Graphical abstract
Keywords: Drug repurposing, Antiparasitic, Malaria, Leishmaniasis, Cryptosporidium, Trypanosomiasis, Toxoplasmosis
Highlights
-
•
Anti-protozoan drug discovery is challenging, time consuming and expensive.
-
•
Drug repurposing has historically played a role in anti-protozoan drug discovery.
-
•
Drug repurposing can result in significant time and cost savings.
-
•
Here we review drug repurposing for major human protozoan diseases.
Abstract
Parasitic diseases have an enormous health, social and economic impact and are a particular problem in tropical regions of the world. Diseases caused by protozoa and helminths, such as malaria and schistosomiasis, are the cause of most parasite related morbidity and mortality, with an estimated 1.1 million combined deaths annually. The global burden of these diseases is exacerbated by the lack of licensed vaccines, making safe and effective drugs vital to their prevention and treatment. Unfortunately, where drugs are available, their usefulness is being increasingly threatened by parasite drug resistance. The need for new drugs drives antiparasitic drug discovery research globally and requires a range of innovative strategies to ensure a sustainable pipeline of lead compounds. In this review we discuss one of these approaches, drug repurposing or repositioning, with a focus on major human parasitic protozoan diseases such as malaria, trypanosomiasis, toxoplasmosis, cryptosporidiosis and leishmaniasis.
1. Major human parasitic protozoan diseases
Protozoan parasites that are infectious to humans represent a significant threat to health and cause more than a million deaths annually (Lozano et al., 2012). They also threaten the lives of billions world-wide and are associated with significant morbidity and large economic impacts (World Health Organization, 2010b; Murray et al., 2012b). Three of the most important human diseases caused by protozoan parasites have disability adjusted life years (DALY’s) in the millions and have far reaching global distributions (Fig. 1) (World Health Organization, 2008, 2010b; Murray et al., 2012b). While there is little doubt that the protozoan disease burden is focussed in tropical and subtropical regions of the world, more temperate regions of our globe, including North America and the Asia Pacific region, are also impacted by protozoan diseases (Fig. 1). The significant burden of human protozoan infections has been exacerbated by the lack of a licensed vaccine for any of the diseases these parasites cause. Treatment and prophylaxis has therefore been dependent on drugs, many of which have become less effective necessitating the search for replacements. The repurposing of drugs originally licensed for alternative indications has contributed to antiparasitic drug discovery, with some current antiparasitics having arisen via this approach. This strategy has numerous advantages including potential reductions in development times and costs. This review focuses on drug repurposing for the human parasitic protozoan diseases malaria, trypanosomiasis, leishmaniasis, toxoplasmosis, and cryptosporidiosis. Topics covered include an overview of drugs that have been successfully repurposed for use against these pathogens, repurposed or extended uses of clinically used antiprotozoal drugs, an analysis of recent whole-cell anti-protozoan screening campaigns that have used libraries of clinically available drugs, and a discussion of current drug repurposing and drug rescue strategies and resources.
Fig. 1.
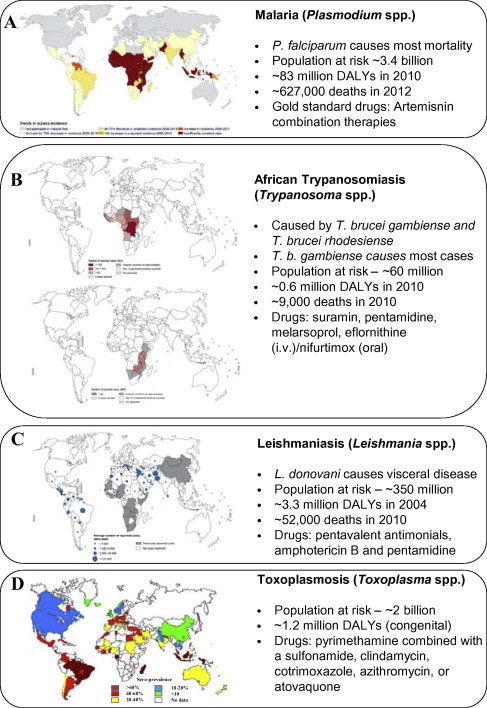
Distribution and disease impact of major human diseases caused by parasitic protozoa. (A–C) Reproduced, with the permission of the publisher, the World Health Organization, Global Health Observatory Map Gallery (World Health Organization). (D) Data reprinted, with minor modification, from Ref. Torgerson and Mastroiacovo (2013) with permission from Elsevier [license# 3266830155236]. Deaths and disability adjusted life years (DALYs) data taken from Lozano et al. (2012), Murray et al. (2012b), Torgerson and Mastroiacovo (2013), World Health Organization (2013).
1.1. Malaria
Malaria is the most significant of the protozoan parasites that infect man. Found in tropical and sub-tropical regions of the world, malaria parasites threaten the lives of 3.3 billion and cause ∼0.6–1.1 million deaths annually (Fig. 1A) (World Health Organization, 2008; Lozano et al., 2012; Murray et al., 2012a). Malaria also results in significant morbidity and economic loss (Murray et al., 2012a). While pregnant women and children are particularly vulnerable to the threat of malaria, severe disease is also a threat for naïve travellers to malaria endemic regions and immunocompromised people. Six Plasmodium species are responsible for malaria in humans (Sutherland et al., 2010), however, the two most significant are Plasmodium falciparum and Plasmodium vivax. Most malaria related deaths are caused by P. falciparum and occur in sub-Saharan Africa, while P. vivax is responsible for significant morbidity particularly in South America and the Asia-Pacific region (World Health Organization, 2010; Murray et al., 2012a). Similar to other protozoan infections of man, the treatment of malaria is dependent on chemotherapy. While substantial funds have been invested in producing a malarial vaccine, to date poor efficacy has been achieved for those that have been trialled clinically, including the leading candidate RTS,S (Agnandji et al., 2011; Abdulla et al., 2013). This means that drugs remain a mainstay for malaria prevention and treatment. The WHO currently recommends three malaria prevention strategies for high risk groups: intermittent preventative treatment in pregnancy (IPTp) with sulfadoxine–pyrimethamine (SP); intermittent preventive treatment in infants (IPTi) with SP at second and third diphtheria–tetanus–pertussis vaccination; and seasonal chemoprevention with amodiaquine plus SP for children aged 3–59 months in some areas of highly seasonal malaria transmission (World Health Organization, 2013a). Since 2012, artemisinin combination therapies (ACTs) have been adopted as first line treatment for uncomplicated malaria in most endemic countries, while chloroquine is now only used in some countries in the Americas due to widespread drug resistance (World Health Organization, 2013b). Recommended treatment for severe malaria is quinine, or the artemisinin derivatives artemether or artesuante (World Health Organization, 2013a). Primaquine is the only drug available for radical cure of P. vivax, but unfortunately this drug suffers from side effects in people with glucose-6-phosphate dehydrogenase (G6PD) deficiency, necessitating pre-screening where it is used (Howes et al., 2012; World Health Organization, 2013b). Unfortunately, all of the drugs currently available for the treatment of malaria have been associated with drug resistant parasites. Even artemisinin and its derivatives, arguably our last line of defense against this devastating disease, have been associated with decreased efficacy and emerging drug resistance (Dondorp et al., 2009; Dondorp and Ringwald, 2013; Ariey et al., 2014). The ongoing need to discover and develop new antimalarial drugs which are effective against multi-drug resistant parasites has spurred the research community to invest in various drug development and discovery strategies. As discussed in Section 3, drug repurposing has played a role in malaria chemotherapy to date and has been the subject of renewed interest in recent high throughput screening campaigns (Section 5).
1.2. African trypanosomiasis
African trypanosomiasis, also known as African sleeping sickness, is caused by parasitic protozoan of the genus Trypanosoma. The two forms, West African and East African trypanosomiasis, are caused by Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense, respectively (Brun et al., 2011). T. b. gambiense accounts for more than 95% of cases (Brun et al., 2011; Simarro et al., 2011) and causes chronic infection which can emerge as severe disease many years after parasite infection (Checchi et al., 2008). T. b. rhodesiense causes acute infection, which can rapidly result in central nervous system involvement with parasites crossing the blood–brain barrier (Nikolskaia et al., 2006; Grab and Kennedy, 2008; Barrett et al., 2011). African trypanosomiasis threatens the lives of ∼60 million people in sub-Saharan Africa and, if left untreated, is fatal. In 2010 this disease was responsible for around 9000 deaths (Fig. 1B) (Lozano et al., 2012). While these deaths are lower than in 2004 (World Health Organization, 2008), there are concerns that drug resistance will impact this position (Vincent et al., 2010; Baker et al., 2013). There is no vaccine available for trypanosomiases with treatment relying on chemotherapy. The drug of choice for treatment depends on the infecting species and the stage of infection. In early stages, T. b. gambiense and T. b. rhodesiense infections can be treated with pentamidine or suramin, respectively (Lourie and Yorke, 1937; Steverding, 2010; Murthy et al., 2013). While both of these drugs are less toxic than late-infection (second-stage) treatments they are still associated with significant adverse effects and require parenteral administration (Barrett et al., 2011; Brun et al., 2011). If disease has progressed, treatment relies on melarsoprol or eflornithine (Friedheim, 1949; Pepin et al., 1987; Burri and Brun, 2003). Melarsoprol, an arsenical drug and one of the most noxious licensed for administration to humans, can cause severe adverse effects including reactive encephalopathy, heart failure and death (Brun et al., 2011). While eflornithine is less toxic, the treatment regimen with this drug is expensive, strict and difficult to administer (Barrett et al., 2011; Simarro et al., 2012). Eflornithine, while having recently been introduced as part of a combination therapy (nifurtimox–eflornithine combination therapy; NECT) is not effective against T. b. rhodesiense (Iten et al., 1997; Priotto et al., 2007, 2009; Murthy et al., 2013). In addition to these serious limitations it is likely that parasites have developed resistance to melarsoprol (Pepin and Milord, 1994; Burri and Keiser, 2001; Robays et al., 2008; Barrett et al., 2011). While treatment failures with pentamidine, suramin and efornithine are rare, resistance to these drugs has also been reported (Brun et al., 2001; Bernhard et al., 2007; Barrett et al., 2011). As chemotherapy is central to the treatment of sleeping sickness and there are serious limitations of current therapies, research efforts to identify new agents with different modes of action are essential to continual control and disease elimination efforts.
1.3. Chagas disease
Chagas disease, or American trypanosomiasis, is a serious health concern in Latin America and as a result of migration is an emerging disease in traditionally non-endemic countries (Coura and Vinas, 2010; Gascon et al., 2010). Chagas disease is caused by infection with Trypanosoma cruzi and threatens the lives of millions primarily in Mexico, Latin American and the United States. The World Health Organization estimates that 8–10 million people are infected annually (World Health Organization, 2010a). Chagas disease presents as an initial acute phase which is followed by a chronic phase. The acute phase of the disease can be severe in a small number of individuals (<1%), but is generally asymptomatic (Nunes et al., 2013). While infection can remain asymptomatic for many years, progression of the disease into its chronic phase often results (30–40%) in cardiomyopathy, progressive myocardium damage and heart disease (Nunes et al., 2013). Loss of life can occur (10,000 people in 2010) (Lozano et al., 2012), however, the disease also results in significant DALYs (∼0.5 million DALYs in 2010) and economic loss (estimated $7.2 billion/year globally) (World Health Organization, 2010b; Murray et al., 2012a; Lee et al., 2013). There is no vaccine for Chagas disease, with treatment currently depending on only two chemotherapeutics – benznidazole and nifurtimox. Both of these drugs are very effective if given soon after infection, however treatment failures (Munoz et al., 2013; Pinto et al., 2013) are not uncommon and drug resistant parasites have been identified (Murta et al., 1998). In addition both drugs cause adverse effects in large numbers of patients (Wegner and Rohwedder, 1972; Coura et al., 1997; Jackson et al., 2010). Benznidazole and nifurtimox are contraindicated in pregnant women and during kidney or liver failure. Nifurtimox is also contraindicated in people with neurological or psychiatric disorders. Given the deficiencies of the current treatment options there is a desperate need for drug discovery research. While numerous strategies to identify new treatment options are being pursued arguably the biggest hurdle is the lack of financial incentive for pharmaceutical companies to develop new treatments. In this climate the implementation of drug repurposing offers significant benefits.
1.4. Leishmaniasis
Leshmaniases are complex diseases caused by different species of the genus Leishmania. Leishmania parasites exist as exflagellated promastigotes in the female phlebotomine sandfly vector and as amastigotes in their mammalian hosts. Amastigotes are obligate intracellular parasites that generally reside in a phagolysosome compartment within macrophages. There are three different clinical forms of leishmaniasis; visceral leishmaniasis (VL), cutaneous leishmaniasis (CL) and mucocutaneous leishmaniasis (MCL). All have different immunopathologies and cause varying degrees of mortality and morbidity. Each year there are ∼12 million cases of leishmaniasis, with 0.5 million cases of VL and 1.5 million of CL (Fig. 1D). Drugs used to treat leishmaniasis include pentavalent antimonials (sodium stibogluconate (Pentostam™; GSK) and meglumine antimoniate (Glucantime, Aventis)), amphotericin B and its lipid formulations, pentamidine, and ketoconazole (Sundar and Chakravarty, 2013). The anitmonials require up to 28 days parenteral administration and have variable efficacy against the different forms of leishmaniasis. Treatment failure with pentavalent antimonial drugs has also been demonstrated (Lira et al., 1999; Sundar et al., 2000; Sundar, 2001; Rijal et al., 2003). The efficacy of ketoconazole is also variable (Navin et al., 1992; Ozgoztasi and Baydar, 1997). Diamidine pentamidine use is limited by issues of toxicity. While amphotericin B is very effective against antimonial-resistant Leishmania donovani VL (Thakur et al., 1999), it is toxic and requires slow parenteral infusion over four hours. Lipid-associated formulations of amphotericin B with reduced toxicity and increased plasma half-life, such as AmBisome®, are effective and have been approved for clinical use, but are expensive (Berman et al., 1998; Meyerhoff, 1999). A significant advance in leishmaniasis therapy was the introduction of miltefosine which, as discussed in more detail below (Section 3.3), was originally developed as an anticancer drug (Croft et al., 2003). Antileishmanial drug discovery has been fuelled in recent years by a variety of factors including whole genome sequencing (Ivens et al., 2005; Peacock et al., 2007) and the establishment of the neglected tropical diseases program (Hotez, 2011). However, discovery and development efforts are still hampered by problems including the differential chemosensitivities of Leishmania species and the different pharmacokinetic requirements for drugs for VL versus CL (Croft and Coombs, 2003).
1.5. Toxoplasmosis
Toxoplasmosis is caused by an obligate intracellular protozoan Toxoplasma gondii. It has a world-wide epidemiology and is believed to infect 25–30% of the world’s population (Montoya and Liesenfeld, 2004). As a zoonosis, humans usually become infected by horizontal transmission through the consumption of tissue cysts in contaminated meat (Kapperud et al., 1996; Baril et al., 1999; Cook et al., 2000). However other methods of transmission including vertical transmission from mother to child also occur. If infection occurs for the first time during pregnancy foetal death or congenital defects can result (Montoya and Liesenfeld, 2004). Toxoplasmosis can also be particularly serious in immunosuppressed individuals, such as those with HIV/AIDS, where it can result in severe disease (Luft and Remington, 1992). In otherwise healthy individuals the symptoms of toxoplasmosis are often mild or not apparent however clinical symptoms such as ocular disease (e.g. uveitis) can occur. There is some evidence to suggest that Toxoplasma infection is associated with a high incidence of traffic accidents (Flegr et al., 2002, 2009) and diseases such as epilepsy and schizophrenia (Palmer, 2007; Torrey et al., 2007), which may mean the global burden of this disease is higher than current estimates for congenital toxoplasmosis (1.2 million DALYs; Fig. 1D) (Torgerson and Mastroiacovo, 2013). Current treatment options for toxoplasmosis are limited. While asymptomatic toxoplasmosis is rarely treated, therapy of the more severe forms of the disease often consists of pyrimethamine in combination with a sulfonamide. To reduce transmission of parasites from mother to foetus pregnant women can also be treated with spiramycin and leucovorin (Desmonts and Couvreur, 1974b). Additional drugs available for use include clindamycin, co-trimoxazole, azithromycin, or atovaquone (Georgiev, 1994). Adverse side effects to pyrimethamine combinations (Dannemann et al., 1992) and concerns about the efficacy of other the macrolides such as azithromycin (Farthing et al., 1992) highlight the need for alternative therapeutic options.
1.6. Cryptosporidiosis
Crytoptosporidium parvum and Cryptosporidium hominis are the most common etiological agents of human cryptosporidiosis. Cryptosporidiosis causes self-limited disease in immunocompetent hosts (Chen et al., 2002), but is a major cause of recurrent diarrhoea in children under 5 years of age. A recent study investigating the cause and effect of diarrhoea in children (under 5 years) residing in four African and three Asian study sites identified cryptosporidiosis as the second most common infection in infants with infection associated with death in toddlers (12–23 months) (Kotloff et al., 2013). Globally cryptosporidiosis is estimated to be responsible for 30–50% of the deaths in under 5 year olds (Ochoa et al., 2004; Snelling et al., 2007). Infection in this age group is also associated with developmental problems (Guerrant et al., 1999). Current treatment options for cryptosporidiosis are limited, with the current gold standard drug nitazoxanide (NTZ) having only moderate clinical efficacy in children and immunocompetent people, and none in people with HIV (Abubakar et al., 2007; Amadi et al., 2009). Problems associated with the discovery and development of new drugs for cryptosporidiosis includes the lack of a widely accepted method to culture Cryptosporidium and an inability to genetically manipulate the organism. While this limits target-based drug discovery approaches, as discussed in Section 5, recent advances in cell-based approaches have meant that they are now amenable to HTS (Bessoff et al., 2013).
2. Anti-parasitic drug discovery approaches
The discovery and development of effective new drugs that target parasitic protozoa in humans is challenging. Differences in the clinical forms of each disease, pharmacokinetic requirements, pre-existing drug resistance, limited abilities to culture some organisms, and difficulties in genetically manipulating some parasites all add to the complexity of the problem. To address these difficulties a range of approaches are used to identify new antiparasitic agents. Strategies include extending the usefulness of existing drugs by generating new formulations with varying strengths/combinations/dosing regimens, de novo drug discovery, “piggy-back” drug discovery approaches (Nwaka and Hudson, 2006), and label extension or drug repurposing.
Development of new formulations and combinations of existing drugs is a clever way of extending the life of important biological actives. The reformulation of drugs can improve compliance, pharmacodynamics and pharmacokinetics making them more effective in vivo (Ogutu, 2013). Combination treatments are also believed to prevent the development of drug resistance and for this reason are now recommended standard practice for malaria chemotherapy and other diseases where multiple drugs are available (Abdulla and Sagara, 2009; Burrows et al., 2013).
De novo drug discovery focuses on identifying active new chemical entities (NCEs). This process usually involves screening strategies to identify “hits” that inhibit parasite growth/development in whole-cell screens or a specific validated target in target-based assays. Target based approaches have not been as successful as promised (Wells, 2010) with many now advocating whole-organism screening approaches. This has led to the identification of thousands of anti-parasitic “hit” compounds in recent years, particularly for diseases such as malaria (Gamo et al., 2010). However, in most cases progression of “hit” compounds through the developmental pipeline has been poor, or is only just beginning to yield leads for clinical progression (Anthony et al., 2012; Jones and Avery, 2013).
In contrast to de novo drug discovery, the “piggy-back” approach involves taking a validated molecular target for one disease and exploiting it as a drug discovery starting point for another disease (Nwaka and Hudson, 2006). Various parasitic protozoan experimental targets identified via this process are currently being pursued. For example histone deacetylase (HDAC) enzymes, inhibitors of which are clinically approved for T cell lymphoma, are being pursued as potential drug targets for malaria, leishmaniasis, trypanosomiasis (Kelly et al., 2012), and other parasitic diseases (reviewed in (Andrews et al., 2012a,b)). Primary sulphonamide compounds that primarily target carbonic anhydrase (Supuran, 2008b) are also being studied as potential antimalarial (Reungprapavut et al., 2004; Krungkrai et al., 2005, 2008; Andrews et al., 2013), anti-trypanasomal and anti-leishmanial agents (Guzel-Akdemir et al., 2013; Pan et al., 2013; Syrjanen et al., 2013). Kinase inhibitors, several of which are clinically approved for human use (O’Brien and Fallah Moghaddam, 2013), are under development as a potential new drug target class for parasitic diseases such as malaria, African trypanosomiasis and schistosomiasis (Beckmann et al., 2012; Lucet et al., 2012; Katiyar et al., 2013; Ochiana et al., 2013; Patel et al., 2013). Phosphodiesterase inhibitors, compounds that regulate signalling pathways, have been used to treat aliments including rectile dysfunction and schizophrenia and are also under investigation as anti-parasitic agents (Bland et al., 2011; de Koning et al., 2012).
Drug repurposing strategies have the potential advantage of facilitating rapid and cost effective drug development. As the cost associated with discovering and developing a NCE may be in the realm of $US800 million and may take 1–2 decades, this is a considerable advantage (Ashburn and Thor, 2004; Taylor and Wainwright, 2005; Nwaka and Hudson, 2006). Benefits can include reduced time to market if preclinical and clinical development can build on already available safety data, and potential new applications for candidates that may have failed preclinical or clinical testing for the primary indication. In the following sections we discuss the impact of drug repurposing in the context of the major human parasitic protozoan diseases discussed above.
3. Anti-protozoan drugs repurposed from other applications
Drug repurposing, repositioning, redirecting or re-profiling is best described as applying known drugs or compounds with proven clinical safety to new diseases or indications. As repurposed drugs are typically approved for clinical use, this discovery process can result in significant time and cost savings. Such savings are particularly appealing for rare diseases or those where there is little financial incentive to invest in “de novo” drug discovery. The discovery and development of drugs for parasitic diseases, for example, often provides little financial incentive to big pharma and as a result is an area that can benefit from drug repurposing. The sensitivity profile of parasites to known drugs is also rapidly changing and “de novo” drug discovery often leaves us ill equipped to combat drug resistance. Drug repurposing strategies have already had some success in this field, and it is likely that as techniques and tools for drug repositioning become further developed additional agents with useful clinical activity for these disease will be discovered. The following sections provide examples of drugs that have been successfully repurposed for use against major parasitic protozoan diseases and discuss the resources and techniques currently available to repurpose drugs in this arena.
3.1. Drugs repurposed for malaria
Several broad spectrum antibiotics have been shown to be effective antimalarial drugs in certain settings. For example, synthetically derived tetracyclines such as doxycycline have been used successfully for chemoprophylaxis and treatment of malaria. Doxycycline was first approved for clinical use in 1967 (Vibramcyin) as a once-a-day broad-spectrum antibiotic. This drug is a partially efficacious causal prophylactic against liver stages of the malaria parasite and has been shown to have slow acting schizonticidal activity (Clyde et al., 1971; Rieckmann et al., 1971). It is used as a malaria prophylactic and is effective as a treatment in combination with fast acting agents such as quinine and quinidine (Tan et al., 2011) (Table 1). Likewise clindamycin, a lincosamide antibiotic which was originally used in the 1940–1950s for acne treatment (Christian and Krueger, 1975), with numerous applications since, is used clinically for malaria (and toxoplasmosis – see Section 1.5), with antimalarial activity first described in the 1970’s (Miller et al., 1974) (Table 1).
Table 1.
Examples of drugs repurposed for use against parasitic protozoal diseases.
| Drug | Structure | Initial use(s) | Repurposed use(s) | References |
|---|---|---|---|---|
| Clindamycin | 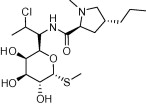 |
Anti-bacterial (e.g. acne) | Malaria – slow acting blood schizontocidal agent; used in combination with fast acting antimalarials; Toxoplasmosis | Miller et al. (1974), Christian and Krueger (1975), Montoya and Liesenfeld (2004) |
| Doxycycline | 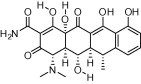 |
Broad-spectrum bacteriostatic agent | Malaria; causal prophylactic (liver stage) and slow acting blood schizontocidal agent; used with fast acting antimalarials | Tan et al. (2011) |
| Co-trimoxazole (trimethoprim/sulfamethoxazole) | 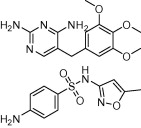 |
Urinary-tract infection, otitis media, shigellosis, and P. carinii pneumonia | P. falciparum malaria in non-pregnant adults and children; dihydrofolate reductase inhibitor; toxoplasmosis | Gleckman et al. (1979), Montoya and Liesenfeld (2004), Manyando et al. (2013) |
| Elfornithine (DFMO) | 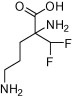 |
Antitumour agent/P. carinii infection in AIDS patients/hirsutism | Human African sleeping sickness – effective against T. b. gambiense but not T. b. rhodesiense | Abeloff et al. (1986), Paulson et al. (1992), Hickman et al. (2001), Shapiro and Lui (2001), Burri and Brun (2003), Kennedy (2008, 2013), Casero and Woster (2009), Simarro et al. (2011, 2012) |
| Miltefosine | 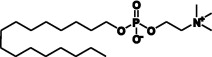 |
Skin metastases (breast cancer) | Vischeral leishmaniasis | Smorenburg et al. (2000), Paladin Labs Inc (2010), Dorlo et al. (2012) |
| Paromomycin | 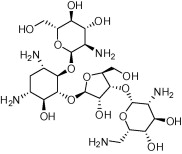 |
Antibiotic; acute and chronic intestinal amebiasis; adjunctive management of hepatic coma. | Parenteral treatment of visceral leishmaniasis; cutaneous leishmaniasis | Ben Salah et al. (2013) |
| Amphotericin B (AmBiome structure shown) | 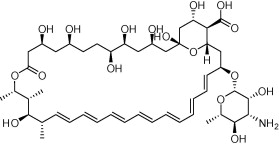 |
Antifungal infections such as aspergillosis, cryptococcosis, North American blastomycosis, systemic candidiasis, histoplasmosis, and zygomycosis | Visceral leishmaniasis; AmBD now superseded by liposomal formulations of amphotericin B such as AmBiome (structure shown) | Meyerhoff (1999), Hu et al. (2003), Ostrosky-Zeichner et al. (2003), Sundar and Chakravarty (2010) |
| Sulphadiazine | 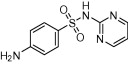 |
Bacterial infections (e.g. streptococcal and staphylococcal infections); topical use against gram −ve and +ve bacterial infections in burns victims | Used in combination with pyrimethamine for prevention and treatment of toxoplasmosis | British Medical Journal (1942), Coloviras et al. (1942), Leport et al. (1988), Guerina et al. (1994), Montoya and Liesenfeld (2004), Rorman et al. (2006) |
| Spiramycin | 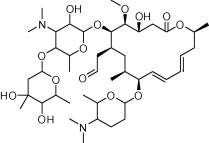 |
Antimicrobial (gram-positive organisms) | Congenital toxoplasmosis | Desmonts and Couvreur (1974a), Couvreur et al. (1988), Descotes et al. (1988), Robert-Gangneux and Darde (2012) |
The sulphonamide group is a key component of many drugs used to treat a wide range of human diseases, including kidney disease, glaucoma, epilepsy and obesity and more recently as candidates for cancer therapy (Supuran, 2008a,b, 2010). Whilst there are over 100 FDA-approved sulphonamide containing drugs (Smith and Jones, 2008) only a handful have been exploited as antiparasitic therapies. The antibacterial sulphonamides, sulphadiazine and sulphadoxine, that target folate synthesis, have been used in combination therapies to treat T. gondii and P. falciparum infections (Doberstyn et al., 1976; Guerina et al., 1994) (Table 1). The anti-folate sulfa drug co-trimoxazole (Bactrim and other names), a combination of trimethoprim and sulfamethoxazole, first used to treat chronic urinary-tract infection, otitis media, shigellosis, and Pneumocystis carinii pneumonia (Gleckman et al., 1979) is also used to treat toxoplasmosis (Section 1.5) and P. falciparum malaria in non-pregnant adults and children (Gleckman et al., 1979; Manyando et al., 2013).
Dapsone, a sulfone antibiotic that was first used to treat leprosy and then other diseases such as dermatitis herpetiformis, and P. carinii pneumonia in AIDS patients (reviewed in (Wolf et al., 2002)) was introduced to treat malaria in the late 1990’s. Dapsone was marketed as Lapdap (GlaxoSmithKline) in combination with chlorproguanil (Lapudrine) (Winstanley, 2001) and, while effective against parasite folate metabolism, was withdrawn from the market 2008 (Merckx et al., 2003). Unfortunately dapsone was found to result in haemolytic anaemia in ∼20% of patients, being a particular problem in individuals with glucose 6-phosphate dehydrogenase (G6PD) deficiency (Puavilai et al., 1984).
3.2. Drugs repurposed for trypanosomiasis
Drugs used to treat African Tryanosomiasis have primarily been developed for that purpose rather than being repurposed from other indications. An exception is eflornithine (difluoromethyl ornithine; DFMO), an inhibitor of the ornithine decarboxylase enzyme which is required for polyamine biosynthesis. This drug was identified as an antitumour agent (reviewed in (Casero and Woster, 2009)), however, clinical studies were discontinued due to problems with adverse side-effects (Abeloff et al., 1986). Eflornithine has also been used clinically against P. carinii in AIDS patients (Paulson et al., 1992) and was FDA-approved (Vaniqa®) for treatment of Hirsutism in women in 2001 (Hickman et al., 2001; Shapiro and Lui, 2001). In 1990, eflornithine (Ornidyl®) was approved as an orphan drug for intravenous use against forms of African sleeping sickness (Burri and Brun, 2003; Kennedy, 2008), and as a result of partnership between the WHO and pharma, intravenous eflornithine became widely available to patients around 2001 (Kennedy, 2008, 2013). Since then eflornithine has been combined with a drug originally used for Chagas diseases, nifurtimox, for first-line treatment for CNS-stage T. b. gambiense human African tyrpanosomiasis (Simarro et al., 2011, 2012). Eflornithine is, however, ineffective against T. b. rhodesiense human African tyrpanosomiasis. It must also be given intravenously, can have many side-effects, and has issues of potential drug resistance.
3.3. Drugs repurposed for leishmaniasis
Drugs active against leishmaniasis that have arisen via repurposing include Amphotericin B, paromomycin, and miltefosine. Amphotericin B deoxycholate (AmBD) was first licensed as an infusion in 1959 for use against life threatening fungal infection then used in India for treatment of visceral leishmaniasis (Hu et al., 2003; Ostrosky-Zeichner et al., 2003). While this drug has an excellent cure rate it needs to be administered by intravenous infusion and patients can suffer from adverse infusion reactions such as fever, chills, and thrombophlebitis. More toxic adverse reactions including nephrotoxicity, myocarditis, and death can also occur. The administration mode and associated adverse effects of AmBD mean that its use requires monitoring and hospitalization, increasing the cost of this therapy. New formulations of AmBD, including liposomal amphotericin B (AmBisome; Gilead Sciences), amphotericin B lipid complex (ABLC; Abelcet®, Enzon Pharmaceuticals), and amphotericin B cholesterol dispersion (ABCD; Amphotec™, InterMune Corp.) which package AmBD with cholesterol and other phospholipids to overcome some of these issues, have been produced. Liposomal AmBDs are now used to treat fungal infections caused by Aspergillus, Candida and/or Cryptococcus. They are particularly beneficial in patients with renal impairment (precludes the use of amphotericin B deoxycholate) and those with febrile neutropenia (Sundar and Chakravarty, 2010). In 1997 AmBisome became the first FDA-approved drug for leishmaniasis (Meyerhoff, 1999).
Paromomycin (aminosidine) is an aminoglycoside–aminocyclitol antibiotic that is used for parenteral treatment of visceral leishmaniasis, and more recently cutaneous leishmaniasis (Ben Salah et al., 2013). First isolated in the 1950s (Davidson et al., 2009), paromomycin has broad-spectrum antibiotic activity against gram-positive and gram-negative bacteria. It is also effective against cestodes and other protozoa, including Giardia and Entamoeba histolytica (Davidson et al., 2009). The antileishmanial activities of paromomycin were established in the 1960s (Kellina, 1961; Neal, 1968; Neal et al., 1995). While paromomycin remains an option for the treatment of giardiasis, leishmaniasis and amebiasis, it is no longer used as an antibiotic with the more popular cephalosporin and quinolone compounds forcing this drug out of the market in the 1980s (Davidson et al., 2009). Paromomycin acts by inhibiting protein synthesis, however, its anti-protozoan activity may be more complex (Maarouf et al., 1997).
The only drug registered for oral treatment of leishmaniasis is miltefosine. Miltefosine and related alkylphosphocholine drugs were simultaneously, but independently, discovered to have anti-trypanosomal and antineoplastic activity in the 1980’s (Croft et al., 2003; Dorlo et al., 2012). Priority was given to the development of miltefosine for local treatment of cutaneous metastases of breast cancer, with a topical formulation (Miltex®, Baxter, UK) being approved for this indication (Smorenburg et al., 2000). While this compound was also assessed for use against other types of tumours, it was ultimately discontinued due to dose-limiting gastrointestinal side effects (reviewed in (Dorlo et al., 2012)). These anti-cancer studies did however lead to miltefosine being re-investigated for leishmaniasis and, after several clinical trials, to its approval for oral treatment for leishmaniasis (Paladin Labs Inc, 2010). Today miltefosine can be used to treat all of the clinical leishmaniases (Berman, 2008), however, it is associated with common gastrointestinal side effects. It is also limited by its relatively high cost (Sundar and Chakravarty, 2013), teratogenicity and growing concerns in relation to increases in clinical isolate susceptibility (Prajapati et al., 2012).
3.4. Drugs repurposed for Toxoplasmosis
As a first choice, eye disease or lymphadenitis resulting from Toxoplasma infection can be treated with the antimalarial drug pyrimethamine (Table 2) combined with the antibacterial agent sulfadiazine (Table 1) (British Medical Journal, 1942; Coloviras et al., 1942; Leport et al., 1988; Guerina et al., 1994; Rorman et al., 2006) and folinic acid (Montoya and Liesenfeld, 2004). For patients who cannot tolerate sulfa drugs, sulfadiazine can be replaced with clindamycin (Table 1) or azithromycin, both of which were first used as antibacterial agents (Christian and Krueger, 1975; Montoya and Liesenfeld, 2004). Spiramycin (Rovamycin) is a macrolide antimicrobial agent with activity against gram-positive organisms that was first used in France in 1955 (Descotes et al., 1988). In the 1970’s spiramycin was shown to have benefit in preventing congenital toxoplasmosis (Desmonts and Couvreur, 1974a; Couvreur et al., 1988), and is now used when maternal infection is acquired or suspected during pregnancy (Montoya and Liesenfeld, 2004; Robert-Gangneux and Darde, 2012). As discussed below (Section 4, Table 2), the antimalarial drug atovaquone has also been repurposed for Toxoplasmosis.
Table 2.
Examples of antiprotozoal drugs repurposed for use against other diseases.
| Drug name | Structure | Approved use | Repurposed/investigational use | References |
|---|---|---|---|---|
| Quinine | 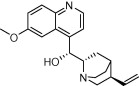 |
Malaria | Lupus, arthritis, and leg cramps | Bird et al. (1995), Barrocas and Cymet (2007), Ben-Zvi et al. (2012), Murphy et al. (2013) |
| Chloroquine |  |
Malaria | Extraintestinal amebiasis (E. histolytica); intrathoracic and cutaneous sarcoidosis; cancer treatment | Basnuevo and Gutierrez Estarli (1951), Scaffidi and Astuto (1959), Cohen and Reynolds (1975), Zic et al. (1991), Baltzan et al. (1999), Sotelo et al. (2006), Amaravadi et al. (2011) |
| Atovaquone | 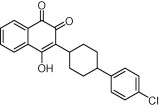 |
Malaria | P. carinii pneumonia; toxoplasmosis | Araujo et al. (1991), Baggish and Hill (2002), Montoya and Liesenfeld (2004) |
| Artemisinin (and derivatives) | 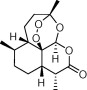 |
Malaria | Cancers, pathogenic microbes (e.g. Trypanosoma spp., Leishmania), metazoan helminths (Schistosoma spp.), fungi, and viruses | Xiao (2005), Mishina et al. (2007), Efferth et al. (2008), Krishna et al. (2008), Sen et al. (2010), Njuguna et al. (2012) |
| Pyrimethamine | 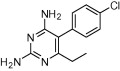 |
Malaria | Toxoplasmosis | Wilson and Edeson (1953), British Medical Journal (1957), Montoya and Liesenfeld (2004) |
| Nifurtimox | 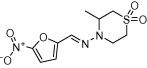 |
Chagas disease (American typanosomiasis) | Second-stage human African trypanosomiasis (in combination with Elfornithine (DFMO)); cancer therapy (neuroblastoma) | Moens et al. (1984), Van Nieuwenhove (1992), Saulnier Sholler et al. (2006, 2011), Priotto et al. (2009), Lutje et al. (2013) |
| Nitazoxanide (Alinia®) |  |
Antiprotozoal agent; approved for use against diarrhoea caused by C. parvum and G. lamblia | Anaerobic bacteria and parasites residing in bowel, hepatitis C, cancer cells (e.g. bowel and breast), and Leishmania parasites | White (2004), Muller et al. (2008a,b), Elazar et al. (2009), Di Santo and Ehrisman (2013), Fan-Minogue et al. (2013), Mesquita et al. (2013) |
4. Antiprotozoan drugs under investigation for use against other diseases
Examples of drugs that have been approved for protozoa and then repurposed to treat other diseases (Table 2) further highlight the potential of drug repurposing as a drug discovery mechanism. Quinine, an antimalarial first developed in the 17th century, was the mainstay of malaria therapy until chloroquine was introduced in the 1940’s (Achan et al., 2011). In addition to being used for malaria, quinine and related members of the quinolone family have also been used to treat lupus, arthritis, and leg cramps (Bird et al., 1995; Barrocas and Cymet, 2007; Ben-Zvi et al., 2012; Murphy et al., 2013). Chloroquine has been investigated for use against amebiases such as E. histolytica and sarcoidosis (Zic et al., 1991; Baltzan et al., 1999), HIV (Savarino et al., 2004; Brouwers et al., 2008), and for cancer, with evidence suggesting that this compound sensitizes cancer cells to radiation and chemotherapeutic agents (Sotelo et al., 2006; Amaravadi et al., 2011). The antimalarial drug atovaquone, a hydroxy-1,4-naphthoquinone and structural analogue of the protozoan mitochondrial electron transport protein ubiquinone, has also been repurposed. Atovaquone was first released for malaria in 1992 (Baggish and Hill, 2002) and is primarily used in combination with proguanil for malaria prophylaxis, however, it has also been used against diseases including P. carinii pneumonia, toxoplasmosis, and bovine babesiosis (Araujo et al., 1991; Baggish and Hill, 2002). Likewise, the antimalarial artemisinin, a sesquiterpene endoperoxide lactone natural product from the sweet wormwood plant, Artemisia annua L. (Asteraceae) (Klayman, 1985), has been extensively studied as a potential therapy for other diseases, including cancer, pathogenic microbes, helminths, fungi, and viruses (Krishna et al., 2008; Njuguna et al., 2012). The anticancer activity of artemisinin derivatives was first reported in the early 1990’s and has since been shown to have various effects including growth arrest, induction of apoptosis, alteration of hormone responsive properties, and inhibition of cancer cell angiogenesis (reviewed in (Njuguna et al., 2012)). Artemether, an artemisinin derivative, has also been used for Schistosoma mansoni prophylaxis and in combination with praziquantel for treatment of Schistosoma japonica (Xiao, 2005). Artesunate, another artemisinin derivative, has been shown to have inhibitory activity against human cytomegalovirus, herpes simplex type-1, hepatitis B and C, and HIV-1 viruses (Efferth et al., 2008). Artemisinin, and various derivatives, also inhibit the growth of parasitic protozoa such as T. cruzi, T. b. rhodesiense, and Leishmania parasites (Mishina et al., 2007; Sen et al., 2010).
As discussed in Section 1.3, while nifurtimox was first used to treat American trypanosomiasis (Chagas disease), this drug has since been repurposed for use against human African trypanosomiasis (Moens et al., 1984; Van Nieuwenhove, 1992) where it is useful in combination with eflornithine (NECT) (Priotto et al., 2009) (Table 2). A recent meta-analysis of nine clinical trials on treatment of second-stage human African trypanosomiasis caused by T. b. gambiense indicated that, while melarsoprol monotherapy resulted in fewer relapses than pentamidine or nifurtimox, more adverse events were seen. Nifurtimox combined with eflornithine was found to give rise to fewer relapses, to be well tolerated, and had the benefit of reducing the frequency and number of eflornithine slow infusions to two a day (Priotto et al., 2009; Lutje et al., 2013). Studies have also indicated that nifurtimox may also have benefit as a cancer therapy for treatment of neuroblastoma (Saulnier Sholler et al., 2006, 2011).
Another example of an anti-protozoan agent that has been repurposed is nitazoxanide. Nitazoxanide (Alinia®, Romark), the only clinically approved drug to treat diarrhoea caused by C. parvum, is also used to treat Giardia lamblia. In addition, nitazoxanide has been used to treat diseases caused by other parasites (including Leishmania) and anaerobic bacteria, has been shown to promote hepatitis C elimination, and to have effects against some types of cancer cells (White, 2004; Muller et al., 2008b; Elazar et al., 2009; Di Santo and Ehrisman, 2013; Fan-Minogue et al., 2013; Mesquita et al., 2013) (Table 2).
5. Screening of clinical drug libraries to identify new repurposing candidates
A number of whole-cell based high throughput screening campaigns utilizing compound libraries containing registered drugs have recently been carried out (Table 3). Antimalarial screens have been carried out by different groups, most against asexual blood stage P. falciparum parasites (Chong et al., 2006; Weisman et al., 2006; Yuan et al., 2011), but also against the less assay-tractable liver stage using mouse malaria parasites (da Cruz et al., 2012). In 2006, two screens were carried out using laboratory lines of P. falciparum, yielding numerous hits including the antihistamine astemizole which was also shown to have some activity in vivo in mouse models of malaria (Chong et al., 2006). This compound has since been shown to also have activity against Plasmodium liver stages (Derbyshire et al., 2012) and is being pursued as a starting point for new antimalarial compounds (Roman et al., 2013). Recently, Yuan et al. (2011) screened a panel of 61 P. falciparum lines with different drug sensitivities against the NIH Chemical Genomics Center Pharmaceutical Collection which contains 2816 compounds registered or approved for human or animal use (Huang et al., 2011). Results of this extensive screen yielded 32 compounds with potent in vitro activity (IC50 <1 μM), most of which are clinically approved. It is reported that 10 of these compounds had not previously been shown to have antimalarial activity (Yuan et al., 2011).
Table 3.
Examples of whole-cell-based HTS campaigns using clinical drug libraries.
| Parasite | Library (details) | # Screened | # “Hits” ⁎ | Comments | References |
|---|---|---|---|---|---|
| P. falciparum (asexual) | MicroSource Spectrum and Killer Collectionsa | 2160 | 72 (at 1 μM) | 19 drugs identified not been previously shown to inhibit P. falciparum | Weisman et al. (2006) |
| P. falciparum (asexual) | Johns Hopkins Clinical Compound Library (JHCCL)b | 2687 | 189 (at 10 μM) | Antihistamine astemizole and its metabolite identified as new antimalarials | Chong et al. (2006) |
| P. falciparum (asexual) | NIH National Chemical Genomics Center (NCGC) Pharmaceutical Collectionf | 2816 | 32 (IC50 <1 μM) | Screened against 61 P. falciparum lines; also identified genetic loci and genes associated with differential compound phenotypes | Yuan et al. (2011) |
| P. berghei (liver-stage)⁎⁎ | US Drug Collection Libraryd | 1037 | 116 (at 10 μM) | Decoquinate identified (mitochondrial bc1 complex inhibitor) | da Cruz et al. (2012) |
| T. brucei | MicroSource Spectrum and Killer Collectionsa | 2160 | 35 (at 1 μM) | 17 novel trypanocidal agents identified | Mackey et al. (2006) |
| T. cruzi | Iconix libraryc | 909 | 55 (at 10 μM) | Antibacterial, antifungal, anticancer, and allergy drugs with >∼50× selectivity for parasite vs normal cells | Engel et al. (2010) |
| C. parvum | NIH Clinical Collectione | 727 | 24 (at 10 μM) | Statin class of HMG-CoA reductase inhibitors identified as potent inhibitors | Bessoff et al. (2013) |
| E. histolytica | Iconix libraryc | 910 | 11 (at 5 μM) | Rheumatoid arthritis drug auranofin identified (EC50 0.5 μM (10×> metronidazole) | Debnath et al. (2012) |
| T. vaginalis | US Drug Collection Libraryd | 1040 | 47 (at 20 μM) | 11 Drugs with activity ⩾ metronidazol at 20 μM | Goodhew and Secor (2013) |
Known drugs, bioactive compounds, and natural products (http://www.msdiscovery.com).
1937 FDA-approved drugs and 750 compounds approved outside USA or undergoing phase 2 clinical trials (http://www.nature.com/nchembio/journal/v2/n8/extref/nchembio806-S1.pdf).
FDA-approved and unapproved bioactive compounds/Ref. Wishart et al. (2008).
Microsource Discovery Systems, USA – drugs approved for human use in the USA or that are undergoing clinical trials.
Evotec, USA – FDA-approved drugs and drug-like molecules.
Ref. Huang et al. (2011).
Definition of “hit” varied, but was usually ⩾50% inhibition at the indicated test concentration.
In vitro infection model combining P. berghei parasites and the Huh7 human hepatoma cell line.
Fewer high throughput studies have been carried out for other protozoan pathogens, largely due to problems associated with robust culture methods and assay limitations. However there has been some recent activity in this area (Table 3). Screens of drug libraries against T. brucei and T. cruzi have identified almost 100 hits, some of which are novel actives for trypanosomes and with promising parasite-specific selectivity (Mackey et al., 2006; Engel et al., 2010). To search for drugs for cryptosporidiosis, Bessoff et al. (2013) screened a library of FDA approved compounds with a history of clinical use against C. parvum. This screen yielded 21 hits, 15 of which are FDA approved for clinical use, including compounds classified as topical antibacterials, channel inhibitors, a microtubule agent, pyrimidine analogues, and tetracycline analogues (Bessoff et al., 2013). Further work arising from this screening campaign identified Itavastatin as a potent C. parvum inhibitor. The activity of this drug is believed to be, at least partly, due to the inhibition of human HMG-CoA reductase (Bessoff et al., 2013). Whole cell screening against the intestinal parasite E. histolytica, which causes human amebiasis, yielded eleven hit compounds including the rheumatoid arthritis drug auranofin which displayed approximately ten-fold greater activity than the control amebiasis drug metronidazole (Debnath et al., 2012). Likewise, screening of a different library against Trichomonas vaginalis, which causes the common sexually transmitted diseases trichomoniasis, yielded hits with better activity than metronidazole (Goodhew and Secor, 2013), which is used to treat this parasitic disease.
A comparison of hit compounds in common between different parasite species resulting from the clinical library screens in Table 3 is shown in Fig. 2. While these data do not take into account variations in library composition or assay conditions, they suggest significant cross-over of “hits” between parasitic protozoan species. While many compounds showed activity against two parasite species, some inhibited the growth of three or more species, including antineoplastic agents (cycloheximide, daunorubicin/daunorubicin hydrochloride, docetaxel, doxorubicin/doxorubicin hydrochloride, idarubicin/idarubicin hydrochloride, mitomycin C, paclitaxel, and vinorelbine/vinorelbine bitatrate), antiseptic agents (gentian violet, phenylmercuric acetate, and thimerosal), and antibiotic/anti-infective agents (doxycycline/doxycycline hydrochloride and emetine/emetine HCl). Although potentially complicated by library bias, it is perhaps not surprising that antibiotics and antifungals, together with tubulin inhibitors, are highly represented in these hits. As discussed above (Section 3), antibiotics have a history of repurposing success for diseases such as malaria, and drugs such as the benzimidazoles target tubulin in the protozoan parasite Giardia lamblia and are also active against P. falciparum (Skinner-Adams et al., 1997), helminths and other parasite pathogens of humans (Canete et al., 2009). Data in Fig. 2 demonstrating significant bioactivity cross-over between parasite species, together with the work discussed above on repurposing drugs between parasitic diseases (Section 4), indicates that there may be potential value in cross-screening compound libraries between protozoa species. This could also include leads arising from “de novo” or piggyback approaches.
Fig. 2.
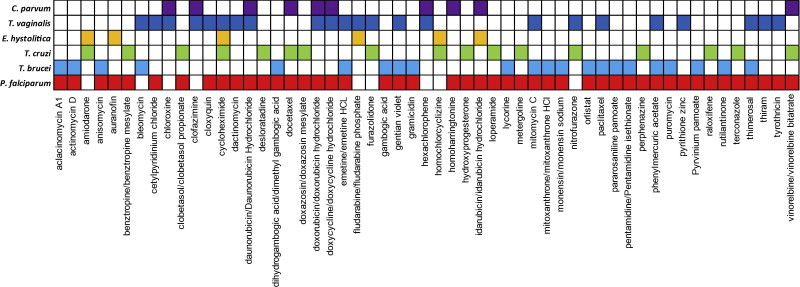
Common antiprotozoal “hits” from whole-cell-based screening of clinical drug libraries. “Hits” present in two or more different parasite species from studies shown in Table 3 are shown. For P. falciparum, data are from the three asexual blood stage screens (Chong et al., 2006; Weisman et al., 2006; Yuan et al., 2011). White boxes – either not defined as a “hit” or not present in library screened.
6. Resources and tools to facilitate drug repurposing and drug rescue
Drug repurposing and rescue campaigns rely on obtaining access to compounds that have either been approved for clinical use, or for which some clinical data is available such as compounds that have been abandoned by big pharma. In 2011 Huang et al. (Huang et al., 2011) calculated that the “universe” of compounds available (including veterinary compounds) for repurposing and chemical genomic studies was 8969, comprising 4034 unique approved molecular entities and 4935 unique registered molecular entities. However, access to these compounds is limited, often complicated by commercial concerns, particularly issues involving intellectual property. While some of these chemical entities may be available from open access and commercially available clinical compound libraries (including those discussed above and in Table 3), the potential value of obtaining access to additional compounds in this “universe” is clear. Funding agencies in the United Kingdom and the United States, recognizing the difficulties often encountered by academia in accessing such compounds, are investing in strategies to link academics with industry to enable investigation of pre-worked or abandoned molecular entities (Mullard, 2011, 2012). In 2011, the UK Medical Research Council (MRC) and AstraZeneca announced an alliance (AZ-MRC alliance) giving British researchers’ access AstraZeneca compounds, including some with prior clinical data, as tools to probe molecular mechanisms of disease. Likewise, in 2012 the United States National Institutes of Health “National Center for Advancing Translation” launched its New Therapeutic Uses (NTU) program which aims to discover new uses for compounds from industry that have undergone significant investigation including human safety testing. While these public private partnerships are encouraging and may lead to new opportunities, they currently include small numbers of compounds (22 and 58, respectively, for AZ-MRC and NTU pilot programs), do not include infectious disease agents, and could be of additional benefit if expanded in the future.
While this review has focused on repurposing human therapeutics, another attractive approach might be to repurpose veterinary therapeutics for human use, particularly given the genetic similarity between many of human and animal infecting protozoa. Unfortunately, there is currently limited information available on this approach, probably due to concerns of poor tolerability and toxicity. However some open access and commercial compound libraries do include agents used for veterinary purposes. The NIH NCGC Pharmaceutical Collection (Huang et al., 2011) contains a small number of veterinary compounds and, as discussed above (Table 3), this library has been screened against multiple P. falciparum lines. One of the hits identified was Tylosin, a veterinary drug used to treat bacterial infections (Yuan et al., 2011).
In addition to whole cell and target based screens of chemical libraries, in silico profiling or pre-screening of compound libraries may also be a useful approach to identify new drug leads for parasitic diseases. These approaches may also be a cost effective alternative to whole-library screening, guiding selection of appropriate compounds for further assessment. Pre-screening can involve compound prioritization based on activity against similar organisms (as discussed above) or, if target or mode-of-action data are available, may involve more detailed in silico assessment (Ekins et al., 2007). In silico screening may be pharmacophore, network or target based and although all of these screening techniques have disadvantages, including precluding potential hits, they may improve the success of drug repurposing campaigns. In a recent study by Sateriale et al. (2013) data mining was used to identify drug repurposing opportunities for protozoan parasites. In this study protein sequences were used in a target-based screening approach to enrich libraries for potential bioactive compounds. A total of 13 parasite proteomes were examined, including Plasmodium, Leishmania, and Trypanosoma species, with hits being compared to C. parvum in vitro whole cell screening data for validation. This approach resulted in significantly higher hit rates in the pre-screened library than in the un-screened cohort of compounds. The in silico screening results from that study are available online and are likely to be a useful resource for drug repurposing efforts (Sateriale et al., 2013). In addition databases and analysis tools such as DrugBank (Wishart et al., 2008), PubChem (Bolton et al., 2008; Wang et al., 2014), ChEMBL (Bento et al., 2014), NCGC Pharmaceutical Collection (NPC) (Huang et al., 2011), TDR Targets (Magarinos et al., 2012) and PROMISCUOUS (von Eichborn et al., 2011) are freely available for alternative pre-screening strategies.
7. Overview and perspectives
Diseases caused by parasitic protozoa cause enormous health, social, and economic impact, in particular to tropical regions of the world, many of which are resource constrained. The lack of a licensed vaccine for any human parasitic disease, together with a lack of affordable, safe and effective drugs for some diseases, or problems of parasite drugs resistance where drugs are available, is driving the search for new antiparasitic agents. Despite recent advances that are helping to fuel drug discovery efforts, such as the sequencing of several parasites genomes and the establishment of public–private partnerships (PPPs) to specifically focus on tropical disease drug discovery and development (Chatelain and Ioset, 2011; Jakobsen et al., 2011), there are numerous challenges facing research in this area. In particular, the large cost associated with progressing compounds through the drug discovery pipeline, together with the poor financial incentives to big pharma, are bottlenecks that need to be overcome. In this arena, repurposing or repositioning existing drugs could have potential benefits such as reduced costs during discovery, development and clinical phases; an accelerated drug development process; possible extension of patent life; the potential to recover and repurpose previously failed compounds; and lower overall risk. With evidence to suggest bioactive cross-over particularly with antibiotics, fungicides, HDAC inhibitors and kinase inhibitors, to name a few, drug repurposing is likely a promising avenue for the discovery and development of new anti-parasitic agents. A potential problem with this approach does need to be acknowledged – the reluctance of some pharmaceutical companies to allow testing of existing drugs against “non-commercial” diseases indications in case unwanted toxicities are discovered that could potentially compromise the commercial value of their products (Urbina and Docampo, 2003; Pink et al., 2005; Nwaka and Hudson, 2006). It is, however, encouraging that some pharmaceutical companies are now opening up their own compound collections and bioactivity data to benefit drug discovery for diseases like malaria (Gamo et al., 2010).
Acknowledgements
This work was supported by the Australian Research Council (FT0991213 to K.T.A.), Griffith University (NRG to T.S.A.), and the Australian National Health and Medical Research Council (PhD Scholarship to G.F.).
Contributor Information
Katherine T. Andrews, Email: k.andrews@griffith.edu.au.
Tina S. Skinner-Adams, Email: t.skinner-adams@griffith.edu.au.
References
- Abdulla S., Sagara I. Dispersible formulation of artemether/lumefantrine: specifically developed for infants and young children. Malar. J. 2009;8(Suppl. 1):S7. doi: 10.1186/1475-2875-8-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulla S., Salim N., Machera F., Kamata R., Juma O., Shomari M., Kubhoja S., Mohammed A., Mwangoka G., Aebi T., Mshinda H., Schellenberg D., Carter T., Villafana T., Dubois M.C., Leach A., Lievens M., Vekemans J., Cohen J., Ballou W.R., Tanner M. Randomized, controlled trial of the long term safety, immunogenicity and efficacy of RTS, S/AS02D malaria vaccine in infants living in a malaria-endemic region. Malar. J. 2013;12:11. doi: 10.1186/1475-2875-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeloff M.D., Rosen S.T., Luk G.D., Baylin S.B., Zeltzman M., Sjoerdsma A. Phase II trials of alpha-difluoromethylornithine, an inhibitor of polyamine synthesis, in advanced small cell lung cancer and colon cancer. Cancer Treat. Rep. 1986;70(7):843–845. [PubMed] [Google Scholar]
- Abubakar I., Aliyu S.H., Arumugam C., Hunter P.R., Usman N.K. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst. Rev. 2007;1:CD004932. doi: 10.1002/14651858.CD004932.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achan J., Talisuna A.O., Erhart A., Yeka A., Tibenderana J.K., Baliraine F.N., Rosenthal P.J., D’Alessandro U. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar. J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnandji S.T., Lell B., Soulanoudjingar S.S., Fernandes J.F., Abossolo B.P., Conzelmann C., Methogo B.G., Doucka Y., Flamen A., Mordmuller B., Issifou S., Kremsner P.G., Sacarlal J., Aide P., Lanaspa M., Aponte J.J., Nhamuave A., Quelhas D., Bassat Q., Mandjate S., Macete E., Alonso P., Abdulla S., Salim N., Juma O., Shomari M., Shubis K., Machera F., Hamad A.S., Minja R., Mtoro A., Sykes A., Ahmed S., Urassa A.M., Ali A.M., Mwangoka G., Tanner M., Tinto H., D’Alessandro U., Sorgho H., Valea I., Tahita M.C., Kabore W., Ouedraogo S., Sandrine Y., Guiguemde R.T., Ouedraogo J.B., Hamel M.J., Kariuki S., Odero C., Oneko M., Otieno K., Awino N., Omoto J., Williamson J., Muturi-Kioi V., Laserson K.F., Slutsker L., Otieno W., Otieno L., Nekoye O., Gondi S., Otieno A., Ogutu B., Wasuna R., Owira V., Jones D., Onyango A.A., Njuguna P., Chilengi R., Akoo P., Kerubo C., Gitaka J., Maingi C., Lang T., Olotu A., Tsofa B., Bejon P., Peshu N., Marsh K., Owusu-Agyei S., Asante K.P., Osei-Kwakye K., Boahen O., Ayamba S., Kayan K., Owusu-Ofori R., Dosoo D., Asante I., Adjei G., Chandramohan D., Greenwood B., Lusingu J., Gesase S., Malabeja A., Abdul O., Kilavo H., Mahende C., Liheluka E., Lemnge M., Theander T., Drakeley C., Ansong D., Agbenyega T., Adjei S., Boateng H.O., Rettig T., Bawa J., Sylverken J., Sambian D., Agyekum A., Owusu L., Martinson F., Hoffman I., Mvalo T., Kamthunzi P., Nkomo R., Msika A., Jumbe A., Chome N., Nyakuipa D., Chintedza J., Ballou W.R., Bruls M., Cohen J., Guerra Y., Jongert E., Lapierre D., Leach A., Lievens M., Ofori-Anyinam O., Vekemans J., Carter T., Leboulleux D., Loucq C., Radford A., Savarese B., Schellenberg D., Sillman M., Vansadia P. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011;365(20):1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- Amadi B., Mwiya M., Sianongo S., Payne L., Watuka A., Katubulushi M., Kelly P. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect. Dis. 2009;9:195. doi: 10.1186/1471-2334-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaravadi R.K., Lippincott-Schwartz J., Yin X.M., Weiss W.A., Takebe N., Timmer W., DiPaola R.S., Lotze M.T., White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 2011;17(4):654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews K.T., Haque A., Jones M.K. HDAC inhibitors in parasitic diseases. Immunol. Cell Biol. 2012;90(1):66–77. doi: 10.1038/icb.2011.97. [DOI] [PubMed] [Google Scholar]
- Andrews K.T., Tran T.N., Fairlie D.P. Towards histone deacetylase inhibitors as new antimalarial drugs. Curr. Pharm. Des. 2012;18(24):3467–3479. [PubMed] [Google Scholar]
- Andrews K.T., Fisher G.M., Sumanadasa S.D., Skinner-Adams T., Moeker J., Lopez M., Poulsen S.A. Antimalarial activity of compounds comprising a primary benzene sulfonamide fragment. Bioorg. Med. Chem. Lett. 2013;23(22):6114–6117. doi: 10.1016/j.bmcl.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Anthony M.P., Burrows J.N., Duparc S., Moehrle J.J., Wells T.N. The global pipeline of new medicines for the control and elimination of malaria. Malar. J. 2012;11:316. doi: 10.1186/1475-2875-11-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F.G., Huskinson J., Remington J.S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob. Agents Chemother. 1991;35(2):293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariey F., Witkowski B., Amaratunga C., Beghain J., Langlois A.C., Khim N., Kim S., Duru V., Bouchier C., Ma L., Lim P., Leang R., Duong S., Sreng S., Suon S., Chuor C.M., Bout D.M., Menard S., Rogers W.O., Genton B., Fandeur T., Miotto O., Ringwald P., Le Bras J., Berry A., Barale J.C., Fairhurst R.M., Benoit-Vical F., Mercereau-Puijalon O., Menard D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Disc. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Baggish A.L., Hill D.R. Antiparasitic agent atovaquone. Antimicrob. Agents Chemother. 2002;46(5):1163–1173. doi: 10.1128/AAC.46.5.1163-1173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., de Koning H.P., Maser P., Horn D. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol. 2013;29(3):110–118. doi: 10.1016/j.pt.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltzan M., Mehta S., Kirkham T.H., Cosio M.G. Randomized trial of prolonged chloroquine therapy in advanced pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 1999;160(1):192–197. doi: 10.1164/ajrccm.160.1.9809024. [DOI] [PubMed] [Google Scholar]
- Baril L., Ancelle T., Goulet V., Thulliez P., Tirard-Fleury V., Carme B. Risk factors for Toxoplasma infection in pregnancy: a case-control study in France. Scand. J. Infect. Dis. 1999;31(3):305–309. doi: 10.1080/00365549950163626. [DOI] [PubMed] [Google Scholar]
- Barrett M.P., Vincent I.M., Burchmore R.J., Kazibwe A.J., Matovu E. Drug resistance in human African trypanosomiasis. Future Microbiol. 2011;6(9):1037–1047. doi: 10.2217/fmb.11.88. [DOI] [PubMed] [Google Scholar]
- Barrocas A.M., Cymet T. Cinchonism in a patient taking Quinine for leg cramps. Compr. Ther. 2007;33(3):162–163. doi: 10.1007/s12019-007-0013-1. [DOI] [PubMed] [Google Scholar]
- Basnuevo J.G., Gutierrez Estarli E. Four cases of amebic liver abscess cured with chloroquine. Rev. Kuba Med. Trop. Parasitol. 1951;7(1–2):19–20. [PubMed] [Google Scholar]
- Beckmann S., Leutner S., Gouignard N., Dissous C., Grevelding C.G. Protein kinases as potential targets for novel anti-schistosomal strategies. Curr. Pharm. Des. 2012;18(24):3579–3594. [PubMed] [Google Scholar]
- Ben Salah A., Ben Messaoud N., Guedri E., Zaatour A., Ben Alaya N., Bettaieb J., Gharbi A., Belhadj Hamida N., Boukthir A., Chlif S., Abdelhamid K., El Ahmadi Z., Louzir H., Mokni M., Morizot G., Buffet P., Smith P.L., Kopydlowski K.M., Kreishman-Deitrick M., Smith K.S., Nielsen C.J., Ullman D.R., Norwood J.A., Thorne G.D., McCarthy W.F., Adams R.C., Rice R.M., Tang D., Berman J., Ransom J., Magill A.J., Grogl M. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N. Engl. J. Med. 2013;368(6):524–532. doi: 10.1056/NEJMoa1202657. [DOI] [PubMed] [Google Scholar]
- Bento A.P., Gaulton A., Hersey A., Bellis L.J., Chambers J., Davies M., Kruger F.A., Light Y., Mak L., McGlinchey S., Nowotka M., Papadatos G., Santos R., Overington J.P. The ChEMBL bioactivity database: an update. Nucleic Acids Res. 2014;42(1):D1083–D1090. doi: 10.1093/nar/gkt1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi I., Kivity S., Langevitz P., Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin. Rev. Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J.J. Treatment of leishmaniasis with miltefosine: 2008 status. Expert Opin. Drug Metab. Toxicol. 2008;4(9):1209–1216. doi: 10.1517/17425255.4.9.1209. [DOI] [PubMed] [Google Scholar]
- Berman J.D., Badaro R., Thakur C.P., Wasunna K.M., Behbehani K., Davidson R., Kuzoe F., Pang L., Weerasuriya K., Bryceson A.D. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull. World Health Organ. 1998;76(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- Bernhard S.C., Nerima B., Maser P., Brun R. Melarsoprol- and pentamidine-resistant Trypanosoma brucei rhodesiense populations and their cross-resistance. Int. J. Parasitol. 2007;37(13):1443–1448. doi: 10.1016/j.ijpara.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Bessoff K., Sateriale A., Lee K.K., Huston C.D. Drug repurposing screen reveals FDA-approved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth. Antimicrob. Agents Chemother. 2013;57(4):1804–1814. doi: 10.1128/AAC.02460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M.R., O’Neill A.I., Buchanan R.R., Ibrahim K.M., Des Parkin J. Lupus anticoagulant in the elderly may be associated with both quinine and quinidine usage. Pathology. 1995;27(2):136–139. doi: 10.1080/00313029500169742. [DOI] [PubMed] [Google Scholar]
- Bland N.D., Wang C., Tallman C., Gustafson A.E., Wang Z., Ashton T.D., Ochiana S.O., McAllister G., Cotter K., Fang A.P., Gechijian L., Garceau N., Gangurde R., Ortenberg R., Ondrechen M.J., Campbell R.K., Pollastri M.P. Pharmacological validation of Trypanosoma brucei phosphodiesterases B1 and B2 as druggable targets for African sleeping sickness. J. Med. Chem. 2011;54(23):8188–8194. doi: 10.1021/jm201148s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton E., Wang Y., Thiessen P.A., Bryant S.H. PubChem: integrated platform of small molecules and biological activities. Annu. Rep. Comput. Chem. 2008;4 (Chapter 12) [Google Scholar]
- British Medical Journal Sulphadiazine. Br. Med. J. 1942;1(4228):76–77. [PMC free article] [PubMed] [Google Scholar]
- British Medical Journal TOXOPLASMIC uveitis and pyrimethamine (author not listed) Br. Med. J. 1957;2(5052):1042–1044. [PMC free article] [PubMed] [Google Scholar]
- Brouwers J., Vermeire K., Schols D., Augustijns P. Development and in vitro evaluation of chloroquine gels as microbicides against HIV-1 infection. Virology. 2008;378(2):306–310. doi: 10.1016/j.virol.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun R., Schumacher R., Schmid C., Kunz C., Burri C. The phenomenon of treatment failures in Human African Trypanosomiasis. Trop. Med. Int. Health. 2001;6(11):906–914. doi: 10.1046/j.1365-3156.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- Brun R., Don R., Jacobs R.T., Wang M.Z., Barrett M.P. Development of novel drugs for human African trypanosomiasis. Future Microbiol. 2011;6(6):677–691. doi: 10.2217/fmb.11.44. [DOI] [PubMed] [Google Scholar]
- Burri C., Brun R. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 2003;90(Supp 1):S49–S52. doi: 10.1007/s00436-002-0766-5. [DOI] [PubMed] [Google Scholar]
- Burri C., Keiser J. Pharmacokinetic investigations in patients from northern Angola refractory to melarsoprol treatment. Trop. Med. Int. Health. 2001;6(5):412–420. doi: 10.1046/j.1365-3156.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- Burrows J.N., van Huijsduijnen R.H., Mohrle J.J., Oeuvray C., Wells T.N. Designing the next generation of medicines for malaria control and eradication. Malar. J. 2013;12:187. doi: 10.1186/1475-2875-12-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canete R., Escobedo A.A., Almirall P., Gonzalez M.E., Brito K., Cimerman S. Mebendazole in parasitic infections other than those caused by soil-transmitted helminths. Trans. R. Soc. Trop. Med. Hyg. 2009;103(5):437–442. doi: 10.1016/j.trstmh.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Casero R.A., Jr., Woster P.M. Recent advances in the development of polyamine analogues as antitumor agents. J. Med. Chem. 2009;52(15):4551–4573. doi: 10.1021/jm900187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain E., Ioset J.R. Drug discovery and development for neglected diseases: the DNDi model. Drug Des. Dev. Ther. 2011;5:175–181. doi: 10.2147/DDDT.S16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchi F., Filipe J.A., Haydon D.T., Chandramohan D., Chappuis F. Estimates of the duration of the early and late stage of gambiense sleeping sickness. BMC Infect. Dis. 2008;8:16. doi: 10.1186/1471-2334-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.M., Keithly J.S., Paya C.V., LaRusso N.F. Cryptosporidiosis. N. Engl. J. Med. 2002;346(22):1723–1731. doi: 10.1056/NEJMra013170. [DOI] [PubMed] [Google Scholar]
- Chong C.R., Chen X., Shi L., Liu J.O., Sullivan D.J., Jr. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat. Chem. Biol. 2006;2(8):415–416. doi: 10.1038/nchembio806. [DOI] [PubMed] [Google Scholar]
- Christian G.L., Krueger G.G. Clindamycin vs placebo as adjunctive therapy in moderately severe acne. Arch. Dermatol. 1975;111(8):997–1000. [PubMed] [Google Scholar]
- Clyde D.F., Miller R.M., DuPont H.L., Hornick R.B. Antimalarial effects of tetracyclines in man. J. Trop. Med. Hyg. 1971;74(11):238–242. [PubMed] [Google Scholar]
- Cohen H.G., Reynolds T.B. Comparison of metronidazole and chloroquine for the treatment of amoebic liver abscess. Control. Trial Gastroenterol. 1975;69(1):35–41. [PubMed] [Google Scholar]
- Coloviras G.J., West W.T., Armour J.C. The local treatment of burns with sulfadiazine spray. Can. Med. Assoc. J. 1942;47(6):505–514. [PMC free article] [PubMed] [Google Scholar]
- Cook A.J., Gilbert R.E., Buffolano W., Zufferey J., Petersen E., Jenum P.A., Foulon W., Semprini A.E., Dunn D.T. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. European research network on congenital toxoplasmosis. BMJ. 2000;321(7254):142–147. doi: 10.1136/bmj.321.7254.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura J.R., Vinas P.A. Chagas disease: a new worldwide challenge. Nature. 2010;465(7301):S6–S7. doi: 10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- Coura J.R., de Abreu L.L., Willcox H.P., Petana W. Comparative controlled study on the use of benznidazole, nifurtimox and placebo, in the chronic form of Chagas’ disease, in a field area with interrupted transmission. I. Preliminary evaluation. Rev. Soc. Bras. Med. Trop. 1997;30(2):139–144. doi: 10.1590/s0037-86821997000200009. [DOI] [PubMed] [Google Scholar]
- Couvreur J., Desmonts G., Thulliez P. Prophylaxis of congenital toxoplasmosis. Effects of spiramycin on placental infection. J. Antimicrob. Chemother. 1988;22(Suppl. B):193–200. doi: 10.1093/jac/22.supplement_b.193. [DOI] [PubMed] [Google Scholar]
- Croft S.L., Coombs G.H. Leishmaniasis – current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19(11):502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Croft S.L., Seifert K., Duchene M. Antiprotozoal activities of phospholipid analogues. Mol. Biochem. Parasitol. 2003;126(2):165–172. doi: 10.1016/s0166-6851(02)00283-9. [DOI] [PubMed] [Google Scholar]
- da Cruz F.P., Martin C., Buchholz K., Lafuente-Monasterio M.J., Rodrigues T., Sonnichsen B., Moreira R., Gamo F.J., Marti M., Mota M.M., Hannus M., Prudencio M. Drug screen targeted at plasmodium liver stages identifies a potent multistage antimalarial drug. J. Infect. Dis. 2012;205(8):1278–1286. doi: 10.1093/infdis/jis184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannemann B., McCutchan J.A., Israelski D., Antoniskis D., Leport C., Luft B., Nussbaum J., Clumeck N., Morlat P., Chiu J. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann. Intern. Med. 1992;116(1):33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- Davidson R.N., den Boer M., Ritmeijer K. Paromomycin. Trans. R. Soc. Trop. Med. Hyg. 2009;103(7):653–660. doi: 10.1016/j.trstmh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- de Koning H.P., Gould M.K., Sterk G.J., Tenor H., Kunz S., Luginbuehl E., Seebeck T. Pharmacological validation of Trypanosoma brucei phosphodiesterases as novel drug targets. J. Infect. Dis. 2012;206(2):229–237. doi: 10.1093/infdis/jir857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath A., Parsonage D., Andrade R.M., He C., Cobo E.R., Hirata K., Chen S., Garcia-Rivera G., Orozco E., Martinez M.B., Gunatilleke S.S., Barrios A.M., Arkin M.R., Poole L.B., McKerrow J.H., Reed S.L. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 2012;18(6):956–960. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire E.R., Prudencio M., Mota M.M., Clardy J. Liver-stage malaria parasites vulnerable to diverse chemical scaffolds. Proc. Natl. Acad. Sci. USA. 2012;109(22):8511–8516. doi: 10.1073/pnas.1118370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descotes J., Vial T., Delattre D., Evreux J.C. Spiramycin: safety in man. J. Antimicrob. Chemother. 1988;22(Suppl. B):207–210. doi: 10.1093/jac/22.supplement_b.207. [DOI] [PubMed] [Google Scholar]
- Desmonts G., Couvreur J. Congenital toxoplasmosis. A prospective study of 378 pregnancies. N. Engl. J. Med. 1974;290(20):1110–1116. doi: 10.1056/NEJM197405162902003. [DOI] [PubMed] [Google Scholar]
- Desmonts G., Couvreur J. Toxoplasmosis in pregnancy and its transmission to the fetus. Bull. N.Y. Acad. Med. 1974;50(2):146–159. [PMC free article] [PubMed] [Google Scholar]
- Di Santo N., Ehrisman J. Research perspective: potential role of nitazoxanide in ovarian cancer treatment. Old drug, new purpose? Cancers (Basel) 2013;5(3):1163–1176. doi: 10.3390/cancers5031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doberstyn E.B., Hall A.P., Vetvutanapibul K., Sonkon P. Single-dose therapy of Falciparum malaria using pyrimethamine in combination with diformyldapsone or sulfadoxine. Am. J. Trop. Med. Hyg. 1976;25(1):14–19. doi: 10.4269/ajtmh.1976.25.14. [DOI] [PubMed] [Google Scholar]
- Dondorp A.M., Ringwald P. Artemisinin resistance is a clear and present danger. Trends Parasitol. 2013;29(8):359–360. doi: 10.1016/j.pt.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Dondorp A.M., Nosten F., Yi P., Das D., Phyo A.P., Tarning J., Lwin K.M., Ariey F., Hanpithakpong W., Lee S.J., Ringwald P., Silamut K., Imwong M., Chotivanich K., Lim P., Herdman T., An S.S., Yeung S., Singhasivanon P., Day N.P.J., Lindegardh N., Socheat D., White N.J. Artemisinin resistance in plasmodium falciparum malaria. N. Engl. J. Med. 2009;361(5):455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorlo T.P., Balasegaram M., Beijnen J.H., de Vries P.J. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012;67(11):2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- Efferth T., Romero M.R., Wolf D.G., Stamminger T., Marin J.J., Marschall M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008;47(6):804–811. doi: 10.1086/591195. [DOI] [PubMed] [Google Scholar]
- Ekins S., Mestres J., Testa B. In silico pharmacology for drug discovery: methods for virtual ligand screening and profiling. Br. J. Pharmacol. 2007;152(1):9–20. doi: 10.1038/sj.bjp.0707305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazar M., Liu M., McKenna S.A., Liu P., Gehrig E.A., Puglisi J.D., Rossignol J.F., Glenn J.S. The anti-hepatitis C agent nitazoxanide induces phosphorylation of eukaryotic initiation factor 2alpha via protein kinase activated by double-stranded RNA activation. Gastroenterology. 2009;137(5):1827–1835. doi: 10.1053/j.gastro.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Engel J.C., Ang K.K., Chen S., Arkin M.R., McKerrow J.H., Doyle P.S. Image-based high-throughput drug screening targeting the intracellular stage of Trypanosoma cruzi, the agent of Chagas’ disease. Antimicrob. Agents Chemother. 2010;54(8):3326–3334. doi: 10.1128/AAC.01777-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan-Minogue H., Bodapati S., Solow-Cordero D., Fan A., Paulmurugan R., Massoud T.F., Felsher D.W., Gambhir S.S. A c-Myc activation sensor-based high-throughput drug screening identifies an antineoplastic effect of nitazoxanide. Mol. Cancer Ther. 2013;12(9):1896–1905. doi: 10.1158/1535-7163.MCT-12-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farthing C., Rendel M., Currie B., Seidlin M. Azithromycin for cerebral toxoplasmosis. Lancet. 1992;339(8790):437–438. doi: 10.1016/0140-6736(92)90132-m. [DOI] [PubMed] [Google Scholar]
- Flegr J., Havlicek J., Kodym P., Maly M., Smahel Z. Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study. BMC Infect. Dis. 2002;2:11. doi: 10.1186/1471-2334-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegr J., Klose J., Novotna M., Berenreitterova M., Havlicek J. Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect. Dis. 2009;9:72. doi: 10.1186/1471-2334-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedheim E.A. Mel B in the treatment of human trypanosomiasis. Am. J. Trop. Med. Hyg. 1949;29(2):173–180. doi: 10.4269/ajtmh.1949.s1-29.173. [DOI] [PubMed] [Google Scholar]
- Gamo F.J., Sanz L.M., Vidal J., de Cozar C., Alvarez E., Lavandera J.L., Vanderwall D.E., Green D.V., Kumar V., Hasan S., Brown J.R., Peishoff C.E., Cardon L.R., Garcia-Bustos J.F. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465(7296):305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- Gascon J., Bern C., Pinazo M.J. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115(1–2):22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Georgiev V.S. Management of toxoplasmosis. Drugs. 1994;48(2):179–188. doi: 10.2165/00003495-199448020-00005. [DOI] [PubMed] [Google Scholar]
- Gleckman R., Alvarez S., Joubert D.W. Drug therapy reviews: trimethoprim–sulfamethoxazole. Am. J. Hosp. Pharm. 1979;36(7):893–906. [PubMed] [Google Scholar]
- Goodhew E.B., Secor W.E. Drug library screening against metronidazole-sensitive and metronidazole-resistant Trichomonas vaginalis isolates. Sex. Transm. Infect. 2013;89(6):479–484. doi: 10.1136/sextrans-2013-051032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grab D.J., Kennedy P.G. Traversal of human and animal trypanosomes across the blood–brain barrier. J. Neurovirol. 2008;14(5):344–351. doi: 10.1080/13550280802282934. [DOI] [PubMed] [Google Scholar]
- Guerina N.G., Hsu H.W., Meissner H.C., Maguire J.H., Lynfield R., Stechenberg B., Abroms I., Pasternack M.S., Hoff R., Eaton R.B. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The new England regional Toxoplasma working group. N. Engl. J. Med. 1994;330(26):1858–1863. doi: 10.1056/NEJM199406303302604. [DOI] [PubMed] [Google Scholar]
- Guerrant D.I., Moore S.R., Lima A.A., Patrick P.D., Schorling J.B., Guerrant R.L. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four–seven years later in a poor urban community in northeast Brazil. Am. J. Trop. Med. Hyg. 1999;61(5):707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- Guzel-Akdemir O., Akdemir A., Pan P., Vermelho A.B., Parkkila S., Scozzafava A., Capasso C., Supuran C.T. A class of sulfonamides with strong inhibitory action against the alpha-carbonic anhydrase from Trypanosoma cruzi. J. Med. Chem. 2013 doi: 10.1021/jm400418p. [DOI] [PubMed] [Google Scholar]
- Hickman J.G., Huber F., Palmisano M. Human dermal safety studies with eflornithine HCl 13.9% cream (Vaniqa), a novel treatment for excessive facial hair. Curr. Med. Res. Opin. 2001;16(4):235–244. doi: 10.1185/030079901750176735. [DOI] [PubMed] [Google Scholar]
- Hotez P. Enlarging the “Audacious Goal”: elimination of the world’s high prevalence neglected tropical diseases. Vaccine. 2011;29(Suppl. 4):D104–D110. doi: 10.1016/j.vaccine.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Howes R.E., Piel F.B., Patil A.P., Nyangiri O.A., Gething P.W., Dewi M., Hogg M.M., Battle K.E., Padilla C.D., Baird J.K., Hay S.I. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9(11):e1001339. doi: 10.1371/journal.pmed.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E., Dul E., Sung C.M., Chen Z., Kirkpatrick R., Zhang G.F., Johanson K., Liu R., Lago A., Hofmann G., Macarron R., de los Frailes M., Perez P., Krawiec J., Winkler J., Jaye M. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J. Pharmacol. Exp. Ther. 2003;307(2):720–728. doi: 10.1124/jpet.103.055541. [DOI] [PubMed] [Google Scholar]
- Huang R., Southall N., Wang Y., Yasgar A., Shinn P., Jadhav A., Nguyen D.T., Austin C.P. The NCGC pharmaceutical collection: a comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci. Transl. Med. 2011;3(80):80ps16. doi: 10.1126/scitranslmed.3001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iten M., Mett H., Evans A., Enyaru J.C., Brun R., Kaminsky R. Alterations in ornithine decarboxylase characteristics account for tolerance of Trypanosoma brucei rhodesiense to d,l-alpha-difluoromethylornithine. Antimicrob. Agents Chemother. 1997;41(9):1922–1925. doi: 10.1128/aac.41.9.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens A.C., Peacock C.S., Worthey E.A., Murphy L., Aggarwal G., Berriman M., Sisk E., Rajandream M.A., Adlem E., Aert R., Anupama A., Apostolou Z., Attipoe P., Bason N., Bauser C., Beck A., Beverley S.M., Bianchettin G., Borzym K., Bothe G., Bruschi C.V., Collins M., Cadag E., Ciarloni L., Clayton C., Coulson R.M., Cronin A., Cruz A.K., Davies R.M., De Gaudenzi J., Dobson D.E., Duesterhoeft A., Fazelina G., Fosker N., Frasch A.C., Fraser A., Fuchs M., Gabel C., Goble A., Goffeau A., Harris D., Hertz-Fowler C., Hilbert H., Horn D., Huang Y., Klages S., Knights A., Kube M., Larke N., Litvin L., Lord A., Louie T., Marra M., Masuy D., Matthews K., Michaeli S., Mottram J.C., Muller-Auer S., Munden H., Nelson S., Norbertczak H., Oliver K., O’Neil S., Pentony M., Pohl T.M., Price C., Purnelle B., Quail M.A., Rabbinowitsch E., Reinhardt R., Rieger M., Rinta J., Robben J., Robertson L., Ruiz J.C., Rutter S., Saunders D., Schafer M., Schein J., Schwartz D.C., Seeger K., Seyler A., Sharp S., Shin H., Sivam D., Squares R., Squares S., Tosato V., Vogt C., Volckaert G., Wambutt R., Warren T., Wedler H., Woodward J., Zhou S., Zimmermann W., Smith D.F., Blackwell J.M., Stuart K.D., Barrell B., Myler P.J. The genome of the kinetoplastid parasite. Leishmania Major Sci. 2005;309(5733):436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Y., Alirol E., Getaz L., Wolff H., Combescure C., Chappuis F. Tolerance and safety of nifurtimox in patients with chronic chagas disease. Clin. Infect. Dis. 2010;51(10):e69–e75. doi: 10.1086/656917. [DOI] [PubMed] [Google Scholar]
- Jakobsen P.H., Wang M.W., Nwaka S. Innovative partnerships for drug discovery against neglected diseases. PLoS Negl. Trop. Dis. 2011;5(9):e1221. doi: 10.1371/journal.pntd.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.J., Avery V.M. Whole-organism high-throughput screening against Trypanosoma brucei brucei. Expert Opin. Drug Discov. 2013;8(5):495–507. doi: 10.1517/17460441.2013.783816. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Jenum P.A., Stray-Pedersen B., Melby K.K., Eskild A., Eng J. Risk factors for Toxoplasma gondii infection in pregnancy. Results of a prospective case-control study in Norway. Am. J. Epidemiol. 1996;144(4):405–412. doi: 10.1093/oxfordjournals.aje.a008942. [DOI] [PubMed] [Google Scholar]
- Katiyar S., Kufareva I., Behera R., Thomas S.M., Ogata Y., Pollastri M., Abagyan R., Mensa-Wilmot K. Lapatinib-binding protein kinases in the African trypanosome: identification of cellular targets for kinase-directed chemical scaffolds. PLoS ONE. 2013;8(2):e56150. doi: 10.1371/journal.pone.0056150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellina O.I. A study of experimental cutaneous leishmaniasis in white mice. Med. Parazitol. (Mosk). 1961;30:684–691. [PubMed] [Google Scholar]
- Kelly J.M., Taylor M.C., Horn D., Loza E., Kalvinsh I., Bjorkling F. Inhibitors of human histone deacetylase with potent activity against the African trypanosome Trypanosoma brucei. Bioorg. Med. Chem. Lett. 2012;22(5):1886–1890. doi: 10.1016/j.bmcl.2012.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P.G. The continuing problem of human African trypanosomiasis (sleeping sickness) Ann. Neurol. 2008;64(2):116–126. doi: 10.1002/ana.21429. [DOI] [PubMed] [Google Scholar]
- Kennedy P.G. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness) Lancet Neurol. 2013;12(2):186–194. doi: 10.1016/S1474-4422(12)70296-X. [DOI] [PubMed] [Google Scholar]
- Klayman D.L. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228(4703):1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., Faruque A.S., Zaidi A.K., Saha D., Alonso P.L., Tamboura B., Sanogo D., Onwuchekwa U., Manna B., Ramamurthy T., Kanungo S., Ochieng J.B., Omore R., Oundo J.O., Hossain A., Das S.K., Ahmed S., Qureshi S., Quadri F., Adegbola R.A., Antonio M., Hossain M.J., Akinsola A., Mandomando I., Nhampossa T., Acacio S., Biswas K., O’Reilly C.E., Mintz E.D., Berkeley L.Y., Muhsen K., Sommerfelt H., Robins-Browne R.M., Levine M.M. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Krishna S., Bustamante L., Haynes R.K., Staines H.M. Artemisinins: their growing importance in medicine. Trends Pharmacol. Sci. 2008;29(10):520–527. doi: 10.1016/j.tips.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krungkrai J., Scozzafava A., Reungprapavut S., Krungkrai S.R., Rattanajak R., Kamchonwongpaisan S., Supuran C.T. Carbonic anhydrase inhibitors. Inhibition of Plasmodium falciparum carbonic anhydrase with aromatic sulfonamides: towards antimalarials with a novel mechanism of action? Bioorg. Med. Chem. 2005;13(2):483–489. doi: 10.1016/j.bmc.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Krungkrai J., Krungkrai S.R., Supuran C.T. Carbonic anhydrase inhibitors: inhibition of Plasmodium falciparum carbonic anhydrase with aromatic/heterocyclic sulfonamides-in vitro and in vivo studies. Bioorg. Med. Chem. Lett. 2008;18(20):5466–5471. doi: 10.1016/j.bmcl.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Lee B.Y., Bacon K.M., Bottazzi M.E., Hotez P.J. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect. Dis. 2013;13(4):342–348. doi: 10.1016/S1473-3099(13)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leport C., Raffi F., Matheron S., Katlama C., Regnier B., Saimot A.G., Marche C., Vedrenne C., Vilde J.L. Treatment of central nervous system toxoplasmosis with pyrimethamine/sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome. Efficacy of long-term continuous therapy. Am. J. Med. 1988;84(1):94–100. doi: 10.1016/0002-9343(88)90014-9. [DOI] [PubMed] [Google Scholar]
- Lira R., Sundar S., Makharia A., Kenney R., Gam A., Saraiva E., Sacks D. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 1999;180(2):564–567. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]
- Lourie E., Yorke W. Studies in chemotherapy. XVI. The trypanocidal action of synthalin. Ann. Trop. Med. Parasitol. 1937;31:435–445. [Google Scholar]
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Barker-Collo S., Bartels D.H., Bell M.L., Benjamin E.J., Bennett D., Bhalla K., Bikbov B., Bin Abdulhak A., Birbeck G., Blyth F., Bolliger I., Boufous S., Bucello C., Burch M., Burney P., Carapetis J., Chen H., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., de Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahodwala N., De Leo D., Degenhardt L., Delossantos A., Denenberg J., Des Jarlais D.C., Dharmaratne S.D., Dorsey E.R., Driscoll T., Duber H., Ebel B., Erwin P.J., Espindola P., Ezzati M., Feigin V., Flaxman A.D., Forouzanfar M.H., Fowkes F.G., Franklin R., Fransen M., Freeman M.K., Gabriel S.E., Gakidou E., Gaspari F., Gillum R.F., Gonzalez-Medina D., Halasa Y.A., Haring D., Harrison J.E., Havmoeller R., Hay R.J., Hoen B., Hotez P.J., Hoy D., Jacobsen K.H., James S.L., Jasrasaria R., Jayaraman S., Johns N., Karthikeyan G., Kassebaum N., Keren A., Khoo J.P., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Lipnick M., Lipshultz S.E., Ohno S.L., Mabweijano J., MacIntyre M.F., Mallinger L., March L., Marks G.B., Marks R., Matsumori A., Matzopoulos R., Mayosi B.M., McAnulty J.H., McDermott M.M., McGrath J., Mensah G.A., Merriman T.R., Michaud C., Miller M., Miller T.R., Mock C., Mocumbi A.O., Mokdad A.A., Moran A., Mulholland K., Nair M.N., Naldi L., Narayan K.M., Nasseri K., Norman P., O’Donnell M., Omer S.B., Ortblad K., Osborne R., Ozgediz D., Pahari B., Pandian J.D., Rivero A.P., Padilla R.P., Perez-Ruiz F., Perico N., Phillips D., Pierce K., Pope C.A., 3rd, Porrini E., Pourmalek F., Raju M., Ranganathan D., Rehm J.T., Rein D.B., Remuzzi G., Rivara F.P., Roberts T., De Leon F.R., Rosenfeld L.C., Rushton L., Sacco R.L., Salomon J.A., Sampson U., Sanman E., Schwebel D.C., Segui-Gomez M., Shepard D.S., Singh D., Singleton J., Sliwa K., Smith E., Steer A., Taylor J.A., Thomas B., Tleyjeh I.M., Towbin J.A., Truelsen T., Undurraga E.A., Venketasubramanian N., Vijayakumar L., Vos T., Wagner G.R., Wang M., Wang W., Watt K., Weinstock M.A., Weintraub R., Wilkinson J.D., Woolf A.D., Wulf S., Yeh P.H., Yip P., Zabetian A., Zheng Z.J., Lopez A.D., Murray C.J., AlMazroa M.A., Memish Z.A. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucet I.S., Tobin A., Drewry D., Wilks A.F., Doerig C. Plasmodium kinases as targets for new-generation antimalarials. Future Med. Chem. 2012;4(18):2295–2310. doi: 10.4155/fmc.12.183. [DOI] [PubMed] [Google Scholar]
- Luft B.J., Remington J.S. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 1992;15(2):211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- Lutje V., Seixas J., Kennedy A. Chemotherapy for second-stage human African trypanosomiasis. Cochrane Database Syst. Rev. 2013;6:CD006201. doi: 10.1002/14651858.CD006201.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarouf M., Lawrence F., Brown S., Robert-Gero M. Biochemical alterations in paromomycin-treated Leishmania donovani promastigotes. Parasitol. Res. 1997;83(2):198–202. doi: 10.1007/s004360050232. [DOI] [PubMed] [Google Scholar]
- Mackey Z.B., Baca A.M., Mallari J.P., Apsel B., Shelat A., Hansell E.J., Chiang P.K., Wolff B., Guy K.R., Williams J., McKerrow J.H. Discovery of trypanocidal compounds by whole cell HTS of Trypanosoma brucei. Chem. Biol. Drug Des. 2006;67(5):355–363. doi: 10.1111/j.1747-0285.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- Magarinos M.P., Carmona S.J., Crowther G.J., Ralph S.A., Roos D.S., Shanmugam D., Van Voorhis W.C., Aguero F. TDR Targets: a chemogenomics resource for neglected diseases. Nucleic Acids Res. 2012;40(Database issue):D1118–D1127. doi: 10.1093/nar/gkr1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyando C., Njunju E.M., D’Alessandro U., Van Geertruyden J.P. Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS ONE. 2013;8(2):e56916. doi: 10.1371/journal.pone.0056916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx A., Le Roch K., Nivez M.P., Dorin D., Alano P., Gutierrez G.J., Nebreda A.R., Goldring D., Whittle C., Patterson S., Chakrabarti D., Doerig C. Identification and initial characterization of three novel cyclin-related proteins of the human malaria parasite Plasmodium falciparum. J. Biol. Chem. 2003;278(41):39839–39850. doi: 10.1074/jbc.M301625200. [DOI] [PubMed] [Google Scholar]
- Mesquita J.T., Tempone A.G., Reimao J.Q. Combination therapy with nitazoxanide and amphotericin B, Glucantime, miltefosine and sitamaquine against Leishmania (Leishmania) infantum intracellular amastigotes. Acta Trop. 2013 doi: 10.1016/j.actatropica.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Meyerhoff A. U.S. Food and Drug Administration approval of Am Bisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin. Infect. Dis. 1999;28(1):42–48. doi: 10.1086/515085. discussion 49–51. [DOI] [PubMed] [Google Scholar]
- Miller L.H., Glew R.H., Wyler D.J., Howard W.A., Collins W.E., Contacos P.G., Neva F.A. Evaluation of clindamycin in combination with quinine against multidrug-resistant strains of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1974;23(4):565–569. doi: 10.4269/ajtmh.1974.23.565. [DOI] [PubMed] [Google Scholar]
- Mishina Y.V., Krishna S., Haynes R.K., Meade J.C. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrob. Agents Chemother. 2007;51(5):1852–1854. doi: 10.1128/AAC.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens F., De Wilde M., Ngato K. Clinical trial of nifurtimox in human African trypanosomiasis. Ann. Soc. Belg. Med. Trop. 1984;64(1):37–43. [PubMed] [Google Scholar]
- Montoya J.G., Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Mullard A. Could pharma open its drug freezers? Nat. Rev. Drug Discov. 2011;10(6):399–400. doi: 10.1038/nrd3473. [DOI] [PubMed] [Google Scholar]
- Mullard A. Drug repurposing programmes get lift off. Nat. Rev. Drug Discov. 2012;11(7):505–506. doi: 10.1038/nrd3776. [DOI] [PubMed] [Google Scholar]
- Muller J., Naguleswaran A., Muller N., Hemphill A. Neospora caninum: functional inhibition of protein disulfide isomerase by the broad-spectrum anti-parasitic drug nitazoxanide and other thiazolides. Exp. Parasitol. 2008;118(1):80–88. doi: 10.1016/j.exppara.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Muller J., Sidler D., Nachbur U., Wastling J., Brunner T., Hemphill A. Thiazolides inhibit growth and induce glutathione-S-transferase Pi (GSTP1)-dependent cell death in human colon cancer cells. Int. J. Cancer. 2008;123(8):1797–1806. doi: 10.1002/ijc.23755. [DOI] [PubMed] [Google Scholar]
- Munoz C., Zulantay I., Apt W., Ortiz S., Schijman A.G., Bisio M., Ferrada V., Herrera C., Martinez G., Solari A. Evaluation of nifurtimox treatment of chronic chagas disease by means of several parasitological methods. Antimicrob. Agents Chemother. 2013;57(9):4518–4523. doi: 10.1128/AAC.00227-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Lisnevskaia L., Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet. 2013;382(9894):809–818. doi: 10.1016/S0140-6736(13)60889-2. [DOI] [PubMed] [Google Scholar]
- Murray C.J., Rosenfeld L.C., Lim S.S., Andrews K.G., Foreman K.J., Haring D., Fullman N., Naghavi M., Lozano R., Lopez A.D. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379(9814):413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- Murray C.J., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V., Abraham J., Ackerman I., Aggarwal R., Ahn S.Y., Ali M.K., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Bahalim A.N., Barker-Collo S., Barrero L.H., Bartels D.H., Basanez M.G., Baxter A., Bell M.L., Benjamin E.J., Bennett D., Bernabe E., Bhalla K., Bhandari B., Bikbov B., Bin Abdulhak A., Birbeck G., Black J.A., Blencowe H., Blore J.D., Blyth F., Bolliger I., Bonaventure A., Boufous S., Bourne R., Boussinesq M., Braithwaite T., Brayne C., Bridgett L., Brooker S., Brooks P., Brugha T.S., Bryan-Hancock C., Bucello C., Buchbinder R., Buckle G., Budke C.M., Burch M., Burney P., Burstein R., Calabria B., Campbell B., Canter C.E., Carabin H., Carapetis J., Carmona L., Cella C., Charlson F., Chen H., Cheng A.T., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., de Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahiya M., Dahodwala N., Damsere-Derry J., Danaei G., Davis A., De Leo D., Degenhardt L., Dellavalle R., Delossantos A., Denenberg J., Derrett S., Des Jarlais D.C., Dharmaratne S.D., Dherani M., Diaz-Torne C., Dolk H., Dorsey E.R., Driscoll T., Duber H., Ebel B., Edmond K., Elbaz A., Ali S.E., Erskine H., Erwin P.J., Espindola P., Ewoigbokhan S.E., Farzadfar F., Feigin V., Felson D.T., Ferrari A., Ferri C.P., Fevre E.M., Finucane M.M., Flaxman S., Flood L., Foreman K., Forouzanfar M.H., Fowkes F.G., Fransen M., Freeman M.K., Gabbe B.J., Gabriel S.E., Gakidou E., Ganatra H.A., Garcia B., Gaspari F., Gillum R.F., Gmel G., Gonzalez-Medina D., Gosselin R., Grainger R., Grant B., Groeger J., Guillemin F., Gunnell D., Gupta R., Haagsma J., Hagan H., Halasa Y.A., Hall W., Haring D., Haro J.M., Harrison J.E., Havmoeller R., Hay R.J., Higashi H., Hill C., Hoen B., Hoffman H., Hotez P.J., Hoy D., Huang J.J., Ibeanusi S.E., Jacobsen K.H., James S.L., Jarvis D., Jasrasaria R., Jayaraman S., Johns N., Jonas J.B., Karthikeyan G., Kassebaum N., Kawakami N., Keren A., Khoo J.P., King C.H., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Laden F., Lalloo R., Laslett L.L., Lathlean T., Leasher J.L., Lee Y.Y., Leigh J., Levinson D., Lim S.S., Limb E., Lin J.K., Lipnick M., Lipshultz S.E., Liu W., Loane M., Ohno S.L., Lyons R., Mabweijano J., MacIntyre M.F., Malekzadeh R., Mallinger L., Manivannan S., Marcenes W., March L., Margolis D.J., Marks G.B., Marks R., Matsumori A., Matzopoulos R., Mayosi B.M., McAnulty J.H., McDermott M.M., McGill N., McGrath J., Medina-Mora M.E., Meltzer M., Mensah G.A., Merriman T.R., Meyer A.C., Miglioli V., Miller M., Miller T.R., Mitchell P.B., Mock C., Mocumbi A.O., Moffitt T.E., Mokdad A.A., Monasta L., Montico M., Moradi-Lakeh M., Moran A., Morawska L., Mori R., Murdoch M.E., Mwaniki M.K., Naidoo K., Nair M.N., Naldi L., Narayan K.M., Nelson P.K., Nelson R.G., Nevitt M.C., Newton C.R., Nolte S., Norman P., Norman R., O’Donnell M., O’Hanlon S., Olives C., Omer S.B., Ortblad K., Osborne R., Ozgediz D., Page A., Pahari B., Pandian J.D., Rivero A.P., Patten S.B., Pearce N., Padilla R.P., Perez-Ruiz F., Perico N., Pesudovs K., Phillips D., Phillips M.R., Pierce K., Pion S., Polanczyk G.V., Polinder S., Pope C.A., 3rd, Popova S., Porrini E., Pourmalek F., Prince M., Pullan R.L., Ramaiah K.D., Ranganathan D., Razavi H., Regan M., Rehm J.T., Rein D.B., Remuzzi G., Richardson K., Rivara F.P., Roberts T., Robinson C., De Leon F.R., Ronfani L., Room R., Rosenfeld L.C., Rushton L., Sacco R.L., Saha S., Sampson U., Sanchez-Riera L., Sanman E., Schwebel D.C., Scott J.G., Segui-Gomez M., Shahraz S., Shepard D.S., Shin H., Shivakoti R., Singh D., Singh G.M., Singh J.A., Singleton J., Sleet D.A., Sliwa K., Smith E., Smith J.L., Stapelberg N.J., Steer A., Steiner T., Stolk W.A., Stovner L.J., Sudfeld C., Syed S., Tamburlini G., Tavakkoli M., Taylor H.R., Taylor J.A., Taylor W.J., Thomas B., Thomson W.M., Thurston G.D., Tleyjeh I.M., Tonelli M., Towbin J.A., Truelsen T., Tsilimbaris M.K., Ubeda C., Undurraga E.A., van der Werf M.J., van Os J., Vavilala M.S., Venketasubramanian N., Wang M., Wang W., Watt K., Weatherall D.J., Weinstock M.A., Weintraub R., Weisskopf M.G., Weissman M.M., White R.A., Whiteford H., Wiebe N., Wiersma S.T., Wilkinson J.D., Williams H.C., Williams S.R., Witt E., Wolfe F., Woolf A.D., Wulf S., Yeh P.H., Zaidi A.K., Zheng Z.J., Zonies D., Lopez A.D., AlMazroa M.A., Memish Z.A. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Murta S.M., Gazzinelli R.T., Brener Z., Romanha A.J. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol. Biochem. Parasitol. 1998;93(2):203–214. doi: 10.1016/s0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]
- Murthy S., Keystone J., Kissoon N. Infections of the developing world. Crit. Care Clin. 2013;29(3):485–507. doi: 10.1016/j.ccc.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Navin T.R., Arana B.A., Arana F.E., Berman J.D., Chajon J.F. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J. Infect. Dis. 1992;165(3):528–534. doi: 10.1093/infdis/165.3.528. [DOI] [PubMed] [Google Scholar]
- Neal R.A. The effect of antibiotics of the neomycin group on experimental cutaneous leishmaniasis. Ann. Trop. Med. Parasitol. 1968;62(1):54–62. doi: 10.1080/00034983.1968.11686529. [DOI] [PubMed] [Google Scholar]
- Neal R.A., Allen S., McCoy N., Olliaro P., Croft S.L. The sensitivity of Leishmania species to aminosidine. J. Antimicrob. Chemother. 1995;35(5):577–584. doi: 10.1093/jac/35.5.577. [DOI] [PubMed] [Google Scholar]
- Nikolskaia O.V., de A.L.A.P., Kim Y.V., Lonsdale-Eccles J.D., Fukuma T., Scharfstein J., Grab D.J. Blood–brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease. J. Clin. Invest. 2006;116(10):2739–2747. doi: 10.1172/JCI27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njuguna N.M., Ongarora D.S., Chibale K. Artemisinin derivatives: a patent review (2006 – present) Expert Opin. Ther. Pat. 2012;22(10):1179–1203. doi: 10.1517/13543776.2012.724063. [DOI] [PubMed] [Google Scholar]
- Nunes M.C., Dones W., Morillo C.A., Encina J.J., Ribeiro A.L., Council on Chagas Disease of the Interamerican Society of Cardiology Chagas disease: an overview of clinical and epidemiological aspects. J. Am. Coll. Cardiol. 2013;62(9):767–776. doi: 10.1016/j.jacc.2013.05.046. [DOI] [PubMed] [Google Scholar]
- Nwaka S., Hudson A. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 2006;5(11):941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- O’Brien Z., Fallah Moghaddam M. Small molecule kinase inhibitors approved by the FDA from 2000 to 2011: a systematic review of preclinical ADME data. Expert Opin. Drug Metab. Toxicol. 2013;9(12):1597–1612. doi: 10.1517/17425255.2013.834046. [DOI] [PubMed] [Google Scholar]
- Ochiana S.O., Pandarinath V., Wang Z., Kapoor R., Ondrechen M.J., Ruben L., Pollastri M.P. The human Aurora kinase inhibitor danusertib is a lead compound for anti-trypanosomal drug discovery via target repurposing. Eur. J. Med. Chem. 2013;62:777–784. doi: 10.1016/j.ejmech.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa T.J., Salazar-Lindo E., Cleary T.G. Management of children with infection-associated persistent diarrhea. Semin. Pediatr. Infect. Dis. 2004;15(4):229–236. doi: 10.1053/j.spid.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Ogutu B. Artemether and lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Expert Opin. Pharmacother. 2013;14(5):643–654. doi: 10.1517/14656566.2013.771167. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L., Marr K.A., Rex J.H., Cohen S.H. Amphotericin B: time for a new “gold standard”. Clin. Infect. Dis. 2003;37(3):415–425. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- Ozgoztasi O., Baydar I. A randomized clinical trial of topical paromomycin versus oral ketoconazole for treating cutaneous leishmaniasis in Turkey. Int. J. Dermatol. 1997;36(1):61–63. doi: 10.1046/j.1365-4362.1997.00022.x. [DOI] [PubMed] [Google Scholar]
- Paladin Labs Inc, 2010. Application for Inclusion of Miltefosine on WHO. Model List of Essential Medicines.
- Palmer B.S. Meta-analysis of three case controlled studies and an ecological study into the link between cryptogenic epilepsy and chronic toxoplasmosis infection. Seizure. 2007;16(8):657–663. doi: 10.1016/j.seizure.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Pan P., Vermelho A.B., Capaci Rodrigues G., Scozzafava A., Tolvanen M.E., Parkkila S., Capasso C., Supuran C.T. Cloning, characterization, and sulfonamide and thiol inhibition studies of an alpha-carbonic anhydrase from Trypanosoma cruzi, the causative agent of Chagas disease. J. Med. Chem. 2013;56(4):1761–1771. doi: 10.1021/jm4000616. [DOI] [PubMed] [Google Scholar]
- Patel G., Karver C.E., Behera R., Guyett P.J., Sullenberger C., Edwards P., Roncal N.E., Mensa-Wilmot K., Pollastri M.P. Kinase scaffold repurposing for neglected disease drug discovery: discovery of an efficacious, lapatinib-derived lead compound for trypanosomiasis. J. Med. Chem. 2013;56(10):3820–3832. doi: 10.1021/jm400349k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson Y.J., Gilman T.M., Heseltine P.N., Sharma O.P., Boylen C.T. Eflornithine treatment of refractory Pneumocystis carinii pneumonia in patients with acquired immunodeficiency syndrome. Chest. 1992;101(1):67–74. doi: 10.1378/chest.101.1.67. [DOI] [PubMed] [Google Scholar]
- Peacock C.S., Seeger K., Harris D., Murphy L., Ruiz J.C., Quail M.A., Peters N., Adlem E., Tivey A., Aslett M., Kerhornou A., Ivens A., Fraser A., Rajandream M.A., Carver T., Norbertczak H., Chillingworth T., Hance Z., Jagels K., Moule S., Ormond D., Rutter S., Squares R., Whitehead S., Rabbinowitsch E., Arrowsmith C., White B., Thurston S., Bringaud F., Baldauf S.L., Faulconbridge A., Jeffares D., Depledge D.P., Oyola S.O., Hilley J.D., Brito L.O., Tosi L.R., Barrell B., Cruz A.K., Mottram J.C., Smith D.F., Berriman M. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat. Genet. 2007;39(7):839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin J., Milord F. The treatment of human African trypanosomiasis. Adv. Parasitol. 1994;33:1–47. doi: 10.1016/s0065-308x(08)60410-8. [DOI] [PubMed] [Google Scholar]
- Pepin J., Milord F., Guern C., Schechter P.J. Difluoromethylornithine for arseno-resistant Trypanosoma brucei gambiense sleeping sickness. Lancet. 1987;2(8573):1431–1433. doi: 10.1016/s0140-6736(87)91131-7. [DOI] [PubMed] [Google Scholar]
- Pink R., Hudson A., Mouries M.A., Bendig M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005;4(9):727–740. doi: 10.1038/nrd1824. [DOI] [PubMed] [Google Scholar]
- Pinto A.Y., Valente Vda C., Coura J.R., Valente S.A., Junqueira A.C., Santos L.C., Ferreira A.G., Jr., de Macedo R.C. Clinical follow-up of responses to treatment with benznidazol in Amazon: a cohort study of acute Chagas disease. PLoS ONE. 2013;8(5):e64450. doi: 10.1371/journal.pone.0064450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati V.K., Mehrotra S., Gautam S., Rai M., Sundar S. In vitro antileishmanial drug susceptibility of clinical isolates from patients with Indian visceral leishmaniasis–status of newly introduced drugs. Am. J. Trop. Med. Hyg. 2012;87(4):655–657. doi: 10.4269/ajtmh.2012.12-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priotto G., Kasparian S., Ngouama D., Ghorashian S., Arnold U., Ghabri S., Karunakara U. Nifurtimox–eflornithine combination therapy for second-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Congo. Clin. Infect. Dis. 2007;45(11):1435–1442. doi: 10.1086/522982. [DOI] [PubMed] [Google Scholar]
- Priotto G., Kasparian S., Mutombo W., Ngouama D., Ghorashian S., Arnold U., Ghabri S., Baudin E., Buard V., Kazadi-Kyanza S., Ilunga M., Mutangala W., Pohlig G., Schmid C., Karunakara U., Torreele E., Kande V. Nifurtimox–eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet. 2009;374(9683):56–64. doi: 10.1016/S0140-6736(09)61117-X. [DOI] [PubMed] [Google Scholar]
- Puavilai S., Chutha S., Polnikorn N., Timpatanapong P., Tasanapradit P., Charuwichitratana S., Boonthanom A., Wongwaisayawan H. Incidence of anemia in leprosy patients treated with dapsone. J. Med. Assoc. Thai. 1984;67(7):404–407. [PubMed] [Google Scholar]
- Reungprapavut S., Krungkrai S.R., Krungkrai J. Plasmodium falciparum carbonic anhydrase is a possible target for malaria chemotherapy. J. Enzyme Inhib. Med. Chem. 2004;19(3):249–256. doi: 10.1080/14756360410001689577. [DOI] [PubMed] [Google Scholar]
- Rieckmann K.H., Powell R.D., McNamara J.V., Willerson D., Jr., Lass L., Frischer H., Carson P.E. Effects of tetracycline against chloroquine-resistant and chloroquine-sensitive Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1971;20(6):811–815. doi: 10.4269/ajtmh.1971.20.811. [DOI] [PubMed] [Google Scholar]
- Rijal S., Chappuis F., Singh R., Bovier P.A., Acharya P., Karki B.M., Das M.L., Desjeux P., Loutan L., Koirala S. Treatment of visceral leishmaniasis in south-eastern Nepal: decreasing efficacy of sodium stibogluconate and need for a policy to limit further decline. Trans. R. Soc. Trop. Med. Hyg. 2003;97(3):350–354. doi: 10.1016/s0035-9203(03)90167-2. [DOI] [PubMed] [Google Scholar]
- Robays J., Nyamowala G., Sese C., Betu Ku., Mesu Kande V., Lutumba P., Van der Veken W., Boelaert M. High failure rates of melarsoprol for sleeping sickness, Democratic Republic of Congo. Emerg. Infect. Dis. 2008;14(6):966–967. doi: 10.3201/eid1406.71266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Gangneux F., Darde M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012;25(2):264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G., Crandall I.E., Szarek W.A. Synthesis and anti-plasmodium activity of benzimidazole analogues structurally related to astemizole. ChemMedChem. 2013 doi: 10.1002/cmdc.201300172. [DOI] [PubMed] [Google Scholar]
- Rorman E., Zamir C.S., Rilkis I., Ben-David H. Congenital toxoplasmosis–prenatal aspects of Toxoplasma gondii infection. Reprod. Toxicol. 2006;21(4):458–472. doi: 10.1016/j.reprotox.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Sateriale A., Bessoff K., Sarkar I.N., Huston C.D. Drug repurposing: mining protozoan proteomes for targets of known bioactive compounds. J. Am. Med. Inform. Assoc. 2013 doi: 10.1136/amiajnl-2013-001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier Sholler G.L., Kalkunte S., Greenlaw C., McCarten K., Forman E. Antitumor activity of nifurtimox observed in a patient with neuroblastoma. J. Pediatr. Hematol. Oncol. 2006;28(10):693–695. doi: 10.1097/01.mph.0000212994.56812.f2. [DOI] [PubMed] [Google Scholar]
- Saulnier Sholler G.L., Bergendahl G.M., Brard L., Singh A.P., Heath B.W., Bingham P.M., Ashikaga T., Kamen B.A., Homans A.C., Slavik M.A., Lenox S.R., Higgins T.J., Ferguson W.S. A phase 1 study of nifurtimox in patients with relapsed/refractory neuroblastoma. J. Pediatr. Hematol. Oncol. 2011;33(1):25–30. doi: 10.1097/MPH.0b013e3181f47061. [DOI] [PubMed] [Google Scholar]
- Savarino A., Lucia M.B., Rastrelli E., Rutella S., Golotta C., Morra E., Tamburrini E., Perno C.F., Boelaert J.R., Sperber K., Cauda R. Anti-HIV effects of chloroquine: inhibition of viral particle glycosylation and synergism with protease inhibitors. J. Acquir. Immune Defic. Syndr. 2004;35(3):223–232. doi: 10.1097/00126334-200403010-00002. [DOI] [PubMed] [Google Scholar]
- Scaffidi V., Astuto R. Treatment of amoebic hepatitis with emetine and chloroquine. Panminerva Med. 1959;1:162. [PubMed] [Google Scholar]
- Sen R., Ganguly S., Saha P., Chatterjee M. Efficacy of artemisinin in experimental visceral leishmaniasis. Int. J. Antimicrob. Agents. 2010;36(1):43–49. doi: 10.1016/j.ijantimicag.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Shapiro J., Lui H. Vaniqa–eflornithine 13.9% cream. Skin Therapy Lett. 2001;6(7):1–3, 5. [PubMed] [Google Scholar]
- Simarro P.P., Diarra A., Ruiz Postigo J.A., Franco J.R., Jannin J.G. The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000–2009: the way forward. PLoS Negl. Trop. Dis. 2011;5(2):e1007. doi: 10.1371/journal.pntd.0001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simarro P.P., Franco J., Diarra A., Postigo J.A., Jannin J. Update on field use of the available drugs for the chemotherapy of human African trypanosomiasis. Parasitology. 2012;139(7):842–846. doi: 10.1017/S0031182012000169. [DOI] [PubMed] [Google Scholar]
- Skinner-Adams T.S., Davis T.M., Manning L.S., Johnston W.A. The efficacy of benzimidazole drugs against Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 1997;91(5):580–584. doi: 10.1016/s0035-9203(97)90035-3. [DOI] [PubMed] [Google Scholar]
- Smith D.A., Jones R.M. The sulfonamide group as a structural alert: A distorted story? Curr. Opin. Drug Discov. Devel. 2008;11(1):72–79. [PubMed] [Google Scholar]
- Smorenburg C.H., Seynaeve C., Bontenbal M., Planting A.S., Sindermann H., Verweij J. Phase II study of miltefosine 6% solution as topical treatment of skin metastases in breast cancer patients. Anticancer Drugs. 2000;11(10):825–828. doi: 10.1097/00001813-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Snelling W.J., Xiao L., Ortega-Pierres G., Lowery C.J., Moore J.E., Rao J.R., Smyth S., Millar B.C., Rooney P.J., Matsuda M., Kenny F., Xu J., Dooley J.S. Cryptosporidiosis in developing countries. J. Infect. Dev. Ctries. 2007;1(3):242–256. [PubMed] [Google Scholar]
- Sotelo J., Briceno E., Lopez-Gonzalez M.A. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2006;144(5):337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- Steverding D. The development of drugs for treatment of sleeping sickness: a historical review. Parasit. Vectors. 2010;3(1):15. doi: 10.1186/1756-3305-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health. 2001;6(11):849–854. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- Sundar S., Chakravarty J. Liposomal amphotericin B and leishmaniasis: dose and response. J. Global Infect. Dis. 2010;2(2):159–166. doi: 10.4103/0974-777X.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S., Chakravarty J. Leishmaniasis: an update of current pharmacotherapy. Expert Opin. Pharmacother. 2013;14(1):53–63. doi: 10.1517/14656566.2013.755515. [DOI] [PubMed] [Google Scholar]
- Sundar S., More D.K., Singh M.K., Singh V.P., Sharma S., Makharia A., Kumar P.C., Murray H.W. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 2000;31(4):1104–1107. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- Supuran C.T. Carbonic anhydrases as drug targets. Curr. Pharm. Des. 2008;14(7):601–602. doi: 10.2174/138161208783877910. [DOI] [PubMed] [Google Scholar]
- Supuran C.T. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008;7(2):168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- Supuran C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010;20(12):3467–3474. doi: 10.1016/j.bmcl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Sutherland C.J., Tanomsing N., Nolder D., Oguike M., Jennison C., Pukrittayakamee S., Dolecek C., Hien T.T., do Rosario V.E., Arez A.P., Pinto J., Michon P., Escalante A.A., Nosten F., Burke M., Lee R., Blaze M., Otto T.D., Barnwell J.W., Pain A., Williams J., White N.J., Day N.P., Snounou G., Lockhart P.J., Chiodini P.L., Imwong M., Polley S.D. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J. Infect. Dis. 2010;201(10):1544–1550. doi: 10.1086/652240. [DOI] [PubMed] [Google Scholar]
- Syrjanen L., Vermelho A.B., de Almeida Rodrigues I., Corte-Real S., Salonen T., Pan P., Vullo D., Parkkila S., Capasso C., Supuran C.T. Cloning, characterization, and inhibition studies of a beta-carbonic anhydrase from Leishmania donovani chagasi, the protozoan parasite responsible for leishmaniasis. J. Med. Chem. 2013;56(18):7372–7381. doi: 10.1021/jm400939k. [DOI] [PubMed] [Google Scholar]
- Tan K.R., Magill A.J., Parise M.E., Arguin P.M., Centers for Disease C. and Prevention Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am. J. Trop. Med. Hyg. 2011;84(4):517–531. doi: 10.4269/ajtmh.2011.10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G.J., Wainwright P. Open label extension studies: research or marketing? BMJ. 2005;331(7516):572–574. doi: 10.1136/bmj.331.7516.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur C.P., Singh R.K., Hassan S.M., Kumar R., Narain S., Kumar A. Amphotericin B deoxycholate treatment of visceral leishmaniasis with newer modes of administration and precautions: a study of 938 cases. Trans. R. Soc. Trop. Med. Hyg. 1999;93(3):319–323. doi: 10.1016/s0035-9203(99)90037-8. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Mastroiacovo P. The global burden of congenital toxoplasmosis: a systematic review. Bull. World Health Organ. 2013;91(7):501–508. doi: 10.2471/BLT.12.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey E.F., Bartko J.J., Lun Z.R., Yolken R.H. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr. Bull. 2007;33(3):729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina J.A., Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 2003;19(11):495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhove S. Advances in sleeping sickness therapy. Ann. Soc. Belg. Med. Trop. 1992;72(Suppl. 1):39–51. [PubMed] [Google Scholar]
- Vincent I.M., Creek D., Watson D.G., Kamleh M.A., Woods D.J., Wong P.E., Burchmore R.J., Barrett M.P. A molecular mechanism for eflornithine resistance in African trypanosomes. PLoS Pathog. 2010;6(11):e1001204. doi: 10.1371/journal.ppat.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eichborn J., Murgueitio M.S., Dunkel M., Koerner S., Bourne P.E., Preissner R. PROMISCUOUS: a database for network-based drug-repositioning. Nucleic Acids Res. 2011;39(Database issue):D1060–D1066. doi: 10.1093/nar/gkq1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Suzek T., Zhang J., Wang J., He S., Cheng T., Shoemaker B.A., Gindulyte A., Bryant S.H. PubChem BioAssay: 2014 update. Nucleic Acids Res. 2014;42(1):D1075–D1082. doi: 10.1093/nar/gkt978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner D.H., Rohwedder R.W. Experience with nifurtimox in chronic Chagas’ infection. Preliminary report. Arzneimittelforschung. 1972;22(9):1635–1641. [PubMed] [Google Scholar]
- Weisman J.L., Liou A.P., Shelat A.A., Cohen F.E., Guy R.K., DeRisi J.L. Searching for new antimalarial therapeutics amongst known drugs. Chem. Biol. Drug Des. 2006;67(6):409–416. doi: 10.1111/j.1747-0285.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T.N. Microbiology. Is the tide turning for new malaria medicines? Science. 2010;329(5996):1153–1154. doi: 10.1126/science.1194923. [DOI] [PubMed] [Google Scholar]
- White C.A., Jr. Nitazoxanide: a new broad spectrum antiparasitic agent. Expert Rev. Anti Infect. Ther. 2004;2(1):43–49. doi: 10.1586/14787210.2.1.43. [DOI] [PubMed] [Google Scholar]
- Wilson T., Edeson J.F. Treatment of acute malaria with pyrimethamine. Br. Med. J. 1953;1(4804):253–255. doi: 10.1136/bmj.1.4804.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley P. Chlorproguanil–dapsone (LAPDAP) for uncomplicated falciparum malaria. Trop. Med. Int. Health. 2001;6(11):952–954. doi: 10.1046/j.1365-3156.2001.00751.x. [DOI] [PubMed] [Google Scholar]
- Wishart D.S., Knox C., Guo A.C., Cheng D., Shrivastava S., Tzur D., Gautam B., Hassanali M. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36(Database issue):901–906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R., Matz H., Orion E., Tuzun B., Tuzun Y. Dapsone. Dermatol. Online J. 2002;8(1):2. [PubMed] [Google Scholar]
- World Health Organization, 2008. The global burden of disease: 2004 update.
- World Health Organization 2010, Global report on antimalarial drug efficacy and drug resistance: 2000–2010.
- World Health Organization, 2010a. Chagas Disease: control and elimination. World Health Assembly Report.
- World Health Organization, 2010b. Working to overcome the global impact of neglected tropical diseases. First WHO report on neglected tropical diseases.
- World Health Organization, Global Health Observatory Map Gallery. Retrieved 15th November, 2013, from http://gamapserver.who.int/mapLibrary/.
- World Health Organization, 2013. World Malaria Report 2013.
- Xiao S.H. Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemisinins. Acta Trop. 2005;96(2–3):153–167. doi: 10.1016/j.actatropica.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Yuan J., Cheng K.C., Johnson R.L., Huang R., Pattaradilokrat S., Liu A., Guha R., Fidock D.A., Inglese J., Wellems T.E., Austin C.P., Su X.Z. Chemical genomic profiling for antimalarial therapies, response signatures, and molecular targets. Science. 2011;333(6043):724–729. doi: 10.1126/science.1205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zic J.A., Horowitz D.H., Arzubiaga C., King L.E., Jr. Treatment of cutaneous sarcoidosis with chloroquine. Review of the literature. Arch. Dermatol. 1991;127(7):1034–1040. [PubMed] [Google Scholar]


