Abstract
Background and Objectives:
The purpose of this study was to compare the efficacy of bovine-derived xenograft (Bio-Oss Collagen) and Type I collagen membrane (Bio-Gide) with bovine-derived xenograft (Bio-Oss Collagen) and fibrin fibronectin sealing system (TISSEEL) in the treatment of periodontal infrabony defects.
Materials and Methods:
Fourteen healthy patients in the age range of 20 to 60 years, showing bilateral or contralateral infrabony defects were selected. The defects were assigned randomly to Site A (bovine-derived xenograft [Bio-Oss Collagen] with bioresorbable Type I collagen membrane [Bio-Gide]) and Site B (fibrin fibronectin sealing system [TISSEEL] with bovine-derived xenograft [Bio-Oss Collagen]). The radiographic parameters were recorded at baseline, 6 months, and 9 months postoperatively.
Results:
All fourteen patients returned for recall at regular intervals till the completion of the study. Both the experimental groups showed clinically and radiographically statistically significant reduction in probing pocket depth and gain in clinical attachment level.
Conclusion:
Both groups showed potential for enhancing the periodontal regeneration with no statistically significant between the two groups; however, on comparison the Bio-Oss Collagen and TISSEEL group were slightly better.
Keywords: Bio-Gide, Bio-Oss Collagen, fibrin fibronectin sealing system, growth factors, guided tissue regeneration, periodontal regeneration
INTRODUCTION
Periodontal regeneration refers to the restoration of bone, cementum, and periodontal ligament to their original levels before they were damaged by the periodontal disease process. Periodontal regeneration can be achieved by a variety of surgical procedures including bone grafts, bone substitutes, guided tissue regeneration (GTR) or a combination of bone grafts or bone substitutes and GTR.[1]
Clinical research performed in periodontal regeneration has suggested that one of the most predictable techniques in improving clinical attachment levels and bone fill is achieved when using a combination of a graft material and GTR.[2]
The ideal graft material is one that is osteoinductive, easy to manipulate, absorbable, and abundant. Bovine porous bone mineral (Bio-Oss Collagen) is a relatively new material used in periodontal regeneration. This material is prepared by protein extraction of bovine bone which results in a structure similar to human cancellous bone and has the ability to enhance bone formation.
Bone formation has been shown with bovine bone mineral in sinus elevation procedures around endosseous implants and in critical size osseous defects.[1]
Mechanical barriers are used with the objective of controlling early periodontal surgical wound-healing dynamics in order to promote periodontal regeneration. Basically, barrier membrane serves the purpose of preventing epithelial downgrowth, thereby allowing progenitor cells of the bone and periodontal ligament to regenerate desirable tissues from the base of the defect.
Several materials have been used as barriers for GTR and shown to be well tolerated by the gingival tissues.
A bilayer collagen membrane (Bio-Gide) of porcine origin has been shown to be a promising alternative in guided tissue regeneration since it is slowly absorbable, (possibly lasting as much as 9 months)[3] and therefore having the potential to promote regeneration of the periodontal tissues. This membrane has been shown to be as effective as expanded polytetrafluoroethylene membranes in guided bone regeneration around dental implants.[4]
TISSEEL is a highly concentrated fibrin glue (FG) which has been widely used in many surgical disciplines including periodontal surgery. Beside its hemostatic capabilities, FG has been used in periodontal surgery to fixate gingival grafts to recipient sites[5] and to enhance treatment outcome of different reconstructive procedures. TISSEEL contains factors, such as thrombin, fibrin, fibronectin, and PDGF, which are known to retain biological activities, in particular on cell proliferation and differentiation, and which may also stimulate wound healing by acting as a scaffold for the proliferation of mesenchymal and endothelial cells. This in turn may lead to an increased granulation tissue formation, deposition of collagen, and enhance the restoration of a severed tissue compartment of the attachment apparatus of the tooth, which have been lost due to periodontal disease.[6]
The present study was undertaken to compare clinically and radiographically, the regenerative potential of a bovine-derived xenograft (Bio-Oss Collagen) with either a bioresorbable type I collagen membrane (Bio-Gide) or fibrin fibronectin sealing system, TISSEEL in treating periodontal infrabony defects.
MATERIALS AND METHODS
A total of 14 healthy patients, fulfilling the selection criteria were enrolled for this study. Twenty eight sites in these 14 patients showing bilateral/contralateral infrabony defects were randomly selected according to the split mouth design and divided into experimental site A and experimental site B.
Experimental Site A: 14 sites were treated with open flap surgery followed by placement of bovine derived xenograft (Bio-Oss Collagen) with a bioresorbable type I collagen membrane (Bio-Gide).
Experimental Site B: 14 sites were treated with open flap surgery followed by placement of a bovine derived xenograft (Bio-Oss Collagen) with fibrin fibronectin sealing system (TISSEEL).
Inclusion criteria included patients with the age group of 20 to 60 years, with moderate to severe chronic periodontitis with pocket depth of ≥5 mm, having at least two infrabony defects ≥3 mm, on any two quadrants or on contralateral sides of the same arch. Radiographic evidence of the infrabony defect had to exist. The exclusion criteria consisted of patients with uncontrolled diabetes; on anticoagulant/immunosuppressive therapy and/or with other systemic disease; patients unable to perform routine oral hygiene procedures; patients with history of drug allergy, and who are known smokers. Patients were informed as to the character and purpose of the study and were required to sign an informed consent. The study design and consent was approved by the University Institutional Review Board.
Initial therapy consisted of oral hygiene instructions which were repeated until patients achieved an O’Leary plaque score (O’Leary et al. 1972)[7] of 25% or below followed by scaling and root planing. A re-evaluation was performed after 6 to 8 weeks to confirm the indication for periodontal surgery.
The following calculations were made from the clinical measurement recorded:
Pocket depth = Fixed Reference Point to Base of the Pocket – Fixed Reference Point to Gingival Margin
Clinical attachment level = Fixed Reference Point to Base of the Pocket-Fixed Reference Point to CEJ
Gingival margin position = Fixed Reference Point to CEJ - Fixed Reference Point to Gingival Margin.
The following clinical parameters were recorded at baseline, 3 months, 6 months, and 9 months post surgery - Plaque Index (Silness and Loe 1964),[8] Probing Pocket Depth using William's graduated periodontal probe (Lynch, 1992),[9] Gingival Recession (Oreamuno et al. 1990),[10] Relative Clinical Attachment Level (Clark et al., 1987),[11] Radiographic evaluation by Long Cone Paralleling Technique.
The probing pocket depth, clinical attachment level, gingival recession, and radiographic parameters were recorded at baseline and at the end of 6 and 9 months.
The surgical procedure was performed by local infiltration of 2% lidocaine containing epinephrine at a concentration of 1:80,000. Buccal and lingual sulcular incisions were given and mucoperiosteal flaps were elevated. Care was exercised to preserve as much interproximal soft tissue as possible. Complete debridement of the defects as well as scaling and root planing were achieved. The defects were randomly assigned before surgery to the two treatment groups with randomized block approach. At the control site, the defect was filled with a composite bovine-derived xenograft Bio-Oss Collagen (Geistlich, Wolhusen Switzerland). The graft material was moistened in sterile saline for 5 minutes before placement into the defect. Following grafting, a bioresorbable collagen membrane of porcine origin (Bio-Gide) was cut according to the morphology of the defect using a template. The membrane was carried to the defect site and adapted over the entire defect so as to cover 2 to 3 mm of the surrounding alveolar bone and to ensure stability of the graft material. Neither sutures nor pins were used for membrane stabilization. Finally, the mucoperiosteal flaps were repositioned coronally and fixed with interrupted or horizontal mattress sutures [Figures 1–5].
Figure 1.
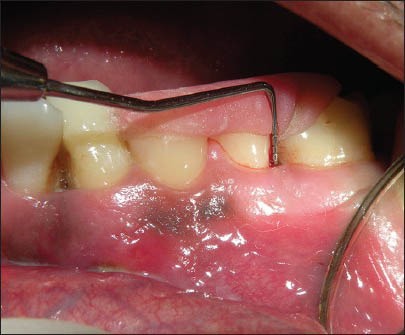
Probing pocket depth for Bio-Oss Collagen with Bio- Gide Membrane
Figure 5.
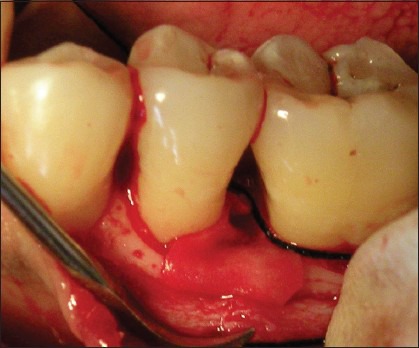
Placement of Bio- Gide Membrane
Figure 2.
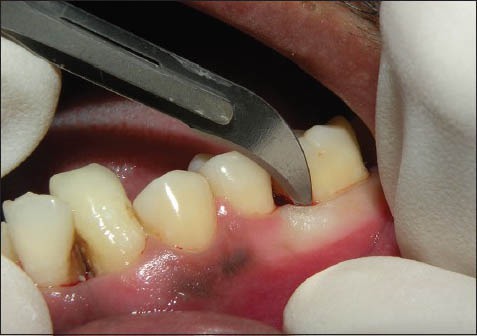
Incision
Figure 3.
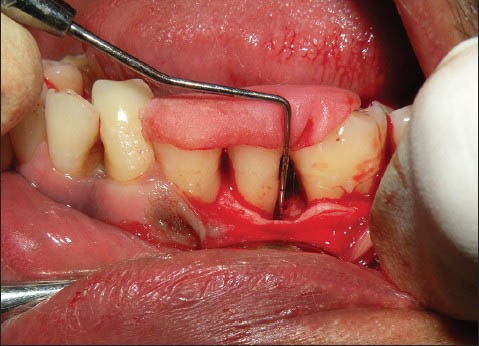
Defect depth
Figure 4.
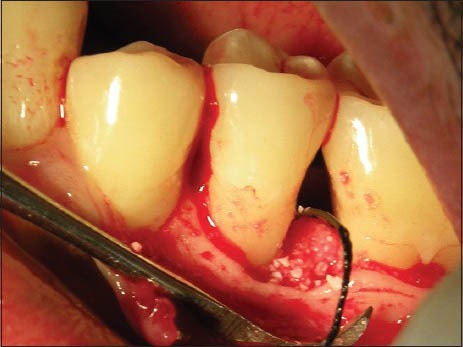
Placement of graft material (Bio-Oss Collagen)
The same protocol was followed for the site B except that the membrane is not placed and instead the fibrin fibronectin sealing system (TISSEEL) was combined with Bio-Oss Collagen [Figures 6–8]. The flaps were approximated with very little tension and periodontal dressings were placed (Coe – pac). All the patients were prescribed systemic antibiotics – amoxicillin 500 mg t.i.d for 5 days, along with an analgesic Piroxicam 20 mg b.i.d for 3 days.
Figure 6.
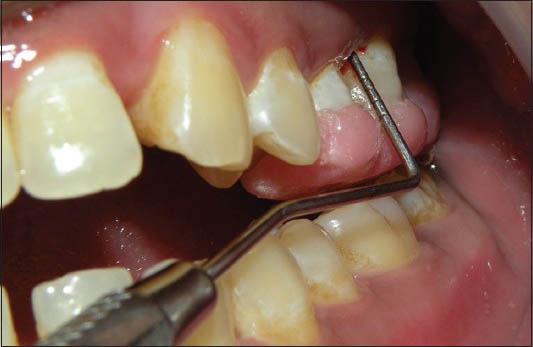
Probing pocket Depth with stent for Bio-Oss + TISSEEL group
Figure 8.
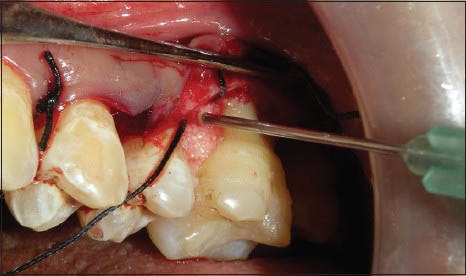
Placement of graft material and TISSEEL in the defect
Postoperative instructions were given to all the patients and they were instructed to report to the department 24 hours after the surgery and then after 10 days. On the 10th day following surgery, the periodontal dressing and the sutures were removed, and the surgical site was irrigated with normal saline. Symptoms regarding discomfort, pain, and sensitivity were enquired of the patient. Recall appointments for patients were made after 3, 6, and finally at 9 months. At each visit, oral hygiene instructions were reinforced and supragingival scaling was done if required. Patients were advised chlorhexidine mouth rinse twice daily for another 3 weeks. Measurements of the osseous defects were made utilizing the same grooves previously employed to record pocket depth and attachment levels. The distance between the most apical end of the stent and the point at which the groove adapted probe made contact with the bottom of the defect was the one recorded on the buccal and lingual sites.
Intraoral periapical radiographs were taken for each site before the surgical procedure and at intervals of 6 and 9 months using long cone paralleling technique [Figures 9 and 10]. The IOPA radiographs were digitalized into 3648 × 2736 pixel images [Figures 11–14] using Sony digital camera (Japan) and were then analyzed using the Image J Software. All the scanned radiographs which were in pixels were converted to a gray scale of 8 bytes. The distance was then converted and standardized from pixels to millimeters (mm), thereby setting a standard scale for all the linear radiographic measurements.
Figure 9.
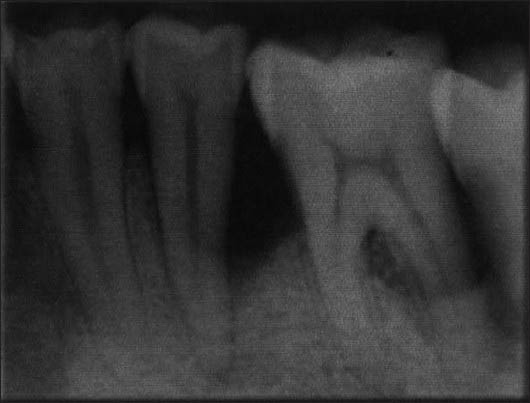
Preoperative radiograph (Bio-Oss + Bio-Gide)
Figure 10.
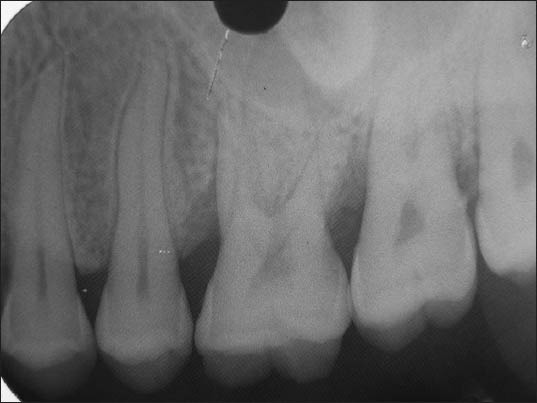
Preoperative radiograph of Bio-Oss + TISSEEL group
Figure 11.
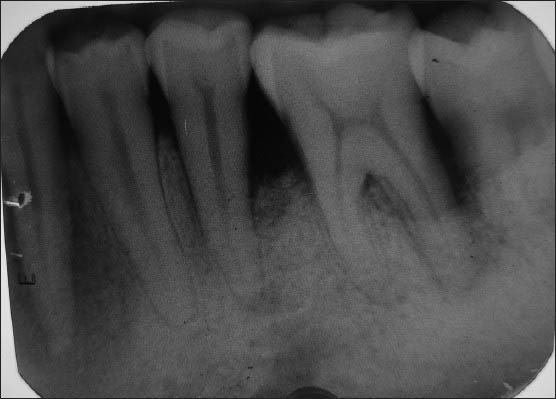
6 months postoperative (Bio-Oss + Bio-Gide)
Figure 14.
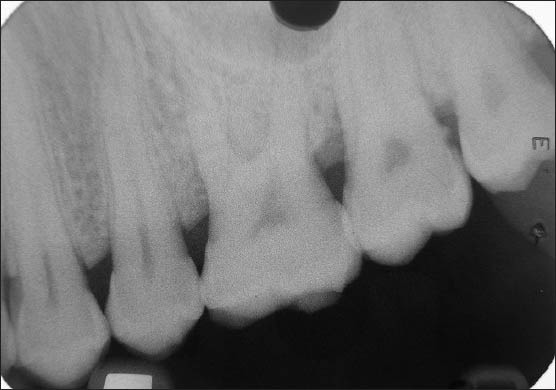
9 months postoperative (Bio-Oss + TISSEEL group)
Figure 7.
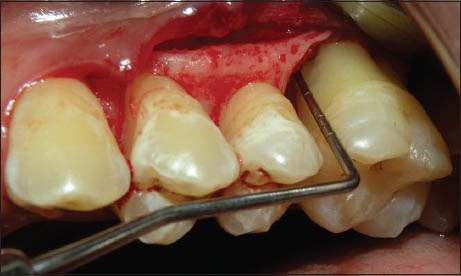
Defect Depth (Bio-Oss + TISSEEL)
Figure 12.
9 months postoperative (Bio-Oss + Bio-Gide)
Figure 13.
6 months postoperative radiograph (Bio-Oss + TISSEEL group)
RESULTS
All 14 patients completed the study. Surgical sites - control and experimental, healed uneventfully. No cases of flap dehiscence or infection were detected. Clinical evaluation of post grafting surgical healing revealed an excellent soft tissue response to both materials with no adverse complications. All patients demonstrated plaque control consistent with that achieved prior to initiation of surgical therapy.
A total of 28 defects were treated in 14 patients, 14 defects were treated with Bio-Oss Collagen and Bio-Gide membrane and the other 14 defects were treated with Bio-Oss Collagen and TISSEEL. Initial clinical measurements were similar for both the experimental and control sites. Following collection of data on all patients, a statistical analysis was completed to assess those parameters discussed as primary objectives.
Statistical analysis was performed using SPSS 20.00 software, with one-way ANNOVA, ANCOVA, and t test.
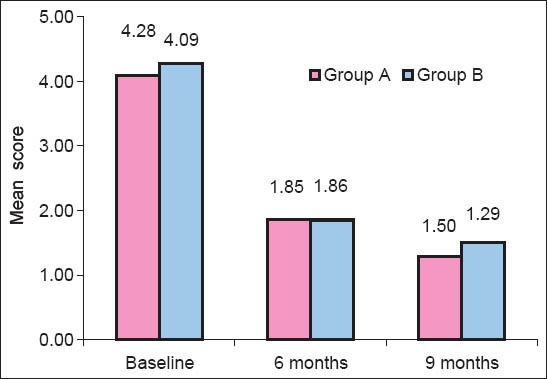
Plaque Index [Graph 1]: At baseline, the mean plaque index score was 1.04 ± 0.31. At 6 months, the mean plaque index score was reduced to 0.68 ± 0.06 with mean reduction of 0.35 ± 0.31 (34.08%). At 9 months, the mean plaque index score was reduced to 0.67 ± 0.04 with mean reduction of 0.36 ± 0.32 (35.45%). All mean values were statistically highly significant (P < 0.05).
Graph 1.
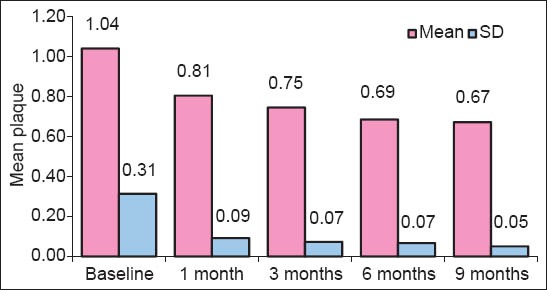
Comparison of baseline, 6 months, and 9 months with respect to plaque index scores
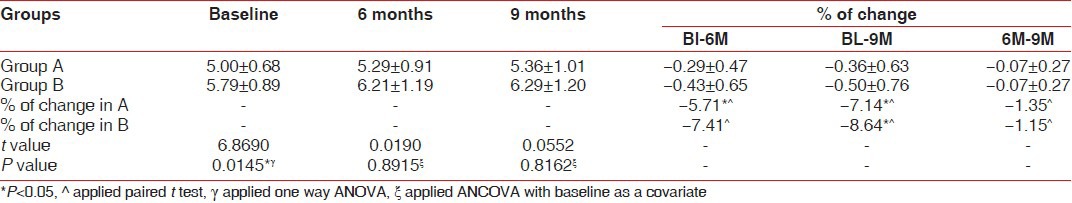
Pocket depth (PD) was significantly decreased as compared to baseline values in both the groups [Table 1], but the difference was not significant between the groups. The reduction in the PD for the site A (BDX and GTR) was 4.42 ± 1.22 mm, while in the BDX and TISSEEL group PD reduction was 4.00 ± 1.41. With regards to clinical attachment level gain, both groups demonstrated significant improvement over baseline findings [Table 2], but a comparison of treatment revealed no significant difference between the two graft materials. The gain in CAL for site A (BDX and GTR) was 4.21 ± 1.29 mm, while BDX and TISSEEL group showed a gain of 3.74 ± 1.43 mm. An evaluation of the free gingival margin at 6 months and 9 months revealed that none of the groups experienced any recession as compared to baseline [Table 3].
Table 1.
Summary of reduction in probing pocket depth (in mm) scores at baseline, 6 months, and 9 months for subjects who completed the study for Group A and B
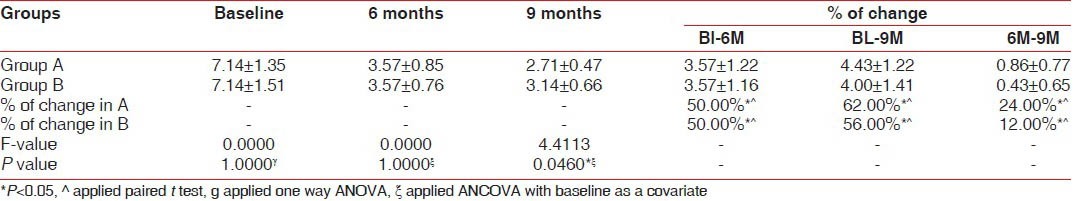
Table 2.
Summary of reduction in clinical attachment level (in mm) scores at baseline, 6 months, and 9 months for subjects who completed the study for Group A and B
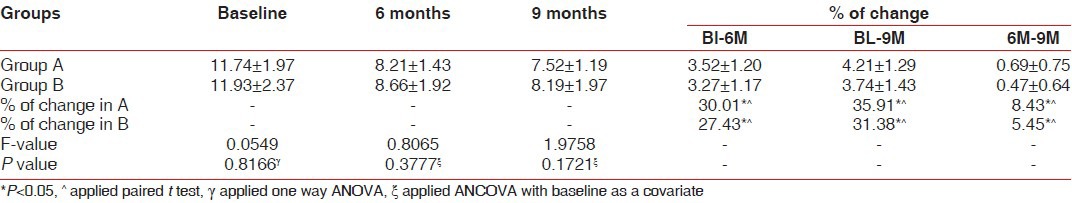
Table 3.
Summary of reduction in gingival recession (in mm) scores at baseline, 6 months, and 9 months for subjects who completed the study for Group A and B
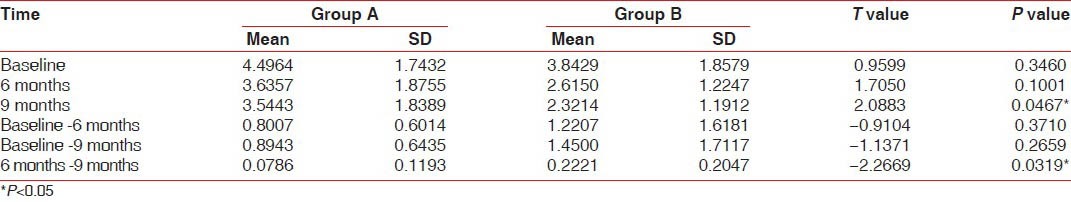
The radiographic evaluation of hard tissue findings at 6 and 9 months revealed that both treatment groups had improved significantly over baseline values with regard to bone fill and % defect resolution [Graph 2]. The average bone fill in BDX and GTR group was 3.74 ± 1.68 mm, while the BDX and TISSEEL group demonstrated an average bone fill of 4.24 ± 1.90 mm. This average bone fill corresponds to 43.66% bone fill for BDX and GTR and 52.75% for BDX and TISSEEL group. The average defect resolution for BDX and GTR was 68.56% and 64.91% for BDX and TISSEEL group, respectively. Finally, change in alveolar crestal height gain was significant in both groups. The comparison between the two groups at 6 months, 9 months postoperatively revealed the differences of −0.9, −1.1, respectively, which were statistically not significant. At 6 and 9 months postoperatively, there was a difference of -2.2, which was statistically significant (P < 0.03) [Table 4].
Graph 2.
Comparison of Group A and Group B with respect to bone fill and defect resolution
Table 4.
Comparison of Group A and Group B with respect to distance from CEJ to the alveolar crest (in mm) at baseline, 6 months, and 9 months by unpaired t-test
Statistical findings revealed similar results between BDX + GTR and BDX + TISSEEL for both hard and soft tissue analysis.
DISCUSSION
This study compared Bovine-Derived Xenograft (Bio-Oss Collagen) and Type I Collagen Membrane (Bio-Gide) With Bovine-Derived Xenograft (Bio-Oss Collagen) and Fibrin Fibronectin Sealing System (TISSEEL) in the treatment of human infrabony defects in patients with moderate to severe periodontitis. The results of the statistical analysis of this experiment found no significant difference between the 2 groups with regards to the outcome of treatment, while both groups demonstrated an overall significant improvement in all clinical parameters.
In the present study, only those patients were considered for periodontal surgery who showed good oral hygiene maintenance during the Phase-I therapy. Oral hygiene status was assessed by taking the plaque index at baseline, 1 month, 3 months, 6 months, and 9 months post-surgery.
The reduction in the PD for the group A (BDX and GTR) was 4.42 ± 1.22 mm, these findings being in agreement with those of Lekovic et al.[12] and Mellonig et al.[13] while in the BDX and TISSEEL group, PD reduction was 4.00 ± 1.41 which were similar with the findings of Cortellini et al.[14]
Clinical attachment level is another important parameter which has become widely accepted as one of the primary clinical end points of regenerative attempts around the natural teeth.[15] Both groups demonstrated significant improvement over baseline findings, but a comparison of treatment revealed no significant difference between the two graft materials. The gain in CAL for site A (BDX and GTR) was 4.21 ± 1.29 mm, which were in accordance with the studies of Camelo et al.,[3] Lekovic et al.,[16] Stavropoulos et al.,[17] and Sculean et al.[18] While the BDX and TISSEEL group showed a gain of 3.74 ± 1.43 mm, which was in accordance with the studies of Cortellini et al.,[14] Stefania et al.[19]
Radiographic parameters for the changes in the level of alveolar bone were done as per the method explained by Meffert et al.[20]
The defects which were treated with the bovine-derived xenograft in combination with TISSEEL showed an average of 9% of greater bone fill than the Bio-Oss Collagen and Bio-Gide group, but the difference was not statistically significant. These findings were in accordance with those of Hong et al.,[21] Stefania et al.[19]
Defect resolution was calculated as the distance between the crest of the alveolar bone to the base of the defect. In site A, the mean distance from alveolar crest to base of the defect was 4.09 ± 2.01 mm which was reduced to 2.80 ± 1.89 mm (68.56%) post surgery, showing statistically significant improvement in defect resolution. These findings were in accordance with those of Sculean et al.,[18] Stavropoulos et al.,[17] Tonnetti et al.,[22] Lekovic et al.,[12] Yamada et al.,[23] and Camelo et al.[3] In site B, the mean distance from the alveolar crest to the base of the defect was 4.28 ± 1.38 mm, which was reduced to 2.77 ± 1.6 mm (64.9%) at the end of 9 months which was statistically significant. These findings were in accordance with the studies of Hong et al.,[21] Stefania et al.,[19] Cortellini et al.[14] The inter group comparisons showed no statistical relevance.
After periodontal surgery, there is remodeling of alveolar bone leading to crestal resorption. The defect resolution occurs by defect fill as well as increase in alveolar crest height. Hence, recording the changes in the alveolar crest height is important.
In the present study, a gain in the alveolar crest height of 0.86 ± 0.68 mm (19.1%) at 6 months was observed which increased even more at 9 months postoperatively to 0.95 ± 0.68 mm, which was statistically highly significant. Similar trend was observed in site B also. The gain in the alveolar crest height was 1.2 ± 01.6 mm (31.9%) at 6 months and was further increased to 1.52 ± 1.61 mm (39.59%) postoperatively. The findings were statistically highly significant.
The comparison between the two experimental groups showed that there was higher increase in the alveolar crest height in Experimental site B where a combination of Bio-Oss Collagen and TISSEEL was used. The difference was statistically significant.
In the present study, a favorable response was observed in all the clinical and radiological parameters recorded at different intervals for both Site A and Site B, and were statistically highly significant (P < 0.001).
The placement and suturing of the membrane was time consuming as compared to bone grafting along with fibrin fibronectin sealing system, even though the FFS system added to the cost of the treatment.
No postoperative complications other than those considered normal following any surgical procedure were noticed in either of the two groups. Also, no antigenic reactions were observed in any of the patients, thereby indicating the safety of Bio-Oss Collagen as a bone graft material, TISSEEL as a carrier material, and Bio-Gide as bioresorbable membrane.
Application of fibrin glue provides an artificial clot that is very stable after 3 minutes and persists longer than the neutral clot because of the presence of anti-fibrinolytic substances.[24] The prolonged fixation of the fibrin glue into the surgical wound is the property that allows its use as a sustained local delivery carrier. In addition, human fibrin glue is rich in fibronectin, which has been reported to favor new attachment formation by adhering to root surfaces and promoting fibroblasts and periodontal ligament cells migration.[25]
Caffesse et al.[26] used fibrin and fibronectin in combination with barrier membranes to treat periodontal defects in beagle dogs. The authors showed slightly better results in sites treated with the combinations of fibrin-fibronectin and GTR when compared with sites treated with GTR alone. Furthermore, no detrimental effects of the fibrin/fibronectin system on histologic new attachment formation were observed.
The results of the present study confirm that infrabony defects can be successfully treated with bone graft and fibrin glue. Clinically and statistically significant amounts of probing attachment and bone gains were observed at the 9-month follow up. The comparison between the test and the control sites, however, did not reach statistical significance. Nevertheless, slightly better clinical results were obtained in the sites treated with fibrin glue in combination with bone graft in terms of gain in alveolar crest height. This can be partly explained by the greater depth of the infrabony component of the defects in the site B.[27] The lack of a significant difference may be due to the small number of subjects enrolled in the study.
CONCLUSION
The following conclusions were drawn from the present study:
Highly significant reduction in probing pocket depth took place in both the groups, which were almost identical. There was significant gain in the clinical attachment level in both the groups. Radiographically, a greater percentage of defect fill was noticed in both the groups.
There was a significant increase in the alveolar crest height in the group treated with Bio-Oss Collagen and TISSEEL combination. This might be because of the growth factors in the TISSEEL and the prolonged fixation of the fibrin glue into the surgical wound that allows its use as a sustained local delivery carrier. The regenerative materials (bovine-derived xenograft (Bio-Oss Collagen), Type I collagen membrane (Bio-Gide) and fibrin fibronectin sealing system (TISSEEL)) were safe to use. From the current study, it appears that both the treatment modalities were successful in showing the promising results in the treatment of periodontal infrabony defects.
LIMITATIONS
Surgical re-entry and histological evaluation was not done due to ethical considerations
The long-term effects of these treatment options need to be assessed with larger sample size and longer study period.
Materials used
Bio-Oss Collagen and Bio-Gide Membrane-Geistlich Pharma, Switzerland
TISSEEL-Baxter Healthcare Limited, UK.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Camargo PM, Lekovic V, Weinlaender M, Nedic M, Vasilic N, Wolinsky LE, et al. A controlled re-entry study on the effectiveness of bovine porous bone mineral used in combination with a collagen membrane of porcine origin in the treatment of intrabony defects in humans. J Clin Periodontol. 2000;27:889–96. doi: 10.1034/j.1600-051x.2000.027012889.x. [DOI] [PubMed] [Google Scholar]
- 2.Cortellini P, Bowers GM. Periodontal regeneration of intrabony defects: And evidence-based treatment approach. Int J Periodont Rest Dent. 1995;15:128–45. [PubMed] [Google Scholar]
- 3.Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, et al. Clinical, radiographic and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodont Rest Dent. 1998;18:321–31. [PubMed] [Google Scholar]
- 4.Zitzmann NU, Naef R, Scharer P. Resorbable versus non-resorbable membranes in combination with Bio-Oss for guided bone regeneration. Int J Maxillofac Implants. 1997;12:844–52. [PubMed] [Google Scholar]
- 5.Bartolucci EP, Pini Prato GP. Preliminary observations on the use of a biologic sealing system (Tissucol®) in periodontal surgery. J Periodontol. 1982;53:731–5. doi: 10.1902/jop.1982.53.12.731. [DOI] [PubMed] [Google Scholar]
- 6.Fabris G, Trombelli L, Schincaglia GP, Cavallini R, Calura G, del Senno L. Effects of a fibrin-fibronectin sealing system on proliferation and type I collagen synthesis of human PDL fihroblasts in vitro. J Clin Periodontol. 1998;25:11–4. doi: 10.1111/j.1600-051x.1998.tb02357.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Leary TJ, Drake R, Naylon JE. The Plaque control record. J Periodontol. 1972;43:38–8. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 8.Silness J, Loe H Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal conditions. Acta Odont Scand. 1964;24:747–59. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 9.Lynch SE. Methods for evaluation of regenerative procedures. J Periodontol. 1992;63:1085–92. doi: 10.1902/jop.1992.63.12s.1085. [DOI] [PubMed] [Google Scholar]
- 10.Oreamuno S, Lekovic V, Kenney EB, Carranza FA, Takei HH, Prokic B. Comparative clinical study of porous hydroxyapatite and decalcified freezedried bone in human periodontal defects. J Periodontol. 1990;61:399–404. doi: 10.1902/jop.1990.61.7.399. [DOI] [PubMed] [Google Scholar]
- 11.Clark DC, Quee TC, Bergeon MJ, Chan ECS, Lautar-Lemay C, de Gruchy K. Reliability of attachment level measurements using the cementoenamel junction and plastic stent. J Periodontol. 1987;58:115–8. doi: 10.1902/jop.1987.58.2.115. [DOI] [PubMed] [Google Scholar]
- 12.Camargo PM, Lekovic V, Weinlaender M, Nedic M, Vasilic N, Wolinsky LE, et al. A controlled re-entry study on effectiveness of bovine porous bone mineral used in combination with a collagen membrane of porcine origin in the treatment of intrabony defects in humans. J Clin Periodontol. 2000;27:889–96. doi: 10.1034/j.1600-051x.2000.027012889.x. [DOI] [PubMed] [Google Scholar]
- 13.Mellonig JT. Human histologic evaluation of a bovine-derived bone xenograft in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 2000;20:19–29. [PubMed] [Google Scholar]
- 14.Cortellini P, Pini Prato GP, Tonetti MS. No detrimetital effect of fibrin glue on the regeneration of intrabony defects. A controlled clinical trial. J Clin Periodontol. 1995;22:697–702. doi: 10.1111/j.1600-051x.1995.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 15.Caton JG, Greenstein G. Factors related to periodontal regeneration. Periodontol 2000. 1993;1:9–15. [PubMed] [Google Scholar]
- 16.Lekovic V, Camargo PM, Weinlaender M, Kenney EB, Vasilic N. Combination use of Bovine porous bone mineral, enamel matrix proteins, and a bioabsorbable membrane in intrabony periodontal defects in humans. J Periodontol. 2001;72:583–9. doi: 10.1902/jop.2001.72.5.583. [DOI] [PubMed] [Google Scholar]
- 17.Stavropoulos A, Karring T. Five-year results of guided tissue regeneration in combination with deproteinized bovine bone (Bio-Oss®) in the treatment of intrabony periodontal defects: A case series report. Clin Oral Invest. 2005;9:271–7. doi: 10.1007/s00784-005-0002-7. [DOI] [PubMed] [Google Scholar]
- 18.Sculean A, Schwarz F, Chiantella GC, Donos N, Arweiler NB, Brecx M, et al. Five-year results of a prospective, randomized, controlled study evaluating treatment of intra-bony defects with a natural bone mineral and GTR. J Clin Periodontol. 2007;34:72–7. doi: 10.1111/j.1600-051X.2006.01007.x. [DOI] [PubMed] [Google Scholar]
- 19.Re S, Corrente G, Abundo R, Cardaropoli D. Orthodontic movement into bone defects augmented with bovine bone mineral and fibrin sealer: A Re-entry case report. Int J Periodontics Rest Dent. 2002;22:138–45. [PubMed] [Google Scholar]
- 20.Meffert RM, Thomas JR, Hamilton KM, Brownstein CN. Hydroxylapatite as an alloplastic graft in the treatment of human periodontal osseous defects. J Periodontol. 1985;56:63–73. doi: 10.1902/jop.1985.56.2.63. [DOI] [PubMed] [Google Scholar]
- 21.Hong SJ, Kim CS, Han DK, Cho IH, Jung UW, Choi SH, et al. The effect of a fibrin-fibronectin/β-tricalcium phosphate/recombinant human bone morphogenetic protein-2 system on bone formation in rat calvarial defects. Biomaterials. 2006;27:3810–6. doi: 10.1016/j.biomaterials.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 22.Cortellini P, Tonetti MS. Long-term tooth survival following regenerative treatment of intrabony defects. J Periodontol. 2004;75:672–8. doi: 10.1902/jop.2004.75.5.672. [DOI] [PubMed] [Google Scholar]
- 23.Yamada S, Shima N, Kitamura H, Sugito H. Effect of porous xenographic bone graft with collagen barrier membrane on periodontal regeneration. Int J Periodont Rest Dent. 2002;22:389–97. [PubMed] [Google Scholar]
- 24.Pini Prato GP, Cortellini P, Agudio G, Clauser C. Human fibrin glue versus sutures in periodontal surgery. J Periodontol. 1987;58:426–31. doi: 10.1902/jop.1987.58.6.426. [DOI] [PubMed] [Google Scholar]
- 25.Poison AM, Proye MP. Fibrin linkage: A precursor for new attachment. J Periodontol. 1983;54:141–7. doi: 10.1902/jop.1983.54.3.141. [DOI] [PubMed] [Google Scholar]
- 26.Caffesse RG, Nasjleti CE, Anderson GB, Lopatin DE, Smith BA, Morrison EC. Periodontal healing following guided tissue regeneration with citric acid and fibronectin application. J Periodontol. 1991;62:21–9. doi: 10.1902/jop.1991.62.1.21. [DOI] [PubMed] [Google Scholar]
- 27.Tonetti M, Pini-Prato G, Cortellini P. Periodontal regeneration of human infrabony defects (IV). Determinants of the healing response. J Periodontol. 1993;64:934–40. doi: 10.1902/jop.1993.64.10.934. [DOI] [PubMed] [Google Scholar]


