Abstract
Background
Resveratrol exhibits beneficial effects against numerous degenerative diseases at different stages of pathogenesis. This study investigated potential mechanisms and resveratrol effects on high glucose (HG)-induced oxidative stress (30 mM d-glucose, 30 min) and cell proliferation (30 mM d-glucose, 24 h) in vascular smooth muscle cells (VSMCs).
Material/Methods
Intracellular reactive oxygen species (ROS) generation was detected by 2′,7′-dichlorofluorescein diacetate (DCFH-DA). Total antioxidant capacity (TAC), malonyldialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) were measured to evaluate oxidative stress. VSMC proliferation was measured by CCK-8 assays and through propidium iodide-based cell cycle analysis. Expression of NAD(P)H oxidase, proliferation proteins, and cell signalling were assessed by immunoblot analysis.
Results
Co-treatment of primary cultures of VSMCs with 1–100 μM resveratrol decreased HG-induced ROS overproduction (P<0.05). Resveratrol also abolished HG-induced phosphorylation of oxidase subunit p47 phox and reduced HG-induced cyclin D1, cyclin E, and PCNA expression in a concentration-dependent manner. Furthermore, resveratrol (10 μM) attenuated HG-induced phosphorylation of Akt, p38 mitogen-activated protein kinase (MAPK), ERK 1/2, and JNK1/2 without affecting total levels. HG stimulation enhanced downstream IκB-α phosphorylation and NF-κB activity, and resveratrol repressed these effects.
Conclusions
Resveratrol inhibits HG-induced oxidative stress and VSMC proliferation by suppressing ROS generation, NADPH oxidase, Akt phosphorylation, p38 MAPK/JNK/ERK phosphorylation, and IκB-α and NF-κB activities.
MeSH Keywords: Cell Proliferation, Grape Seed Extract, Hyperglycemia, Muscle, Smooth, Vascular, Oxidative Stress
Background
Diabetes (DM) is, by common consent, a serious global public health problem. DM and its complications (e.g., nephropathy, retinopathy, neuropathy, impaired wound healing, and accelerated atherosclerosis) are associated with many cellular and subcellular changes in the vessels [1]. It is generally accepted that vessels affected by diabetes exhibit altered vasomotor function [2]. In particular, vascular smooth-muscle cells (VSMCs) in patients with type 2 diabetes play a critical role in numerous cardiovascular pathologies [3].
VSMCs of blood vessel walls, through their inherent ability to switch between a contractile quiescent phenotype and an active secretory state, maintain vascular homoeostasis in health and development [4]. This plasticity also gives VSMCs the essential capacity to adapt and be remodelled in pathological states. Emerging clinical and experimental evidence suggests that VSMCs in diabetes may be functionally impaired and thus contribute to the increased incidence of macrovascular complications [5,6].
The pathogenesis of vascular complications in type 2 diabetes is multifactorial, with the principal contributors being oxidative stress, dyslipidemia, and hyperglycemia [7]. Elevated blood glucose levels drive production of reactive oxygen species (ROS) via multiple pathways, resulting in uncoupling of mitochondrial oxidative phosphorylation and endothelial NO synthase (eNOS) activity, reducing NO availability and generating further ROS [8]. These lipid abnormalities contribute to increasing oxidative stress and may directly inhibit eNOS activity. Furthermore, lipid deposition and oxidative stress will result in vascular damage, which triggers an inflammatory reaction, and the subsequent release of chemoattractants and cytokines increases vascular dysfunction.
Resveratrol, a potent Sirtuin1 (SIRT1) activator, has been reported to produce beneficial effects against numerous degenerative diseases at different stages of pathogenesis [9,10] and can act at multiple levels (e.g., cellular signalling, enzymatic pathways, apoptosis, and gene expression) [11]. Despite extensive evidence of the role of resveratrol in the prevention and treatment of cardiovascular diseases, the mechanism and precise link between resveratrol and its protective effects on VSMCs in diabetes are not completely understood. In the present study, we hypothesized that resveratrol can attenuate high glucose (HG)-induced oxidative stress and cell proliferation, and we attempted to explore the molecular mechanisms underlying such a potential protective effect of resveratrol.
Material and Methods
Material
Unless otherwise stated, all the chemical reagents used in this study were purchased from Sigma Chemical Co. (St. Louis, USA).
Cell culture
Protocols involving isolation and culture of VSMCs from rat aorta were approved by the Laboratory Centre of Shanghai Tenth People’s Hospital in accordance with guidelines for animal experiments from the Committee of Medical Ethics at the National Health Department of China. VSMCs were isolated from the aorta of 6- to 8-week old male SD rats by enzymatic digestion method [12]. In vitro primary culture of VSMCs was validated by assessing for expression of α-smooth muscle cell actin. Cells were grown in Dulbecco modified Eagle’s medium containing 25 mM HEPES (Gibco, Invitrogen, Carlsbad, CA) with 10% penicillin-streptomycin-amphotericin B and 10% fetal bovine serum (FBS, HyClone, Logan, UT) at 37°C in 5% CO2. Experiments were conducted on VSMCs between passages 6–10. Resveratrol was dissolved in dimethylsulphoxide (DMSO) at a stock concentration of 104 M and stored at −20°C. Further dilutions were made in cell culture medium. The final working concentration of DMSO in cell culture experiments did not exceed 0.125%. All control conditions for in vitro experiments contained 0.125% DMSO as vehicle control.
Cell viability assay
The viability of VSMCs was determined by using the Cell Count Kit-8 (CCK-8), according to the manufacturer’s directions (Beyotime Institute of Biotechnology, Jiangsu, China). CCK-8, being nonradioactive, utilizes sensitive colorimetric assays to determine the number of viable cells, cell proliferation, and cytotoxicity assays. VSMC samples (100 μL) were seeded in 96-well plates at a density of 1–2×104 cells/mL for 24 h followed by resveratrol treatment for 24 h. Then, 10 μL of CCK-8 reagent was added to each well for 2 h, and the absorbance at 450 nm was measured using a microplate reader (BioTek, Winooski, VT, USA).
Measurement of ROS
The level of intracellular ROS was measured with the fluorescent probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA, Beyotime Institute of Biotechnology, Jiangsu, China). VSMCs were seeded onto a 24-well plate at a density of 1–2×105 cells per well. After synchronization for 24 h, the cells were treated with or without resveratrol for 24 h and stimulated with 30 mM glucose (d-glucose) for 30 min. The fluorescent probe DCFH-DA was added to each well at a final concentration of 10 μM and incubated at 37°C for 20 min in the dark. The plate was washed 3 times with PBS, trypsinized, re-suspended, and then immediately subjected to fluorescence microscopy and flow cytometry (FCM) analyses. Fluorescence was subsequently imaged using a fluorescent microscope (Leica DMI6000, Leica, Germany) and detected using a microplate reader (Tecan Infinite 200) and FCM (EPICS-XL, Beckman Coulter, Fullerton, USA). The wavelengths of excitation and emission used to detect the ROS were 514 nm and 529 nm, respectively.
Measurement of biomarkers of oxidative stress
Cells were pretreated with or without 1–100 μM resveratrol for 24 h followed by stimulation with 30 mM glucose (d-glucose) for 30 min. The VSMCs were collected and incubated on ice in 500 μL of cell lysis buffer (1 mM EDTA, 10 mg/mL aprotinin, 0.5 mg/mL leupeptin, 0.7 mg/mL pepstatin, and 0.5 mM PMSF, pH 7.0). After centrifugation at 10 000 × g for 5 min, supernatant was collected to determine malonyldialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH), and the total antioxidant capacity (TAC) levels by using commercial kits (Jian Cheng Biological Engineering Institute, Nanjing, China).
Protein extraction and Western blot analysis
Both total and nuclear protein contents were analyzed by Western blotting. Nuclear NF-κB p65 was extracted from VSMCs by using a Nuclear Extraction Kit (Beyotime Biotech Inc., Jiangsu, China). To perform the Western blot experiments, VSMCs were removed from plates after treatments using lysis solution with 4% SDS, 2 mM EDTA, and 50 mM Tris-HCl, pH 6.8. The homogenates were centrifuged at 15 000 × g for 20 min at 4°C, following which the supernatants were collected and removed for protein quantification using the Bradford method. Equal amounts of protein (50 μg) were separated by 8% or 10% SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes, which were then washed with Tris-buffered saline, blocked with 5% non-fat dry milk (except for phosphorylated myosin phosphatase targeting protein, which was blocked using 5% BSA) in Tris-buffered saline Tween-20 for 1 h, and incubated with the appropriate primary antibody at dilutions recommended by the supplier.
The membrane was then washed, and primary antibodies were detected with a secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. The blots were then developed with SuperSignal-enhanced chemiluminescent substrate solution (Pierce Chemical Company, Rockford, USA). Anti-β-actin, anti-phosphorylated p47 phox (anti-p-p47 phox), anti-phosphorylated ERK 1/2 (anti-p-ERK 1/2), anti-ERK 1/2, anti-phosphorylated JNK 1/2 (anti-p-JNK 1/2), anti-JNK 1/2, anti-phosphorylated p38 mitogen-activated protein kinase (MAPK) (anti-p-p38 MAPK), anti-p38 MAPK, anti-phosphorylated AKT (anti-p-Akt), anti-AKT, anti-phosphorylated IκB-α (anti-p-IκB-α), and anti-NF-κB antibodies were purchased from Cell Signaling Technology (Danvers, Massachusetts, USA).
Cell cycle analysis
Cellular DNA content was measured by FCM by using propidium iodide. In total, 4×105 cells were treated with HG (30 mM, d-glucose) in the presence or absence of resveratrol for 24 h. Cells were then harvested by centrifugation and fixed with 70% ethanol at 4°C overnight. Cells were subsequently resuspended in PBS with 0.1% FBS and were washed twice with PBS. Finally, cells were suspended in PBS-EDTA containing propidium iodide (10 μg/mL), incubated at 37°C for 10 min following a 1-h treatment with RNase (0.1 mg/mL), and analyzed by flow cytometry. DNA histograms were analyzed by FlowJo software (version 7.6.5).
Statistical analysis
All quantitative data and experiments described in this study were repeated at least 3 times. Results are expressed as mean ± SD. Differences between groups were analyzed statistically by 2-way analysis of variance (ANOVA) followed by Tukey post hoc test using SPSS (Statistical Package for the Social Sciences software, version 16.0 for Windows). Values of P<0.05 were considered statistically significant.
Results
Effect of resveratrol on cell viability
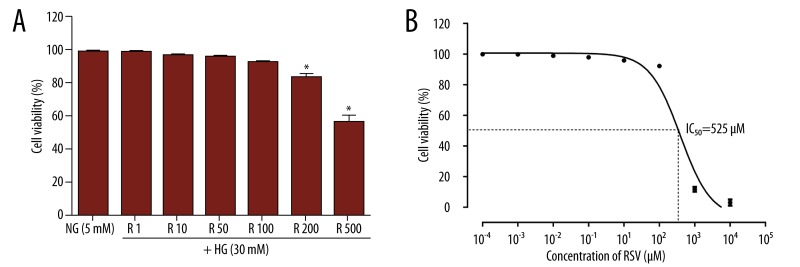
In experiments performed with media containing 10% FBS, no significant differences were observed in cell viability of VSMCs containing 1–100 μM resveratrol compared with the control (Figure 1A). However, VSMC viability was markedly inhibited in the presence of 200 and 500 μM resveratrol compared with control VSMCs or VSMCs treated with 100 μM resveratrol. Additionally, resveratrol alone reduced cell number by 50% at a concentration of 525 μM (Figure 1B). Hence, for all subsequent experiments, primary cultures of VSMCs were treated with 1–100 μM resveratrol.
Figure 1.
Parameters of viability in the VSMCs incubated with different concentrations of resveratrol. (A) Cells were incubated for 24 h with resveratrol from 1 to 500 μM in the incubation medium, as described in the Materials and Methods section, and viability was assessed by CCK-8 assay. (B) Cytotoxic effects of resveratrol on VSMCs at various concentrations. Values are expressed as means ±S.D. of triplicate experiments. R: resveratrol. * P<0.05 indicates significant differences from the NG (5 mM) group.
Resveratrol reduced HG-induced ROS formation and oxidative stress in cultured VSMCs
Intracellular ROS production in VSMCs was remarkably increased in the presence of HG for 30 min (Figure 2A). However, resveratrol pretreatment resulted in a significant decrease in ROS generation (Figures 2A, 2B). In VSMCs treated with different concentrations of resveratrol (1–100 μM), the fluorescence intensity was significantly reduced (Figure 2B, all P<0.05).
Figure 2.
Inhibitory effect of resveratrol on HG-induced ROS overproduction and p47 phox expression in VSMCs. Cells were pretreated with resveratrol for 24 h prior to HG exposure for 30 min. ROS were detected using a 2′,7′-dichlorofluorescein diacetate (DCFH-DA) probe. (A) Representative images of ROS fluorescence in different groups: NG (5 mM) group, HG (30 mM) group, and groups of VSMCs treated with different concentrations of resveratrol (1, 10, 50, and 100 mM) prior to HG exposure. (B) Fluorescence intensity measured by spectrofluorometer. (C) Blotting data for phospho-p47 phox and β-actin protein expressions from the VSMCs. Values are expressed as means ±S.D. from 3 independent experiments. Bar: 200 μm. # P<0.05 indicates significant difference from the HG (30 mM) group. * P<0.01 indicates significant difference from the HG (30 mM) group.
We next evaluated the effect of resveratrol on HG-induced NAD(P)H oxidative stress. Because NAD(P)H oxidase subunit activation is required for HG-evoked ROS production in vascular cells, we examined the phosphorylation status of the oxidase subunits as reported in a previous study [13]. Serine phosphorylation of the p47 phox subunits increased after HG stimulation, compared with normal glucose levels, and this increase was markedly repressed by resveratrol treatment (Figure 2C).
FCM results showed that stimulation with high glucose induced a massive ROS production in the VSMCs (Figures 3A, 3B), while pretreatment with resveratrol significantly inhibited ROS overproduction in a dose-dependent manner (Figure 3B, all P<0.05).
Figure 3.
FCM results of intracellular ROS production and effects of resveratrol on other redox parameters after HG stimulation. ROS were detected using a 2′,7′-dichlorofluorescin diacetate (DCFH-DA) probe by FCM. (A) Representative images of FCM in VSMCs treated with or without different concentrations of resveratrol (1, 10, and 100 mM) prior to HG exposure. (B) Quantitative analysis of the ratio of DCFH-DA probe positive cells. (C) SOD, (D) MDA, (E) GSH and (F) TAC were assessed as described in the Material and Methods section. R: resveratrol. * P<0.05 indicates significant difference from the HG (30 mM) group. # P<0.01 indicates significant difference from the HG (30 mM) group.
Changes in oxidative stress biomarkers
The levels of oxidative stress biomarkers of VSMCs were significantly different (all P<0.05). Figure 3 shows the comparison of MDA, SOD, GSH, and TAC levels in the supernatant of both resveratrol-treated and non-treated VSMCs. The levels of SOD, GSH, and TAC were significantly lower in the VSMCs incubated with HG than in resveratrol-treated VSMCs, whereas the levels of MDA were significantly higher in VSMCs incubated with HG compared with VSMCs treated with resveratrol (P<0.01).
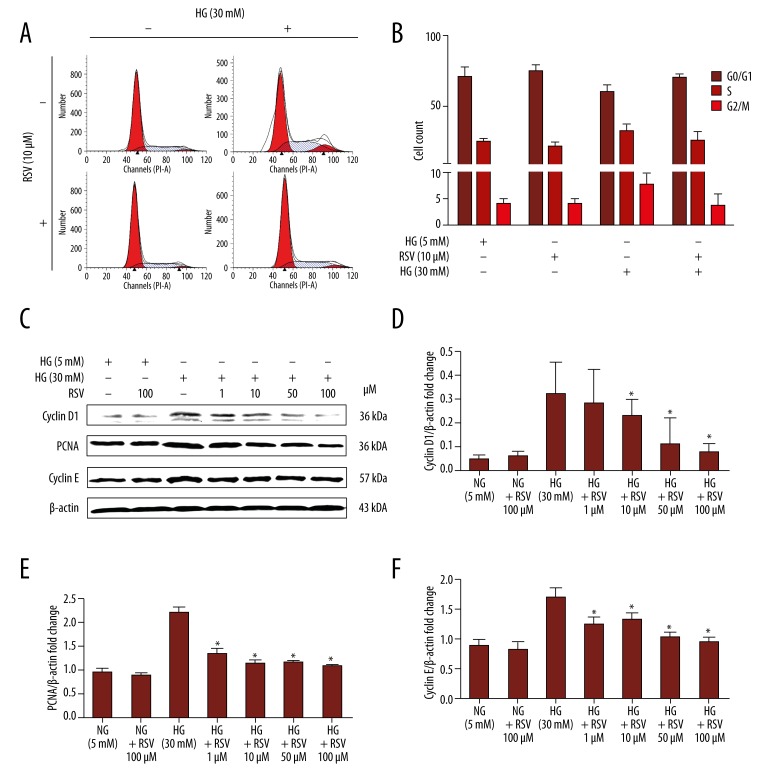
Effect of resveratrol on cell cycle
To further elucidate the mechanism by which treatment with resveratrol resulted in a relative decrease in cell numbers, the effect of resveratrol on cell cycle was examined. The percentages of cells in phases G1, S, and G2/M of the cell cycle were determined in trypsinized cells from VSMC cultures treated with various concentrations of resveratrol for 24 h. The percentage of cells in S and G2/M phases increased to 32.1±1.6% and 7.6±1.4%, respectively, in VSMCs treated with HG (Table 1). Resveratrol significantly reduced the percentages of cells in S phase to 28.7±1.4% and 25.9±1.7% after HG stimulation at 10–100 μM (all P<0.05). The percentage of cells in G2/M phase was reduced from 7.6% for the HG group to 5.5% with 10 μM resveratrol (P<0.05) and to 3.7% with 100 μM resveratrol (P<0.01). Figure 4A shows flow cytometry tracings from a representative experiment. Significant arrests in the S phase were observed following treatment with 10 μM resveratrol (Figure 4A, 4B). A concomitant decrease in the percentage of cells in G2/M phase was also observed (Figure 4A, 4B, P<0.01).
Table 1.
Effect of resveratrol on the cell cycle of VSMCs.
| Resveratrol (μM) | Glucose (mM) | % G1 (SD) | % S (SD) | % G2/M (SD) |
|---|---|---|---|---|
| 0 | 5 | 70.6 (7.1) | 25.4 (2.2) | 4.0 (1.1) |
| 0 | 30 | 60.3 (4.8) | 32.1 (1.6) | 7.6 (1.4) |
| 1 | 30 | 61.5 (3.3) | 30.8 (2.1) | 7.7 (1.5) |
| 10 | 30 | 65.8 (6.2) | 28.7 (1.4)# | 5.5 (1.1)# |
| 50 | 30 | 69.1 (1.1) | 27.8 (1.3)# | 4.8 (1.6)# |
| 100 | 30 | 70.4 (2.3) | 25.9 (1.7)* | 3.7 (1.2)* |
Values are mean (SD) of the results from 4 separate experiments, each with duplicate or triplicate cultures.
P<0.05 indicates significant differences from the HG (30 mM) group;
P<0.01 indicates significant differences from the HG (30 mM) group.
Figure 4.
Effect of resveratrol on HG-induced VSMC proliferation and expression of proliferation-related proteins. (A) Representative histograms showing cell cycle distribution upon HG treatment (30 mM, 24 h) in the presence or absence of resveratrol (10 μM). (B) Bar graph summarizing cell cycle data. (C) Representative immunoblot detecting levels of expression of cyclin D1, PCNA, and cyclin E in HG-treated VSMCs in the presence or absence of resveratrol (10 μM). (D–F) Quantified band density for cyclin D1, PCNA, and cyclin E. Values are expressed as means ±S.D. from 3 independent experiments. * P<0.01 indicates significant difference from the HG (30 mM) group.
Additionally, results demonstrated that resveratrol-induced cell cycle arrest is associated with a decrease of kinase activities associated with cyclins and PCNA (Figure 4C–4F). Resveratrol treatment of the VSMCs at 24 h resulted in a dose-dependent decrease in the expression of cyclin D1 and proliferating cell nuclear antigen (PCNA), as well as cyclin E (P<0.05).
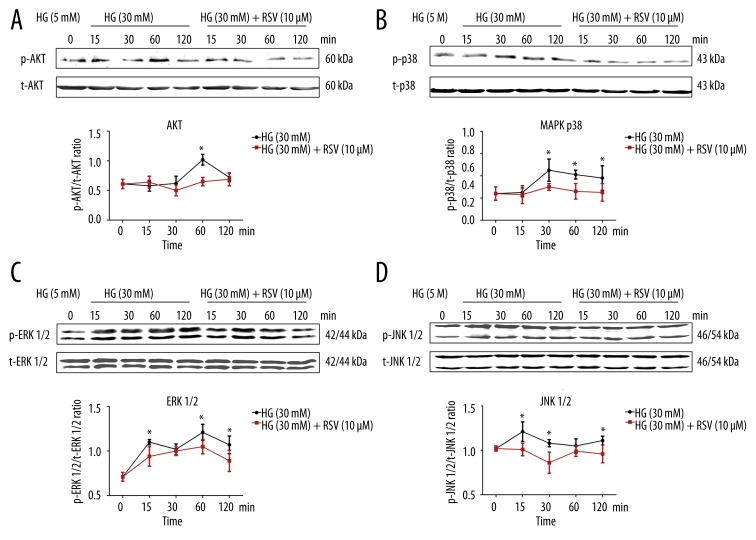
Effect of resveratrol on HG-induced cell signalling
To investigate the effect of resveratrol on the PI3K/Akt signalling pathway, protein expression of phospho-Akt was measured by immunoblotting (Figure 5A). The protein expression of phospho-Akt was markedly increased by incubation of cells with 30 mM glucose (d-glucose) for 1 h. This effect was attenuated by pretreatment with 10 μM resveratrol.
Figure 5.
Western blot data showing the effects of 10 μM resveratrol on phosphorylation of Akt (A), p38 MAPK (B), ERK 1/2 (C), and JNK 1/2 (D) in 30 mM HG-exposed VSMCs. Total cell protein extracts were blotted with a primary antibody against phosphorylated Akt, p38 MAPK, ERK 1/2, and phosphorylated JNK 1/2. β-actin protein was used as an internal control. Values are expressed as means ±S.D. from 3 independent experiments. * P<0.01 indicates significant differences from the HG (30 mM) group.
In VSMCs, the protein expressions of phospho-p38 MAPK, phospho-ERK 1/2 and phosphor-JNK 1/2 increased after incubation with HG (30 mM, d-glucose) for 15, 30, 60, or 120 min (Figure 5B–5D). The protein expression of phospho-p38 MAPK in VSMCs incubated with HG increased at 30, 60, and 120 min. The expression of phospho-ERK 1/2 in VSMCs incubated with HG increased at 15, 60, and 120 min. The protein expression of phospho-JNK 1/2 in VSMCs incubated with HG increased at 15, 30, and 120 min. Conversely, the expressions of total ERK, JNK, and p38 MAPK were not affected by resveratrol treatment, and the expressions of phospho-ERK, phospho-JNK, and phospho-p38 MAPK were reduced following treatment with 100 μM resveratrol for 24 h (Figure 5B–5D, all P<0.05).
The downstream NF-κB signalling pathway was also assessed in our study, and the expression of phospho-IκB-α and NF-κB were measured by immunoblotting (Figure 6). Phospho-IκB-α expression was markedly increased by incubation with 30 mM glucose (d-glucose) at 6 and 12 h. Phospho-NF-κB p65 activities were significantly increased by incubation of cells with 30 mM glucose at 12 and 24 h. These effects were attenuated by resveratrol pretreatment (Figure 6A, 6B).
Figure 6.
Inhibition of phosphorylation of IκB-α (A) and NF-κB (B) by resveratrol in HG-exposed VSMCs. Western blot analysis was performed with a primary antibody against phosphorylated IκB-α and NF-κB. Values are expressed as means ±S.D. from 3 independent experiments. * P<0.01 indicates significant difference from the HG (30 mM) group.
Discussion
The main aim of this study was to investigate whether the SIRT1 activator resveratrol could protect VSMCs against HG-induced ROS formation and proliferation in vitro. The potential mechanisms were also explored. These data complement findings from our previous study [10], which suggested that SIRT1 activator resveratrol might have therapeutic potential in preventing diabetes associated with vascular diseases and atherogenesis.
Diabetes-related vascular diseases, including coronary artery disease (CAD) and cerebrovascular and peripheral vascular diseases, are the leading cause of mortality and morbidity in industrialized countries [14]. VSMCs contribute to the pathogenesis of vascular lesions, since their proliferation and migration are critical events for progressive intima thickening and development of arterial wall sclerosis. Although an immediate connection between DM and VSMC phenotype remains to be established, it is widely believed that hyperglycemia can directly produce detrimental changes in phenotype and function, and can accelerate cardiovascular complications [8]. Thus, inhibition of VSMC proliferation may have a beneficial effect in retarding the development of atherosclerotic disease.
Resveratrol (trans-3,5,49-trihydroxystilbene) is a non-flavonoid polyphenolic compound found in a wide variety of plant species, a number of which are components of the human diet, including mulberries, peanuts, grapes, and red wines [15]. It has been reported that resveratrol produces an effect similar to that of caloric restriction, which may inhibit phenomena associated with aging [16,17]. Several studies have evaluated resveratrol as a protective factor against degenerative diseases or aging. Resveratrol possesses a myriad of beneficial cardiovascular effects and can act at multiple levels, particularly those related to cellular signalling, enzymatic pathways, apoptosis, and gene expression.
Although numerous animal and in vitro studies suggest that resveratrol can improve cardiovascular and metabolic health in humans [18–21], a recent epidemiological study reported that resveratrol levels in an older population were not significantly associated with inflammation biomarkers, cardiovascular disease, or cancer [22]. However, the concentration of resveratrol in red wine is not very high and the bioavailable resveratrol presents a high interindividual variability [23]. Furthermore, other clinical trials have confirmed that resveratrol exerts beneficial effects on patients with CAD or on those at high risk of developing CAD [24–26]. Thus, more clinical studies in humans may be needed to confirm whether resveratrol really has beneficial effects on individuals with risks.
HG can increase intracellular ROS generation and is involved in diabetic cellular injury. Additionally, oxidative stress mediated by ROS has been observed to contribute significantly to the development and progression of diabetes and related vascular complications [27]. NAD(P)H oxidase has been implicated as the major source of vascular ROS generation in response to HG and advanced glycation end-products (AGEs) [28]. Long-term activation of NAD(P)H oxidase in diabetes may diminish intracellular levels of NADPH, an essential cofactor for endothelial NO synthase (eNOS) and several antioxidant systems. The NADPH oxidase complex comprises a membrane-associated low-potential cytochrome b558 composed of one p22 phox and one gp91phox subunit, and several cytosolic regulatory subunits (p47 phox, p40 phox, p67 phox, and Rac1 or Rac2) [29]. To date, evidence has shown that deletion or inhibition of p47 phox might help attenuate the progression of diabetic nephropathy and diabetic endothelial dysfunction [30,31]. In the present study, we found that resveratrol treatment could reduce high-glucose-induced intracellular ROS formation and could also down-regulate the expression of p47 phox in VSMCs. Results from this study confirmed that resveratrol might be useful as an antioxidant, and further explained the benefits of resveratrol in the treatment of DM.
Frankel et al. [32] initially reported that resveratrol could reduce the oxidation of human low-density lipoproteins (LDL). Owing to the hydroxylated structure of resveratrol, it can form a radical derivative stabilized by the delocalization of 2 electrons between the 2 aromatic cycles and the methylene bridge joining them [11]. Other studies have also confirmed that resveratrol was able to induce cellular antioxidants and phase2 enzymes and normalize the activity levels of oxidized glutathione reductase, superoxide dismutase (SOD), and myeloperoxidase (MPO) [33–37].
Thus, increased intracellular ROS subsequently activate downstream kinases, which in turn activate p38 MAPK, JNK, and ERK 1/2 through phosphorylation [38–42]. Furthermore, activation of MAPK is followed by its nuclear localization, as well as interaction with the nuclear factor κB (NF-κB) pathway [43], causing enhanced expression of proliferation-related genes. A study by Chan reported [44] that resveratrol inhibited cell migration via PI3K/Akt and MAPK pathways. Similarly, Zhang et al. [45] also found that resveratrol repressed neointimal hyperplasia, induced by balloon injury through inhibition of oxidative stress and inflammation, by blocking the ERK1/2/NF-kappa B pathway. Additionally, Poussier et al. demonstrated that VSMC proliferation could be inhibited by resveratrol through a block on G1-S phase and by an increase in apoptosis, although the exact mechanisms were not elucidated [46]. In this study, we found that the inhibition of MAPK pathway by resveratrol was involved in blocking HG-induced VSMC proliferation.
The Akt family of serine threonine kinases is of critical importance with regard to growth factor signalling, cell proliferation, survival, and oncogenesis [47]. Park et al. reported a natural dimethylated analog of resveratrol, which inhibited rat aortic vascular smooth muscle cell proliferation by blocking the Akt-dependent pathway [48], while Schreiner et al. [49] reported that resveratrol blocked Akt activation in angiotensin II- or EGF-stimulated VSMCs in a redox-independent manner. Furthermore, research suggests that the Akt pathway is involved in resveratrol regulation of both autophagy and apoptosis [50,51].
NF-κB has been identified as a transcription factor that plays an important role in inflammatory response, cell proliferation, and apoptosis [52,53]. Once NF-κB is activated, it is translocated to the nucleus and activates several anti-apoptotic genes to prevent apoptosis against oxidative stress [54]. NF-κB nuclear translocation can also activate pro-inflammatory genes that promote inflammation, cell death, and tumorigenesis [55,56]. Yeung et al. [57] reported that SIRT1 can physically interact with and deacetylate the RelA/p65 subunit of NF-κB and inhibit its transcriptional activity. Zhang et al. [58] reported that HG enhances mesangial cell proliferation through the JNK/NF-κB/NADPH oxidase/ROS pathway, which can be inhibited by resveratrol. In our study, resveratrol markedly reduced phospho-Akt and phospho-IκB-α activities in a time-dependent manner. Therefore, we provide novel evidence that resveratrol repressed VSMC proliferation induced by HG, possibly via inhibition of the Akt/NF-κB/ROS pathway.
Although we have observed diversiform bioactivity changes of resveratrol in the present study, the effects of resveratrol are believed to be more profound than previously reported. Little is known about the contributions of resveratrol or SIRT1 during VSMC development and interaction with other signalling networks in response to HG-induced cell proliferation. Further identification of resveratrol in VSMCs under HG conditions will be required to gain comprehensive information about the beneficial effects of resveratrol on VSMCs. Furthermore, d-glucose and l-glucose may exert a different effect on VSMCs proliferation due to osmotic difference. The osmotic control was not performed in our study.
Conclusions
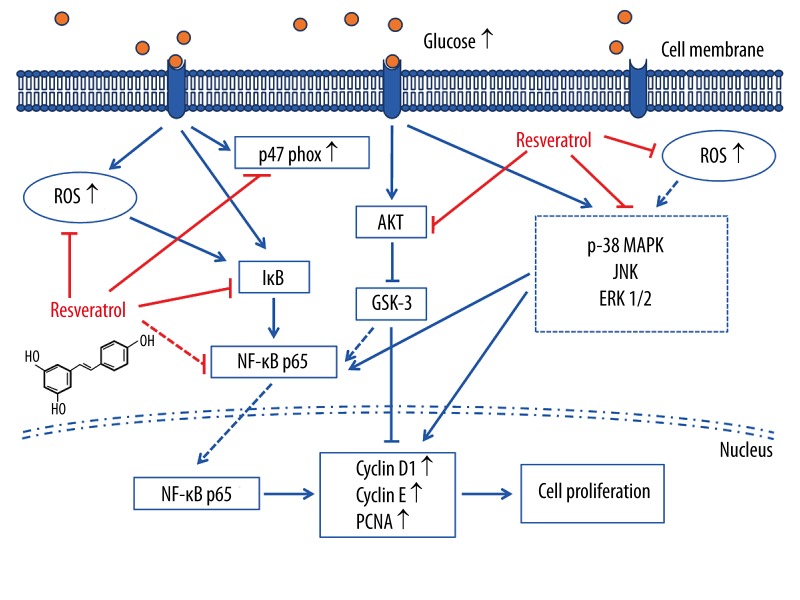
In summary (Figure 7), our present study indicates that resveratrol can inhibit HG-induced oxidative stress and VSMC proliferation by suppressing ROS overproduction, NADPH oxidative stress, Akt phosphorylation, p38 MAPK/JNK/ERK phosphorylation, and downstream NF-κB activities. In addition, our work provides new insights into the protective properties of resveratrol on VSMCs, which may constitute a novel mechanism for the clinical application of resveratrol for treatment of diabetic vascular complications.
Figure 7.
Sketch of the signal cascade involved in HG-induced intracellular oxidative stress and VSMCs proliferation, as well as the effects of resveratrol. Elevated glucose concentration initiates the downstream signalling cascade. This subsequently promotes ROS overproduction; NAD(P)H oxidative stress; phosphorylation of Akt, p38 MAPK, ERK 1/2, and JNK 1/2; and NF-κB nuclear translocation. On the other hand, ROS also can phosphorylate Akt, p38 MAPK, ERK 1/2, and JNK 1/2 and initiate NF-κB nuclear translocation. The activated NF-κB then interferes with the gene expression of some target genes such as cyclin D1, PCNA, and cyclin E, directly or indirectly. Resveratrol, either by binding directly to the kinases or upon entering the cytoplasm, inhibits ROS generation and downstream signalling. The symbol “⊥” indicates inhibition or blockade.
Acknowledgments
The authors thank Dr. Wenhui Peng for assistance with research design.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by grants from the National Natural Science Foundation of China (no. 81070107; 81200198), and partly supported by the Foundation for Distinguished Graduate Students of Shanghai Tenth People’s Hospital
References
- 1.Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92(22):1037–45. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Woodman RJ, Chew GT, Watts GF. Mechanisms, significance and treatment of vascular dysfunction in type 2 diabetes mellitus: focus on lipid-regulating therapy. Drugs. 2005;65(1):31–74. doi: 10.2165/00003495-200565010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Williams SB, Cusco JA, Roddy MA, et al. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27(3):567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 4.Farmer DG, Kennedy S. RAGE, vascular tone and vascular disease. Pharmacol Ther. 2009;124(2):185–94. doi: 10.1016/j.pharmthera.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Lipskaia L, Hadri L, Lopez JJ, et al. Benefit of SERCA2a gene transfer to vscular endothelial and smooth muscle cells: a new aspect in therapy of cardiovascular diseases. Curr Vasc Pharmacol. 2013;11(4):465–79. doi: 10.2174/1570161111311040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo R, Su Y, Yan J, et al. Fasudil improves short-term echocardiographic parameters of diastolic function in patients with type 2 diabetes with preserved left ventricular ejection fraction: a pilot study. Heart Vessels. 2014 doi: 10.1007/s00380-013-0458-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Galassetti P. Inflammation and oxidative stress in obesity, metabolic syndrome, and diabetes. Exp Diabetes Res. 2012;2012:943706. doi: 10.1155/2012/943706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter KE, Riches K. The vascular smooth muscle cell: a therapeutic target in Type 2 diabetes? ClinSci (Lond) 2013;125(4):167–82. doi: 10.1042/CS20120413. [DOI] [PubMed] [Google Scholar]
- 9.Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol. 2012;22(10):546–54. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo R, Liu B, Wang K, et al. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-κB pathway. Diab Vasc Dis Res. 2014;11(2):92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- 11.Delmas D, Jannin B, Latruffe N. Resveratrol: preventing properties against vascular alterations and ageing. Mol Nutr Food Res. 2005;49(5):377–95. doi: 10.1002/mnfr.200400098. [DOI] [PubMed] [Google Scholar]
- 12.Scott-Burden T, Resink TJ, Baur U, et al. Epidermal growth factor responsiveness in smooth muscle cells from hypertensive and normotensive rats. Hypertension. 1989;13(4):295–304. doi: 10.1161/01.hyp.13.4.295. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Zhu LH, Jiang H, et al. Grape seed proanthocyanidins attenuate vascular smooth muscle cell proliferation via blocking phosphatidylinositol 3-kinase-dependent signaling pathways. J Cell Physiol. 2010;223(3):713–26. doi: 10.1002/jcp.22080. [DOI] [PubMed] [Google Scholar]
- 14.Desilles JP, Meseguer E, Labreuche J, et al. Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke. 2013;44(7):1915–23. doi: 10.1161/STROKEAHA.111.000813. [DOI] [PubMed] [Google Scholar]
- 15.de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49(5):405–30. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 16.Burzynski SR. Aging: gene silencing or gene activation? Med Hypotheses. 2005;64(1):201–8. doi: 10.1016/j.mehy.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–89. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 18.Tomayko EJ, Cachia AJ, Chung HR, et al. Resveratrol supplementation reduces aortic atherosclerosis and calcification and attenuates loss of aerobic capacity in a mouse model of uremia. J Med Food. 2014;17(2):278–83. doi: 10.1089/jmf.2012.0219. [DOI] [PubMed] [Google Scholar]
- 19.Zheng X, Zhu S, Chang S, et al. Protective effects of chronic resveratrol treatment on vascular inflammatory injury in steptozotocin-induced type 2 diabetic rats: role of NF-kappa B signaling. Eur J Pharmacol. 2013;720(1–3):147–57. [PubMed] [Google Scholar]
- 20.Sabe AA, Elmadhun NY, Dalal RS, et al. Resveratrol regulates autophagy signaling in chronically ischemic myocardium. J Thorac Cardiovasc Surg. 2014;147(2):792–8. doi: 10.1016/j.jtcvs.2013.06.062. Discussion 798–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrizzo A, Puca A, Damato A, et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension. 2013;62(2):359–66. doi: 10.1161/HYPERTENSIONAHA.111.01009. [DOI] [PubMed] [Google Scholar]
- 22.Semba RD, Ferrucci L, Bartali B, et al. Resveratrol Levels and All-Cause Mortality in Older Community-Dwelling Adults. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2014.1582. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitaglione P, Sforza S, Galaverna G, et al. Bioavailability of trans-resveratrol from red wine in humans. Mol Nutr Food Res. 2005;49(5):495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 24.Tomé-Carneiro J, Gonzálvez M, Larrosa M, et al. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27(1):37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bo S, Ciccone G, Castiglione A, et al. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem. 2013;20(10):1323–31. doi: 10.2174/0929867311320100009. [DOI] [PubMed] [Google Scholar]
- 26.Tomé-Carneiro J, Gonzálvez M, Larrosa M, et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: a triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res. 2012;56(5):810–21. doi: 10.1002/mnfr.201100673. [DOI] [PubMed] [Google Scholar]
- 27.Ha H, Lee HB. Reactive oxygen species and matrix remodeling in diabetic kidney. J Am Soc Nephrol. 2003;14(8 Suppl 3):S246–49. doi: 10.1097/01.asn.0000077411.98742.54. [DOI] [PubMed] [Google Scholar]
- 28.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82(1):9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 29.Babior BM. NADPH oxidase: an update. Blood. 1999;93(5):1464–76. [PubMed] [Google Scholar]
- 30.Youn JY, Gao L, Cai H. The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia. 2012;55(7):2069–79. doi: 10.1007/s00125-012-2557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu GC, Fang F, Zhou J, et al. Deletion of p47phox attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouse. Diabetologia. 2012;55(9):2522–32. doi: 10.1007/s00125-012-2586-1. [DOI] [PubMed] [Google Scholar]
- 32.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341(8852):1103–4. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 33.Yen GC, Duh PD, Lin CW. Effects of resveratrol and 4-hexylresorcinol on hydrogen peroxide-induced oxidative DNA damage in human lymphocytes. Free Radic Res. 2003;37(5):509–14. doi: 10.1080/1071576031000083099. [DOI] [PubMed] [Google Scholar]
- 34.Shigematsu S, Ishida S, Hara M, et al. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic Biol Med. 2003;34(7):810–17. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Cao Z, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res. 2006;53(1):6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Spanier G, Xu H, Xia N, et al. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60(Suppl 4):111–16. [PubMed] [Google Scholar]
- 37.Rahangdale S, Yeh SY, Malhotra A, Veves A. Therapeutic interventions and oxidative stress in diabetes. Front Biosci. 2009;14:192–209. doi: 10.2741/3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H, Ichikawa S, Tani C, et al. Docosahexaenoic acid induces ERK1/2 activation and neuritogenesis via intracellular reactive oxygen species production in human neuroblastoma SH-SY5Y cells. Biochim Biophys Acta. 2009;1791(1):8–16. doi: 10.1016/j.bbalip.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Bhagatte Y, Lodwick D, Storey N. Mitochondrial ROS production and subsequent ERK phosphorylation are necessary for temperature preconditioning of isolated ventricular myocytes. Cell Death Dis. 2012;3:e345. doi: 10.1038/cddis.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao L, Pimentel DR, Wang J, et al. Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol. 2002;282(4):C926–34. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- 41.de Bernardo S, Canals S, Casarejos MJ, et al. Role of extracellular signal-regulated protein kinase in neuronal cell death induced by glutathione depletion in neuron/glia mesencephalic cultures. J Neurochem. 2004;91(3):667–82. doi: 10.1111/j.1471-4159.2004.02744.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Papa S, Bubici C, Zazzeroni F, et al. Mechanisms of liver disease: cross-talk between the NF-kappaB and JNK pathways. Biol Chem. 2009;390(10):965–76. doi: 10.1515/BC.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan CM, Chang HH, Wang VC, et al. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and MAPK pathways. PLoS One. 2013;8(2):e56819. doi: 10.1371/journal.pone.0056819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Chen J, Yang J, et al. Resveratrol attenuates oxidative stress induced by balloon injury in the rat carotid artery through actions on the ERK1/2 and NF-kappa B pathway. Cell Physiol Biochem. 2013;31(2–3):230–41. doi: 10.1159/000343364. [DOI] [PubMed] [Google Scholar]
- 46.Poussier B, Cordova AC, Becquemin JP, et al. Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J Vasc Surg. 2005;42(6):1190–97. doi: 10.1016/j.jvs.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Altomare DA, Khaled AR. Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr Med Chem. 2012;19(22):3748–62. doi: 10.2174/092986712801661130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park ES, Lim Y, Hong JT, et al. Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway. Vascul Pharmacol. 2010;53(1–2):61–67. doi: 10.1016/j.vph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Schreiner CE, Kumerz M, Gesslbauer J, et al. Resveratrol blocks Akt activation in angiotensin II- or EGF-stimulated vascular smooth muscle cells in a redox-independent manner. Cardiovasc Res. 2011;90(1):140–47. doi: 10.1093/cvr/cvq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alayev A, Sun Y, Snyder RB, et al. Resveratrol prevents rapamycin-induced upregulation of autophagy and selectively induces apoptosis in TSC2-deficient cells. Cell Cycle. 2014;13(3):371–82. doi: 10.4161/cc.27355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai T, Dong DS, Pei L. Resveratrol mitigates isoflurane-induced neuroapoptosis by inhibiting the activation of the Akt-regulated mitochondrial apoptotic signaling pathway. Int J Mol Med. 2013;32(4):819–26. doi: 10.3892/ijmm.2013.1464. [DOI] [PubMed] [Google Scholar]
- 52.Pechanova O, Simko F. The role of nuclear factor kappa B and nitric oxide interaction in heart remodelling. J Hypertens. 2010;(Suppl 1):S39–44. doi: 10.1097/01.hjh.0000388493.81578.b1. [DOI] [PubMed] [Google Scholar]
- 53.Queisser N, Schupp N. Aldosterone, oxidative stress, and NF-κB activation in hypertension-related cardiovascular and renal diseases. Free Radic Biol Med. 2012;53(2):314–27. doi: 10.1016/j.freeradbiomed.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19(2):263–72. [PMC free article] [PubMed] [Google Scholar]
- 55.Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci. 2008;28(9):2221–30. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maiese K, Chong ZZ, Hou J, et al. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5(2):125–42. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–80. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Pang S, Deng B, et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int J Biochem Cell Biol. 2012;44(4):629–38. doi: 10.1016/j.biocel.2012.01.001. [DOI] [PubMed] [Google Scholar]