Abstract
This review presents the most thoroughly studied bacterial fatty acid synthetic pathway, that of Escherichia coli and then discusses the exceptions to the E. coli pathway present in other bacteria. The known interrelationships between the fatty acid and polyketide synthetic pathways are also assessed, mainly in the Streptomyces group of bacteria. Finally, we present a compendium of methods for analysis of bacterial fatty acid synthetic pathways.
1. INTRODUCTION
The primary role of bacterial fatty acids is to act as the hydrophobic component of the membrane lipids (generally phospholipids). In a number of bacteria fatty acids also are found as components of storage lipids, the most prevalent being polyhydroxyalkanoic acids (although the lengths of the alkanoic chains are often too short to be considered fatty acids). Other bacteria accumulate storage forms reminiscent of eukaryotic lipids. Actinomycetes such as Streptomyces, Mycobacterium and Rhodococcus accumulate triglycerides (often having unusual acyl chains) whereas, upon growth on hydrocarbons, Acinetobacter and a variety of other hydrocarbon-utilizing bacteria accumulate wax esters in which a long chain fatty acid is esterified to a long chain fatty alcohol. Because accumulation of storage lipids usually depends on exogenous sources of acyl chains rather than de novo fatty acid synthesis, storage lipid fatty acids will not be further discussed. For the same reason post-synthetic modifications of fatty acyl chains such as cyclopropane ring formation (Grogan and Cronan, 1997) and cis-trans isomerization (Cronan, 2002) will also not be discussed.
2. BACTERIAL FATTY ACIDS
The fatty acids synthesized by bacteria (Fig. 1) are similar to the most abundant species present in eukaryotic cells except that the bacterial acids tend to be slightly shorter, generally lack polyunsaturation and the monoenoic C18 acids have different double bond positions. Moreover, some bacteria make branched chain fatty acids whereas others make 3-hydroxyacyl acids.
Figure 1.
Typical bacterial fatty acids. Structure A is hexadecanoic (palmitic) acid, a very abundant saturated fatty acid whereas structure B is cis-9-hexadecenoic (palmitoleic) acid which together with the product formed by one additional spin of the elongation cycle, cis-11-octadecenoic (cis-vaccenic) acid, are the dominant unsaturated species found in bacteria. Structure C is the cyclopropane fatty acid cis-9,10-methylene hexadecanoic acid formed by methylene incorporation from S-adenosyl-L-methionine into phospholipid-bound palmitoleic acid. This together with its C19 homologue are distributed very widely in bacteria. Structures D and E are C15 branched chain fatty acids where D is the anteiso species and E is the iso species. Structure F is 3-hydroxytetadecanoic acid, a major component of the lipid A of most gram negative bacteria.
3. ACYL CARRIER PROTEIN, THE KEY COMPONENT OF BACTERIAL FATTY ACID SYNTHESIS
The mechanism of the synthesis of saturated fatty acids is strongly conserved between bacteria and eukaryotes (the archaea synthesize isoprenoid-derived lipids) although the catalytic entities reside in markedly different protein arrangements. The pathway proceeds in two stages, initiation and cyclic elongation. Intermediates of the pathway are diverted to introduce the double bond of the unsaturated fatty acid species, to provide the 3-hydroxy and short chain fatty acids of the lipid A component of the outer membrane of typical gram negative bacteria (Raetz and Whitfield, 2002), the octanoyl-ACP used in the lipoic acid synthetic pathway, the unknown early intermediates of the biotin synthetic pathway and the acyl homoserine lactones of quorum sensing. A key feature of the fatty acid synthetic pathway is that all of the intermediates are covalently bound to a protein called acyl carrier protein (ACP), a small, very acidic and extremely soluble protein (Prescott and Vagelos, 1972). The carboxyl groups of the fatty acyl intermediates are in thioester linkage to the thiol of the 4'-phosphopanthetheine (4’-PP) prosthetic group which in turn is linked to Ser-36 of ACP through a phosphodiester bond. Such ACP thioesters are the substrates for the enzymes of the pathway. ACP is one of the most abundant proteins in E. coli and constitutes about 0.25% of the total soluble protein (6–8×104 molecules/cell) (Lu et al., 2007; Rock and Cronan, 1981). Indeed, ACP is reported to be the third most abundant protein present in E. coli (being slightly exceeded by RplL and TufB, two translation apparatus proteins) (Lu et al., 2007). The ACP secondary structure predicted from the amino acid sequence (Rock and Cronan, 1979) has been largely confirmed by high-resolution nuclear (NMR) magnetic resonance spectroscopy and x-ray crystallography. ACP (MW 8,860) is composed of a preponderance of acidic residues largely grouped into three β-helices (helices I, II and IV) oriented in an up-down-down topological arrangement to form a helical bundle plus a short fourth helix (helix III) that seems of lower stability and is found both almost parallel and almost perpendicular to the three helix bundle in the various structures now available (Kim and Prestegard, 1990; Roujeinikova et al., 2002b; Roujeinikova et al., 2007).
The structural plasticity of ACP seen in the absence of acylation of the prosthetic group thiol is also seen, albeit to a lesser extent, in the acylated forms. The current picture is that ACP and its acylated derivatives can adopt many different structures by sliding and twisting the helices relative to one another and by rearranging the prosthetic group and loops. This plasticity may allow acyl groups to slide in and out of the hydrophobic cavity such that a compromise is achieved between shielding the acyl chain from solvent and allowing access to the thioester-proximal acyl carbon atoms such that fatty acid synthesis can proceed. If so, the dynamics of this process will depend on the polarity and length of the acyl chain and on interactions with the other fatty acid synthetic proteins.
The prosthetic group of ACP undergoes metabolic turnover (Fig. 2) (Jackowski and Rock, 1983; Thomas and Cronan, 2005) and the apo-protein is not only inactive in fatty acid synthesis but at high levels can be growth inhibitory (Keating et al., 1995). The primary enzyme catalyzing attachment of the prosthetic group is AcpS (De Lay and Cronan, 2006; Flugel et al., 2000) although a back-up enzyme, AcpT, is also present (De Lay and Cronan, 2006; Flugel et al., 2000). AcpS and AcpT are 4'-phosphopantetheine transferases (4'-PP transferases) that transfer the 4'-phosphopantetheine portion from CoA to apo ACP to give holo-ACP plus 3’, 5’-ADP. Although the known 4'-PP transferases comprise a single protein superfamily, the quaternary structures of these proteins vary from monomers to trimers with E. coli AcpS reported to be dimeric (Lambalot and Walsh, 1997; McAllister et al., 2006). Unlike the enzymes that attach biotin and lipoic acid to their cognate proteins, the 4'-PP transferases are generally quite specific for their protein substrates (Lambalot et al., 1996) (see below). Although E. coli AcpS attaches the prosthetic group to many fatty acid ACPs, some other fatty acid ACPs are not substrates (De Lay and Cronan, 2007) and it fails to modify the structurally related carrier proteins of polyketide and nonribosomal polypeptide synthesis (Lambalot et al., 1996). However, Bacillus subtilis Sfp which is responsible for modification of the carrier protein used in synthesis of surfactin, a nonribosomal peptide, readily modifies most carrier proteins regardless of origin or metabolic role (Lambalot et al., 1996). It is thought that ACP helix II ois a major determinant of this substrate specificity (Mofid et al., 2002)
Figure 2.
ACP prosthetic group metabolism in E. coli. Apo-ACP, the product of the acpP gene, is non-functional in fatty acid synthesis. It is post-translationally activated by the attachment of a 4’phosphopantetheine arm derived from coenzyme A, a reaction catalyzed by the holo-ACP synthase AcpS. Turnover of ACP involves removal of the prosthetic group by ACP hydrolyase, AcpH which can then be used in the synthesis of CoA.
Surprisingly, all known 4'-PP transferases accept acylated-CoA substrates and transfer acyl-4’-PP moieties to apo-ACP. E. coli AcpS utilizes acetyl-ACP in place of ACP at about half the catalytic efficiency and is also active with butyryl-, acetoacetyl-, and malonyl-CoAs (McAllister et al., 2006). Again, Sfp is much more promiscuous and transfers acyl-4’-PP moieties modified with molecules such as biotin and fluorescein (Mercer and Burkart, 2007). It is unclear whether or not transfer of acyl-4’-PP moieties is physiologically relevant. However, the low levels of AcpS in E. coli argue that acyl-4’-PP transfer should be unable to replace a fatty acid synthetic enzyme such as FabD.
Soon after the identification of the prosthetic group of ACP as 4’-PP and of the AcpS 4’-PP transferase, Vagelos and Larrabee (Vagelos and Larrabee, 1967) reported an E. coli activity that removed the 4’-PP moiety of ACP (Fig. 2). The enzyme (called ACP hydrolyase and ACP phosphodiesterase) was purified (Fischl and Kennedy, 1990). ACP hydrolyase was shown to be an unusually stable enzyme of molecular weight 25,000 and an N-terminal sequence was reported. Unfortunately, the N-terminal sequence was found to be that of AzoR, a flavin-containing protein later shown to be an azoreductase (Nakanishi et al., 2001). Hence, it is clear that the N-terminal sequence determined was the sequence of a major contaminating protein rather than that of the phosphodiesterase. Regrettably this erroneous sequence attribution has led to many genes (often called acpD) being annotated in bacterial genomes as encoding ACP phosphodiesterase rather than azoreductase (Nakanishi et al., 2001). Thus, in order to prevent confusion with the mistaken AcpD annotations the recently identified gene was named acpH (Thomas and Cronan, 2005) based on the original enzyme name (ACP hydrolyase) given by Vagelos and Larrabee (Vagelos and Larrabee, 1967). The acpH gene was identified by expression cloning and was found to encode a protein of 23 kDa that readily aggregates upon overexpression (Thomas and Cronan, 2005; Thomas et al., 2007). Active enzyme was recovered by folding solubilized inclusion bodies and AcpH was found to be active on acyl-ACPs of fatty acyl chain lengths from C6 to C16. AcpH was active on Bacillus subtilis ACP, but inactive on Lactococcus lactis ACP. Strains carrying deletions of acpH are fully viable, but the ACP prosthetic group is metabolically stable unlike that of wild type strains (Thomas and Cronan, 2005). Upon AcpH overproduction all of the cellular ACP is converted to the apo form (Thomas and Cronan, 2005). AcpH is not essential either in the laboratory or in the natural habitat (Thomas and Cronan, 2005). AcpH has been shown to be a non-canonical member of the HD phosphatase/phosphodiesterase family (Thomas et al., 2007).
3. OVERVIEW OF THE REACTIONS OF FATTY ACID BIOSYNTHESIS
The fatty acid synthesis system of E. coli is the archetype of the Type II or dissociated fatty acid synthesis systems and the E. coli gene names are very often used for their homologues in other organisms. The precursors for fatty acid biosynthesis are derived from the acetyl-CoA pool. Malonyl-CoA is required for all the elongation steps and is formed by the first step in fatty acid biosynthesis catalyzed by acetyl-CoA carboxylase. Malonyl-CoA is utilized for fatty acid biosynthesis only following its conversion to malonyl-ACP by malonyl-CoA:ACP transacylase (Fig. 3). Acetyl-CoA carboxylase will not be further discussed in this review since strictly speaking the enzyme is not a dedicated fatty acid synthesis enzyme because malonyl-CoA is used in synthesis of other molecules (e.g., polyketides). Moreover, a recent review of the E. coli and other multisubunit acetyl-CoA carboxylase is available (Cronan and Waldrop, 2002).
Figure 3.
The initiation steps in the type II fatty acid synthesis pathway of E. coli. Malonyl-CoA is converted to malonyl-ACP by malonyl transacylase (FabD). Fatty acid synthesis is initiated by FabH, which condenses malonyl-ACP with acetyl-CoA.
There are three known mechanisms for the initiation of fatty acid biosynthesis in E. coli. First, FabH (3-ketoacyl-ACP synthase III) catalyzes the condensation of acetyl-CoA with malonyl-ACP to yield acetoacetyl-ACP (Fig. 3). In the second pathway, the acetate moiety is first transferred from acetyl-CoA to acetyl-ACP by the transacylase activity of FabH. The acetyl-ACP is then condensed with malonyl-ACP by FabB (synthase I) or alternatively by FabF (synthase II). The third pathway involves the decarboxylation of malonyl-ACP by FabH, FabB or FabF to form acetyl-ACP followed by subsequent condensation with malonyl-ACP. The evidence for the existence of these pathways and their relative contributions to the initiation of fatty acid biosynthesis is an area of current interest and is discussed in more detail below.
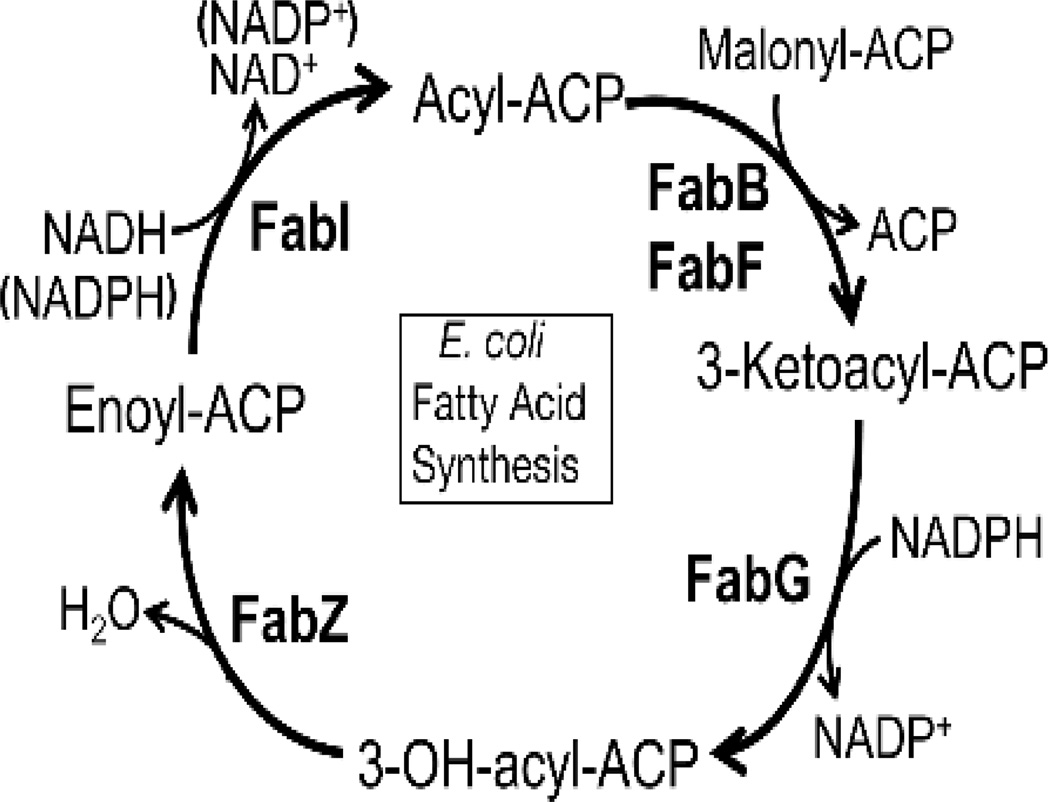
The reactions of the fatty acid elongation cycle are outlined in Fig. 4. The first step is the Claisen condensation of malonyl-ACP with a growing acyl chain catalyzed by a 3-ketoacyl-ACP synthase (either FabB or FabF). This is the only irreversible step in the elongation cycle and thus it is not surprising that the 3-ketoacyl-ACP synthases play key roles in regulating the product distribution of the pathway. The 3-keto-thioester produced is reduced by FabG, an NADPH-dependent 3-ketoacyl-ACP reductase, followed by removal of a water molecule by FabZ, a 3-hydroxyacyl-ACP dehydratase. The final reduction is catalyzed by FabI, an enoyl-ACP reductase, to give an acyl-ACP, which can serve as a substrate for another round of elongation or, if of sufficient chain length, be transferred into complex lipids. The equilibrium of the FabI enoyl-ACP reductase reaction acts to pull the reversible 3-ketoacyl-ACP reductase and 3-hydroxyacyl-ACP dehydratase reactions to the right (Heath and Rock, 1995).
Figure 4.
The elongation cycle. There are four steps in fatty acid elongation. Each new cycle of 2-carbon elongation is initiated by the condensation of acyl-ACP and malonyl-ACP by one of the elongation condensing enzymes, FabB or FabF. The next step is the reduction of the 3-ketoacyl-ACP by the NADPH-dependent FabG. This 3-hydroxyacyl-ACP is dehydrated to trans-2-acyl-ACP by FabZ. The final step is the NADH-dependent reduction of enoyl-ACP to acyl-ACP by FabI.
Two of the reactions of the cycle can be carried out by multiple discrete enzymes. As noted above in γ–proteobacteria such as E. coli there are three 3-ketoacyl-ACP synthases and two 3-hydroxyacyl-ACP dehydratases are also present with one of each playing a key role in unsaturated fatty acid synthesis. Due to their differing substrate specificities, each isozyme makes a unique contribution to the regulation of the distribution of products from the pathway (see below). In most other bacteria that use the type II pathway there are two 3-ketoacyl-ACP synthases and two 3-hydroxyacyl-ACP dehydratases. However, in bacteria that synthesize branched chain fatty acids there can be three 3-ketoacyl-ACP synthases.
The fatty acid biosynthetic pathway ends in the transfer of the acyl chains of the acyl-ACP end products by various acyltransferase systems. In general, transfer of the acyl chain is to a glycerol-based backbone molecule to form membrane lipids.
4. THE INITATION STEPS OF FATTY ACID SYNTHESIS
The first step in fatty acid synthesis is condensation of a primer acyl-CoA with malonyl-ACP (Fig. 3). The conversion of malonyl-CoA to malonyl-ACP is catalyzed by malonyl-CoA:ACP transacylase, the product of the fabD gene (Magnuson et al., 1992; Verwoert et al., 1992). FabD is a monomeric protein that accepts the malonyl moiety from malonyl-CoA to form a stable malonyl enzyme intermediate in which the malonyl moiety is in ester linkage to a serine hydroxyl (Oefner et al., 2006; Serre et al., 1995). Nucleophilic attack of this intermediate by the sulfhydryl of ACP gives malonyl-ACP, the major building block of fatty acids. Crystal structures of FabD, FabD complexed with malonate and the FabD-malonyl-CoA complex have been reported (Oefner et al., 2006; Serre et al., 1995). It should be noted that several polyketide ACPs have been reported to self-malonate (Arthur et al., 2005; Arthur et al., 2006; Hitchman et al., 1998). That is, the malonyl moiety is transferred from CoA to ACP in the absence of malonyl-CoA:ACP transacylase. This reaction will be discussed below.
In contrast to the reactions that produce malonyl-ACP, the reactions whereby the methyl carbon atom and the immediately adjacent carbon atom (the last two carbons of the fatty acid chain in chemical nomenclature) are incorporated into fatty acid remain somewhat unclear. In straight chain acids the methyl and penultimate carbons are derived from acetyl-CoA. Acetyl-CoA is a substrate for FabH (3-ketoacyl-ACP synthase III which has also been called acetoacetyl-ACP synthase) and is incorporated directly to form the first four-carbon fatty acyl-ACP species (Jackowski and Rock, 1987; Tsay et al., 1992a). In E. coli malonyl-ACP is utilized only in the elongation steps in fatty acid biosynthesis. However, FabH, FabB, and FabF are capable of initiating fatty acid synthesis in vitro in the absence of an added acetyl-ACP or acetyl-CoA as the primer. This synthesis occurs through a side reaction; decarboxylation of malonyl- ACP to give acetyl-ACP. This reaction is readily demonstrated in vitro (Alberts et al., 1972; McGuire et al., 2001) but its role in initiation in vivo awaits experimental verification. FabH has been shown to be essential for fatty acid synthesis in Lactococcus lactis by construction of a deletion mutant (Lai and Cronan, 2004). However, the deleted strain retains about 10% of the normal fatty acid synthetic ability indicating a partial bypass of FabH activity (Lai and Cronan, 2004). This seems likely to be due to malonyl-ACP decarboxylation followed by FabF-catalyzed condensation of the resulting acetyl-ACP with malonyl-ACP. However, this scenario requires experimental testing. Another possible source of acetyl-ACP would be transfer of acetyl-4’-PP from acetyl-CoA to apo ACP catalyzed by AcpS (see above). However, the intracellular level of AcpS activity seems far too low to provide the needed carbon flow.
In the synthesis of branched chain acids acetyl-CoA is replaced by isovaleryl-CoA, isobutyryl-CoA, or 2-methylbutyryl-CoA which are derived from the metabolic pathways for the amino acids, valine, leucine and isoleucine (Wallace et al., 1995). The enzyme responsible for the synthesis of these acyl-CoAs is a specialized branched-chain-keto acid dehydrogenase complex, and mutations in the activity of this enzyme system result in strains that are auxotrophic for branched-chain acids (Cropp et al., 2000). Bacillus subtilis has two FabH homologues that accept the branched acyl-CoAs (Choi et al., 2000a) whereas Streptomyces glaucescens has only a single such enzyme (Han et al., 1998). It should be noted that the branched chain organisms also contain some straight chain acids and this is reflected in the fact that their FabH enzymes accept acetyl-CoA, although branched chain primers are the preferred substrates. In contrast, E. coli FabH ignores branched chain primers (Choi et al., 2000a).
5. The ENZYMES OF THE FATTY ACID ELONGATION CYCLE
5.1 The 3-Ketoacyl-ACP Synthase Reaction (FabB, FabF, FabH)
The first reaction of each cycle is the Claisen condensation of an acyl thioester (acyl-ACP or for FabH, acetyl-CoA) with malonyl-ACP to form a 3-ketoacyl-ACP plus ACP. Three E. coli enzymes are known to catalyze 3-ketoacyl-ACP synthase reactions. These enzymes were referred to as synthases I, II and III, but more recently have come to be called FabB, FabF and FabH, respectively, after their gene names. The fabH and fabF genes are located within the fatty acid synthetic gene cluster (Rawlings and Cronan, 1992) whereas fabB maps alone at a distant site. Siggard-Andersoen and coworkers (Siggaard-Andersen et al., 1994) reported a putative fourth KAS activity in E. coli and assigned an open reading frame to this activity. However, this report was based on a series of indirect inferences and was in error; the gene sequenced was the fabF gene (Magnuson et al., 1995; Rawlings and Cronan, 1992) and the enzyme activity described seems likely to be a mixture of FabH with FabB and/or FabF. The three E. coli 3-ketoacyl-ACP synthases represent two classes of decarboxylating Claisen condensing enzymes (Heath and Rock, 2002; White et al., 2005). The FabB and FabF enzymes have Cys-His-His active sites whereas FabH has a Cys-His-Asn active site triad. These differences are reflected in the rest of the primary sequences of the proteins. FabB and FabF are about 37% identical whereas alignment of either FabB or FabF with FabH gives only scattered alignments of very low quality. High-resolution crystal structures of all three enzymes are available (White et al., 2005).
The functional FabB is composed of two identical subunits (Garwin et al., 1980b) and contains both malonyl-ACP and fatty acyl-ACP binding sites (D'Agnolo et al., 1975b). In the condensation reaction, the acyl group becomes covalently linked to a specific FabB cysteine sulfhydryl (D'Agnolo et al., 1975b). The acyl-enzyme undergoes condensation with malonyl-ACP to form 3-ketoacyl-ACP, CO2, holo-ACP and free enzyme. Inhibition studies using cerulenin (see below) show the active site cysteine to be Cys-163 (Kauppinen et al., 1988).
Further investigation revealed the presence of a second long chain synthase activity in E. coli, FabF (D'Agnolo et al., 1975a). Like FabB, FabF has a dimeric structure and is inhibited by cerulenin (Ulrich et al., 1983), although FabF is less sensitive to the antibiotic than is FabB and is not essential for growth of E. coli. Both synthases are both capable of participating in saturated and unsaturated fatty acid synthesis. The enzymes have been shown, in vitro, to function similarly with all substrates except palmitoleoyl-ACP; palmitoleoyl-ACP is an excellent substrate for FabF, but not for FabB (Edwards et al., 1997b; Garwin et al., 1980b). This observation is consistent with the role of FabF in the regulation of fatty acid composition of the membrane phospholipid in response to temperature (see below). Mutant strains having fabB missense or null mutations (Garwin et al., 1980a), however, require unsaturated fatty acids for growth. Therefore, in vivo FabB must catalyze a key reaction in unsaturated fatty acid synthesis that FabF cannot. This reaction is probably the elongation of cis-3-decenoyl-ACP, although this has not been demonstrated experimentally. This step is the rate-limiting step in unsaturated fatty acid synthesis (Clark et al., 1983).
FabB overproduction has two known effects. First, overproduction of the enzyme overcomes its poor ability to elongate palmitoleoyl-ACP and an increased amount of cis-vaccenic acid is incorporated into phospholipid (de Mendoza et al., 1983). The increase, however, has no effect on the temperature regulation of fatty acid composition (de Mendoza et al., 1983). Secondly, excess cellular FabB renders E. coli resistant to the antibiotic thiolactomycin which is an inhibitor of all three 3-ketoacyl-ACP synthases. It was thought that FabB catalyzed decarboxylation of malonyl-ACP offered the cell an alternative initiation pathway for fatty acid biosynthesis; malonyl-ACP decarboxylation to give acetyl-ACP that was used as a primer for chain elongation. The isolation of thiolactomycin-resistant strains that have an resistant FabB has borne out this hypothesis in vivo (Jackowski et al., 2002). Moreover, in the presence of the antibiotic, excess FabB appears to allow the cell to bypass the standard FabH initiation pathway probably by decarboxylation of malonyl-ACP to form acetyl-ACP (Jackowski et al., 2002; Tsay et al., 1992b). Given the above observations, FabB appears to be the only E. coli 3-ketoacyl-ACP synthase absolutely required for growth (Jackowski et al., 2002; Tsay et al., 1992b).
FabH is a dimeric protein of 33.5 kDa first detected as a condensation activity resistant to cerulenin both in vivo and in vitro (Jackowski and Rock, 1987). Although cerulenin blocks the synthesis of long chain fatty acids, short chain (C4-C8) acids linked to ACP are accumulated both in vivo and in cell extracts. The fabH gene has been shown to encode KAS III. As noted above the FabH sequence has no similarity to those of FabB or FabF, although there is good alignment with other enzymes known to catalyze condensation reactions. From the chain length of the acyl-ACPs produced and the behavior of fabB fabF double mutants mentioned above, it is clear that FabH does not participate in the progressive condensation steps of fatty acid synthesis that produce the long chain acids found in the phospholipids and lipid A. However, the enzyme could produce the fatty acid synthetic intermediates used in lipoic acid (and perhaps biotin) synthesis. However, the high activity of the enzyme suggests that it does not exclusively function in this pathway (E. coli requires only a few hundred lipoic acid molecules per cell).
3-Ketoacyl-ACP reductase (FabG)
An open reading frame encoding a protein with strong similarities to several acetoacetyl-CoA reductases and particularly plant 3-ketoacyl-ACP reductases (>50 % identical residues) was found between the fabD and acpP genes in the fab gene cluster (Rawlings and Cronan, 1992). This gene was designated as fabG and is obligatorily cotranscribed with the upstream genes (Zhang and Cronan, 1996; Zhang and Cronan, 1998). Blocking fabG transcription blocked cell growth indicating that it is an essential gene (Zhang and Cronan, 1998). However, no mutants in this gene were available until the recent isolation of temperature-sensitive mutants of both E. coli and Salmonella enterica (Lai and Cronan, 2004). Strikingly, all of the mutations were located in or near the subunit interfaces of the FabG homotetramer suggesting that monomers and dimers of the enzyme are inactive. At the nonpermissive temperature fatty acids synthesis was blocked in the fabG(Ts) mutants following the initial condensation such that only four carbon acyl-ACP species accumulated. Thus, only a single NADPH-specific 3-ketoacyl-ACP reductase exists in E. coli and it functions with all acyl chain lengths (Lai and Cronan, 2004). Crystal structures of FabG are available (White et al., 2005).
5.3. The 3-Hydroxyacyl-ACP dehydratase (FabZ)
This enzyme is not to be confused with the 3-hydroxydecanoyl-ACP dehydratase specifically required for introduction of the double bond of the unsaturated acids in E. coli and related bacteria. Prior biochemical data gave a puzzling picture of this reaction. One group reported that this step is catalyzed by a single enzyme active with substrates of all chain lengths (Birge et al., 1967) whereas another laboratory reported the presence of three enzymes specific for short, medium, and long chain length substrates (Mizugaki et al., 1968). More recent work with recombinant FabZ showed that the dehydratase efficiently catalyzed the dehydration of short chain 3-hydroxyacyl-ACPs and long chain saturated and unsaturated 3-hydroxyacyl-ACPs (Heath and Rock, 1996). The fabZ gene was isolated as a suppressor of a mutation in lipid A biosynthesis. The suppression is thought to be due to increased intracellular levels of 3-hydroxymyristoyl-ACP (Mohan et al., 1994). Unfortunately, the phenotype of the original mutant strain is too feeble for physiological studies. Although no structure of E. coli FabZ is presently available, several FabZ homologue structures have been reported the most closely related of which is that of the Pseudomonas aeruginosa protein (Kimber et al., 2004). This FabZ shows sequence homology to FabA (which was a key to its discovery) and thus it was no surprise that the monomers have a hot-dog fold (see below) and the active sites lie at the dimer interface. FabZ has a Glu residue in place of the active site Asp residue of FabA. This substitution was thought to play an important role in the differing specificities of the two enzymes. However, the discovery of a FabZ homologue (FabN) that has FabA activity and has a Glu active site residue (Wang and Cronan, 2004) indicates that this simple hypothesis is incorrect.
5.4. The Enoyl-ACP reductase (FabI)
Two forms of enoyl-ACP reductase, the last enzyme of the fatty acid cycle, were originally reported, one dependent on NADH and the other on NADPH (Weeks and Wakil, 1968). However, both activities were subsequently shown to be due to the same enzyme, FabI (Bergler et al., 1996), the sole enoyl-ACP reductase of E. coli (Heath and Rock, 1995). The identification of the protein encoded by this gene, now called fabI, was the result of studying mutants of E. coli and S. enterica resistant to diazaborines, a class of potent antimicrobial agents that inhibit lipid synthesis (Turnowsky et al., 1989). FabI is also the target of the widely used antimicrobial compound, triclosan (Heath et al., 1999; McMurry et al., 1998). FabI plays a role in the fatty acid synthesis cycle in that its action pulls the other reversible steps of the cycle (FabG, and FabZ) to the right such that each cycle of fatty acid biosynthesis is completed (Heath and Rock, 1995). Several FabI crystal structures (White et al., 2005) and a useful fabI temperature sensitive mutant (Bergler et al., 1994) are available. It should be noted that E. coli FabI has been reported to be inhibited by palmitoyl-CoA (Bergler et al., 1996). Although this has been ascribed a physiological role, it remains to be demonstrated that the observed inhibition is not due to the well-known detergent properties of long chain acyl-CoAs.
6. UNSATURATED FATTY ACID SYNTHESIS IN E. COLI
6.1. The 3-Hydroxydecanoyl-ACP Dehydratase (FabA)
This enzyme essentially extracts a major fraction of the 3-hydroxydecanoyl-ACP from the elongation cycle and introduces a cis double bond (Fig. 5). Following elongation of the cis-3-decenoyl-ACP by FabB (Fig. 4), these modified acyl chains are returned to the cycle for elongation to the long chain unsaturated acids needed for phospholipid function (Bloch, 1971). 3-Hydroxydecanoyl-ACP dehydratase specifically catalyzes the dehydration of 3-hydroxydecanoyl-ACP to a mixture of trans-2-decenoyl-ACP and cis-3-decenoyl-ACP (Bloch, 1971). The reaction proceeds via the formation of trans-2-decenoyl-ACP as an enzyme-bound intermediate which can disassociate from the enzyme (Bloch, 1971). When disassociation occurs, the trans-2 intermediate is reduced by an enoyl-ACP reductase and subsequently converted to saturated fatty acids as in the standard elongation cycle (Clark et al., 1983; Guerra and Browse, 1990; Heath and Rock, 1996). Enzyme-bound trans-2-decenoyl-ACP, however, is isomerized to cis-3-decenoyl-ACP. The double bond is preserved and the cis-3 intermediate is elongated to the unsaturated fatty acids of E. coli, palmitoleic acid and cis-vaccenic acid. The enzyme, a homodimer of 18 kDa subunits (Leesong et al., 1996), is distinct from the elongation cycle FabZ dehydratase discussed above, although the two enzymes contain sequences in common.
Figure 5.
Production of unsaturated fatty acids. Unsaturated fatty acids arise from a branch in the biosynthetic pathway at the 3-hydroxydecanoyl-ACP intermediate. FabA is a unique 3-hydroxyacyl-ACP dehydratase that is capable for forming trans-2-decenoyl-ACP and isomerizing this intermediate to cis-3-decenoyl-ACP. FabA is very selective for the 10-carbon substrates in vivo. FabB is absolutely required for the subsequent elongation of cis-3-decenoyl-ACP to 16:1-ACP. Interestingly, 16:1-ACP is a poor substrate for FabB, and its elongation to 18:1-ACP is controlled by the activity of FabF. FabF is a naturally temperature-sensitive enzyme, and this property account for the greater proportion of 18:1 in bacteria grown at low temperatures compared to those grown at higher temperatures. The trans-3-decenoyl-ACP is used by FabI followed by elongation by either FabB or FabF to form palmitic acid, the major saturated fatty acid in E. coli. The parentheses show isozymes from other bacteria that catalyze the reaction shown.
The first mutants isolated that were blocked in fatty acid biosynthesis, called fabA, lacked 3-hydroxydecanoyl-ACP dehydratase (Silbert and Vagelos, 1967). These mutants are unable to synthesize unsaturated fatty acids, but synthesize saturated fatty acids normally. In vitro, mutant fabA enzymes could form neither the cis-3 nor trans-2-decenoyl products (Cronan and Gelmann, 1973). This finding, along with the observation that saturated fatty acid synthesis continues in vivo indicated that another dehydratase was available for saturated fatty acid synthesis, the enzyme, now called FabZ, which catalyzes the formation of trans-2-decenoyl-ACP, but cannot catalyze the isomerase reaction (Heath and Rock, 1996). In the absence of thermal regulation, the ratio of unsaturated to saturated fatty acids in E. coli is dependent on the levels of FabA and FabB. It was shown that overproduction of FabA in vivo failed to increase the level of unsaturated fatty acids, but significantly increased the amount of saturated fatty acids incorporated into membrane phospholipids (Clark et al., 1983). This indicated that, although FabA is required for the synthesis of unsaturated fatty acids, the level of enzyme activity does not limit the rate of unsaturated fatty acid synthesis. Introduction of multiple copies of the fabB gene (encoding synthase I) reversed the effect of dehydratase overproduction resulting in wild type fatty acid compositions (Clark et al., 1983). Thus, the step more likely to limit the rate of unsaturated fatty acid synthesis is the elongation of cis-3-decenoyl-ACP catalyzed by FabB. The levels of expression of the fabA and fabB genes, therefore, appear to establish a basal ratio of unsaturated to saturated fatty acid synthesis in the absence of thermal regulation. Modulation of the fatty acid composition of membrane phospholipid in response to temperature shift is discussed below.
FabA functions with acyl chain lengths from C4 to C12 in an in vitro fatty acid synthesis system reconstituted from purified enzymes (Heath and Rock, 1996). This is a considerably wider range of FabA substrates than originally reported in studies using synthetic model substrates (Bloch, 1971). The reasons for this disagreement are unclear, but it should be noted that the ratios of products produced by the enzyme using acyl-ACP substrates (Guerra and Browse, 1990) differs from that obtained using model substrates (Bloch, 1971).
The mechanism of FabA has been thoroughly studied (Bloch, 1971; Schwab and Henderson, 1990) since it was the first enzyme for which a mechanism-activated ("suicide") inhibitor was described. This inhibitor, 3-decynoyl-N-acetylcysteamine, forms a covalent adduct with the active site histidine residue resulting in loss of all of the partial reactions of the enzyme (Bloch, 1971; Cronan et al., 1988; Leesong et al., 1996) The x-ray crystal structure of E. coli FabA was the first structure determined for an E. coli fatty acid synthetic protein. FabA was the first example of the “hot dog” fold in which a long central β-helix is wrapped by a six-stranded antiparallel β-sheet (Leesong et al., 1996). FabA forms an unusually stable dimer and it seems that the dimerization is essential for activity since the two active sites are formed along the dimer interface with the critical His and Asp active site residues being contributed by different monomers (Leesong et al., 1996).
7. THE ABUNDANT EXCEPTIONS TO THE E. COLI FATTY ACID SYNTHESIS PARADIGM
7.1 Branched Chain Fatty Acids
The synthesis of branched chain fatty acids by use of FabH homologues has been discussed above. Such fatty acids occur widely in bacteria (e.g., Bacilli, Streptomyces, Staphylococci).
7.2 Anaerobic synthesis of unsaturated fatty acids
The classical pathway of anaerobic unsaturated fatty acid synthesis in E. coli is not widely distributed in bacteria. Genomic analyses indicate that only the β- and γ-proteobacteria encode the proteins of this pathway. Most other bacteria including many pathogens make unsaturated fatty acids under anaerobic conditions, but lack recognizable homologues of the key E. coli unsaturated fatty acid synthetic enzymes, FabA and FabB. Thus far, Streptococcus pneumoniae has been shown to introduce the cis double bond using FabM (Marrakchi et al., 2002), an isomerase of sequence unrelated to FabA, that performs only one of the FabA reactions (FabA is both an isomerase and a dehydratase). In contrast Enterococcus faecalis was shown to have proteins that are homologues of FabZ and FabF that perform the roles of FabA and FabB, respectively (Wang and Cronan, 2004). This work has been extended (Altabe et al., 2007; Lu et al., 2005). However, riddles remain in a number of bacteria (e.g., the clostridia and the lactic acid bacteria). Pseudomonas aeruginosa has not only the classical FabA-FabB pathway, but also two oxygen-requiring desaturase pathways one of which uses endogenous phospholipid acyl chains whereas the other uses exogenous saturated fatty acids (Cronan, 2006a; Zhu et al., 2006). The bacilli also have desaturases that are induced by low growth temperatures that utilize endogenous phospholipid acyl chains (Mansilla and de Mendoza, 2005). Other bacteria lack FabM, FabA, FabB and desaturases and lack extra copies of genes encoding FabZ and FabF homologues. Therefore, it seems that other pathways for unsaturated synthesis exist.
7.3. Diversity of Enoyl-ACP Reductases
A curious and important divergence is seen in the conservation of bacterial fatty acid synthetic (FAS) proteins. Most FAS II enzymes are strongly conserved among bacteria both in sequence and (where data are available) in structure and most domains of the FAS I proteins are clearly derived from FAS II proteins (Cronan, 2006b; Cronan, 2004). An exception is the last step of the elongation cycle, formation of a saturated acyl-ACP by reduction (generally NAD(P)H-dependent) of the enoyl-ACP double bond. As mentioned above in E. coli this reaction is catalyzed by the product of the fabI gene that was discovered as the target for the antibacterial action of a set of diazaborine compounds (Bergler et al., 1994). FabI was later shown to also be the site of the antibacterial action of triclosan (McMurry et al., 1998), a compound used in antibacterial hand soaps and a large variety of other everyday products. Although FabI homologues are widely distributed in bacteria and other FAS II-containing organisms, the existence of a number of bacterial species naturally resistant to triclosan was soon recognized (Heath et al., 1999; Marrakchi et al., 2003). In these bacteria triclosan resistance was due to the presence of enoyl-ACP reductase isozymes of varying resistance to triclosan. Bacillus subtilis contains two isozymes, a FabI homologue and an isozyme called FabL that is moderately resistant to triclosan (Heath et al., 2000) and, like FabI, is a member of the short chain dehydrogenase reductase superfamily. Streptococcus pneumoniae contains a single enoyl-ACP reductase, FabK, that is refractory to triclosan and is a TIM barrel flavoprotein unrelated to the short chain dehydrogenase reductase isozymes (Marrakchi et al., 2003). A fourth class of enoyl-ACP reductase is present in vibrios and related bacteria (Massengo-Tiasse and Cronan, 2008). The diversity of the bacterial enoyl-ACP reductases when compared to the lack of structural and mechanistic diversity seen in the other enzymes of the FAS II elongation cycle argues that naturally occurring compounds exist that selectively inhibit one or another of these enzymes. This hypothesis has recently been confirmed by the recent discoveries of natural enoyl-ACP reductase inhibitors of fungal origin that specifically target FabI (Cephalochromin) (Zheng et al., 2007) and FabK (Atromentin and Leucomelone) (Zheng et al., 2006).
7.4 Type I Megasynthase Fatty Acid Synthesis
The most striking divergence from the E. coli type II fatty acid synthetic pathway is the type I megasynthase pathway found in the mycolic acid-producing branch of the Actimomycetales (mycobacteria, corynebacteria, rhodococci, and nocardiae) (Schweizer and Hofmann, 2004). Mycolic acids are high-molecular-weight α-alkyl β-hydroxy fatty acids (70 to 90 carbon atoms in length) composed of a species-specific "short" arm of 22 to 26 carbon atoms and a, "long" meromycolic acid arm of 50 to 60 carbon atoms (although this arm is shorter in corynebacteria) (Gokhale et al., 2007; Schweizer and Hofmann, 2004). These essential molecules are found in the matrix of the cell wall and are formed by head to head Claisen-type condensations of two long-chain fatty acyl thioesters followed by reduction of the keto group. De novo synthesis of the long chain fatty acid esters is accomplished by several strategies. In mycobacteria, the type II system is unable to perform de novo synthesis which is the function of the type I system. The type one system synthesizes C16 and C26 saturated acyl-CoAs. The C16-CoA is elongated by the type II FabH protein and successive condensation (by two very long chain FabF-like enzymes) and reduction cycles to give the C56-CoA. This is condensed with the C26-CoA by the Pks13 polyketide synthase that catalyzes the Claisen condensation. Reduction of the resulting ketone by specific reductase gives the mycolic acids (Gokhale et al., 2007). It should be noted that the type I system may play only a minor role in the growth of pathogenic mycobacteria since they readily acquire host fatty acids. Excepting its specificity for long chain lengths, the mycobacterial type II system is fairly typical.
The multifunctional fatty acid synthesis (FAS) polypeptides of mycobacterial and corynebacteria contain all the functional domains required for de novo fatty acid synthesis (Schweizer and Hofmann, 2004). These domains are organized in the following order: acyltransferase, enoyl reductase, dehydratase, malonyl/palmitoyl transferase, acyl carrier protein, 3-ketoacyl reductase and 3-ketoacyl synthase. All intermediates generated remain enzyme-bound during the process of elongation and undergo transacylation to other catalytic sites within the enzyme. FAS I generates short-chain fatty acyl-CoA primers that are further elongated by the type II system. It is remarkable that the domain order of the bacterial type I protein is precisely that given by in silico fusion of the yeast β and β fatty acid synthase subunits with β being the N-terminal partner (Schweizer and Hofmann, 2004). Moreover, both the bacterial type I protein and yeast enzymes are functional hexamers. It will be interesting to see if the bacterial type I protein has the fantastic porous barrel architecture of the fungal enzyme (Cronan, 2006c). Corynebacteria synthesize unsaturated fatty acids by use of a second type I synthase that contains a FabA-like domain (Schweizer and Hofmann, 2004). Mutational inactivation of the gene encoding this second synthase results in an unsaturated fatty acid requirement. However, some corynebacteria lack this second enzyme and make only saturated species (Schweizer and Hofmann, 2004) whereas others lack a type I protein and require fatty acids for growth (Tauch et al., 2005). Finally, it should be noted that proteins annotated as fatty acid synthetic proteins based on sequence homologies with the E. coli proteins can have no involvement in fatty acid synthesis. For example a Pseudomonas aeruginosa FabH homologue catalyzes a key step in the synthesis of an extracellular quinoline derivative (Zhang et al., 2008) that acts as a quorum-sensing molecule and a Mesorhizobium FabH homologue plays a role in biotin synthesis (Sullivan et al., 2001).
8. RELATIONSHIPS BETWEEN FATTY ACID SYNTHESIS AND POLYKETIDE SYNTHESIS
There seems no doubt that polyketide synthesis is an evolutionary descendent of fatty acid synthesis. The fatty acid synthase (FAS-I) responsible for de novo fatty acid synthesis in the cytosol of animal cells is homologous in sequence and analogous in architecture with the very large family of type I modular polyketide synthases (PKSs). Both “megasynthase” assembly lines use homologous domains (ACP or PCP) to carry the growing fatty acid or polyketide via a pantetheine-linked thioester (Smith and Sherman, 2008). The common thioester chemistry, similar structures and the adaptable architecture have resulted in the proliferation of hybrid PKS-FAS pathways found in phylogenetically diverse bacteria (Smith and Sherman, 2008). Indeed, the very complex lipids of mycobacteria are made via a partnership between an FAS and a modular PKS (Gokhale et al., 2007) and a class of fatty acids are produced by modular PKS systems (Metz et al., 2001; Wallis et al., 2002). This close relationship raises the question of whether or not FAS and PKS systems share enzymatic components. The two systems clearly share the precursors, acetyl-CoA and malonyl-CoA, but this is probably not a competitive situation because fatty acid synthesis proceeds primarily in growing cells whereas polyketides are secondary metabolites whose synthesis generally occurs after a culture ceases net growth. Indeed, ectopic expression of sets of whiE-PKS genes presumed sufficient to assemble a carbon chain caused inhibition of early growth of the strains. This growth inhibition was not a nonspecific effect of the protein expression because it required all three genes to be expressed (subsets of two of the proteins gave no inhibition of growth). It has been reasonably proposed that this growth inhibition is due to competition between fatty acid synthesis and the inappropriately timed polyketide synthesis for precursors or proteins common common to the two pathways (Yu and Hopwood, 1995). This hypothesis remains to be tested.
The more interesting question is whether or not the fatty acid and polyketide synthetic pathways share protein components. This question has been addressed almost exclusively in bacteria of the genus Streptomyces due to the remarkable diversity of polyketides made by these bacteria and has focused on type II polyketide synthases which are composed of discrete monofunctional proteins (analogous to type II fatty acid synthesis) rather than the large, highly modular Type I polyketide synthases in which interactions are “hard-wired”. The early finding that S. coelicolor contained three different ACP–like proteins, rather than the single protein expected if the fatty acid ACP (AcpP) could also function in polyketide synthesis, suggested that ACP was not a shared component (Revill et al., 1996). AcpP was identified by its constitutive expression, its genomic clustering with genes encoding other fatty acid synthetic proteins and its ability to function in E. coli fatty acid synthesis. In contrast the remaining two proteins were developmentally regulated and one of these was shown to be required for synthesis of the polyketide, actinorhodin (Revill et al., 1996). Moreover, Streptomyces AcpPs were shown to only very weakly complement mutants lacking the actinorhodin ACP–like protein; only traces of the polyketide were produced (Khosla et al., 1992; Revill et al., 1996). Later work showed that AcpP and some PKS ACPs are modified by distinct phosphopantetheinyl transferases (the AcpS reaction of Fig. 2), although other PKS ACPs are modified by AcpS (Cox et al., 2002; Lu et al., 2008; Shen et al., 1992a; Walsh et al., 1997). Hence, some AcpS proteins are less specific for their acceptor protein than E. coli AcpS. Moreover, a few phosphopantetheinyl transferases such as B. subtilis Sfp are known to modify a wide diversity of ACP-like proteins (Finking et al., 2002; Mootz et al., 2001).
Recently a new approach to test the compatibility of the ACPs of type II FAS and PKS systems has been developed (Worthington et al., 2008). This approach uses modified ACP species that carry a reactive electrophile in place of the thiol of the 4’-PP moiety. Interaction between one of these modified ACPs and a 3-ketoacyl-ACP synthase results in formation of a covalent bound between the ACP prosthetic group and the active site cysteine of a synthase. The systems compared were the E. coli FAS AcpP together with the E. coli FabB and FabF synthases and EncC (the ACP) and EncAB synthase of the Streptomyces maritimus polyketide, enterocin. Two different 4’-PP analogues were tested for each ACP. One analogue mimicked a short chain acyl-ACP whereas the other mimicked a longer chain species. As expected both the E. coli and S. maritimus ACP species reacted with their cognate synthase. Both versions of E. coli AcpP also reacted with S. maritimus EncAB. However, only a partially reciprocal result was seen in that FabF failed to react with either version of EncC whereas FabB reacted only with EncC carrying the shorter analogue (Worthington et al., 2008). These findings raise the question of whether or not EncC functions in both the FAS and entericin pathways of S. maritimus even though the ACP is encoded in the gene cluster of the latter pathway. However, no S. maritimus genome sequence is available and thus the possibility of an FAS ACP cannot be evaluated at present. The other fatty acid synthetic protein suspected to play a role in polyketide synthesis is FabD, the malonyl-CoA:AcpP transacylase (Fig. 3). FabD proteins purified from Streptomyces were shown to be active on both AcpPs and Type II PKS ACPs (Florova et al., 2002; Revill et al., 1995; Summers et al., 1995). These enzymes were demonstrated to be fatty acid synthetic proteins by their genomic clustering with other fatty acid genes and in one case the ability to functionally replace the E. coli FabD protein when expressed in E. coli (Summers et al., 1995). There seems only a single discrete malonyl-CoA:AcpP transacylase encoded in the S. coelicolor genome sequence (although domains of similar sequence are found in the modular type I PKS genes) and this is also the case in S. avermitilis. However, the genome of S. griseus subsp. griseus encodes three FabD homologues. Hence, there is a possibility that there may be malonyl-CoA:ACP transacylases targeted to specific PKS ACPs in some Streptomyces but not others. The malonyl-CoA:AcpP transacylase of S. glaucescens has been reported to play an indirect role in providing a building block for synthesis of the polyketide tetracenomycin C (Florova et al., 2002). In vivo studies with deuterium labeled acetate indicates that, in direct contrast to fatty acid synthesis, the starter acetate unit is not derived directly from acetyl-CoA as shown by loss of deuterium upon incorporation into the polyketide. The starter unit is thought to be formed indirectly by synthesis of malonyl-CoA from acetyl-CoA followed by transfer of the malonyl moiety to the tetracenomycin ACP (TcmM) (Florova et al., 2002. The resulting malonyl-TcmM would then be decarboxylated to give acetyl-TcmM, the acetate moiety of which is the proposed starter unit (Florova et al., 2002). Although malonyl-ACP decarboxylation is a known side reaction of 3-ketoacyl-ACP synthases, the identity of the enzyme catalyzing decarboxylation is unclear. However, FabH seems a strong possibility (Smirnova and Reynolds, 2001; Wallace et al., 1995).
In mycobacteria the FAS II 3-ketoacyl-ACP synthase (KasA) has been found to interact with PpsB and PpsD, two polyketide modules involved in the biosynthesis of the virulence lipid, phthiocerol in E. coli (Kruh et al., 2008). The interaction with PpsB, a four-module PKS protein containing an ACP domain, was examined in detail. When PspB labeled with a long chain fatty acid was incubated with KasA and AcpM, the Mycobacterium AcpP, the acyl chain was transferred to both proteins and could be elongated when other FAS components were present. Hence, transfer of a PKS product to an FAS for further elongation seems possible. The reverse transfer did not occur. The PKS to FAS transfer was proposed to serve to increase the diversity of mycobacterial lipids (Kruh et al., 2008).
9. METHODS FOR STUDY OF BACTERIAL FATTY ACID SYNTHESIS
9.1 Preparation of the holo and apo forms of E. coli ACP
ACP is one of the most abundant proteins of E. coli and can readily be purified from wild type cells. However, modern day preparations utilize high-level overexpression of the protein. In some cases the acpP gene has been modified by addition of N-terminal or C-terminal tags to aid purification of the encoded fusion protein. However, bacterial ACPs have atypical and highly conserved properties that make purification of the native proteins very facile. First, ACP is very acidic (isoelectric point of 4.1), small and extremely soluble (solutions of >40 mg/ml can be made). This solubility allows bulk purification steps such as precipitation of most other proteins with 50% 2-propanol or saturated ammonium sulfate while ACP remains in solution. We favor the 50% 2-propanol treatment since the 2-propanol does not interfere with ion exchange chromatography, the final step of the purification. The acidic nature of ACP results in very tight binding of ACP to strongly basic anion exchanger resins such as Q (quaternary ammonium) or the weakly basic anion exchanger, DEAE (often abbreviated D). We favor the DEAE matrix because it binds fewer contaminating proteins than Q. ACP is bound to the DEAE column at pH 6.1. At this pH the strongly acidic ACP binds tightly whereas most other proteins bind weakly (if at all) because the pH is below their isoelectric points. Second, ACP readily and quantitatively refolds following denaturation with heat, urea, guanidine or trichloroacetic acid (TCA). In the procedures below ACP species are routinely recovered and concentrated by TCA precipitation. The TCA can be removed by dialysis as below by washing the ACP precipitate with a pH 4.1 buffer followed by dissolution in a buffer of higher pH (Cronan and Klages, 1981). Both procedures remove any deoxycholate (used as a co-precipitant) remaining from the precipitation step. The procedure outlined below is modified from those described previously (Keating et al., 1995; Rock and Cronan, 1980; Rock and Cronan, 1981) and applies to Escherichia coli ACP, but may be adaptable to the ACPs of other organisms provided they have similar sizes and isoelectric points.
9.2 Strains and plasmids used
The E. coli K-12 strain used for ACP expression is DK574 (Keating et al., 1995) which is strain SJ16 (Jackowski and Rock, 1981) containing plasmid pMR19 (Rawlings, 1993) which encodes the structural gene for ACP from E. coli under control of of a tac promoter and the LacI repressor overexpression plasmid pMS421 (Grana et al., 1988)The strain also has a chromosomal lesion in the gene encoding the aspartate decarboxylase, panD. This mutation does not affect growth in rich medium but imposes a requirement for exogenous supplementation with the CoA precursor β-alanine in minimal medium (Cronan, 1982). This permits the isolation of radiolabeled ACP by supplementation with radioactive β-alanine. Plasmid pJT93 carrying the holo-ACP synthase gene acpS under control of a lac promoter was constructed as follows: acpS was amplified from E. coli K-12 genomic DNA. The PCR product was digested with restriction enzymes KpnI and PstI and ligated into the vector pDHC30 (Phillips et al., 2000) digested with the same enzymes. Plasmid pJT94 encoding acpH under lac promoter control was constructed in a similar fashion. The acpH gene was amplified from E. coli K-12 genomic DNA, the PCR product digested with enzymes KpnI and PstI and ligated into pDHC30 digested with the same enzymes.
9.3 Holo-ACP
At high levels of expression acyl carrier protein is isolated primarily in the apo-form (Keating et al., 1995). To ensure complete phosphopantetheinylation of the protein, strain DK574 is additionally transformed with the plasmid pJT93 expressing the Escherichia coli AcpS 4’-PP transferase under tac promoter control, a system similar to the BAP1 system for the purification of PKS ACPs discussed by Zhang and Tang in this section. Strain DK574 carrying pJT93 is cultured overnight in LB medium supplemented with 15 µg/ml kanamycin, 50 µg/ml streptomycin and 10 µg/ml chloramphenicol. This starter culture is diluted 100-fold into 500 ml of the same medium and incubated with shaking at 37°C until it reaches an optical density of 0.8.
When ACP labeled in the 4’-PP arm is desired, the following changes are made: the expression strain is cultured overnight in 5 ml M9 medium supplemented with 15 µg/ml kanamycin, 50 µg/ml streptomycin and 10 µg/ml chloramphenicol, 0.4% glucose and limiting (0.5 µM) β-alanine in order to reduce the CoA pools. These β-alanine starved cultures are then diluted 100-fold into 100–500 ml of M9 medium containing 0.4% glucose and 8 µM [3H] β-alanine (American Radiolabeled Chemicals) and grown to an optical density of 0.8.
The expression of ACP and AcpS are simultaneously induced by the addition of 100 µM IPTG followed by incubation for a further 3–4 h. The cells are harvested by centrifugation at 17,700 × g for 10 min and washed in an equal volume of 50 mM Tris-HCl, pH 8.8. The cells are then resuspended in 5 ml AcpS reaction buffer (50 mM Tris-HCl pH 8.8, 10 mM MgCl2, 5 mM dithiothreitol) and lysed by sonication (six 20 second pulses) or by two passages through a French pressure cell. The cell lysate is cleared by centrifugation at 18,000 × g for 20 minutes at 4°C, 1 mM CoA is added to the supernatant and incubated at 37 °C for 4 hours. The alkaline pH and high concentration of dithiothreitol (DTT) also serve to hydrolyze acyl-ACP thioesters that may be present. At this stage, complete conversion to holo-ACP is verified by conformationally sensitive gel electrophoresis on a non-denaturing 20% polyacrylamide gel containing 0.5 M Urea (Rock et al., 1981b) followed by staining with Coomassie Brilliant Blue 250.
The treated cell extract is dialyzed against 50 mM potassium-2-(N-morpholino)ethanesulfonic acid (K-MES) (pH 6.1) overnight or subjected to ultrafiltration using a centrifugal filter of MWCO 5000 with three buffer replacements. An equal volume of ice-cold isopropanol is added to the dialyzed extract and incubated with stirring at 4 °C for 2–14 h to allow precipitation of cellular protein. The suspension is cleared by centrifugation at 18,000 × g for 20 minutes at 4 °C. A Vivaspin D Maxi H DEAE centrifuge column (Sartorius) is equilibrated by two washes with 10 ml of 0.5 M K-MES, pH 6.1 and one wash with 10 ml of 25 mM K-MES, pH 6.1 at 2,000 × g. The supernatant resulting from centrifugation of the isopropanol treatment is applied to the Vivaspin column followed by two washes of the column with 10 ml 25 mM potassium-MES containing 0.3 M LiCl. ACP is eluted from the Vivaspin column with 10 ml of 25 mM K-MES containing 0.5 M LiCl. LiCl is preferred over NaCl as the latter results in the formation of sodium adducts of ACP which may interfere with downstream analyses such as mass spectrometry. ACP is precipitated from the eluate by the addition of TCA to a concentration of 5% together with sodium deoxycholate to 0.02% as a co-precipitant (Bensadoun and Weinstein, 1976) followed by incubation at 4°C for 30 min. This suspension is centrifuged at 18,000 × g for 30 minutes and ACP is resuspended in 1 ml 0.5 M Tris-HCl, pH 8.0. The buffer containing the concentrated protein is replaced by dialysis against 25 mM K-MES, pH 6.1 containing 1 mM DTT and visualized by conformationally sensitive gel electrophoresis (see below) followed by staining with Coomassie Brilliant Blue 250. A 500 ml culture gives about 2 mg of pure ACP (the poorer medium used in preparation of tritium-labeled ACP results in about half this yield).
9.4 Apo-ACP
Apo-ACP is often required in the study of 4’-PP transferases or as a substrate for the preparation of acyl-ACP thioesters using a 4’-PP transferase. In this case, the strain DK574 is transformed with the plasmid pJT94 expressing the AcpH ACP hydrolyase under tac control. Culture of the expression strain and co-expression of ACP and AcpH are carried out as described above. When expression is complete, cells are lysed by sonication or passage through a French pressure cell in 5 ml of AcpH buffer: 50 mM Tris-HCl pH 8.8, 25 mM MgCl2, 1 mM DTT, 0.2 mM MnCl2 and incubated for 4 hours at 37° C. The purification follows that of holo-ACP.
9.5 Synthesis of Acyl-ACP substrates
It is often necessary to synthesize acyl-ACP thioesters as substrates for fatty acid synthesis enzymes. Acyl ACP thioesters may be enzymatically synthesized by three complementary methods. The first utilizes E. coli acyl ACP synthetase (Aas) to form an acyl ACP thioester using holo-ACP and free fatty acid as substrates. The acyl ACP synthetase of E. coli (Ray and Cronan, 1976) is a membrane protein later discovered to have two active sites, an Aas active site and 2-acylglycerophosphoethanolamine acyltransferase active site (Jackowski et al., 1994). The overall reaction salvages lysophospholipids formed during lipoprotein synthesis by acylation (Jackowski et al., 1994). The Aas activity of the protein has been extensively used for making acyl-ACPs (Rock et al., 1981b; Shanklin, 2000). More recently, the gene encoding acyl ACP synthetase from Vibrio harveyi was identified and the protein (AasS) expressed and purified (Jiang et al., 2006). AasS is a soluble cytoplasmic enzyme readily purified in hexahistidine-tagged form and so presents another attractive choice for synthesis of acyl-ACP thioesters. A typical reaction mixture consists of 20 µM ACP, 200 µM fatty acid and 170 nM AasS in a buffer containing 100 mM Tris-HCl (pH 7.8), 10 mM MgCl2, 1 mM TCEP and 10 mM ATP in a reaction volume of 1 ml. The reaction is allowed to proceed for 4 h at 37 °C and stopped by addition of 2 volumes of acetone and the proteins allowed to precipitate at −20°C overnight. The precipitate is centrifuged at 18,000 × g for 20 minutes followed by two washes with three volumes of acetone. The pellet is air dried and resuspended in 20 mM Tris-HCl (pH 7.4) containing 1 mM Tris [2-carboxyethyl] phosphine (TCEP). For most purposes the acetone precipitation product will suffice, although it maybe further purified by chromatography on octyl-Sepharose, if the acyl chain is C8 or longer (Rock et al., 1981b). It should be noted that the preferred substrates for AasS are saturated fatty acids of 5 to 16 carbon atoms chain length (Shen et al., 1992b). For preparation of unsaturated acyl-ACPs E. coli Aas may be required. It should also be noted that AasS is active on only a subset of bacterial ACPs (Jiang et al., 2006) whereas E. coli Aas has a broader specificity (Shanklin, 2000; Sharma et al., 2005). A second method for acyl-ACP synthesis is the use of a phosphopantetheinyl transferase such as the Sfp of Bacillus subtilis to transfer an acyl-phosphopantetheine group from acyl coenzyme A to apo ACP as discussed by Zhang & Tang in this volume, although the use of CoA thioesters makes this an expensive synthetic route and many acyl-CoAs are not commercially available. The third method is specific chemical acylation of the thiol of the ACP prosthetic group (Cronan and Klages, 1981). In the presence of high concentrations of imidazole at pH 6.5 N-acylimidazoles specifically acylate only the prosthetic group thiol; there is no acylation of the other nucleophilic groups of the protein and the reactions are often quantitative (Cronan and Klages, 1981; Roujeinikova et al., 2002a; Sharma et al., 2005). N- Acylimidazoles are readily synthesized and can be used without purification. There, also, is a fourth synthetic method that is very specialized, but that fulfills a shortcoming of the other methods. Synthesis of 3-ketothioesters of ACP is problematical due to the instability of these species. As noted above M. tuberculosis FabH uses saturated acyl-CoAs of chain length C8-C12 as primers in the condensation reaction with malonyl-ACP to give 3-ketoacyl-ACPs two carbon atoms longer than the primer (Choi et al., 2000b). Therefore, M. tuberculosis FabH together with the appropriate acyl-CoA can be added to the reconstructed fatty acid synthesis system in place of the generic FabH and will generate the needed 3-ketoacyl-ACP in situ (Marrakchi et al., 2002).
It should be noted that due to the lack of tryptophan and the paucity of other aromatic residues in E. coli ACP (and most other ACPs) standard protein colorimetric assays often seriously underestimate the concentrations of ACP and acyl-ACP solutions. For E. coli ACP a molar extinction coefficent of 1.8×103 at 278 nm has been determined based on amino acid analysis (Rock and Cronan, 1980) and a similar value is found in the older literature (Sauer et al., 1964). This value is accurate unless the ACP sample is grossly contaminated with nucleic acid (this is mainly tRNA that elutes from the DEAE matrix with ACP). Given overexpression of ACP this is not a problem, but when ACP was purified from wild type cells (where the ratio of tRNA to ACP is much higher) this contamination was readily detected by inspection of the UV spectrum of the sample and the tRNA was readily removed by isoelectric point precipitation (Rock and Cronan, 1980). It should also be noted that Coomassie Blue staining of ACP also underestimates (by 5 to10-fold depending on the staining protocol) the amount of ACP present because of a lack of basic groups to bind the dye.
9.6 Purification of fatty acid synthetic enzymes
Although each of the E. coli fatty acid synthetic enzymes has been purified from wild type cells using classical chromatographic procedures, the enzymes are now almost invariably purified as fusion proteins carrying various purification tags, most often N-terminal hexahistidine tags (Heath and Rock, 1995; Hoang et al., 2002). Each of the E. coli and Pseudomonas aeruginosa fatty acid synthetic enzymes remains active with an N-terminal hexahistidine tag (although in most cases the activities of the tagged and native forms have not been compared directly). Similarly the fatty acid synthetic enzymes of a wide variety of organisms retain activity when hexahistidine tagged. Purification of soluble hexahistidine tagged proteins by metal chelate chromatography is routine in most laboratories and will not be described here. Although the tagging approach has also been successful with a wide variety of Type II fatty acid synthetic enzymes, occasional problems have arisen. For example a C-terminally hexahistidine tagged version of the Vibrio cholerae enoyl-ACP reductase, FabV) was several orders of magnitude less active that the native protein (Massengo-Tiasse and Cronan, 2008). In contrast the N-terminal hexahistidine tagged version was much more active than the C-terminally tagged version (although it was somewhat less active than the native protein in vivo). It should be noted that the nickel ions that bleed from the standard metal chelate chromatographic columns and the imidazole used for elution must be efficiently removed from the enzyme preparations. Ni++ can catalyze air oxidation of the thiol (usually DTT) added to insure reduction of ACP and enzyme cysteine residues. Oxidation results in inactive enzymes and inactivates ACP by formation of ACP disulfide-linked dimers. Imidazole can attack acyl thioesters to form acyl-imidazoles (Jencks and Carriuolo, 1959).
9.7 Direct assays of fatty acid synthetic enzyme activities
Although the acyl substrates of fatty acid synthesis are bound to ACP, some of the fatty acid synthetic enzymes show activity with acyl-CoA and acyl-N-acetylcysteamine substrates (Bloch, 1971; Heath et al., 2000; Massengo-Tiasse and Cronan, 2008). These latter substrates are generally less active than the cognate ACP substrates and have lower VMAX and higher KM values consistent with their serving as model compounds. The simplest of the assays are determination of the 3-ketoacyl-ACP reductase (FabG) and enoyl-ACP reductase (FabI, FabL, FabV) activities because the progress of these reactions is readily monitored by oxidation of the reduced pyridine nucleotide substrate by spectrophotometry. Moreover, these enzymes are often active with commercially available acyl-CoA substrates (Choi et al., 2000b; Heath et al., 2000; Massengo-Tiasse and Cronan, 2008).
Two of the enzymes, 3-ketoacyl-ACP synthase III (FabH) and malonyl-CoA:ACP transacylase (FabD) may be assayed by TCA precipitation assays (Alberts et al., 1974; Han et al., 1998). Short chain acyl-CoAs are soluble in TCA whereas acyl-ACPs are TCA insoluble. Hence, the conversion of a 14C-labeled acyl-CoA substrate to a 14C-labeled, ACP-bound acyl chain results in conversion of a TCA soluble compound to an insoluble compound. Therefore, a simple precipitation step followed by scintillation counting of the washed precipitates gives quantitative data.
The 3-hydroxyacyl-ACP dehydratases (FabZ, FabA) may be assayed by hydration of the enoyl-thioester trans-2 double bond, the reversal of the physiological reaction. Loss of the characteristic adsorption of enoyl-thioesters at 263 nm can be followed spectrophotometrically (Bloch, 1971). This assay can be problematical using ACP thioesters and purified enzymes due to protein adsorption at this wavelength and CoA thioesters cannot be used since they absorb strongly at this wavelength. Moreover, assay of crude extracts is precluded by protein and nucleic acid absorption. For these reasons N-acetylcysteamine thioesters are the preferred substrates providing the enzyme will accept these model compounds. The reaction can also be run in the forward reaction by following the increase in absorption at 263 nm. The double bond isomerization reactions catalyzed by FabA and FabM can likewise be followed by gain or loss of absorption at 263 nm (depending on the substrate used) since cis-3-acylthioesters do not absorb at this wavelength (Bloch, 1971; Marrakchi et al., 2002; Schwab et al., 1985).
Determination of the long chain 3-ketoacyl-ACP synthase (FabF, FabB) activity requires the most complicated of the assays since the enzymes generally require that both substrates (malonyl-ACP and the acyl-ACP) are ACP thioesters which precludes TCA precipitation assays. The reaction uses malonyl-ACP labeled with 14C either at malonate carbon 2 of or at carbons 1 and 3 plus an unlabeled acyl-ACP. Following incubation with the long chain 3-ketoacyl-ACP synthase the assay is stopped by addition of buffered sodium borohydride which cleaves the thioester bonds and reduces both the acyl thioester moiety and the newly introduced keto moiety to alcohol moieties (Edwards et al., 1997a; Garwin et al., 1980b). The products are radioactive 1,3-acyl diols, a stable product that is readily extracted into toluene, a solvent compatible with direct scintillation counting (the product of malonyl-ACP reduction remains in the aqueous phase). It should be noted that both the 3-ketoacyl-ACP synthase and 3-hydroxyacyl-ACP dehydratase reactions can be followed spectrophotometrically using assays coupled to pyridine nucleotide conversion (oxidation or reduction, respectively) by 3-ketoacyl-ACP reductase (FabG (Birge et al., 1967)). However, this enzyme is not commercially available. We have chosen to give literature references on a range of bacteria rather than specific assay conditions since the assays used for the E. coli enzyme may not suffice for enzymes from other bacteria. For example, the FabH of Mycobacterium tuberculosis uses long chain acyl-CoA substrates rather than acetyl-CoA (Choi et al., 2000b). Long chain acyl-CoAs are insoluble in TCA and thus the E. coli FabH TCA precipitation assay will not work. Instead, the appropriate assay would be the borohydride reduction assay used for FabF and FabB. A second example is the FabH proteins of Bacilli and Streptomyces where short branched-chain acyl-CoAs are used in place of acetyl-CoA (Heath et al., 2000; Li et al., 2005; Smirnova and Reynolds, 2001).
9.8 Reconstituted fatty acid synthesis systems
Crude extracts of E. coli synthesize fatty acids when the extracts are supplemented with a thiol reducing agent, NADPH, malonyl-CoA, acetyl-CoA and ACP (Lennarz et al., 1962). An NADPH regenerating system is often included due to the potent NADH oxidase activity of membrane fragments in the crude extract (the pyridine nucleotide transhydrogenase present in the extracts interconverts NADPH and NADH). ACP is added to augment that of the extract and this is probably needed to compensate for the massive dilution relative to in vivo conditions resulting from extract preparation. If the malonyl-CoA is 14C labeled, the reaction can be followed by extraction of the synthesized fatty acids followed by scintillation counting (alternatively the acetyl-CoA can be labeled but incorporation is much less efficient). The reaction can also be followed by use of the conformationally sensitive gel electrophoresis system described below.
If an extract deficient in the enzymatic step of interest is available, such extracts can be used to assay activity of an enzyme that catalyzes that step of fatty acid synthesis. Extracts deficient in specific enzymes can be obtained from a mutant strain lacking the enzyme to be assayed (Cronan et al., 1969; Gelmann and Cronan, 1972; Silbert and Vagelos, 1967). Another method is by treatment of the extract with an inhibitor that specifically and irreversibly inactivates the enzyme of interest (followed by removal of excess inhibitor before assay) (McGuire et al., 2001). In both cases the purified enzyme is then added to the deficient extract and its ability to restore fatty acid synthetic ability is measured. Similarly, if one has two mutant strains defective in fatty acid synthesis that have the same phenotype, the two extracts can be mixed. If the mutations are in the same gene (protein) normal fatty acid synthesis will not be restored whereas if the strains are mutant in different genes, normal synthesis results. Such “extract complementation” assays were used to show that the E coli unsaturated fatty acid auxotrophs, fabA and fabB, had lesions in two different genes (Cronan et al., 1969) and demonstrated the enoyl-ACP reductase activities of FabV and FabK (Marrakchi et al., 2003; Massengo-Tiasse and Cronan, 2008).
Another more flexible approach takes advantage of the ease with which the bacterial fatty acid synthesis proteins can be purified. In this approach (called “reconstructed fatty acid synthesis”) each of the proteins is purified as the hexahistidine-tagged species and is then used to assemble a system that synthesizes fatty acids from 14C-labeled malonyl-CoA and acetyl-CoA using NADH and NADPH as cofactors (Heath and Rock, 1995; Heath and Rock, 1996). The products of the reaction have generally been assayed by the conformationally sensitive gel electrophoresis system described below although the solvent extraction used with extract complementation could also be used. Since the purity of each of component is readily tested by its omission, this system can be used to test functionality of any FAS candidate protein. A criticism of this approach is that in general equal masses of each enzyme were added which may not reflect the in vivo situation. However, this approach has subsequently been validated by mass spectrophotometric analysis of the levels of the fatty acid synthetic proteins in E. coli that indicate that the proteins (except ACP) are each present in a few thousands of molecules per cell (Lu et al., 2007). Since the subunit molecular weights of the E. coli fatty acid synthetic enzymes vary by only about 2-fold, use of equal masses of the enzymes in the reconstructed fatty acid synthesis system is not a great departure from the in vivo situation. Moreover, the chosen ratio of ACP to the synthetic enzymes also approximates the in vivo ratio. A similar reconstructed system was later assembled from the P. aeruginosa proteins (Hoang et al., 2002).
9.9 Resolution of ACP species by conformationally sensitive gel electrophoresis
With the advent of facile synthesis of acyl-ACP species comparisons of the properties of E. coli ACP and long chain acyl-ACPs showed that acyl-ACPs were more resistant to partial denaturation at elevated pH values (pH 9–9.5) than the nonacylated species (Rock and Cronan, 1979). The two protein species coeluted from size exclusion chromatography columns at neutral pH whereas at pH 9.4 the nonacylated species eluted first. Since the two protein species had essentially the same mass and charge, the earlier elution indicated that the nonacylated species had a larger hydrodynamic radius and that the acyl group acted to stabilize ACP structure towards this pH-induced expansion. A greater degree of separation was obtained by polyacrylamide gel electrophoresis using the standard Tris-based buffer system generally used for SDS gels but with the SDS omitted (Cronan, 1982; Rock et al., 1981b). The pH of the separating gel in this system increases from pH 8.8 to about pH 9.5 during electrophoresis (Cronan, 1982) resulting in good resolution of acylated and nonacylated ACP species with the acylated species migrating more rapidly. Moreover, it was found that the acetyl-, butyryl-, hexanoyl- and octanoyl-ACPs could be resolved with a direct relationship between electrophoretic mobility and acyl chain length (Cronan, 1982). During these investigations it was found that heating of the gel by electrical resistance increased resolution and when commercial temperature-controlled electrophoresis units became available, the system was standardized to 20% polyacrylamide gels run at 37°C (Jackowski and Rock, 1987). However, workers in the plant lipid field found that addition of various concentrations of urea to the gels markedly improved resolution of ACP species (Post-Beittenmiller et al., 1991), such that the separations could be done in a minigel format. Short chain acyl-ACP species were separated at low urea concentrations (0.5–1 M) whereas resolution of longer chain (C16-C18) species required higher urea concentrations (up to 5 M).
Apo-, holo- and acyl-ACP may be distinguished from each other by conformationally sensitive gel electrophoresis. This method takes advantage of the differential partial denaturation of ACP species under alkaline conditions in the presence of urea. ACP species carrying a hydrophobic acyl chain are more stable than holo-ACP which in turn is more stable than apo-ACP and ACP species acylated with hydrophilic acyl groups (e.g., malonyl-ACP) (Rock et al., 1981a). In the gel systems commonly used to resolve ACP species, partially denaturing conditions are maintained by urea and alkaline pH and the mobility of a protein is inversely related to its hydrodynamic radius (all species of a given ACP have essentially the same net charge). Apo- and holo-ACP are best distinguished from each other by using a resolving gel composed of 20% acrylamide, 0.67% N, N’-methylenebisacrylamide, 375 mM Tris-HCl (pH 8.8), 0.5 M Urea and a stacking gel of 5% acrylamide, 0.17% N, N’-methylenebisacrylamide, 125 mM Tris-HCl, (pH 6.8). Both gels contain N, N, N’, N’-tetramethylethylenediamine, and polymerization is initiated by addition of ammonium persulfate to 0.04%. The minigels are loaded and the samples are subjected to electrophoresis at 120 V for 100–110 min in a running buffer containing 25 mM Tris-HCl and 0.19 M glycine. To resolve acyl-ACP thioesters, the concentration of urea is increased to 2.5 M and the gel is run at 120V for 120 – 150 minutes. The proteins is then visualized by staining the gel in a solution of 50% methanol, 10% acetic acid and 0.1% Coomassie Brilliant Blue R250 followed by destaining in a solution of 10% methanol and 10% acetic acid. If the protein samples are radioactive, the gel is dried and subjected to autoradiography or phosphorimaging.
These gel systems have been very useful in analysis of fatty acid synthesis in vivo and in vitro and allow detection of each of the intermediates of the early cycles of fatty acid synthesis. However, they must be augmented with other analytical techniques such at mass spectroscopy. This is because some intermediates are labile (such as 3-ketoacyl-ACPs) and others are difficult to resolve (trans-2-enoic and saturated species of the same chain length). Moreover, the presence of hydrophilic groups such as a 3-hydroxy moiety can be offset by greater hydrophobicity (increased chain length) which can complicate interpretation. The x-ray (Roujeinikova et al., 2002b; Roujeinikova et al., 2007) and NMR (Kim and Prestegard, 1989; Kim and Prestegard, 1990) structures of ACP provide some rationale for the observed properties of ACP and its acyl thioesters. These data confirmed the original prediction that ACP is structured into a four helix bundle although certain of the helixes are plastic and the protein probably exists in at least two conformations. The acyl chain stabilizes the structure mainly through hydrophobic interactions between the hydrocarbon portion of the acyl chain and residues of the hydrophobic cavity primarily formed by the last three helices of the protein. The prosthetic group also interacts with residues other than that to which it is attached accounting for the separation of the holo and apo forms of ACP.
It must be noted that the system described above is for E. coli ACP and its acyl species. Despite the strong sequence conservation, bacterial ACPs differ markedly in their electrophoretic mobilities on these gels (de la Roche et al., 1997; De Lay and Cronan, 2007) and the urea concentrations must be optimized for each ACP. For example separation of the acyl species of the ACP-I and ACP-II of spinach (62% identity) required markedly different urea concentrations (Post-Beittenmiller et al., 1991). It should be noted that in the absence of urea this gel system resolves ACP from the other proteins present in crude extracts of E. coli (Flugel et al., 2000). This is because other proteins are either too large to enter the gel or lack sufficient negative charge to migrate as rapidly as ACP (or both).
9.10. ACPs behave abnormally in SDS-polyacrylamide gel electrophoresis and gel filtration
ACPs generally migrate abnormally in standard SDS-polyacrylamide gel electrophoresis systems because the highly charged nature of the protein results in aberrantly low SDS binding. Indeed, both E. coli and V. harveyi ACPs migrate as 20–22 kDa proteins despite having molecular weights of 8.6 kDa (de la Roche et al., 1997; Rock and Cronan, 1979). Long chain acyl-ACPs migrate more rapidly than ACP due to increased binding of SDS to the acyl group which imparts greater negative charge (Rock and Cronan, 1979). However, the thioester linkage of acyl-ACPs can be hydrolyzed during SDS gel electrophoresis. E. coli ACP migrates on gel filtration columns as though a protein of 19–20 kDa (Rock and Cronan, 1979). This is not due to dimer formation, but rather to the atypically large hydrodynamic radius of this very flexible and dynamic protein.
REFERENCES
- Alberts AA, Majerus PW, Vagelos PR. Malonyl-CoA acyl carrier protein transacylase. Meth. Enzymol. 1974;14:53–56. [Google Scholar]
- Alberts AW, Bell RM, Vagelos PR. Acyl carrier protein. XV. Studies of β-ketoacyl-acyl carrier protein synthetase. J. Biol. Chem. 1972;247:3190–3198. [PubMed] [Google Scholar]
- Altabe S, Lopez P, de Mendoza D. Isolation and characterization of unsaturated fatty acid auxotrophs of Streptococcus pneumoniae and Streptococcus mutans . J. Bacteriol. 2007;189:8139–8144. doi: 10.1128/JB.01275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur CJ, Szafranska A, Evans SE, Findlow SC, Burston SG, Owen P, Clark-Lewis I, Simpson TJ, Crosby J, Crump MP. Self-malonylation is an intrinsic property of a chemically synthesized type II polyketide synthase acyl carrier protein. Biochemistry. 2005;44:15414–15421. doi: 10.1021/bi051499i. [DOI] [PubMed] [Google Scholar]
- Arthur CJ, Szafranska AE, Long J, Mills J, Cox RJ, Findlow SC, Simpson TJ, Crump MP, Crosby J. The malonyl transferase activity of type II polyketide synthase acyl carrier proteins. Chem. Biol. 2006;13:587–596. doi: 10.1016/j.chembiol.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bergler H, Fuchsbichler S, Hogenauer G, Turnowsky F. The enoyl-[acyl-carrier-protein] reductase (FabI) of Escherichia coli which catalyzes a key regulatory step in fatty acid biosynthesis, accepts NADH and NADPH as cofactors and is inhibited by palmitoyl-CoA. Eur. J. Biochem. 1996;242:689–694. doi: 10.1111/j.1432-1033.1996.0689r.x. [DOI] [PubMed] [Google Scholar]
- Bergler H, Wallner P, Ebeling A, Leitinger B, Fuchsbichler S, Aschauer H, Kollenz G, Hogenauer G, Turnowsky F. Protein EnvM is the NADH-dependent enoyl-ACP reductase (FabI) of Escherichia coli . J. Biol. Chem. 1994;269:5493–5496. [PubMed] [Google Scholar]
- Birge CH, Silbert DF, Vagelos PR. A β-hydroxydecanoyl-ACP dehydrase specific for saturated fatty acid biosynthesis in E. coli . Biochem. Biophys. Res. Commun. 1967;29:808–814. doi: 10.1016/0006-291x(67)90291-4. [DOI] [PubMed] [Google Scholar]
- Bloch K. β-hydroxydecanoyl thioester dehydrase. In: Boyer PD, editor. The Enzymes. New York: Academic Press; 1971. pp. 441–464. [Google Scholar]
- Choi KH, Heath RJ, Rock CO. β-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J. Bacteriol. 2000a;182:365–370. doi: 10.1128/jb.182.2.365-370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Kremer L, Besra GS, Rock CO. Identification and substrate specificity of β-ketoacyl (acyl carrier protein) synthase III (mtFabH) from Mycobacterium tuberculosis. J Biol Chem. 2000b;275:28201–28207. doi: 10.1074/jbc.M003241200. [DOI] [PubMed] [Google Scholar]
- Clark DP, DeMendoza D, Polacco ML, Cronan JE., Jr β-Hydroxydecanoyl thio ester dehydrase does not catalyze a rate-limiting step in Escherichia coli unsaturated fatty acid synthesis. Biochemistry. 1983;22:5897–5902. doi: 10.1021/bi00294a032. [DOI] [PubMed] [Google Scholar]
- Cox RJ, Crosby J, Daltrop O, Glod F, Jarzabek ME, Nicholson TP, Reed M, Simpson TJ, Smith LH, Soulas F, Szafranska AE, Westcott J. Streptomyces coelicolor phosphopantetheinyl transferase: a promiscuous activator of polyketide and fatty acid synthase acyl carrier proteins. J. Chem. Soc. Perkin Trans. 2002;1:1644–1649. [Google Scholar]
- Cronan JE. A bacterium that has three pathways to regulate membrane lipid fluidity. Mol Microbiol. 2006a;60:256–259. doi: 10.1111/j.1365-2958.2006.05107.x. [DOI] [PubMed] [Google Scholar]
- Cronan JE. Avant garde fatty acid synthesis by trypanosomes. Cell. 2006b;126:641–643. doi: 10.1016/j.cell.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan JE. Remarkable structural variation within fatty acid megasynthases. Nat Chem Biol. 2006c;2:232–234. doi: 10.1038/nchembio0506-232. [DOI] [PubMed] [Google Scholar]
- Cronan JE., Jr Molecular properties of short chain acyl thioesters of acyl carrier protein. J Biol Chem. 1982;257:5013–5017. [PubMed] [Google Scholar]
- Cronan JE., Jr Phospholipid modifications in bacteria. Curr Opin Microbiol. 2002;5:202–205. doi: 10.1016/s1369-5274(02)00297-7. [DOI] [PubMed] [Google Scholar]
- Cronan JE., Jr The structure of mammalian fatty acid synthase turned back to front. Chem Biol. 2004;11:1601–1602. doi: 10.1016/j.chembiol.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Cronan JE, Jr, Birge CH, Vagelos PR. Evidence for two genes specifically involved in unsaturated fatty acid biosynthesis in Escherichia coli . J. Bacteriol. 1969;100:601–604. doi: 10.1128/jb.100.2.601-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan JE, Jr, Gelmann EP. An estimate of the minimum amount of unsaturated fatty acid required for growth of Escherichia coli . J. Biol. Chem. 1973;248:1188–1195. [PubMed] [Google Scholar]
- Cronan JE, Jr, Klages AL. Chemical synthesis of acyl thioesters of acyl carrier protein with native structure. Proc Natl Acad Sci U S A. 1981;78:5440–5444. doi: 10.1073/pnas.78.9.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan JE, Jr, Li WB, Coleman R, Narasimhan M, de Mendoza D, Schwab JM. Derived amino acid sequence and identification of active site residues of Escherichia coli β-hydroxydecanoyl thioester dehydrase. J. Biol. Chem. 1988;263:4641–46416. [PubMed] [Google Scholar]
- Cronan JE, Jr, Waldrop GL. Multi-subunit acetyl-CoA carboxylases. Prog. Lipid Res. 2002;41:407–435. doi: 10.1016/s0163-7827(02)00007-3. [DOI] [PubMed] [Google Scholar]
- Cropp TA, Smogowicz AA, Hafner EW, Denoya CD, McArthur HA, Reynolds KA. Fatty-acid biosynthesis in a branched-chain alpha-keto acid dehydrogenase mutant of Streptomyces avermitilis . Can J Microbiol. 2000;46:506–514. [PubMed] [Google Scholar]
- D'Agnolo G, Rosenfeld IS, Vagelos PR. Multiple forms of β-ketoacyl-acyl carrier protein synthetase in Escherichia coli . J. Biol. Chem. 1975a;250:5289–5294. [PubMed] [Google Scholar]
- D'Agnolo G, Rosenfeld IS, Vagelos PR. β-Ketoacyl-acyl carrier protein synthetase. Characterization of the acyl-enzyme intermediate. J. Biol. Chem. 1975b;250:5283–5288. [PubMed] [Google Scholar]
- de la Roche MA, Shen Z, Byers DM. Hydrodynamic properties of Vibrio harveyi acyl carrier protein and its fatty-acylated derivatives. Arch Biochem Biophys. 1997;344:159–164. doi: 10.1006/abbi.1997.0203. [DOI] [PubMed] [Google Scholar]
- De Lay NR, Cronan JE. A genome rearrangement has orphaned the Escherichia coli K-12 AcpT phosphopantetheinyl transferase from its cognate Escherichia coli O157:H7 substrates. Mol. Microbiol. 2006;61:232–242. doi: 10.1111/j.1365-2958.2006.05222.x. [DOI] [PubMed] [Google Scholar]
- De Lay NR, Cronan JE. In vivo functional analyses of the type II acyl carrier proteins of fatty acid biosynthesis. J. Biol. Chem. 2007;282:20319–20328. doi: 10.1074/jbc.M703789200. [DOI] [PubMed] [Google Scholar]
- de Mendoza D, Klages Ulrich A, Cronan JE., Jr Thermal regulation of membrane fluidity in Escherichia coli Effects of overproduction of β-ketoacyl-acyl carrier protein synthase I. J. Biol. Chem. 1983;258:2098–2101. [PubMed] [Google Scholar]
- Edwards P, Nelsen JS, Metz JG, Dehesh K. Cloning of the fabF gene in an expression vector and in vitro characterization of recombinant fabF and fabB encoded enzymes from Escherichia coli . FEBS Lett. 1997a;402:62–66. doi: 10.1016/s0014-5793(96)01437-8. [DOI] [PubMed] [Google Scholar]
- Edwards P, Nelsen JS, Metz JG, Dehesh K. Cloning of the the fabF gene in an expression vector and in vitro characterization of recombinant fabF and fabB encoded enzymes from Escherichia coli . FEBS Lett. 1997b;402:62–66. doi: 10.1016/s0014-5793(96)01437-8. [DOI] [PubMed] [Google Scholar]
- Finking R, Solsbacher J, Konz D, Schobert M, Schafer A, Jahn D, Marahiel MA. Characterization of a new type of phosphopantetheinyl transferase for fatty acid and siderophore synthesis in Pseudomonas aeruginosa . J. Biol Chem. 2002;277:50293–50302. doi: 10.1074/jbc.M205042200. [DOI] [PubMed] [Google Scholar]
- Fischl AS, Kennedy EP. Isolation and properties of acyl carrier protein phosphodiesterase of Escherichia coli . J. Bacteriol. 1990;172:5445–5449. doi: 10.1128/jb.172.9.5445-5449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florova G, Kazanina G, Reynolds KA. Enzymes involved in fatty acid and polyketide biosynthesis in Streptomyces glaucescens : role of FabH and FabD and their acyl carrier protein specificity. Biochemistry. 2002;41:10462–10471. doi: 10.1021/bi0258804. [DOI] [PubMed] [Google Scholar]
- Flugel RS, Hwangbo Y, Lambalot RH, Cronan JE, Jr, Walsh CT. Holo-(acyl carrier protein) synthase and phosphopantetheinyl transfer in Escherichia coli . J. Biol. Chem. 2000a;275:959–968. doi: 10.1074/jbc.275.2.959. [DOI] [PubMed] [Google Scholar]
- Flugel RS, Hwangbo Y, Lambalot RH, Cronan JE, Jr, Walsh CT. Holo-(acyl carrier protein) synthase and phosphopantetheinyl transfer in Escherichia coli . J. Biol Chem. 2000b;275:959–968. doi: 10.1074/jbc.275.2.959. [DOI] [PubMed] [Google Scholar]
- Garwin JL, Klages AL, Cronan JE., Jr β-ketoacyl-acyl carrier protein synthase II of Escherichia coli. Evidence for function in the thermal regulation of fatty acid synthesis. J. Biol. Chem. 1980a;255:3263–3265. [PubMed] [Google Scholar]
- Garwin JL, Klages AL, Cronan JE., Jr Structural, enzymatic, and genetic studies of β-ketoacyl-acyl carrier protein synthases I and II of Escherichia coli . J. Biol. Chem. 1980b;255:11949–11956. [PubMed] [Google Scholar]
- Gelmann EP, Cronan JE., Jr Mutant of Escherichia coli deficient in the synthesis of cis -vaccenic acid. J Bacteriol. 1972;112:381–387. doi: 10.1128/jb.112.1.381-387.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale RS, Saxena P, Chopra T, Mohanty D. Versatile polyketide enzymatic machinery for the biosynthesis of complex mycobacterial lipids. Nat Prod Rep. 2007;24:267–277. doi: 10.1039/b616817p. [DOI] [PubMed] [Google Scholar]
- Grana D, Gardella T, Susskind MM. The effects of mutations in the ant promoter of phage P22 depend on context. Genetics. 1988;120:319–327. doi: 10.1093/genetics/120.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan DW, Cronan JE., Jr Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev. 1997;61:429–441. doi: 10.1128/mmbr.61.4.429-441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra DJ, Browse JA. Escherichia coli β-hydroxydecanoyl thioester dehydrase reacts with native C10 acyl-acyl-carrier proteins of plant and bacterial origin. Arch. Biochem. Biophys. 1990;280:336–345. doi: 10.1016/0003-9861(90)90339-z. [DOI] [PubMed] [Google Scholar]
- Han L, Lobo S, Reynolds KA. Characterization of β-ketoacyl-acyl carrier protein synthase III from Streptomyces glaucescens and its role in initiation of fatty acid biosynthesis. J Bacteriol. 1998;180:4481–4486. doi: 10.1128/jb.180.17.4481-4486.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RJ, Rock CO. Enoyl-acyl carrier protein reductase ( fabI ) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli . J. Biol. Chem. 1995;270:26538–26542. doi: 10.1074/jbc.270.44.26538. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Rock CO. Roles of the FabA and FabZ β-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J. Biol. Chem. 1996;271:27795–27801. doi: 10.1074/jbc.271.44.27795. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Rock CO. The Claisen condensation in biology. Nat. Prod. Rep. 2002;19:581–596. doi: 10.1039/b110221b. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 1999;274:11110–11114. doi: 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Su N, Murphy CK, Rock CO. The enoyl-[acyl-carrier-protein] reductases FabI and FabL from Bacillus subtilis . J. Biol. Chem. 2000;275:40128–40133. doi: 10.1074/jbc.M005611200. [DOI] [PubMed] [Google Scholar]
- Hitchman TS, Crosby J, Byrom KJ, Cox RJ, Simpson TJ. Catalytic self-acylation of type II polyketide synthase acyl carrier proteins. Chem. Biol. 1998;5:35–47. doi: 10.1016/s1074-5521(98)90085-0. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Sullivan SA, Cusick JK, Schweizer HP. β-ketoacyl acyl carrier protein reductase (FabG) activity of the fatty acid biosynthetic pathway is a determining factor of 3-oxo-homoserine lactone acyl chain lengths. Microbiology. 2002;148:3849–3856. doi: 10.1099/00221287-148-12-3849. [DOI] [PubMed] [Google Scholar]
- Jackowski S, Jackson PD, Rock CO. Sequence and function of the aas gene in Escherichia coli . J. Biol Chem. 1994;269:2921–2928. [PubMed] [Google Scholar]
- Jackowski S, Rock CO. Regulation of coenzyme A biosynthesis. J Bacteriol. 1981;148:926–932. doi: 10.1128/jb.148.3.926-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S, Rock CO. Ratio of active to inactive forms of acyl carrier protein in Escherichia coli . J. Biol. Chem. 1983;258:15186–15191. [PubMed] [Google Scholar]
- Jackowski S, Rock CO. Acetoacetyl-acyl carrier protein synthase, a potential regulator of fatty acid biosynthesis in bacteria. J. Biol. Chem. 1987;262:7927–7931. [PubMed] [Google Scholar]
- Jackowski S, Zhang YM, Price AC, White SW, Rock CO. A missense mutation in the fabB β-ketoacyl-acyl carrier protein synthase I) gene confers thiolactomycin resistance to Escherichia coli . Antimicrob Agents Chemother. 2002;46:1246–1252. doi: 10.1128/AAC.46.5.1246-1252.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jencks WP, Carriuolo J. Imidazole catalysis. III. General base catalysis and the reactions of acetyl imidazole with thiols and amines. J Biol Chem. 1959;234:1280–1285. [PubMed] [Google Scholar]
- Jiang Y, Chan CH, Cronan JE. The soluble acyl-acyl carrier protein synthetase of Vibrio harveyi B392 is a member of the medium chain acyl-CoA synthetase family. Biochemistry. 2006;45:10008–10019. doi: 10.1021/bi060842w. [DOI] [PubMed] [Google Scholar]
- Kauppinen S, Siggaard-Anderson M, van Wettstein-Knowles P. β-Ketoacyl-ACP synthase I of Escherichia coli: nucleotide sequence of the fabB gene and identification of the cerulenin binding residue. Carlsberg Res. Commun. 1988;53:357–370. doi: 10.1007/BF02983311. [DOI] [PubMed] [Google Scholar]
- Keating DH, Carey MR, Cronan JE., Jr The unmodified (apo) form of Escherichia coli acyl carrier protein is a potent inhibitor of cell growth. J. Biol. Chem. 1995;270:22229–22235. doi: 10.1074/jbc.270.38.22229. [DOI] [PubMed] [Google Scholar]
- Khosla C, Ebert-Khosla S, Hopwood DA. Targeted gene replacements in a Streptomyces polyketide synthase gene cluster: role for the acyl carrier protein. Mol Microbiol. 1992;6:3237–3249. doi: 10.1111/j.1365-2958.1992.tb01778.x. [DOI] [PubMed] [Google Scholar]
- Kim Y, Prestegard JH. A dynamic model for the structure of acyl carrier protein in solution. Biochemistry. 1989;28:8792–8797. doi: 10.1021/bi00448a017. [DOI] [PubMed] [Google Scholar]
- Kim Y, Prestegard JH. Refinement of the NMR structures for acyl carrier protein with scalar coupling data. Proteins. 1990;8:377–385. doi: 10.1002/prot.340080411. [DOI] [PubMed] [Google Scholar]
- Kimber MS, Martin F, Lu Y, Houston S, Vedadi M, Dharamsi A, Fiebig KM, Schmid M, Rock CO. The structure of (3R)-hydroxyacyl-acyl carrier protein dehydratase (FabZ) from Pseudomonas aeruginosa . J. Biol. Chem. 2004;279:52593–52602. doi: 10.1074/jbc.M408105200. [DOI] [PubMed] [Google Scholar]
- Kruh NA, Borgaro JG, Ruzsicska BP, Xu H, Tonge PJ. A novel interaction linking the FAS-II and Pdim biosynthetic pathways. J Biol Chem. 2008;283:31719–31725. doi: 10.1074/jbc.M802169200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CY, Cronan JE. Isolation and characterization of β-ketoacyl-acyl carrier protein reductase ( fabG) mutants of Escherichia coli and Salmonella enterica serovar Typhimurium. J. Bacteriol. 2004;186:1869–1878. doi: 10.1128/JB.186.6.1869-1878.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambalot RH, Gehring AM, Flugel RS, Zuber P, LaCelle M, Marahiel MA, Reid R, Khosla C, Walsh CT. A new enzyme superfamily - the phosphopantetheinyl transferases. Chem. Biol. 1996;3:923–936. doi: 10.1016/s1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- Lambalot RH, Walsh CT. Holo-[acyl-carrier-protein] synthase of Escherichia coli . Methods Enzymol. 1997;279:254–262. doi: 10.1016/s0076-6879(97)79029-3. [DOI] [PubMed] [Google Scholar]
- Leesong M, Henderson BS, Gillig JR, Schwab JM, Smith JL. Structure of a dehydratase-isomerase from the bacterial pathway for biosynthesis of unsaturated fatty acids: two catalytic activities in one active site. Structure. 1996;4:253–264. doi: 10.1016/s0969-2126(96)00030-5. [DOI] [PubMed] [Google Scholar]
- Lennarz WJ, Light RJ, Bloch K. A fatty acid synthetase from E. coli . Proc Natl Acad Sci U S A. 1962;48:840–846. doi: 10.1073/pnas.48.5.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Florova G, Reynolds KA. Alteration of the fatty acid profile of Streptomyces coelicolor by replacement of the initiation enzyme 3-ketoacyl acyl carrier protein synthase III (FabH) J Bacteriol. 2005;187:3795–3799. doi: 10.1128/JB.187.11.3795-3799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Vogel C, Wang R, Yao X, Marcotte EM. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat. Biotechnol. 2007;25:117–124. doi: 10.1038/nbt1270. [DOI] [PubMed] [Google Scholar]
- Lu YJ, White SW, Rock CO. Domain swapping between Enterococcus faecalis FabN and FabZ proteins localizes the structural determinants for isomerase activity. J. Biol. Chem. 2005;280:30342–30348. doi: 10.1074/jbc.M504637200. [DOI] [PubMed] [Google Scholar]
- Lu YW, San Roman AK, Gehring AM. Role of phosphopantetheinyl transferase genes in antibiotic production by Streptomyces coelicolor . J. Bacteriol. 2008;190:6903–6908. doi: 10.1128/JB.00865-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson K, Carey MR, Cronan JE., Jr The putative fabJ gene of Escherichia coli fatty acid synthesis is the fabF gene. J. Bacteriol. 1995;177:3593–3595. doi: 10.1128/jb.177.12.3593-3595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson K, Oh W, Larson TJ, Cronan JE., Jr Cloning and nucleotide sequence of the fabD gene encoding malonyl coenzyme A-acyl carrier protein transacylase of Escherichia coli . FEBS Lett. 1992;299:262–266. doi: 10.1016/0014-5793(92)80128-4. [DOI] [PubMed] [Google Scholar]
- Mansilla MC, de Mendoza D. The Bacillus subtilis desaturase: a model to understand phospholipid modification and temperature sensing. Arch Microbiol. 2005;183:229–235. doi: 10.1007/s00203-005-0759-8. [DOI] [PubMed] [Google Scholar]
- Marrakchi H, Choi KH, Rock CO. A new mechanism for anaerobic unsaturated fatty acid formation in Streptococcus pneumoniae . J. Biol. Chem. 2002;277:44809–44816. doi: 10.1074/jbc.M208920200. [DOI] [PubMed] [Google Scholar]
- Marrakchi H, Dewolf WE, Jr, Quinn C, West J, Polizzi BJ, So CY, Holmes DJ, Reed SL, Heath RJ, Payne DJ, Rock CO, Wallis NG. Characterization of Streptococcus pneumoniae enoyl-(acyl-carrier protein) reductase (FabK) Biochem J. 2003;370:1055–1062. doi: 10.1042/BJ20021699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massengo-Tiasse RP, Cronan JE. Vibrio cholerae FabV defines a new class of enoyl-acyl carrier protein reductase. J Biol Chem. 2008;283:1308–1316. doi: 10.1074/jbc.M708171200. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Peery RB, Zhao G. Acyl carrier protein synthases from gram-negative, gram-positive, and atypical bacterial species: Biochemical and structural properties and physiological implications. J. Bacteriol. 2006;188:4737–4748. doi: 10.1128/JB.01917-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire KA, Siggaard-Andersen M, Bangera MG, Olsen JG, von Wettstein-Knowles P. β-Ketoacyl-[acyl carrier protein] synthase I of Escherichia coli: aspects of the condensation mechanism revealed by analyses of mutations in the active site pocket. Biochemistry. 2001;40:9836–9845. doi: 10.1021/bi0105577. [DOI] [PubMed] [Google Scholar]
- McMurry LM, Oethinger M, Levy SB. Triclosan targets lipid synthesis. Nature. 1998;394:531–532. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- Mercer AC, Burkart MD. The ubiquitous carrier protein--a window to metabolite biosynthesis. Nat. Prod. Rep. 2007;24:750–773. doi: 10.1039/b603921a. [DOI] [PubMed] [Google Scholar]
- Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science. 2001;293:290–293. doi: 10.1126/science.1059593. [DOI] [PubMed] [Google Scholar]
- Mizugaki M, Swindell AC, Wakil SJ. Intermediate- and long-chain β-hydroxyacyl-ACP dehydrases from E. coli fatty acid synthetase. Biochem. Biophys. Res. Commun. 1968;33:520–527. doi: 10.1016/0006-291x(68)90607-4. [DOI] [PubMed] [Google Scholar]
- Mofid MR, Finking R, Marahiel MA. Recognition of hybrid peptidyl carrier proteins/acyl carrier proteins in nonribosomal peptide synthetase modules by the 4'-phosphopantetheinyl transferases AcpS and Sfp. J Biol Chem. 2002;277:17023–17031. doi: 10.1074/jbc.M200120200. [DOI] [PubMed] [Google Scholar]
- Mohan S, Kelly TM, Eveland SS, Raetz CR, Anderson MS. An Escherichia coli gene (fabZ) encoding (3R)-hydroxymyristoyl acyl carrier protein dehydrase. Relation to fabA and suppression of mutations in lipid A biosynthesis. J. Biol. Chem. 1994;269:32896–32903. [PubMed] [Google Scholar]
- Mootz HD, Finking R, Marahiel MA. 4'-Phosphopantetheine transfer in primary and secondary metabolism of Bacillus subtilis . J. Biol Chem. 2001;276:37289–37298. doi: 10.1074/jbc.M103556200. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Yatome C, Ishida N, Kitade Y. Putative ACP phosphodiesterase gene (acpD) encodes an azoreductase. J. Biol. Chem. 2001;276:46394–46399. doi: 10.1074/jbc.M104483200. [DOI] [PubMed] [Google Scholar]
- Oefner C, Schulz H, D'Arcy A, Dale GE. Mapping the active site of Escherichia coli malonyl-CoA-acyl carrier protein transacylase (FabD) by protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 2006;62:613–618. doi: 10.1107/S0907444906009474. [DOI] [PubMed] [Google Scholar]
- Phillips GJ, Park SK, Huber D. High copy number plasmids compatible with commonly used cloning vectors. Biotechniques. 2000;28:400–402. 404. doi: 10.2144/00283bm02. 406 passim. [DOI] [PubMed] [Google Scholar]
- Post-Beittenmiller D, Jaworski JG, Ohlrogge JB. In vivo pools of free and acylated acyl carrier proteins in spinach. Evidence for sites of regulation of fatty acid biosynthesis. J Biol Chem. 1991;266:1858–1865. [PubMed] [Google Scholar]
- Prescott DJ, Vagelos PR. Acyl carrier protein. Adv. Enzymol. Relat. Areas Mol. Biol. 1972;36:269–311. doi: 10.1002/9780470122815.ch8. [DOI] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings M. Isolation and characterization of a gene and a genomic clone encoding acyl carrier protein from Escherichia coli. Urbana-Champaign: University of Illinois; 1993. [Google Scholar]
- Rawlings M, Cronan JE., Jr The gene encoding Escherichia coli acyl carrier protein lies within a cluster of fatty acid biosynthetic genes. J. Biol. Chem. 1992;267:5751–5754. [PubMed] [Google Scholar]
- Ray TK, Cronan JE., Jr Activation of long chain fatty acids with acyl carrier protein: demonstration of a new enzyme, acyl-acyl carrier protein synthetase, in Escherichia coli. Proc Natl Acad Sci U S A. 1976;73:4374–4378. doi: 10.1073/pnas.73.12.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill WP, Bibb MJ, Hopwood DA. Purification of a malonyltransferase from Streptomyces coelicolor A3(2) and analysis of its genetic determinant. J Bacteriol. 1995;177:3946–3952. doi: 10.1128/jb.177.14.3946-3952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill WP, Bibb MJ, Hopwood DA. Relationships between fatty acid and polyketide synthases from Streptomyces coelicolor A3(2): characterization of the fatty acid synthase acyl carrier protein. J Bacteriol. 1996;178:5660–5667. doi: 10.1128/jb.178.19.5660-5667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CO, Cronan JE., Jr Re-evaluation of the solution structure of acyl carrier protein. J. Biol. Chem. 1979;254:9778–9785. [PubMed] [Google Scholar]
- Rock CO, Cronan JE., Jr Improved purification of acyl carrier protein. Anal Biochem. 1980;102:362–364. doi: 10.1016/0003-2697(80)90168-2. [DOI] [PubMed] [Google Scholar]
- Rock CO, Cronan JE., Jr Acyl carrier protein from Escherichia coli . Methods Enzymol. 1981;71:341–351. doi: 10.1016/0076-6879(81)71043-7. [DOI] [PubMed] [Google Scholar]
- Rock CO, Cronan JE, Jr, Armitage IM. Molecular properties of acyl carrier protein derivatives. J Biol Chem. 1981a;256:2669–2674. [PubMed] [Google Scholar]
- Rock CO, Garwin JL, Cronan JE., Jr Preparative enzymatic synthesis of acyl-acyl carrier protein. Methods Enzymol. 1981b;72:397–403. doi: 10.1016/s0076-6879(81)72029-9. [DOI] [PubMed] [Google Scholar]
- Roujeinikova A, Baldock C, Simon WJ, Gilroy J, Baker PJ, Stuitje AR, Rice DW, Rafferty JB, Slabas AR. Crystallization and preliminary X-ray crystallographic studies on acyl-(acyl carrier protein) from Escherichia coli. Acta Crystallogr D Biol Crystallogr. 2002a;58:330–332. doi: 10.1107/s0907444901020091. [DOI] [PubMed] [Google Scholar]
- Roujeinikova A, Baldock C, Simon WJ, Gilroy J, Baker PJ, Stuitje AR, Rice DW, Slabas AR, Rafferty JB. X-ray crystallographic studies on butyryl-ACP reveal flexibility of the structure around a putative acyl chain binding site. Structure. 2002b;10:825–835. doi: 10.1016/s0969-2126(02)00775-x. [DOI] [PubMed] [Google Scholar]
- Roujeinikova A, Simon WJ, Gilroy J, Rice DW, Rafferty JB, Slabas AR. Structural studies of fatty acyl-(acyl carrier protein) thioesters reveal a hydrophobic binding cavity that can expand to fit longer substrates. J. Mol. Biol. 2007;365:135–145. doi: 10.1016/j.jmb.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Sauer F, Pugh EL, Wakil SJ, Delaney R, Hill RL. 2-Mercaptoethylamine and β-alanine as components of acyl carrier protein. Proc Natl Acad Sci U S A. 1964;52:1360–1366. doi: 10.1073/pnas.52.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J, Henderson J. Enzyme-catalyzed allylic rearrangements. Chem. Rev. 1990;90:1203–1245. [Google Scholar]
- Schwab JM, Klassen JB, Lin DC. β-Hydroxydecanoylthioester dehydrase: a rapid, convenient, and accurate product distribution assay. Anal Biochem. 1985;150:121–124. doi: 10.1016/0003-2697(85)90449-x. [DOI] [PubMed] [Google Scholar]
- Schweizer E, Hofmann J. Microbial type I fatty acid synthases (FAS): major players in a network of cellular FAS systems. Microbiol Mol Biol Rev. 2004;68:501–517. doi: 10.1128/MMBR.68.3.501-517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre L, Verbree EC, Dauter Z, Stuitje AR, Derewenda ZS. The Escherichia coli malonyl-CoA:acyl carrier protein transacylase at 1.5-A resolution. Crystal structure of a fatty acid synthase component. J. Biol. Chem. 1995;270:12961–12964. doi: 10.1074/jbc.270.22.12961. [DOI] [PubMed] [Google Scholar]
- Shanklin J. Overexpression and purification of the Escherichia coli inner membrane enzyme acyl-acyl carrier protein synthase in an active form. Protein Expr Purif. 2000;18:355–360. doi: 10.1006/prep.2000.1206. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Modak R, Sharma S, Sharma AK, Sarma SP, Surolia A, Surolia N. A novel approach for over-expression, characterization, and isotopic enrichment of a homogeneous species of acyl carrier protein from Plasmodium falciparum . Biochem Biophys Res Commun. 2005;330:1019–1026. doi: 10.1016/j.bbrc.2005.03.094. [DOI] [PubMed] [Google Scholar]
- Shen B, Summers RG, Gramajo H, Bibb MJ, Hutchinson CR. Purification and characterization of the acyl carrier protein of the Streptomyces glaucescens tetracenomycin C polyketide synthase. J Bacteriol. 1992a;174:3818–3821. doi: 10.1128/jb.174.11.3818-3821.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Fice D, Byers DM. Preparation of fatty-acylated derivatives of acyl carrier protein using Vibrio harveyi acyl-ACP synthetase. Anal Biochem. 1992b;204:343–349. doi: 10.1016/0003-2697(92)90135-t. [DOI] [PubMed] [Google Scholar]
- Siggaard-Andersen M, Wissenbach M, Chuck JA, Svendsen I, Olsen JG, von Wettstein-Knowles P. The fabJ-encoded β-ketoacyl-[acyl carrier protein] synthase IV from Escherichia coli is sensitive to cerulenin and specific for short-chain substrates. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11027–11031. doi: 10.1073/pnas.91.23.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert DF, Vagelos PR. Fatty acid mutant of E. coli lacking a β-hydroxydecanoyl thioester dehydrase. Proc. Natl. Acad. Sci. U.S.A. 1967;58:1579–1586. doi: 10.1073/pnas.58.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova N, Reynolds KA. Engineered fatty acid biosynthesis in Streptomyces by altered catalytic function of beta-ketoacyl-acyl carrier protein synthase III. J Bacteriol. 2001;183:2335–2342. doi: 10.1128/JB.183.7.2335-2342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Sherman DH. Biochemistry. An enzyme assembly line. Science. 2008;321:1304–1305. doi: 10.1126/science.1163785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Brown SD, Yocum RR, Ronson CW. The bio operon on the acquired symbiosis island of Mesorhizobium sp. strain R7A includes a novel gene involved in pimeloyl-CoA synthesis. Microbiology. 2001;147:1315–1322. doi: 10.1099/00221287-147-5-1315. [DOI] [PubMed] [Google Scholar]
- Summers RG, Ali A, Shen B, Wessel WA, Hutchinson CR. Malonyl-coenzyme A:acyl carrier protein acyltransferase of Streptomyces glaucescens: a possible link between fatty acid and polyketide biosynthesis. Biochemistry. 1995;34:9389–9402. doi: 10.1021/bi00029a015. [DOI] [PubMed] [Google Scholar]
- Tauch A, Kaiser O, Hain T, Goesmann A, Weisshaar B, Albersmeier A, Bekel T, Bischoff N, Brune I, Chakraborty T, Kalinowski J, Meyer F, Rupp O, Schneiker S, Viehoever P, Puhler A. Complete genome sequence and analysis of the multiresistant nosocomial pathogen Corynebacterium jeikeium K411, a lipid-requiring bacterium of the human skin flora. J Bacteriol. 2005;187:4671–4682. doi: 10.1128/JB.187.13.4671-4682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Cronan JE. The enigmatic acyl carrier protein phosphodiesterase of Escherichia coli: genetic and enzymological characterization. J. Biol. Chem. 2005;280:34675–34683. doi: 10.1074/jbc.M505736200. [DOI] [PubMed] [Google Scholar]
- Thomas J, Rigden DJ, Cronan JE. Acyl carrier protein phosphodiesterase (AcpH) of Escherichia coli is a non-canonical member of the HD phosphatase/phosphodiesterase family. Biochemistry. 2007;46:129–136. doi: 10.1021/bi061789e. [DOI] [PubMed] [Google Scholar]
- Tsay JT, Oh W, Larson TJ, Jackowski S, Rock CO. Isolation and characterization of the β-ketoacyl-acyl carrier protein synthase III gene (fabH) from Escherichia coli K-12. J. Biol. Chem. 1992a;267:6807–6814. [PubMed] [Google Scholar]
- Tsay JT, Rock CO, Jackowski S. Overproduction of β-ketoacyl-acyl carrier protein synthase I imparts thiolactomycin resistance to Escherichia coli K-12. J. Bacteriol. 1992b;174:508–513. doi: 10.1128/jb.174.2.508-513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnowsky F, Fuchs K, Jeschek C, Hogenauer G. envM genes of Salmonella typhimurium and Escherichia coli . J. Bacteriol. 1989;171:6555–6565. doi: 10.1128/jb.171.12.6555-6565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich AK, de Mendoza D, Garwin JL, Cronan JE., Jr Genetic and biochemical analyses of Escherichia coli mutants altered in the temperature-dependent regulation of membrane lipid composition. J. Bacteriol. 1983;154:221–230. doi: 10.1128/jb.154.1.221-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagelos PR, Larrabee AR. Acyl carrier protein. IX. Acyl carrier protein hydrolase. J. Biol. Chem. 1967;242:1776–1781. [PubMed] [Google Scholar]
- Verwoert II, Verbree EC, van der Linden KH, Nijkamp HJ, Stuitje AR. Cloning, nucleotide sequence, and expression of the E scherichia coli fabD gene, encoding malonyl coenzyme A-acyl carrier protein transacylase. J. Bacteriol. 1992;174:2851–2857. doi: 10.1128/jb.174.9.2851-2857.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KK, Zhao B, McArthur HA, Reynolds KA. In vivo analysis of straight-chain and branched-chain fatty acid biosynthesis in three actinomycetes. FEMS Microbiol Lett. 1995;131:227–234. doi: 10.1111/j.1574-6968.1995.tb07781.x. [DOI] [PubMed] [Google Scholar]
- Wallis JG, Watts JL, Browse J. Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem Sci. 2002;27:467. doi: 10.1016/s0968-0004(02)02168-0. [DOI] [PubMed] [Google Scholar]
- Walsh CT, Gehring AM, Weinreb PH, Quadri LE, Flugel RS. Post-translational modification of polyketide and nonribosomal peptide synthases. Curr Opin Chem Biol. 1997;1:309–315. doi: 10.1016/s1367-5931(97)80067-1. [DOI] [PubMed] [Google Scholar]
- Wang H, Cronan JE. Functional replacement of the FabA and FabB proteins of Escherichia coli fatty acid synthesis by Enterococcus faecalis FabZ and FabF homologues. J. Biol. Chem. 2004;279:34489–34495. doi: 10.1074/jbc.M403874200. [DOI] [PubMed] [Google Scholar]
- Weeks G, Wakil SJ. Studies on the mechanism of fatty acid synthesis. 18. Preparation and general properties of the enoyl acyl carrier protein reductases from Escherichia coli . J. Biol. Chem. 1968;243:1180–1189. [PubMed] [Google Scholar]
- White SW, Zheng J, Zhang YM, Rock The structural biology of type II fatty acid biosynthesis. Annu. Rev. Biochem. 2005;74:791–831. doi: 10.1146/annurev.biochem.74.082803.133524. [DOI] [PubMed] [Google Scholar]
- Worthington AS, Hur GH, Meier JL, Cheng Q, Moore BS, Burkart MD. Probing the compatibility of type II ketosynthase-carrier protein partners. Chembiochem. 2008;9:2096–2103. doi: 10.1002/cbic.200800198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TW, Hopwood DA. Ectopic expression of the Streptomyces coelicolor whiE genes for polyketide spore pigment synthesis and their interaction with the act genes for actinorhodin biosynthesis. Microbiology. 1995;141:2779–2791. doi: 10.1099/13500872-141-11-2779. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cronan JE., Jr Polar allele duplication for transcriptional analysis of consecutive essential genes: application to a cluster of Escherichia coli fatty acid biosynthetic genes. J. Bacteriol. 1996;178:3614–3620. doi: 10.1128/jb.178.12.3614-3620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cronan JE., Jr Transcriptional analysis of essential genes of the Escherichia coli fatty acid biosynthesis gene cluster by functional replacement with the analogous S almonella typhimurium gene cluster. J. Bacteriol. 1998;180:3295–3303. doi: 10.1128/jb.180.13.3295-3303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Frank MW, Zhu K, Mayasundari A, Rock CO. PqsD is responsible for the synthesis of DHQ, an extracellular metabolite produced by Pseudomonas aeruginosa . J. Biol Chem. 2008;283:28788–28794. doi: 10.1074/jbc.M804555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CJ, Sohn MJ, Kim WG. Atromentin and leucomelone, the first inhibitors specific to enoyl-ACP reductase (FabK) of Streptococcus pneumoniae . J. Antibiot.(Tokyo) 2006;59:808–812. doi: 10.1038/ja.2006.108. [DOI] [PubMed] [Google Scholar]
- Zheng CJ, Sohn MJ, Lee S, Hong YS, Kwak JH, Kim WG. Cephalochromin, a FabI-directed antibacterial of microbial origin. Biochem. Biophys. Res. Commun. 2007;362:1107–1112. doi: 10.1016/j.bbrc.2007.08.144. [DOI] [PubMed] [Google Scholar]
- Zhu K, Choi KH, Schweizer HP, Rock CO, Zhang YM. Two aerobic pathways for the formation of unsaturated fatty acids in Pseudomonas aeruginosa . Mol Microbiol. 2006;60:260–273. doi: 10.1111/j.1365-2958.2006.05088.x. [DOI] [PubMed] [Google Scholar]