Abstract
Background: Stable-isotope ratios of carbon (13C/12C, expressed as δ13C) and nitrogen (15N/14N, or δ15N) have been proposed as potential nutritional biomarkers to distinguish between meat, fish, and plant-based foods.
Objective: The objective was to investigate dietary correlates of δ13C and δ15N and examine the association of these biomarkers with incident type 2 diabetes in a prospective study.
Design: Serum δ13C and δ15N (‰) were measured by using isotope ratio mass spectrometry in a case-cohort study (n = 476 diabetes cases; n = 718 subcohort) nested within the European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk population-based cohort. We examined dietary (food-frequency questionnaire) correlates of δ13C and δ15N in the subcohort. HRs and 95% CIs were estimated by using Prentice-weighted Cox regression.
Results: Mean (±SD) δ13C and δ15N were −22.8 ± 0.4‰ and 10.2 ± 0.4‰, respectively, and δ13C (r = 0.22) and δ15N (r = 0.20) were positively correlated (P < 0.001) with fish protein intake. Animal protein was not correlated with δ13C but was significantly correlated with δ15N (dairy protein: r = 0.11; meat protein: r = 0.09; terrestrial animal protein: r = 0.12, P ≤ 0.013). δ13C was inversely associated with diabetes in adjusted analyses (HR per tertile: 0.74; 95% CI: 0.65, 0.83; P-trend < 0.001], whereas δ15N was positively associated (HR: 1.23; 95% CI: 1.09, 1.38; P-trend = 0.001).
Conclusions: The isotope ratios δ13C and δ15N may both serve as potential biomarkers of fish protein intake, whereas only δ15N may reflect broader animal-source protein intake in a European population. The inverse association of δ13C but a positive association of δ15N with incident diabetes should be interpreted in the light of knowledge of dietary intake and may assist in identifying dietary components that are associated with health risks and benefits.
INTRODUCTION
With the growing recognition of the importance of diet in the prevention of chronic disease (1), interest in identifying nutritional biomarkers that offer objective assessment of dietary intake to overcome limitations of imprecision, bias, and measurement error posed by the self-report of diet has increased. As a proof of principle, for incident type 2 diabetes (T2D)4, we previously showed a stronger inverse association with greater plasma vitamin C concentration as a marker of higher fruit and vegetable intake compared with dietary assessment by using a food-frequency questionnaire (FFQ) (2). Many other foods have been implicated in diabetes etiology, such as meat intake (3–6), fish intake (7–11), and the intake of sweetened beverages (12, 13), and the identification of biomarkers of intake of these foods has the potential to advance the field of nutritional epidemiology.
Carbon and nitrogen stable-isotope ratios (13C/12C ratio, expressed as δ13C, and 15N/14N ratio, expressed as δ15N) were recently proposed as potential nutritional biomarkers for distinguishing between meat, fish, and plant-based foods, which are isotopically distinct. The technique has a long history in archeology and ecology as a method of dietary analysis (14, 15), but few epidemiologic studies in human populations have been conducted. The most frequent application of these biomarkers to date has been described for the assessment of dietary animal protein intake, which showed that individuals consuming animal protein have higher hair δ15N than do those consuming vegetarian or vegan diets. For example, in the German Verbundstudie Ernährungserhebung und Risikofaktoren-Analytik study (n = 121 adults), hair δ13C and δ15N ratios were marginally lower among vegetarians than omnivores (16), and, in a small UK study (n = 28), individuals consuming diets characterized by high quantities of fish/animal protein (ie, omnivores) were more likely to have higher δ15N values (17). In a short-term feeding study, we showed that urinary and fecal δ13C and δ15N for individuals consuming fish and meat diets were elevated relative to those of individuals consuming a vegetarian diet, which raises the potential for their use as nutritional biomarkers of animal-derived protein intake (18). In a sample of native Alaskan Eskimos, O'Brien et al showed that δ15N values are strongly correlated with dietary and erythrocyte omega-3 (n−3) fatty acids (19).
The ability of the δ13C values of foods to distinguish between those derived from C4 plants (a photosynthetically distinct group of plants ie corn/maize, cane) and those from other foods derived from the more abundant C3 plants (eg, beets, fruit and vegetables, rice, wheat, nuts, and seeds) (20) has enabled serum δ13C values to be used to distinguish between individuals with a high compared with a low intake of sugar-sweetened beverages (SSBs) containing C4 sugars, such as high-fructose corn syrup (21). More recently, in Yup'ik Alaskan Eskimos, both erythrocyte δ13C and δ15N values predicted sweetener intake (22).
To date, the distribution of carbon and nitrogen stable isotopes in European populations has been limited to small studies of human hair (16, 17, 23, 24), whereas their potential use as nutritional biomarkers in association with disease etiology remains unexplored. The aim of this study was to 1) describe the distribution and correlates of serum δ13C and δ15N in a UK population to determine their utility as biomarkers of dietary intake, and 2) to assess the prospective association between δ13C and δ15N and incident T2D.
SUBJECTS AND METHODS
Study participants and design
The European Prospective Investigation into Cancer (EPIC)–Norfolk study recruited 25,639 men and women, aged 40–79 y at baseline (1993–1997), as described previously (25). In brief, since the baseline health check, there have been 3 follow-up assessments, including a postal questionnaire at 18 mo, a repeat (second) health-check visit (1998–2000) including a questionnaire, and a further postal questionnaire (2002–2004). The current analysis used data from a case-cohort design (26, 27) with 1530 participants, in which a random subcohort was selected to be representative of the whole cohort, and all incident cases were ascertained. We excluded participants with prevalent (n = 29) or missing diabetes status (n = 81); those with prevalent cardiovascular disease or cancer (n = 162); those without duplicate measurements of δ15N or δ13C (n = 7); those with >10 lines missing from the FFQ (n = 13); those missing data on fish, meat, or protein intake (n = 26); or those with missing covariate information (n = 34). A case-cohort study included overlap between subcohort and incident cases by design, and the final sample included 1178 participants with 476 total incident T2D cases and 718 subcohort participants (including 16 incident T2D cases and 702 noncase participants). Informed consent was obtained from all study participants, and ethical approval was granted by the Norwich District Ethics Committee.
Baseline measurements
Health and lifestyle information was collected by using a questionnaire, including the participants’ personal and family health information, demographic circumstances, lifestyle, social circumstances, and diet (25). A standardized health check was conducted by trained nurses. Physical activity was assessed by self-report, and a validated 4-scale index was derived (inactive, moderately inactive, moderately active, and active) by combining levels of occupational and recreational physical activity (28). Smoking status was recorded in 3 categories (current, former, and never). Nonfasting venous blood samples were collected and stored overnight in a dark container at 4–7°C and then centrifuged at 2100 × g for 15 min at 4°C, transported, and subsequently stored in liquid nitrogen at −196°C. For plasma vitamin C measurement, venous blood was drawn into citrate-containing bottles and kept overnight in a dark container at 4–7°C, samples were centrifuged, and plasma was stabilized by using a standardized volume of metaphosphoric acid. Plasma vitamin C concentrations were measured within 1 wk of sampling by using a fluorometric assay. Serum samples were stored for an average of 12 y at −196°C before stable-isotope analysis.
Dietary assessment
The participants completed a validated 130-item semiquantitative FFQ for food items consumed in the preceding year (29). Nutrient intake (g/d) was calculated from the frequency and amount (medium serving size) of each reported food by using in-house software (30). Protein intake from different food sources (fish, meat, dairy products, egg, vegetables, and cereal) was calculated by using McCance and Widdowson's Composition of Foods database. For instance, for fish protein, each of the 6 fish variables (fried fish in batter, fish fingers/fish cakes, other white fish, oily fish, shellfish, and fish roe) were multiplied by their corresponding protein content (protein/per gram of fish) from food codes in UK food-composition tables and were summed to obtain total fish protein. The same method was used to derive protein from other sources. Animal protein (g/d) was derived as the sum of protein from meat, fish, dairy products, and egg intakes. Terrestrial animal protein (secondary animal protein) is the sum of protein from meat, dairy products, and egg and is nonmarine (primary animal protein). Vegetable protein (g/d) was derived in a manner similar to that for fish protein by using 26 vegetable food lines. Dairy protein was derived by summing protein from milk, cream, yogurt, cheese, and dairy desserts (g/d) by using 8 dairy food lines.
Definition of dietary exposures
Dietary groups defined predominantly by plant, fish, or terrestrial animal intake were derived to enable comparisons with previous studies. Consumption of a vegetarian diet was addressed as a question in the health and lifestyle questionnaire (“Do you follow any particular diets?”, of which a list of diets included “vegetarian”). Participants who reported a vegetarian diet or who reported consuming no meat and no fish in the FFQ were considered to be vegetarian. Two types of consumer groups were derived to capture variation in stable isotope values: fish consumers and terrestrial animal and animal products consumers. For the fish-consumption groups, we included only lower meat consumers (<100 g/d) and then classified participants on the basis of their levels of fish consumption (based on the number of portions of fish consumed per week, assuming a portion size of 100 g); the levels were as follows: nonconsumer, low consumer (>0 to ≤1 portion/wk); medium consumer (>1 to ≤2 portions/wk); high consumer (>2 to ≤3 portions/wk); and very high consumer (>3 portions/wk). For the terrestrial animal and animal product consumption groups, we included only lower fish consumers (<200 g/wk) and then classified participants according to their level of combined intakes of meat, dairy products, and eggs; the levels were as follows: low consumer (<250 g/d), medium consumer (250 to ≤500 g/d), high consumer (500 to ≤750 g/d), and very high consumer (>750 g), with 250-g/d increments being half of the mean consumption among low fish eaters (mean = 508 g/d; range = 0–1089 g/d). Because of homogeneity in consumption of fish and meat in this study population, the consumer groups were not mutually exclusive. In addition, consumer groups were defined to create strata that contained sufficient data that would allow for comparisons. For these reasons, the analyses including these definitions should be interpreted with caution.
Fatty acid analysis
To enable the examination of the association between isotopic values (δ13C and δ15N) and omega-3 fatty acids, as previously described for δ15N (19), we used a subset of our sample (n = 147) with measures of plasma phospholipid fatty acids (31) for α-linolenic acid (ALA), EPA, docosapentaenoic acid, and DHA expressed as a percentage of the total fatty acid concentrations (31).
Stable isotopic analysis
Sample preparation was carried out at the MRC Epidemiology Unit, University of Cambridge, United Kingdom. Duplicate 10-μL aliquots of serum were drawn from thawed samples, transferred into high-purity tin capsules (3.5 × 3.75 mm), and then dried in a speed vacuum concentrator (miVAC; Genevac Ltd). Samples were prepared in duplicate batches and transported within 1 wk of preparation to the McDonald Institute for Archaeological Research, University of Cambridge. Isotopic analyses were performed by using a Costech automated elemental analyzer coupled in continuous-flow mode to an isotope ratio–monitoring mass spectrometer (Thermo Finnigan MAT253) at the Godwin Laboratory, Department of Earth Sciences, University of Cambridge. Stable isotope concentrations are measured as the ratio of the heavier isotope to the lighter isotope relative to an internationally defined scale, Vienna Pee Dee Belemnite for carbon and mean atmospheric nitrogen for nitrogen, according to standard convention (32). The natural abundance stable-isotope composition of δ13C and δ15N are reported in the delta (δ) scale in parts per thousand or “per mil” (expressed as ‰) values, where δX = [(Rsample/Rstandard) − 1] × 1000, where R is the ratio of heavy to light isotope (for both nitrogen and carbon). The δ13C and δ15N values in this study are reported as the mean of samples analyzed in duplicate. Based on replicate analyses of international and laboratory standards, measurement errors were ≤0.2‰ for δ13C and δ15N for 92% of the study population (n = 1,178) and ≤0.9‰ for the remaining 8%.
Diabetes case ascertainment
Incident cases of diabetes occurring until 31 July 2006 were ascertained by using multiple sources, including self-report of doctor-diagnosed diabetes or diabetes-specific medication, linkage to primary or secondary care registers, hospital admissions data, or mortality data. Follow-up began at the date of recruitment and ended at either 31 July 2006, date of diabetes diagnosis, or date of death (if the individual died before this date), whichever occurred first. Individuals with prevalent diabetes or for whom the diabetes status could not be confirmed were excluded from analysis.
Statistical analysis
Distribution of δ13C and δ15N values and baseline characteristics
We examined the mean and SD of δ13C and δ15N values in the entire subcohort population and described the sociodemographic and dietary characteristics of participants by tertiles of both biomarkers separately by using means (±SDs), medians (IQRs), or frequencies. We created bag plots (33) to examine pictorially the combined distribution of δ13C and δ15N in the subcohort by dietary status characterized by vegetarian diet, fish-consumer status, and terrestrial animal consumer status and tested joint differences in isotope ratios across these groups by using a multivariate test of means (mvtest command).
Correlation between stable isotopes and dietary intake
Spearman correlation coefficients (Spearman's rho, r) were calculated to examine the relation between δ13C and δ15N values and dietary intake, including dietary protein intake (total and from fish, meat, dairy products, terrestrial animal, vegetables, and cereals) and sugar intake (total, fructose, glucose, galactose, lactose, and sucrose). In the subgroup with plasma phospholipid measures available (n = 147 in the subcohort), we also examined Spearman correlations with total omega-3 fatty acids, ALA, EPA, docosapentaenoic acid, and DHA.
Association between stable isotopes, dietary factors, and incident diabetes
To estimate the association between stable isotopes and types of dietary protein intake and diabetes, we used Prentice-weighted Cox proportional hazards analysis, which accounts for the case-cohort design, and with age as the underlying time variable (26, 27).
HRs and corresponding 95% CIs for diabetes comparing tertiles of δ13C and δ15N were examined for the association between stable isotopes and incident diabetes. We also examined the association of total protein and protein from fish, meat, terrestrial animal, dairy products, vegetables, and cereals with diabetes. We used the following modeling strategy to adjust for potential confounders: in model 1 we adjusted for sex, family history of diabetes (2 categories), smoking status (3 categories), educational level (4 categories), physical activity level (4 categories), and δ13C (for δ15N analyses) or δ15N (for δ13C analyses). In model 2 we additionally adjusted for dietary risk factors, including total energy intake (kcal/d), alcohol intake (g/d), and plasma vitamin C (μmol/L) (as an objective marker of fruit and vegetable intakes). Model 3 was further adjusted for BMI (continuous) and waist circumference (continuous). In model 4 we also adjusted for terrestrial animal protein intake to examine the possibility that intake may mediate the association between δ13C and δ15N and diabetes. In sensitivity analyses we also 1) adjusted for fish protein in model 4 to test the independent association between δ13C, δ15N, meat- and terrestrial animal-protein, and diabetes and 2) excluded individuals with δ13C or δ15N measurement >0.2‰ (ie, the cutoff used for analytic uncertainty in δ13C or δ15N measurement, n = 97 of 1178). All statistical analyses were performed by using STATA version 11.2 (StataCorp). Bag plots were created by using version 2.12.1 of the statistical package R (34).
RESULTS
The mean (±SD) age of the subcohort participants was 57.7 ± 9.3 y, the mean (±SD) BMI (in kg/m2) was 25.9 ± 3.6, 41% were men, 13% reported being current smokers and 38% former smokers, 47% reported being physically or moderately physically active, and 37% reported having finished education after compulsory schooling (age 16 y). The mean (±SD) value for δ13C was −22.8 ± 0.4‰ (a negative delta value for δ13C means that the isotopic ratio of the sample is lower than that of the standard) and for δ15N was 10.2 ± 0.4‰. The Pearson correlation (r) value between δ13C and δ15N was r = 0.28 (P < 0.001) in the subcohort and was r = 0.26 (P < 0.001) in the cases.
Baseline demographic and dietary characteristics by δ13C and δ15N distribution
Smoking frequency was higher across increasing tertiles of both δ13C and δ15N, and participants were older across increasing tertiles of δ15N; however, no other sociodemographic factors varied with isotopic values (Tables 1 and 2). Of the dietary factors, dietary fish protein had the strongest correlations (r = 0.22 and P < 0.001 for δ13C; r = 0.20 and P < 0.001 for δ15N), which were not materially different among incident cases only (results not shown). Whereas increasing δ13C values were positively correlated with fish intake and fish protein intake, no significant association of intakes of meat, meat protein, terrestrial animal, or terrestrial animal protein intake with δ13C was observed. With increasing δ15N values, there were higher intakes of fish and dairy products, higher terrestrial animal intakes, a lower frequency of a vegetarian diet, and higher intakes of animal-source protein (including meat protein, dairy protein, and terrestrial animal protein). Of the sugars, only lactose intake was higher across increasing δ15N, with a modest correlation (r = 0.12, P = 0.001); no variation across δ13C values was observed. Intakes of total breakfast cereals, fizzy drinks, or fruit juice did not differ by tertiles of δ13C or δ15N (data not shown), whereas a higher alcohol intake was observed with increasing δ13C. Cereal protein intake was inversely associated with both δ13C (r = −0.13, P < 0.001) and δ15N (r = −0.10, P = 0.011).
TABLE 1.
Baseline sociodemographic and dietary characteristics by tertiles of δ13C values in the subcohort (n = 718): the EPIC-Norfolk study1
| Tertiles of δ13C values |
Correlation analyses2 |
|||||
| 1 | 2 | 3 | P-trend3 | Spearman's r | P value | |
| n | 245 | 234 | 239 | — | — | — |
| δ13C (‰) | −23.2 ± 0.24 | −22.8 ± 0.1 | −22.4 ± 0.3 | <0.001 | — | — |
| Range | −23.83 to −22.97 | −22.96 to −22.68 | −22.67 to −21.12 | — | — | — |
| δ15N (‰) | 10.1 ± 0.4 | 10.3 ± 0.4 | 10.4 ± 0.4 | <0.001 | — | — |
| Range | 8.57–11.29 | 9.16–11.36 | 8.03–11.67 | — | — | — |
| Sociodemographic characteristics | ||||||
| Age (y) | 57.0 ± 9.7 | 57.7 ± 9.3 | 58.3 ± 9.0 | 0.36 | 0.05 | 0.16 |
| Male sex [n (%)] | 92 (37.6) | 91 (38.9) | 111 (46.4) | 0.10 | — | — |
| BMI (kg/m2) | 25.7 ± 3.4 | 26.1 ± 3.5 | 25.9 ± 3.8 | 0.47 | 0.01 | 0.75 |
| Waist circumference (cm) | ||||||
| Men | 92.9 ± 9.0 | 95.5 ± 9.0 | 94.9 ± 10.4 | 0.16 | 0.09 | 0.10 |
| Women | 81.0 ± 9.9 | 80.9 ± 9.2 | 80.4 ± 10.7 | 0.87 | −0.03 | 0.53 |
| Family history of diabetes [n (%)] | 32 (13.1) | 19 (8.1) | 28 (11.7) | 0.21 | — | — |
| Smoking status [n (%)] | 0.047 | |||||
| Never | 125 (51.0) | 124 (53.0) | 99 (41.4) | — | — | |
| Former | 92 (37.6) | 85 (36.3) | 98 (41.0) | — | — | |
| Current | 28 (11.4) | 25 (10.7) | 42 (17.6) | — | — | |
| Educational level [n (%)] | 0.33 | — | — | |||
| Compulsory schooling | 100 (40.8) | 84 (35.9) | 80 (33.5) | — | — | |
| Up to O level | 16 (6.5) | 25 (10.7) | 29 (12.1) | — | — | |
| Up to A level | 99 (40.4) | 90 (38.5) | 95 (39.8) | — | — | |
| Up to degree level | 30 (12.2) | 35 (15.0) | 35 (14.6) | — | — | |
| Physical activity [n (%)] | 0.09 | — | — | |||
| Active | 55 (22.5) | 55 (23.5) | 41 (17.2) | — | — | |
| Moderately active | 54 (22.0) | 55 (23.5) | 78 (32.6) | — | — | |
| Moderately inactive | 75 (30.6) | 69 (29.5) | 58 (24.3) | — | — | |
| Inactive | 61 (24.9) | 55 (23.5) | 62 (25.9) | — | — | |
| Dietary characteristics | ||||||
| Total energy intake (kcal/d) | 2083 ± 604 | 2056 ± 645 | 1999 ± 560 | 0.30 | −0.04 | 0.26 |
| Fat (g/d) | 79.9 ± 31.7 | 77.6 ± 31.7 | 73.4 ± 30.0 | 0.07 | −0.08 | 0.03 |
| Carbohydrate (g/d) | 261.9 ± 81.2 | 258.7 ± 87.7 | 247.4 ± 75.2 | 0.12 | −0.06 | 0.12 |
| Alcohol intake (g/d) | 2.4 (0.8, 7.0)5 | 4.6 (0.8, 10.6) | 6.1 (1.3, 13.0) | <0.001 | 0.20 | <0.001 |
| Fruit intake (g/d) | 221 (132, 338) | 193 (112, 344) | 218 (124, 333) | 0.44 | −0.02 | 0.54 |
| Vegetable intake (g/d) | 256 (195, 348) | 241 (180, 349) | 248 (186, 323) | 0.35 | −0.04 | 0.28 |
| Fish intake (g/d) | 27 (16, 39) | 32 (20, 44) | 39 (25, 57) | <0.001 | 0.22 | <0.001 |
| Meat intake (g/d) | 100 (65, 136) | 94 (65, 126) | 99 (69, 125) | 0.75 | −0.008 | 0.83 |
| Dairy intake (g/d) | 371 (291, 508) | 362 (298, 527) | 399 (308, 528) | 0.29 | 0.06 | 0.13 |
| Terrestrial animal intake (g/d)6 | 510 (375, 626) | 480 (375, 663) | 510 (405, 655) | 0.41 | 0.05 | 0.18 |
| Follows a vegetarian diet [n (%)] | 16 (6.5) | 7 (3.0) | 14 (5.9) | 0.18 | — | — |
| Total sugars (g/d) | 126.1 (97.4, 157.2) | 124.7 (95.0, 159.1) | 125.6 (97.9, 154.4) | 0.90 | 0.01 | 0.77 |
| Fructose (g/d) | 22.9 (16.9, 31.3) | 21.7 (15.9, 30.0) | 22.7 (16.8, 29.2) | 0.62 | −0.02 | 0.56 |
| Glucose (g/d) | 21.1 (15.7, 27.2) | 20.3 (14.9, 27.6) | 20.7 (15.2, 27.0) | 0.69 | −0.03 | 0.44 |
| Galactose (g/d) | 0.2 (0.0, 0.8) | 0.2 (0.0, 0.8) | 0.2 (0.0, 0.8) | 0.25 | 0.05 | 0.16 |
| Lactose (g/d) | 19.9 (15.1, 26.5) | 19.0 (14.8, 28.4) | 20.5 (16.4, 27.6) | 0.28 | 0.05 | 0.22 |
| Sucrose (g/d) | 50.4 (34.9, 71.0) | 49.2 (35.4, 71.9) | 52.0 (34.4, 70.3) | 0.99 | 0.03 | 0.48 |
| Total protein (g/d) | 82.2 (66.1, 95.6) | 81.2 (66.6, 95.8) | 80.8 (67.2, 94.2) | 0.95 | 0.007 | 0.86 |
| Fish protein (g/d) | 5.1 (3.3, 7.4) | 5.6 (4.1, 8.2) | 7.2 (4.7, 10.3) | <0.001 | 0.22 | <0.001 |
| Meat protein (g/d) | 24.1 (14.8, 32.4) | 23.2 (16.0, 31.1) | 24.3 (16.6, 30.0) | 0.92 | 0.009 | 0.81 |
| Dairy protein (g/d) | 16.4 (12.6, 21.0) | 16.7 (11.8, 22.2) | 17.2 (13.2, 21.6) | 0.54 | 0.04 | 0.24 |
| Terrestrial animal protein (g/d)6 | 41.8 (32.5, 53.0) | 43.0 (33.3, 51.6) | 43.0 (34.5, 52.2) | 0.88 | 0.04 | 0.33 |
| Vegetable protein (g/d) | 5.6 (4.2, 7.8) | 5.5 (4.2, 7.5) | 5.3 (3.8, 7.2) | 0.21 | −0.05 | 0.14 |
| Cereal protein (g/d) | 9.2 (6.1, 13.4) | 8.9 (5.7, 12.6) | 7.4 (5.2, 11.0) | 0.003 | −0.13 | <0.001 |
EPIC, European Prospective Investigation into Cancer and Nutrition; FFQ, food-frequency questionnaire; δ13C, carbon stable-isotope ratio (13C/12C ratio); δ15N, nitrogen stable-isotope ratio (15N/14N ratio).
Spearman's rho, r, between δ13C values (‰) and total and types of FFQ-derived dietary intake (g/d). Differences across tertiles are denoted by P values, which correspond to Kruskal-Wallis test.
P-trend across tertiles of δ13C.
Mean ± SD (all such values).
Median; IQR in parentheses (all such values).
Terrestrial animal protein intake is the sum of meat, dairy, and egg protein (g/d).
TABLE 2.
Baseline sociodemographic and dietary characteristics by tertiles of δ15N values in the subcohort (n = 718): the EPIC-Norfolk study1
| Tertiles δ15N values |
Correlation analyses2 |
|||||
| 1 | 2 | 3 | P-trend3 | Spearman's r | P value | |
| n | 248 | 235 | 235 | — | — | — |
| δ15N (‰) | 9.8 ± 0.34 | 10.3 ± 0.10 | 10.7 ± 0.2 | <0.001 | — | — |
| Range | 8.03 to 10.09 | 10.10 to 10.43 | 10.44 to 11.67 | — | — | — |
| δ13C (‰) | −22.9 ± 0.4 | −22.8 ± 0.4 | −22.7 ± 0.3 | <0.001 | — | — |
| Range | −23.83 to −21.43 | −23.76 to −21.12 | −23.69 to −21.28 | — | — | — |
| Sociodemographic characteristics | — | — | ||||
| Age (y) | 56.1 ± 9.7 | 58.0 ± 9.2 | 59.0 ± 8.8 | 0.002 | 0.14 | <0.001 |
| Male sex [n (%)] | 111 (44.8) | 92 (39.2) | 91 (38.7) | 0.32 | — | — |
| BMI (kg/m2) | 25.7 ± 3.6 | 26.0 ± 3.6 | 26.0 ± 3.5 | 0.58 | 0.06 | 0.09 |
| Waist circumference (cm) | ||||||
| Men | 93.3 ± 9.2 | 95.2 ± 10.6 | 95.3 ± 9.0 | 0.24 | 0.07 | 0.26 |
| Women | 80.9 ± 10.8 | 80.9 ± 10.0 | 80.5 ± 8.7 | 0.93 | 0.04 | 0.37 |
| Family history of diabetes [n (%)] | 30 (12.1) | 22 (9.4) | 27 (11.5) | 0.61 | — | — |
| Smoking status [n (%)] | 0.002 | — | — | |||
| Never | 111 (44.8) | 128 (54.5) | 109 (46.4) | — | — | |
| Former | 113 (45.6) | 81 (34.5) | 81 (34.5) | — | — | |
| Current | 24 (9.7) | 26 (11.1) | 45 (19.2) | — | — | |
| Educational level [n (%)] | 0.56 | — | — | |||
| Compulsory schooling | 84 (33.9) | 93 (39.6) | 87 (37.0) | — | — | |
| Up to O level | 22 (8.9) | 27 (11.5) | 21 (8.9) | — | — | |
| Up to A level | 106 (42.7) | 81 (34.5) | 97 (41.3) | — | — | |
| Up to degree level | 36 (14.5) | 34 (14.5) | 30 (12.8) | — | — | |
| Physical activity [n (%)] | 0.22 | — | — | |||
| Active | 57 (23.0) | 51 (21.7) | 43 (18.3) | — | — | |
| Moderately active | 63 (25.4) | 58 (24.7) | 66 (28.1) | — | — | |
| Moderately inactive | 71 (28.6) | 75 (31.9) | 56 (23.8) | — | — | |
| Inactive | 57 (23.0) | 51 (21.7) | 70 (29.8) | — | — | |
| Dietary characteristics | ||||||
| Total energy intake (kcal/d) | 2059 (570) | 2016 (596) | 2065 (647) | 0.63 | −0.02 | 0.55 |
| Fat (g/d) | 77.4 (29.9) | 74.5 (29.1) | 79.1 (34.4) | 0.27 | −0.002 | 0.96 |
| Carbohydrate (g/d) | 260.6 (79.2) | 253.7 (80.2) | 253.6 (85.6) | 0.56 | −0.06 | 0.10 |
| Alcohol intake (g/d) | 3.3 (0.8, 9.7)5 | 4.1 (0.8, 9.9) | 4.7 (0.8, 11.5) | 0.82 | 0.03 | 0.45 |
| Fruit intake (g/d) | 213 (123, 341) | 219 (133, 353) | 204 (115, 330) | 0.63 | −0.02 | 0.51 |
| Vegetable intake (g/d) | 250 (182, 340) | 242 (187, 338) | 248 (187, 333) | 0.90 | −0.02 | 0.65 |
| Fish intake (g/d) | 27 (16, 43) | 32 (22, 46) | 38 (27, 51) | <0.001 | 0.21 | <0.001 |
| Meat intake (g/d) | 94 (61, 124) | 98 (65, 132) | 103 (73, 136) | 0.16 | 0.07 | 0.06 |
| Dairy intake (g/d) | 335 (249, 515) | 367 (304, 508) | 431 (312, 560) | 0.004 | 0.13 | <0.001 |
| Terrestrial animal intake (g/d)6 | 464 (355, 631) | 502 (382, 649) | 543 (418, 670) | 0.004 | 0.13 | <0.001 |
| Follows a vegetarian diet [n (%)] | 21 (8.5) | 11 (4.7) | 5 (2.1) | 0.006 | — | — |
| Total sugars (g/d) | 126.4 (95.6, 156.1) | 125.1 (96.9, 153.6) | 125.6 (99.9, 159.1) | 0.70 | 0.003 | 0.94 |
| Fructose (g/d) | 22.6 (16.2, 30.0) | 22.1 (16.2, 30.2) | 22.6 (16.5, 29.7) | 0.97 | −0.007 | 0.85 |
| Glucose (g/d) | 21.1 (15.2, 27.2) | 20.5 (15.3, 27.8) | 19.9 (15.2, 26.5) | 0.58 | −0.04 | 0.34 |
| Galactose (g/d) | 0.2 (0.0, 0.8) | 0.2 (0.0, 0.8) | 0.2 (0.0, 0.8) | 1.00 | 0.002 | 0.96 |
| Lactose (g/d) | 18.2 (13.6, 27.1) | 19.9 (15.8, 26.3) | 21.6 (16.5, 29.6) | 0.005 | 0.12 | 0.001 |
| Sucrose (g/d) | 51.9 (34.8, 74.4) | 48.7 (35.8, 66.8) | 50.7 (34.9, 70.3) | 0.61 | −0.02 | 0.65 |
| Total protein (g/d) | 79.3 (66.4, 93.2) | 81.8 (65.6, 95.7) | 83.7 (68.5, 97.9) | 0.21 | 0.07 | 0.08 |
| Fish protein (g/d) | 5.1 (3.3, 8.2) | 5.7 (3.6, 8.3) | 6.9 (4.7, 9.2) | <0.001 | 0.20 | <0.001 |
| Meat protein (g/d) | 22.2 (14.0, 29.6) | 23.7 (15.5, 31.9) | 24.8 (16.9, 31.7) | 0.051 | 0.09 | 0.013 |
| Dairy protein (g/d) | 15.9 (11.8, 21.1) | 16.7 (13.0, 21.1) | 18.0 (13.2, 22.0) | 0.016 | 0.11 | 0.003 |
| Terrestrial animal protein (g/d)6 | 41.5 (32.7, 48.9) | 43.0 (31.9, 52.4) | 43.9 (35.3, 54.3) | 0.010 | 0.12 | 0.002 |
| Vegetable protein (g/d) | 5.5 (4.1, 8.1) | 5.6 (4.0, 7.5) | 5.2 (4.0, 7.2) | 0.20 | −0.07 | 0.07 |
| Cereal protein (g/d) | 8.8 (5.9, 14.0) | 9.2 (5.6, 12.0) | 7.5 (5.2, 11.6) | 0.020 | −0.10 | 0.011 |
EPIC, European Prospective Investigation into Cancer and Nutrition; FFQ, food-frequency questionnaire; δ13C, carbon stable-isotope ratio (13C/12C ratio); δ15N, nitrogen stable-isotope ratio (15N/14N ratio).
Spearman's rho, r, between δ15N values (‰) and total and types of FFQ-derived dietary intake (g/d). Differences across tertiles are denoted by P values, which correspond to Kruskal-Wallis test.
P-trend across tertiles of δ15N.
Mean ± SD (all such values).
Median; IQR in parentheses (all such values).
Terrestrial animal protein intake is the sum of meat, dairy, and egg protein (g/d).
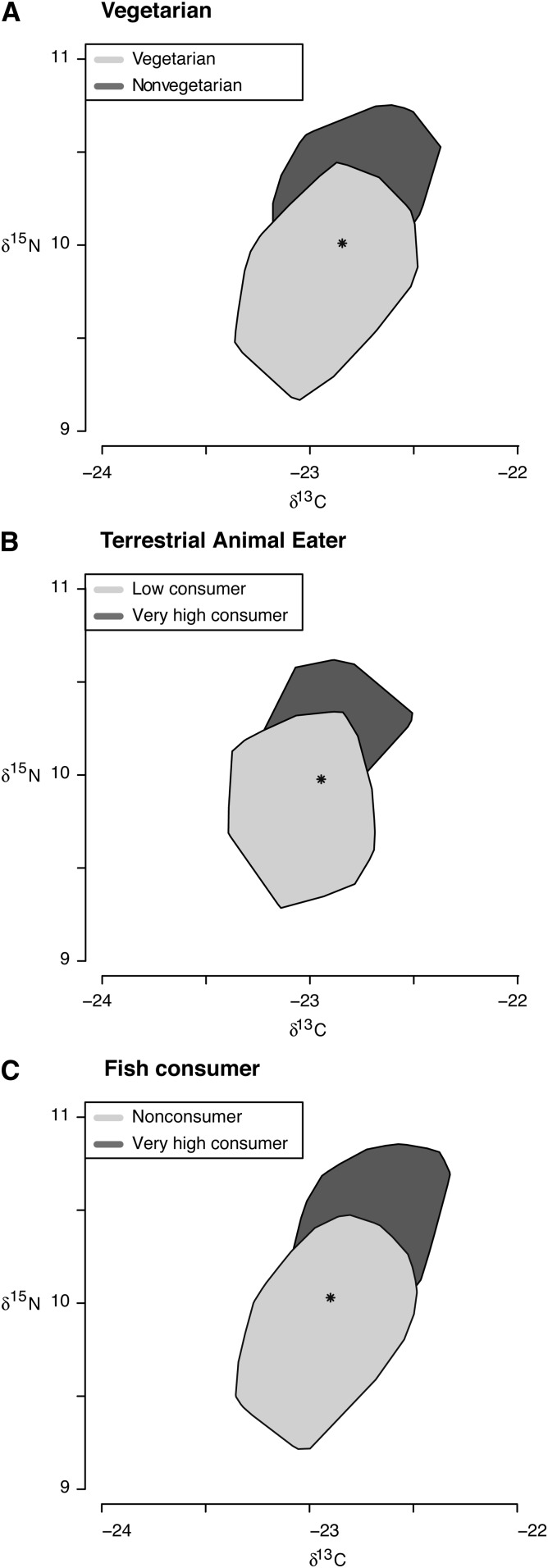
Both mean δ13C and δ15N values were higher with greater fish-consumer status, whereas only δ15N values were higher with greater terrestrial animal and animal product consumption (see Supplementary Table 1 under “Supplemental data” in the online issue). The bag plots in Figure 1 visually compare the combined variation in mean δ13C and δ15N values by dietary status, showing statistically significant differences between nonvegetarians and vegetarians (A; P < 0.001), low and very high terrestrial animal products consumers (B; P < 0.001), and non- and very high fish consumers (C; P < 0.001). P values represent significant differences across 4 categories for terrestrial animal consumption and across 5 categories for fish consumption.
FIGURE 1.
Bag plots of δ13C and δ15N (‰) distribution by vegetarian status (yes, n = 37; no, n = 681) (A), terrestrial animal product consumer (very high consumption, n = 32; low consumption, n = 28) (B), and fish consumer (very high consumption, n = 92; no consumption, n = 43) (C). Bag plots were generated to examine pictorially the combined distribution of δ13C and δ15N by dietary status characterized by vegetarian compared with nonvegetarian diet, terrestrial animal consumer status, and fish-consumer status. Joint differences in isotope ratios across consumption groups were tested by using a multivariate test of means (P < 0.001 for all comparisons). δ13C, carbon stable-isotope ratio (13C/12C ratio); δ15N, nitrogen stable-isotope ratio (15N/14N ratio).
Association of stable isotopes with plasma phospholipid fatty acids
Of the plasma phospholipid fatty acids (n = 147 participants), positive correlations were observed for EPA with δ13C (r = 0.23, P < 0.01) and δ15N (r = 0.22, P < 0.01) and for total omega-3 fatty acids (r = 0.26, P < 0.01) and DHA (r = 0.30, P < 0.001) with δ15N. In addition, ALA was inversely associated with δ13C (r = −0.24, P < 0.01) and δ15N (−0.21, P < 0.01). Median (IQR) fatty acids relative to total fatty acids were as follows: total omega-3, 7.4% (6.4, 8.7%); ALA, 0.2% (0.2, 0.3%); EPA, 1.1% (0.7, 1.4%); and DHA, 4.8% (4.0, 5.6%).
Association between stable isotopes, dietary factors, and the risk of diabetes
Compared with the subcohort, persons who developed diabetes were older, were more likely to be male and to have a family history of diabetes, had a higher BMI and waist circumference, were more physically inactive, were less likely to smoke, and were less likely to be educated to a degree level (see Supplementary Table 2 under “Supplemental data” in the online issue). The case participants reported higher intakes of meat (P < 0.001) but lower intakes of fruit (P = 0.01) and fruit juice (P = 0.04) and had lower plasma vitamin C concentrations (P < 0.001). Mean (±SD) δ15N values were slightly but significantly higher in cases (10.3 ± 0.4‰) than in the subcohort (10.2‰ ± 0.4; P = 0.006). Conversely, δ13C values were slightly but significantly lower (P = 0.009) in cases (−22.9 ± 0.4‰) than in the subcohort (−22.8 ± 0.4‰).
The δ13C values were inversely associated with diabetes in adjusted analyses (HR per tertile: 0.74; 95% CI: 0.65, 0.83; P-trend < 0.001; model 3), as shown in Table 3. In contrast, δ15N values were positively associated with diabetes (HR per tertile: 1.23; 95% CI: 1.09, 1.38; P-trend = 0.001, model 3). Additional adjustment for terrestrial animal protein intake did not materially change the associations observed between δ13C, δ15N, and diabetes (model 4). Fish protein intake was inversely associated with diabetes, whereas terrestrial animal and meat protein intakes were positively associated with diabetes; dairy product, vegetable, and cereal protein intakes were not associated with diabetes. In sensitivity analyses, adjustment for fish protein intake (model 4) did not materially change the associations between δ13C, δ15N, meat protein, or terrestrial animal protein and diabetes. In addition, exclusion of samples with a >0.2‰ range (n = 97) in δ13C and δ15N duplicate measurements did not alter our findings.
TABLE 3.
The association between tertiles of δ13C and δ15N values, types of dietary protein intake, and type 2 diabetes: the EPIC-Norfolk Study1
| Tertiles of stable isotopes and dietary protein types2 |
|||||
| 1 | 2 | 3 | Effect per tertile | P-trend | |
| δ13C | |||||
| Cases/subcohort | 205/245 | 126/234 | 145/239 | — | |
| Median (‰) | −23.1 | −22.8 | −22.5 | — | |
| Model 1 | 1 | 0.66 (0.53, 0.83) | 0.60 (0.48, 0.75) | 0.77 (0.69, 0.86) | <0.001 |
| Model 2 | 1 | 0.64 (0.50, 0.80) | 0.60 (0.48, 0.76) | 0.77 (0.68, 0.86) | <0.001 |
| Model 3 | 1 | 0.66 (0.52, 0.84) | 0.55 (0.43, 0.69) | 0.74 (0.65, 0.83) | <0.001 |
| Model 4 | 1 | 0.68 (0.54, 0.87) | 0.55 (0.43, 0.70) | 0.75 (0.65, 0.83) | <0.001 |
| δ15N | |||||
| Cases/subcohort | 131/248 | 170/235 | 175/235 | — | |
| Median (‰) | 9.9 | 10.3 | 10.7 | — | |
| Model 1 | 1 | 1.27 (1.01, 1.61) | 1.23 (0.97, 1.55) | 1.10 (0.98, 1.23) | 0.10 |
| Model 2 | 1 | 1.32 (1.05, 1.67) | 1.22 (0.96, 1.54) | 1.09 (0.98, 1.23) | 0.12 |
| Model 3 | 1 | 1.52 (1.19, 1.95) | 1.55 (1.22, 1.99) | 1.23 (1.09, 1.38) | 0.001 |
| Model 4 | 1 | 1.53 (1.19, 1.96) | 1.54 (1.21, 1.97) | 1.22 (1.09, 1.38) | 0.001 |
| Fish protein | |||||
| Cases/subcohort | 179/240 | 140/240 | 157/238 | — | |
| Median (g/d) | 3.3 | 5.9 | 10.4 | — | |
| Model 1 | 1 | 0.75 (0.59, 0.93) | 0.95 (0.76, 1.18) | 0.97 (0.86, 1.08) | 0.56 |
| Model 2 | 1 | 0.74 (0.59, 0.92) | 1.00 (0.80, 1.25) | 0.99 (0.88, 1.11) | 0.85 |
| Model 3 | 1 | 0.63 (0.50, 0.80) | 0.73 (0.57, 0.92) | 0.84 (0.74, 0.95) | 0.006 |
| Model 4 | 1 | 0.63 (0.50, 0.79) | 0.72 (0.57, 0.92) | 0.84 (0.74, 0.95) | 0.005 |
| Terrestrial animal protein 3 | |||||
| Cases/subcohort | 133/239 | 152/240 | 191/239 | — | |
| Median (g/d) | 29.7 | 42.6 | 58.5 | — | |
| Model 1 | 1 | 1.05 (0.82, 1.33) | 1.46 (1.16, 1.83) | 1.22 (1.09, 1.37) | 0.001 |
| Model 2 | 1 | 1.14 (0.89, 1.46) | 1.65 (1.27, 2.13) | 1.29 (1.14, 1.47) | <0.001 |
| Model 3 | 1 | 0.87 (0.68, 1.12) | 1.56 (1.19, 2.05) | 1.27 (1.10, 1.46) | 0.001 |
| Model 4 | 1 | 0.87 (0.68, 1.12) | 1.58 (1.20, 2.07) | 1.28 (1.11, 1.47) | 0.001 |
| Meat protein | |||||
| Cases/subcohort | 118/239 | 174/240 | 184/239 | — | |
| Median (g/d) | 12.5 | 23.7 | 36.2 | — | |
| Model 1 | 1 | 1.28 (1.00, 1.63) | 1.40 (1.10, 1.77) | 1.17 (1.05, 1.32) | 0.006 |
| Model 2 | 1 | 1.29 (1.01, 1.65) | 1.43 (1.10, 1.85) | 1.19 (1.05, 1.35) | 0.008 |
| Model 3 | 1 | 1.01 (0.78, 1.29) | 1.35 (1.04, 1.75) | 1.17 (1.02, 1.34) | 0.021 |
| Model 4 | 1 | 1.02 (0.80, 1.32) | 1.37 (1.06, 1.78) | 1.18 (1.03, 1.35) | 0.014 |
| Dairy protein | |||||
| Cases/subcohort | 167/239 | 155/240 | 154/239 | — | |
| Median (g/d) | 10.8 | 16.8 | 23.8 | — | |
| Model 1 | 1 | 0.91 (0.72, 1.14) | 1.07 (0.85, 1.35) | 1.03 (0.92, 1.16) | 0.56 |
| Model 2 | 1 | 0.92 (0.73, 1.16) | 1.25 (0.98, 1.58) | 1.11 (0.98, 1.25) | 0.10 |
| Model 3 | 1 | 0.87 (0.69, 1.09) | 1.07 (0.84, 1.37) | 1.03 (0.91, 1.17) | 0.66 |
| Vegetable protein | |||||
| Cases/subcohort | 158/239 | 158/240 | 160/239 | — | |
| Median (g/d) | 3.6 | 5.5 | 8.7 | — | |
| Model 1 | 1 | 1.05 (0.84, 1.32) | 1.17 (0.93, 1.46) | 1.08 (0.96, 1.21) | 0.18 |
| Model 2 | 1 | 1.07 (0.85, 1.34) | 1.33 (1.05, 1.67) | 1.15 (1.02, 1.29) | 0.018 |
| Model 3 | 1 | 0.97 (0.77, 1.23) | 1.04 (0.82, 1.32) | 1.02 (0.91, 1.15) | 0.75 |
| Cereal protein | |||||
| Cases/subcohort | 167/239 | 148/240 | 161/239 | — | |
| Median (g/d) | 4.5 | 8.4 | 14.4 | — | |
| Model 1 | 1 | 0.78 (0.62, 0.99) | 0.78 (0.62, 0.97) | 0.88 (0.79, 0.99) | 0.030 |
| Model 2 | 1 | 0.87 (0.69, 1.10) | 0.95 (0.74, 1.21) | 0.97 (0.86, 1.10) | 0.67 |
| Model 3 | 1 | 0.94 (0.74, 1.19) | 0.97 (0.75, 1.25) | 0.98 (0.86, 1.12) | 0.80 |
HRs (95% CIs) and P-trend values across tertiles of δ13C and δ15N and dietary protein types were estimated by Prentice-weighted Cox regression, adjusted for the following: model 1 [sex, family history of diabetes, smoking, education level, physical activity (results for δ13C values and δ15N values mutually adjusted for each other)], model 2 (model 1 + total energy intake, alcohol intake, and plasma vitamin C), model 3 (model 2 + BMI and waist circumference), and model 4 (model 3 + terrestrial animal protein for δ13C, δ15N, and fish protein and fish protein for meat protein and terrestrial animal protein). EPIC, European Prospective Investigation into Cancer and Nutrition; δ13C, carbon stable-isotope ratio (13C/12C ratio); δ15N, nitrogen stable-isotope ratio (15N/14N ratio).
Total cases: n = 476 and subcohort = 718 (includes 16 cases). Median values of isotope and protein tertiles are based on the subcohort only.
Terrestrial animal protein is the sum of meat, dairy, and egg protein.
DISCUSSION
We have described the distribution and correlates of serum carbon (δ13C) and nitrogen (δ15N) stable-isotope values as potential nutritional biomarkers in a European population-based study and have generated novel findings by examining the association of these measures with incident T2D.
The distribution of carbon and nitrogen isotope values in a European population
The distribution of serum δ13C and δ15N in the current study (mean values of −22.8‰ and 10.2‰, respectively) is broadly similar to that reported from small European studies using hair samples (17, 23, 35), with differences in isotopic values being generally attributable to the sample type analyzed. Our (EPIC-Norfolk) population had a relatively low mean (±SD) δ13C value (−22.8 ± 0.4‰) compared with North American populations: Atherosclerosis Risk In Communities study (−19.34‰; range: −17.21 to −21.71) and Center for Alaska Native Health Research study (∼−19.8 ± 0.6‰) (21, 22), which was likely attributable to the abundance of C4 plants in the food chain in North America (21, 36–38). Conversely, we observed higher mean (±SD) nitrogen isotope values (10.2 ± 0.4‰) than in the CANHR population (8.8 ± 1.4‰) (19), which, given the high intake of marine foods in the CANHR population's diet, was most likely a result of differences in baseline isotope values between the food webs of these populations (39, 40).
Carbon and nitrogen stable isotope values as nutritional biomarkers
Both δ13C and δ15N isotope values were most strongly positively associated with dietary fish and fish protein intakes, consistent also with the positive correlation we found with the plasma phospholipid omega-3 fatty acids EPA and DHA. Our observed correlations (ranging between 0.20 and 0.30) are modest in comparison with those observed between EPA, DHA, and erythrocyte δ15N (r ∼ 0.75) in the CANHR study (19). This was likely explained by the much higher marine-based diet of Yup'ik Eskimo populations than of a general UK population, which typically has a modest fish intake. Overall, our correlations between both δ13C and δ15N and fish/fish protein in a general population are notably similar in magnitude to those of other biomarkers such as plasma vitamin C as a biomarker of fruit/vegetable intake (41, 42). Our current findings in an observational study of blood-based isotope values also confirm what we previously reported in a small feeding study, ie, higher δ13C and δ15N were found in urine and fecal samples from individuals after a fish diet when compared with all other diets (18).
Our finding that fish protein intake was associated with δ13C and δ15N values, which suggests that these are likely biomarkers of fish protein intake, is supported by the elevation of the marine food chain in both δ13C and δ15N relative to C3 terrestrial foods (43, 44). Whereas fish protein in this study only contributed a mean of ∼8% of total dietary protein, consumption varied from 0% to 22%, which allowed for sufficient variation in using it in our analyses. The stronger correlations observed for fish protein than for other types of protein may have been attributable to fish having a very different isotopic signature than other types of protein, with studies of food δ13C and δ15N values in Europe and Alaska clearly showing much higher δ13C and δ15N values in marine foods than in nonmarine foods (39, 40).
Whereas δ13C values were not associated with animal-source protein intake, δ15N values were modestly positively associated with dairy product intake and with lower vegetarian consumer status and with dairy protein, meat protein, and overall terrestrial animal protein intakes—consistent with previous reports that δ15N values are markers of primary and secondary animal protein intakes (17, 23, 35). As in the ARIC study (21), we found no significant correlations between δ13C and δ15N and dietary sugars, except for a modest association of δ15N with lactose, which likely reflects that dairy products are higher in δ15N than are the sources of other sugars. In contrast with erythrocyte δ13C and δ15N as potential markers of sweetener intake in the CANHR population (22), and serum δ13C as a biomarker of SSB intake in ARIC (21), we did not find an association between SSBs (juice and fizzy drinks) and isotope values. This was most likely explained by the difference in predominant sugar source, which in the United Kingdom, unlike in the United States, is C3-derived because the European Union limits the production of high-fructose corn syrup to ∼5% of total sugar production (45, 46). The correlation between alcohol intake and δ13C (r = 0.20, P < 0.001) in our study was unexpected and, to our knowledge, has not been reported previously. This requires further research to elucidate potential mechanisms, because most alcohol consumed in Europe is C3-derived (47, 48). A possibility is that alcohol intake may be a marker of other foods rich in δ13C (49, 50).
Association with diabetes
There are several possible explanations for our novel findings of an inverse association of δ13C but a positive association of δ15N with incident diabetes. It is possible that these opposing associations are driven through the differential dietary intakes reflected by the 2 isotopic values. We noted that only δ15N, and not δ13C, was a biomarker of terrestrial animal intake and animal protein intake (meat, dairy, and overall terrestrial animal protein intake). A compelling body of evidence exists showing that meat intake, particularly red and processed meat intake, is strongly associated with an increased risk of diabetes (3–6), and it is possible that the positive association of δ15N with diabetes in our study largely reflected δ15N as a biomarker of meat-related dietary factors. This was supported by our observed positive association of both meat protein intake and terrestrial animal protein intake with diabetes. In contrast, the inverse association of δ13C with diabetes was supported by the inverse association of fish protein intake with diabetes in our study and was in line with our previous observation of an inverse association between fish intake and diabetes (10) and with δ13C predominantly reflecting its status as a biomarker of fish-related dietary factors. However, there are also issues of measurement error in self-reported intake, which further highlights the need to identify objective nutritional biomarkers. Past research on the association between fish intake and diabetes risk has been inconclusive, showing null associations (8, 9), decreased risk (10), increased risk (51, 52), or variation by geographic location (7, 8), and there has been acknowledgment of the influence of fish-cooking methods and factors other than fish protein, such as omega-3 fatty acid and vitamin D contents that might affect the risk of diabetes. It is also possible that δ15N provides a measure of other unknown factors, eg, toxins within fish (53, 54). Hence, toxins may be a correlate of δ15N, which could contribute to the positive δ15N-diabetes association. Further work is needed to clarify to what extent serum δ13C and δ15N differentially reflect fish protein intake and whether serum δ15N could be used as a biomarker to distinguish the metabolic availability of primary and secondary animal protein (fish, meat, and dairy) in the diet and their isotopic signatures in human blood and tissues.
Strengths and limitations
This was the largest study to date that examined the distribution and correlates of both δ13C and δ15N values in a representative European population. It was also the first study that examined the potential use of δ13C and δ15N values in relation to disease risk. Our findings may be generalizable to other European populations with similar intakes of fish and meat. The limitations of this study merit consideration. Measurement error in FFQ-derived dietary intake may have contributed to the modest correlations with stable isotope values. However, in a subset we examined the association between δ13C and δ15N and objectively measured plasma phospholipid omega-3 fatty acids, which have been found to be valid biomarkers of fish intake (55). We observed modest correlations between δ15N and EPA and DHA of a magnitude similar to that of serum δ15N and FFQ-derived fish protein. Measurement error in stable-isotope measures cannot be excluded. We measured stable isotopes in serum, which does not reflect long-term dietary intake, as do commonly used tissues such as hair and nails. However, serum samples were taken at the time the FFQ was administered; therefore, it is unlikely that differences in time between these measures would have had a substantial effect on our findings. Any influence of using nonfasted serum samples on isotope values is unknown, but, if present at all, is likely to be small. For analyses of associations with diabetes, we accounted for a range of confounders, including age and smoking status, which were related to isotopic values, but we cannot exclude the possibility that unmeasured or unknown confounding factors may have influenced our findings. A priori we set out to study associations with diabetes, but it would be of interest to examine the associations of these biomarkers with other chronic disease endpoints in future work.
Conclusion
We showed in a European population that carbon and nitrogen stable isotope values are potential biomarkers of fish and meat-related intakes that can be measured from stored blood samples in large population-based studies, extending previous findings from small studies of hair samples, from feeding studies, and from specialist populations. We provided the first available evidence that these isotope ratios have potential utility as nutritional biomarkers for investigating disease etiology in relation to diabetes incidence. The divergent diabetes associations with increasing values of δ13C (inverse) and δ15N (positive) need to be interpreted in light of knowledge of dietary intake and may assist in identifying dietary components that are associated with health risks and benefits. Future research is warranted to help increase our understanding of associations of isotopic signatures with dietary patterns and to establish these biomarkers for dietary assessment in nutritional epidemiology.
Supplementary Material
Acknowledgments
We thank all EPIC-Norfolk study participants and staff for their contribution to the study and Mike Hall and James Rolfe (Godwin Laboratory, University of Cambridge) for assistance with the isotope ratio mass spectrometry analysis.
The authors’ responsibilities were as follows—PSP, AJMC, and NGF: had access to all data for the study and take responsibility for the contents of the manuscript; NGF (guarantor), NJW, and TCO: conceptualized and designed the study; NJW and K-TK: were the EPIC-Norfolk Principal Investigators; PSP and AJMC: provided sample preparations and analyzed the data; CKK: assisted with sample preparation and carried out all isotopic analyses; GGCK: provided technical and analytical chemistry expertise; AMM and RNL : processed the FFQ data and provided expertise on the EPIC-Norfolk FFQ; and PSP, AJMC, TCO, SB, and NGF: drafted the manuscript. All authors contributed to the interpretation of the data, critical revision of the article for important intellectual content, and final approval of the version to be published. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: ALA, α-linolenic acid; EPIC, European Prospective Investigation into Cancer and Nutrition; FFQ, food-frequency questionnaire; SSB, sugar-sweetened beverage; T2D, type 2 diabetes; δ13C, carbon stable-isotope ratio (13C/12C ratio); δ15N, nitrogen stable-isotope ratio (15N/14N ratio).
REFERENCES
- 1.World Health Organisation. Diet, nutrtion, and the prevention of chronic diseases. World Health Organ Tech Rep Ser 2003;916. [PubMed]
- 2.Harding AH, Wareham NJ, Bingham SA, Khaw K, Luben R, Welch A, Forouhi NG. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the European Prospective Investigation of Cancer-Norfolk prospective study. Arch Intern Med 2008;168:1493–9. [DOI] [PubMed] [Google Scholar]
- 3.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 2011;94:1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia 2009;52:2277–87. [DOI] [PubMed] [Google Scholar]
- 6.InterAct Consortium. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct study. Diabetologia 2013;56:47–59. [DOI] [PubMed] [Google Scholar]
- 7.Wallin A, Di Giuseppe D, Orsini N, Patel PS, Forouhi NG, Wolk A. Fish consumption, dietary long-chain n−3 fatty acids, and risk of type 2 diabetes: systematic review and meta-analysis of prospective studies. Diabetes Care 2012;35:918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xun P, He K. Fish consumption and incidence of diabetes: meta-analysis of data from 438,000 individuals in 12 independent prospective cohorts with an average 11-year follow-up. Diabetes Care 2012;35:930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu JH, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, Djousse L, Hu FB, Mozaffarian D. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 2012;107(suppl 2):S214–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel PS, Sharp SJ, Luben RN, Khaw KT, Bingham SA, Wareham NJ, Forouhi NG. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes: the European Prospective Investigation of Cancer (EPIC)-Norfolk cohort study. Diabetes Care 2009;32:1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel PS, Forouhi NG, Kuijsten A, Schulze MB, van Woudenbergh GJ, Ardanaz E, Amiano P, Arriola L, Balkau B, Barricarte A, et al. The prospective association between total and type of fish intake and type 2 diabetes in 8 European countries: EPIC-InterAct Study. Am J Clin Nutr 2012;95:1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.InterAct Consortium. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia 2013;56:1520–30. [DOI] [PubMed] [Google Scholar]
- 14.Lee-Thorp JA. On isotopes and old bones. Archaeometry 2008;50:925–50. [Google Scholar]
- 15.Gannes LZ, Martinez del Rio C, Koch P. Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comp Biochem Physiol A Mol Integr Physiol 1998;119:725–37. [DOI] [PubMed] [Google Scholar]
- 16.Petzke KJ, Boeing H, Metges CC. Choice of dietary protein of vegetarians and omnivores is reflected in their hair protein 13C and 15N abundance. Rapid Commun Mass Spectrom 2005;19:1392–400. [DOI] [PubMed] [Google Scholar]
- 17.O'Connell TC, Hedges REM. Investigations into the effect of diet on modern human hair isotopic values. Am J Phys Anthropol 1999;108:409–25. [DOI] [PubMed] [Google Scholar]
- 18.Kuhnle GG, Joosen AM, Kneale CJ, O'Connell TC. Carbon and nitrogen isotopic ratios of urine and faeces as novel nutritional biomarkers of meat and fish intake. Eur J Nutr 2013;52:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien DM, Kristal AR, Jeannet MA, Wilkinson MJ, Bersamin A, Luick B. Red blood cell δ15N: a novel biomarker of dietary eicosapentaenoic acid and docosahexaenoic acid intake. Am J Clin Nutr 2009;89:913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahren AH, Saudek C, Yeung EH, Kao WH, Kraft RA, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr 2006;84:1380–4. [DOI] [PubMed] [Google Scholar]
- 21.Yeung EH, Saudek CD, Jahren AH, Kao WH, Islas M, Kraft R, Coresh J, Anderson CA. Evaluation of a novel isotope biomarker for dietary consumption of sweets. Am J Epidemiol 2010;172:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash SH, Kristal AR, Bersamin A, Hopkins SE, Boyer BB, O'Brien DM. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup'ik study population. J Nutr 2013;143:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bol R, Pflieger C. Stable isotope (13C, 15N and 34S) analysis of the hair of modern humans and their domestic animals. Rapid Commun Mass Spectrom 2002;16:2195–200. [DOI] [PubMed] [Google Scholar]
- 24.Huelsemann F, Flenker U, Koehler K, Schaenzer W. Effect of a controlled dietary change on carbon and nitrogen stable isotope ratios of human hair. Rapid Commun Mass Spectrom 2009;23:2448–54. [DOI] [PubMed] [Google Scholar]
- 25.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, Wareham N. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 1999;80(suppl 1):95–103. [PubMed] [Google Scholar]
- 26.Prentice R. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73:1–11. [Google Scholar]
- 27.Onland-Moret NC, van der AD, van der Schouw YT, Buschers W, Elias SG, van Gils CH, Koerselman J, Roest M, Grobbee DE, Peeters PH. Analysis of case-cohort data: a comparison of different methods. J Clin Epidemiol 2007;60:350–5. [DOI] [PubMed] [Google Scholar]
- 28.Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, Day NE. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 2003;6:407–13. [DOI] [PubMed] [Google Scholar]
- 29.Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S, Lubin R, Thurnham DI, Key TJ, Roe L, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol 1997;26(suppl 1):S137–51. [DOI] [PubMed] [Google Scholar]
- 30.Welch AA, Luben R, Khaw KT, Bingham SA. The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. J Hum Nutr Diet 2005;18:99–116. [DOI] [PubMed] [Google Scholar]
- 31.Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 2010;92:1214–22. [DOI] [PubMed] [Google Scholar]
- 32.Hoefs J. Stable isotope geochemistry. Berlin, Germany: Springer, 1997. [Google Scholar]
- 33.Rousseeuw PJ, Ruts I, Tukey JW. The bagplot: a bivariate boxplot. Am Stat 1999; (53):382–7. [Google Scholar]
- 34.R Core team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2012. Available from: http://www.R-project.org/ (cited 1 December 2013).
- 35.Petzke KJ, Boeing H, Klaus S, Metges CC. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr 2005;135:1515–20. [DOI] [PubMed] [Google Scholar]
- 36.Nash SH, Kristal AR, Boyer BB, King IB, Metzgar JS, O'Brien DM. Relation between stable isotope ratios in human red blood cells and hair: implications for using the nitrogen isotope ratio of hair as a biomarker of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr 2009;90:1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jahren AH, Kraft RA. Carbon and nitrogen stable isotopes in fast food: signatures of corn and confinement. Proc Natl Acad Sci USA 2008;105:17855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinelli LA, Nardoto GB, Chesson LA, Rinaldi FD, Ometto JPHB, Cerling TE, Ehleringer JR. Worldwide stable carbon and nitrogen isotopes of Big Mac patties: an example of a truly “glocal” food. Food Chem 2011;127:1712–8. [Google Scholar]
- 39.Nash SH, Bersamin A, Kristal AR, Hopkins SE, Church RS, Pasker RL, Luick BR, Mohatt GV, Boyer BB, O'Brien DM. Stable nitrogen and carbon isotope ratios indicate traditional and market food intake in an indigenous circumpolar population. J Nutr 2012;142:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huelsemann F, Koehler K, Braun H, Schaenzer W, Flenker U. Human dietary delta(15)N intake: representative data for principle food items. Am J Phys Anthropol 2013;152:58–66. [DOI] [PubMed] [Google Scholar]
- 41.Khaw KT, Bingham S, Welch A, Luben R, Wareham N, Oakes S, Day N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 2001;357:657–63. [DOI] [PubMed] [Google Scholar]
- 42.Dehghan M, Akhtar-Danesh N, McMillan CR, Thabane L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr J 2007;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchardt B, Bunch V, Helin P. Fingernails and diet: stable isotope signatures of a marine hunting community from Ummannaq, North Greenland. Chem Geol 2007;244:316–29. [Google Scholar]
- 44.Schoeninger MJ, DeNiro MJ. Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim Cosmochim Acta 1984;48:625–39. [Google Scholar]
- 45.Cooper JC, Giruad-Heraud E, Requillart V. Economic impacts of isoglucose deregulation on the European sweetener market. Eur Rev Agric Econ 1995;22:425–45. [Google Scholar]
- 46.European Food Information Council. Frequently asked questions. Available from: http://www.eufic.org/page/en/page/FAQ/faqid/glucose-fructose-syrup/ (cited 1 December 2013).
- 47.Brooks JR, Buchmann N, Phillips S, Ehleringer B, Evans RD, Lott M, Martinelli LA, Pockman WT, Sandquist D, Sparks JP, et al. Heavy and light beer: a carbon isotope approach to detect C(4) carbon in beers of different origins, styles, and prices. J Agric Food Chem 2002;50:6413–8. [DOI] [PubMed] [Google Scholar]
- 48.Bréas O, Reniero F, Serrini G, Martin GJ, Rossmann A. Isotope ratio mass spectrometry: analysis of wines from different European countries. Rapid Commun Mass Spectrom 1994;8:967–70. [Google Scholar]
- 49.Breslow RA, Guenther PM, Juan W, Graubard BI. Alcoholic beverage consumption, nutrient intakes, and diet quality in the US adult population, 1999-2006. J Am Diet Assoc 2010;110:551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kesse E, Clavel-Chapelon F, Slimani N, van Liere M. Do eating habits differ according to alcohol consumption? Results of a study of the French cohort of the European Prospective Investigation into Cancer and Nutrition (E3N-EPIC). Am J Clin Nutr 2001;74:322–7. [DOI] [PubMed] [Google Scholar]
- 51.Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr 2009;90:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Tian C, Jia C. Association of fish and n−3 fatty acid intake with the risk of type 2 diabetes: a meta-analysis of prospective studies. Br J Nutr 2012;108:408–17. [DOI] [PubMed] [Google Scholar]
- 53.Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, Lin-Shiau SY, Liu SH. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes 2006;55:1614–24. [DOI] [PubMed] [Google Scholar]
- 54.Yoshinaga J, Suzuki T, Hongo T, Minagawa M, Ohtsuka R, Kawabe T, Inaoka T, Akimichi T. Mercury concentration correlates with the nitrogen stable isotope ratio in the animal food of Papuans. Ecotoxicol Environ Saf 1992;24:37–45. [DOI] [PubMed] [Google Scholar]
- 55.Fekete K, Marosvolgyi T, Jakobik V, Decsi T. Methods of assessment of n−3 long-chain polyunsaturated fatty acid status in humans: a systematic review. Am J Clin Nutr 2009;89:2070S–84S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.