Abstract
Low levels of the molecular inotrope S100A1 are sufficient to rescue post-ischemic heart failure (HF). As a prerequisite to clinical application and to determine the safety of myocardial S100A1 DNA-based therapy, we investigated the effects of high myocardial S100A1 expression levels on the cardiac contractile function and occurrence of arrhythmia in a preclinical large animal HF model. At 2 weeks after myocardial infarction domestic pigs presented significant left ventricular (LV) contractile dysfunction. Retrograde application of AAV6-S100A1 (1.5 × 1013 tvp) via the anterior cardiac vein (ACV) resulted in high-level myocardial S100A1 protein peak expression of up to 95-fold above control. At 14 weeks, pigs with high-level myocardial S100A1 protein overexpression did not show abnormalities in the electrocardiogram. Electrophysiological right ventricular stimulation ruled out an increased susceptibility to monomorphic ventricular arrhythmia. High-level S100A1 protein overexpression in the LV myocardium resulted in a significant increase in LV ejection fraction (LVEF), albeit to a lesser extent than previously reported with low S100A1 protein overexpression. Cardiac remodeling was, however, equally reversed. High myocardial S100A1 protein overexpression neither increases the occurrence of cardiac arrhythmia nor causes detrimental effects on myocardial contractile function in vivo. In contrast, this study demonstrates a broad therapeutic range of S100A1 gene therapy in post-ischemic HF using a preclinical large animal model.
Keywords: heart failure, DNA-based therapy, S100A1, AAV, therapeutic window, translational science
Introduction
Despite advances in clinical therapy, heart failure (HF) is not curable as available treatments only alleviate symptoms and cannot reverse underlying causes of the disease.1–4 Molecular abnormalities in cardiomyocyte calcium (Ca2+) handling and beta-adrenergic receptor signal transduction have been identified as the main key factors of HF pathogenesis and transition to failure and death.5–7 Cardiomyocyte-targeted correction of distinct intracellular molecular defects by viral-based therapeutic DNA delivery offers the opportunity to address these pathways in the diseased myocardium and reverse the HF phenotype in clinically relevant large animal models.8–11
The Ca2+ sensor protein S100A1 has emerged as an attractive target for genetically targeted HF therapy in various in vivo HF models because of its molecular profile.12 The S100A1 protein regulates a network in cardiomyocytes that controls sarcoplasmic reticulum Ca2+ cycling and mitochondrial function through interaction with the ryanodine receptor, sarcoplasmic reticulum Ca2+-ATPase (SERCA2) and mitochondrial F1-ATPase activity, causing antihypertrophic, positive inotrope and antiarrhythmic effects and reducing energy depletion in HF.13–19 Importantly, the S100A1 protein is significantly downregulated in human endstage HF, rendering S100A1 an appropriate target for cardiac gene therapy.20,21
The first-ever clinical HF gene therapy phase I/II (CUPID) trial addressing abnormal intracellular Ca2+ handling by overexpressing SERCA2a in HF patients was recently initiated, whereas a second phase I/II HF gene therapy trial using adenylyl cyclase VI currently seeks the Food and Drug Administration investigational drug status.22,23 However, there is still legitimate concern about potential cardiac adverse effects of myocardial gene therapy. First, adverse cardiac effects due to modulation of intracellular Ca2+ cycling and beta-adrenergic signal transduction have been already demonstrated in transgene animal models in terms of ventricular arrhythmia, deterioration of cardiac function and increased mortality.24–28 Second, myocardial gene delivery appeared largely inhomogenous in various rodent and preclinical animal models, potentially increasing susceptibility to malignant ventricular arrhythmia and limiting therapeutic effects.6,29,30 Third, high-level overexpression of a gene product might even impair cardiac contractile function as target protein expression levels might be beyond the therapeutic window and beneficial effects might be dose dependent. As therapeutic effects of S100A1 in HF are partly mediated by increased SERCA2a activity, it is important to mention that Mercadier's group showed that SERCA2a-mediated delay of HF after myocardial infarction is at a cost of increased acute arrhythmia.31 Thus, as a prerequisite to clinical application, a careful analysis of cardiac adverse effects especially at high vector doses is necessary prior to clinical translation of myocardial S100A1 gene therapy trials. In the present study the AAV6-S100A1 construct was used to achieve high-level myocardial S100A1 protein overexpression in order to investigate the therapeutic window and safety profile of cardiac S100A1 gene therapy in HF.
Investigation of adverse cardiac effects as well as the therapeutic window of myocardial S100A1 gene therapy needs to be accomplished in a preclinical large animal model closely approximating human physiology, function and anatomy.10,32 As HF is mainly caused by ischemic cardiomyopathy, we used a preclinical post-myocardial infarction pig model enabling investigation of cardiac arrhythmia and contractile function, as sarcomeric proteins, heart rate and, most importantly, ratio of SERCA2a/NCX activity are closer to humans as compared with rodent models.33,34 Overall, this study provides a profound and essential preclinical safety analysis of high-level myocardial S100A1 protein overexpression on cardiac contractile function and susceptibility to malignant arrhythmia using a preclinical model of post-ischemic HF.
Results
Model of porcine post-ischemic HF
By taking advantage of a model of percutaneous catheter-based intermittent balloon occlusion of the proximal left circumflex coronary artery, lateral left ventricular (LV) transmural myocardial infarction was achieved, as shown by triphenyltetrazolium chloride staining (Figures 1a and b). Reproducibility of the myocardial area at risk during occlusion of the left circumflex coronary artery has been demonstrated by myocardial perfusion echocardiography.10 At 2 weeks after myocardial infarction, post-myocardial infarction (MI) pigs (n=30) demonstrated systolic LV dysfunction with significantly reduced LV ejection fraction (LVEF) and enlargement of the LV (Figures 1c–e) as compared with sham pigs (n=11). Post-MI pigs (n=30) were randomized to saline (n=7), control virus (AAV6-luc, n=11) and AAV6-S100A1 treatment (n=12) 2 weeks post MI. A time course of the study protocol is given in Figure 1f.
Figure 1.
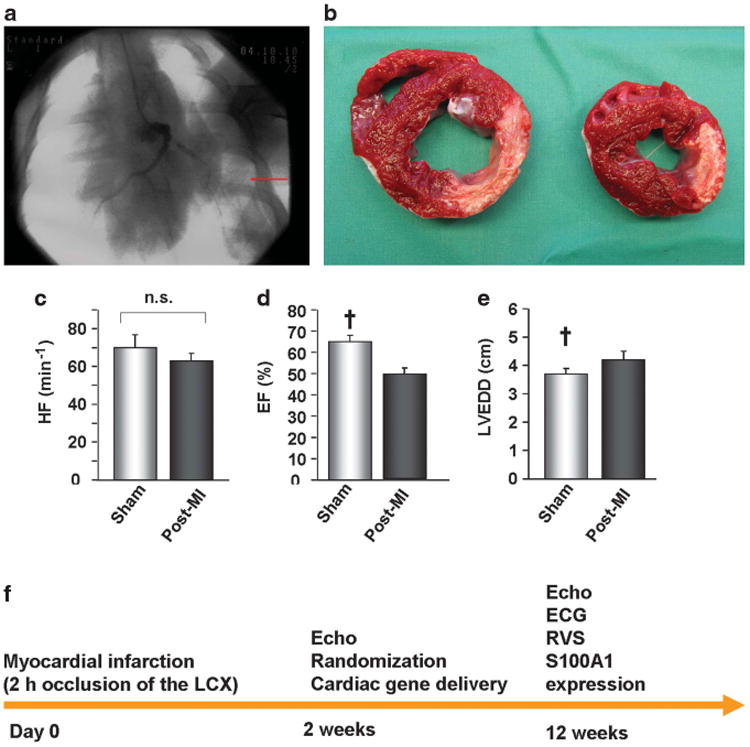
Preclinical model of pig post-ischemic heart failure. (a) Radioscopic image showing contrast dye in the left anterior descending (LAD) coronary artery as well as (arrow) catheter-based left circumflex coronary artery occlusion. (b) Representative triphenyltetrazolium chloride staining of a mid-ventricular section and a section below demonstrating scar formation 14 weeks after MI. (c–e) Echocardiography revealed significantly decreased left LVEF as well as significant LV dilation (EDD, end-diastolic diameter) 2 weeks after MI (n=30) as compared with sham-operated pigs (n=11), whereas heart rate (HR) remained unaltered. (f) Study protocol and time flow. RVS; right ventricular stimulation. ECG; electrocardiogram. †P<0.05 vs post-MI. Data are presented as mean±s.e.m.
High-level myocardial S100A1 overexpression after AAV6-S100A1 gene transfer
High-level S100A1 myocardial overexpression after AAV6-S100A1 gene delivery (1.5 × 1013 tvp) was compared with low-level S100A1 control samples obtained from a previous study using AAV9-S100A1 (1.5 × 1013 tvp)-treated pigs and the same pig in the post-ischemic HF model.10 Because of retrograde AAV-mediated S100A1 gene delivery via the anterior cardiac vein (ACV) into failing pig hearts, myocardial S100A1 overexpression was localized in the anterior and septal remote myocardium (Figures 2a–d). S100A1 overexpression was pronounced close to the ACV running in parallel to the LAD irrespective of whether AAV6 or AAV9 was used as the vector (Figures 2c and d). No significant S100A1 overexpression occurred in the posterior segments of the heart (Figures 2c and d). Distinct differences were observed in the magnitude of S100A1 expression between AAV6-S100A1- and AAV9-S100A1-treated failing pig hearts. Although the same dosage of AAV-S100A1 vectors was applied (1.5 × 1013 tvp per animal; ∼5×1011 tvp per kg) and the same cardiomyocyte-specific promoter (CMV-MLC0.26) was used to express the human S100A1 cDNA, average LV S100A1 overexpression was significantly increased following AAV6-S100A1 gene delivery as compared with AAV9-S100A1 (22.9 ± 3.2-fold vs 3.2 ± 0.3-fold of appropriate AAV-luciferase control; P<0.01) (Figures 2c and d). Moreover, AAV6-S100A1-mediated myocardial S100A1 protein overexpression showed various regions with 50-fold and higher (up to 95-fold) S100A1 overexpression (Figures 2c and d). Of note, these ‘hot spots’ were frequently located close to an area with missing S100A1 or modest S100A1 overexpression in AAV6-S100A1-treated hearts (Figures 2c and d). In contrast, AAV9-S100A1 resulted in an up to 5.4-fold myocardial S100A1 overexpression, and thus the gradient of S100A1 expression between neighboring myocardial areas was mitigated (Figures 2c and d). Western blot analysis revealed that the level of chronic myocardial S100A1 overexpression does not affect the expression of distinct proteins involved in intracellular Ca2+ cycling, such as SERCA2a, ryanodine receptor and phospholamban (PLN), as well as Ser16-PLN, Thr17-PLN and Ser2808-RyR phosporylation sites (Figure 2e).
Figure 2.
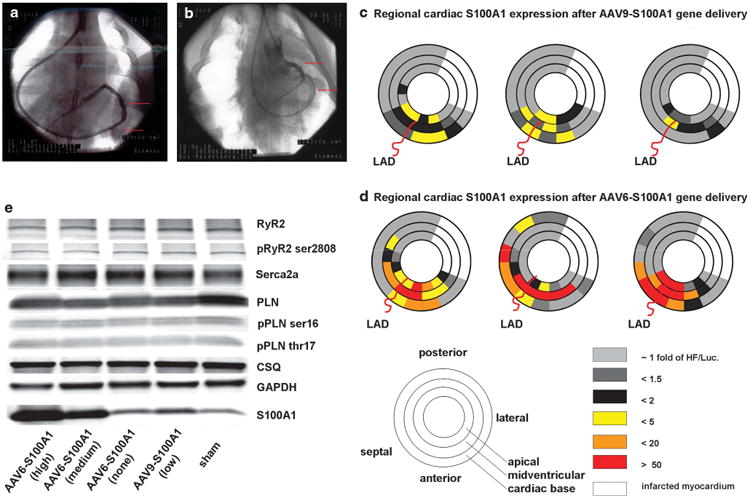
Cardiac AAV/S100A1 gene therapy. (a) Radioscopic image showing the retroperfusion catheter (lower arrow) inserted into the coronary sinus as well as contrast dye in the ACV (upper arrow) for retrograde delivery of the AAV vectors. (b) Retroperfusion catheter inserted into the ACV (lower arrow) and contrast dye within the LAD (upper arrow), which runs in parallel to the ACV. (c and d) Myocardial S100A1 protein expression pattern of three representative pigs 12 weeks after retrograde delivery of (c) 1.5 × 1013 tvp of AAV9-S100A1 and (d) 1.5 × 1013 tvp of AAV6-S100A1 via the ACV using western blot analysis in 36 segments of the heart. S100A1 overexpression was high and locally excessive in AAV6-S100A1-treated myocardium as compared with AAV9-S100A1. (e) Representative western blot analysis showing S100A1 protein expression in the myocardium 12 weeks after AAV6-S100A1 gene delivery. S100A1 protein expression is significantly increased in anterior (high-level; 48±7.1-fold) and anteroseptal (medium level; 12 ± 2.4-fold) myocardium as compared with AAV6-luciferase (average S100A1 overexpression: 22.9 ± 3.2-fold; P< 0.001; n=36 regions of 12 animals each), whereas S100A1 protein overexpression could not be observed in the posterior (none) myocardium. Low-level S100A1 overexpression was found in AAV9-S100A1-treated anterior myocardium (3.2 ± 0.3-fold vs AAV9-luciferase; n=36 regions of 6 animals each). Myocardial S100A1 overexpression does not alter the expression and phosporylation state of representative proteins involved in intracellular Ca2+ cycling (n=5).
High-level myocardial S100A1 overexpression does not abet the occurrence of monomorphic ventricular tachyarrhythmia
Using a clinically relevant protocol of right ventricular stimulation in pigs 14 weeks after myocardial infaction (12 weeks after cardiac gene delivery), we found that the percentage of pigs with inducible monomorphic ventricular tachyarrhythmia (MVT) was not statistically different between AAV6-luc, AAV6-S100A1 and saline groups (Figure 3a). Despite locally extreme myocardial S100A1 expression, there was a nonsignificant trend toward an increased inducibility of MVT in saline pigs 14 weeks post MI (Figure 3a; Supplementary Table 3). Moreover, high-level myocardial S100A1 expression did not abet the occurrence of right ventricular stimulation-induced MVT (Figure 3b). As expected, MVT could not be induced in non-infarcted saline control pigs (Figure 3a). Importantly, extreme myocardial S100A1 expression did not cause significant alterations in heart rate, PQ interval, QRS interval and corrected QT interval (QTc) (Table 1) as compared with failing and non-failing control groups.
Figure 3.
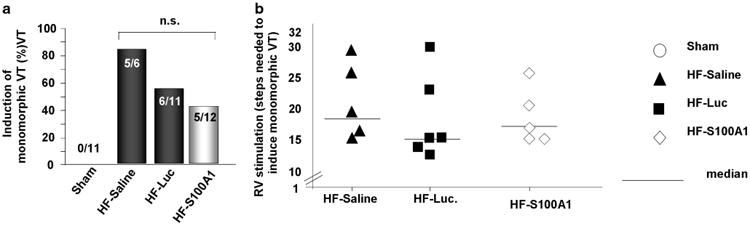
High-Level AAV6/S100A1 gene therapy does not abet the inducibility of monomorphic ventricular tachyarrhythmia. (a) Absolute numbers of pigs with inducible MVT 12 weeks after gene therapy (14 weeks after myocardial infarction). Neither AAV6/S100A1 nor AAV6/luciferase increased the occurrence of MVTs, whereas MVTs were not inducible in non-infarcted sham-operated pigs. (b) Of note, high-level cardiac AAV6/S100A1 gene therapy does not abet right ventricular stimulation-induced occurrence of MVTs.
Table 1.
Excessive and inhomogenous myocardial S100A1 expression does not impact characteristics of the electrocardiogram at rest 12 weeks after cardiac AAV6-S100A1 gene delivery (n=12) as compared with control (n=11 for sham; n=11 for HF/luc and n=6 for HF/saline)
| Sham | HF-Saline | HF-Luc. | HF-S100A1 | |
|---|---|---|---|---|
| Electrocardiogram at rest | ||||
| HR min −1 | 64±3 | 70±4 | 67±3 | 66±3 |
| PQ interval | 121±3 | 119±5 | 110±3 | 125±5 |
| QRS interval | 91±2 | 92±3 | 89±2 | 94±2 |
| QTc interval | 507±10 | 515±8 | 494±8 | 488±8 |
Data are presented as mean±s.e.m.
High-level myocardial S100A1 overexpression increases cardiac function and attenuates LV remodeling in failing pig myocardium in vivo
Despite the patchy nature and local extreme myocardial S100A1 expression after AAV6-S100A1 gene delivery, LVEF in failing pig hearts was significantly improved as compared with HF control groups (LVEF: Sham: 67±2%, HF-Saline: 45±4%, HF-AAV6-Luc: 44±2%, HF-AAV6-S100A1: 55±3%), whereas HR remained unchanged (Figures 4a and b). Plus dP/dt, as a further marker of LV contractile function, showed a strong trend toward increased values as compared with HF control groups (Figure 4c). In line with the improvement in global cardiac function, enlargement of the end-diastolic LV diameter (LVEDD: Sham: 4.9±0.1 cm, HF-Saline: 5.3±0.2 cm, HF-AAV6-Luc: 5.4±0.3 cm, HF-AAV6-S100A1: 5.0±0.2 cm), and thus cardiac remodeling, was significantly attenuated because of high-level AAV6-S100A1 gene therapy as compared with HF control groups (Figure 4d). Markers of chronic cardiac failure, such as BNP and NCX expression, were significantly reduced in AAV6-S100A1-treated failing pig hearts compared with HF control groups (BNP expression (fold of Sham): Sham: 1.0±0.3-fold, HF-Saline: 12.3±2-fold, HF-AAV6-Luc: 8.4±1.8-fold, HF-AAV6-S100A1: 2.6±1.5-fold) (Figure 4e and Supplementary Figure 6, online only supplement). Of note, high-level AAV6-S100A1 overexpression showed a nonsignificant trend toward increased mitochondrial F1-ATP synthase activity, whereas low-level AAV9-S100A1 overexpression significantly increased F1-ATP synthase activity as compared with the control (Figure 4f).
Figure 4.
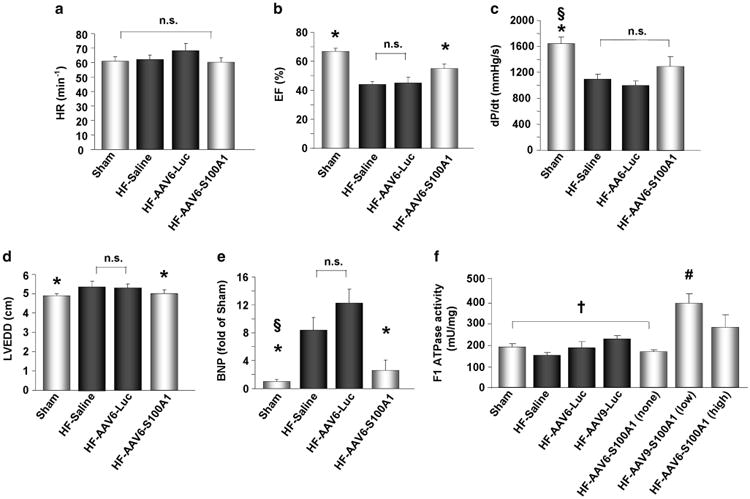
AAV6/S100A1 gene therapy increases global cardiac function and attenuates myocardial remodeling in the failing myocardium. (a–e) Twelve weeks after myocardial infarction, contractile function of post-ischemic failing hearts was significantly reduced as shown by a significant reduction in + dP/dt and LVEF, whereas HR remained unchanged as compared with sham-operated controls (n=11). AAV6/S100A1 treatment (n=12) significantly increased LVEF as compared with HF/AAV6-luciferase (n=11) and HF/saline (n=6) controls. Plus dP/dt showed a nonsignificant trend toward increased values in AAV6/S100A1-treated failing hearts. (d and e) Myocardial remodeling of failing pig hearts was significantly attenuated 10 weeks after AAV6/S100A1 treatment as LV end-diastolic diameter (LVEDD) and expression of the brain natriuretic peptide (BNP) were significantly reduced as compared with HF/saline and HF/AAV6-luciferase controls. (f) F1-ATPase activity was significantly increased in low-level AAV9/S100A1 myocardium and showed a nonsignificant trend in high-level AAV6/S100A1 samples as compared with appropriate controls (n=5). *P<0.05 vs AAV6-luciferase and HF/saline. §P<0.05 vs AAV6-S100A1. #P<0.05 vs †. Data are presented as mean±s.e.m.
Level of S100A1 expression determines intracellular Ca2+ cycling in neonatal cardiomyocytes
Low (4-fold) and moderate (10-fold) S100A1 overexpression significantly increases intracellular Ca2+ transients in isolated neonatal rat cardiomyocytes (NRCM), whereas this effect was abrogated under extreme (45-fold) S100A1 protein overexpression (Supplementary Figure 7, online-only supplement). Of note, increase in intracellular Ca2+ cycling was pronounced at low S100A1 overexpression.
AAV6-S100A1 biodistribution and safety profile after high-level myocardial S100A1 overexpression
As myocardial S100A1 expression was locally extreme after AAV6-S100A1 gene delivery, we were interested in investigating a potential ‘spill-over’ of the AAV6-S100A1 vector to non-cardiac organs. As S100A1 was expressed in a cardio-selective manner employing the CMV-MLC2 promoter, we analyzed S100A1 cDNA in genomic DNA. Human S100A1 cDNA in isolated genomic DNA was detectable by PCR in liver, skeletal muscle and cardiac tissue, demonstrating AAV6 infection in these organs (Figure 5). However, the majority of AAVs were still delivered to the myocardium as human S100A1 cDNA was 17±8-fold higher as compared with the liver (206±66-fold for skeletal muscle; n=5 pigs). Human S100A1 cDNA in isolated genomic DNA was not detectable in lung and brain tissue (Figure 5).
Figure 5.
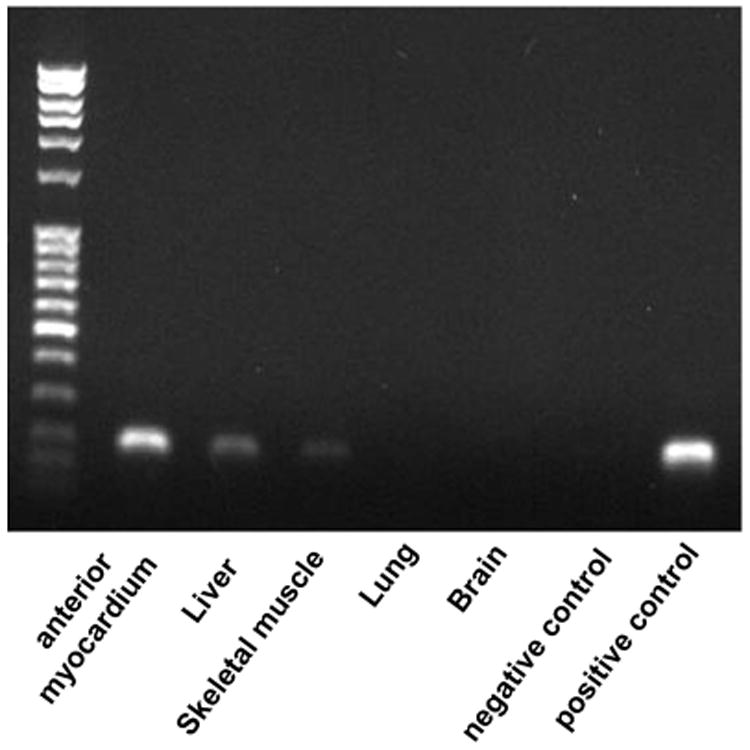
AAV biodistribution. Human S100A1 DNA delivered by AAVs was detectable using PCR in isolated genomic DNA from liver, skeletal muscle and cardiac tissue, demonstrating AAV infection of these organs. However, the majority of AAVs were delivered to the myocardium as human S100A1 DNA isolated from genomic DNA was 206±66-fold higher in cardiac tissue (anterior wall) as compared with skeletal muscle and 17±8-fold higher compared with the liver (n=5 pigs). Human S100A1 DNA was not detectable in lung and brain tissue. Representative gel analysis of human S100A1 PCR products (PCR run at holded at cycle 27).
Three months after AAV6-S100A1 gene delivery, leukocyte, erythrocyte and platelet counts as well as hemoglobin concentrations were unchanged between sham and HF animals treated with either saline, AAV6-luc or AAV6-S100A1 (Table 2). Accordingly, in all groups, sodium, potassium and glucose levels did not show any differences, and pancreas enzymes, kidney retention parameters and serum liver enzymes were also found within the physiological normal range for pigs (Table 2).
Table 2.
Preserved blood biomarkers 12 weeks after AAV6-S100A1 gene therapy (n=9) as compared with HF/AAV6-luciferase (n=6), HF/saline (n=7) and sham operation (n=8) controls
| Sham | HF-Saline | HF-Luc. | HF-S100A1 | |
|---|---|---|---|---|
| Blood parameters: | ||||
| Sodium (139–152 mmol l −1) | 141 ±0.6 | 140 ± 1.2 | 138 ±2.2 | 140 ± 1.4 |
| Potassium (3.5–4.5 mmol l −1) | 3.9 ± 0.07 | 4.1 ±0.25 | 4.9 ± 0.45 | 3.7 ± 0.08 |
| Leukocytes (11–22 nl) | 15.4 ± 0.96 | 15.1 ±0.5 | 15.9 ± 1.8 | 17.0± 1.1 |
| Erythrocytes (5.0–7.0 pl) | 5.9 ± 0.2 | 6.0 ±0.1 | 5.8 ±0.1 | 6.2 ±0.12 |
| Platelets (200–500 nl) | 376 ± 38 | 430 ± 33 | 302 ± 42 | 455 ± 37 |
| Hemoglobin (9.0–13.0 g dl−1) | 9.8 ± 0.2 | 10.1 ±0.2 | 9.8 ± 0.2 | 10.0 ±0.1 |
| Creatinine (0.8–2.3 mg dl −1) | 1.7 ±0.13 | 1.8 ±0.11 | 1.4 ± 0.05 | 1.9 ± 0.09 |
| Urea (20–53 mg dl −1) | 26 ± 2.6 | 24±1.9 | 30 ±2.1 | 31 ±2.6 |
| Glucose (mg dl−1) | 82 ±6 | 83 ±7 | 73 ±4 | 76 ±7 |
| Lipase (15–60u l−1) | 14±0.4 | 15 ± 1.3 | 15 ± 1.4 | 17 ±2.1 |
| Glutamate-Pyruvate-Transaminase (GPT) (22–47 U l−1) | 49 ± 4.8 | 42 ± 2.3 | 46 ±2.1 | 46 ± 3.3 |
| Glutamate–Oxalacetate-Transferase (GOT) (20–66 U l −1) | 38 ± 4.8 | 33 ± 3.3 | 33 ± 4.0 | 35 ± 2.2 |
Discussion
Cardiac S100A1 gene therapy enters the stage of clinical translation as it was proven to have additive beneficial effects beyond that in current pharmacological HF treatment of β-adrenergic receptor blockade and to provide long-term therapeutic effects using a preclinical large animal HF model.10,12,35 Moreover, S100A1 gene therapy was shown to rescue HF in small and large animal models as well as in isolated failing human cardiomyocytes.21,30,35,36 However, potential cardiac adverse effects such as contractile dysfunction and ventricular arrhythmia due to high-level myocardial S100A1 overexpression are of potential concern.6,24,26–28,31 Therefore, we decided to investigate the therapeutic window and safety of high-level S100A1 overexpression prior to clinical use.
In order to systematically assess regional myocardial S100A1 protein expression 12 weeks after AAV-S100A1 gene delivery, the non-infarcted remote myocardium was divided into 36 segments to perform western blot analysis. Retrograde delivery of 1.5 × 1013 tvp of AAV6-S100A1 (1.5 × 1013 tvp of AAV9-S100A1) via the ACV resulted in robust myocardial S100A1 overexpression in the anterior and anteroseptal target area (22.9 ± 3.2-fold compared with AAV6-Luc control), whereas AAV9-S100A1 gene delivery was shown to result in modest S100A1 overexpression (3.2 ± 0.3-fold of AAV9-Luc control).10 Detailed analysis of S100A1 expression pattern in the myocardium showed inhomogenous S100A1 expression, which is in line with previous cardiac in vivo gene transfer studies.29,30,35,36 Moreover, use of AAV6-S100A1 caused ‘hot spots’ of cardiac S100A1 overexpression (up to 95-fold) next to segments without S100A1 overexpression, whereas the maximal gradient of S100A1 expression was 5.4-fold in AAV9-S100A1-treated pigs. Of note, AAV vector production and quantification was carried out in the same vector production core lab and the same cardio-selective promoter was used. AAV6 and AAV9 serotypes exhibit an inherent cardiotropism, which is due to distinct capsid characteristics causing transductional efficacy, the ability to overcome the endothelial barrier and the interstitial space efficiently, and escape neutralizing antibodies, which might in part explain different myocardial S100A1 overexpression, although the same AAV particle number was used.7
Despite the high-level and locally extreme myocardial S100A1 overexpression, AAV6-S100A1 significantly increased LVEF, whereas + dP/dt showed a nonsignificant trend toward improved LV contractile function. Thus, S100A1 gene therapy showed a broad therapeutic window without detrimental effects on the contractile function. This might be explained in part by indirect effects such as reduction in myocardial wall stress and regression of maladaptive hypertrophy mediated by the myocardium with moderate S100A1 overexpression and affecting the myocardium with extreme or absence of S100A1 overexpression. Data from isolated cardiomyocytes might support this hypothesis as non-S100A1-treated cardiomyocytes isolated from AAV-S100A1-treated failing rat hearts (∼40% infection rate) displayed increased functional properties.35 Consistently, high-level patchy S100A1 overexpression significantly attenuated LV enlargement, normalized NCX expression and reduced BNP expression, which is in line with studies on less-efficient S100A1 overexpression.10,15,17,21,30,35,36 However, low-level S100A1 expression (3.2±0.3-fold) without local ‘hot spots’ of excessive myocardial S100A1 overexpression mediated by AAV9-S100A1 rescued +dP/dt and showed a pronounced increase in LVEF as compared with AAV6-S100A1.10 In line, mitochondrial F1-ATPase activity was significantly increased in low-level S100A1-overexpressing myocardial samples, whereas high-level S100A1-overexpressing specimens showed a nonsignificant trend. Low and moderate S100A1 protein overexpression significantly increased intracellular Ca2+ transients in NRCM, whereas extreme S100A1 overexpression abrogated this effect. However, even extreme S100A1 overexpression had no deleterious effects on intracellular Ca2+ cycling as compared with sham. Notably, expression and phosphorylation status of proteins involved in intracellular Ca2+ cycling, such as PLN, ryanodine receptor and SERCA2a, remained unaltered after S100A1 overexpression. Therefore, S100A1/SERCA2a, S100A1/RyR and S100A1/PLN ratios are altered and we might speculate that extreme ratios are beyond the optimum. However, dose-dependent effects of S100A1 are not fully explained but are in line with previous results from isolated skeletal muscle fibers demonstrating a smaller increase in peak Ca2+ released from the sarcoplasmic reticulum at high S100A1 concentration.37
Occurrence of ventricular tachyarrhythmia is a potential major cardiac adverse effect of myocardial gene delivery that might be triggered by high-level and inhomogenous myocardial S100A1 protein overexpression. The ‘multicenter unsustained tachycardia trial’ demonstrated the possibility of electrophysiologically guided risk stratification to identify patients at risk for sudden cardiac death.38,39 Although the multicenter unsustained tachycardia trial included patients with ischemic cardiomyopathy and moderately reduced LV function, we investigated the inducibility of MVT in post-ischemic pigs with moderately reduced LV function as a marker of potential occurrence of malignant ventricular arrhythmia. Inducibility of MVTs was not increased following high-level myocardial S100A1 overexpression in HF as comparedwith HF control groups, whereas, as expected, MVTs could not be induced in sham-operated non-infarcted pigs. Moreover, repolarization and depolarization intervals of the ecg, including the QTc interval, which might be used as a biomarker of the occurrence of ventricular tachyarrhythmia, were not statistically altered in AAV6-S100A1-treated pigs. Of note, Völkers et al.10,19,36 demonstrated that S100A1 functions as an inhibitory modulator of ryanodine receptor function, reducing Ca2+-spark frequency at diastolic cytosolic Ca2+ concentration and thus susceptibility to malignant ventricular arrhythmia.
We further analyzed the biodistribution of S100A1 cDNA in isolated genomic DNA of non-cardiac organs. Retrograde high-dosage AAV6-S100A1 gene therapy via the ACV resulting in an average of more than 20-fold myocardial S100A1 protein expression mainly targets the heart, which is line with previous studies using AAV9-S100A1.10 However, AAV biodistribution ratios between the myocardium and liver (AAV6: 17:1 vs AAV9: 63:1) and between the myocardium and skeletal muscle (AAV6: 206:1 vs AAV9: 2564:1) were reduced in AAV6-S100A1-treated pigs as compared with AAV9-S100A1-treated pigs, suggesting an increased spill-over and reduced myocardial absorption of AAV6-S100A1 as compared with AAV9-S100A1.10 Despite wider AAV vector biodistribution to non-cardiac organs in AAV6-S100A1-treated pigs, blood count, sodium, potassium and glucose blood concentrations were similar and pancreas enzymes, kidney retention parameters and serum liver enzymes were within the physiological normal range for pigs, suggesting no major detrimental impact of the AAV6 vector on the functional properties of these organs.
Overall, high-level myocardial S100A1 protein expression mediated by AAV6-S100A1 does not cause cardiac adverse effects, such as an increased susceptibility to ventricular tachyarrhythmia or impairment of contractile function in vivo. The current study demonstrates a broad therapeutic window of S100A1 gene therapy in post-ischemic HF, which might mitigate potential concern of cardiac adverse effects prior to clinical use of S100A1 gene therapy to treat end-stage HF.
Materials and Methods
All animal procedures and experiments were performed in accordance with the ‘Guide for the Care and Use of Laboratory Animals’ (NIH) and were approved by the local ‘Animal Care and Use Committee’ of Baden-Württemberg.
Model of post-ischemic HF and catheter-based cardiac gene delivery
The model of post-ischemic HF and cardiac gene delivery has been previously described in detail.10,11 Detailed information is given in the online-only methods.
AAV vector production and vector titration
Extensive information about AAV vector production, harvesting and purifying is given in the online-only methods.
Myocardium treated with AAV9
AAV9-S100A1- and AAV9-Luc-treated myocardial samples were obtained from a previous study using the same pig preclinical HF model, the same AAV-generation/production core laboratory, the same cardio-selective promoter, the same myocardial AAV delivery approach and the same sample collection method.10 AAV9-treated cardiac samples were used to systematically analyze regional S100A1 expression as well as expression of proteins involved in intracellular calcium cycling and F1-ATPase activity.
Regional western blot analysis
Detailed information about membrane preparation and western blotting procedures are given in the online-only methods.
Real-time RT-PCR and AAV-S100A1 biodistribution
Genomic DNA and total RNA from myocardial samples as well as from the liver, lung, skeletal muscle and brain were isolated and analyzed as described.30,36 Further information is given in the online-only methods.
Occurrence of MVT by right ventricular stimulation in pigs with HF
Electrophysiological examination is described extensively in the online-only methods.
Hematology, electrolytes and clinical chemistry
Analyses were carried out according to the International Federation of Clinical Chemistry primary reference procedures for the measurement of catalytic activity of enzymes at 37°C as well as with standard methods using indirect potentiometry for electrolytes.
Statistical analysis
Data are expressed as means±s.e.m. The unpaired Student t test was used when appropriate. To compare means among three or more independent groups, one-way ANOVA (analysis of variance) was performed for statistical comparisons. The Bonferroni test was applied whenever multiple comparisons were conducted. For statistical analysis, Graph PadPrism software was used and the P-values shown are adjusted in function for the number of comparisons. For all tests, a value of P<0.05 was accepted as being statistically significant. S100A1 expression levels were compared with appropriate AAV6-luc or AAV9-luc control groups.
Supplementary Material
Acknowledgments
We thank Barbara Leuchs and the DKFZ vector core production unit for generating high-titer AAV vector stocks. This work was supported by the following NIH grants: R01HL92130 and R01HL92130-02S1 (PM); P01HL075443, R01HL56205 and R01HL061690 (WJK); Deutsche Forschungsgemeinschaft: 1654/3-2 (OJM), 562/1-1 (PM and STP); the Bundesministerium für Bildung und Forschung: 01GU0527 (PM, OJM, HAK); Deutsche Gesellschaft für Kardiologie Otto Hess Promotionsstipendium (CW); and the German Cardiovascular Research Center (DZHK to PM, HAK).
Footnotes
Conflict of Interest: P Most and HA Katus report potential competing interests as they have filed US and EU patent applications on the therapeutic use of the S100A1 protein to treat heart failure. CW, IN, BK, PS, PR, JR, AJ, WJK, OJM and STP declare no conflict of interest.
References
- 1.AHA. Heart disease and stroke statistics: 2010 update. Circulation. 2010;21:e1–e170. [Google Scholar]
- 2.Flather MD, Yusuf S, Køber L, Pfeffer M, Hall A, Murray G, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet. 2000;6:1575–1581. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- 3.Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR. Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol. 2000;36:2072–2080. doi: 10.1016/s0735-1097(00)01006-8. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med. 2010;362:228–238. doi: 10.1056/NEJMcp0909392. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 6.Pleger ST, Boucher M, Most P, Koch WJ. Targeting myocardial beta-adrenergic receptor signaling and calcium cycling for heart failure gene therapy. J Card Fail. 2007;13:401–414. doi: 10.1016/j.cardfail.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Pleger ST, Brinks H, Ritterhof J, Raake P, Koch WJ, Katus HA, et al. Heart failure gene therapy: the path to clinical practice. Circ Res. 2013;113:792–809. doi: 10.1161/CIRCRESAHA.113.300269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–1557. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 9.Lai NC, Roth DM, Gao MH, Tang T, Dalton N, Lai YY, et al. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004;110:330–336. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 10.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, et al. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3:92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, et al. AAV6.βARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34:1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraus C, Rohde D, Weidenhammer C, Qiu G, Pleger ST, Voelkers M, et al. S100A1 in cardiovascular health and disease: closing the gap between basic science and clinical therapy. J Mol Cell Cardiol. 2009;47:445–455. doi: 10.1016/j.yjmcc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boerries M, Most P, Gledhill JR, Walker JE, Katus HA, Koch WJ, et al. Ca2+-dependent interaction of S100A1 with F1-ATPase leads to an increased ATP content in cardiomyocytes. Mol Cell Biol. 2007;27:4365–4373. doi: 10.1128/MCB.02045-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gusev K, Ackermann GE, Heizmann CW, Niggli E. Ca2 + signaling in mouse cardiomyocytes with ablated S100A1 protein. Gen Physiol Biophys. 2009;28:371–383. doi: 10.4149/gpb_2009_04_371. [DOI] [PubMed] [Google Scholar]
- 15.Most P, Bernotat J, Ehlermann P, Pleger ST, Reppel M, Börries M, et al. S100A1: a regulator of myocardial contractility. Proc Natl Acad Sci USA. 2001;98:13889–13894. doi: 10.1073/pnas.241393598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Most P, Remppis A, Pleger ST, Löffler E, Ehlermann P, Bernotat J, et al. Transgenic overexpression of the Ca2+ binding protein S100A1 in the heart leads to increased in vivo myocardial contractile performance. J Biol Chem. 2003;278:33809–33817. doi: 10.1074/jbc.M301788200. [DOI] [PubMed] [Google Scholar]
- 17.Most P, Seifert H, Gao E, Funakoshi H, Völkers M, Heierhorst J, et al. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114:1258–1268. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- 18.Tsoporis JN, Marks A, Zimmer DB, McMahon C, Parker TG. The myocardial protein S100A1 plays a role in the maintenance of normal gene expression in the adult heart. Mol Cell Biochem. 2003;242:27–33. [PubMed] [Google Scholar]
- 19.Völkers M, Loughrey CM, Macquaide N, Remppis A, DeGeorge BR, Jr, Wegner FV, et al. S100A1 decreases calcium spark frequency and alters their spatial characteristics in permeabilized adult ventricular cardiomyocytes. Cell Calcium. 2007;41:135–143. doi: 10.1016/j.ceca.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Remppis A, Greten T, Schafer BW, Hunziker P, Erne P, Katus HA, et al. Altered expression of the Ca(2 +)-binding protein S100A1 in human cardiomyopathy. Biochim Biophys Acta. 1996;1313:253–257. doi: 10.1016/0167-4889(96)00097-3. [DOI] [PubMed] [Google Scholar]
- 21.Brinks H, Rohde D, Volkers M, Qiu G, Pleger ST, Herzog N, et al. S100A1 genetically-targeted therapy reverses dysfunction of human failing cardiomyocytes. J Am Coll Cardiol. 2011;58:966–973. doi: 10.1016/j.jacc.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jessup M, Greenberg B, Mancini D, Cappola TP, Pauly DF, Greenberg B, et al. CUPID-a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2 + ATPase (SERCA2a) in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang T, Gao MH, Hammond HK. Prospects for gene transfer for clinical heart failure. Gene Ther. 2012;19:606–612. doi: 10.1038/gt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dybkova N, Sedej S, Napolitano C, Neef S, Rokita AG, Hünlich M, et al. Overexpression of CaMKIIδc in RyR2R4496C +/− knock-in mice leads to altered intracellular Ca2 + handling and increased mortality. J Am Coll Cardiol. 2011;57:469–479. doi: 10.1016/j.jacc.2010.08.639. [DOI] [PubMed] [Google Scholar]
- 25.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelhardt S, Hein L, Dyachenkow V, Kranias EG, Isenberg G, Lohse MJ. Altered calcium handling is critically involved in the cardiotoxic effects of chronic beta-adrenergic stimulation. Circulation. 2004;109:1154–1160. doi: 10.1161/01.CIR.0000117254.68497.39. [DOI] [PubMed] [Google Scholar]
- 27.Pott C, Muszynski A, Ruhe M, Bogeholz N, Schulte JS, Milberg P, et al. Proarrhythmia in a non-failing murine model of cardiac-specific Na+/Ca2+ exchanger overexpression: whole heart and cellular mechanisms. Basic Res Cardiol. 2012;107:247. doi: 10.1007/s00395-012-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wittköpper K, Fabritz L, Neef S, Ort KR, Grefe C, Unsöld B, et al. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest. 2010;120:617–626. doi: 10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurice JP, Hata JA, Shah AS, White DC, McDonald PH, Dolber PC, et al. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary beta2-adrenergic receptor gene delivery. J Clin Invest. 1999;104:21–29. doi: 10.1172/JCI6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pleger ST, Remppis A, Heidt B, Völkers M, Chuprun JK, Kuhn M, et al. S100A1 gene therapy preserves in vivo cardiac function after myocardial infarction. Mol Ther. 2005;12:1120–1129. doi: 10.1016/j.ymthe.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Escoubet B, Prunier F, Amour J, Simonides WS, Vivien B, et al. Constitutive cardiac overexpression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase delays myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmia. Circulation. 2004;109:1898–1903. doi: 10.1161/01.CIR.0000124230.60028.42. [DOI] [PubMed] [Google Scholar]
- 32.Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2:262–271. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hove-Madsen L, Bers DM. Sarcoplasmic reticulum Ca2+ uptake and thapsigargin sensitivity in permeabilized rabbit and rat ventricular myocytes. Circ Res. 1993;73:820–828. doi: 10.1161/01.res.73.5.820. [DOI] [PubMed] [Google Scholar]
- 34.James J, Zhang Y, Wright K, Witt S, Glascock E, Osinska H, et al. Transgenic rabbits expressing mutant essential light chain do not develop hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2002;34:873–882. doi: 10.1006/jmcc.2002.2025. [DOI] [PubMed] [Google Scholar]
- 35.Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, et al. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 36.Most P, Pleger ST, Völkers M, Heidt B, Boerries M, Weichenhan D, et al. Cardiac adenoviral S100A1 gene transfer rescues failing myocardium. J Clin Invest. 2004;114:1550–1563. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Most P, Remppis A, Weber C, Bernotat J, Ehlermann P, Pleger ST, et al. The C terminus (amino acids 75–94) and the linker region (amino acids 42–54) of the Ca2 + -binding protein S100A1 differentially enhance sarcoplasmic Ca2 + release in murine skinned skeletal muscle fibers. J Biol Chem. 2003;18:26356–26364. doi: 10.1074/jbc.M303338200. [DOI] [PubMed] [Google Scholar]
- 38.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 39.Buxton AE, Lee KL, DiCarlo L, Gold MR, Greer JS, Prystowsky N, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter unsustained tachycardia trial investigators. N Engl J Med. 2000;342:1937–1945. doi: 10.1056/NEJM200006293422602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


