Abstract
Background: Breakfast is associated with lower body weight in observational studies. Public health authorities commonly recommend breakfast consumption to reduce obesity, but the effectiveness of adopting these recommendations for reducing body weight is unknown.
Objective: We tested the relative effectiveness of a recommendation to eat or skip breakfast on weight loss in adults trying to lose weight in a free-living setting.
Design: We conducted a multisite, 16-wk, 3-parallel-arm randomized controlled trial in otherwise healthy overweight and obese adults [body mass index (in kg/m2) between 25 and 40] aged 20–65 y. Our primary outcome was weight change. We compared weight change in a control group with weight loss in experimental groups told to eat breakfast or to skip breakfast [no breakfast (NB)]. Randomization was stratified by prerandomization breakfast eating habits. A total of 309 participants were randomly assigned.
Results: A total of 283 of the 309 participants who were randomly assigned completed the intervention. Treatment assignment did not have a significant effect on weight loss, and there was no interaction between initial breakfast eating status and treatment. Among skippers, mean (±SD) baseline weight-, age-, sex-, site-, and race-adjusted weight changes were −0.71 ± 1.16, −0.76 ± 1.26, and −0.61 ± 1.18 kg for the control, breakfast, and NB groups, respectively. Among breakfast consumers, mean (±SD) baseline weight-, age-, sex-, site-, and race-adjusted weight changes were −0.53 ± 1.16, −0.59 ± 1.06, and −0.71 ± 1.17 kg for the control, breakfast, and NB groups, respectively. Self-reported compliance with the recommendation was 93.6% for the breakfast group and 92.4% for the NB group.
Conclusions: A recommendation to eat or skip breakfast for weight loss was effective at changing self-reported breakfast eating habits, but contrary to widely espoused views this had no discernable effect on weight loss in free-living adults who were attempting to lose weight. This trial was registered at clinicaltrails.gov as NCT01781780.
See corresponding editorial on page 503.
INTRODUCTION
A common public health message from reputable sources is that eating breakfast is important to achieve and maintain a healthy weight (1). Observational evidence suggests that there is an association of breakfast with body weight and weight loss. However, there is little causal evidence to support this conjecture. The need for evidence to determine whether there is a causal effect of regular breakfast consumption on weight loss was recently emphasized (2, 3).
The association of breakfast consumption with lower body weight is well established (4–8); however, observational evidence does not preclude the possibility that breakfast eaters tend to weigh less because of other weight-related factors associated with breakfast eating. Short-term studies highlight potential physiologic mechanisms by which breakfast may influence appetite, energy expenditure, fat oxidation, and body weight (9–12). Nevertheless, whether the proposed physiologic mechanisms translate to long-term effects on energy intake and body weight is unclear.
Some hypotheses with regard to breakfast consumption and lower body weight are based on the conjecture that breakfast consumption is important for the regulation of energy intake. Some studies suggest that skipping breakfast results in higher energy intake at lunch compared with when breakfast is consumed (9, 13–15). Others suggest that skipping breakfast may not be compensated for through increasing energy intake later in the day, resulting in net negative energy balance relative to when breakfast is consumed (16, 17). Energy balance may be maintained through successive compensations over several days (18), but long-term studies of the influence of breakfast consumption compared with prolonged fasting on energy intake under free-living, ad libitum conditions have not been conducted. Therefore, the long-term impact of breakfast on energy intake and weight loss is not clear.
An extensive review (3) showed only one randomized controlled trial (RCT) pertinent to the effects of breakfast eating compared with breakfast skipping on weight in a nonmalnourished industrialized population. This RCT tested the impact of eating or skipping breakfast on weight loss (19). Their findings suggested that the effect of breakfast on weight loss might depend on breakfast eating habits before the study, such that switching breakfast eating habits as a result of study assignment increased weight loss. However, the study was not well powered to detect this interaction, and the result was only suggestive of significance (P = 0.06).
Herein, we conducted an RCT to determine whether advising good nutrition habits is more effective at producing weight loss if paired with advice to skip or eat breakfast. We tested the effect of breakfast recommendations on weight loss in free-living adults who were attempting to lose weight, because this population is likely affected by public health breakfast recommendations. On the basis of previous findings (19), we stratified randomization by baseline breakfast eating habits and hypothesized that individuals who were advised to switch their usual breakfast eating habits due to their group assignment would lose more weight than the control group.
SUBJECTS AND METHODS
Study design
We conducted a multisite, 3-parallel-arm RCT. Study sites included the University of Alabama at Birmingham, University of Copenhagen (Denmark), Boston Medical Center, Columbia University, and the University of Colorado, Denver. All sites conducted the study in a clinical research setting. After screening, participants were randomly assigned to 1 of 3 groups [control, breakfast, or no breakfast (NB)], and randomization was stratified by typical breakfast eating habits at baseline. The study duration was 16 wk, and the primary outcome measure was weight change from baseline. Weight was measured at baseline and after 16 wk, and additional contacts included phone calls at weeks 4, 8, and 12. All study visits occurred between January 2013 and January 2014. The trial was stopped when recruitment goals were met and all participants had completed the 16-wk duration. The study was registered at clinicaltrials.gov (registry no. NCT01781780).
Study sample
Inclusion criteria were as follows: aged 20–65 y, BMI (in kg/m2) >25 but <45, interested in weight loss, and beginning the day by 0900 h at least 5 d/wk. Exclusion criteria were participation in a weight-reduction program in the past 3 mo, weight loss or gain ≥5% of body weight in the past 6 mo, taking medication that affects appetite (weight-loss drugs or antidepressant, steroid, or thyroid medication, unless dosage has been stable for at least 6 mo), taking medication that requires eating food in the morning, a history of prior surgical procedure for weight control, current smoker or had smoked <6 mo before the start of the study, any major disease, a score on the Brief Symptom Inventory that exceeded the 90th percentile (20) or a T-score ≥63 on the global severity index or on 2 of the 9 symptom scales on the Symptom Checklist 90-R (21), current eating disorder [a 26-item Eating Attitudes Test (22) score >20], a recent or ongoing problem with drug abuse or addiction, excessive alcohol intake, night shift work, pregnancy, or breastfeeding.
Participants were recruited from cities of participating sites through e-mail, campus newspaper advertisements, flyers, and word of mouth. Participants were screened over the phone for initial eligibility criteria and asked to come into the clinic for a second level of screening. After informed consent, weight and height, a pregnancy test for all women, the Brief Symptom Inventory–18 (20), or Symptom Checklist 90-R21 (Copenhagen site only), and the Eating Attitudes Test (22) were all administered at a second screening to determine final eligibility. If participants passed the second level of screening and were interested, they underwent random assignment. The institutional review board, or ethical committee, of each participating institution approved the protocol, and all participants provided written informed consent.
Intervention
Participants enrolled in the study were randomly assigned to 1 of 3 groups at the baseline visit. The control group received a USDA pamphlet “Let's Eat for the Health of It,” describing general good nutrition habits (with no mention of breakfast) (23), along with a handout emphasizing the main points of the pamphlet. The breakfast group received the USDA pamphlet with a handout instructing participants to consume breakfast before 1000 h every day. The breakfast handout also provided suggestions of food items that might constitute a healthy breakfast; however, no specific restrictions were given on types of foods that could be consumed for the breakfast meal. The NB group received the USDA pamphlet with a handout instructing participants not to consume any calories before 1100 h every day, and that only water or zero-calorie beverages could be consumed from the time of waking until 1100 h.
The study coordinators reviewed the USDA pamphlet and the appropriate handout documents with participants in detail by reading them aloud and answering any questions. It was recommended that participants incorporate the suggestions as best they could into their daily life. Follow-up phone calls occurred at weeks 4, 8, and 12 to enhance participant compliance and attrition. During these calls, participants were asked if they were following the recommendations, keeping their compliance diary (breakfast and NB groups), and if they were experiencing any barriers to incorporating the recommendations into their diet. Weight and height were measured at baseline, and body weight was measured again at the end of the trial period. Participants were not blinded to their treatment condition; however, all study staff who measured final weights were blinded to participants’ group assignment.
Randomization
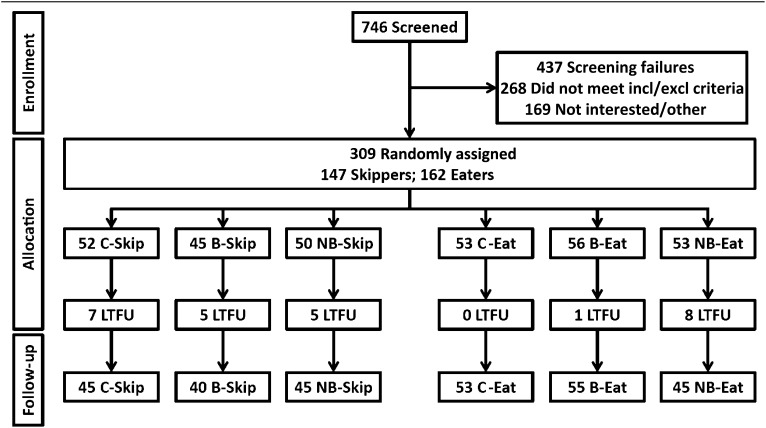
Participants who ate breakfast regularly (≥4 times/wk) were randomly assigned separately from those who did not frequently eat breakfast (≤3 times/wk); hereafter, this distinction will be referred to as “prerandomization status,” with participants classified as either breakfast “eaters” or “skippers.” Participants were randomly assigned to control, breakfast, or NB in a manner enforcing equal (1:1:1) allocation, specifically a single block size of n = 27, with 9 control, 9 breakfast, and 9 NB assignments. This was done within each site and prerandomization status classification. Because of implementation errors or sites not meeting recruitment goals, perfect balance was achieved at only one site; however, these issues did not cause treatment assignments to be unduly unbalanced across sites or prerandomization statuses (see Figure 1). The assigned treatment groups were specified on cards contained in sequentially numbered, opaque, sealed envelopes that were prepared by the Office of Energetics at the University of Alabama at Birmingham. Sealed envelopes were sent to participating sites, and study coordinators enrolled participants and opened the next consecutively numbered envelope in the appropriate site-by-initial-status series in the presence of the participant. Randomization was performed with R, version 2.15.3 (24), by using a pseudo-random number generator with an arbitrary but fixed seed.
FIGURE 1.
Flow of participants through enrollment, allocation, and follow-up: CONSORT diagram. “Eat” indicates participants who were breakfast eaters before the study (≥4 times/wk); “Skip” indicates participants who were breakfast skippers before the study (≤3 times/wk). B, breakfast group; C, control group; CONSORT, Consolidated Standards of Reporting Trials; incl/excl, inclusion/exclusion; LTFU, lost to follow-up; NB, no-breakfast group.
Measures
Participants’ height was measured at baseline without shoes by using a wall-mounted stadiometer. Weight was measured at baseline and at the final study visit after 16 wk by using electronic scales to the nearest 0.1 kg with participants wearing light clothing without shoes. Weight change from baseline to follow-up was the primary outcome of interest. BMI was calculated as kilograms divided by meters squared by using measured weight and height for the purpose of screening. Compliance with the recommendations in the breakfast and NB groups was tracked by using a self-report diary that asked participants to circle “yes” or “no” for each day they were enrolled in the study to indicate if they had complied with the breakfast recommendation. Compliance with the intervention was calculated as the percentage of days participants complied with the breakfast recommendation, and this was a secondary outcome.
Statistical analyses
All analyses were performed by using R (version 2.15.3) (24). An intent-to-treat analysis of change in body weight over the 16-wk intervention period (n = 309) was used to determine the primary outcome of this RCT. Multiple imputation was used to account for missing data. A completers-only analysis was also conducted (n = 283). Ordinary least-squares linear regression models, adjusted for baseline measurements and including initial weight as a covariate, were used to compare participants across assignment groups. Means and SDs were calculated on the basis of data at the baseline and week 16 time points, as well as for changes in the outcome measures between these 2 time points.
As a secondary analysis, data from the diaries of completers were used to assess the impact of compliance on the breakfast and NB arms, to perform an as-treated analysis (n = 185). Participants in the control group did not complete diaries, because they were not given specific instructions with respect to breakfast consumption. For the breakfast and NB participants, their group indicator was multiplied by the proportion of days in compliance and treated as a continuous covariate. During an exit interview with participants, it was determined that circled diary days were sometimes used by participants to indicate breakfast had been eaten on that day, rather than compliance with their group instructions, as was instructed (see Supplemental Figure 1, A and B, under “Supplemental data” in the online issue). Therefore, because it was recorded for most participants during their exit interview if they filled out the diary according to breakfast consumption, or compliance with instructions, compliance proportions were discerned through participant intent when filling out the diary as best as possible (see Supplemental Figure 1, C and D, under “Supplemental data” in the online issue). Because this information was not available for every participant and our efforts did not completely ameliorate the compliance question (note the tails bounded by the dashed boxes in panels C and D), we performed the secondary analysis twice, once with compliance as given in panels C and D and once with the compliance “yes” and “no” counts swapped for boxed individuals. In cases in which compliance proportion could not be ascertained, either due to missing diaries or an inability to discern intent, this “missingness” was handled by multiple imputation.
As a tertiary analysis, we examined whether or not participants randomly assigned to the control group changed their self-reported breakfast eating habits, with specific interest in prerandomization status. Logistic regression was used to perform this analysis of completers (n = 105).
To accommodate missing values in the participants who did not complete the week 16 follow-up or who were missing compliance information, multiple imputation was performed. In the imputation process, a total of 10 imputed data sets were created and analyzed. For this trial, given the very small amount of missing data (8.4% of weight-change data and 12% of compliance data), 10 imputations were sufficient for imputation (25). The software package used to implement the multiple imputation and pooling procedure was Multivariate Imputation by Chained Equations library, version 2.18 (26), found in R, version 2.15.3 (24). As a sensitivity analysis, we also conducted the same overall modeling with the use of completers only. Tests of significance were conducted at the 0.05 2-tailed significance level.
The study sample was calculated such that we had at least 90% power at a 2-tailed α of 0.05 for pairwise comparisons between groups to detect an interaction between treatment and stratification group. We did this by first using the Schlundt et al (19) study interaction effect to determine sample size with the use of the calculation in Tiwari et al (27). Because the Schlundt et al study was a 2-group study, and we had 3 treatment groups, the sample size of 177 was multiplied by 1.5 to account for the extra group, making 266 participants necessary for our study. To account for a dropout rate of 13.5% as reported in the Schlundt et al article, a total of 308 participants were expected to be recruited. This would leave an adequate sample size of 266 for 90% power to detect a small to moderate effect size of r = 0.22–0.34 reported in Schlundt et al.
RESULTS
Demographic characteristics
Of the 309 participants who were randomly assigned, 283 completed the study. Fourteen participants were lost to follow-up, and 12 participants discontinued the study for various reasons (pregnancy, time or work constraints, or did not wish to participate in the control group). Comparisons of baseline covariates across treatment groups are given in Table 1. There were no significant differences in baseline covariates between the treatment groups at baseline.
TABLE 1.
Baseline demographic characteristics1
| Group |
||||
| Control (n = 105) | Breakfast (n = 101) | No breakfast (n = 103) | P | |
| Height (cm) | 168.2 ± 8.12 | 167.0 ± 7.3 | 166.7 ± 10.0 | 0.42 |
| Weight (kg) | 90.8 ± 16.8 | 89.6 ± 13.9 | 91.9 ± 15.9 | 0.57 |
| Women (n) | 79 | 74 | 81 | 0.66 |
| Age (y) | 42.1 ± 11.2 | 40.6 ± 12.0 | 42.0 ± 12.4 | 0.58 |
| Race (n) | 0.39 | |||
| White non-Hispanic | 54 | 45 | 48 | |
| Black non-Hispanic | 32 | 40 | 34 | |
| White Hispanic | 6 | 5 | 12 | |
| Black Hispanic | 2 | 5 | 3 | |
| Other | 11 | 6 | 6 | |
| Site (n) | 1.00 | |||
| UAB3 | 24 | 25 | 23 | |
| Columbia University | 24 | 20 | 23 | |
| Colorado | 15 | 14 | 15 | |
| Copenhagen | 18 | 18 | 18 | |
| Boston | 24 | 24 | 24 | |
| Breakfast skippers (n) | 52 | 45 | 50 | 0.73 |
n = 309. There were no missing data for these variables. P values were calculated by using either an F test or Fisher's exact test, as appropriate.
Mean ± SD (all such values).
UAB, University of Alabama at Birmingham.
Effect of treatment on weight loss
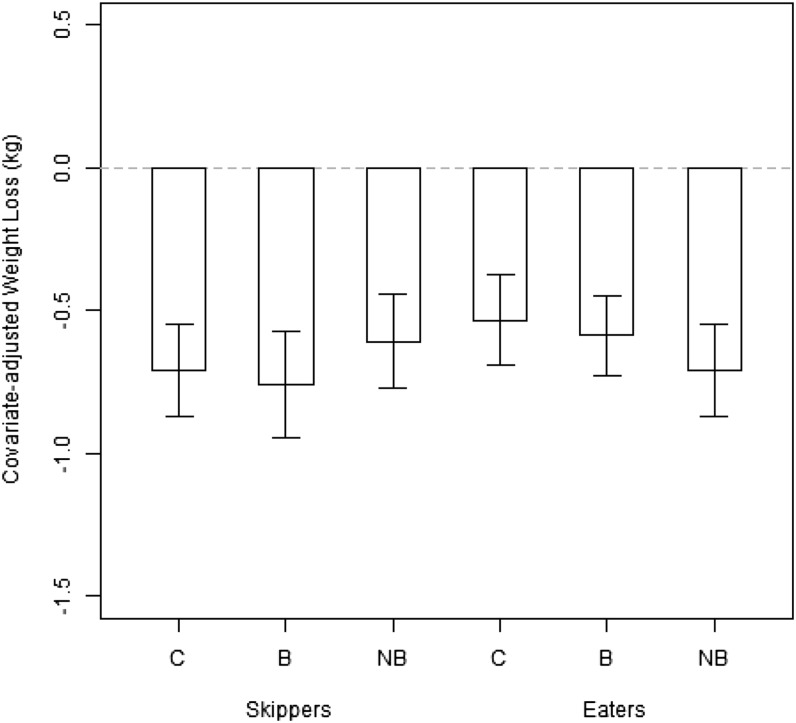
For the completers-only analysis (n = 283), there was no effect of treatment assignment on weight loss (P = 0.77; see Table 2 and Figure 2). Among breakfast skippers, baseline mean (±SD) weight-, age-, sex-, site-, and race-adjusted weight changes were −0.71 ± 1.16, −0.76 ± 1.26, and −0.61 ± 1.18 kg for the control, breakfast, and NB groups, respectively. Among breakfast eaters, baseline mean (±SD) weight-, age-, sex-, site-, and race-adjusted weight changes were −0.53 ± 1.16, −0.59 ± 1.06, and −0.71 ± 1.17 kg for the control, breakfast, and NB groups, respectively.
TABLE 2.
Completer interaction model: weight-loss type II ANOVA1
| Sum of squares | df | F | P | |
| Initial weight | 10.47 | 1 | 1.05 | 0.31 |
| Age | 31.16 | 1 | 3.12 | 0.079 |
| Race | 112.25 | 4 | 2.81 | 0.026 |
| Site | 107.45 | 4 | 2.69 | 0.032 |
| Male sex | 42.57 | 1 | 4.26 | 0.040 |
| Prerandomization status | 0.73 | 1 | 0.07 | 0.79 |
| Assignment | 5.13 | 2 | 0.26 | 0.77 |
| Site × assignment | 7.12 | 2 | 0.36 | 0.70 |
| Prerandomization status × assignment | 92.98 | 8 | 1.16 | 0.32 |
| Residuals | 2578.91 | 258 |
n = 283. Values were determined by type II ANOVA for the analysis of weight loss as a function of initial weight, age, race, site, sex, prerandomization status, and experimental assignment under a “completers only” analysis. In addition, interactions of assignment by site and by prerandomization status were considered. No effect involving assignment was significant.
FIGURE 2.
Mean (±SD) covariate-adjusted weight changes by treatment and stratification. The gray dashed line represents “0” or no weight change from baseline. ANOVA found no significant effects of treatment or stratification on weight change. Among breakfast skippers, baseline mean (±SD) weight-, age-, sex-, site-, and race-adjusted weight changes were −0.71 ± 1.16, −0.76 ± 1.26, and −0.61 ± 1.18 kg for the C, B, and NB groups, respectively. Among breakfast eaters, baseline mean (±SD) weight-, age-, sex-, site-, and race-adjusted weight changes were −0.53 ± 1.16, −0.59 ± 1.06, and −0.71 ± 1.17 kg for the C, B, and NB groups, respectively. B, breakfast group; C, control group; NB, no-breakfast group.
After adjustment for age, race, site, sex, prerandomization status, and baseline weight, there was no evidence of an interaction between treatment assignment and site (P = 0.32) nor between treatment assignment and prerandomization status (P = 0.70) on weight change. With restriction to a strictly additive model (see Table 3), weight change was not significantly affected by prerandomization status (P = 0.71). Subject sex was nominally significant, with men losing, on average, 0.99 kg more than women (P = 0.040). The age of the subject was suggestive of a linear trend, with older individuals losing more weight, on average, than their younger counterparts (a difference of 0.34 kg for every 10 y of age; P = 0.050). Significant differences in weight change by race (P = 0.034) were primarily driven by evidence that black Hispanics tended to lose less weight than other ethnicities (weight change shifted by +3.4 kg), although the sample size for this subgroup was quite small (n = 10). Significant differences in weight change by site (P = 0.035) were primarily driven by the University of Copenhagen site, where participants lost more weight, on average, than those at other sites (weight change shifted by −1.75 kg). All of the aforementioned effects in the additive model were calculated simultaneously (type II ANOVA). The multiple imputation produced comparable results, with no differences in weight change by treatment assignment or interactions of treatment assignment with site or with prerandomization status. Findings with regard to adjusted covariates were also similar, with no change in nominal significance.
TABLE 3.
Completer additive model: weight-loss type II ANOVA1
| Sum of squares | df | F | P | |
| Initial weight | 6.43 | 1 | 0.64 | 0.42 |
| Age | 38.69 | 1 | 3.87 | 0.050 |
| Race | 105.99 | 4 | 2.65 | 0.034 |
| Site | 104.74 | 4 | 2.62 | 0.035 |
| Male sex | 42.70 | 1 | 4.27 | 0.040 |
| Prerandomization status | 1.38 | 1 | 0.13 | 0.71 |
| Assignment | 5.13 | 2 | 0.26 | 0.77 |
| Residuals | 2679.77 | 268 |
n = 283. Values were determined by type II ANOVA for the analysis of weight loss as a function of initial weight, age, race, site, sex, prerandomization status, and experimental assignment in a strictly additive model (no interactions allowed) under a “completers only” analysis. Assignment was not significant.
Compliance-adjusted treatment effects
On average, self-reported compliance (calculated as percentage of days that participants followed breakfast recommendation after determining intent when filling out the diary) was 93.6% for the breakfast group and 92.4% for the NB group in completers. Adjustment of treatment assignment by compliance did not result in a significant effect of treatment on weight loss (P = 0.82) when using the compliance information “as is” in a completers-only analysis (Table 4). Results were not different under multiple imputation, “fixing” the compliance information, or both.
TABLE 4.
Completer additive model: compliance-adjusted weight loss1
| Sum of squares | df | F | P | |
| Initial weight | 11.72 | 1 | 1.14 | 0.28 |
| Age | 27.36 | 1 | 2.65 | 0.10 |
| Race | 93.69 | 4 | 2.27 | 0.062 |
| Site | 76.34 | 4 | 1.85 | 0.12 |
| Male sex | 41.51 | 1 | 4.03 | 0.046 |
| Prerandomization status | 0.87 | 1 | 0.08 | 0.77 |
| Compliance-adjusted assignment | 4.05 | 2 | 0.20 | 0.82 |
| Residuals | 2574.32 | 250 |
n = 183. Values were determined by type II ANOVA for the analysis of weight loss as a function of initial weight, age, race, site, sex, prerandomization status, and compliance-adjusted assignment in a strictly additive model (no interactions allowed) under a “completers only” analysis. Compliance-adjusted assignment was not significant.
Effect of control condition on breakfast eating habits
Among those assigned to the control group, there were 44 a priori breakfast skippers and 52 breakfast eaters. Over the course of the experiment, 15 of 44 breakfast skippers began eating breakfast regularly, whereas only 4 of 52 regular breakfast eaters began skipping breakfast. The difference in odds of changing behaviors cannot be explained by initial weight, age, race, study site, or sex (Table 5); thus, the proclivity of changing breakfast eating habits differed significantly by prerandomization status, with skippers more likely to become breakfast eaters during the intervention than vice versa (covariate-adjusted OR: 6.1; 95% CI: 1.72, 27.65; P = 0.01) (Table 5). Results were not different under multiple imputation.
TABLE 5.
Control group breakfast habit changes: completers1
| Likelihood ratio χ2 | df | P | |
| Initial weight | 1.71 | 1 | 0.19 |
| Age | 0.030 | 1 | 0.86 |
| Race | 8.94 | 4 | 0.063 |
| Site | 9.11 | 4 | 0.058 |
| Male sex | 0.15 | 1 | 0.70 |
| Prerandomization status | 6.65 | 1 | 0.010 |
n = 105. Values were determined by type II analysis of deviance for the analysis of spontaneous changing of breakfast eating habits among the control group. The binary outcome of habit change was considered in a logistic regression, regressing on prerandomization status while adjusting for initial weight, age, race, site, and sex under a “completers only” analysis. There was a significant effect for prerandomization status (15 of 44 breakfast skippers became breakfast eaters, whereas only 4 of 52 breakfast eaters became breakfast skippers).
DISCUSSION
Although breakfast consumption can help ensure adequate nutrient intake and may have several health benefits (28–30), this RCT showed no effect of a recommendation to eat or skip breakfast on weight loss. This experiment was not designed to test the efficacy of a particular breakfast [eg, protein quality (31)] or of the precise timing of isocaloric amounts of food on weight loss (32), and conclusions with regard to the influence of breakfast type or meal timing on weight loss cannot be drawn from this study. Rather, we intended to test the effectiveness of a public health recommendation to skip or eat breakfast in causing weight loss in free-living individuals attempting to lose weight over a 16-wk period.
Compliance with recommendations to eat or skip breakfast, at least as judged by self-report, was high (93.6% for the breakfast group and 92.4% for the NB group), suggesting that the recommendation was effective at producing the intended effects on breakfast consumption. Therefore, we tested the effect of public health breakfast recommendations among individuals who wanted to lose weight and who took the recommendation seriously and made an attempt to follow that recommendation. It should also be noted that some individuals interpreted the control general nutrition recommendations to mean that they should begin eating breakfast, particularly if they were skippers before the study, even though breakfast was not mentioned in the control group instructions. This may have weakened our power to detect differences between the control and breakfast groups but not between the breakfast-skipping group and the other groups.
Contrary to the near-significant interaction found by Schlundt et al (19) between initial breakfast eating habits and treatment assignment that suggested that individuals who switch their breakfast eating habits may lose more weight, we saw no interaction of prerandomization status and treatment assignment. They tested the impact of breakfast on weight loss as part of a clinical weight-loss program that involved specific caloric intake reduction targets and counseling to help participants meet that target. Our findings suggest that the observed interaction in the Schlundt et al study may have been a type 1 error.
Alternatively, intake was considerably more ad libitum in our study than in the Schlundt et al study and reflective of what individuals would do in a free-living condition if exposed to a public health recommendation to eat more healthfully and either skip or eat breakfast. It is likely that participants were freer to compensate for any changes in energy intake that would have resulted from changes in breakfast eating habits in our study compared with the Schlundt et al study, which may account for a lack of treatment by prerandomization status interaction in our study. Our findings do not preclude the possibility that in a more controlled, clinical weight-loss setting, switching breakfast eating habits would produce more weight loss and only suggest that in a free-living less controlled setting, breakfast eating habits do not influence weight loss.
Several strengths of our study design should be considered. This is the largest RCT designed to determine the effectiveness of breakfast recommendations on weight loss. The large sample size and multiple sites ensure both adequate power and reasonable generalizability. In addition, our randomized design and rigorous statistical analysis strengthen inferences about causation.
Several limitations of our study should also be considered. We did not measure body composition or other metabolic variables. Previous findings suggest that breakfast consumption may affect endocrine regulation of appetite and body fatness (9–12), In addition, our study was 16 wk in duration, which may not have been long enough to detect an effect on body weight if the overall change in energy intake resulting from the breakfast habit changes is very small.
Because a general recommendation to eat or skip breakfast did not influence weight loss in this study, future research might assess whether more specific recommendations with regard to the timing and quantity of meals or meal compositions might improve weight-loss outcomes. In addition, our findings can only be applied to free-living adults who are attempting to lose weight, and therefore trials to determine the effectiveness of breakfast recommendations in a clinical weight-loss setting, or in children and adolescents, merit consideration. This is an important area in need of investigation to ensure that public health recommendations are effective and not unnecessarily using public resources or discouraging public trust of authorities in the efforts to reduce the population prevalence of obesity.
Supplementary Material
Acknowledgments
We thank registered dieticians Bettina Belmann Mirasola and Maria Roed Andersen who helped collect data at the University of Copenhagen.
The authors’ responsibilities were as follows—DBA and EJD: designed the research; EJD, A Alcorn, MC, ACB, EAT, and LHL: conducted the research; JMS, CMA, JOH, DBA, M-PS-O, and A Astrup: provided essential materials and facilities; JD: performed statistical analysis; EJD and JD: wrote the manuscript (only authors who made a major contribution); and EJD: had primary responsibility for final content. The authors disclose that, although not for the support of this study, the authors or their institutions have received gifts, grants, or consulting fees from multiple organizations that market products commonly consumed at breakfast, including Kraft Foods (DBA), Kellogg Company (DBA), Cooking Light magazine (DBA), Quaker (Pepsi; DBA), the Dairy Research Institute (DBA), the Egg Board (DBA), Global Dairy Platform (A Astrup), Arla (A Astrup), Cargill (LHL), McDonalds (JOH), General Mills (JOH), Walt Disney Company (JOH), Retrofit (JOH), Nutrisystem (CMA), MetaProteomics (CMA), Dunkin’ Donuts (CMA), Au Bon Pain (CMA), Bay State Milling Company (CMA), and Post Cereals (M-PS-O). None of these organizations were involved in the support, design, analysis, or interpretation of this study. EJD, JD, A Alcorn, EAT, MC, ACB, and JMS had no conflicts of interest to declare.
REFERENCES
- 1.Zeratsky K. Why does eating a healthy breakfast help control weight? Available from: http://www.mayoclinic.org/food-and-nutrition/expert-answers/FAQ-20058449 (cited 12 February 2014).
- 2.Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, Bohan Brown MM, Durant N, Dutton G, Foster EM, Heymsfield SB, et al. Myths, presumptions, and facts about obesity. N Engl J Med 2013;368:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AW, Bohan Brown MM, Allison DB. Belief beyond the evidence: using the proposed effect of breakfast on obesity to show 2 practices that distort scientific evidence. Am J Clin Nutr 2013;98:1298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horikawa C, Kodama S, Yachi Y, Heianza Y, Hirasawa R, Ibe Y, Saito K, Shimano H, Yamada N, Sone H. Skipping breakfast and prevalence of overweight and obesity in Asian and Pacific regions: a meta-analysis. Prev Med 2011;53:260–7. [DOI] [PubMed] [Google Scholar]
- 5.Mesas AE, Munoz-Pareja M, Lopez-Garcia E, Rodriguez-Artalejo F. Selected eating behaviours and excess body weight: a systematic review. Obesity Rev 2012;13(2):106-35. [DOI] [PubMed] [Google Scholar]
- 6.Rampersaud GC, Pereira MA, Girard BL, Adams J, Metzl JD. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc 2005;105:743–60; quiz 61–2. [DOI] [PubMed] [Google Scholar]
- 7.Szajewska H, Ruszczynski M. Systematic review demonstrating that breakfast consumption influences body weight outcomes in children and adolescents in Europe. Crit Rev Food Sci Nutr 2010;50:113–9. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh-Taskar PR, Nicklas TA, O'Neil CE, Keast DR, Radcliffe JD, Cho S. The relationship of breakfast skipping and type of breakfast consumption with nutrient intake and weight status in children and adolescents: the National Health and Nutrition Examination Survey 1999-2006. J Am Diet Assoc 2010;110:869–78. [DOI] [PubMed] [Google Scholar]
- 9.Astbury NM, Taylor MA, Macdonald IA. Breakfast consumption affects appetite, energy intake, and the metabolic and endocrine responses to foods consumed later in the day in male habitual breakfast eaters. J Nutr 2011;141:1381–9. [DOI] [PubMed] [Google Scholar]
- 10.Smeets AJ, Westerterp-Plantenga MS. Acute effects on metabolism and appetite profile of one meal difference in the lower range of meal frequency. Br J Nutr 2008;99:1316–21. [DOI] [PubMed] [Google Scholar]
- 11.Leidy HJ, Racki EM. The addition of a protein-rich breakfast and its effects on acute appetite control and food intake in 'breakfast-skipping’ adolescents. Int J Obes (Lond) 2010;34:1125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr 2005;81:388–96. [DOI] [PubMed] [Google Scholar]
- 13.Geliebter A, Yahav E, Haq S, Hashim SA. Cholesterol and weight change following daily high or low fiber breakfast cereals. Obes Res 2000;8:25S-S. [Google Scholar]
- 14.Hamedani A, Akhavan T, Samra RA, Anderson GH. Reduced energy intake at breakfast is not compensated for at lunch if a high-insoluble-fiber cereal replaces a low-fiber cereal. Am J Clin Nutr 2009;89:1343–9. [DOI] [PubMed] [Google Scholar]
- 15.Geliebter A, Yahav E, Forbes G, Hashim SA. Lunch meal intake following high and low glycemic breakfast cereals. FASEB J 1999;13(5):A871-A. [Google Scholar]
- 16.Kral TV, Whiteford LM, Heo M, Faith MS. Effects of eating breakfast compared with skipping breakfast on ratings of appetite and intake at subsequent meals in 8- to 10-y-old children. Am J Clin Nutr 2011;93:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levitsky DA, Pacanowski CR. Effect of skipping breakfast on subsequent energy intake. Physiol Behav 2013;119:9–16. [DOI] [PubMed] [Google Scholar]
- 18.Champagne CM, Han H, Bajpeyi S, Rood J, Johnson WD, Lammi-Keefe CJ, Flatt JP, Bray GA. Day-to-day variation in food intake and energy expenditure in healthy women: the Dietitian II Study. J Acad Nutr Diet 2013;113:1532–8. [DOI] [PubMed] [Google Scholar]
- 19.Schlundt DG, Hill JO, Sbrocco T, Pope-Cordle J, Sharp T. The role of breakfast in the treatment of obesity: a randomized clinical trial. Am J Clin Nutr 1992;55:645–51. [DOI] [PubMed] [Google Scholar]
- 20.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983;13:595–605. [PubMed] [Google Scholar]
- 21.Derogatis LR, Unger R. Corsini encyclopedia of psychology. Hoboken, NJ; John Wiley amp Sons, Inc., 2010..
- 22.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The Eating Attitudes Test: psychometric features and clinical correlates. Psychol Med 1982;12:871–8. [DOI] [PubMed] [Google Scholar]
- 23. USDA. Letaposs eat for the health of it. Fayetteville, AR: University of Arkansas Division of Agriculture Research amp Extension, 2011. (Department of Health and Human Services publication HHS-ODPHP-2010-01-DGA-B.).
- 24.Team RCR. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. [Google Scholar]
- 25.Schafer JL. Analysis of incomplete multivariate data. London, United Kingdom: Chapman & Hall, 1997. [Google Scholar]
- 26.Stef van Buuren KG-O. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 27.Tiwari HK, Birkner T, Moondan A, Zhang S, Page GP, Patki A, Allison DB. Accurate and flexible power calculations on the spot: applications to genomic research. Stat Interface 2011;4:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicklas TA, O'Neil C, Myers L. The importance of breakfast consumption to nutrition of children, adolescents, and young adults. Nutr Today 2004;39:30–9. [DOI] [PubMed] [Google Scholar]
- 29.Kleinman RE, Hall S, Green H, Korzec-Ramirez D, Patton K, Pagano ME, Murphy JM. Diet, breakfast, and academic performance in children. Ann Nutr Metab 2002;46(suppl 1):24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berner LA, Keast DR, Bailey RL, Dwyer JT. Fortified foods are major contributors to nutrient intakes in diets of US children and adolescents . J Acad Nutr Diet 2014. (in press). [DOI] [PubMed] [Google Scholar]
- 31.Vander Wal JS, Gupta A, Khosla P, Dhurandhar NV. Egg breakfast enhances weight loss. Int J Obes (Lond) 2008;32:1545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr 2007;85:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.