Abstract
Objectives
Lipoprotein(a), Lp(a), represents an apolipoprotein (apo) B-carrying lipoprotein, yet the relationship between Lp(a) and apoB levels has not been fully explored.
Methods
We addressed the relationship between Lp(a) and apoB-containing lipoprotein levels in 336 Caucasians and 224 African-Americans. Our approach takes unique molecular properties of Lp(a) as well as contribution of Lp(a) to the levels of these lipoproteins into account.
Results
Levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apoB and apoB/apoA-1 did not differ across ethnicity. African-Americans had higher levels of Lp(a) and high-density lipoprotein cholesterol and lower triglyceride levels compared to Caucasians. Lp(a) levels were correlated with levels of TC (p<0.005), LDL-C (p<0.001), apoB (p<0.05) or apoB/apoA-1 (p<0.05) in both ethnic groups. These associations remained significant only in African-Americans after adjustments for the contribution of Lp(a)-cholesterol or Lp(a)-apoB. Furthermore, taking Lp(a)-apoB into account, allele-specific apo(a) levels were significantly associated with apoB levels and the apoB/apoA-1 ratio in African-Americans. The latter associations in African-Americans remained significant for allele-specific apo(a) levels for smaller apo(a) sizes (<26 K4 repeats), after controlling for the effects of age, sex, and BMI.
Conclusions
Although TC, LDL-C, and apoB levels were comparable between African-Americans and Caucasians, the associations of these parameters with Lp(a) and allele specific apo(a) levels differed between these two ethnic groups. In African-Americans, apoB and apoB/apoA-1 remained consistently and positively associated with both Lp(a) and allele-specific apo(a) levels after adjustments for the contribution of Lp(a)-apoB. The findings suggest an interethnic difference with a closer relationship between Lp(a) and apoB among African-Americans.
Keywords: plasma lipids, ethnicity, corrections for the contribution of Lp(a), apo(a) sizes, K4 repeats
INTRODUCTION
Accumulating evidence, including recent Mendelian randomization and genetic studies, support the role of lipoprotein(a), Lp(a), as an independent causal risk factor for atherosclerotic cardiovascular disease [1–3]. Based on this evidence, clinical guidelines have recommended screening for elevated Lp(a) levels [4]. Besides a cholesterol-rich lipid core and one molecule of apolipoprotein (apo) B-100, like an LDL particle, Lp(a) contains a unique glycoprotein, i.e., apo(a) bound to apoB-100 with a single disulfide bond. Apo(a) has repeated loop structures, referred to as Kringles (K), where one motif, K4 type 2, is present in three to more than 40 copies [5]. This extensive size polymorphism in the apo(a) gene is a major predictor of Lp(a) levels, and in general small apo(a) sizes are associated with high Lp(a) levels [6–8]. However, for any given apo(a) size, Lp(a) levels vary across individuals, families, and ethnic groups with most profound differences between individuals of African versus non-African descent [9–11]. We have previously demonstrated a concept of allele-specific apo(a) level which determines the amount of Lp(a) associated with a defined apo(a) allele/isoform size, and reported that these levels were important in assessing the Lp(a)-associated cardiovascular risk across ethnicity [12].
Environmental and lifestyle-related factors and currently available lipid-lowering medications have no or minimal effects on Lp(a) levels [13–18] with exception of niacin [19] and hormone replacement therapy [20]. New evidence on the interactions between Lp(a) and inflammation [21–23] or chronic immune activation [24] has emerged, as studies have suggested an impact of these factors on Lp(a) with a variable effect across ethnicity. Notably, the relationship of Lp(a) with the plasma lipid profile, particularly, apoB-containing atherogenic lipoproteins, has attracted considerable attention, producing inconsistent results. While many studies focused on this topic have reported a positive correlation between Lp(a) and low-density lipoprotein cholesterol (LDL-C) or total cholesterol (TC) [25–31], others report no such association [16, 32, 33].
Furthermore, current assessments of TC, LDL-C and apoB generally do not take the contribution of Lp(a) to the levels of these lipoproteins into account. Considering that cholesterol corresponds to ~30% and apoB contributes to ~16% of Lp(a) mass [34, 35], the contribution of Lp(a) to overall TC, LDL-C and apoB levels can be substantial. This potential is particularly prominent in individuals with elevated Lp(a) levels. In addition, the effects of apo(a) size polymorphism (number of K4 repeats) and ethnicity/race on this association remain elusive.
In the present study, we investigated the relationship between Lp(a), allele-specific apo(a) and apoB-containing atherogenic lipoprotein levels, while taking into account the contribution of Lp(a) to the latter. The inclusion of two ethnic groups with very different Lp(a) and allele-specific apo(a) levels, but with similar apoB-containing lipoprotein levels (Caucasians and African-Americans) allowed us to directly assess the relationship between apoB and Lp(a) under a range of conditions.
MATERIALS AND METHODS
Subjects
Subjects were recruited from a patient population scheduled for diagnostic coronary angiography either at Harlem Hospital Center in New York City or at the Mary Imogene Bassett Hospital in Cooperstown, NY. The clinical characteristics of the study population and the study design including inclusion and exclusion criteria have been described previously, and notably, exclusion criteria included use of lipid lowering drugs, as well as hormone replacement therapies [12, 36]. Briefly, a total of 648 patients, self-identified as Caucasian (n=344), African American (n=232), or other (n=72) were enrolled. The present report is based on the findings in 560 subjects (336 Caucasians, 224 African Americans); 16 subjects were excluded due to incomplete data. The apo(a) allele sizes, circulating apo(a) isoforms, and allele-specific apo(a) levels were available on 426 subjects (167 African Americans, 259 Caucasians). This cohort has been extensively studied with regard to Lp(a) and apo(a) size distributions and detailed information can be found in our previous studies [12, 21–23, 37]. The study was approved by the Institutional Review Boards at Harlem Hospital, the Mary Imogene Bassett Hospital, Columbia University College of Physicians and Surgeons, and University of California, Davis, and informed consent was obtained from all subjects.
Measurement of plasma lipids and lipoproteins
Participants were asked to fast for 12 hours, and blood samples were drawn approximately 2 to 4 hours before the catheterization procedure. Serum and plasma samples were separated and stored at −80°C prior to analysis. Concentrations of triglycerides (Sigma Diagnostics, St. Louis, MO), TC and high density lipoprotein cholesterol (HDL-C) (Roche, Sommerville, NJ) were determined using standard enzymatic procedures [38, 39]. HDL-C levels were measured after precipitation of apoB-containing lipoproteins with dextran sulfate [40], and LDL-C levels were calculated in subjects with triglyceride levels of <400 mg/dL with the formula of Friedewald [41]. ApoB-100 levels were determined by rate immunonephelometry (Array 360, Beckman, Brea, CA) using manufacturer’ supplied calibrator and bi-level quality controls which were within the recommended precision for each test [42]. The interassay coefficient of variation was consistently less than 6%. These biochemical assays were performed at a core laboratory certified in lipid and lipoprotein measurements by the Center for Disease Control (Mary Imogene Basset Research Institute, Cooperstown, NY). Plasma Lp(a) levels were measured by an apo(a) size insensitive sandwich enzyme-linked immunosorbent assay (ELISA) (Sigma Diagnostics, St Louis, MO) and expressed as nmol/L [12]. The interassay coefficient of variation was 8.4% at an apo(a) level of 19.9 nM and 9.0% at an apo(a) level of 67.1nM [12, 36].
As Lp(a) levels were measured in nmol/L and other plasma lipids and lipoproteins were expressed in mg/dL, we converted Lp(a) values into mg/dL by use of a conversion factor of 2.4 nmol/L=1 mg/dL [12]. TC, LDL-C, and apoB levels were adjusted for the Lp(a) contribution [25], according to compositional data in which cholesterol accounts for ~30% and apoB-100 for ~16% of total Lp(a) mass [34, 35]. Thus, the level of Lp(a) mass (mg/dL) multiplied by 0.3 was subtracted from TC and LDL-C values in all subjects. Similarly, total Lp(a) mass multiplied by 0.16 was subtracted from apoB-100 values. For corrected apoB-100/ApoA-1 ratio, the latter values were divided by apoA-1 levels.
To verify our findings on the relationship between Lp(a) and apoB-100 levels, we performed additional analyses. First, we converted apoB-100 values in mg/dL into nmol/L, using a molecular weight of approximately 512 kDa for ApoB-100 [43]. Thus, we recalculated particle concentration of apoB-100 by use of a conversion factor of 0.0512 mg/dL = 1 nmol. Second, Lp(a) values expressed as nmol/L were subtracted from the apoB-100 values expressed as nmol/L to obtain non-Lp(a)-apoB values. These values were used to describe the relationship between Lp(a) and apoB-100 at a molecular level.
Determinations of apo(a) isoform size and allele-specific apo(a) levels
Apo(a) isoform sizes were determined by Western blotting technique with SDS-agarose gel electrophoresis of plasma samples, followed by immonoblotting as previously described [7, 37]. The protein isoform dominance pattern was assessed by optical analyses of the apo(a) protein bands on the Western blots, and the visual estimations were validated by computerized scanning. To determine allele-specific apo(a) levels, for each of the apo(a) protein bands, Lp(a) levels were apportioned according to the degree of intensity of the bands on the Western blot as previously described [37].
Statistics
Statistical analysis was performed with SPSS software (SPSS Inc, Chicago, IL). Results were expressed as mean ± standard deviation (SD) for normally distributed variables, or median with interquartile range for non-normally distributed variables. Triglyceride levels were logarithmically transformed, and Lp(a) and allele-specific apo(a) levels were square root transformed to achieve normal distributions. Both the larger and smaller apo(a) isoforms of subjects with two distinguishable bands and one isoform of subjects with a single band were considered for statistical analyses. Unadjusted and adjusted (partial) Pearson’s correlation coefficients for age, sex, and body mass index (BMI) were calculated to describe the magnitude and direction of the association of Lp(a) or allele-specific apo(a) levels with other variables for each ethnic group, respectively. Group means were compared using Student’s t-test. All analyses were two-tailed, and p-values less than 0.05 were considered statistically significant.
RESULTS
Clinical characteristics of study population
Caucasians were slightly older and obese as compared with African-Americans (Table 1). There was no difference in the gender distribution between the two ethnic groups. Levels of TC, LDL-C or apoB did not differ significantly between Caucasians and African-Americans. However, African-Americans had significantly higher levels of HDL-C (p<0.001) and apoA-1 (p=0.003) and significantly lower levels of triglycerides (p<0.001) and apoB/apoA-1 ratio (p=0.008) compared to Caucasians. Furthermore, as expected, Lp(a) levels were significantly higher in African-Americans compared to Caucasians (p<0.001).
Table 1.
Characteristics of study population
| Characteristics | Caucasians† (n=336) | African-Americans (n=224) | p-value |
|---|---|---|---|
| Men/women (n) | 217/119 | 126/98 | NS |
| Age (years) | 56.8±10.3 | 54.8±9.2 | 0.025 |
| Body mass index (kg/m2) | 29.7±6.0 | 28.5±6.1 | 0.043 |
| Total cholesterol (mg/dL) | 197±41 | 198±45 | NS |
| LDL cholesterol (mg/dL) | 122±35 | 126±42 | NS |
| HDL cholesterol (mg/dL) | 41±12 | 49±17 | <0.001 |
| Lipoprotein(a) (nmol/L) | 24 (7–79) | 108 (59–180) | <0.001 |
| Triglycerides (mg/dL) | 153 (114–222) | 106 (80–144) | <0.001 |
| ApoB-100 (mg/dL) | 136±36 | 134±40 | NS |
| ApoA-1 (mg/dL) | 122±23 | 130±28 | 0.003 |
| ApoB/ApoA-1 | 1.15±0.33 | 1.07±0.37 | 0.008 |
Data are expressed as mean ± standard deviation or median (interquartile range) for non-normally distributed variables. Logarithmically transformed triglyceride and square root transformed Lp(a) values were used for statistical analyses.
The results for Lp(a), apoB, apoA-1, and apoB/apoA-1 ratio were based on the data of 304 subjects.
Abbreviations: Apo, apolipoprotein; HDL, high-density lipoprotein; LDL, low-density lipoprotein, NS, not significant.
Relationship of Lp(a) with plasma lipid and lipoprotein levels before and after correction
First, we assessed the relationship of Lp(a) with plasma lipid and lipoprotein levels before corrections for each ethnic group separately. In both ethnic groups, Lp(a) levels were significantly and positively correlated with levels of TC, LDL-C, apoB and apoB/apoA-1 ratio regardless of adjustment for the covariates of age, sex, and BMI (Table 2). In addition, triglyceride levels were weakly but significantly associated with Lp(a) levels in African-Americans (p=0.028) before adjustment for the covariates. However, analyses controlling for the effects of the covariates resulted in disappearance of the latter significant association (p=0.052). HDL-C or apoA-1 levels were not significantly associated with Lp(a) levels in either ethnic group.
Table 2.
Pearson’s correlation coefficients on the relationship between plasma Lp(a) levels (mg/dL) and other lipid and lipoproteins with and without adjustment for the covariates of age, sex, and body mass index
| Caucasians | African-Americans | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
|
| ||||||||
| r | p | r | p | r | p | r | p | |
| Before correction | ||||||||
| Total cholesterol (mg/dL) | 0.159 | 0.004 | 0.179 | 0.002 | 0.391 | <0.001 | 0.377 | <0.001 |
| LDL cholesterol (mg/dL) | 0.217 | <0.001 | 0.220 | <0.001 | 0.411 | <0.001 | 0.401 | <0.001 |
| ApoB-100 (mg/dL) | 0.127 | 0.021 | 0.142 | 0.013 | 0.415 | <0.001 | 0.405 | <0.001 |
| ApoB/ApoA-1(mg/dL) | 0.138 | 0.012 | 0.153 | 0.007 | 0.334 | <0.001 | 0.330 | <0.001 |
| HDL cholesterol (mg/dL) | −0.061 | NS | −0.067 | NS | −0.022 | NS | −0.025 | NS |
| Triglycerides (mg/dL) | −0.005 | NS | 0.029 | NS | 0.145 | 0.028 | 0.130 | 0.052 |
| ApoA-1 | −0.039 | NS | −0.039 | NS | 0.016 | NS | −0.022 | NS |
|
| ||||||||
| After correction | ||||||||
| Total cholesterol (mg/dL) | −0.083 | NS | −0.079 | NS | 0.139 | 0.034 | 0.117 | NS |
| LDL cholesterol (mg/dL) | −0.073 | NS | −0.078 | NS | 0.138 | 0.036 | 0.118 | NS |
| ApoB-100 (mg/dL) | −0.022 | NS | −0.010 | NS | 0.272 | <0.001 | 0.258 | <0.001 |
| ApoB/ApoA-1 | 0.005 | NS | 0.014 | NS | 0.213 | 0.001 | 0.206 | 0.002 |
Lp(a) levels were measured in nmol/L and converted to mg/dL by use of a conversion factor of 2.4 nmol/L = 1 mg/dL. Corrected levels were calculated as described in Methods [34, 35]. The formulas used were for TC, TC (mg/dL) − [Lp(a) (mg/dL) × 0.3]; for LDL, LDL (mg/dL) − [Lp(a) (mg/dL) × 0.3], and for apoB-100, apoB-100 (mg/dL) − [Lp(a) (mg/dL) × 0.16], respectively. For corrected apoB/apoA-1 ratio, the latter apoB-100 values were divided by apoA-1 values. Logarithmically transformed triglyceride and square root transformed Lp(a) values were used for statistical analyses. For abbreviations see the footnote of Table 1.
Second, we tested the effects of correction for the contribution of Lp(a) to apoB-containing lipoprotein levels on the relationship. Corrected values of TC, LDL-C, apoB and apoB/apoA-1 were calculated according to the formulas as described in Methods. As seen in Figure 1, the largest differences between corrected and uncorrected levels were seen for African-Americans. Thus, percentage changes in TC, LDL-C, and apoB levels from before correction levels were significantly greater in African-Americans than Caucasians (p<0.001 for all). We observed an interethnic difference as Lp(a) remained significantly associated with levels of TC, LDL-C, apoB and apoB/apoA-1 ratio in African-Americans, but not in Caucasians (Table 2). Furthermore, analyses controlling for the effects of covariates of age, sex, and BMI revealed a loss of significant associations between Lp(a) levels with TC or LDL-C levels in African-Americans.
Figure 1. The effects of correction for the contribution of Lp(a)-cholesterol or -apoB content on apoB-containing atherogenic lipoprotein levels in Caucasians versus African-Americans.
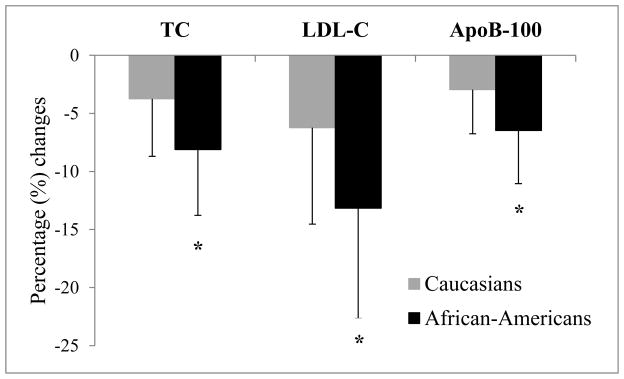
Differences in TC, LDL-C and ApoB levels before and after correction for the contribution of Lp(a)-cholesterol or -apoB content were calculated per each individual, and expressed as a percentage (%) change from the before correction level. Based on the individual percentage change, the mean percentage changes in TC, LDL-C, and apoB levels per each ethnic group were computed, and are shown in the graph. For details on calculations of corrected levels, see the footnote of Table 2 or the Methods section.
*: p<0.001 versus Caucasians
Abbreviations: TC, total cholesterol, LDL-C, low-density lipoprotein cholesterol, apoB, apolipoprotein B;
To verify our finding on the relationship between Lp(a) and apoB levels at a molecular basis, we first converted apoB levels in mg/dL into nmol/L, and then recalculated non-Lp(a)-apoB (nmol/L) values as described in Methods. As shown in Figure 2, particle concentrations of Lp(a) were correlated with particle concentrations of apoB in African-Americans, but not in Caucasians, after correction for the contribution of Lp(a)-apoB content (Figure 2C and D), confirming the findings when using mg/dL.
Figure 2. Association of Lp(a) (nmol/L) level with apoB (nmol/L) level before and after correction for the contribution of Lp(a)-apoB content in Caucasians versus African-Americans.
The relationship between apoB and square root transformed Lp(a) levels before (A and B) and after (C and D) correction for the contribution of Lp(a)-apoB content is described by partial correlation coefficients controlling for the effects of age, sex, and BMI. At molecular level, Lp(a) remained significantly and positively associated with apoB levels after corrections in African-Americans (D), but not in Caucasians (C). For conversion of apoB levels in mg/dL into nmol/L and calculations of non-Lp(a) apoB levels, see the Methods section.
Abbreviations: SqRt, square root; apoB, apolipoprotein B;
Relationship of allele-specific apo(a) with plasma lipid and lipoprotein levels before and after correction
To assess an impact of apo(a) size on the relationship between Lp(a) and apoB, we analyzed the associations between Lp(a) levels associated with a defined apo(a) allele/isoform size, i.e., allele-specific apo(a) levels and other plasma lipid and lipoproteins levels. Before corrections, reflecting the Lp(a) findings, allele-specific apo(a) levels were significantly and positively correlated with levels of TC (p<0.001), LDL-C (p<0.001), apoB (p<0.05) and apoB/apoA-1 (p<0.05) ratio in both Caucasians and African-Americans (Supplementary Table 1). Adjustments for the effects of covariates (age, sex, and BMI) did not alter the findings, except for triglyceride in African-Americans. When corrected levels were taken into account, significant associations between allele-specific apo(a) levels and the above parameters were no longer seen in Caucasians. In African-Americans, however, allele-specific apo(a) levels remained significantly and positively associated with apoB (p<0.001) and apoB/apoA-1 (p=0.001) levels, but not with TC and LDL-C levels (Supplementary Table 1). Again, these finding were not impacted by adjustments for the covariates. The correlations of allele-specific apo(a) levels (nmol/L) with apoB levels (nmol/L) before and after correction for the contribution of Lp(a)-apoB content across ethnicity are shown in Supplementary Figure 1. As for the findings in mg/dL, particle concentrations of allele-specific apo(a) were associated with particle concentrations of apoB even after taking the contribution of Lp(a)-apoB into account.
Relationship of allele-specific apo(a) levels for smaller versus larger apo(a) sizes with plasma lipid and lipoprotein levels before and after correction
To assess the impact of apo(a) size on the relationship between allele-specific apo(a) levels and apoB in more depth, we divided study subjects into two groups based on the median apo(a) size, i.e., 26 K4 repeats (for both ethnic groups). For smaller apo(a) sizes (<26 K4 repeats), allele-specific apo(a) levels were significantly and positively correlated with levels of TC, LDL-C and apoB, but not with apoB/apoA-1 in Caucasians (Table 3). In African-Americans, all four parameters (TC, LDL-C, apoB and apoB/apoA-1) were consistently associated with allele-specific apo(a) levels for smaller apo(a) sizes. For larger apo(a) sizes (≥26 K4 repeats), we observed an interethnic difference. Thus, allele-specific apo(a) levels for larger apo(a) sizes did not correlate with any of the parameters in Caucasians, but significantly correlated with all four parameters (TC, LDL-C, apoB and apoB/apoA-1) in African-Americans. Notably, as seen in Table 3, these results remained essentially the same when adjusted for the covariates of age, sex, and BMI.
Table 3.
Pearson’s correlation coefficients on the relationship of allele-specific apo(a) levels (mg/dL) for smaller (<26 K4) versus larger (≥26 K4) apo(a) sizes with apoB-containing lipoproteins with and without adjustment for the covariates of age, sex, and body mass index
| Correction | Caucasians | African-Americans | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| ≥26 K4 | <26 K4 | ≥26 K4 | <26 K4 | |||||||||||||
|
| ||||||||||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||||
|
| ||||||||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| Before | ||||||||||||||||
| TC | 0.002 | NS | 0.014 | NS | 0.178 | 0.020 | 0.227 | 0.004 | 0.232 | 0.003 | 0.278 | <0.001 | 0.280 | 0.002 | 0.276 | 0.002 |
| LDL-C | 0.065 | NS | 0.051 | NS | 0.250 | 0.001 | 0.265 | 0.001 | 0.244 | 0.002 | 0.288 | <0.001 | 0.311 | <0.001 | 0.307 | 0.001 |
| ApoB-100 | −0.021 | NS | −0.005 | NS | 0.183 | 0.017 | 0.234 | 0.003 | 0.243 | 0.002 | 0.289 | <0.001 | 0.310 | 0.001 | 0.302 | 0.001 |
| ApoB/ApoA-1 | 0.024 | NS | 0.043 | NS | 0.135 | NS | 0.199 | 0.013 | 0.165 | 0.038 | 0.196 | 0.014 | 0.326 | <0.001 | 0.323 | <0.001 |
|
| ||||||||||||||||
| After | ||||||||||||||||
| TC | −0.067 | NS | −0.058 | NS | −0.051 | NS | −0.034 | NS | 0.069 | NS | 0.119 | NS | 0.111 | NS | 0.096 | NS |
| LDL-C | −0.017 | NS | −0.033 | NS | −0.022 | NS | −0.023 | NS | 0.064 | NS | 0.113 | NS | 0.140 | NS | 0.127 | NS |
| ApoB-100 | −0.060 | NS | −0.043 | NS | 0.034 | NS | 0.080 | NS | 0.151 | NS | 0.200 | 0.013 | 0.220 | 0.015 | 0.208 | 0.023 |
| ApoB/ApoA-1 | −0.015 | NS | 0.003 | NS | 0.011 | NS | 0.071 | NS | 0.093 | NS | 0.125 | NS | 0.245 | 0.007 | 0.239 | 0.009 |
Square root transformed allele-specific apo(a) values were used for statistical analyses. For calculations of corrected levels, please see the footnote of Table 2 or the Methods section.
Abbreviations: Apo, apolipoprotein; K, kringle; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol;
We then assessed the effects of correction on the relationship. Not surprisingly, the corrections eliminated previously observed significant associations of TC, LDL-C and apoB with allele-specific apo(a) levels for smaller (<26 K4 repeats) apo(a) sizes in Caucasians (Table 3). In African-Americans, a similar effect was observed for larger (≥26 K4 repeats) apo(a) sizes as the previously observed significant associations of all four parameters vanished after corrections. For smaller apo(a) sizes, however, both apoB and apoB/apoA-1 remained consistently associated with allele-specific apo(a) levels in African-Americans even after corrections, and these associations were independent of the effects of covariates.
DISCUSSION
In the present study, we demonstrate a differential association of plasma Lp(a) and allele-specific apo(a) levels with other apoB-containing atherogenic lipoproteins across African-American-Caucasian ethnicity, despite similar levels of these apoB-containing lipoproteins in the two groups. Lp(a) and allele-specific apo(a) levels were significantly and positively correlated with TC, LDL-C, apoB and apoB/apoA-1 levels in both ethnic groups. However, after adjustment for the contribution of Lp(a)-cholesterol or -apoB content, only apoB levels and apoB/apoA-1 ratio remained consistently and positively associated with both Lp(a) and allele-specific apo(a) levels in African-Americans. These findings were independent of the effects of age, sex, and BMI. Notably, we were able to verify our findings on the relationship between Lp(a) and apoB levels at a molecular basis. In addition, in African-Americans, there was a significant association between apoB and allele-specific Lp(a) levels for smaller, but not for larger apo(a) sizes.
The relationship of Lp(a) with other traditional cardiovascular risk factors, including atherogenic plasma lipids and lipoproteins levels, has attracted considerable attention and has been a focus of investigation in many studies. Studies focused on the relationship between Lp(a) and the plasma lipid profile, however, have produced conflicting results, and notably, most studies have not taken the contribution of Lp(a) cholesterol content to TC or LDL-C levels into account. Furthermore, limited information is available regarding any roles of apo(a) size polymorphism or ethnicity on the relationship of Lp(a) with apoB-containing lipoproteins. A positive correlation of Lp(a) with TC, LDL-C and/or triglyceride levels has been reported in non-diabetics [25–28] as well as diabetics [29]. Lp(a) levels were significantly associated with TC, LDL-C and apoB levels in patients with chronic renal failure [44], or with LDL size in patients with coronary artery disease [30]. In both Caucasians and African-Americans, Lp(a) levels were correlated with apoB but not with other lipoprotein levels [16]. Another study conducted among Caucasians and African-Americans from the Seychelles Island reported a positive correlation of Lp(a) with TC, LDL-C, and apoB levels regardless of ethnic background [27]. In line with these findings, prior to any correction for the contribution of Lp(a), we observed significant and positive correlations of Lp(a) with TC, LDL-C and apoB levels in both Caucasians and African-Americans.
Compositional data indicates that cholesterol accounts for ~30% and apoB for ~16% of Lp(a) mass [34, 35]. Therefore, in particular, among individuals with elevated Lp(a) levels, such as subjects of African descent, the contribution of Lp(a) to TC, LDL-C or apoB levels can be substantial. Among Caucasians enrolled in the Framingham Offspring Study, the observed positive correlations of Lp(a) with TC and LDL-C levels were abolished after appropriate adjustments [25]. Our findings in Caucasians were in line with this observation as the significant associations of Lp(a) with TC and LDL-C disappeared after correction for Lp(a)-cholesterol content. These findings suggest that the observed significant associations were mainly due to the contribution of Lp(a) cholesterol to TC or LDL-C levels. However, relatively little attention has been paid to the impact of any contribution of Lp(a) on the relationship between Lp(a) and apoB-containing lipoproteins across ethnicity. In contrast to findings in Caucasians, Lp(a) remained significantly associated with TC, LDL-C, apoB and apoB/apoA-1 in our African-American subjects even after adjustment for Lp(a)-cholesterol or -apoB. These findings are notable as African-Americans, in general, have higher Lp(a) levels than Caucasians. One might therefore expect that a subtraction of an amount equal to 30% of Lp(a) mass from TC or LDL-C (or 16% of Lp(a)-apoB mass from apoB) would result in a much greater degree of level change (Figure 1), and thus consequently might lead to a lack of correlation of Lp(a) levels with these parameters. Our results were, however, contrary to this assumption.
Similar to our results for Lp(a) levels, allele-specific apo(a) levels were significantly correlated with apoB-containing lipoproteins in both ethnic groups. In Caucasians, corrections for the contribution of Lp(a) led to a loss of significant associations. However, in African-Americans, the findings were somewhat different as allele-specific apo(a) levels remained significantly associated with apoB and apoB/apoA-1 levels. Further analyses stratified by the median apo(a) size provided an in-depth insight into this relationship as circulating apoB levels showed the strongest correlation with allele-specific apo(a) levels carried by smaller apo(a) sizes in African-Americans.
The underlying mechanisms through which these associations are mediated are unclear, although many factors, including differences in hepatic apo(a) synthesis rate, Lp(a) assembly mechanisms, and recruitment of various apoB sources in the formation of Lp(a) may contribute. In this context, the question of assembly site for Lp(a) has been a subject of much debate, and it is unclear to what extent intracellular, extracellular, and/or plasma membrane-associated assembly contributes and whether differences might occur between individuals [45–47]. An in vivo kinetic study conducted in human subjects reported two sources for Lp(a)-apoB with about equal portions derived from preformed lipoproteins, such as IDL or LDL, and from newly synthesized hepatic apoB [46]. Another study using multi-compartmental modelling in healthy controls and patients undergoing hemodialysis suggested that the majority (>90%) of Lp(a)-apoB is synthesized from the liver [48]. It is tempting to suggest that the differences noted for the relationship between Lp(a) and apoB levels across ethnicity and/or apo(a) size groups might be related to differences in synthetic pathway. Further studies aimed at elucidating Lp(a) synthesis in different population groups are needed. It is in this context of interest that the production rate for apoB from LDL differed substantially from the production rate for apoB from Lp(a), suggesting different apoB kinetic pools for the formation of LDL and Lp(a) [47]. In contrast, Demant et al., reported results supporting an extracellular assembly [46]. Our findings of a differential association of apoB with allele-specific apo(a) levels with smaller versus larger apo(a) sizes in African-Americans may provide additional insights, and lend support for the concept that the source of apoB for Lp(a) production may vary between different groups and/or conditions.
The findings of the current study have several important implications. First, they demonstrate an interethnic difference in the relationship between Lp(a) and apoB-containing lipoproteins. Second, they emphasize the importance of taking into account the contribution of Lp(a)-cholesterol and -apoB content to apoB-containing lipoprotein levels. This could potentially be clinically relevant for individuals with high Lp(a) levels due to a higher contribution of Lp(a) to LDL-C levels. As currently available lipid-lowering drugs, except niacin, do not appreciably impact Lp(a) levels, a failure to reduce LDL-C levels in some individuals despite an aggressive lipid-lowering treatment, including statins, might partially be explained by the contribution of Lp(a)-cholesterol and -apoB to LDL. It was therefore interesting to note the closer relationship between Lp(a) and corrected apoB levels in one ethnic group, but not in the other. Furthermore, the present study was the first to investigate the relationship of apoB-containing atherogenic lipoprotein levels with allele-specific apo(a) levels, i.e., Lp(a) levels associated with a defined apo(a) allele size.
We acknowledge some limitations of this study as subjects in our study were recruited from patients scheduled for coronary angiography, and are likely more typical of a high-risk patient group than the healthy population at large. The mean ApoB level in our study was higher than that of reported for the general population [49], but was closer to that of reported for diabetic adults enrolled in the National Health and Nutrition Examination Survey (115 mg/dL) [50]. However, other clinical and laboratory parameters were in agreement with differences generally observed between healthy African-American and Caucasian populations from other studies. Moreover, the generalizibilty of these findings to other ethnic groups is unknown.
In conclusion, although TC, LDL-C, and apoB levels were comparable between African-Americans and Caucasians, the associations of these parameters with Lp(a) and allele specific apo(a) levels differed between these two ethnic groups. In African-Americans, apoB and apoB/apoA-1 remained consistently and positively associated with both Lp(a) and allele-specific apo(a) levels after adjustments for the contribution of Lp(a)-apoB. The findings suggest a close relationship between Lp(a) and apoB among African-Americans.
Supplementary Material
Association of allele-specific apo(a) (nmol/L) level with apoB (nmol/L) level before and after correction for the contribution of Lp(a)-apoB content in Caucasians versus African-Americans.
The relationship between apoB and square root transformed allele-specific apo(a) level before (A and B) and after (C and D) correction for the contribution of Lp(a)-apoB content is described by partial correlation coefficients controlling for the effects of age, sex, and BMI. Allele-specific apo(a) level remained significantly and positively associated with apoB level after correction in African-Americans (D), but not in Caucasians (C). For conversion of apoB levels in mg/dL into nmol/L, see the Methods section.
Abbreviations: SqRt, square root; apoB, apolipoprotein B;
HIGHLIGHTS.
Lp(a) levels associate with TC, LDL-C and ApoB levels across ethnicity.
After appropriate corrections, Lp(a) levels correlate with ApoB in African-Americans.
Apo(a) sizes may potentially influence the associations of Lp(a) with apoB levels.
Contribution of Lp(a) to apoB-containing lipoprotein levels can be substantial.
Consideration of Lp(a) is important for risk assessment and treatment modification.
Acknowledgments
We thank Dr. Kyoungmi Kim from the Department of Public Health Sciences, UC Davis, for valuable consultations regarding statistical approaches.
SOURCES OF FUNDING
These studies were supported by grants #62705 (Berglund, L, Principal Investigator) from the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute and the UC Davis NIH-funded Clinical and Translational Science Center (CTSC) base operating grant (#TR000002, #RR024146). Dr. Byambaa is a recipient of the UC Davis CTSC Highly Innovative Pilot Award and the Mentored Clinical and Population Research Program Award from the American Heart Association (#14CRP17930014), and a current Building Interdisciplinary Research Careers in Women’s Health/K12 Training Program scholar (#NIH 2K12HD051958).
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, et al. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–9. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 3.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–28. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLean JW, Tomlinson JE, Kuang WJ, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330:132–7. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 6.Koschinsky ML, Beisiegel U, Henne-Bruns D, et al. Apolipoprotein(a) size heterogeneity is related to variable number of repeat sequences in its mRNA. Biochem. 1990;29:640–4. doi: 10.1021/bi00455a007. [DOI] [PubMed] [Google Scholar]
- 7.Lackner C, Cohen JC, Hobbs HH. Molecular definition of the extreme size polymorphism in apolipoprotein(a) Hum Mol Genet. 1993;2:933–40. doi: 10.1093/hmg/2.7.933. [DOI] [PubMed] [Google Scholar]
- 8.van der Hoek YY, Wittekoek ME, Beisiegel U, et al. The apolipoprotein(a) kringle IV repeats which differ from the major repeat kringle are present in variably-sized isoforms. Hum Mol Genet. 1993;2:361–6. doi: 10.1093/hmg/2.4.361. [DOI] [PubMed] [Google Scholar]
- 9.Boerwinkle E, Leffert CC, Lin J, et al. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest. 1992;90:52–60. doi: 10.1172/JCI115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcovina SM, Albers JJ, Jacobs DR, Jr, et al. Lipoprotein[a] concentrations and apolipoprotein[a] phenotypes in Caucasians and African Americans. The CARDIA study. Arterioscler Thromb. 1993;13:1037–45. doi: 10.1161/01.atv.13.7.1037. [DOI] [PubMed] [Google Scholar]
- 11.Parra HJ, Luyeye I, Bouramoue C, et al. Black-white differences in serum Lp(a) lipoprotein levels. Clin Chim Acta. 1987;168:27–31. doi: 10.1016/0009-8981(87)90263-4. [DOI] [PubMed] [Google Scholar]
- 12.Paultre F, Pearson TA, Weil HF, et al. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler Thromb Vasc Biol. 2000;20:2619–24. doi: 10.1161/01.atv.20.12.2619. [DOI] [PubMed] [Google Scholar]
- 13.Wood RJ, Volek JS, Davis SR, et al. Effects of a carbohydrate-restricted diet on emerging plasma markers for cardiovascular disease. Nutr Metab (Lond) 2006;3:19. doi: 10.1186/1743-7075-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sofi F, Fatini C, Sticchi E, et al. Fish intake and LPA 93C>T polymorphism: gene-environment interaction in modulating lipoprotein (a) concentrations. Atherosclerosis. 2007;195:e147–54. doi: 10.1016/j.atherosclerosis.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Catena C, Novello M, Dotto L, et al. Serum lipoprotein(a) concentrations and alcohol consumption in hypertension: possible relevance for cardiovascular damage. J Hypertens. 2003;21:281–8. doi: 10.1097/00004872-200302000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Guyton JR, Dahlen GH, Patsch W, et al. Relationship of plasma lipoprotein Lp(a) levels to race and to apolipoprotein B. Arteriosclerosis. 1985;5:265–72. doi: 10.1161/01.atv.5.3.265. [DOI] [PubMed] [Google Scholar]
- 17.Corsetti JP, Sterry JA, Sparks JD, et al. Effect of weight loss on serum lipoprotein(a) concentrations in an obese population. Clin Chem. 1991;37:1191–5. [PubMed] [Google Scholar]
- 18.Lobo RA, Notelovitz M, Bernstein L, et al. Lp(a) lipoprotein: relationship to cardiovascular disease risk factors, exercise, and estrogen. Am J Obstet Gynecol. 1992;166:1182–8. discussion 88–90. [PubMed] [Google Scholar]
- 19.Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med. 1989;226:271–6. doi: 10.1111/j.1365-2796.1989.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 20.Shewmon DA, Stock JL, Rosen CJ, et al. Tamoxifen and estrogen lower circulating lipoprotein(a) concentrations in healthy postmenopausal women. Arterioscler Thromb. 1994;14:1586–93. doi: 10.1161/01.atv.14.10.1586. [DOI] [PubMed] [Google Scholar]
- 21.Anuurad E, Rubin J, Chiem A, et al. High Levels of Inflammatory Biomarkers Are Associated with Increased Allele-Specific Apolipoprotein(a) Levels in African-Americans. J Clin Endocrinol Metab. 2008;93:1482–8. doi: 10.1210/jc.2007-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enkhmaa B, Anuurad E, Zhang W, et al. Association of Lp-PLA(2) activity with allele-specific Lp(a) levels in a bi-ethnic population. Atherosclerosis. 2010;211:526–30. doi: 10.1016/j.atherosclerosis.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enkhmaa B, Anuurad E, Ozturk Z, et al. Differential associations of serum amyloid A and pentraxin-3 with allele-specific lipoprotein(a) levels in African Americans and Caucasians. Transl Res. 2011;158:92–8. doi: 10.1016/j.trsl.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enkhmaa B, Anuurad E, Zhang W, et al. HIV Disease Activity as a Modulator of Lipoprotein(a) and Allele-Specific Apolipoprotein(a) Levels. Arterioscler Thromb Vasc Biol. 2013;33:387–92. doi: 10.1161/ATVBAHA.112.300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenner JL, Ordovas JM, Lamon-Fava S, et al. Effects of age, sex, and menopausal status on plasma lipoprotein(a) levels. The Framingham Offspring Study. Circulation. 1993;87:1135–41. doi: 10.1161/01.cir.87.4.1135. [DOI] [PubMed] [Google Scholar]
- 26.Slunga L, Asplund K, Johnson O, et al. Lipoprotein (a) in a randomly selected 25–64 year old population: the Northern Sweden Monica Study. J Clin Epidemiol. 1993;46:617–24. doi: 10.1016/0895-4356(93)90034-x. [DOI] [PubMed] [Google Scholar]
- 27.Bovet P, Rickenbach M, Wietlisbach V, et al. Comparison of serum lipoprotein(a) distribution and its correlates among black and white populations. Int J Epidemiol. 1994;23:20–7. doi: 10.1093/ije/23.1.20. [DOI] [PubMed] [Google Scholar]
- 28.Contois JH, Lammi-Keefe CJ, Vogel S, et al. Plasma lipoprotein(a) distribution in the Framingham Offspring Study as determined with a commercially available immunoturbidimetric assay. Clin Chim Acta. 1996;253:21–35. doi: 10.1016/0009-8981(96)06341-3. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez C, Chacon P, Garcia-Pascual L, et al. Differential influence of LDL cholesterol and triglycerides on lipoprotein(a) concentrations in diabetic patients. Diabetes Care. 2001;24:350–5. doi: 10.2337/diacare.24.2.350. [DOI] [PubMed] [Google Scholar]
- 30.Moon JY, Kwon HM, Kwon SW, et al. Lipoprotein(a) and LDL particle size are related to the severity of coronary artery disease. Cardiology. 2007;108:282–9. doi: 10.1159/000099097. [DOI] [PubMed] [Google Scholar]
- 31.Pedreno J, Fernandez R, Ballester A, et al. Lack of association of serum lipoprotein (a) levels with type-2 diabetes mellitus in patients with angiographically defined coronary artery disease. Int J Cardiol. 2000;74:159–67. doi: 10.1016/s0167-5273(00)00304-1. [DOI] [PubMed] [Google Scholar]
- 32.Gaw A, Docherty G, Brown EA, et al. Predictors of plasma lipoprotein(a) concentration in the West of Scotland Coronary Prevention Study cohort. Atherosclerosis. 1999;143:445–50. doi: 10.1016/s0021-9150(98)00305-0. [DOI] [PubMed] [Google Scholar]
- 33.Zlatohlavek L, Zidkova K, Vrablik M, et al. Lipoprotein(a) and its position among other risk factors of atherosclerosis. Physiol Res. 2008;57:777–83. doi: 10.33549/physiolres.931133. [DOI] [PubMed] [Google Scholar]
- 34.Kamstrup PR, Benn M, Tybjaerg-Hansen A, et al. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–84. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 35.Seman LJ, Breckenridge WC. Isolation and partial characterization of apolipoprotein (a) from human lipoprotein (a) Biochem Cell Biol. 1986;64:999–1009. doi: 10.1139/o86-133. [DOI] [PubMed] [Google Scholar]
- 36.Anuurad E, Rubin J, Lu G, et al. Protective effect of apolipoprotein E2 on coronary artery disease in African Americans is mediated through lipoprotein cholesterol. J Lipid Res. 2006;47:2475–81. doi: 10.1194/jlr.M600288-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Rubin J, Paultre F, Tuck CH, et al. Apolipoprotein [a] genotype influences isoform dominance pattern differently in African Americans and Caucasians. J Lipid Res. 2002;43:234–44. [PubMed] [Google Scholar]
- 38.McGowan MW, Artiss JD, Strandbergh DR, et al. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983;29:538–42. [PubMed] [Google Scholar]
- 39.Allain CC, Poon LS, Chan CS, et al. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 40.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–88. [PubMed] [Google Scholar]
- 41.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 42.Maciejko JJ, Levinson SS, Markyvech L, et al. New assay of apolipoproteins A-I and B by rate nephelometry evaluated. Clin Chem. 1987;33:2065–9. [PubMed] [Google Scholar]
- 43.Kovar J, Havel RJ. Sources and properties of triglyceride-rich lipoproteins containing apoB-48 and apoB-100 in postprandial blood plasma of patients with primary combined hyperlipidemia. J Lipid Res. 2002;43:1026–34. doi: 10.1194/jlr.m100435-jlr200. [DOI] [PubMed] [Google Scholar]
- 44.Kimak E, Solski J. ApoA- and apoB-containing lipoproteins and Lp(a) concentration in non-dialyzed patients with chronic renal failure. Ren Fail. 2002;24:485–92. doi: 10.1081/jdi-120006775. [DOI] [PubMed] [Google Scholar]
- 45.Jenner JL, Seman LJ, Millar JS, et al. The metabolism of apolipoproteins (a) and B-100 within plasma lipoprotein (a) in human beings. Metabolism. 2005;54:361–9. doi: 10.1016/j.metabol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Demant T, Seeberg K, Bedynek A, et al. The metabolism of lipoprotein(a) and other apolipoprotein B-containing lipoproteins: a kinetic study in humans. Atherosclerosis. 2001;157:325–39. doi: 10.1016/s0021-9150(00)00732-2. [DOI] [PubMed] [Google Scholar]
- 47.Frischmann ME, Ikewaki K, Trenkwalder E, et al. In vivo stable-isotope kinetic study suggests intracellular assembly of lipoprotein(a) Atherosclerosis. 2012;225:322–7. doi: 10.1016/j.atherosclerosis.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 48.Frischmann ME, Kronenberg F, Trenkwalder E, et al. In vivo turnover study demonstrates diminished clearance of lipoprotein(a) in hemodialysis patients. Kidney Int. 2007;71:1036–43. doi: 10.1038/sj.ki.5002131. [DOI] [PubMed] [Google Scholar]
- 49.Bachorik PS, Lovejoy KL, Carroll MD, et al. Measurement of apolipoproteins A-I and B during the National Health and Nutrition Examination Survey (NHANES) III. Clin Chem. 1994;40:1915–20. [PubMed] [Google Scholar]
- 50.Ford ES, Li C, Sniderman A. Temporal changes in concentrations of lipids and apolipoprotein B among adults with diagnosed and undiagnosed diabetes, prediabetes, and normoglycemia: findings from the National Health and Nutrition Examination Survey 1988–1991 to 2005–2008. Cardiovasc Diabetol. 2013;12:26. doi: 10.1186/1475-2840-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association of allele-specific apo(a) (nmol/L) level with apoB (nmol/L) level before and after correction for the contribution of Lp(a)-apoB content in Caucasians versus African-Americans.
The relationship between apoB and square root transformed allele-specific apo(a) level before (A and B) and after (C and D) correction for the contribution of Lp(a)-apoB content is described by partial correlation coefficients controlling for the effects of age, sex, and BMI. Allele-specific apo(a) level remained significantly and positively associated with apoB level after correction in African-Americans (D), but not in Caucasians (C). For conversion of apoB levels in mg/dL into nmol/L, see the Methods section.
Abbreviations: SqRt, square root; apoB, apolipoprotein B;



