Abstract
Objectives
This study was conducted to investigate the following: (1) the effects of chewing honey on plaque formation in orthodontic patients, (2) the effect of chewing honey on dental plaque bacterial counts, (3) determine if honey possesses antibacterial effects on bacteria recovered from plaques.
Methods
Female orthodontic patients (n = 20, 12–18 years of age) participated in this randomized controlled study. The effects of honey were compared to treatment with either 10% sucrose or 10% sorbitol that served as positive and negative controls, respectively. The pH of plaque was measured using a digital pH meter prior to baseline and at 2, 5, 10, 20, and 30 min after chewing honey or rinsing with control solutions and the numbers of Streptococcus mutans, Lactobacilli, and Prophymonas gingivalis in respective plaques were determined. The antibacterial activity of honey was tested against commonly used antibiotics using the disk diffusion method.
Results
Significant differences in pH were observed in the honey and sucrose groups compared to the pH observed in the sorbitol group (p ⩽ 0.001). The maximum pH drop occurred at 5 min in both the honey and sucrose groups; however the pH in the honey group rapidly recovered 10–20 min after exposure and did not drop below the critical decalcification pH of 5.5. On the other hand, the pH following sucrose exposure fell <5.5 and was associated with a 30 min recovery time. The pH observed for the sorbitol group did not change over time. Bacterial counts were significantly reduced in the honey group compared to the other treatment groups (p ⩽ 0.001) and honey significantly inhibited the growth of all studied strains compared to inhibition observed with antibiotics (p ⩽ 0.001).
Conclusions
Honey can be used as an alternative to traditional remedies for the prevention of dental caries and gingivitis following orthodontic treatment.
Keywords: Honey, Plaque control, Bacterial counting, pH measurement, Orthodontic treatment
1. Introduction
Clinicians have been attempting to reduce gingivitis and enamel demineralization during orthodontic treatments (Agerbaek et al., 1975; Mizrahi, 1988; O’Reilly and Featherstone, 1987; Patel et al., 2010; Shannon, 1980; Underwood et al., 1989) by using of fluoride dentifrices and fluoride solutions (Mizrahi, 1988; O’Reilly and Featherstone, 1987; Shannon, 1980; Underwood et al., 1989), antibacterial mouth rinses (Agerbaek et al., 1975), and antibiotics (Park et al., 1998).
Recently, much attention has been given to natural products with health-promoting benefits. Historically, honey has been used as an eco-friendly medicine for many years in the treatment of burns, infected wounds (Cutting, 2007; Moore et al., 2001), peptic ulcers, bacterial gastroenteritis (Ali, 2003; Salem, 1981), and ophthalmic infections (Emarah, 1982). In addition, honey possesses potent broad-spectrum antibacterial activity and studies have demonstrated that manuka honey have anti-cancer properties (English et al., 2004).
In vitro studies have shown that exposure to a honey solution affected monocyte activity (Fischer et al., 2007). It was found that mouth washes containing propolis (present in bee products) possessed antimicrobial activity against Streptococcus mutans and can be used as an alternative treatment in dental caries prevention (Duailibe et al., 2007) and in the reduction of plaque accumulation and polysaccharide formation (Koo et al., 2002). Recently it was reported that periodontal pockets irrigated with 10% propolis solution had a 95% decrease in gingivitis (do Amaral et al., 2006) suggesting (based on both clinical and microbiological parameters) that subgingival irrigation with a propolis extract as an adjunct to periodontal treatment and more effective than scaling and root planing (Ahuja and Ahuja, 2011; Coutinho, 2012). Topical application of a propolis extract on oral Candida albicans lesions resulted in remission within three weeks and treatment efficacy was comparable to treatment with nystatin, the standard antifungal product used to treat these infections (Santos et al., 2005). Another study conducted on premolars for direct pulp capping also showed that propolis was as effective as calcium hydroxide. Based on these observations it has been concluded that propolis can be used along with calcium hydroxide as an intra-canal treatment (de Rezende et al., 2008).
A study conducted by Patel et al. (2010) on bacterial isolates obtained from patients undergoing orthodontic treatment showed that the honey was a more effective antibacterial than some of the common antibiotics tested, further suggesting that honey might inhibit dental plaque formation and aid in controlling gingivitis associated with orthodontic procedures (Coutinho, 2012; Nayak et al., 2010; Patel et al., 2010; Steinberg et al., 1996). Therefore, the present study was conducted to determine the following: (1) the effect of chewing honey on plaque pH in orthodontic patients, (2) the effect of chewing honey on bacterial counts present in dental plaques and, (3) the in vitro effects of honey on the growth of plaque bacteria.
2. Materials and methods
Female patients (n = 20, 12–18 years of age) undergoing orthodontic treatment were enrolled in the present study. The study was reviewed and approved by the Ethical Committee of the Faculty of Dental Medicine, Al-Azhar University, Cairo, Egypt and informed consent was obtained from each subject after the study was explained. Treatment constituted of fixed orthodontic therapy with extraction of the first bicuspids followed by individual canine retraction (maintaining maximum anchorage conservation) and space closure. Subjects having received antibiotic therapy 2-weeks prior to the start of the study or subjects with xerostomia were excluded.
2.1. Assessment of plaque pH
The pH of the honey (Imtenan Co. Ltd., Elnozha Elgededa, Cairo, Egypt), sucrose (Al Monairy Corn Products. Cairo, Egypt), and sorbitol (Sorbidex™, Cargill Middle East, Dubai, United Arab Emirates) was measured using a digital pH meter (Orion model 230A, Thermo Scientific Inc., Tokyo, Japan). The electrode was calibrated before measurement using standard buffers of pH 4.0 and 7.0 and the pH read after allowing the reading to stabilize for 30 s. Measurements were taken 3 times and the means were recorded.
2.2. Plaque collection
Plaque samples were collected for pH measurement as described with modifications described below (Rugg-Gunn et al., 1975). All subjects were required to refrain from brushing their teeth or using any oral hygiene products for 24 h and to abstain from consuming any food or drink (except water) for at least 2 h before each test session. These criteria conformed to the guidelines of the Plaque Acidity Working Group of the Food, Nutrition, and Dental Health Committee of the American Dental Association (Harper et al., 1986). Patients were asked to return on a weekly basis and at each visit, baseline plaque samples were collected with a spoon excavator from all accessible surfaces of the upper central incisors, buccal surfaces of upper first molars and premolars, and the lingual surfaces of lower molars and incisors. The subjects were asked to swallow immediately before plaque collection to minimize salivary contamination, and during sample collection care was taken to avoid contamination with blood or saliva. Patients were then asked to chew and ingest 10 g of pure undiluted honey in 2 min or rinse with 15 ml of 10% sucrose or sorbitol solutions (positive and negative controls, respectively) for 1 min (Nayak et al., 2010; Parsons, 2011). Post consumption plaque samples were collected at 2, 5, 10, 20, and 30 min and the pH determined in the same manner (Table 1). Only one substance (honey, sucrose, or sorbitol) was tested at each visit in a randomized order with at least 7 days between each test to avoid any carryover effects.
Table 1.
Changes in pH over time.
| Groups | pH |
ANOVA |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 2 min | 5 min | 10 min | 20 min | 30 min | F | Sig. | |
| Honey | 6.85 ± (.45)a | 6.42 ± (.40)a | 5.86 ± (.20)c | 6.23 ± (.20)b | 6.54 ± (.20)a | 6.84 ± (.20)a | ||
| Sucrose | 6.82 ± (.45)a | 6.22 ± (.34)b | 5.28 ± (.29)c | 5.71 ± (.18)c | 6.33 ± (.16)b | 6.79 ± (.24)a | 17.756 | .000⁎⁎⁎ |
| Sorbitol | 6.88 ± (.45)a | 6.74 ± (.45)a | 6.72 ± (.48)a | 6.67 ± (.47)a | 6.82 ± (.48)a | 6.86 ± (.48)a | ||
Data are expressed as the mean ± SD of 3 observations.
Means with similar letters are not significantly different.
(a, b) and (b, c) are significant at p ⩽ 0.05, (a, c) are significant at p ⩽ 0.01, ⁎⁎⁎p ⩽ 0.001.
Collected plaque samples were mixed with 20 μl of distilled water and the pH was measured with a micro-combination electrode (Orion model 9802BN, Thermo Scientific Inc., Tokyo, Japan) in conjunction with a portable pH meter (Orion model 230A, Thermo Scientific Inc., Tokyo, Japan). Calibration of the system was carried out before each test and the electrode was cleaned with distilled water and placed in a standard solution of pH 7.0.
2.3. Bacterial counts
Base-line and 30 min plaque samples were collected in 2 ml of sterile thioglycollate broth transport media in screw capped vials and immediately transferred to the laboratory (the regional Center for Myology and Biotechnology, Culture and Sensitivity Unit, Al-Azhar University, Cairo, Egypt). S. mutans, Lactobacillus acidophilus, and Prophyromonas gingivalis were isolated from respective samples (Cowan et al., 2004) and bacterial counts determined using the standard pour plate method (Norden and Kass, 1968).
2.4. Bacterial sensitivity testing
The antimicrobial activity of honey was studied using the disk diffusion method (Cruickshank, 1968). Antibiotic discs (5 mm diameter) were placed in the center of the agar plates. Disks (5 mm diameter) containing honey were prepared from Whatman’s filter paper and were autoclaved. Respective disks were placed equidistant from each other on the streaked nutrient agar plates and incubated for 24 h at 37 °C. The zone of inhibition to the nearest millimeter (including the diameter of the disk) was measured using a ruler.
2.5. Statistical analysis
Data were analyzed by ANOVA and Tukey’s post hoc tests using SPSS Version V.17 (SPSS Inc, Chicago, Illinois). Data are expressed as the mean ± standard deviation (SD) or standard error (SE) of triplicate readings and p-values < 0.05 were considered statistically significant.
3. Results
There were significant differences in the plaque pH between the honey and sucrose groups compared to the pH observed in the sorbitol group (Fig. 1 and Table 1; p ⩽ 0.001). The maximum decrease in pH occurred at 5 min in both the honey and sucrose groups with the pH in the honey group recovering in 20 min. The critical value for decalcification (pH 5.5) was not reached for in either the honey or sorbitol groups, however, the pH in the sucrose group fell below the critical value but recovered by 30 min. The pH curve for sorbitol was almost linear with no significant drop over time during the test period (Fig. 1). Although the pH was affected by exposure to either honey, sucrose, or sorbitol only exposure to honey significantly reduced (p < 0.001) the number of bacteria that were recovered from plaques 30 min after exposure (Fig. 2 and Table 2).
Figure 1.
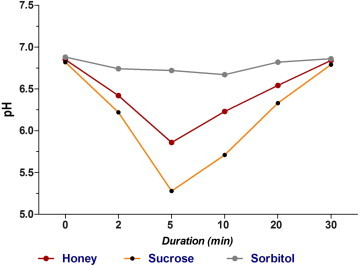
Mean plaque pH differences between groups over time.
Figure 2.

Mean bacterial counts (CFU/10 μl) before and one hour after honey consumption.
Table 2.
Bacterial counts before and 1 h after chewing honey.
| Mean | SD |
t – Test |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | SD | SE | t Value | Sig. | ||||
| Streptococci | Before | 255.6 | 90.8 | 151.20 | 47.51 | 15.03 | 10.063 | .000⁎⁎⁎ |
| After | 104.4 | 51.7 | ||||||
| Lactobacilli | Before | 100.2 | 63.5 | 58.00 | 33.83 | 10.69 | 5.422 | .000⁎⁎⁎ |
| After | 42.2 | 33 | ||||||
| P. gingivalis | Before | 56.4 | 24.4 | 14.40 | 7.44 | 2.35 | 6.119 | .000⁎⁎⁎ |
| After | 42 | 17.8 | ||||||
CFU = Colony Forming Unit.
CFUs determined from 10 μl.
SD, standard deviation; SE, standard error.
p ⩽ 0.001.
The antibacterial properties of honey were assessed by comparing the zones of inhibition resulting from the culture of S. mutans, P. gingivalis, and L. acidophilus in the presence of either honey or various antibiotics (Table 3 and Fig. 3). Honey significantly inhibited the growth of all the strains studied compared to zones of inhibition resulting from growth in the presence of antibiotics (p ⩽ 0.001).
Table 3.
The effect of honey on bacterial growth.
| Mean | SD | Tukey’s HSD test |
|||
|---|---|---|---|---|---|
| Mean difference | Std. error | Sig. | |||
| Streptococcus mutans | |||||
| Honey | 27.6 | 3.1 | |||
| Penicillin | 18.2 | 3.5 | 9.40 | 1.37073 | .000⁎⁎⁎ |
| Oxytetracycline | 20.4 | 3.1 | 7.20 | 1.37073 | .000⁎⁎⁎ |
| Chloramphenicol | 19.2 | 2.0 | 8.40 | 1.37073 | .000⁎⁎⁎ |
| Cefaclor | 16.4 | 2.9 | 11.20 | 1.37073 | .000⁎⁎⁎ |
| Prophymonas gingivalis | |||||
| Honey | 24.5 | 2.7 | |||
| Penicillin | 16.8 | 3.4 | 7.70 | 1.26139 | .000⁎⁎⁎ |
| Oxytetracycline | 19.3 | 2.4 | 5.20 | 1.26139 | .002⁎⁎ |
| Chloramphenicol | 18.3 | 2.1 | 6.20 | 1.26139 | .000⁎⁎⁎ |
| Cefaclor | 15.6 | 2.6 | 8.90 | 1.26139 | .000⁎⁎⁎ |
| Lactobacillus acidophilus | |||||
| Honey | 31.9 | 2.9 | |||
| Penicillin | 21.4 | 3.1 | 10.50 | 1.25536 | .000⁎⁎⁎ |
| Oxytetracycline | 21.5 | 2.8 | 10.40 | 1.25536 | .000⁎⁎⁎ |
| Chloramphenicol | 15.2 | 2.0 | 16.70 | 1.25536 | .000⁎⁎⁎ |
| Cefaclor | 19.2 | 2.6 | 12.70 | 1.25536 | .000⁎⁎⁎ |
p ⩽ 0.01.
p ⩽ 0.001.
Figure 3.
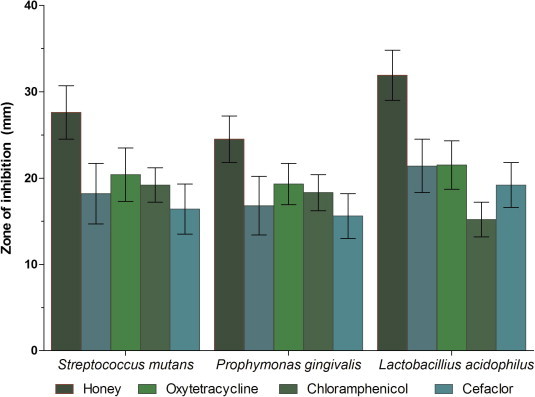
Bactericidal properties of honey compared to antibiotics.
4. Discussion
Although honey is acidic (endogenous pH 4.2) both sucrose and sorbitol were used at pH 7. Data presented in this study demonstrated that eating honey was associated with a greater reduction in pH compared to changes observed following sucrose consumption. Exposure to sorbitol was not associated with a significant drop in pH suggesting that the inherent pH of liquids was not indicative of pH changes to the plaque or of their erosive potential (Edwards et al., 1999; Grenby et al., 1989). The plaque pH measurement method used in this study has been used successfully in previous studies and shown to be a reliable method of determining the cariogenicity of food (Lehl et al., 1993). These methods have been shown to satisfactorily identify non acidogenic foods compared to appropriate positive (sucrose) and negative (sorbitol) controls (Curzon and Hefferren, 2001). Although acidic plaques have been correlated with increase in the numbers of caries, (Tahmassebi and Duggal, 1997) few studies have examined the effect of honey on plaque pH. Since honey is an important sweetening agent it represents a source of fermentable sugar for oral bacteria and studies have suggested that honey was as cariogenic as sucrose (Bowen and Lawrence, 2005; Rells and Nizell, 1973; Shannon et al., 1979) or as in other studies which labeled it as more cariogenic than sucrose (Konig, 1967; Wakeman et al., 1948). In contrast, other reports have shown that honey was less cariogenic than sucrose (Molan, 2001) and that honey and other bee products (e.g., propolis) were non-cariogenic, in addition to suggesting that honey be used for control of dental caries (Kujumgiev et al., 1999; Menezes et al., 1997; Park et al., 1998).
The present study demonstrated that the mean minimum plaque pH 5 min after exposure to honey was higher than the pH observed following sucrose exposure (5.86 and 5.28, respectively) although this difference was not statistically significant. In contrast, at 10 and 20 min the plaque pH after chewing honey was significantly higher than after rinsing with sucrose (p ⩽ 0.05). Unlike sucrose, chewing honey did not result in a decrease in pH below the critical value of 5.5 associated with enamel demineralization. Perhaps the antibacterial activity of honey against cariogenic bacteria overcame its pH reducing effects based on previous observations demonstrating that honey possessed antibacterial properties against medically important bacteria (Bonvehí and Coll, 1994; Digrak et al., 1995; Gebara et al., 1996; Grange and Davey, 1990; Ikeno et al., 1991; Lindenfelser, 1967; Steinberg et al., 1996). However, few studies have investigated its activity against oral pathogens (Burdock, 1998; Gebara et al., 1996; Lindenfelser, 1967; Steinberg et al., 1996).
Previous studies suggested that the antibacterial spectrum of honey is fairly broad, acting against Gram-positive and -negative rods and cocci, yeast, and fungi (Duailibe et al., 2007). Results described in the present study demonstrated that the number of S. mutans, P. gingivalis, and L. acidophilus were significantly reduced after chewing honey, supporting previously published reports (Hayacibara et al., 2005; Ozan et al., 2007; White et al., 1963).
Antibiotic susceptibility testing demonstrated that the isolated microorganisms in the present study were more sensitive to honey than antibiotics. At this time, however, the mechanism associated with the antibacterial effects of honey remain unknown, although the presence of hydrogen peroxide (Havsteen, 1983), flavonoids (Mundo et al., 2004), and hypertonic sugar concentrations (Cai and Wu, 1996) have been put forth as possibilities. More specifically, hydrogen peroxide has been shown to form in honey by the action of the enzyme glucose oxidase that produces gluconic acid and hydrogen peroxide from glucose. Flavonoids have been shown to have antibacterial properties (Mundo et al., 2004), and high sugar concentrations produce a hypertonic condition causing plasmolysis of microbial cells resulting in growth inhibition and death (Cai and Wu, 1996).
5. Conclusions
Within the limitations of the study it can be concluded that topical application of honey can modify the pH, reduce bacterial counts, and inhibit bacterial growth. These data suggested that topical application/chewing of honey might help prevent gingivitis and caries in patients undergoing orthodontic treatment. Further studies will be required to substantiate these preliminary observations.
Conflict of interest
The authors declare no conflict of interest and/or financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agerbaek N., Melsen B., Rolla G. Application of chlorhexidine by oral irrigation systems. Scand. J. Dent. Res. 1975;83:284–287. doi: 10.1111/j.1600-0722.1975.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Ahuja V., Ahuja A. Apitherapy—a sweet approach to dental diseases. Part II: propolis. J. Acad. Adv. Dent. Res. 2011;2:1–8. [Google Scholar]
- Ali A.M. Prevention of ammonia-induced gastric lesions in rats by natural honey. J. Nutr. Environ. Med. 2003;13:239–246. [Google Scholar]
- Bonvehí J.S., Coll F.V. The composition, active components and bacteriostatic activity of propolis in dietetics. J. Am. Oil. Chem. Soc. 1994;71:529–532. [Google Scholar]
- Bowen W.H., Lawrence R.A. Comparison of the cariogenicity of cola, honey, cow milk, human milk, and sucrose. Pediatrics. 2005;116:921–926. doi: 10.1542/peds.2004-2462. [DOI] [PubMed] [Google Scholar]
- Burdock G.A. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem. Toxicol. 1998;36:347–363. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- Cai L., Wu C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996;59:987–990. doi: 10.1021/np960451q. [DOI] [PubMed] [Google Scholar]
- Coutinho A. Honeybee propolis extract in periodontal treatment: a clinical and microbiological study of propolis in periodontal treatment. Indian J. Dent. Res. 2012;23:294. doi: 10.4103/0970-9290.100449. [DOI] [PubMed] [Google Scholar]
- Cowan S.T., Steel K.J., Barrow G., Feltham R. Cambridge University Press; 2004. Cowan and Steel’s Manual for the Identification of Medical Bacteria. [Google Scholar]
- Cruickshank R. 11th ed. E and S Livingston Ltd.; Edinburgh and London: 1968. Medical Microbiology: A Guide to Diagnosis and Control of Infection. 888. [Google Scholar]
- Curzon M., Hefferren J. Nutrition: modern methods for assessing the cariogenic and erosive potential of foods. Br. Dent. J. 2001;191:41–46. doi: 10.1038/sj.bdj.4801087. [DOI] [PubMed] [Google Scholar]
- Cutting K.F. Honey and contemporary wound care: an overview. Ostomy Wound Manage. 2007;53:49–54. [PubMed] [Google Scholar]
- de Rezende G.P., da Costa L.R., Pimenta F.C., Baroni D.A. In vitro antimicrobial activity of endodontic pastes with propolis extracts and calcium hydroxide: a preliminary study. Braz. Dent. J. 2008;19:301–305. doi: 10.1590/s0103-64402008000400003. [DOI] [PubMed] [Google Scholar]
- Digrak M., Yilmaz O., Ozcelik S. In vitro antimicrobial effect of propolis collected in Elazig region. Turk. J. Biol. 1995;19:249–257. [Google Scholar]
- do Amaral R.C., Gomes R.T., Rocha W.M.S., Lemos S., Abreu R., Santos V.R. Periodontitis treatment with Brazilian green propolis gel. Pharmacology (online) 2006:336–341. [Google Scholar]
- Duailibe S.A., Goncalves A.G., Ahid F.J. Effect of a propolis extract on Streptococcus mutans counts in vivo. J. Appl. Oral Sci. 2007;15:420–423. doi: 10.1590/S1678-77572007000500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M., Creanor S.L., Foye R.H., Gilmour W.H. Buffering capacities of soft drinks: the potential influence on dental erosion. J. Oral Rehabil. 1999;26:923–927. doi: 10.1046/j.1365-2842.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- Emarah M.H.A. Clinical study of the topical use of bee honey in the treatment of some ocular diseases. Bull. Islam. Med. 1982;2:422–425. [Google Scholar]
- English H.K., Pack A.R., Molan P.C. The effects of manuka honey on plaque and gingivitis: a pilot study. J. Int. Acad. Periodontol. 2004;6:63–67. [PubMed] [Google Scholar]
- Fischer G., Conceicao F.R., Leite F.P., Dummer L.A., Vargas G.D., Hubner Sde O., Dellagostin O.A., Paulino N., Paulino A.S., Vidor T. Immunomodulation produced by a green propolis extract on humoral and cellular responses of mice immunized with SuHV-1. Vaccine. 2007;25:1250–1256. doi: 10.1016/j.vaccine.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Gebara E.C.E., Zardetto C.G.D.C., Mayer M.P.A. Estudo in vitro da açäo antimicrobiana de substâncias naturais sobre S. mutans e S. sobrinus; in vitro study of the antimicrobial activity of natural substances against S. mutans and S. sobrinus. Rev. Odontol. Univ. Säo Paulo. 1996;10:251–256. [Google Scholar]
- Grange J.M., Davey R.W. Antibacterial properties of propolis (bee glue) J. R. Soc. Med. 1990;83:159–160. doi: 10.1177/014107689008300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenby T.H., Phillips A., Desai T., Mistry M. Laboratory studies of the dental properties of soft drinks. Br. J. Nutr. 1989;62:451–464. doi: 10.1079/bjn19890045. [DOI] [PubMed] [Google Scholar]
- Harper D., Abelson D., Jensen M. Human plaque acidity models. J. Dent. Res. 1986;65:1503–1510. [Google Scholar]
- Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. 1983;32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Hayacibara M.F., Koo H., Rosalen P.L., Duarte S., Franco E.M., Bowen W.H., Ikegaki M., Cury J.A. In vitro and in vivo effects of isolated fractions of Brazilian propolis on caries development. J. Ethnopharmacol. 2005;101:110–115. doi: 10.1016/j.jep.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ikeno K., Ikeno T., Miyazawa C. Effects of propolis on dental caries in rats. Caries Res. 1991;25:347–351. doi: 10.1159/000261390. [DOI] [PubMed] [Google Scholar]
- Konig K.G. Caries induced in laboratory rats. Post-eruptive effect of sucrose and of bread of different degrees of refinement. Br. Dent. J. 1967;123:585–589. [PubMed] [Google Scholar]
- Koo H., Cury J.A., Rosalen P.L., Ambrosano G.M., Ikegaki M., Park Y.K. Effect of a mouthrinse containing selected propolis on 3-day dental plaque accumulation and polysaccharide formation. Caries Res. 2002;36:445–448. doi: 10.1159/000066535. [DOI] [PubMed] [Google Scholar]
- Kujumgiev A., Tsvetkova I., Serkedjieva Y., Bankova V., Christov R., Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999;64:235–240. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- Lehl G., Taneja J.R., Chopra S.L. Evaluation of the cariogenicity of sugar containing drinks by estimating changes in pH of human dental plaque and saliva. J. Indian Soc. Pedod. Prev. Dent. 1993;11:9–14. [PubMed] [Google Scholar]
- Lindenfelser L. Antimicrobial activity of propolis. Am. Bee J. 1967;107:90–92. [Google Scholar]
- Menezes H., Bacci M., Jr, Oliveira S., Pagnocca F. Antibacterial properties of propolis and products containing propolis from Brazil. Apidologie. 1997;28:71–76. [Google Scholar]
- Mizrahi E. Glass ionomer cements in orthodontics—an update. Am. J. Orthod. Dentofac. Orthop. 1988;93:505–507. doi: 10.1016/0889-5406(88)90079-0. [DOI] [PubMed] [Google Scholar]
- Molan P.C. The potential of honey to promote oral wellness. Gen. Dent. 2001;49:584–589. [PubMed] [Google Scholar]
- Moore O.A., Smith L.A., Campbell F., Seers K., McQuay H.J., Moore R.A. Systematic review of the use of honey as a wound dressing. BMC Complement. Altern. Med. 2001;1:2. doi: 10.1186/1472-6882-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundo M.A., Padilla-Zakour O.I., Worobo R.W. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int. J. Food Microbiol. 2004;97:1–8. doi: 10.1016/j.ijfoodmicro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Nayak P.A., Nayak U.A., Mythili R. Effect of Manuka honey, chlorhexidine gluconate and xylitol on the clinical levels of dental plaque. Contemp. Clin. Dent. 2010;1:214–217. doi: 10.4103/0976-237X.76386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden C.W., Kass E.H. Bacteriuria of pregnancy–a critical appraisal. Annu. Rev. Med. 1968;19:431–470. doi: 10.1146/annurev.me.19.020168.002243. [DOI] [PubMed] [Google Scholar]
- O’Reilly M.M., Featherstone J.D. Demineralization and remineralization around orthodontic appliances: an in vivo study. Am. J. Orthod. Dentofacial Orthop. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- Ozan F., Sumer Z., Polat Z.A., Er K., Ozan U., Deger O. Effect of mouthrinse containing propolis on oral microorganisms and human gingival fibroblasts. Eur. J. Dent. 2007;1:195–201. [PMC free article] [PubMed] [Google Scholar]
- Park Y.K., Koo M.H., Abreu J.A., Ikegaki M., Cury J.A., Rosalen P.L. Antimicrobial activity of propolis on oral microorganisms. Curr. Microbiol. 1998;36:24–28. doi: 10.1007/s002849900274. [DOI] [PubMed] [Google Scholar]
- Parsons E.K. University of Otago; 2011. Manuka Honey: An Investigation into the Effect of Manuka Honey on Oral Mucositis in Patients Receiving Radiation Therapy to the Head and Neck. [Google Scholar]
- Patel R., Thaker V., Patel V., Shukla P., Bhatnagar P., Patel A. In-vitro study of changing antibiotic sensitivity and resistance by honey on gingival inflammation during orthodontic treatment-a preliminary report. Orthodontic Cyber J. 2010:3–8. [Google Scholar]
- Rells G.K., Nizell A.E. Cariogenicity of honey. J. Am. Dent. Assoc. 1973;87:29. [PubMed] [Google Scholar]
- Rugg-Gunn A.J., Edgar W.M., Geddes D.A., Jenkins G.N. The effect of different meal patterns upon plaque pH in human subjects. Br. Dent. J. 1975;139:351–356. doi: 10.1038/sj.bdj.4803614. [DOI] [PubMed] [Google Scholar]
- Salem S. Honey regimen in gastrointestinal disorders. Bull. Islam. Med. 1981;1:358–362. [Google Scholar]
- Santos V.R., Pimenta F.J., Aguiar M.C., do Carmo M.A., Naves M.D., Mesquita R.A. Oral candidiasis treatment with Brazilian ethanol propolis extract. Phytother. Res. 2005;19:652–654. doi: 10.1002/ptr.1715. [DOI] [PubMed] [Google Scholar]
- Shannon I.L. Comparison of orthodontic cements containing sodium fluoride or stannous flouride. Am. J. Orthod. 1980;78:640–645. doi: 10.1016/0002-9416(80)90203-1. [DOI] [PubMed] [Google Scholar]
- Shannon I.L., Edmonds E.J., Madsen K.O. Honey: sugar content and cariogenicity. ASDC J. Dent. Child. 1979;46:29–33. [PubMed] [Google Scholar]
- Steinberg D., Kaine G., Gedalia I. Antibacterial effect of propolis and honey on oral bacteria. Am. J. Dent. 1996;9:236–239. [PubMed] [Google Scholar]
- Tahmassebi J.F., Duggal M.S. The effect of different methods of drinking on the pH of dental plaque in vivo. Int. J. Paediatr. Dent. 1997;7:249–254. doi: 10.1046/j.1365-263x.1997.00054.x. [DOI] [PubMed] [Google Scholar]
- Underwood M.L., Rawls H.R., Zimmerman B.F. Clinical evaluation of a fluoride-exchanging resin as an orthodontic adhesive. Am. J. Orthod. Dentofac. Orthop. 1989;96:93–99. doi: 10.1016/0889-5406(89)90250-3. [DOI] [PubMed] [Google Scholar]
- Wakeman E.J., Smith J.K. Microorganisms associated with dental caries in the cotton rat. J. Dent. Res. 1948;27:489–492. doi: 10.1177/00220345480270040701. [DOI] [PubMed] [Google Scholar]
- White J.W., Jr., Subers M.H., Schepartz A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta (BBA) 1963;73:57–70. doi: 10.1016/0006-3002(63)90359-7. [DOI] [PubMed] [Google Scholar]



