Abstract
Background: Higher whole-grain (WG) intake is associated with a lower prevalence of metabolic syndrome (MetS); however, there is inconsistent clinical evidence with regard to the benefit of WGs compared with refined grains (RGs) on MetS.
Objective: We hypothesized that consuming WGs in the place of RGs would improve MetS criteria in individuals with or at risk of MetS.
Design: A randomized, controlled, open-label parallel study was conducted in 50 overweight and obese individuals with increased waist circumference and one or more other MetS criteria. Participants consumed a controlled weight-loss diet containing either WG or RG (control) products for 12 wk. Body composition, MetS criteria and related markers, and plasma alkylresorcinols (compliance marker of WG intake) were measured at baseline and at 6 and 12 wk. A subgroup (n = 28) underwent magnetic resonance imaging to quantify subcutaneous and visceral adipose tissue (AT).
Results: Baseline variables were not significantly different between groups; however, the RG group tended to have higher triglycerides and lower high-density lipoprotein (HDL) cholesterol (P = 0.06). Alkylresorcinols increased with consumption of the WG diet and did not change with consumption of the RG diet (time × treatment, P < 0.0001), which showed dietary compliance. There were no differences in anthropometric changes between groups; however, weight, body mass index, and percentage of body AT decreased at both 6 and 12 wk (P < 0.05), and reductions in percentage of abdominal AT occurred by 6 wk and did not change between 6 and 12 wk (P = 0.09). Both glucose (P = 0.02) and HDL cholesterol (P = 0.04) were lower with the consumption of the WG compared with the RG diet. However, when noncompliant individuals (n = 3) were removed, the glucose effect was stronger (P = 0.01) and the HDL-cholesterol effect was no longer significant (P = 0.14).
Conclusions: Replacing RGs with WGs within a weight-loss diet does not beneficially affect abdominal AT loss and has modest effects on markers of MetS. WGs appear to be effective at normalizing blood glucose concentrations, especially in those individuals with prediabetes. This trial was registered at www.clinicaltrials.gov as NCT00924521.
INTRODUCTION
One-third of US adults have diagnosed metabolic syndrome (MetS)4, 90% of whom are overweight or obese (1). MetS is a composite of risk factors associated with increased risk of type 2 diabetes and cardiovascular disease (CVD) (2). The criteria for MetS include having ≥3 of the following clinical indicators: increased waist circumference, fasting glucose, triglycerides, and blood pressure (BP) and decreased HDL cholesterol (2). Excess abdominal adipose tissue (AT) and accompanying insulin resistance are believed to be the underlying causes of MetS (3).
Nutritional approaches to achieve ideal body weight are important for the prevention of obesity-related diseases. In addition to weight loss, dietary advice often includes replacing refined-grain (RG) with whole-grain (WG) foods (4). WGs contain fibrous bran, starchy endosperm, and nutrient-rich germ. Removing the bran and germ, or “refining” the grain, reduces the fiber, vitamin, mineral, and phytochemical contents of the grain product (4).
Epidemiologic research consistently links increased WG consumption to lower incidence of metabolic diseases [ie, MetS (5), type 2 diabetes (6), and CVD (7)] and reduced abdominal adiposity [estimated by waist circumference (8) and percentage of abdominal (9) and visceral (10) AT]. Although this literature is consistently positive for WGs, the magnitude of effect is often small. For example, every 40 g WGs consumed per day was related to a reduction in weight gain of 0.49 kg over 10 y (11). A recent meta-analysis of randomized controlled trials comparing WGs and RGs showed that WGs may accelerate body AT loss (although not weight loss) (12). Moreover, replacing RGs with WGs during weight loss for 12 wk caused a greater decrease in percentage of abdominal AT in a clinical trial in free-living individuals with MetS (13), but this was not shown in a similar study (14). The consumption of WGs may reduce the risk of MetS via preferential reduction in abdominal AT during weight loss.
Clinical studies evaluating the effects of WGs on MetS characteristics have yielded mixed findings, with beneficial effects being reported for different MetS components in each study (13–16). This discrepancy between epidemiologic and clinical studies has been attributed, in part, to inconsistent adherence in free-living studies and the variety, type, and form in which the grain was consumed (13, 15). To control this variability, we first used a controlled-feeding study design and assessed a biomarker of WG wheat intake, plasma alkylresorcinols (17), to improve participant adherence. Second, the diets differed only in the type of grain products used (WGs or RGs). Third, we designed diets with grain products that were composed primarily of wheat, which make up a majority of the US WG intake. We hypothesized that the WG diet would improve MetS criteria, particularly abdominal obesity, more than the RG diet in overweight and obese participants with increased waist circumference. Primary endpoints were percentage of abdominal fat and MetS characteristics, and secondary endpoints included insulin, total and LDL cholesterol, inflammatory markers, adipokines, and other measurements of body composition.
SUBJECTS AND METHODS
Subjects
Eligible participants were overweight or obese [BMI (in kg/m2): 25–42], 35–55 y of age, and were required to have a waist circumference ≥102 cm (men) or ≥88 cm (women) and at least one other criterion of MetS (18), as follows: fasting plasma glucose ≥100 mg/dL, fasting serum triglycerides ≥150 mg/dL, BP ≥130/≥85 mm Hg, and/or fasting serum HDL cholesterol <50 mg/dL (women) or <40 mg/dL (men). Exclusion criteria included use of medications affecting glucose or lipid metabolism, frequent (>4 times/wk) use of anti-inflammatory medications, pregnancy or lactation, smoking, high alcohol intake (>14 drinks/wk), and diagnosed CVD, diabetes, or inflammatory disease. Two alcoholic drinks per week were allowed for participants during the study, but alcohol was restricted for 48 h before any clinic visits. BP medications were allowed (n = 2); however, participants taking BP medications still needed to have at least 2 other markers of MetS to qualify for the study. Participants provided informed consent before any clinical procedures were performed at the screening appointment. This study was conducted according to the guidelines in the Declaration of Helsinki, and the Institutional Review Board at the Pennsylvania State University approved all procedures involving human participants. The trial was registered at clinicaltrials.gov (identifier: NCT00924521).
Study design and intervention
This was a 12-wk, randomized, parallel-arm, open-label controlled-feeding trial comparing the effects of WGs with RGs (control) on markers of MetS. Participants were screened at the clinical research center (CRC) on the Pennsylvania State University campus within 6 mo before starting the study to determine eligibility. Eligible individuals (n = 60) were randomly assigned to either the WG or RG diet group for the entire 12 wk by using a computer-generated random-number assignment. Participants could not be blinded to their group assignment. An unblinded study coordinator stratified participants by age, sex, and BMI and conducted all data analyses. Outcome assessors (ie, nurses and technicians) were blinded. Diets were tailored to individual energy requirements by using the Harris-Benedict equation with a low activity factor of 1.3 (19). Energy levels ranged from 1600 to 3600 kcal/d; the WG diets contained between 163 and 301 g WGs/d, and the RG diets contained 0 g WGs/d (Table 1). All participants were placed on (what was intended to be) an isocaloric, weight-maintenance diet for the first 6 wk, followed by a hypocaloric diet (∼500 kcal energy deficit/d) for the second 6 wk. Average caloric intake during the isocaloric phase was 3200 kcal/d (range: 2700–3900 kcal/d) for men and 2350 kcal/d (range: 2100–3500 kcal/d) for women. During the hypocaloric period, average intake was 2500 kcal/d (range: 1600–3400 kcal/d) for men and 1750 kcal/d (range: 1600–2800 kcal/d) for women. During the second phase, the meals were composed of the same foods but were proportionally smaller, with the exception of some prepackaged foods (chip bags, granola bars). Significant weight loss occurred on both calorie levels in both the WG and RG groups; therefore, the isocaloric and hypocaloric phases were pooled to compare the difference between WGs and RGs after 12 wk of weight loss on controlled diets. To enhance compliance, participants were given the option to take a 1- to 2-wk break after the first 6-wk diet period. Nineteen (WG group: n = 7; RG group: n = 12) of 50 participants who completed the study chose to take the break; the remainder transitioned to the next phase of the study immediately. There was no difference in total weight lost between those who took the compliance break and those who did not [break (−4.1 ± 0.5 kg) compared with no break (−5.0 ± 0.4 kg); P = 0.2]. Participants completed a variety of clinical assessments over 2 consecutive days before the intervention and after 6 and 12 wk of consuming the diets. The first participants began the study in March 2009, and the final participants completed the study by May 2011.
TABLE 1.
WGs and alkylresorcinols in the WG diet at each energy level1
| Energy level2 | Servings of WGs | Amount of WGs | Average dietary alkylresorcinols3 |
| servings/d | g/d | g/d | |
| 16004 | 5.8 | 163 | — |
| 1800 | 5.9 | 164 | 56.3 ± 8.2 |
| 2100 | 6.7 | 187 | 65.7 ± 9.6 |
| 2400 | 7.8 | 213 | 75.1 ± 11.0 |
| 2700 | 8.6 | 228 | 84.5 ± 12.3 |
| 3000 | 9.7 | 258 | 93.9 ± 13.7 |
| 3300 | 10.5 | 280 | 103.3 ± 15.1 |
| 3600 | 11.5 | 301 | 112.7 ± 16.4 |
WG, whole grain.
Values are kcal/d.
Estimated alkylresorcinol amounts for each calorie level were based on alkylresorcinol amounts measured in grain products and the amount of grain consumed for that calorie level; the average of 6 daily menus was calculated. Values are means ± SDs.
Alkylresorcinol amounts for the 1600-kcal menu were not calculated because WG amounts were very similar to the 1800-kcal amount.
The WG and RG diets used the same 6-d cycle menus with the exception of the grain products. Participants assigned to the WG diet consumed all WG products from a variety of grain types, whereas those receiving the RG diet consumed the RG counterpart. An example of the grain product substitutions in a sample 1-d menu is shown in Supplemental Table 1 under “Supplemental data” in the online issue. The American Association of Cereal Chemists and the Food and Drug Administration define WG as “the intact, ground, cracked, or flaked caryopsis, whose principal anatomical components—the starchy endosperm, germ and bran—are present in the same relative proportions as they exist in the intact caryopsis” (20). WG products made from milled flour (eg, bread, pasta) were required to have >51% of dry weight from WG flour. When possible, WG products carrying the 100% Whole Grain stamp were selected, which indicated that each grain serving contained at least 16 g WG and used 100% WG flour. The WG and alkylresorcinol contents of the WG products in the study are described in Supplemental Table 2 under “Supplemental data” in the online issue. The top 3 grains consumed were wheat, oats, and rice with the WG diet and wheat, rice, and corn with the RG diet; wheat products represented 77% and 63% of the grains consumed with the WG and RG diets, respectively.
Both diets had the same macronutrient composition and were designed to meet National Cholesterol Education Program guidelines (18) for saturated fat (<7% total energy), mono- and polyunsaturated fats (∼10% and ∼7% total energy, respectively), total cholesterol (<200 mg/d), and total fiber (>20 g/d) (Table 2). The low-fat diet was selected as a safe, healthy background diet that would be appropriate in both weight-maintenance and weight-loss settings. All meals and snacks were prepared at 1 of 2 metabolic kitchens on the university campus. Participants were required to come to either of the metabolic kitchens on Monday through Friday to pick up and/or eat their meals (weekend meals were packed and provided on Friday), fill out compliance forms, and record their weight (self-report).
TABLE 2.
Nutritional composition of WG and RG diets at the 2100-kcal level1
| Nutrients | RG | WG |
| Total energy (kcal) | 2023 | 2079 |
| Carbohydrates [g (% of total energy)] | 280 (55) | 299 (57) |
| Protein [g (% of total energy)] | 90 (17) | 97 (18) |
| Fat [g (% of total energy)] | 64 (28) | 62 (26) |
| Saturated fat [g (% of total energy)] | 15 (7) | 14 (6) |
| Monounsaturated fat [g (% of total energy)] | 23 (10) | 23 (10) |
| Polyunsaturated fat [g (% of total energy)] | 16 (7) | 16 (7) |
| Cholesterol (mg) | 104 | 88 |
| Total fiber (g) | 22 | 38 |
| Sugars (g) | 100 | 114 |
| Magnesium (mg) | 262 | 411 |
| Calcium (mg) | 1031 | 1491 |
| Sodium (mg) | 4026 | 3928 |
| Potassium (mg) | 3028 | 3445 |
| Whole grains [servings/d (g)] | 0 (0) | 7 (187) |
The average of the nutritional composition from 6 daily menus is presented. Average grams of whole grains were calculated manually (from food labels); all other nutrients were analyzed by Food Processor SQL. RG, refined grain; WG, whole grain.
Clinical assessments
Body weight was measured with subjects wearing light clothing and no shoes every 3 wk at the CRC after a 12-h overnight fast. Only body weights recorded at the CRC were used for statistical comparisons. Blood was drawn in the morning after a 12-h fast according to standardized protocol. Screening results were analyzed with the use of fresh blood by a clinical diagnostic testing center (Quest Diagnostics); serum and plasma aliquots in EDTA-coated tubes were stored at −80°C until time of analysis. Waist circumference was measured in accordance with the National Heart, Lung, and Blood Institute guidelines (21). BMI was calculated by using weight from each clinical visit and height measured at baseline. Percentage of abdominal AT was measured by using dual-energy X-ray absorptiometry (DXA) within a 50-cm2 area around the center point of the midline between the lateral iliac crests and the lowest rib margins. Participants weighing <157 kg (n = 46) were measured with a hologic DXA (QDR-4500W; Hologic Corporation), and those ≥157 kg (n = 3) were measured with a GE Lunar iDXA (General Electric). The abdominal region of interest could only be measured with hologic DXA; therefore, 3 individuals receiving the WG (n = 2) and RG (n = 1) diets could not be analyzed for the primary endpoint. Percentage of AT was used as the primary body composition endpoint to minimize variability in output by the 2 DXA scanners. BP was measured by trained nurses with the use of a standing mercury manometer (Baumanometer). Participants sat for ≥5 min with their legs uncrossed before repeated measurements, which were taken ≥1 min apart; the average of 3 measurements was used.
Resting metabolic rate was measured at baseline and at the end of the study by using a Cosmed FitMate. The test was performed after participants fasted for 12 h, refrained from vigorous exercise for 24 h, and abstained from alcoholic and caffeinated beverages for 48 h. Participants rested for 30 min in a supine position shortly after arriving at the clinic. A mask connected to the Cosmed FitMate was then placed over their nose and mouth for 15 min to measure oxygen consumption and energy expenditure. A trained technician stayed with the participants during the test, noting changes in breath depth and frequency, fixing air leaks in the mask, and ensuring that participants were awake and comfortable.
Biochemical analyses
Plasma glucose, total cholesterol, HDL cholesterol, and triglycerides from fasted samples taken on consecutive days were measured by using enzymatic procedures and spectrophotometry (Quest Diagnostics). LDL cholesterol was determined by using Friedewald's equation: LDL cholesterol = total cholesterol (TC) – HDL cholesterol − (triglycerides ÷ 5) (18). Serum insulin and high-sensitivity C-reactive protein (CRP) were measured with an immunoassay technique and latex-enhanced immunonephelometry, respectively (Quest Diagnostics). CRP values >10 mg/L were not used for analysis, because this concentration indicates infection. HOMA-IR was calculated as follows: fasting glucose (mg/dL) × fasting insulin (mU/L)/405 (22). Serum total adiponectin, high-molecular-weight adiponectin, leptin, IL-6, and TNF-α were measured with ELISAs (R&D Systems) in duplicate (assay CVs <10%) according to the manufacturer's protocols. Plasma alkylresorcinols were analyzed by using normal-phase liquid chromatography–tandem mass spectrometry (23) after extraction with diethyl ether. Intrabatch repeatability for each standard was <10%, and interbatch repeatability was <15%.
Subgroup: abdominal imaging scans
A subset (n = 28/60) of participants agreed to undergo abdominal MRI scans to assess changes in subcutaneous and visceral AT. MRI data were acquired with a Siemens Magnetom Trio 3.0 Tesla scanner. Single-slice axial abdominal fast spin echo images were acquired (repetition time/echo time = 100/2.46 ms; flip angle = 70° number of excitations =1; bandwidth = 280 kHz; matrix = 256 × 192; slice thickness = 8 mm; echo train length = 4) by using a Siemens Magnetom Trio body coil. Three consecutive slices surrounding the third lumbar vertebra (L3) were analyzed for subcutaneous and visceral AT volume by using a program developed in-house (Matlab; MathWorks). Two trained technicians manually traced all slices, and the average of the 2 was used. The sum of 3 slices surrounding L3 was chosen because excess AT in this area of the abdomen has been shown to have the highest correlation with metabolic dysfunction (24, 25).
Statistical methods
All statistical analyses were performed on SAS software (version 9.2; SAS Institute). Nonparametric t tests (PROC NPAR1WAY) and chi-square tests were used to assess baseline differences in continuous and categorical variables, respectively. For biochemical variables measured on 2 consecutive days, an average was used. Linear-mixed models (PROC MIXED) were used to analyze both raw values and change score models. Log-transformed alkylresorcinol values at 0, 6, and 12 wk were analyzed to assess compliance (time × treatment) and are presented as geometric means (95% CIs). Changes in body weight and composition were analyzed at 6 and 12 wk in models adjusted for age and sex. All other biomarkers were analyzed by comparing the total change over 12 wk between groups (WG compared with RG) in unadjusted and adjusted models. The first adjusted model included age, sex, and baseline BMI; the second included these and weight loss. Reassessment without the “noncompliant” individuals (as determined by alkylresorcinol concentrations at 12 wk), interactions by sex (men compared with women), and compliance break status (those who took a 1–2 wk break after the first 6 wk of the diet compared with those who continued with the diet uninterrupted) were conducted for primary endpoints by using the model adjusted for age, sex, and baseline BMI. To determine the normality of the data, the skewness of the residuals from the models was assessed. Statistical outliers (± >3 SDs from mean or data points that skewed residuals) were removed as well as all CRP values >10 mg/L, which is indicative of infection. Geometric means (95% CIs) of baseline values and change scores ± SEMs from the unadjusted model are presented for the nonnormally distributed variables. Baseline means ± SDs and change scores ± SEMs are presented for variables with models having normally distributed residuals. Freeman-Halton adjustment of the Fisher's exact test was used for the prediabetes subgroup analysis. Regression outputs (R2, P value) between variables associated with alkylresorcinol changes are presented as exploratory analyses. Differences were considered significant at P < 0.05 and as tendencies at P < 0.10. P values for secondary outcomes were corrected for false discovery rate by using the Benjamini-Hochberg procedure.
The primary endpoint used to determine sample size was the change in percentage of abdominal AT over 12 wk. For 80% power to detect a difference of 1.75 ± 2.2% in percentage of abdominal AT loss between the 2 diet groups at an α level of 0.05, a final sample size of 50 participants was needed. Power calculations were based on data from a previous study in MetS participants consuming WG and RG diets (12). Per protocol analysis included only the data from participants who completed both phases of the study (n = 50). An uneven randomization was attributable to higher dropouts in the RG group. MetS criteria and percentage of abdominal AT were primary endpoints; secondary endpoints included lipoproteins, insulin, inflammatory markers, adipokines, and other measures of body composition.
RESULTS
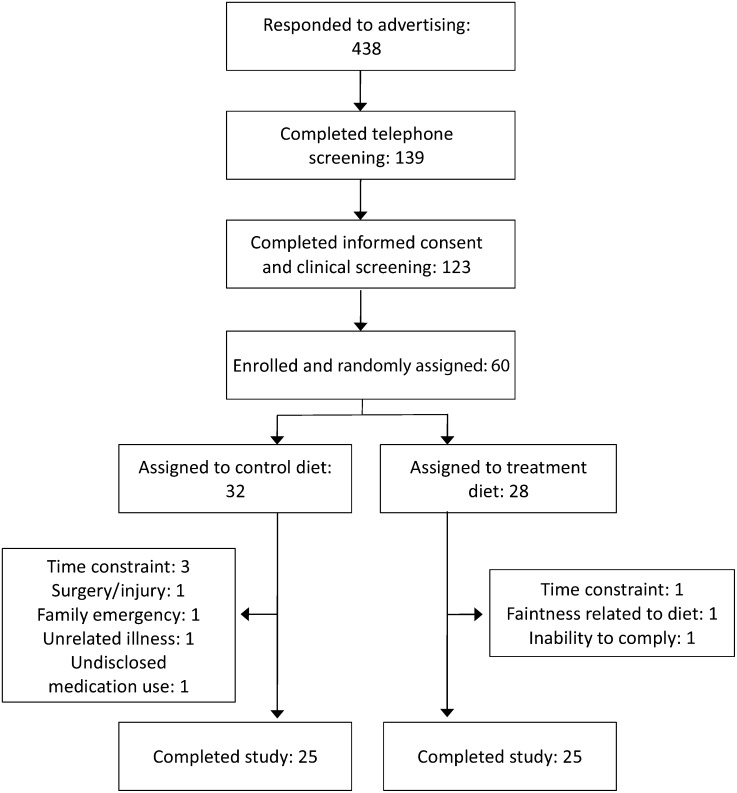
Of the 438 respondents, 28% (n = 123) underwent clinical screening, 14% (n = 60) were randomly assigned, and 12% (n = 50) completed the study (Figure 1). Ten individuals discontinued participation in the study because of an inability to comply with the time commitment (n = 5), family emergency (n = 1), adverse reaction to the WG diet (n = 1), unrelated illness/injury (n = 2), or undisclosed or newly prescribed medications (n = 1). Plasma alkylresorcinol concentrations of <80 nmol/L identify a probable low–WG wheat or rye consumer (RG group), whereas concentrations >100 nmol/L identify a probable frequent consumer (WG group) (20). Nine individuals began the study at concentrations >80 nmol/L, but after 12 wk only 3 participants (all in the WG group) had alkylresorcinol concentrations that did not meet the cutoffs set for their assigned group. Outcome variable analyses without these 3 participants did not differ from results from the complete study population, with the exception of the glucose and HDL-cholesterol effects (see Metabolic syndrome). The retention rates for the RG and WG diets were 78% (n = 25/32) and 89% (n = 25/28), respectively. A total of 50 participants were included in the final analysis. Baseline demographic characteristics were not significantly different between groups (Table 3); however, the RG group tended to have higher triglyceride and lower HDL concentrations (P = 0.06; Table 4) and had an elevated TC:HDL-cholesterol ratio (P = 0.05; Table 5).
FIGURE 1.
Flow diagram of the study.
TABLE 3.
Baseline characteristics of treatment and control groups1
| RG | WG | |
| n | 25 | 25 |
| Sex (M/F) | 13/12 | 12/13 |
| Age (y) | 45.8 ± 6.02 | 46.4 ± 5.9 |
| BMI (kg/m2) | 33.5 ± 4.0 | 32.9 ± 3.5 |
| Weight (kg) | 99.5 ± 16.5 | 99.7 ± 17.9 |
| Body AT (%) | 36.3 ± 8.0 | 36.0 ± 8.1 |
| Abdominal AT (%) | 40.6 ± 5.5 | 38.0 ± 6.7 |
| Obese: BMI (kg/m2) >30 [n (%)] | 21 (84) | 18 (72) |
| Metabolic syndrome: ≥3 criteria [n (%)] | 19 (76) | 14 (56) |
| Medication use3 [n (%)] | 10 (40) | 12 (48) |
| Postmenopausal34 [n (%)] | 4 (33) | 3 (23) |
| Alkylresorcinols (nmol/L)5 | 32.3 (21.7, 48.1) | 34.0 (23.1, 50.0) |
Group differences were tested by nonparametric t tests (quantitative) and chi-square tests (qualitative). There were no significant differences between groups at baseline. AT, adipose tissue; RG, refined grain; WG, whole grain.
Mean ± SD (all such values).
Percentage of women per group (RG group, n = 12; WG group, n = 13).
Self-reported data.
Values are geometric means (95% CIs) because of skewed data.
TABLE 4.
Metabolic syndrome characteristics: baseline values and total change scores in RG and WG groups1
| RG |
WG |
Group differences, P2 |
||||||
| RG/WG | Baseline3 | Δ12 wk4 | Baseline3 | Δ12 wk4 | Unadjusted | Model 1 | Model 2 | |
| n | ||||||||
| Waist circumference (cm) | 25/25 | 112 ± 10 | −3.9 ± 0.75 | 107 ± 8 | −2.3 ± 0.75 | 0.11 | 0.13 | 0.06 |
| Triglycerides (mg/dL)67 | 25/24 | 151 (126, 182) | −17 ± 9 | 118 (94, 149) | −2 ± 9 | 0.27 | 0.19 | 0.12 |
| HDL cholesterol (mg/dL)7 | 25/25 | 41 ± 15 | −2.5 ± 0.95 | 45 ± 11 | −5.2 ± 0.95 | 0.04 | 0.04 | 0.04 |
| Glucose (mg/dL)6 | 25/24 | 96 (93, 98) | −1.0 ± 1.1 | 98 (95, 102) | −4.3 ± 1.15 | 0.03 | 0.02 | 0.03 |
| Systolic BP (mm Hg) | 25/25 | 125 ± 12 | −9.2 ± 1.95 | 123 ± 12 | −7.2 ± 1.95 | 0.43 | 0.42 | 0.42 |
| Diastolic BP (mm Hg) | 25/25 | 85 ± 6 | −5.9 ± 1.45 | 83 ± 10 | −2.4 ± 1.4 | 0.09 | 0.09 | 0.08 |
BP, blood pressure; RG, refined grain; WG, whole grain.
Unadjusted model compares the raw change over 12 wk between WG and RG groups; model 1 adjusted for age, sex, and baseline BMI; model 2 adjusted as in model 1 + weight loss (group difference, P < 0.05).
Values are means ± SDs for normally distributed variables and geometric means (95% CIs) for nonnormally distributed variables.
Values are change scores ± SEMs from the unadjusted model.
P < 0.05, change from baseline.
Statistical outlier removed from analysis.
Baseline group difference trend, P < 0.1; P values from nonparametric 2-sample t test.
TABLE 5.
Secondary outcomes: baseline values and total change scores in RG and WG groups1
| RG |
WG |
Group differences, P2 |
||||||
| RG/WG | Baseline3 | Δ12 wk4 | Baseline3 | Δ12 wk4 | Unadjusted | Model 1 | Model 2 | |
| n | ||||||||
| Total adiponectin (ng/mL) | 25/25 | 7265 ± 4299 | −1019 ± 3045 | 7685 ± 3998 | −1038 ± 3045 | 0.97 | 0.96 | 0.96 |
| HMW adiponectin (ng/mL)6 | 25/24 | 2372 (1768, 3181) | −577 ± 1375 | 2518 (1941, 3268) | −175 ± 140 | 0.30 | 0.30 | 0.30 |
| Leptin (ng/mL)6 | 25/24 | 24 (18, 31) | −7.0 ± 1.65 | 23 (16, 31) | −10.1 ± 1.65 | 0.74 | 0.74 | 0.74 |
| TC:HDL ratio67 | 25/24 | 4.9 (4.4, 5.4) | −0.2 ± 0.1 | 4.4 (3.8, 5.0) | 0.2 ± 0.1 | 0.30 | 0.30 | 0.30 |
| LDL cholesterol (mg/dL) | 24/24 | 124 ± 31 | −12.8 ± 3.75 | 110 ± 36 | −11.6 ± 3.75 | 0.96 | 0.96 | 0.96 |
| CRP (mg/L)68 | 25/17 | 2.1 (1.4, 3.1) | −0.6 ± 0.4 | 3.0 (2.0, 4.6) | −0.6 ± 0.5 | 0.96 | 0.96 | 0.96 |
| IL-6 (pg/mL)6 | 23/23 | 1.7 (1.4, 2.0) | 0.1 ± 0.2 | 1.8 (1.5, 2.2) | 0.3 ± 0.2 | 0.96 | 0.96 | 0.96 |
| TNF-α (pg/mL)6 | 24/24 | 1.4 (1.2, 1.7) | −0.1 ± 0.15 | 1.2 (1.0, 1.3) | 0 ± 0.1 | 0.74 | 0.74 | 0.74 |
| Insulin (uIU/mL)6 | 25/23 | 2.6 (1.7, 4.1) | −0.9 ± 0.7 | 3.4 (2.2, 5.4) | −0.7 ± 0.7 | 0.96 | 0.96 | 0.96 |
| HOMA-IR6 | 25/23 | 0.6 (0.4, 1.0) | −0.2 ± 0.2 | 0.8 (0.5, 1.3) | −0.2 ± 0.2 | 0.96 | 0.96 | 0.96 |
| RMR (kcal/d) | 24/24 | 1616 ± 415 | −148 ± 535 | 1626 ± 384 | −76 ± 52 | 0.74 | 0.74 | 0.74 |
CRP, C-reactive protein; HMW, high-molecular-weight; RG, refined grain; RMR, resting metabolic rate; TC, total cholesterol; WG, whole grain.
Unadjusted model compares the raw change over 12 wk between WG and RG groups; model 1 adjusted for age, sex, and baseline BMI; model 2 adjusted as in model 1 + weight loss (group difference, false discovery rate–adjusted P < 0.05).
Values are means ± SDs for normally distributed variables and geometric means (95% CIs) for nonnormally distributed variables.
Values are change scores ± SEMs from the unadjusted model.
P < 0.05, change from baseline.
Statistical outliers removed from change score analysis.
Baseline group difference, P = 0.05; P values from nonparametric t test.
CRP values >10 mg/L were removed because this concentration indicates infection.
Participant compliance
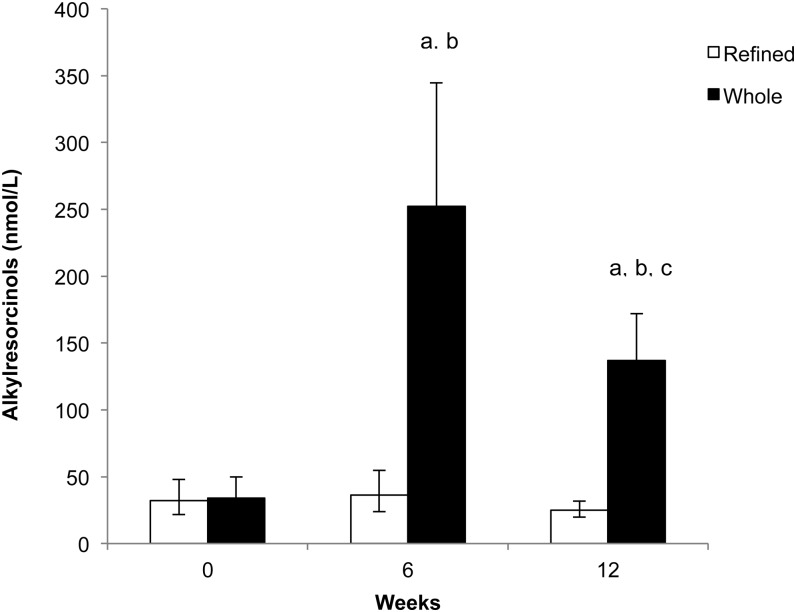
Alkylresorcinol concentrations increased with the WG but not the RG diet over time (time × treatment, P < 0.0001; Figure 2). There was an ∼6-fold increase during the first diet period (640% from baseline) in which participants consumed more WGs compared with the second, lower-energy diet period (297% from baseline). Alkylresorcinol concentrations with the RG diet did not change. On the basis of dietary compliance records, the participants consumed all study foods and did not consume any nonstudy foods on 86% of reported days. Weekly physical activity records showed that participants maintained a stable activity level.
FIGURE 2.
Geometric mean (95% CI) alkylresorcinol concentrations at 0, 6, and 12 wk in participants consuming the whole- and refined-grain diets. Linear mixed models (PROC MIXED; SAS Institute) of log-transformed alkylresorcinol values at 0, 6, and 12 wk were used to determine differences between groups at different time points (time × treatment, P < 0.0001; post hoc differences are Tukey-adjusted). In the whole-grain group, alkylresorcinol values increased significantly from baseline compared with the refined-grain group at 6 and 12 wk. aSignificant difference between groups at time point (P < 0.05). bSignificant within-group difference from baseline (P < 0.05). cSignificant within-group difference from 6 wk (P < 0.05).
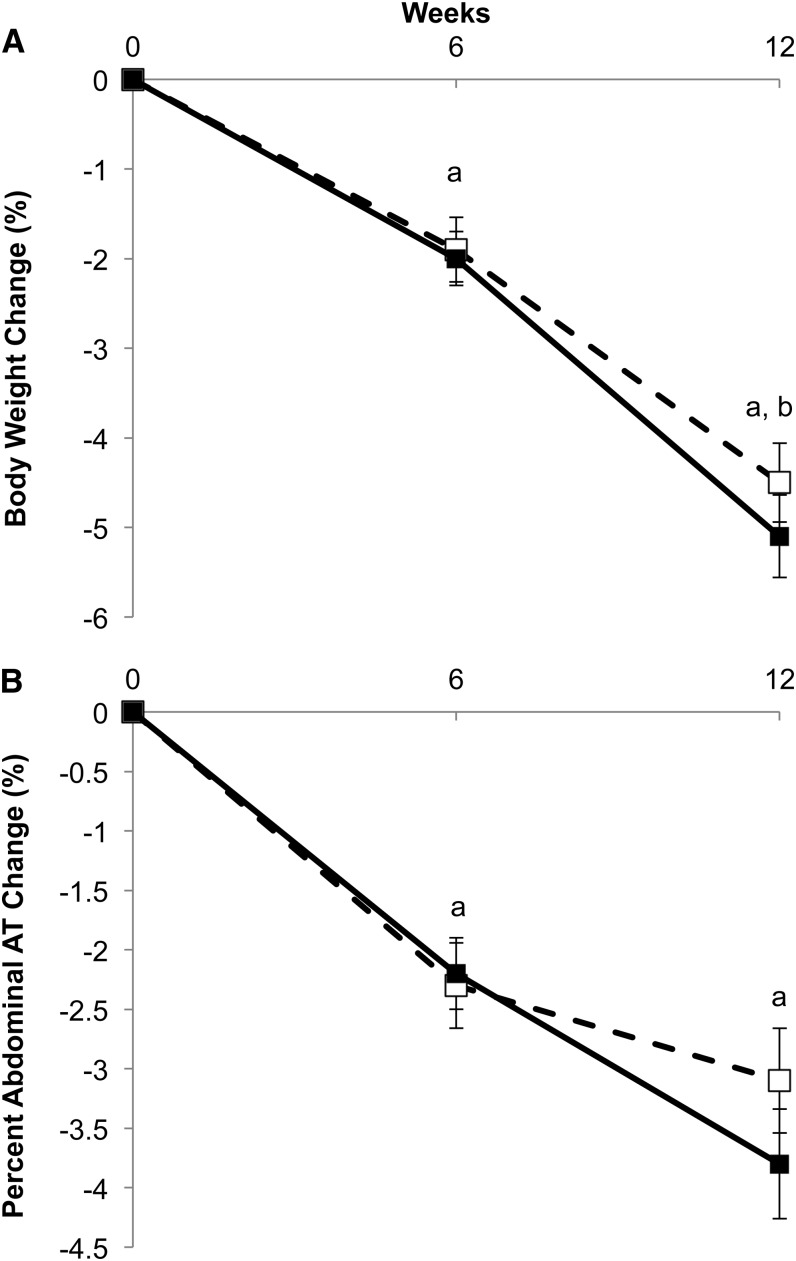
Weight and body composition
Reductions in body weight (RG group compared with WG group: −4.4 ± 0.4 compared with −5.0 ± 0.4 kg; total change from baseline, P < 0.001; Figure 3A), BMI (RG group compared with WG group: −1.5 ± 0.1 compared with −1.7 ± 0.1; P < 0.001), and percentage of body AT (RG group compared with WG group: −1.0 ± 0.2% compared with −0.8 ± 0.2%; P < 0.01) occurred in both diet groups during both the iso- and hypocaloric diet phases (time, P < 0.05). The reduction in percentage of abdominal AT at 6 wk was not significantly different from that at 12 wk (time, P = 0.09), indicating that most of the change in abdominal AT occurred during the first 6 wk in both groups (RG group compared with WG group: −1.2 ± 0.4% compared with −1.5 ± 0.4%; total change from baseline, P < 0.01; Figure 3B).
FIGURE 3.
Percentage of change in total body weight (A) and percentage of abdominal AT (B) at 6 and 12 wk with whole-grain (filled squares) and refined-grain (open squares) diets. Linear mixed models (PROC MIXED; SAS Institute) were used to determine whether change at 6 and 12 wk was significantly different between groups (weight, time × treatment, P = 0.62; % abdominal AT, time × treatment, P = 0.70). Change between groups was not significantly different; therefore, the letters represent significance related to changes for both groups. aSignificant difference from baseline (P < 0.05). bSignificant difference from 6 wk (P < 0.05). AT, adipose tissue.
Metabolic syndrome
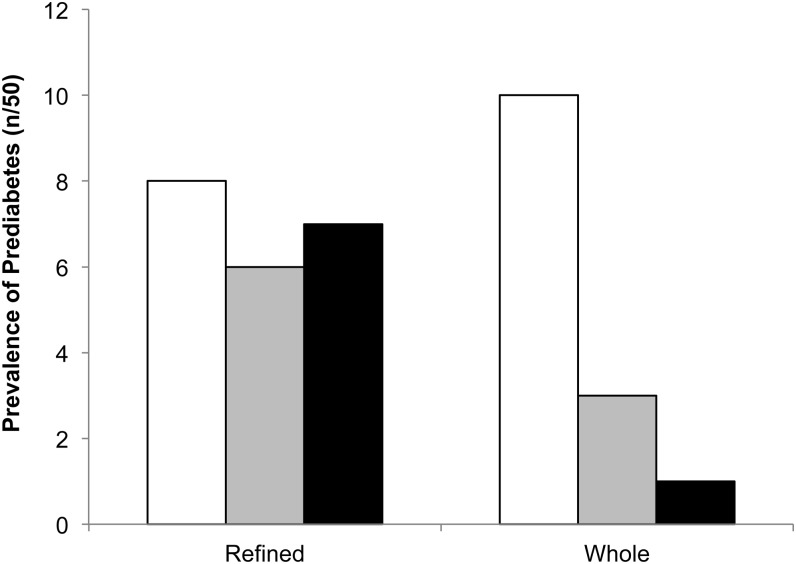
Group baseline values, change scores, and model effects for MetS criteria are shown in Table 4 (graphs in Supplemental Figure 1 under “Supplemental data” in the online issue). The reduction in glucose was greater with the WG diet than with the RG diet in unadjusted and adjusted models (P < 0.05). Removing noncompliant participants (n = 3) strengthened the effect (RG group compared with WG group: −0.9 ± 1.1 compared with −5.0 ± 1.2 mg/dL; P = 0.02). A treatment × break status interaction showed that those who took a break had a smaller reduction in glucose compared with those who did not (break compared with no break: −0.4 ± 1.3 kg compared with −4.0± 1.0 kg; break, P = 0.04), but this was not significantly different between groups (treatment × break, P = 0.75) and the effect of treatment was still significant (treatment, P = 0.01). There was no treatment × sex interaction for glucose. Of the individuals with prediabetes (fasting plasma glucose ≥100 mg/dL) at baseline, 90% (n = 9/10) from the WG group and 13% (n = 1/8) from the RG group had their prediabetes resolved after 12 wk (P = 0.04) (Figure 4). The change in alkylresorcinol concentrations was inversely related to the change in fasting blood glucose (R2 = 0.25, P = 0.003).
FIGURE 4.
Prevalence (n out of 50) of prediabetes in the refined- and whole-grain groups at 0 (open bars), 6 (gray bars), and 12 (filled bars) wk. Difference between groups, P = 0.04 (Freeman-Halton adjustment of the Fisher's exact test).
HDL cholesterol decreased more in the WG group than in the RG group in unadjusted and adjusted models (P < 0.05). However, this effect was no longer significant after removing the noncompliant participants (RG group compared with WG group: −2.6 ± 0.8 compared with −4.3 ± 0.8 mg/dL; P = 0.14). The interactions between treatment and sex or break status were not significant for HDL cholesterol; however, women had a greater reduction in HDL cholesterol than did men (men compared with women: −2.0 ± 0.9 compared with −5.7 ± 0.9 mg/dL; sex, P < 0.01).
Waist circumference and systolic BP decreased in both groups after 12 wk (P < 0.05). Diastolic BP tended to decrease more in the RG group in unadjusted and adjusted models (P < 0.1). Triglyceride concentrations did not change over time in either of the groups. There was a treatment × break interaction, but no post hoc comparisons were significant (treatment × break, P = 0.04). None of the other markers of MetS correlated with changes in alkylresorcinol concentrations.
Secondary endpoints
LDL cholesterol, leptin, and total adiponectin decreased in both groups (P < 0.05; Table 5). There were no group differences for changes in high-molecular-weight adiponectin, TNF-α, and resting metabolic rate. No changes in fasting insulin, HOMA-IR, TC:HDL-cholesterol ratio, CRP, and IL-6 values were observed. Changes in secondary endpoints were not correlated with changes in alkylresorcinols.
MRI subgroup
Changes in both subcutaneous and visceral AT at the L3 level were not significantly different between groups after 12 wk (Table 6). Changes in AT depots were not correlated with changes in alkylresorcinols.
TABLE 6.
Subcutaneous and visceral adipose tissue at the L3 level: baseline values and total change scores in the MRI subgroup1
| RG |
WG |
Group differences, P2 | ||||
| RG/WG | Baseline3 | Δ12 wk4 | Baseline3 | Δ12 wk4 | Model 1 | |
| n | ||||||
| Subcutaneous AT (g) | 12/16 | 782 ± 242 | −51 ± 27 | 668 ± 243 | −44 ± 235 | 0.73 |
| Visceral AT (g) | 12/16 | 571 ± 245 | −51 ± 175 | 482 ± 175 | −63 ± 155 | 0.32 |
n = 28. AT, adipose tissue; L3, third lumbar vertebra; RG, refined grain; WG, whole grain.
Model 1 compares change in abdominal AT over 12 wk between WG and RG groups adjusted for age and sex; secondary outcomes, group difference P < 0.05.
Values are means ± SDs for normally distributed variables and geometric means (95% CIs) for nonnormally distributed variables.
Values are change scores ± SEMs from the unadjusted model.
P < 0.05, change from baseline.
DISCUSSION
Both of the diets resulted in significant weight loss and elicited modest but generally beneficial effects on metabolic variables. Although there were no differences between WG and RG groups over time for the primary endpoint, percentage of abdominal AT, there was a greater reduction in glucose with the WG diet compared with the RG diet. Moreover, the prevalence of prediabetes was markedly reduced with consumption of the WG diet (90%) compared with the RG diet (13%). HDL cholesterol decreased to a greater extent in participants consuming the WG diet, but this effect was no longer significant when removing potentially noncompliant subjects.
Post hoc analysis of data from the Diabetes Prevention Program (26) compared diabetes risk in individuals with prediabetes who achieved a normal fasting glucose concentration during 6 y in a lifestyle modification trial compared with participants with persistent hyperglycemia. Individuals whose glucose concentrations were normal (<100 mg/dL) at least once during the 6 annual visits were 56% less likely to develop type 2 diabetes compared with those whose glucose concentrations stayed in the prediabetic range. Although the magnitude of glucose reduction was modest with consumption of the WG diet, the resolution of prediabetes is a clinically significant finding.
In the WG literature, a variety of grains and endpoints have been the focus of previous randomized controlled trials, such as oats and LDL-cholesterol lowering (27), barley (28) or rye (29) and insulin sensitivity, and wheat and weight loss (13). In the United States, wheat products, especially ready-to-eat cereals and breads, comprise the majority of WGs that are consumed (30); therefore, results from studies using primarily wheat products are the most relevant to the US population. Clinical trials with similar study designs—parallel arm studies lasting 12–16 wk in overweight and obese individuals with the use of primarily wheat products (13–16, 29)—showed that WG diets improve AT loss and BP compared with RG diets with no effects on glucose or insulin. Our findings add to the literature that WGs have generally positive effects on MetS criteria.
Dietary adherence and exclusive consumption of WGs were integral design components of the present study. None of the aforementioned studies controlled the background diet, but 4 of the 5 provided the grain products to their participants (14–16, 29). Our participants consumed more WGs compared with other studies: 6–12 servings/d (∼163–301 g WGs/d depending on the calorie amount) compared with 105 g WGs/d (14), 60–120 g WGs/d (15), 3 servings WGs/d [∼48 g WGs/d (estimated) (16)], or ∼5 servings WGs/d [∼80 g WGs/d (estimated) (13)]. In short-term trials, the threshold for WG intake may need to be higher to detect metabolic changes.
We hypothesized that the glucose effect in our study is related to a variety of mechanisms related to WG structure (31). WG wheat contains fiber (primarily insoluble), minerals (magnesium, calcium), and phytochemicals (ferulic acid) that are in much lower quantities in RGs (31). The mechanisms by which WGs may affect glucose metabolism have not been fully elucidated, despite the abundance of plausible hypotheses. The presence of fiber slows the enzymatic digestion of starch in the small intestine (32). However, increased fiber does not translate to a lower glycemic response because of variability created by different kinds of fiber, starch and food structure, flour particle size, and grain processing (33). Undigested fiber from WGs can contribute to a bifidogenic or prebiotic effect in the lower intestine (34), which may improve glucose metabolism (35). An increase in fecal Bifidobacterium numbers compared with baseline was shown in study subjects consuming WG products in 2 randomized controlled studies (36, 37), but neither showed a concomitant effect on glucose or insulin. Magnesium is important for cellular insulin sensitivity; however, improvements in glucose concentrations were shown with magnesium supplements (not from foods) in diabetic populations only (38). Ferulic acid is a potent antioxidant phytochemical found in high amounts in WGs (39), but its bioavailability is negligible and physiologic effects at these amounts are yet unknown. In sum, consuming WGs as a whole food appears to have many health benefits, although all of the mechanisms involved are not fully understood.
RG and WG groups experienced a similar decrease in all measurements of abdominal AT [% abdominal AT, DXA; subcutaneous and visceral AT (g), MRI]. Paradoxically, the RG group showed a trend for a greater reduction in waist circumference in a fully adjusted model (P = 0.06); however, change over time was parallel between groups (Supplemental Figure 1 under “Supplemental data” in the online issue). These findings contrast with cross-sectional (9, 10) and some clinical (13) evidence that suggests that WGs affect abdominal AT more favorably than RGs. If the effects of WGs on body weight and AT mass are related to enhanced satiety and lower calorie consumption at the next meal, the lack of effect in the present study may be attributable to the controlled-feeding design, which required participants to consume all food provided. It is possible that, in a different setting, subjects in the WG group may have prolonged meals or consumed smaller portions because of increased satiety.
HDL cholesterol decreased more with the WG diet than with the RG diet in unadjusted and partially adjusted models; however, the decrease in and subsequent maintenance of HDL-cholesterol concentrations were parallel between the WG and RG groups (Supplemental Figure 1 under “Supplemental data” in the online issue). Moreover, the removal of potentially noncompliant participants eliminated the effect. The larger reduction in HDL cholesterol with the WG diet may be attributable to a combination of higher baseline concentrations in the WG group and a low-fat, weight-loss background diet (40). Low-fat (and low-saturated-fat) diets typically lower LDL cholesterol, but HDL cholesterol is reduced as well (41). To this point, LDL cholesterol decreased by ∼12% in both groups. The effect of WGs on HDL cholesterol must be interpreted with caution, and future research is warranted. Indeed, MetS-related dyslipidemia did not improve or worsen with WG feeding in related clinical trials (13–16, 29).
A study limitation is the heterogeneous study population (different MetS criteria, both sexes, and women in varying hormonal states); however, there was an equal distribution between the groups for hormonal status and sex, and conclusions can be generalized to a broader population. Another limitation was the inability to maintain the baseline weights of participants during the “isocaloric” phase of the study. Providing a low-energy-dense, isocaloric diet to overweight and obese individuals often will cause a weight fluctuation of 1–2 kg in the first phase; thus, incorporating a run-in period may have ameliorated this issue. Those who took the compliance break had less favorable changes (although not all significant) in some MetS criteria compared with those who did not, which may have affected outcomes. Blinding study personnel who randomly assigned the participants and analyzed the data would have reduced the risk of bias; however, the randomization was successful in matching the groups for age, sex, and BMI. Finally, a reduction in alkylresorcinol concentrations during the second diet phase was expected because of the designed decrease in WG intake. The magnitude of the reduction was relatively larger than could have been expected, indicating possible issues with compliance or an unknown aspect of alkylresorcinol absorption or metabolism. Removing the potentially noncompliant individuals from analyses affected some results, which lends credibility to the functionality of this biomarker, although the apparently large decrease in plasma alkylresorcinols on switching to the lower-energy diet may not only be explained just by the reduced intake of WGs.
The strengths of this study included a high level of participant adherence due to the controlled-feeding protocol and the use of commonly consumed WG products. Our study population is representative of the large sector of individuals in the United States who are highly vulnerable to metabolic diseases.
In conclusion, we showed that although replacing RGs with WGs within a weight-loss diet does not beneficially affect abdominal AT loss, it appears to be effective at normalizing blood glucose concentrations. Moreover, our findings indicate that WGs may improve glucose status in individuals with prediabetes and thereby prevent the onset of diabetes.
Supplementary Material
Acknowledgments
We thank our study participants and the research nursing staff at the Pennsylvania State University Clinical Research Center.
The authors’ responsibilities were as follows—PMK-E, JPVH, SGW, AMH, JAG, and SSJ: designed the research; KHJ and AMH: conducted the research; SKL: supervised the MRI scanning and analysis; ABR: performed alkylresorcinol analysis; KHJ and SGW: performed biochemical and statistical analyses; and KHJ and PMK-E: wrote the manuscript. All of the authors take responsibility for the final content of the manuscript. SSJ worked for the Bell Institute of Health and Nutrition, part of the General Mills Company, and is now affiliated with Kerry Ingredients and Flavours; ABR worked for the Nestlé Research Center, part of the Nestlé company, and now is affiliated with Chalmers University of Technology in Gothenburg, Sweden. Both companies produce a wide range of food products, including those that contain whole grains. None of the other authors declared a conflict of interest.
Footnotes
Abbreviations used: AT, adipose tissue; BP, blood pressure; CRC, clinical research center; CRP, C-reactive protein; CVD, cardiovascular disease; DXA, dual-energy X-ray absorptiometry; L3, third lumbar vertebra; MetS, metabolic syndrome; RG, refined grain; TC, total cholesterol; WG, whole grain.
REFERENCES
- 1.Ervin RB.Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race, and ethnicity, and body mass index:United States, 2003-2006. Hyattsville, MD: National Center for Health Statistics, 2009. (National Health Statistics Report 13.) [PubMed] [Google Scholar]
- 2.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 3.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med 2005;56:45–62. [DOI] [PubMed] [Google Scholar]
- 4.USDA; United States Department of Health and Human Services. Dietary Guidelines for Americans. 7th ed. Washington, DC: US Government Printing Office, 2010. [Google Scholar]
- 5.Sahyoun NR, Jacques PF, Zhang XL, Juan W, McKeown NM. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr 2006;83:124–31. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Manson JE, Stampfer MJ, Hu FB, Giovannucci E, Colditz GA, Hennekens CH, Willet WC. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am J Public Health 2000;90:1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs DR, Jr, Meyer KA, Kushi LH, Folsom AR. Whole-grain intake may reduce the risk of ischemic heart disease death in postmenopausal women: the Iowa Women's Health Study. Am J Clin Nutr 1998;68:248–57. [DOI] [PubMed] [Google Scholar]
- 8.Newby PK, Maras J, Bakun P, Muller D, Ferrucci L, Tucker KL. Intake of whole grains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J Clin Nutr 2007;86:1745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKeown NM, Yoshida M, Shea MK, Jacques PF, Lichtenstein AH, Rogers G, Booth SL, Saltzman E. Whole-grain intake and cereal fiber are associated with lower abdominal adiposity in older adults. J Nutr 2009;139:1950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeown NM, Troy LM, Jacques PF, Hoffmann U, O'Donnell CJ, Fox CS. Whole- and refined-grain intakes are differentially associated with abdominal visceral and subcutaneous adiposity in healthy adults: the Framingham Heart Study. Am J Clin Nutr 2010;92:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh-Banerjee P, Franz M, Sampson L, Liu S, Jacobs DR, Spiegelman D, Willett W, Rimm E. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-yr weight gain among men. Am J Clin Nutr 2004;80:1237–45. [DOI] [PubMed] [Google Scholar]
- 12.Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr 2013;98:872–84. [DOI] [PubMed] [Google Scholar]
- 13.Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, Kris-Etherton PM. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr 2008;87:79–90. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, Bugel S, Tetens I, Astrup A. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr 2012;142:710–6. [DOI] [PubMed] [Google Scholar]
- 15.Brownlee IA, Moore C, Chatfield M, Richardson DP, Ashby P, Kuznesof SA, Jebb SA, Seal CJ. Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart Study, a randomised, controlled dietary intervention. Br J Nutr 2010;104(1):125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, Mutch W, Wahle K, Horgan G, Thies F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr 2010;92:733–40. [DOI] [PubMed] [Google Scholar]
- 17.Ross AB, Bourgeois A, Macharia HN, Macharia HN, Kochhar S, Jebb S, Brownlee I, Seal CJ. Plasma alkylresorcinols as a biomarker of whole-grain food consumption in a large population: results from the WHOLEheart Intervention Study. Am J Clin Nutr 2012;95:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- 19.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Proc Natl Acad Sci USA 1918;4(12):370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Association of Cereal Chemists International. Whole grain definition. Cereal Foods World 1999;45:79. [Google Scholar]
- 21.National Heart Lung and Blood Institute Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obese Adults (US). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res 1998;6:51S–209S. [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 23.Ross AB, Redeuil K, Vigo M, Rezzi S, Nagy K. Quantification of alkylresorcinols in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2010;24:554–60. [DOI] [PubMed] [Google Scholar]
- 24.Abate N, Abhimanyu G, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr 1997;65:403–8. [DOI] [PubMed] [Google Scholar]
- 25.Demerath EW, Shen W, Lee M, Choh AC, Czerwinski SA, Siervogel RM, Towne B. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr 2007;85:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly SA, Summerbell CD, Brynes A, Whittaker V, Frost G. Wholegrain cereals for coronary heart disease. Cochrane Database Syst Rev 2007;CD005051. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson AC, Ostman EM, Granfeldt Y, Bjorck IM. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am J Clin Nutr 2008;87:645–54. [DOI] [PubMed] [Google Scholar]
- 29.Giacco R, Lappi J, Costabile G, Kolehmainen M, Schwab U, Landberg R, Uusitupa M, Poutanen K, Pacini G, Rivellese AA, Riccardi G, Mykkänen H. Effects of rye and whole wheat versus refined cereal foods on metabolic risk factors: a randomised controlled two-centre intervention study. Clin Nutr 2013;32(6):941–9. [DOI] [PubMed] [Google Scholar]
- 30.Bachman JL, Reedy J, Subar AF, Krebs-Smith SM. Sources of food group intakes among the US population, 2001-2002. J Am Diet Assoc 2008;108:804–14. [DOI] [PubMed] [Google Scholar]
- 31.Harris KA, Kris-Etherton PM. Effects of whole grains on coronary heart disease risk. Curr Atheroscler Rep 2010;12:368–76. [DOI] [PubMed] [Google Scholar]
- 32.Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc 2008;108:1716–31. [DOI] [PubMed] [Google Scholar]
- 33.Hallfrisch J, Behall KM. Mechanisms of the effects of grains on insulin and glucose responses. J Am Coll Nutr 2000;19(suppl):320S–5S. [DOI] [PubMed] [Google Scholar]
- 34.Swennen K, Courtin CM, Delcour JA. Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci Nutr 2006;46:459–71. [DOI] [PubMed] [Google Scholar]
- 35.Neyrinck AM, Delzenne NM. Potential interest of gut microbial changes induced by non-digestible carbohydrates of wheat in the management of obesity and related disorders. Curr Opin Clin Nutr Metab Care 2010;13:722–8. [DOI] [PubMed] [Google Scholar]
- 36.Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C, Gibson GR, Tuohy KM. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr 2008;99:110–20. [DOI] [PubMed] [Google Scholar]
- 37.Christensen EG, Licht TR, Kristensen M, Bahl MI. Bifidogenic effect of whole-grain wheat during a 12-week energy-restricted dietary intervention in postmenopausal women. Eur J Clin Nutr 2013;67:1316–21. [DOI] [PubMed] [Google Scholar]
- 38.Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa L, Pineo A, Busardo’ A, Paolisso G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med 2003;24:39–52. [DOI] [PubMed] [Google Scholar]
- 39.Adom KK, Liu RH. Antioxidant activity of grains. J Agric Food Chem 2002;50:6182–7. [DOI] [PubMed] [Google Scholar]
- 40.American Heart Association Nutrition Committee. Dietary guidelines for healthy American adults: a statement for physicians and health professionals by the Nutrition Committee, American Heart Association. Circulation 1988;77:721A–4A. [PubMed] [Google Scholar]
- 41.Wu L, Ma D, Walton-Moss B, He Z. Effects of low-fat diet on serum lipids in premenopausal and postmenopausal women: a meta-analysis of randomized controlled trials. Menopause 2014;21(1):89–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.