Abstract
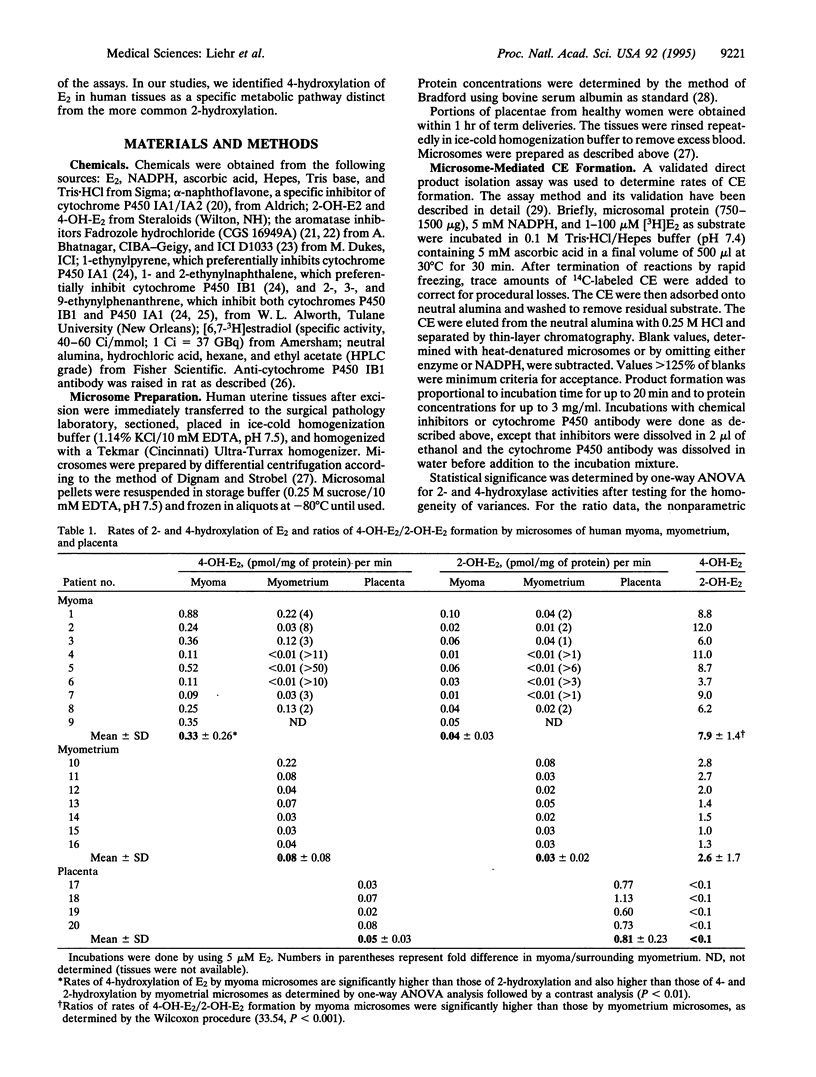
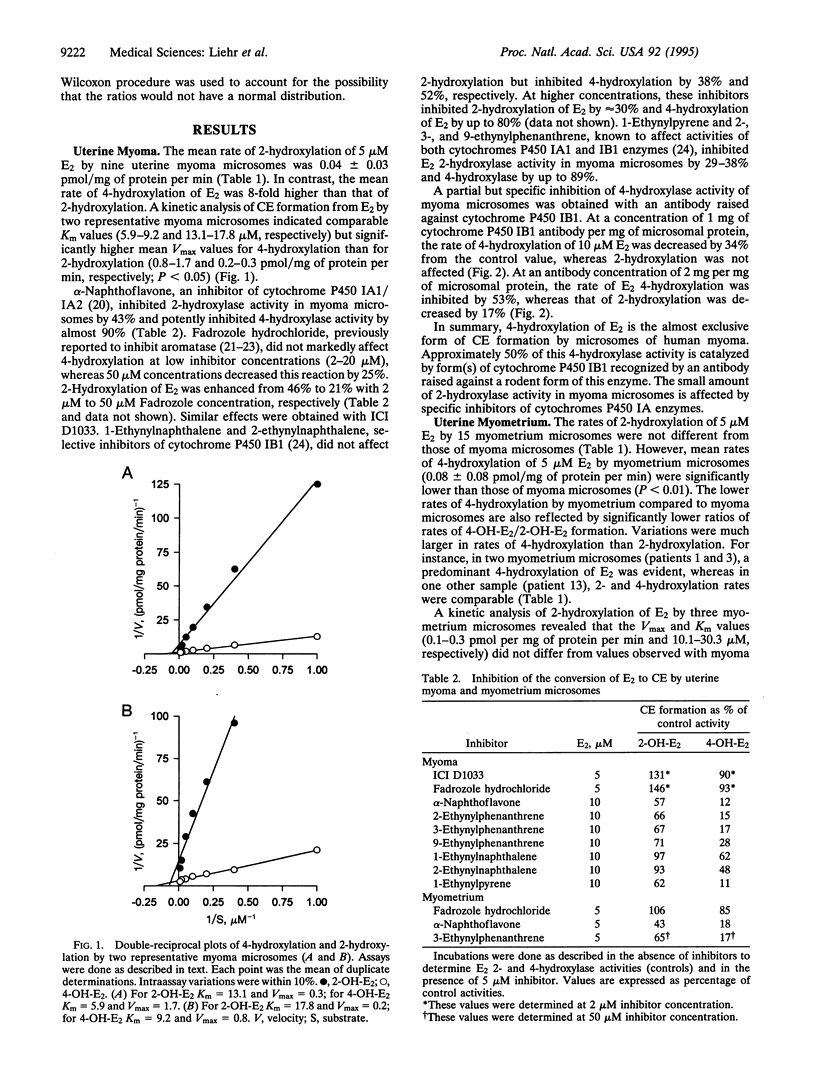
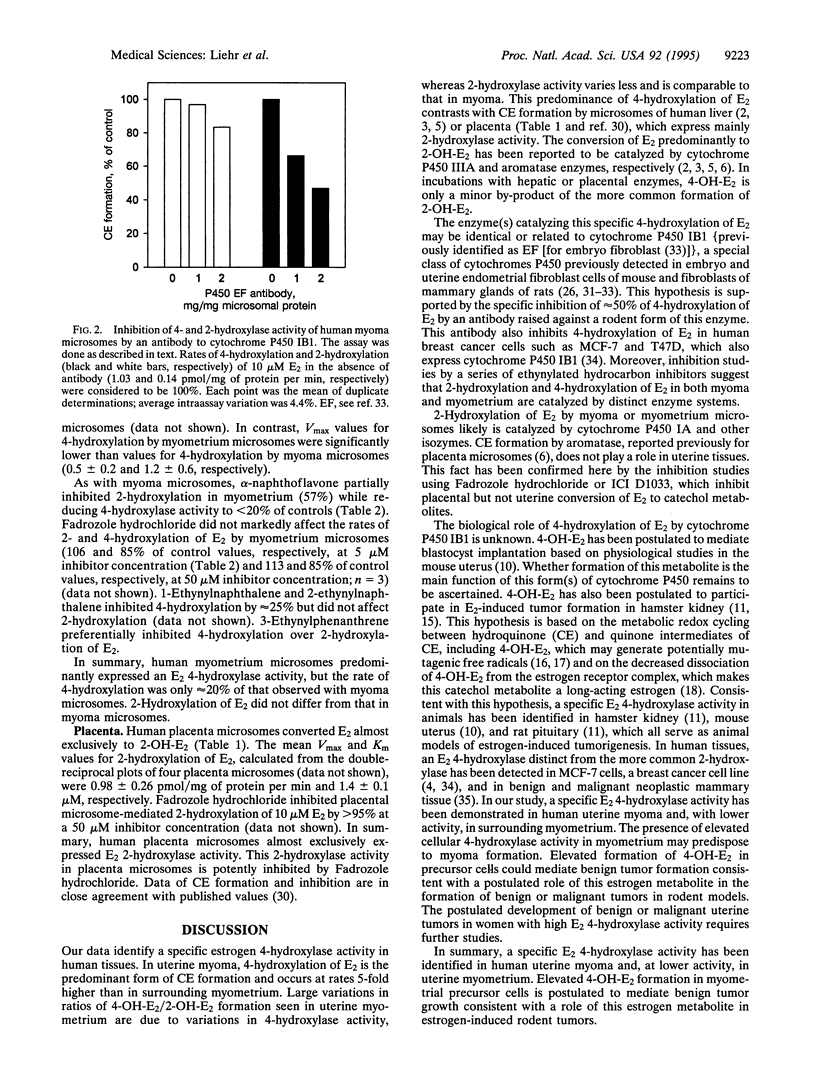
Estradiol is converted to catechol estrogens via 2- and 4-hydroxylation by cytochrome P450 enzymes. 4-Hydroxyestradiol elicits biological activities distinct from estradiol, most notably an oxidant stress response induced by free radicals generated by metabolic redox cycling reactions. In this study, we have examined 2- and 4-hydroxylation of estradiol by microsomes of human uterine myometrium and of associated myomata. In all eight cases studied, estradiol 4-hydroxylation by myoma has been substantially elevated relative to surrounding myometrial tissue (minimum, 2-fold; mean, 5-fold). Estradiol 2-hydroxylation in myomata occurs at much lower rates than 4-hydroxylation (ratio of 4-hydroxyestradiol/2-hydroxyestradiol, 7.9 +/- 1.4) and does not significantly differ from rates in surrounding myometrial tissue. Rates of myometrial 2-hydroxylation of estradiol were also not significantly different from values in patients without myomata. We have used various inhibitors to establish that 4-hydroxylation is catalyzed by a completely different cytochrome P450 than 2-hydroxylation. In myoma, alpha-naphthoflavone and a set of ethynyl polycyclic hydrocarbon inhibitors (5 microM) each inhibited 4-hydroxylation more efficiently (up to 90%) than 2-hydroxylation (up to 40%), indicating > 10-fold differences in Ki (<0.5 microM vs. > 5 microM). These activities were clearly distinguished from the selective 2-hydroxylation of estradiol in placenta by aromatase reported previously (low Km, inhibition by Fadrozole hydrochloride or ICI D1033). 4-Hydroxylation was also selectively inhibited relative to 2-hydroxylation by antibodies raised against cytochrome P450 IB1 (rat) (53 vs. 17%). These data indicate that specific 4-hydroxylation of estradiol in human uterine tissues is catalyzed by a form(s) of cytochrome P450 related to P450 IB1, which contribute(s) little to 2-hydroxylation. This enzyme(s) is therefore a marker for uterine myomata and may play a role in the etiology of the tumor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H., Fotsis T., Höckerstedt K., Hämäläinen E., Bannwart C., Bloigu S., Valtonen A., Ollus A. Diet and urinary estrogen profile in premenopausal omnivorous and vegetarian women and in premenopausal women with breast cancer. J Steroid Biochem. 1989;34(1-6):527–530. doi: 10.1016/0022-4731(89)90138-6. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Gorbach S. L., Goldin B. R., Woods M. N., Dwyer J. T., Hämäläinen E. Estrogen metabolism and excretion in Oriental and Caucasian women. J Natl Cancer Inst. 1994 Jul 20;86(14):1076–1082. doi: 10.1093/jnci/86.14.1076. [DOI] [PubMed] [Google Scholar]
- Alworth W. L., Viaje A., Sandoval A., Warren B. S., Slaga T. J. Potent inhibitory effects of suicide inhibitors of P450 isozymes on 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene initiated skin tumors. Carcinogenesis. 1991 Jul;12(7):1209–1215. doi: 10.1093/carcin/12.7.1209. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bui Q. D., Weisz J. Monooxygenase mediating catecholestrogen formation by rat anterior pituitary is an estrogen-4-hydroxylase. Endocrinology. 1989 Feb;124(2):1085–1087. doi: 10.1210/endo-124-2-1085. [DOI] [PubMed] [Google Scholar]
- Bui Q., Weisz J. Identification of microsomal, organic hydroperoxide-dependent catechol estrogen formation: comparison with NADPH-dependent mechanism. Pharmacology. 1988;36(5):356–364. doi: 10.1159/000138406. [DOI] [PubMed] [Google Scholar]
- CLIFTON K. H., MEYER R. K. Mechanism of anterior pituitary tumor induction by estrogen. Anat Rec. 1956 May;125(1):65–81. doi: 10.1002/ar.1091250106. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Strobel H. W. NADPH-cytochrome P-450 reductase from rat liver: purification by affinity chromatography and characterization. Biochemistry. 1977 Mar 22;16(6):1116–1123. doi: 10.1021/bi00625a014. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Characterization of human microsomal cytochrome P-450 enzymes. Annu Rev Pharmacol Toxicol. 1989;29:241–264. doi: 10.1146/annurev.pa.29.040189.001325. [DOI] [PubMed] [Google Scholar]
- Hersey R. M., Williams K. I., Weisz J. Catechol estrogen formation by brain tissue: characterization of a direct product isolation assay for estrogen-2- and 4-hydroxylase activity and its application to studies of 2- and 4-hydroxyestradiol formation by rabbit hypothalamus. Endocrinology. 1981 Dec;109(6):1912–1920. doi: 10.1210/endo-109-6-1912. [DOI] [PubMed] [Google Scholar]
- KIRKMAN H. Estrogen-induced tumors of the kidney. III. Growth characteristics in the Syrian hamster. Natl Cancer Inst Monogr. 1959 Dec;1:1–57. [PubMed] [Google Scholar]
- Kerlan V., Dreano Y., Bercovici J. P., Beaune P. H., Floch H. H., Berthou F. Nature of cytochromes P450 involved in the 2-/4-hydroxylations of estradiol in human liver microsomes. Biochem Pharmacol. 1992 Nov 3;44(9):1745–1756. doi: 10.1016/0006-2952(92)90068-t. [DOI] [PubMed] [Google Scholar]
- Liehr J. G., Fang W. F., Sirbasku D. A., Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986 Jan;24(1):353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- Liehr J. G., Roy D. Free radical generation by redox cycling of estrogens. Free Radic Biol Med. 1990;8(4):415–423. doi: 10.1016/0891-5849(90)90108-u. [DOI] [PubMed] [Google Scholar]
- Liehr J. G., Ulubelen A. A., Strobel H. W. Cytochrome P-450-mediated redox cycling of estrogens. J Biol Chem. 1986 Dec 25;261(36):16865–16870. [PubMed] [Google Scholar]
- Martucci C. P., Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993 Feb-Mar;57(2-3):237–257. doi: 10.1016/0163-7258(93)90057-k. [DOI] [PubMed] [Google Scholar]
- Murray M., Reidy G. F. Selectivity in the inhibition of mammalian cytochromes P-450 by chemical agents. Pharmacol Rev. 1990 Jun;42(2):85–101. [PubMed] [Google Scholar]
- Newbold R. R., Bullock B. C., McLachlan J. A. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res. 1990 Dec 1;50(23):7677–7681. [PubMed] [Google Scholar]
- Osawa Y., Higashiyama T., Shimizu Y., Yarborough C. Multiple functions of aromatase and the active site structure; aromatase is the placental estrogen 2-hydroxylase. J Steroid Biochem Mol Biol. 1993 Mar;44(4-6):469–480. doi: 10.1016/0960-0760(93)90252-r. [DOI] [PubMed] [Google Scholar]
- Paria B. C., Chakraborty C., Dey S. K. Catechol estrogen formation in the mouse uterus and its role in implantation. Mol Cell Endocrinol. 1990 Feb 12;69(1):25–32. doi: 10.1016/0303-7207(90)90085-m. [DOI] [PubMed] [Google Scholar]
- Pottenger L. H., Christou M., Jefcoate C. R. Purification and immunological characterization of a novel cytochrome P450 from C3H/10T1/2 cells. Arch Biochem Biophys. 1991 May 1;286(2):488–497. doi: 10.1016/0003-9861(91)90070-y. [DOI] [PubMed] [Google Scholar]
- Savas U., Bhattacharyya K. K., Christou M., Alexander D. L., Jefcoate C. R. Mouse cytochrome P-450EF, representative of a new 1B subfamily of cytochrome P-450s. Cloning, sequence determination, and tissue expression. J Biol Chem. 1994 May 27;269(21):14905–14911. [PubMed] [Google Scholar]
- Savas U., Christou M., Jefcoate C. R. Mouse endometrium stromal cells express a polycyclic aromatic hydrocarbon-inducible cytochrome P450 that closely resembles the novel P450 in mouse embryo fibroblasts (P450EF). Carcinogenesis. 1993 Oct;14(10):2013–2018. doi: 10.1093/carcin/14.10.2013. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Eugster H. P., Lincoln D. W., 2nd, Schuetz J. D., Schuetz E. G., Johnson J. A., Kaminsky L. S., Gierthy J. F. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1A1: a comparison of the activities induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in MCF-7 cells with those from heterologous expression of the cDNA. Arch Biochem Biophys. 1992 Mar;293(2):342–348. doi: 10.1016/0003-9861(92)90404-k. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Hayes C. L., Young N. R., Christou M., Sutter T. R., Jefcoate C. R., Gierthy J. F. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estrogen metabolism in MCF-7 breast cancer cells: evidence for induction of a novel 17 beta-estradiol 4-hydroxylase. J Steroid Biochem Mol Biol. 1994 Dec;51(5-6):251–258. doi: 10.1016/0960-0760(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Sutter T. R., Tang Y. M., Hayes C. L., Wo Y. Y., Jabs E. W., Li X., Yin H., Cody C. W., Greenlee W. F. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem. 1994 May 6;269(18):13092–13099. [PubMed] [Google Scholar]
- Weisz J., Bui Q. D., Roy D., Liehr J. G. Elevated 4-hydroxylation of estradiol by hamster kidney microsomes: a potential pathway of metabolic activation of estrogens. Endocrinology. 1992 Aug;131(2):655–661. doi: 10.1210/endo.131.2.1386303. [DOI] [PubMed] [Google Scholar]
- Wozniak A., Holman S. D., Hutchison J. B. In vitro potency and selectivity of the non-steroidal androgen aromatase inhibitor CGS 16949A compared to steroidal inhibitors in the brain. J Steroid Biochem Mol Biol. 1992 Oct;43(4):281–287. doi: 10.1016/0960-0760(92)90162-c. [DOI] [PubMed] [Google Scholar]


