Abstract
Objectives
To develop prediction models to help counsel post-radical prostatectomy patients about functional recovery.
Methods
The study included 2162 patients undergoing radical prostatectomy at a major cancer center who reported urinary and erectile function at one year or at two years and at least 1 prior follow-up at 3, 6, 9, or 12 months. We created logistic regression models predicting function at one or two years on the basis of function at 3, 6, 9, and 12 months (2 years only), with the additional predictors of age, stage, grade, PSA, nerve-sparing status and baseline functional score.
Results
No variable other than current functional score had a consistent, statistically significant relationship with outcome. The area-under-the-curves for predicting function at 2 years based on current function alone at 3, 6, 9, and 12 months were, respectively, 0.796, 0.831, 0.882, and 0.885 for erectile function and 0.789, 0.862, 0.869 and 0.876 for urinary function. Patients using one pad at 6 months had only a 50% probability of being pad free at 2 years; this dropped to 36% for patients using 2 pads. This suggests that there is an opportunity for early identification and possible referral of patients likely to have long-term urinary dysfunction.
Conclusions
Assessment of urinary and erectile function in the first post-operative year is strongly predictive of long-term outcome and can guide patient counseling and decisions about rehabilitative treatments.
Keywords: radical prostatectomy, urinary function, erectile function, prediction
Introduction
The risk of persistent urinary and erectile dysfunction is a major concern for patients undergoing radical prostatectomy and an important source of anxiety in the months following surgery. It is common for patients who do not recover function early to ask clinicians about their likely prognosis.
It seems reasonable to suppose that current function and time since surgery would be strong predictors of eventual recovery. For example, it is likely that a man using one pad a day at 3 months has a better chance of being pad-free at one year than a man still using three pads at 9 months. Yet clinical practice does not seem to have formally incorporated these predictors: patients are typically told only that recovery can take time and that many patients do regain good function even if function is initially poor. Published prediction models for erectile or urinary recovery predominately concern pre-operative function and are intended to be used as tools to aid initial treatment decision making1-7.
We aimed to develop prediction models to help counsel post-radical prostatectomy patients about functional recovery. We hypothesized that current function would be highly predictive of future status and that other patient, surgical and cancer-related variables would not importantly improve predictive accuracy.
Materials and Methods
We aimed to create separate predictive models for the outcomes of erectile and urinary function at one and two years after surgery using patient, cancer and operative variables, as well as current functional score. Models were created for the landmark time points of 3, 6, 9, or 12 months, which are typical times for post-treatment follow-up. Data was obtained under a waiver from the Memorial Sloan-Kettering Cancer Center IRB.
We identified 2162 patients undergoing a radical prostatectomy between 2007 and 2012 who had follow-up data – recorded for routine clinical assessment - on functional status at one year, defined as completion of at least one follow-up survey > 10 months and ≤ 14 months after surgery or two years, defined as >23 months and ≤ 27 months. All patients at MSKCC receive questionnaires with items on urinary and erectile function as a routine part of clinical follow-up. Not all patients complete these questionnaires, particularly patients who do not live in the New York area and who undergo postoperative follow-up at outside institutions. Completion rates at 1 and 2 years during the study period were 44% and 36%, with higher rates in more recent years (e.g. 62% at 1 year for patients treated in 2011) with the implementation of electronic patient-reported outcomes8. There were no statistically significant differences in age, tumor severity or baseline function between patients who did and did not provide data at one and two years. Of these patients, those missing outcome data at each time point were excluded from the corresponding models. Data were considered missing if no questionnaire was completed within 6 weeks of the landmark time. For erectile function this left 956, 1323, and 865 patients for the 3, 6, and 9 month models respectively; for urinary function, 954, 1319, and 868 patients provided data for the 3, 6, and 9 month models. Patients that were missing data on baseline erectile function (n=530), or operative or tumor variables (n=89), were excluded only from those models that included those predictors.
Patients at MSKCC receive relatively standard advice on post-operative rehabilitation. They are advised to undertake pelvic floor muscle exercises (“Kegel exercises”) and are provided written instructions on how to perform them. Patients are not typically referred to formal physical therapy unless urinary incontinence is persistent or they cannot adequately perform Kegel exercises. Patients are advised to use PDE5-inhibitors for cavernosal smooth muscle protection and also for the purposes of sexual activity. When they fail to obtain a penetration hardness erection with PDE5 inhibitors, intracavernosal injection therapy is routinely advised.
Our first goal was to create predictive models for erectile function at one and two years, defined as a score of 22 or greater on the International Index of Erectile Function (IIEF6) questionnaire. We compared two different logistic regression models at each landmark time point. The first model included as predictors age, PSA, pathological stage (pT2A, pT2B, or ≥pT3), pathological Gleason score (≤6, 7 or ≥8), along with baseline erectile function score (poor, moderate or good), nerve sparing status (both preserved vs. one damaged), and patient's current erectile function score (IIEF6 value at 3, 6, 9 or 12 months). Patients' current erectile function score was obtained through questionnaires given at routine clinical checkups. If patients took more than one survey within six weeks of a landmark time point, the survey response closest to the time point of interest was used. Patients' baseline erectile function score was categorized as poor, moderate or good based on surgeon grading. Restricted cubic splines with knots at the tertiles were used to allow for a non-linear association between outcome and both age and current erectile function score. The discrimination of the model was assessed in terms of the area-under-the-curve (AUC) and compared to a model including only current erectile function score.
A similar approach was used for predicting urinary function at one year after surgery. Patients were considered to be continent if they scored greater than 16 on a validated urinary function questionnaire (range 0 – 21) 8.
As a sensitivity analysis, we repeated our analyses removing patients who were functional at each landmark time point from the models. This left 777, 1025, and 636 patients at 3, 6 and 9 months for erectile function, and 539, 488, and 278 patients at these time points for urinary function. The rationale is that a patient who is, say, continent at 6 months, is highly likely to retain good urinary function and may not require counseling as to prognosis.
As an additional analysis, we repeated the above for urinary function but used pad free as the endpoint. At our institution, a validated urinary function questionnaire asks patients about their current pad usage, with responses of None, Occasional, 1, 2, 3+, or Diapers. A response of “None” to this question defined our outcome of interest for this analysis. Any other response was considered as a patient still using pads. As an additional secondary analysis, we repeated our analysis using pad free as the endpoint but only included the patient's current number of pads used per 24-hour period (None, Occasional, 1, 2, or 3+ and diapers) as a predictor in our model at each of the landmark time points. We were interested in determining if the patients current pad usage could also be used as a predictor of recovering urinary continence in place of a full questionnaire. All statistical analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
Results
Patient characteristics are described in Table 1. The cohort reflects the shift towards slightly more aggressive cases treated surgically at major academic centers in recent years. We did not find any significant differences between baseline or follow-up characteristics for patients who were missing functional data at 3, 6, or 9 months compared to those who had data available. For example, 70% of those missing data at 6 months were continent at one year compared to and 72% of patients of those with 6 month data available (p=0.3).
Table 1.
Patient Characteristics. Frequency (%) or Median (IQR)
| n=2162 | |
| Patient Age | 64.2 (58.8, 68.8) |
| Preoperative PSA | 5.0 (3.5, 7.0) |
| Pathological Stage | |
| T2A | 219 (11%) |
| T2B | 1010 (50%) |
| ≥ T3 | 803 (40%) |
| Pathological Gleason Grade | |
| ≤ 6 | 416 (19%) |
| 7 | 1536 (71%) |
| ≥ 8 | 202 (9.4%) |
| Nerve Sparing Status | |
| Both Preserved | 657 (30%) |
| One Damaged | 1498 (70%) |
| Surgical Approach | |
| Open | 859 (40%) |
| Laparoscopic | 546 (25%) |
| Robotic | 757 (35%) |
| Baseline Erectile Function: Surgeon Score | |
| Poor | 333 (21%) |
| Moderate | 326 (20%) |
| Good | 937 (59%) |
| Baseline Urinary Function: Surgeon Score | |
| Poor | 36 (1.7%) |
| Good | 2126 (98%) |
| Regained Erectile Function at 1-Year | 574 (29%) |
| Regained Urinary Function at 1-Year | 1432 (71%) |
| Regained Erectile Function at 2-Years | 440 (36%) |
| Regained Urinary Function at 2-Years | 912 (74%) |
Supplementary Table 1 shows the results of the logistic regression models to predict erectile and urinary function at one year. It is clear that while current function is highly statistically significant, no other variable had a consistent, statistically significant relationship with outcome. As an additional test, we added surgical approach to our model and found that it also did not have a consistent, statistically significant relationship with outcome. The AUCs for the models when only patients' current functional score was included are presented in supplementary Table 2. Similar results were found at both one and two years, therefore we only included current functional score for those models as well.
As a patient's probability of regaining function at one year is largely explained by current functional score, prediction models can be shown graphically (Figures 1 and 2). We can see that as the patient's current functional score increases, their probability of regaining function at 1 year also rises. For example, two patients with IIEF-6 scores of 5 and 18 at 6 months would both be classified as having erectile dysfunction. However, their probability of good function at 12 months is <10% vs. close to 40%, respectively.
Figure 1.
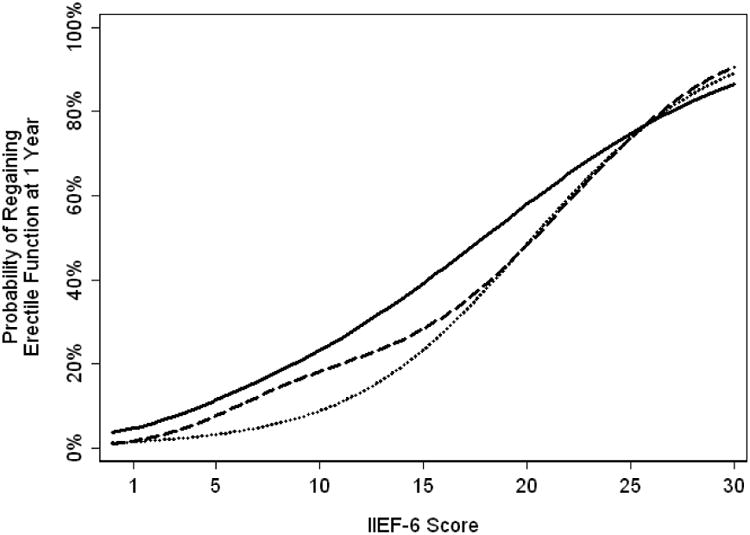
Probability of regaining erectile function at one year based on functional score at 3 months (Solid), 6 months (Dashed) or 9 months (Dotted).
Figure 2.
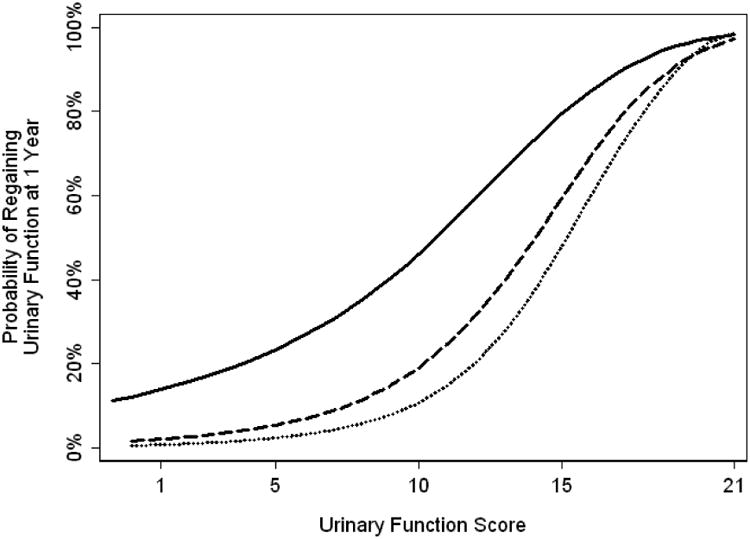
Probability of regaining urinary function at one year based on functional score at 3 months (Solid), 6 months (Dashed) or 9 months (Dotted).
The AUCs for our sensitivity analysis are presented in supplementary Table 2. The lower AUCs found for these models reflect that patients who have already regained function prior to one year will usually retain their function at one year. However, discrimination remains highly acceptable for patient counseling. Similar results were seen for our models at two years.
For our additional analysis using pad free, rather than function score, as the endpoint, results were very similar. AUCs for the one year model were lower by about 0.02 (0.835, 0.859 and 0.878 for 3, 6 and 9 months respectively). When we included the patients current number of pads used per 24-hour period, we found it to be a significant predictor of a patient being pad free at one year for 3, 6, and 9 months (p<0.0005). The AUCs for current number of pads at one and two years are presented in supplementary Table 2. Table 2 shows the probability urinary function at one and two years based on current pad usage. These estimates can be applied irrespective of the urinary function questionnaire used by a urologist. Patients using one pad at 6 months had only a 50% probability of being pad free at 2 years; this dropped to 36% for patients using 2 pads. This suggests that there is an opportunity for early identification and possible referral of patients likely to have long-term urinary dysfunction.
Table 2.
Probability (95% CI) of urinary dysfunction at 1 and 2 years based on number of pads at 3, 6 and 9 months. Cells are left blank where no patients reached continence. For example, no patient in our sample was using 2 pads at 9 months but zero pads at 12 months.
| Number of Pads | Endpoint (months) | 3 months | 6 months | 9 months | 12 months | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pad-free | Pad-free or occasional pad | Pad-free | Pad-free or occasional pad | Pad-free | Pad-free or occasional pad | Pad-free | Pad-free or occasional pad | ||
| None | 12 | 99% (96%, 100%) | 99% (96%, 100%) | 96% (94%, 98%) | 99% (98%, 100%) | 95% (92%, 97%) | 99% (98%, 100%) | - | - |
| 24 | 96% (90%, 98%) | 98% (94%, 100%) | 94% (90%, 96%) | 98% (95%, 99%) | 94% (91%, 96%) | 99% (97%, 100%) | 95% (92%, 97%) | 99% (98%, 100%) | |
| Occasional | 12 | 77% (67%, 85%) | 93% (85%, 97%) | 56% (45%, 66%) | 98% (91%, 99%) | 53% (42%, 64%) | 95% (86%, 98%) | - | - |
| 24 | 78% (64%, 88%) | 96% (84%, 99%) | 64% (50%, 76%) | 98% (87%, 100%) | 58% (44%, 71%) | 96% (85%, 99%) | 54% (40%, 67%) | 90% (78%, 96%) | |
| 1 | 12 | 63% (55%, 70%) | 80% (73%, 86%) | 31% (24%, 39%) | 47% (38%, 55%) | 2% (1%, 8%) | 22% (15%, 31%) | - | - |
| 24 | 73% (63%, 81%) | 83% (74%, 90%) | 50% (38%, 62%) | 62% (50%, 72%) | 22% (14%, 34%) | 40% (28%, 52%) | 17% (10%, 28%) | 34% (24%, 47%) | |
| 2 | 12 | 49% (38%, 60%) | 71% (60%, 80%) | 21% (11%, 36%) | 31% (18%, 47%) | - | 4% (1%, 22%) | - | - |
| 24 | 71% (57%, 82%) | 81% (68%, 90%) | 36% (20%, 55%) | 39% (23%, 58%) | 13% (4%, 32%) | 17% (6%, 37%) | 8% (1%, 39%) | 15% (4%, 45%) | |
| 3+ | 12 | 18% (11%, 28%) | 33% (23%, 44%) | - | 3% (1%, 18%) | - | - | - | - |
| 24 | 23% (12%, 39%) | 33% (20%, 49%) | - | 4% (1%, 25%) | 6% (1%, 31%) | 11% (3%, 35%) | 10% (3%, 32%) | 10% (3%, 32%) | |
Comment
We have found that patient-reported functional outcomes in the months following radical prostatectomy are very strong predictors of long-term outcome, with AUCs typically above 0.85. Moreover, we found that once current function is known, other variables do not aid prediction, even those known to have a strong causal effect, such as nerve-sparing. This illustrates the statistical truism that “how you are” is generally a stronger predictor than “what you are”: as a simple illustration, a runner's time for the marathon is strongly predicted by their time for a shorter distance; predictors such as age, weight or training miles have only marginal additional informative value.
There is a dearth of literature on postoperative prediction of functional recovery after radical prostatectomy. In a review of the literature focusing on erectile function, Briganti et al.1 reported that papers assessing characteristics associated with recovery of function were associated almost exclusively with pre- and perioperative prediction. For example, Alemozaffar et al. built predictive models for recovery of erectile function at two years after surgery, radiotherapy and brachytherapy for prostate cancer based on factors such as pretreatment function, age, body mass index and planned aspects of treatment, such as nerve-sparing and neoadjuvant therapy2. Kilminster et al. similarly used preoperative variables to plot the predicted course of erectile recovery after surgery3. Both Marien et al. 4 and Briganti et al.5 additionally explored comorbidities as predictors of erectile function. Other authors have used comparable approaches to predict urinary outcomes6,7.
We have been able to find only three papers that use postoperative factors to make predictions about long-term outcome. Abdollah et al. took a conditional survival approach noting that a patient's probability of regaining function within the subsequent six months decreases dramatically as time since surgery increases. For example, a patient impotent at six months has about a one in four chance of recovering potency by one year; in contrast, a patient impotent at 18 months has only about a 1 in 12 chance of recovering by two years9. One drawback of these results is that they do not take into account current level of function. For instance, we would predict a higher probability of long-term erectile dysfunction in a patient with no erections at all compared to a patient who did have some erections, although not sufficient for intercourse. Kimura et al. did assess sexual function outcomes on a continuous scale, predicting outcomes at 20 months based on scores at 3 months10. That said, their choice of outcome – percent decrease in function - is problematic. A decline in function of, say, 20%, has markedly different implications for a patient fully potent at baseline compared to borderline function or even poor function. We believe that patients are interested predominately in the probability that they will have adequate function on long-term follow-up. Lastly, Jeong at el.11 found that in patients who were incontinent at 1 year, only the number of pads used at 1 year was significantly associated with further recovery of urinary continence, which is consistent with our results. However, findings were only reported for 1 pads versus more than one pad and only at one year follow-up; here we provide a more complete picture of how current function, both urinary and erectile, predicts follow-up, at both one and two years, based on function at various time-points after surgery.
A possible limitation of our findings is that the models presented in figures 1 and 2 have not been externally validated. It is possible, for example, that either the time course or absolute rates of functional recovery may vary between centers.
Another possible limitation of our study is that baseline function was assessed by surgeons rather than by patients themselves. It is plausible that a more accurate assessment may have lead to baseline function becoming a significant predictor in the model. To test this hypothesis, we created a model predicting erectile function at 24 months. Function at 3 months was not statistically significant after adjusting for function at 12 months. It seems highly improbable that baseline function would be an important predictor if function after surgery was not.
There are two major clinical implications of our findings. First, our models can be used in patient counseling. Patients continue to request information about prognosis after surgery and, given our data, we would question the use of standard bromides about “recovery sometimes taking time”. For instance, a patient who requires pads at 6 months may ask whether he will always have urinary difficulties; use of our models can allow a more quantitative answer. For example, if the man is use an occasional pad, he has a about a 55% chance of being pad free at one year; this probability falls, respectively, to about 30% and 20% for use of 1 and 2 pads daily. Probability of “social continence”, that is, use of no or an occasional pad, is 47%, and 28%, for use of 1, or 2 pads respectively. Second, the models can be used for early intervention. A patient for whom it is known that, given his current status, he is unlikely to recover good function might be referred for sexual rehabilitation or evaluation for incontinence procedures such as a sling or artificial urinary sphincter.
Conclusions
Patient-reported urinary and erectile function in the first months after radical prostatectomy is an extremely strong predictor of whether a patient will eventually regain potency and continence. Other predictors, including age, baseline function, tumor characteristics and nerve-sparing status, do not importantly add predictive value, once current status is known. Assessment of urinary and erectile function in the first post-operative year can guide patient counseling and decisions about rehabilitative treatments
Supplementary Material
Supplementary material
Acknowledgments
Supported in part by funds from David H. Koch provided through the Prostate Cancer Foundation, the Sidney Kimmel Center for Prostate and Urologic Cancers and P50-CA92629 SPORE grant from the National Cancer Institute to Dr. P. T. Scardino.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Briganti A, Capitanio U, Chun FK, et al. Prediction of sexual function after radical prostatectomy. Cancer. 2009;115:3150–9. doi: 10.1002/cncr.24349. [DOI] [PubMed] [Google Scholar]
- 2.Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306:1205–14. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilminster S, Muller S, Menon M, et al. Predicting erectile function outcome in men after radical prostatectomy for prostate cancer. BJU Int. 2012;110:422–6. doi: 10.1111/j.1464-410X.2011.10757.x. [DOI] [PubMed] [Google Scholar]
- 4.Marien T, Sankin A, Lepor H. Factors predicting preservation of erectile function in men undergoing open radical retropubic prostatectomy. J Urol. 2009;181:1817–22. doi: 10.1016/j.juro.2008.11.105. [DOI] [PubMed] [Google Scholar]
- 5.Briganti A, Gallina A, Suardi N, et al. Predicting erectile function recovery after bilateral nerve sparing radical prostatectomy: a proposal of a novel preoperative risk stratification. J Sex Med. 2010;7:2521–31. doi: 10.1111/j.1743-6109.2010.01845.x. [DOI] [PubMed] [Google Scholar]
- 6.Moore KN, Truong V, Estey E, et al. Urinary incontinence after radical prostatectomy: can men at risk be identified preoperatively? J Wound Ostomy Continence Nurs. 2007;34:270–9. doi: 10.1097/01.WON.0000270821.91694.56. quiz 280-1. [DOI] [PubMed] [Google Scholar]
- 7.Sacco E, Prayer-Galetti T, Pinto F, et al. Urinary incontinence after radical prostatectomy: incidence by definition, risk factors and temporal trend in a large series with a long-term follow-up. BJU Int. 2006;97:1234–41. doi: 10.1111/j.1464-410X.2006.06185.x. [DOI] [PubMed] [Google Scholar]
- 8.Vickers AJ, Savage CJ, Shouery M, et al. Validation study of a web-based assessment of functional recovery after radical prostatectomy. Health Qual Life Outcomes. 2010;8:82. doi: 10.1186/1477-7525-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdollah F, Sun M, Suardi N, et al. Prediction of functional outcomes after nerve-sparing radical prostatectomy: results of conditional survival analyses. Eur Urol. 2012;62:42–52. doi: 10.1016/j.eururo.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 10.Kimura M, Banez LL, Schroeck FR, et al. Factors predicting early and late phase decline of sexual health-related quality of life following radical prostatectomy. J Sex Med. 2011;8:2935–43. doi: 10.1111/j.1743-6109.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeong SJ, Kim HJ, Kim JH, et al. Urinary continence after radical prostatectomy: predictive factors of recovery after 1 year of surgery. Int J Urol. 2012;19:1091–8. doi: 10.1111/j.1442-2042.2012.03106.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


