Abstract
Excess thyroid hormone is associated with increased bone loss and fracture risk in older women, but few data exist in men. We sought to determine if thyroid function is independently associated with bone loss and fracture risk in older men. Data were analyzed from the Osteoporotic Fractures in Men (MrOS) Study, a cohort of community-dwelling US men aged 65 years and older. Using a case-cohort design, fasting baseline serum archived at −80C was assayed for thyrotropin (TSH) and free thyroxine (FT4) in 397 men with confirmed non-spine fracture, including 157 hip fractures, and 1420 randomly selected men without fracture. TSH and FT4 were analyzed as continuous variables and as thyroid function categories (subclinical hyperthyroid, euthyroid, and subclinical hypothyroid). Hip DXA (Hologic QDR4500) was measured at baseline and after a mean follow-up of 4.6 yr. Incident nonspine fractures were centrally adjudicated. Bone loss was evaluated with multivariate regression methods and fractures risk was evaluated using hazard models that accounted for the case-cohort sampling, adjusted for age, clinic-site, BMI, race, physical activity, corticosteroid use, smoking, alcohol intake, and thyroid medication use. In fully adjusted analyses, TSH was not associated with risk of nonspine fracture (RH 0.92 per SD decrease in TSH, 95% CI 0.74 – 1.14), but was significantly associated with risk of hip fracture (RH 1.31 [1.01 – 1.71]) which persisted among normal range TSH values (RH 1.21 [1.00 – 1.47]). There was no association between TSH or FT4 and bone loss, and fracture risk did not differ significantly by thyroid function category. We conclude that while neither TSH nor FT4 are associated with bone loss, lower serum TSH may be associated with an increased risk of hip fractures in older men.
Keywords: Thyroid function, osteoporosis, men, bone density, fracture
Introduction
Overt hyperthyroidism is a recognized risk fracture for decreased bone mineral density (BMD) in both men and women, and increased risk of fracture in older women. (1,2) However, the relationship between subclinical hyperthyroidism or subclinical hypothyroidism and skeletal outcomes is less definitive.
One large prospective study found an increased risk for hip and vertebral fractures among women with low thyrotropin (TSH ≤ 0.1 mIU/L) but free thyroxine (FT4) was not measured, thereby limiting definitive determination of thyroid status. (3) Another recent study found an association between subclinical hyperthyroidism and hip fracture in men, but not women. (4) A third prospective study of men and women including participants with subclinical thyroid dysfunction found an association between higher FT4 and decreased femoral neck BMD, but no increase in fracture risk. (5)
Recent evidence also suggests there may be an association between thyroid function and skeletal outcomes even within the euthyroid range, though few prospective data exist. One prospective study found an association between lower TSH and increased risk of nonspine fracture. (6) Cross-sectional studies have similarly found an association between lower TSH and nonspine fractures, (7) but the reported association of different measures of thyroid function with BMD has been inconsistent. (7–10)
Data on the association between thyroid function and skeletal outcomes in men are sparse. Most studies of male cohorts have been cross-sectional and have produced conflicting results. (9,10) Older men in particular represent an important group of individuals at risk for osteoporotic fractures. The lifetime risk of hip fracture in men by age 90 has been reported as close to 20%, and osteoporotic fractures are associated with increased mortality in men as in women. (11,12) Previous data suggest that up to 5% of ambulatory men over 60 have suppressed serum TSH values, (13,14) therefore it is important to determine the relationship between thyroid function and fracture in this population.
Using data from the Osteoporotic Fractures in Men (MrOS) Study, a cohort of community-dwelling older men, we sought to determine the relationship between baseline TSH and FT4 and incident hip and non-spine fractures, and changes in BMD. We also examined these associations among participants with normal range TSH values to determine the relationship between thyroid function and skeletal outcomes in euthyroid individuals.
Participants and Methods
Study Population
The MrOS study is a prospective cohort of 5,994 community-dwelling ambulatory men designed to study healthy aging and fracture risk. Participants were enrolled from March 2000 to April 2002 at one of six US clinical centers. Participants were recruited by mailings to the Department of Motor Vehicles and voter registration databases, community and senior newspaper advertisements, and presentations targeted at seniors in the communities surrounding the six clinical sites. (15) Eligible men were at least 65 years old and able to walk without the assistance of another person and did not have bilateral hip replacements. Details of the MrOS study design and cohort have been previously reported. (15,16) The Institutional Review Board at each clinical center approved the study protocol, and written informed consent was obtained from all participants.
To analyze the prospective relationship between thyroid function, bone loss, and fracture risk, we used a case-cohort design. (17) Fasting serum from the baseline MrOS visit, archived at −80C in all participants, was used to measure TSH and FT4 all men selected for study inclusion. Men without sufficient serum for assays were excluded from all analyses. Of the 5594 eligible participants, we randomly selected 1602 men to serve as the subcohort. (17) Individuals with discordant thyroid function tests (n=33) suggestive of pituitary disease or non-thyroidal illness were excluded from this analysis. Participants using testosterone supplements, bisphosphonates or other osteoporosis treatment medications were also excluded from this analysis due to the effect of these medications on bone density and fracture risk (n=73). Participants using thyroid hormone replacement (n=134) were included, however analyses were also repeated after their exclusion and results (described below) were similar. After these exclusions, the number if men remaining in the random subcohort was 1526.
We observed 397 incident non-spine fractures (including 157 hip fractures) in the entire cohort over the 4.6 years of follow-up. Among these participants with fracture, 112 men also were sampled within the subcohort. The total study sample for the nonspine fracture analysis was 1817 men (1526 subcohort and 397 men with non-spine fractures) and for hip fracture analysis 1635 men (1526 subcohort and 157 hip fractures.).
Thyroid Hormone Measurements
TSH was measured using a third-generation assay (ADVIA Centaur, Siemens Diagnostics, Deerfield, IL). The normal reference range for TSH by this assay is 0.55 – 4.78mIU/L and the coefficient of variation at 2.08mIU/L is 2.4%. FT4 was measured using a competitive immunoassay (Siemens Diagnostics). The normal reference range for FT4 by this assay is 9.0 – 23.8 pmol/L and the coefficient of variation at 14.03 pmol/L is 4.1%.
Thyroid Function Categories
TSH and FT4 were used simultaneously to define thyroid function categories: subclinical hyperthyroid (TSH <0.55 mIU/L, FT4 9.0 – 23.8 pmol/L), euthyroid (TSH 0.55 – 4.78 mIU/L, FT4 9.0 – 23.8 pmol/L), and subclinical hypothyroid (TSH >4.78 mIU/L, FT4 9.0 – 23.8 pmol/L). (18) We were unable to analyze categories of subclinical hyperthyroid participants by degree of TSH suppression (<0.1 vs ≥0.1mIU/L) because so few individuals had a TSH <0.1mIU/L (n=7). Because so few participants had overt hyperthyroidism (TSH<0.55 mIU/L, FT4 > 23.8 pmol/L, n=2) or overt hypothyroidism (TSH >4.78 mIU/L, FT4 <9.0 pmol/L, n=16), these participants were excluded from analyses using thyroid function categories as a predictor because these categories would have been too small to provide meaningful results.
Measurement of Bone Density: DXA
Areal BMD (g/cm2) of the total hip, and hip sub-regions was measured at baseline in all participants using DXA (Hologic QDR4500, Hologic, Bedford, MA, USA). All measurements were made on the right hip unless participants reported a history of right hip replacement or other metal objects in the right leg, and therefore the left hip was imaged. Densitometry operators were certified by Hologic training. Quality control using daily scans of standardized phantoms found no shifts in performance of scanners, and cross-calibration was performed to assess variation between scanners at the different study sites. (19) Of the 1526 men in the random cohort, repeat hip DXA was performed in 1112 men (73%) after a mean follow-up of 4.6 years using the same devices and quality control procedures as for the baseline examination.
Ascertainment of Fractures
Participants were contacted every 4 months by mail to inquire about recent fractures. All reported fractures were centrally adjudicated by physician review of radiology reports or X-rays if reports were not available or definitive. Vertebral fractures, morphometric or clinical, were not included in this analysis.
Assessment of Covariates
Medical history, race, physical activity, fall history, alcohol consumption, and smoking status were obtained using a self-administered questionnaire. Physical activity was quantified using the Physical Activity Score for the Elderly (PASE). (20) During an interview-administered questionnaire, participants were asked to rate their overall health and this response was subsequently dichotomized to “fair” or “poor” health versus “good” or “excellent” health. (21) Prescription medication use at baseline was ascertained by review of all medications taken during the last 30 days and confirmed by review of medication containers during the clinical interview. (16) Medications were classified centrally using a hierarchical drug dictionary based upon the Iowa Drug Information System codes (College of Pharmacy, University of Iowa, Iowa City, IA). (22) Height was measured using a wall-mounted stadiometer and weight by a balance beam or digital scale. Body mass index (BMI) was calculated as kilograms/meter2.
Statistical Analysis
Baseline characteristics of the cohort were compared by incident fracture status, using x2 tests for categorical and t-tests for continuous variables. For variables significantly deviating from a normal distribution, the appropriate non-parametric test was employed.
Covariates were chosen based on the previous literature and biological plausibility. Additional covariates were selected based on significant (p<0.10) univariate association with fracture (BMI, race, smoking status), thyroid function (thyroid hormone use, oral corticosteroid use), or both (age, alcohol use, physical activity score).
After visualization for non-linear relationships, multivariable regression models, adjusted for age, race, clinic site, BMI, smoking status, alcohol use, thyroid hormone use, self-reported history of hyperthyroid/Graves’ disease, oral corticosteroid use, and physical activity score, were used to determine annualized percent change in total hip and femoral neck BMD per standard deviation (SD) decrease in TSH and increase in FT4 in the randomly selected men. Bone loss was also analyzed (in 1052 men without overt hyperthyroidism or hypothyroidism) by thyroid function category using linear regression models. Adjusted mean annualized percent bone loss was compared by thyroid function category and p-value for trend was reported.
The risk of hip and non-spine fractures was determined using hazard models that accounted for the case-cohort sampling and adjusted for the same covariates described above. (23) Initial models used TSH and FT4 as continuous predictors, to examine the risk of fracture per SD decrease in TSH and increase in FT4. Models were then repeated using thyroid function categories: subclinical hyperthyroid, euthyroid, and subclinical hypothyroid.
Because previous literature has suggested a differential effect of age on the association between thyroid function on outcomes such as ischemic heart disease, (24,25) we tested for interaction between thyroid function and age. In addition, we repeated all analyses after excluding thyroid hormone users (n=134). We also tested for an association between TSH within the normal range and bone loss and fracture, by excluding participants with TSH values outside normal limits (0.55 – 4.78 mIU/L). Finally, we tested the proportional hazards assumption and found no evidence of violation.
Results
Baseline Characteristics
Baseline characteristics of the cohort by fracture status are described in Table 1. Overall, participants with fractures (nonspine or hip) were older, more likely to be Caucasian, and had lower total hip and femoral neck BMD than those without fractures. Participants who experienced hip fractures had a significantly lower BMI (p<0.001), lower physical activity score (p=0.01), were more likely to report a history of “high thyroid” or “Graves’ disease” (p=0,05), and consumed, on average, more alcoholic drinks per week (p<0.001) than those without hip fractures. There was no significant difference in mean TSH or FT4 or prevalence of thyroid medication use between participants with hip fractures or non-spine fractures and those who did not fracture. 134 participants were using thyroid hormone replacement, and none of the participants were on anti-thyroidal medications.
Table 1.
Baseline characteristics by non spine fracture status
| Non Spine Fracture | Hip Fractures | |||||
|---|---|---|---|---|---|---|
| No (n=1420) | Yes (n=397) | p-value | No (n=1478) | Yes (n=157) | p-value | |
| Age, years | 73.6 (5.9) | 75.4 (6.4) | <0.001 | 73.6 (5.8) | 78.1 (6.1) | <0.001 |
| BMI, kg/m2 | 27.4 (3.7) | 27.2 (4.1) | 0.4 | 27.4 (3.7) | 26.3 (3.7) | <0.001 |
| Caucasian | 1292 (91.0) | 379 (95.5) | 0.004 | 1345 (91.0) | 151 (96.2) | 0.03 |
| Self reported health | ||||||
| Excellent/good | 1212 (85.4) | 339 (85.4) | 0.99 | 1263 (85.5) | 133 (84.7) | 0.79 |
| Physical Activity Score | 147.8 (68.7) | 144.8 (73.1) | 0.45 | 147.8 (68.6) | 133.8 (69.0) | 0.01 |
| Smoking status | ||||||
| Current | 52 (3.7) | 13 (3.3) | 0.82 | 54 (3.7) | 7 (4.5) | 0.09 |
| Past | 850 (59.9) | 233 (58.7) | 888 (60.1) | 80 (51.0) | ||
| Never | 518 (36.5) | 151 (38.0) | 536 (36.3) | 70 (44.6) | ||
| Alcohol, drinks/week | 4.5 (7.1) | 4.1 (6.3) | 0.09 | 4.6 (7.1) | 2.9 (5.1) | <0.001 |
| Oral corticosteroid use | 33 (2.4) | 6 (1.6) | 0.43 | 33 (2.3) | 2 (1.3) | 0.43 |
| Total hip BMD, gm/cm2 | 0.96 (0.14) | 0.90 (0.15) | <0.001 | 0.96 (0.14) | 0.81 (0.12) | <0.001 |
| Femoral neck BMD, gm/cm2 | 0.79 (0.13) | 0.73 (0.13) | <0.001 | 0.79 (0.13) | 0.66 (0.11) | <0.001 |
| TSH, mIU/L | 2.55 (2.30) | 2.86 (6.29) | 0.88 | 2.55 (2.27) | 2.38 (1.59) | 0.47 |
| FT4, pmol/L | 0.99 (0.16) | 0.99 (0.17) | 0.78 | 0.99 (0.16) | 1.01 (0.18) | 0.35 |
| Thyroid med use | 102 (7.5) | 32 (8.4) | 0.53 | 110 (7.8) | 14 (9.3) | 0.49 |
| Self-reported history of: | ||||||
| Hyperthyroid/Graves’ | 23 (1.6) | 8 (2.0) | 0.59 | 23 (1.6) | 6 (3.8) | 0.05 |
| Low thyroid | 101 (7.1) | 36 (9.1) | 0.19 | 110 (7.4) | 16 (10.2) | 0.22 |
| Fall in past 12 months | 273 (19.2) | 126 (31.7) | <0.001 | 229 (20.3) | 45 (28.7) | 0.01 |
All data presented as mean (SD) or n (%).
Participants with subclinical hypothyroidism were older than their euthyroid counterparts (mean age 76.4 vs. 73.5 years). Oral corticosteroid use was more common among individuals with subclinical hyperthyroidism (8.1% vs. 2.4% of euthyroid participants). Thyroid hormone use was more prevalent among participants with subclinical hyper- and hypothyroidism vs. euthyroid individuals (Table 2). There was no significant association between baseline BMD, prevalent nonspine or hip fracture, or history of fall within the past year and thyroid function category.
Table 2.
Baseline characteristics by thyroid function category
| Subclinical Hyperthyroid (n=37) | Euthyroid (n=1350) | Subclinical Hypothyroid (n=126) | p-value | |
|---|---|---|---|---|
| Age, years | 73.8 (5.9) | 73.5 (5.8) | 76.4 (6.4) | <0.001 |
| BMI, kg/m2 | 28.3 (4.4) | 27.4 (3.7) | 27.2 (3.5) | 0.28 |
| Caucasian | 32 (86.5) | 1231 (91.2) | 118 (93.7) | 0.37 |
| Self reported health | ||||
| Excellent/good | 32 (86.5) | 1158 (85.8) | 104 (82.5) | 0.59 |
| Physical activity score | 124.5 (64.5) | 148.7 (69.1) | 140.9 (66.8) | 0.06 |
| Smoking status | ||||
| Current | 0 (0.0) | 53 (3.9) | 2 (1.6) | 0.23 |
| Past | 25 (67.6) | 813 (60.2) | 70 (55.6) | |
| Never | 12 (32.4) | 484 (35.9) | 54 (42.9) | |
| Alcohol, drinks/week | 4.7 (6.3) | 4.6 (7.1) | 3.1 (5.6) | 0.09 |
| Oral corticosteroid use | 3 (8.1) | 31 (2.4) | 0 (0.0) | 0.02 |
| TSH, mIU/L | 0.32 (0.17) | 2.13 (0.96) | 6.66 (2.28) | |
| FT4, pmol/L | 14.80 (2.83) | 12.87 (1.80) | 11.84 (1.80) | |
| Thyroid med use | 14 (37.8) | 76 (5.9) | 25 (20.7) | <0.001 |
| Self-reported history of: | ||||
| Hyperthyroid/Graves’ | 2 (5.4) | 19 (1.4) | 5 (4.0) | 0.02 |
| Low Thyroid | 14 (37.8) | 78 (5.8) | 23 (18.3) | <0.001 |
| Total hip BMD, g/cm2 | 0.95 (0.13) | 0.95 (0.14) | 0.96 (0.16) | 0.78 |
| Femoral neck BMD, g/cm2 | 0.78 (0.14) | 0.78 (0.13) | 0.80 (0.14) | 0.45 |
| Prevalent nonspine fracture | 21 (56.8) | 715 (53.0) | 62 (49.2) | 0.64 |
| Prevalent hip fracture | 1 (4.8) | 20 (2.7) | 4 (6.5) | 0.24 |
| Fall in past 12 months | 7 (18.9) | 275 (20.4) | 27 (21.4) | 0.94 |
All data presented as mean (SD) or n (%).
TSH, Bone Loss, and Fracture
TSH levels were not associated with change in hip bone density. In multivariable-adjusted models using TSH as a continuous variable, each SD decrease in TSH was associated with a 0.15% increase (95% CI 95% CI −0.06 – 0.37%) in total hip BMD per year, and a 0.19% increase (95% CI −0.09 – 0.47%) in femoral neck BMD per year.
In multivariable-adjusted models using TSH as a continuous variable, there was a statistically significant association between lower TSH levels and risk of hip fracture (relative hazard [RH] 1.31 per SD decrease in TSH, 95% CI 1.01 – 1.71), but no association with nonspine fracture (RH 0.92 per SD decrease in TSH, 95% CI 0.74 – 1.14).
Thyroid Function Category, Bone Loss, and Fracture
Categories of thyroid function did not predict bone loss at the total hip or femoral neck (p=0.12 for trend). For example, in multivariable-adjusted models of total hip BMD, subclinical hyperthyroidism was associated with a 0.87% decrease (95% CI −2.36 – 0.62%), euthyroidism was associated with a 1.61% decrease (95% CI −1.85 – −1.37%), and subclinical hypothyroidism was associated with a 2.13% decrease (95% CI −2.97 - −1.29%) in total hip BMD per year. Results were similarly nonsigificant for bone loss at the femoral neck (p=0.24 for trend). Subclinical hyperthyroidism was associated with a 1.68% decrease (95% CI −3.65 – 0.29%), euthyroidism with a 1.52% decrease (95% CI −1.83 – −1.21%), and subclinical hypothyroidism with a 2.38% decrease (95% CI −3.48 – −1.26%) in femoral neck BMD.
There was no significant difference in risk of hip or non-spine fracture by thyroid function category (Table 3, Figure 1). Compared with euthyroid participants, the multivariable-adjusted RH of nonspine fracture for individuals with subclinical hyperthyroidism was 1.18 (95% CI 0.60 – 2.35) and for individuals with subclinical hypothyroidism was 0.74 (95% CI 0.48 – 1.13). The multivariable-adjusted RH of hip fracture in those with subclinical hyperthyroidism was 0.63 (95% CI 0.15 – 2.69) and for participants with subclinical hypothyroidism was 0.75 (0.40 – 1.41) compared with their euthyroid counterparts.
Table 3.
Fracture Risk by Thyroid Function Category
| Thyroid Function Category | Age and clinic site-adjusted Relative Hazard (95% CI) | MV-Adjusted* Relative Hazard (95% CI) | ||
|---|---|---|---|---|
| Any Nonspine | Hip | Any nonspine | Hip | |
| Subclinical Hyperthyroid | 1.16 (0.60 – 2.23) | 0.57 (0.14 – 2.31) | 1.18 (0.60 – 2.35) | 0.63 (0.15 – 2.69) |
| Euthyroid | Referent | Referent | ||
| Subclinical Hypothyroid | 0.80 (0.53 – 1.21) | 0.85 (0.46 – 1.57) | 0.74 (0.48 – 1.13) | 0.75 (0.40 – 1.41) |
Adjusted for age, clinic site, race, BMI, physical activity score, alcohol intake, smoking status, corticosteroid use, and thyroid hormone use
Figure 1. Kaplan Meier Curve of risk nonspine fracture by thyroid function category.
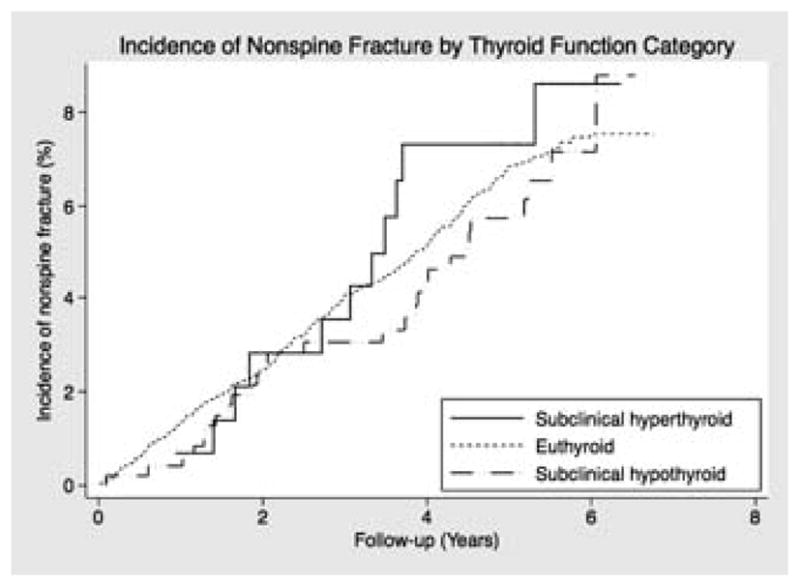
Unadjusted incidence of nonspine fracture compared by thyroid function category with euthyroid as the referent category.
FT4, Bone Loss, and Fracture
There was no association between FT4 levels and bone loss or fracture risk. In multivariable-adjusted models, each SD increase in FT4 was associated with a 0.11% decrease (95% CI −0.34 – 0.13%) in total hip BMD per year, and 0.18% decrease (95% CI −0.50 – 0.13%) in femoral neck BMD per year. Finally, FT4 levels, modeled continuously did not predict nonspine or hip fracture (multivariable-adjusted RH for nonspine fracture = 1.01 per SD increase in FT4, 95% CI 0.89 – 1.15; for hip fracture = 1.14 per SD increase in FT4, 95% CI 0.94 – 1.39).
Subgroup Analyses/Testing for Interaction
Testing for a differential effect of thyroid function on fracture risk by age revealed a statistically significant interaction with non-spine fractures (p-value for interaction = 0.002 between continuous age and continuous TSH). Results stratified above and below the median age (73 years) suggested a protective effect of TSH for nonspine fractures in younger participants (p-value for interaction = 0.03). For participants <73 years old, the age and site-adjusted RH of nonspine fracture per SD decrease in TSH was 0.74 (95% CI 0.68 – 0.80) vs. 1.04 (95% CI 0.84 – 1.30) in participants >73 years old. Age-stratified analyses with hip fracture as the outcome, however, did not reveal a substantial differential effect of age (p-value for interaction = 0.97). For individuals <73 years old, the MV-adjusted RH of hip fracture per SD decrease in TSH was 1.12 (95% CI 0.81 – 1.54) vs. 1.40 (95% CI 0.97 – 2.02) for participants >73 years old.
When examined by quartile of age, models similarly suggested a protective effect of lower TSH on nonspine fracture in the youngest participants (p-value for interaction = 0.006). The age and site-adjusted RH was 0.66 (95% CI 0.59 – 0.74) in the youngest quartile of age (65–68 yrs), vs. 1.10 (95% CI 0.87– 1.39) in the oldest quartile (78–99 yrs). For hip fracture, however, the difference in risk between the youngest and oldest quartile of participants was minimal (p-value for interaction = 0.93). The age and site-adjusted RH for hip fracture was 1.08 (95% CI 0.42 – 2.81) in the youngest quartile of age vs. 1.30 (95% CI 0.84 – 1.99) for the oldest quartile.
Analyses were repeated after excluding thyroid hormone users (n=134) and results were similar; neither TSH, FT4, nor thyroid function categories predicted bone loss or nonspine fracture (data not shown). The association between TSH and risk of hip fracture, became somewhat stronger (RH 1.57, 95% CI 1.15 – 2.12). The association between FT4 and hip fracture became borderline significant, with a RH for hip fracture of 1.20 per SD increase in FT4 (95% CI 0.99 – 1.45) when thyroid hormone users were excluded.
Finally, analyses were repeated including only participants (n=1658) with normal range TSH values (0.55 – 4.78mIU/L) and results were similar. After multivariable adjustment, TSH within the normal range was not associated with annualized percent change in total hip BMD (0.11% decrease per year for each SD decrease in TSH, 95% CI −0.35 – 0.12%) or femoral neck BMD (0.005% increase per year for each SD decrease in TSH, 95% CI −0.31 – 0.32%). Similarly there was no evidence of an association with risk of nonspine fractures (RH 1.01 per SD decrease in TSH, 95% CI 0.90 – 1.14). Within the normal range, we again observed a borderline statistically significant association between TSH and risk of hip fractures (RH 1.21 per SD decrease in TSH, 95% CI 1.00 – 1.47).
Discussion
In this cohort of community-dwelling older men, lower TSH levels were associated with an increased risk of hip fracture but not with bone loss or non-spine fractures. We found no association between subclinical thyroid dysfunction or FT4 levels and bone loss or fracture. We also found some evidence that age may modify the relationship between TSH and fracture, specifically that younger individuals may be less susceptible to the potential adverse effects of low TSH (or higher thyroid function).
Overt hyperthyroidism is recognized as a cause of increased bone turnover and is generally thought to be a risk factor for fractures. (2,26) The association between milder degrees of thyroid dysfunction, such as subclinical hyper- and hypothyroidism, and skeletal health is less clear. In the Study of Osteoporotic Fractures (SOF), a prospective cohort of over 9,000 US women, low TSH levels were significantly associated with vertebral fractures, but only those with TSH ≤ 0.1mIU/L had a significantly increased risk of hip fractures. (3) However, the lack of FT4 measurements in that study precludes determination of whether the risk is present at any degree of TSH suppression or only with overt hyperthyroidism. A recent analysis of the Cardiovascular Health Study (CHS) compared fracture risk in participants with subclinical thyroid disease vs. euthyroid counterparts. Increased risk of hip fracture with subclinical hyperthyroidism and subclinical hypothyroidism was observed in men, but not women. (4) This is in contrast with our study, in which men with subclinical hyper- or hypothyroidism had neither an increased nor decreased risk of fracture.
We observed a statistically significant association between lower TSH levels and hip fracture, which persisted when analyses were restricted to the normal range of TSH values and was stronger after exclusion of participants using exogenous thyroid hormone. However, due to the lack of a relationship between TSH and nonspine fractures, and a lack of association between TSH and BMD the mechanism of this association remains unexplained. One possible hypothesis is that the increased hip fracture risk is due to increased bone turnover that has not yet been detected by BMD changes on DEXA scan. In addition, the fact that hip fracture, but not vertebral fracture risk, was increased could be due to a preferential catabolic effect of thyroid hormone on cortical bone which has been previously described. (27–29)
Previous studies that have also sought to determine the relationship between thyroid function within the normal range and skeletal health have yielded conflicting results. In the Osteoporosis and Ultrasound Study (OPUS), higher TSH levels within the normal range were associated with a 35% reduction in nonvertebral fractures in postmenopausal women, even after adjustment for age, BMI, and BMD. Fracture risk was significantly increased in women with higher FT4 levels, again suggesting that higher thyroid function is a risk factor for osteoporosis and fracture. (6) The OPUS study also found that higher FT4 was associated with lower hip BMD, but interestingly TSH was not associated with BMD, suggesting that the relationship between thyroid function and skeletal health may be mediated by factors besides bone density. In contrast to these findings, the cross-sectional Tromsø Study found no association between serum TSH within the normal range and BMD in a cohort of men and postmenopausal women, although participants with the lowest TSH levels (below the 2.5th percentile) had decreased BMD at the distal radius. (8) Two recent cross-sectional studies in all-male cohorts have also produced inconsistent results. Kim et al., in a study of euthyroid Korean men (mean age 55), found that participants in the highest quintile of TSH had higher BMD at the lumbar spine and femoral neck, though the association with the femoral neck was no longer significant after adjusting for alcohol use and smoking status. (9) Conversely, in a younger cohort of euthyroid Belgian men (mean age 34) Roef et al. reported no association between TSH and BMD or QCT measurements. (10) Higher levels of free and total T3 and total T4, however, were associated with decreased BMD at the hip. In the present study, we found no association between TSH within the normal range and BMD, though, similar to the OPUS study we did observe an increased risk of hip fracture with lower serum TSH values.
The mechanism by which thyroid function affects skeletal health is complex. Biochemical markers of bone turnover, such as osteocalcin and bone specific alkaline phosphatase, are elevated in individuals with thyroid hormone excess. (2,30) Increased bone turnover may contribute to fragility and increased fracture risk even in the absence of bone loss captured by DEXA scan. The increased bone turnover in hyperthyroid states has traditionally been attributed to increased thyroid hormone (T3 and T4) levels; however rodent studies suggest a potential direct effect of TSH on bone. (31) TSH receptors have been found on osteoblasts and osteoclasts, and in one study recombinant TSH had anti-resorptive effects in ovariectamized rats. (31,32) In the present study, we found lower TSH levels to be associated with risk of hip fracture, but no association was seen with FT4 levels. Given that there is a strong inverse correlation between TSH and FT4 levels, this finding is unexpected and may support a T-4-independent effect of TSH on the skeleton. An alternative explanation for this finding is that T4 is a less sensitive indicator of overall thyroid status than is TSH. Another possible mechanism is decreased musculoskeletal function in those with lower TSH (or higher thyroid function), however we did not find an increased prevalence of falls in participants with subclinical hyperthyroidism.
Thyroid hormone use is an important potential confounder and the relationship between thyroid hormone use itself and bone health is also important to determine. In SOF, thyroid hormone use was not independently associated with increased risk of hip fracture. (3) Similarly, the Thyroid Epidemiology Audit and Research Study (TEARS) found that patients on long-term T-4 replacement therapy with low but unsuppressed TSH (0.04 – 0.4mIU/L) did not have an increased risk of fractures. (33) In our study, univariate analysis found no association between thyroid hormone use and non-spine or hip fractures. We adjusted for thyroid hormone use in our models, and models excluding thyroid hormone users showed a stronger association between lower TSH levels and hip fracture risk, suggesting over-suppression with exogenous thyroid hormone was not driving the observed relationship. One possible explanation for the stronger association between TSH and hip fracture after excluding those taking thyroid hormone replacement is that abnormally low TSH in these individuals with known thyroid disease is more likely to be identified by laboratory monitoring and therefore corrected. Individuals with mild endogenous thyroid hyperfunction may not come to medical attention and therefore may endure a longer period of exposure to higher levels of endogenous thyroid hormone.
Our study has several strengths. The MrOS is a well-characterized cohort in which osteoporosis risk fractures are thoroughly assessed and fracture outcomes are carefully measured. (34) It is the first study to evaluate the relationship between thyroid function and fracture risk in a large, entirely-male cohort. One potential limitation is the single measure of thyroid function at baseline. Data suggests that up to 25% of abnormally high or low TSH values will spontaneously normalize. (35–37) Furthermore, the likelihood of normalization of a low TSH depends on the degree of suppression, and mildly suppressed TSH levels (0.1 – 0.4mIU/L) are more likely to revert to euthyroid. (38) In our study, most participants with subclinical hyperthyroidism had low but detectable TSH values (0.1 – 0.4mIU/L), and thus may have been more likely to normalize TSH over time. If these participants with abnormally low TSH at baseline reverted to euthyroidism during the study, this may have biased results toward the null. In addition, thyroid hormone use was only assessed at baseline and not during follow-up, but results were similar when thyroid hormone users were excluded. The bias due to the competing risk of mortality is a potential concern in an older population. However, the mortality rate in this study was low: 1.8 deaths per 100 person years for the subclinical hyperthyroid men; 2.2 deaths per 100 person years in the euthyroid men, and 3.6 deaths per 100 person years for the subclinical hypothyroid men. Thus, aside from the use of proportional hazards models, we did not attempt to account for the competing risk mortality which may bias results when mortality rates are high. (39) Finally, despite a relatively large cohort few participants had abnormal TSH values and confidence intervals were wide, therefore significant associations may have been missed.
In summary, in this large prospective study of older men, we did not find any difference in fracture risk or bone loss among individuals with subclinical hyperthyroidism or hypothyroidism, though we did observe a borderline association between lower TSH and increased risk of hip fracture. The appropriate management of subclinical thyroid dysfunction remains controversial. (18,40), and while our findings support the hypothesis that lower TSH may increase hip fracture risk, further studies are needed to determine whether the treatment of minor thyroid function abnormalities is warranted for fracture prevention.
Summary.
Lower TSH may be associated with increased risk of hip fracture, but not accelerated bone loss.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Footnotes
Disclosure Summary: The authors have nothing to disclose
Availability/Disclosure of Materials and Methods and Author Access to Data
The authors had no restrictions on availability of raw data or statistical analyses.
Authors’ roles: Study design: ACW and DCB. Data analysis: SH and ACW. Data interpretation: ACW, DCB, and SH. Drafting manuscript: ACW and DCB. Revising manuscript content: ACW, DCB, SH, HAF, MHS, PMC, JMZ, and ESO. Approving final version of manuscript: DCB, HAF, MHS, PMC, JMZ, and ESO. ACW takes responsibility for the integrity of the data analysis.
References
- 1.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 2.El Hadidy el HM, Ghonaim M, El Gawad S, El Atta MA. Impact of severity, duration, and etiology of hyperthyroidism on bone turnover markers and bone mineral density in men. BMC Endocr Disord. 2011;11:15. doi: 10.1186/1472-6823-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer DC, Ettinger B, Nevitt MC, Stone KL. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134(7):561–568. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Buzkova P, Fink HA, Vu J, Carbone L, Chen Z, Cauley J, Bauer DC, Cappola AR, Robbins J. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med. 2010;170(21):1876–1883. doi: 10.1001/archinternmed.2010.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Deure WM, Uitterlinden AG, Hofman A, Rivadeneira F, Pols HA, Peeters RP, Visser TJ. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: the Rotterdam Study. Clin Endocrinol (Oxf) 2008;68(2):175–181. doi: 10.1111/j.1365-2265.2007.03016.x. [DOI] [PubMed] [Google Scholar]
- 6.Murphy E, Gluer CC, Reid DM, Felsenberg D, Roux C, Eastell R, Williams GR. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab. 2010;95(7):3173–3181. doi: 10.1210/jc.2009-2630. [DOI] [PubMed] [Google Scholar]
- 7.Mazziotti G, Porcelli T, Patelli I, Vescovi PP, Giustina A. Serum TSH values and risk of vertebral fractures in euthyroid post-menopausal women with low bone mineral density. Bone. 2010;46(3):747–751. doi: 10.1016/j.bone.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jorde R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: the Tromso study. Thyroid. 2008;18(11):1147–1155. doi: 10.1089/thy.2008.0158. [DOI] [PubMed] [Google Scholar]
- 9.Kim BJ, Lee SH, Bae SJ, Kim HK, Choe JW, Kim HY, Koh JM, Kim GS. The association between serum thyrotropin (TSH) levels and bone mineral density in healthy euthyroid men. Clin Endocrinol (Oxf) 2010;73(3):396–403. doi: 10.1111/j.1365-2265.2010.03818.x. [DOI] [PubMed] [Google Scholar]
- 10.Roef G, Lapauw B, Goemaere S, Zmierczak H, Fiers T, Kaufman JM, Taes Y. Thyroid hormone status within the physiological range affects bone mass and density in healthy men at the age of peak bone mass. Eur J Endocrinol. 2011;164(6):1027–1034. doi: 10.1530/EJE-10-1113. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ, 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992;7(9):1005–1010. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- 12.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 13.Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol (Oxf) 1991;34(1):77–83. doi: 10.1111/j.1365-2265.1991.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 14.Bensenor IM, Lotufo PA, Menezes PR, Scazufca M. Subclinical hyperthyroidism and dementia: the Sao Paulo Ageing & Health Study (SPAH) BMC Public Health. 2010;10:298. doi: 10.1186/1471-2458-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Prentice RL. On the design of synthetic case-control studies. Biometrics. 1986;42(2):301–310. [PubMed] [Google Scholar]
- 18.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 19.Cauley JA, Blackwell T, Zmuda JM, Fullman RL, Ensrud KE, Stone KL, Barrett-Connor E, Orwoll ES. Correlates of trabecular and cortical volumetric bone mineral density at the femoral neck and lumbar spine: the osteoporotic fractures in men study (MrOS) J Bone Miner Res. 2010;25(9):1958–1971. doi: 10.1002/jbmr.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 21.Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298(20):2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 22.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 23.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 24.Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab. 2008;93(8):2998–3007. doi: 10.1210/jc.2008-0167. [DOI] [PubMed] [Google Scholar]
- 25.Mariotti S. Mild hypothyroidism and ischemic heart disease: is age the answer? J Clin Endocrinol Metab. 2008;93(8):2969–2971. doi: 10.1210/jc.2008-1237. [DOI] [PubMed] [Google Scholar]
- 26.Vestergaard P, Mosekilde L. Fractures in patients with hyperthyroidism and hypothyroidism: a nationwide follow-up study in 16,249 patients. Thyroid. 2002;12(5):411–419. doi: 10.1089/105072502760043503. [DOI] [PubMed] [Google Scholar]
- 27.Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Ann Intern Med. 1999;130(9):750–758. doi: 10.7326/0003-4819-130-9-199905040-00016. [DOI] [PubMed] [Google Scholar]
- 28.Schneider R, Reiners C. The effect of levothyroxine therapy on bone mineral density: a systematic review of the literature. Exp Clin Endocrinol Diabetes. 2003;111(8):455–470. doi: 10.1055/s-2003-44704. [DOI] [PubMed] [Google Scholar]
- 29.Mosekilde L, Melsen F. A tetracycline-based histomorphometric evaluation of bone resorption and bone turnover in hyperthyroidism and hyperparathyroidism. Acta Med Scand. 1978;204(1–2):97–102. doi: 10.1111/j.0954-6820.1978.tb08406.x. [DOI] [PubMed] [Google Scholar]
- 30.Harvey RD, McHardy KC, Reid IW, Paterson F, Bewsher PD, Duncan A, Robins SP. Measurement of bone collagen degradation in hyperthyroidism and during thyroxine replacement therapy using pyridinium cross-links as specific urinary markers. J Clin Endocrinol Metab. 1991;72(6):1189–1194. doi: 10.1210/jcem-72-6-1189. [DOI] [PubMed] [Google Scholar]
- 31.Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, Davies TF, Zaidi M. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Vukicevic S, Baliram R, Yang G, Sendak R, McPherson J, Zhu LL, Iqbal J, Latif R, Natrajan A, Arabi A, Yamoah K, Moonga BS, Gabet Y, Davies TF, Bab I, Abe E, Sampath K, Zaidi M. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci U S A. 2008;105(11):4289–4294. doi: 10.1073/pnas.0712395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab. 2010;95 (1):186–193. doi: 10.1210/jc.2009-1625. [DOI] [PubMed] [Google Scholar]
- 34.Lewis CE, Ewing SK, Taylor BC, Shikany JM, Fink HA, Ensrud KE, Barrett-Connor E, Cummings SR, Orwoll E. Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22(2):211–219. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 35.Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, Braverman LE. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87 (7):3221–3226. doi: 10.1210/jcem.87.7.8678. [DOI] [PubMed] [Google Scholar]
- 36.Diez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab. 2004;89(10):4890–4897. doi: 10.1210/jc.2003-032061. [DOI] [PubMed] [Google Scholar]
- 37.Rosario PW. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 mIU/l: a prospective study. Clin Endocrinol (Oxf) 2010;72(5):685–688. doi: 10.1111/j.1365-2265.2009.03696.x. [DOI] [PubMed] [Google Scholar]
- 38.Das G, Ojewuyi TA, Baglioni P, Geen J, Premawardhana LD, Okosieme OE. Serum thyrotrophin at baseline predicts the natural course of subclinical hyperthyroidism. Clin Endocrinol (Oxf) 2012;77(1):146–151. doi: 10.1111/j.1365-2265.2012.04345.x. [DOI] [PubMed] [Google Scholar]
- 39.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783–787. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]


