaris suum (Rhoads et al., 1998) and the nematode of fish, Hysterothylacium aduncum (Malagon et al., 2010b).
The third-stage larvae of many parasitic nematodes, including Trichuris suis (Hill et al., 1993), Trichinella spiralis (Lun et al., 2003) and the fish nematode, H. aduncum (Malagon et al., 2010a), secrete metalloproteases that aid in the penetration of the host tissues. The hookworms, A. caninum and Necator americanus, the filarial nematode, O. volvulus, and the gastrointestinal nematode, Strongyloides stercoralis, secrete astacin metalloproteases (Ac-MTP-1, Na-MTP-1, Ov-AST-1 (onchoastacin) and Ss40 (strongylastacin), respectively), that aid in host skin penetration by hookworm L3 (Culley et al., 2000; Williamson et al., 2006) and S. stercoralis L3 (Brindley et al., 1995) and migration through the host tissue by filarial microfilariae (Borchert et al., 2007) to initiate infection. These enzymes have collagenolytic activity to help aid the degradation of the collagen in the host skin so that the parasite can penetrate and infect the host and are thus potential targets for new vaccines or drugs (Zhan et al., 2002; Hotez et al., 2003; Williamson et al., 2006).
ACN-1 is an angiotensin-converting enzyme (ACE)-like protein from C. elegans that is essential for larval development and adult morphogenesis, in particular the ecdysis step in moulting. Expression of acn-1 is regulated by the moulting-associated genes nhr-23 and nhr-25 (Brooks et al., 2003) however, no homologues have been found in parasitic nematodes.
5. Cuticle-associated cysteine and serine proteases and their inhibitors
Cysteine proteases in nematodes (as for serine proteases, metalloproteases and aspartic proteases) have potential roles in digestion of the old cuticle, degradation of cuticular proteins and activation of moulting enzymes by processing their proenzymes. C. elegans encodes a cathepsin-L cysteine protease (Ce-cpl-1) that is similar to the proteases in O. volvulus, B. pahangi, H. contortus, Dictyocaulus viviparus, Toxocara canis, A. caninum, A. suum, and plant parasitic nematodes such as Heteodera glycines (Britton and Murray, 2002), and is believed to be involved in post-embryonic development through degradation of the eggshell and cuticular proteins. Expression of this gene is greatest ∼4 h prior to each moult, indicating a possible role in moulting to degrade the old cuticle whilst processing the next cuticle. This cysteine protease is either involved directly or indirectly by regulating other enzymes involved in the moulting process (Hashmi et al., 2002). B. malayi adults express the cathepsin-like cysteine proteases Bm-cpl-1, Bm-cpl-5 and Bm-cpz-1 that all have potential roles in embryogenesis, larval moulting and eggshell and cuticle remodelling, and share similarities with the C. elegans homologues (Ford et al., 2009). The cathepsin L cysteine proteases, cpl-1, -4 and -5 are secreted from O. volvulus (Guiliano et al., 2004), are involved in the L3 to L4 moult (Lustigman et al., 1992, 1996) and share many characteristics with the CPLs of B. malayi (Guiliano et al., 2004), D. immitis (Richer et al., 1992, 1993) and B. pahangi (Guiliano et al., 2004).
The role of the cathepsin Z-like cysteine protease (Ce-CPZ-1) in C. elegans development is multifunctional, with the mRNA expressed throughout the lifecycle, increasing just prior to the L2/L3, L3/L4 and L4/adult moults, and then decreasing just after each moult. RNAi and gene knock-out result in severe moulting defects, and larval arrest at the L2 to L3 moult, thus suggesting a possible role for this enzyme in the moulting pathway; e.g., degrading cuticular proteins before ecdysis, or controlling proteins with essential roles during moulting (Hashmi et al., 2006). It is similar to Ov-CPZ from O. volvulus and Tc-CPZ from T. canis, indicating that the CPZ-1 function is possibly conserved in other nematodes (Hashmi et al., 2002). N. americanus express an L3 specific cysteine protease that is found in the exsheathing fluid and may therefore play a role in ecdysis (Kumar and Pritchard, 1992).
The cysteine proteases involved in the moulting pathway of C. elegans and parasitic nematodes, such as H. contortus, are developmentally regulated, although their exact role remains to be established. C. elegans CPI-2a is a cystatin-like inhibitor that is present in the cuticle of all post embryonic stages and may have an essential regulatory role during oogenesis and fertilisation. This inhibitor regulates the cysteine proteases, CPL-1 and CPZ-1, during their essential roles in moulting and embryogenesis. The similar expression of cpi-2a to that of cpl-1 and cpz-1 (increasing ∼2.5–3.5 h before each moult then decreasing after the moults), again, suggests that CPI-2a has an important role in moulting to regulate the activities of CPL-1 and/or CPZ-1 (Hashmi et al., 2006). Homologues have been found in B. malayi, Litomosoides sigmodontis, Acanthocheilonema viteae and O. volvulus. Onchocystatin (Ov-CPI-2) is a potentially secreted cysteine protease inhibitor of O. volvulus that is found in the hypodermis and cuticle basal layer of moulting L3 and L4, adults and in the eggshell around the developing microfilariae and may regulate the aforementioned cysteine proteases (Lustigman et al., 1992, 1996; Richer et al., 1993). A homologue of this inhibitor has been described in Anisakis simplex, where it localises to the secretory gland and basal layer of the cuticle of L3 (Rodriguez-Mahillo et al., 2007).
Serine proteases are implicated in the processing of cuticular proteins during moulting and development (see collagen N-terminal processing section), and serine protease inhibitors may regulate or control this class of proteases (Drake et al., 1994). A serine protease inhibitor (Ov-SPI-1) was identified in the L3 stage of O. volvulus, with expression increasing in the moulting larvae and adults. Ov-SPI-1 is located in the basal layer of the cuticle of L3, moulting L3 and L4, and in the sperm and eggshells in adults. RNAi of SPI-1 causes an 84% decrease in moulting and viability of L3. O. volvulus blisterase is found in the same cuticle regions in moulting L3 as Ov-SPI-1, so could be a potential target of Ov-SPI-1 (Ford et al., 2005). Ov-SPI-1 homologues are found in the parasitic nematodes B. malayi, D. immitis, L. sigmodontis, A. suum and Ascaris lumbricoides.
A cross-class inhibitor of serine proteases and cysteine proteases (SRP-2) is present in C. elegans during postembryonic development and is a member of the clade L serpins, of which there are additional genes in C. elegans. It is present in the eggshell and in the intestine, hypodermis and cuticle of larvae and adults, suggesting a potential role in development. Overexpression of this gene leads to slow growth, defective moulting (i.e. failure to shed the old cuticles), and early (L1/L2) larval arrest/death. The cysteine protease, CPL-1, is present in C. elegans in the hypodermis, intestine, pharynx, gonad, eggshell and cuticle, and its expression increases ∼4 h prior to each moult, suggesting a potential role in degrading cuticular proteins. RNAi of cpl-1 results in similar effects as SRP-2 overexpression: embryonic arrest and slow growth (Pak et al., 2004). As yet, no SRP-2 homologues have been identified in parasitic nematodes.
6. Other potential targets of the nematode cuticle
External enzymes, such as papain, bromelain, collagenase, chitinase and lipase, cause structural damage to the cuticle, leading to the death of the plant parasitic nematode, Tylenchorhynchus dubius, but not the plant parasitic nematodes, Hoplolaimus tylenchiformis and Pratylenchus penetrans. These external enzymes do, however, cause a decrease in the motility of these latter two nematodes (Miller and Sands, 1977). Cuticle damage and a decrease in nematode motility also occurred when Heligmosomoides polygyrus, Trichuris muris, Protospirula muricola and the plant parasitic nematodes, M. incognita, Meloidogyne javanica and Globodera rostochiensis, were incubated with plant cysteine proteases, such as papain and bromelain, in vitro. This cuticle damage and loss of activity led to nematode death. This digestion of the cuticle represents a novel potentially exploitable, mechanism of action. Cuticle damage occurred together with loss of worm motility. However, the cuticle proteins that are sensitive to this plant cysteine protease digestion remain unknown, although as only the post-infection stages are affected, this suggests that the susceptible cuticle components are in the parasitic stage cuticles, not the free living stage cuticles (Stepek et al., 2005; Stepek et al., 2006, 2007a,b). Digestion of the cuticle in vivo, just as in vitro, also occurred when H. polygyrus, T. muris and P. muricola infected mice were treated with the plant cysteine proteases, fruit bromelain and crude papaya latex (Stepek et al., 2006, 2007a,b). H. polygyrus adult worms were expelled rapidly within 4 h of treatment if they were damaged, resulting in a decrease in faecal egg count by 87–97% and a decrease in worm burden by 92%, suggesting that cysteine proteases from plants may be a candidate for novel anthelmintics. However, this treatment has no effect against the development of mucosal-dwelling L3 and L4 stages, only the adults in the gut lumen (Stepek et al., 2007b). Proteases from nematophagous bacteria and fungi can penetrate the cuticle of plant parasitic nematodes, causing infection of the nematode (Tian et al., 2009). Serine proteases from Bacillus (Lian et al., 2007; Huang et al., 2009), Hirsutella rhossiliensis (Wang et al., 2009), Paecilomyces lilocinas (Yang et al., 2011) and Pezizomycotina (Li et al., 2010) degrade the nematode cuticle, causing death of the nematode.
Thus, serine proteases from bacteria and fungi, and cysteine proteases from plants degrade nematode cuticles; however, the cuticle substrates for these proteases are still, as yet, unknown, indicating a requirement for further studies to determine these cuticle substrates that are strong potential candidates for novel targets for the much-needed new drugs or vaccines.
7. Concluding remarks
Significant progress has been made regarding our understanding of the structure, assembly and turnover of the C. elegans nematode cuticle. Many of the key enzymes involved in this process have now been identified and described in several important parasitic nematode species (Table 1). It is becoming clear that enzymes involved in the biogenesis of this critical exoskeleton and more significantly, in the moulting process, represent a chink in the armour of these resilient metazoans. With the advent of completed genomes and the development of crucial experimental tools such as RNA interference, research into the enzymology of the cuticle in the parasitic nematodes is entering a promising era that may uncover novel drug targets and their inhibitors.
Table 1.
Summary of the reviewed cuticle biosynthesis and moulting targets in C. elegans and their known parasitic nematode orthologues.
| Cuticle biosynthesis stage | Potential target in C. elegans | Parasitic nematodes with orthologues |
|---|---|---|
| Proline hydroxylation | P4H, (DPY-18, PHY-2) | B. malayi (Bma-PHY-1, -2) |
| PDI-2 | B. malayi (Bma-PDI-2) | |
| Disulphide formation | PDI-2 | B. malayi (Bma-PDI-2) |
| Procollagen trimerisation/proline isomerisation | PPIase (FKB-4, FKB-5) | |
| Collagen N-terminal processing | BLI-4 | O. volvulus (blisterase) |
| BLI-5 | H. contortus, B. malayi (Hc-BLI-5, Bm-BLI-5) | |
| Collagen C-terminal processing | DPY-31 | H. contortus, B. malayi (Hc-DPY-31, Bm-DPY-31) |
| Collagen cross-linking | BLI-3 | Meloidogyne incognita (Miduox) |
| MLT-7 | H. contortus, B. malayi (Hc-MLT-7, Bm-MLT-7) | |
| Moulting | LRP-1 | |
| LET-767 | ||
| NHR-23, NHR-25 | ||
| MLT-10 | B. malayi (Bm-MLT-10) | |
| TRXR-1 | ||
| GSR-1 | ||
| NAS-36 | H. contortus, B. malayi (Hc-NAS-36, Bm-NAS-36) | |
| NAS-37 | ||
| ACN-1 | ||
| CPL-1 | B. malayi (Bm-CPL-1), O volvulus (Ov-CPL-1), H. controtus (Hc-CPL-1), S. vulgaris CPL-1 | |
| CPL-5 | B. malayi (Bm-CPL-5), O volvulus (Ov-CPL-5), | |
| CPZ-1 | O. volvulus (Ov-CPZ), T. canis (Tc-CPZ), B. malayi (Bm-CPZ-1) | |
| CPI-2a | B. malayi, L. sigmodontis, A. viteae, O. volvulus (Ov-CPI-2), A. simplex | |
| SRP-2 |
Acknowledgements
This work is supported by the Biotechnology and Biological Sciences and Biochemical Research Council (UK). The authors have declared that no competing interests exist.
References
- Bachinger H.P. The influence of peptidyl-prolyl cis–trans isomerase on the in vitro folding of type III collagens. J. Biol. Chem. 1987;262:17144–17148. [PubMed] [Google Scholar]
- Bakhetia M., Charlton W., Atkinson H.J., McPherson M.J. RNA interference of dual oxidase in the plant nematode Meloidogyne incognita. Mol. Plant. Microbe Interact. 2005;18:1099–1106. doi: 10.1094/MPMI-18-1099. [DOI] [PubMed] [Google Scholar]
- Bell A., Roberts H.C., Chappell L.H. The antiparasite effects of cyclosporin A: possible drug targets and clinical applications. Gen. Pharmacol. 1996;27:963–971. doi: 10.1016/0306-3623(95)02148-5. [DOI] [PubMed] [Google Scholar]
- Borchert N., Becker-Pauly C., Wagner A., Fischer P., Stocker W., Brattig N.W. Identification and characterization of onchoastacin, an astacin-like metalloproteinase from the filaria Onchocerca volvulus. Microbes Infect. 2007;9:498–506. doi: 10.1016/j.micinf.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Brindley P.J., Gam A.A., McKerrow J.H., Neva F.A. Ss40: the zinc endopeptidase secreted by infective larvae of Strongyloides stercoralis. Exp. Parasitol. 1995;80:1–7. doi: 10.1006/expr.1995.1001. [DOI] [PubMed] [Google Scholar]
- Britton C., Murray L. A cathepsin L protease essential for Caenorhabditis elegans embryogenesis is functionally conserved in parasitic nematodes. Mol. Biochem. Parasitol. 2002;122:21–33. doi: 10.1016/s0166-6851(02)00066-x. [DOI] [PubMed] [Google Scholar]
- Brooks D.R., Appleford P.J., Murray L., Isaac R.E. An essential role in molting and morphogenesis of Caenorhabditis elegans for ACN-1, a novel member of the angiotensin-converting enzyme family that lacks a metallopeptidase active site. J. Biol. Chem. 2003;278:52340–52346. doi: 10.1074/jbc.M308858200. [DOI] [PubMed] [Google Scholar]
- Canty E.G., Kadler K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Charlton W.L., Harel H.Y., Bakhetia M., Hibbard J.K., Atkinson H.J., McPherson M.J. Additive effects of plant expressed double-stranded RNAs on root-knot nematode development. Int. J. Parasitol. 2010;40:855–864. doi: 10.1016/j.ijpara.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Cox G.N., Straprans S., Edgar R.S. The cuticle of Caenorhabditis elegans: II. Stage-specific changes in ultrastructure and protein composition during postembryonic development. Dev. Biol. 1981;86:456–470. doi: 10.1016/0012-1606(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Culley F.J., Brown A., Conroy D.M., Sabroe I., Pritchard D.I., Williams T.J. Eotaxin is specifically cleaved by hookworm metalloproteases preventing its action in vitro and in vivo. J. Immunol. 2000;165:6447–6453. doi: 10.4049/jimmunol.165.11.6447. [DOI] [PubMed] [Google Scholar]
- Davis M.W., Birnie A.J., Chan A.C., Page A.P., Jorgensen E.M. A conserved metalloprotease mediates ecdysis in Caenorhadbitis elegans. Development. 2004;131:6001–6008. doi: 10.1242/dev.01454. [DOI] [PubMed] [Google Scholar]
- Drake L.J., Bianco A.E., Bundy D.A., Ashall F. Characterization of peptidases of adult Trichuris muris. Parasitology. 1994;109:623–630. doi: 10.1017/s0031182000076502. [DOI] [PubMed] [Google Scholar]
- Dunsmore C.J., Malone K.J., Bailey K.R., Wear M.A., Florance H., Shirran S., Barran P.E., Page A.P., Walkinshaw M.D., Turner N.J. Design and synthesis of conformationally constrained cyclophilin inhibitors showing a cyclosporin-A phenotype in C. elegans. Chembiochem. 2011;12:802–810. doi: 10.1002/cbic.201000413. [DOI] [PubMed] [Google Scholar]
- Edens W.A., Sharling L., Cheng G., Shapira R., Kinkade J.M., Lee T., Edens H.A., Tang X., Sullards C., Flaherty D.B., Benian G.M., Lambeth J.D. Tyrosine cross-linking of extracellular matrix is catalysed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91/phox. J. Cell Biol. 2001;154:879–891. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenlauer S.C., Page A.P. The Caenorhabditis elegans ERp60 homolog protein disulfide isomerase-3 has disulfide isomerase and transglutaminase-like cross-linking activity and is involved in the maintenance of body morphology. J. Biol. Chem. 2003;278:4227–4237. doi: 10.1074/jbc.M210510200. [DOI] [PubMed] [Google Scholar]
- Ford L., Guiliano D.B., Oksov Y., Debnath A.K., Liu J., Williams S.A., Blaxter M.L., Lustigman S. Characterization of a novel filarial serine protease inhibitor, Ov-SPI-1, from Onchocerca volvulus, with potential multifunctional roles during development of the parasite. J. Biol. Chem. 2005;280:40845–40856. doi: 10.1074/jbc.M504434200. [DOI] [PubMed] [Google Scholar]
- Ford L., Zhang J., Liu J., Hashmi S., Fuhrman J.A., Oksov Y., Lustigman S. Functional analysis of the cathepsin-like cysteine protease genes in adult Brugia malayi using RNA interference. PLoS Negl. Trop. Dis. 2009;3:e377. doi: 10.1371/journal.pntd.0000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand A.R., Russel S., Ruvkun G. Functional genomic analysis of C. elegans molting. PLoS Biol. 2005;3:1719–1733. doi: 10.1371/journal.pbio.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble H.R., Purcell J.P., Fetterer R.H. Purification of a 44 kilodalton protease which mediates the ecdysis of infective Haemonchus contortus larvae. Mol. Biochem. Parasitol. 1989;33:49–58. doi: 10.1016/0166-6851(89)90041-8. [DOI] [PubMed] [Google Scholar]
- Gamble H.R., Fetterer R.H., Mansfield L.S. Developmentally regulated zinc metalloproteinases from third- and fourth-stage larvae of the ovine nematode Haemonchus contortus. J. Parasitol. 1996;82:197–202. [PubMed] [Google Scholar]
- Ghedin E., Wang S., Spiro D., Caler E., Zhao Q., Crabtree J., Allen J.E., Delcher A.L., Guiliano D.B., Miranda-Saavedra D., Angiuoli S.V., Creasy T., Amedeo P., Haas B., El-Sayed N.M., Wortman J.R., Feldblyum T., Tallon L., Schatz M., Shumway M. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiliano D.B., Hong X., McKerrow J.H., Blaxter M.L., Oksov Y., Liu J., Ghedin E., Lustigman S. A gene family of cathepsin L-like proteases of filarial nematodes are associated with larval molting and cuticle and eggshell remodeling. Mol. Biochem. Parasitol. 2004;136:227–242. doi: 10.1016/j.molbiopara.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Hashmi S., Britton C., Liu J., Guiliano D.B., Oksov Y., Lustigman S. Cathepsin L is essential for embryogenesis and development of Caenorhabditis elegans. J. Biol. Chem. 2002;277:3477–3486. doi: 10.1074/jbc.M106117200. [DOI] [PubMed] [Google Scholar]
- Hashmi S., Zhang J., Oksov Y., Ji Q., Lustigman S. The Caenorhabditis elegans CPI-2a cystatin-like inhibitor has an essential regulatory role during oogenesis and fertilization. J. Biol. Chem. 2006;281:28415–28429. doi: 10.1074/jbc.M600254200. [DOI] [PubMed] [Google Scholar]
- Hawdon J.M., Jones B.F., Perregaux M.A., Hotez P.J. Ancylostoma caninum: metalloprotease release coincides with activation of infective larvae in vitro. Exp. Parasitol. 1995;80:205–211. doi: 10.1006/expr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Hill D.E., Gamble H.R., Rhoads M.L., Fetterer R.H., Urban J.F., Jr. Trichuris suis: a zinc metalloprotease from culture fluids of adult parasites. Exp. Parasitol. 1993;77:170–178. doi: 10.1006/expr.1993.1074. [DOI] [PubMed] [Google Scholar]
- Hong X., Bouvier J., Wong M.M., Yamagata G.Y., McKerrow J.H. Brugia pahangi: identification and characterization of an aminopeptidase associated with larval molting. Exp. Parasitol. 1993;76:127–133. doi: 10.1006/expr.1993.1015. [DOI] [PubMed] [Google Scholar]
- Hotez P., Haggerty J., Hawdon J., Milstone L., Gamble H.R., Schad G., Richards F. Metalloproteases of infective Ancylostoma hookworm larvae and their possible functions in tissue invasion and ecdysis. Infect. Immun. 1990;58:3883–3892. doi: 10.1128/iai.58.12.3883-3892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Ashcom J., Zhan B., Bethony J., Loukas A., Hawdon J., Wang Y., Jin Q., Jones K.C., Dobardzic A., Dobardzic R., Bolden J., Essiet I., Brandt W., Russell P.K., Zook B.C., Howard B., Chacon M. Effect of vaccination with a recombinant fusion protein encoding an astacin like metalloprotease (MTP-1) secreted by host-stimulated Ancylostoma caninum third-stage infective larvae. J. Parasitol. 2003;89:853–855. doi: 10.1645/GE-46R. [DOI] [PubMed] [Google Scholar]
- Huang X., Liu J., Ding J., He Q., Xiong R., Zhang K. The investigation of nematocidal activity in Stenotrophomonas maltophilia G2 and characterization of a novel virulence serine protease. Can. J. Microbiol. 2009;55:934–942. doi: 10.1139/w09-045. [DOI] [PubMed] [Google Scholar]
- Kamath R.S., Fraser A.G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., Welchman D.P., Zipperlen P., Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kostrouchova M., Krause M., Kostrouch Z., Rall J.E. CHR3: a Caenorhabditis elegans orphan nuclear hormone receptor required for proper epidermal development and molting. Development. 1998;125:1617–1626. doi: 10.1242/dev.125.9.1617. [DOI] [PubMed] [Google Scholar]
- Kostrouchova M., Krause M., Kostrouch Z., Rall J.E. Nuclear hormone receptor CHR3 is a critical regulator of all four larval molts of the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2001;98:7360–7365. doi: 10.1073/pnas.131171898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J.M. C. elegans II. CSHL Press; 1997. Extracellular matrix; pp. 471–500. [Google Scholar]
- Kuervers L.M., Jones C.L., O’Neil N.J., Baillie D.L. The sterol modifying enzyme LET-767 is essential for growth, reproduction and development in Caenorhabditis elegans. Mol. Genet. Genom. 2003;270:121–131. doi: 10.1007/s00438-003-0900-9. [DOI] [PubMed] [Google Scholar]
- Kumar S., Pritchard D.I. The partial characterization of proteases present in the excretory/secretory products and exsheathing fluid of the infective (L3) larva of Necator americanus. Int. J. Parasitol. 1992;22:563–572. doi: 10.1016/0020-7519(92)90003-4. [DOI] [PubMed] [Google Scholar]
- Li J., Yu L., Yang J., Dong L., Tian B., Yu Z., Liang L., Zhang Y., Wang X., Zhang K. New insights into the evolution of subtilisin-like serine protease genes in Pezizomycotina. BMC Evol. Biol. 2010;10:68. doi: 10.1186/1471-2148-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L.H., Tian B.Y., Xiong R., Zhu M.Z., Xu J., Zhang K.Q. Proteases from Bacillus: a new insight into the mechanism of action for rhizobacterial suppression of nematode populations. Lett. Appl. Microbiol. 2007;45:262–269. doi: 10.1111/j.1472-765X.2007.02184.x. [DOI] [PubMed] [Google Scholar]
- Lun H.M., Mak C.H., Ko R.C. Characterization and cloning of metallo-proteinase in the excretory/secretory products of the infective-stage larva of Trichinella spiralis. Parasitol. Res. 2003;90:27–37. doi: 10.1007/s00436-002-0815-0. [DOI] [PubMed] [Google Scholar]
- Lustigman S., Brotman B., Huima T., Prince A.M., McKerrow J.H. Molecular cloning and characterization of onchocystatin, a cysteine proteinase-inhibitor of Onchocerca volvulus. J. Biol. Chem. 1992;267:17339–17346. [PubMed] [Google Scholar]
- Lustigman S., McKerrow J.H., Shah K., Lui J., Huima T., Hough M., Brotman B. Cloning of a cysteine protease required for the molting of Onchocerca volvulus third stage larvae. J. Biol. Chem. 1996;271:30181–30189. doi: 10.1074/jbc.271.47.30181. [DOI] [PubMed] [Google Scholar]
- Malagon D., Adroher F.J., Diaz-Lopez M., Benitez R. Collagenolytic activity related to metalloproteases (and serine proteases) in the fish parasite Hysterothylacium aduncum (Nematoda: Anisakidae) Dis. Aquat. Organ. 2010;90:129–134. doi: 10.3354/dao02234. [DOI] [PubMed] [Google Scholar]
- Malagon D., Diaz-Lopez M., Benitez R., Adroher F.J. Cathepsin B- and L-like cysteine protease activities during the in vitro development of Hysterothylacium aduncum (Nematoda: Anisakidae), a worldwide fish parasite. Parasitol. Int. 2010;59:89–92. doi: 10.1016/j.parint.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Meli V.S., Osuna B., Ruvkun G., Frand A.R. MLT-10 defines a family of DUF644 and proline-rich repeat proteins involved in the molting cycle of Caenorhabditis elegans. Mol. Biol. Cell. 2010;21:1648–1661. doi: 10.1091/mbc.E08-07-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P.M., Sands D.C. Effects of hydroclytic enzymes on plant-parasitic nematodes. J. Nematol. 1977;9:192–197. [PMC free article] [PubMed] [Google Scholar]
- Mohrlen F., Hutter H., Zwilling R. The astacin protein family in Caenorhabditis elegans. Eur. J. Biochem. 2003;270:4909–4920. doi: 10.1046/j.1432-1033.2003.03891.x. [DOI] [PubMed] [Google Scholar]
- Muriel J.M., Brannan M., Taylor K., Johnstone I.L., Lithgow G.J., Tuckwell D. M142.2 (cut-6), a novel Caenorhabditis elegans matrix gene important for dauer body shape. Dev. Biol. 2003;260:339–351. doi: 10.1016/s0012-1606(03)00237-9. [DOI] [PubMed] [Google Scholar]
- Myllyharju J., Kivirikko K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Myllyharju J., Kukkola L., Winter A., Page A. The exoskeleton collagens in Caenorhabditis elegans are modified by prolyl 4-hydroxylases with unique combinations of subunits. J. Biol. Chem. 2002;277:29187–29196. doi: 10.1074/jbc.M203824200. [DOI] [PubMed] [Google Scholar]
- Novelli J., Ahmed S., Hodgkin J. Gene interactions in Caenorhabditis elegans define DPY-31 as a candidate procollagen C-proteinase and SQT-3/ROL-4 as its predicted major target. Genetics. 2004;168:1259–1273. doi: 10.1534/genetics.104.027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli J., Page A.P., Hodgkin J. The C terminus of collagen SQT-3 has complex and essential functions in nematode collagen assembly. Genetics. 2006;172:2253–2267. doi: 10.1534/genetics.105.053637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A.P. The nematode cuticle: synthesis, modification and mutants. In: Kennedy M.W., Harnett W., editors. Parasitic Nematodes. CABI; St Albans, UK: 2001. pp. 167–193. [Google Scholar]
- Page A.P., Johnstone I.L. The cuticle. In: WormBook, editor. The C. elegans Research Community. Wormbook; 2007. [Google Scholar]
- Page A.P., Winter A.D. Enzymes involved in the biogenesis of the nematode cuticle. Adv. Parasitol. 2003;53:85–148. doi: 10.1016/s0065-308x(03)53003-2. [DOI] [PubMed] [Google Scholar]
- Page A.P., Kumar S., Carlow C.K.S. Parasite cyclophilins and antiparasite activity of cyclosporin A. Parasitol. Today. 1995;11:385–388. doi: 10.1016/0169-4758(95)80007-7. [DOI] [PubMed] [Google Scholar]
- Page A.P., McCormack G., Birnie A.J. Biosynthesis and enzymology of the Caenorhabditis elegans cuticle: identification and characterization of a novel serine protease inhibitor. Int. J. Parasitol. 2006;36:681–689. doi: 10.1016/j.ijpara.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Pak S.C., Kumar V., Tsu C., Luke C.J., Askew Y.S., Askew D.J., Mills D.R., Bromme D., Silverman G.A. SRP-2 is a cross-class inhibitor that participates in postembryonic development of the nematode Caenorhabditis elegans: initial characterization of the clade L serpins. J. Biol. Chem. 2004;279:15448–15459. doi: 10.1074/jbc.M400261200. [DOI] [PubMed] [Google Scholar]
- Peters K., McDowall J., Rose A. Mutations in the bli-4 (I) locus of Caenorhabditis elegans disrupt both adult cuticle and early larval development. Genetics. 1991;129:95–102. doi: 10.1093/genetics/129.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralanda I., Piessens W.F. Onchocerca volvulus, O. gutturosa, Brugia malayi, and Dirofilaria immitis: a comparative study of the immunochemical properties of cuticular proteins from filarial parasites. Exp. Parasitol. 1991;72:164–173. doi: 10.1016/0014-4894(91)90134-i. [DOI] [PubMed] [Google Scholar]
- Pokharel D.R., Rai R., Kumar P., Chaturvedi C.M., Rathaur S. Tissue localization of collagenase and leucine aminopeptidase in the bovine filarial parasite Setaria cervi. Filaria J. 2006;5:7. doi: 10.1186/1475-2883-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole C.B., Jin J.M., MacReynolds L.A. Cloning and biochemical characterization of blisterase, a subtilisin-like convertase from the filarial parasite, Onchocerca volvulus. J. Biol. Chem. 2003;278:36183–36190. doi: 10.1074/jbc.M302601200. [DOI] [PubMed] [Google Scholar]
- Rajan T.V., Paciorkowski N., Kalajzic I., McGuiness C. Ascorbic acid is a requirement for the morphogenesis of the human filarial parasite Brugia malayi. J. Parasitol. 2003;89:868–870. doi: 10.1645/GE-3137RN. [DOI] [PubMed] [Google Scholar]
- Rhoads M.L., Fetterer R.H., Urban J.F., Jr. Effect of protease class-specific inhibitors on in vitro development of the third- to fourth-stage larvae of Ascaris suum. J. Parasitol. 1998;84:686–690. [PubMed] [Google Scholar]
- Richer J.K., Sakanari J.A., Frank G.R., Grieve R.B. Dirofilaria immitis: proteases produced by third- and fourth-stage larvae. Exp. Parasitol. 1992;75:213–222. doi: 10.1016/0014-4894(92)90181-9. [DOI] [PubMed] [Google Scholar]
- Richer J.K., Hunt W.G., Sakanari J.A., Grieve R.B. Dirofilaria immitis: effect of fluoromethyl ketone cysteine protease inhibitors on the third- to fourth-stage molt. Exp. Parasitol. 1993;76:221–231. doi: 10.1006/expr.1993.1027. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mahillo A.I., Gonzalez-Munoz M., Gomez-Aguado F., Rodriguez-Perez R., Corcuera M.T., Caballero M.L., Moneo I. Cloning and characterisation of the Anisakis simplex allergen Ani s 4 as a cysteine-protease inhibitor. Int. J. Parasitol. 2007;37:907–917. doi: 10.1016/j.ijpara.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Sapio M.R., Hilliard M.A., Cermola M., Favre R., Bazzicalupo P. The Zona Pellucida domain containing proteins, CUT-1, CUT-3 and CUT-5, play essential roles in the development of the larval alae in Caenorhabditis elegans. Dev. Biol. 2005;282:231–245. doi: 10.1016/j.ydbio.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Simmer F., Moorman C., van der Linden A.M., Kuijk E., van den Berghe P.V.E., Kamath R.S., Fraser A.G., Ahringer J., Plasterk R.H.A. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 2003;1:77–84. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.N., Sulston J.E. Some observations on moulting in Caenorhabditis elegans. Nematologica. 1978;24:63–71. [Google Scholar]
- Stenvall J., Fierro-Gonzalez J.C., Swoboda P., Saamarthy K., Cheng Q., Cacho-Valadez B., Arner E.S., Persson O.P., Miranda-Vizuete A., Tuck S. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2011;108:1064–1069. doi: 10.1073/pnas.1006328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepek G., Buttle D.J., Duce I.R., Lowe A., Behnke J.M. Assessment of the anthelmintic effect of natural plant cysteine proteinases against the gastrointestinal nematode, Heligmosomoides polygyrus, in vitro. Parasitology. 2005;130:203–211. doi: 10.1017/s0031182004006225. [DOI] [PubMed] [Google Scholar]
- Stepek G., Lowe A.E., Buttle D.J., Duce I.R., Behnke J.M. In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode, Trichuris muris. Parasitology. 2006;132:681–689. doi: 10.1017/S003118200500973X. [DOI] [PubMed] [Google Scholar]
- Stepek G., Lowe A.E., Buttle D.J., Duce I.R., Behnke J.M. Anthelmintic action of plant cysteine proteinases against the rodent stomach nematode, Protospirura muricola, in vitro and in vivo. Parasitology. 2007;134:103–112. doi: 10.1017/S0031182006001302. [DOI] [PubMed] [Google Scholar]
- Stepek G., Lowe A.E., Buttle D.J., Duce I.R., Behnke J.M. The anthelmintic efficacy of plant-derived cysteine proteinases against the rodent gastrointestinal nematode, Heligmosomoides polygyrus, in vivo. Parasitology. 2007;134:1409–1419. doi: 10.1017/S0031182007002867. [DOI] [PubMed] [Google Scholar]
- Stepek G., McCormack G., Page A.P. Collagen processing and cuticle formation is catalysed by the astacin metalloprotease DPY-31 in free-living and parasitic nematodes. Int. J. Parasitol. 2010;40:533–542. doi: 10.1016/j.ijpara.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Stepek G., McCormack G., Page A.P. The kunitz domain protein BLI-5 plays a functionally conserved role in cuticle formation in a diverse range of nematodes. Mol. Biochem. Parasitol. 2010;169:1–11. doi: 10.1016/j.molbiopara.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Stepek G., McCormack G., Birnie A.J., Page A.P. The astacin metalloprotease moulting enzyme NAS-36 is required for normal cuticle ecdysis in free-living and parasitic nematodes. Parasitology. 2011;138:237–248. doi: 10.1017/S0031182010001113. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Sagoh N., Iwasaki H., Inoue H., Takahashi K. Metalloproteases with EGF, CUB, and thrombospondin-1 domains function in molting of Caenorhabditis elegans. Biol. Chem. 2004;385:565–568. doi: 10.1515/BC.2004.069. [DOI] [PubMed] [Google Scholar]
- Thacker C., Peters K., Srayko M., Rose A.M. The bli-4 locus of Caenorhabditis elegans encodes structurally distinct kex2/subtilisin-like endoproteases essential for early development and adult morphology. Genes Dev. 1995;9:956–971. doi: 10.1101/gad.9.8.956. [DOI] [PubMed] [Google Scholar]
- Thacker C., Sheps J.A., Rose A.M. Caenorhabditis elegans dpy-5 is a cuticle procollagen processed by a proprotein convertase. Cell. Mol. Life Sci. 2006;63:1193–1204. doi: 10.1007/s00018-006-6012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein M.C., McCormack G., Winter A.D., Johnstone I.L., Shoemaker C.B., Page A.P. The Caenorhabditis elegans exoskeleton collagen COL-19: an adult-specific marker for collagen modification, assembly and the analysis of organismal morphology. Dev. Dyn. 2003;226:523–539. doi: 10.1002/dvdy.10259. [DOI] [PubMed] [Google Scholar]
- Thein M.C., Winter A.D., Stepek G., McCormack G., Stapleton G., Johnstone I.L., Page A.P. Combined extracellular matrix cross-linking activity of the peroxidase MLT-7 and the dual oxidase BLI-3 is critical for post-embryonic viability in Caenorhabditis elegans. J. Biol. Chem. 2009;284:17549–17563. doi: 10.1074/jbc.M900831200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Huang W., Huang J., Jiang X., Qin L. Investigation of protease-mediated cuticle-degradation of nematodes by using an improved immunofluorescence-localization method. J. Invertebr. Pathol. 2009;101:143–146. doi: 10.1016/j.jip.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Tzertzinis G., Egana A.L., Palli S.R., Robinson-Rechavi M., Gissendanner C.R., Liu C., Unnasch T.R., Maina C.V. Molecular evidence for a functional ecdysone signaling system in Brugia malayi. PLoS Negl. Trop. Dis. 2010;4:e625. doi: 10.1371/journal.pntd.0000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Liu X., Wu W., Liu X., Li S. Purification, characterization, and gene cloning of an alkaline serine protease from a highly virulent strain of the nematode-endoparasitic fungus Hirsutella rhossiliensis. Microbiol. Res. 2009;164:665–673. doi: 10.1016/j.micres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Williamson A.L., Lustigman S., Oksov Y., Deumic V., Plieskatt J., Mendez S., Zhan B., Bottazzi M.E., Hotez P.J., Loukas A. Ancylostoma caninum MTP-1, an astacin-like metalloprotease secreted by infective hookworm larvae, is involved in tissue migration. Infect. Immun. 2006;74:961–967. doi: 10.1128/IAI.74.2.961-967.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A.D., Page A.P. Prolyl 4-hydroxylase is an essential procollagen-modifying enzyme required for exoskeleton formation and the maintenance of body shape in the nematode Caenorhabditis elegans. Mol. Cell. Biol. 2000;20:4084–4093. doi: 10.1128/mcb.20.11.4084-4093.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A.D., Myllyharju J., Page A.P. A hypodermally expressed prolyl 4-hydroxylase from the filarial nematode Brugia malayi is soluble and active in the absence of protein disulfide isomerase. J. Biol. Chem. 2003;278:2554–2562. doi: 10.1074/jbc.M210381200. [DOI] [PubMed] [Google Scholar]
- Winter A.D., Eschenlauer S.C.P., McCormack G., Page A.P. Loss of secretory pathway FK506-binding proteins results in cold-sensitive lethality and associate extracellular matrix defects in the nematode Caenorhabditis elegans. J. Biol. Chem. 2007;282:12813–12821. doi: 10.1074/jbc.M700274200. [DOI] [PubMed] [Google Scholar]
- Winter A.D., Keskiaho K., Kukkola L., McCormack G., Felix M.A., Myllyharju J., Page A.P. Differences in collagen prolyl 4-hydroxylase assembly between two Caenorhabditis nematode species despite high amino acid sequence identity of the enzyme subunits. Matrix Biol. 2007;26:382–395. doi: 10.1016/j.matbio.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Winter A.D., McCormack G., Page A.P. Protein disulfide isomerase activity is essential for viability and extracellular matrix formation in the nematode Caenorhabditis elegans. Dev. Biol. 2007;308:449–461. doi: 10.1016/j.ydbio.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Winter A.D., McCormack G., Myllyharju J., Page A.P. Prolyl 4-hydroxlase activity is essential for development and cuticle formation in the human infective parasitic nematode Brugia malayi. J. Biol. Chem. 2013;288:1750–1761. doi: 10.1074/jbc.M112.397604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Kramer J.M. In vitro mutagenesis of Caenorhabditis elegans cuticle collagens identifies a potential subtilisin-like protease cleavage site and demonstrates that carboxyl domain disulfide bonding is required for normal function but not assembly. Mol. Cell. Biol. 1994;14:2722–2730. doi: 10.1128/mcb.14.4.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Kramer J.M. Proteolytic processing of Caenorhabditis elegans SQT-1 cuticle collagen is inhibited in right roller mutants whereas cross-linking is inhibited in left roller mutants. J. Biol. Chem. 1999;274:32744–32749. doi: 10.1074/jbc.274.46.32744. [DOI] [PubMed] [Google Scholar]
- Yang Y., Moir E., Kontopidis G., Taylor P., Wear M.A., Malone K., Dunsmore C.J., Page A.P., Turner N.J., Walkinshaw M.D. Structure-based discovery of a family of synthetic cyclophilin inhibitors showing a cyclosporin-A phenotype in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2007;363:1013–1019. doi: 10.1016/j.bbrc.2007.09.079. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhao X., Liang L., Xia Z., Lei L., Niu X., Zou C., Zhang K.Q. Overexpression of a cuticle-degrading protease Ver112 increases the nematicidal activity of Paecilomyces lilacinus. Appl. Microbiol. Biotechnol. 2011;89:1895–1903. doi: 10.1007/s00253-010-3012-6. [DOI] [PubMed] [Google Scholar]
- Yochem J., Tuck S., Greenwald I., Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development. 1999;126:597–606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]
- Zhan B., Hotez P.J., Wang Y., Hawdon J.M. A developmentally regulated metalloprotease secreted by host-stimulated Ancylostoma caninum third-stage infective larvae is a member of the astacin family of proteases. Mol. Biochem. Parasitol. 2002;120:291–296. doi: 10.1016/s0166-6851(01)00453-4. [DOI] [PubMed] [Google Scholar]


