Abstract
Objective
To determine changes in cortical neural networks as defined by resting-state functional connectivity magnetic resonance imaging during voluntary modulation of tinnitus with orofacial maneuvers.
Study Design
Cross-sectional study.
Setting
Academic medical center.
Subjects and Methods
Participants were scanned during the maneuver and also at baseline to serve as their own control. The authors chose, a priori, 58 seed regions to evaluate previously described cortical neural networks by computing temporal correlations between all seed region pairs. Seed regions whose correlations significantly differed between rest and maneuver (P < .05, uncorrected) entered into a second-stage analysis of computing the correlation coefficient between the seed region and time courses in each of the remaining brain voxels. A threshold-free cluster enhancement permutation analysis evaluated the distribution of these correlation coefficients after transformation to Fisher z scores and registration to a surface-based reconstruction using Freesurfer.
Results
The median age for the 16 subjects was 54 years (range, 27–72 years), and all had subjective, unilateral or bilateral, nonpulsatile tinnitus for 6 months or longer. In 9 subjects who could voluntarily increase the loudness of their tinnitus, there were no significant differences in functional connectivity in any cortical networks. A separate analysis evaluated results from 3 patients who decreased the loudness of their tinnitus. Four subjects were excluded because of excessive motion in the scanner.
Conclusion
The absence of significant differences in functional connectivity due to voluntary orofacial maneuvers that increased tinnitus loudness failed to confirm prior reports of altered cerebral blood flows during somatomotor behaviors.
Keywords: tinnitus, imaging
The etiology of persistent, subjective tinnitus remains unknown, despite increased study of the neurobiological origins of tinnitus over the past decade.1 Because of its association with hearing loss and prolonged noise exposure, tinnitus is thought to be related to damage to peripheral auditory receptors and/or the eighth cranial nerve.2,3 There has been some focus on central auditory areas as possible origins for tinnitus and a possible role of neural plasticity on the development and maintenance of tinnitus.4 Results from positron emission tomography (PET)5,6 and magnetic resonance (MR)7,8 imaging studies suggest bothersome tinnitus associates with altered activity in the primary auditory cortex, temporoparietal cortex, inferior colliculus, and medial geniculate body, suggesting maladaptive plasticity or reductions to control the phantom noise.
Some tinnitus patients, perhaps as many as 30%,9 can modulate the loudness and/or pitch of their auditory percept often through voluntary somatomotor actions.10,11 However, the exact neurological explanation(s) for voluntary modulation of tinnitus remains unknown. Voluntary modulation of tinnitus can occur through various orofacial movements,12 such as jaw movement,10,13 cutaneous stimulation,14 and gaze redirection.15–17 In a study by Lockwood et al,18 of 4 patients who controlled their tinnitus with an orofacial maneuver, pure-tone stimuli produced increased regional cerebral blood flow (rCBF) in the primary auditory cortex and also demonstrated abnormal association with areas of the limbic system on PET. In another PET study of patients with gaze-evoked tinnitus, Lockwood and colleagues17 again reported increased activation of the auditory cortex, as well as some abnormal activation of cerebellar and brainstem areas. The subset of tinnitus sufferers who can modulate their tinnitus may represent a unique opportunity to gain insight into possible mechanisms responsible for tinnitus.13
Although both traditional task-based functional magnetic resonance imaging (fMRI) and functional connectivity magnetic resonance imaging (fcMRI) measure the blood oxygen level–dependent (BOLD) activity in the brain, the goals of these 2 methods are different. Whereas in traditional task-based fMRI, one seeks to detect changes in BOLD signals that are secondary to a task or stimulus, in fcMRI, one explores the spontaneous fluctuations of BOLD activity in the brain. In fcMRI, the measured data are spontaneous, slow-frequency (<0.1 Hz) fluctuations in the BOLD signal. These spontaneous BOLD fluctuations were first shown to be informative by Biswal and colleagues,19 who demonstrated functional connectivity, or positive correlations, between the spontaneous BOLD signals in the left and right somatomotor cortices. Functional connectivity reflects structural connections or indirect synaptic linkages between different regions of the brain. Functional connectivity MRI offers an advantage over task-based functional MRI in being unaffected by participant heterogeneity in task-performance ability that may result from genomic, environmental, or behavioral differences.
Similar to the synchrony between the left and right somatomotor areas, fcMRI has also demonstrated other resting state networks. These resting state networks are regions of the brain that are not necessarily spatially contiguous but that show synchrony of spontaneous BOLD fluctuations. Each of these networks resembles those recruited during active behavior.20,21 The strength of activity correlation at rest between nodes of different networks specifically correlates across subjects with behavioral measures. Examples of functional systems delineated using fcMRI are the somatomotor,19,22 attention,21 default-mode,21–23 language,24 memory,25 and command/task control systems.26 Some of these networks have a demonstrated or presumed relationship to tinnitus—primary and secondary auditory cortex,17,18 dorsal lateral prefrontal cortex,27 and components of the dorsal and ventral attention (or cognitive control) networks,28,29 “default mode” (or external vigilance monitory) network,30 and pain-emotion centers in the ventral prefrontal cortex.31
The objective of the current study was to determine whether the functional connectivity associated with any of these brain networks changed in a selected group of patients who could voluntarily modulate their tinnitus loudness. The fcMRI scans were acquired both when patients were at rest and while they sustained their orofacial maneuver. In both resting and maneuver scans, we explored the correlations of spontaneous BOLD activity between selected brain regions of selected resting state networks.
Method
Design and Setting
This was a single-institution (Washington University), cross-sectional study approved by the institutional review board prior to recruitment of participants from July 2009 to November 2010.
Patients
Eligible participants needed to have idiopathic (ie, unknown etiology), subjective, unilateral or bilateral, and chronic, non-pulsatile tinnitus. Chronic was defined as a duration of at least 6 months. Furthermore, patients needed to have reproducible, voluntary control over their tinnitus, whether through attention redirection or an orofacial maneuver compatible with MRI, which is motion sensitive. We excluded patients with a history of hyperacusis, misophonia, or neurological injury or illness. All patients had an audiogram within a year of enrollment and responded to the Tinnitus Handicap Inventory (THI)32 to capture the severity of tinnitus.
Imaging Protocol
All MRI scans were done on a 3 Tesla Siemens scanner (Siemens Medical Solution, Malvern, Pennsylvania). A T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) sequence and a T2-weighted structural image were first obtained. Resting state BOLD data were acquired during 5 scans of 6 minutes’ duration each using a 164-frame echo-planar imaging (EPI) sequence. During the first 2 scans, tinnitus patients performed their tinnitus-altering maneuver, and during the last 2 scans, patients remained at rest with their eyes closed. The third scan served as a wash-out scan and was not included in the analysis.
Image Preprocessing
The EPI images were processed to correct for differences due to interleaved odd-even slice acquisitions and slice timing. The whole-brain signal intensity was normalized to a mode of 1000 across EPI runs. Frames were adjusted for head motion using rigid body correction.22 The images were resampled to 2-mm3 voxels and registered using a 12-parameter affine transformation to an atlas derived from 12 healthy adults.33 Using Analyze (Mayo Research Foundation, Rochester, Minnesota), a software package that can be used to visualize fMRI data, the ventricles and white matter were identified. Variance due to signals from the white matter, ventricles, and whole brain was removed using regression. Images were spatially smoothed using a 6-mm full-width half-maximum Gaussian kernel and temporally band-pass filtered for frequencies less than 0.1 Hz. Head motion was analyzed using the root mean square (RMS) of head movements and the standard deviation (SD) of the mean whole brain signal.
Data Analysis
In each patient, the first 2 and last 2 BOLD time series were concatenated to produce a single time series during performance of the orofacial maneuver and another during rest. Fifty-eight spherical seed regions, each with a diameter of 10 mm, were determined using Talairach coordinates,34 a coordinate system used to locate brain regions, in the default, dorsal attention, ventral attention, cognitive/control, auditory, visual, and somatosensory networks. Temporal correlations were calculated between every pair of seed regions and between each seed region and all other voxels. The correlations were transformed into z scores using Fisher transformation.
Exploratory analysis was first done by searching all seed regions for possible differences between maneuver and resting states. A Kruskal-Wallis test was done to compare the temporal correlation between all possible pairs of seed regions in maneuver vs resting states. Significant seed regions, determined as P < .05, and others presumed to be related to tinnitus (eg, auditory cortex) were then used in the threshold-free cluster enhancement (TFCE) method,35 which uses information from neighboring voxels to determine areas of significance. This eliminates the need for an arbitrary threshold of t statistic maps, which can lead to different results based on the threshold chosen. A surface-based implementation was used as in Hill et al.36 The volume to surface transformation was done using Freesurfer, a software package for MRI analysis.37 Results are displayed using Caret, a software package for visualizing and analyzing neuroimaging data.33
Results
As shown in Table 1, the 16 patients enrolled in this study had a median age of 54 years (range, 27–72 years), 10 had bilateral tinnitus, and the median THI score was 22 (range, 0–78). Four patients excluded from the analysis had excessive head motion. Orofacial maneuvers that were performed included clenching, protruding or shifting the jaw, rolling eyes, or grinding teeth. Nine patients were able to increase the loudness of their tinnitus, and 3, including 1 who performed attention redirection, were able to decrease the loudness of their tinnitus. These nine patients form the focus of our study.
Table 1.
Participant Demographics
| ID | Age, y | Sex | Ear | THI | Louda | y | Audiogram
|
Tinnitus Change/Maneuver | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 kHz, R/L | 2 kHz, R/L | 3 kHz, R/L | 8 kHz, R/L | ||||||||
| V01 | 64 | F | L | 8 | 3 | 5 | 5/Cb | 15/Cb | 10/Cb | 10/Cb | ↑/Roll eyes to the left |
| V03 | 42 | M | R | 24 | 6 | 10 | 5/5 | 5/5 | 5/5 | 5/5 | ↑/Clench jaw |
| V04 | 43 | M | B | 4 | 2 | 15 | 35/35 | 50/55 | 60/60 | 55/50 | ↑/Shift jaw to the right |
| V06 | 53 | F | B | 44 | 7 | 10 | 0/5 | 0/10 | 10/10 | 15/40 | ↑/Clench jaw |
| V07 | 37 | M | B | 58 | 8 | 0.8 | 5/0 | 10/5 | 5/5 | 10/35 | ↑/Protrude lower jaw |
| V10 | 56 | M | B | 28 | 3 | 40 | 55/55 | 55/65 | 55/65 | 55/55 | ↑/Protrude lower jaw |
| V11 | 55 | F | L | 78 | 3 | 11 | 15/10 | 15/15 | 10/40 | 35/50 | ↑/Protrude lower jaw |
| V13 | 52 | F | B | 14 | 5 | 52 | 15/5 | 10/5 | 5/0 | 65/60 | ↑/Clench jaw |
| V16 | 41 | F | R | 54 | 8 | 8 | 75/25 | 70/45 | 65/80 | 80/120 | ↑/Protrude lower jaw |
| V02 | 27 | M | B | 0 | 2 | 17 | 15/15 | 20/15 | 15/20 | 10/20 | ↓/Redirect attention |
| V08 | 57 | M | B | 20 | 8 | 53 | 15/0 | 10/0 | 20/15 | 30/20 | ↓/Retract lower jaw |
| V14 | 44 | F | B | 20 | 5 | 20 | 25/10 | 30/20 | 50/40 | 70/50 | ↓/Close both eyes |
| V05c | 66 | M | L | 12 | 4 | 5 | 10/10 | 5/0 | 25/20 | 60/60 | ↑/Grind teeth |
| V09c | 72 | M | B | 14 | 6 | 47 | 10/10 | 20/25 | 35/40 | 50/50 | ↑/Pressure to left face |
| V12c | 61 | M | R | 64 | 9 | 30 | 10/10 | 30/30 | 45/45 | 55/50 | ↑/Tighten jaw |
| V15c | 62 | M | B | 72 | 8 | 4.5 | 30/40 | 25/25 | 25/30 | 65/60 | ↑/Clench jaw |
| Mean | 53.7 | 32.1 | 5.4 | 20.5 | 20.3/15.7 | 23.1/21.3 | 27.5/31.7 | 41.9/48.3 | |||
| SD | 10.4 | 25.6 | 2.4 | 18.0 | 20.1/16.0 | 19.8/20.0 | 21.2/24.0 | 25.2/25.8 | |||
Abbreviations: B, bilateral; F, female; L, left; M, male; R, right; y, years.
Scale 1 to 10 (loudest).
Could not test.
Scans excluded because of excessive head motion.
Appendix 1 (available at otojournal.org) lists Talairach atlas coordinates for all tested seed regions. For the group of 9 patients who increased the loudness of their tinnitus, several temporal correlations between seed region pairs were significant when comparing maneuver and resting states: left frontal eye fields (LFEF)–right anterior superior occipital gyrus (RV7; P = .03), left inferior temporoparietal junction (LITPJ)–right inferior temporal (RIT; P = .02), left lateral occipital cortex (LLO)–right secondary somatosensory cortex (RBA2; P = .04), right middle frontal gyrus (RMFG)–left postcentral gyrus (LS1; P = .04), right middle frontal gyrus (RMFG)–left parietal operculum (LS2; P = .04), right superior frontal (RSF)–left parieto-occipital sulcus (LPOCS; P = .03), right fusiform gyrus (RV8)–left postcentral gyrus (LS1; P = .03), and anterior cingulate cortex (aCC)–left primary auditory cortex (LAud_Ctx; P = .03). However, the TFCE permutation analysis revealed no significant clusters in the t test contrast maps for maneuver and resting states in any of the networks. Figure 1 shows an example set of connectivity maps for the left auditory cortex seed in a subject whose maneuver increased tinnitus loudness bilaterally. The z scores were similar between the maneuver and resting scans. The 3 patients who decreased the loudness of their tinnitus were analyzed individually because of the smaller group size. Functional connectivity maps for these patients were not visually different between maneuver and resting states (Figure 2).
Figure 1.
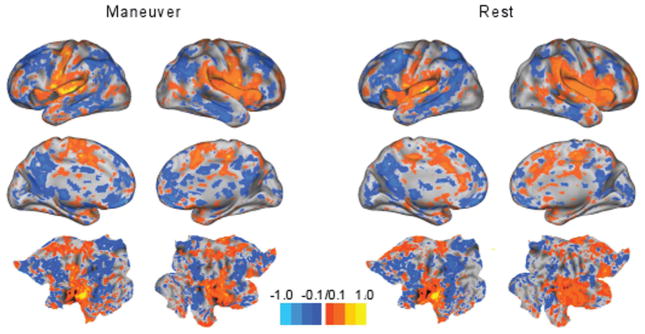
Functional connectivity maps for a subject whose maneuver increased tinnitus loudness show no strong differences between maneuver and rest states. Colors represent the strength of association (z scores). Most z scores were close to zero. Lateral views (top row) and medial views (second row) are shown for the right and left hemispheres of the brain. The bottom row contains flat maps, which are able to show all cortical areas simultaneously.
Figure 2.
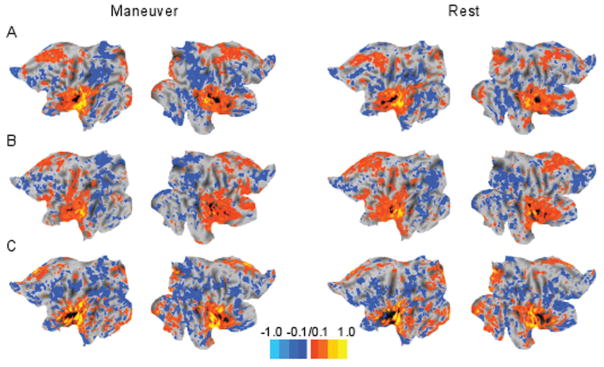
Functional connectivity maps for 3 subjects (rows A, B, C) whose maneuvers decreased tinnitus loudness showed no strong differences between maneuver and rest states. Each subject is shown individually because of the small number of patients recruited that could decrease tinnitus loudness. Most z scores were close to zero, demonstrating no strong associations with the left auditory cortex seed. Flat maps, which are able to show all cortical areas simultaneously, are shown for the left and right hemispheres in each patient.
Discussion
This study found no differences in functional connectivity in various cortical networks when tinnitus patients voluntarily performed orofacial maneuvers to affect tinnitus loudness. These results contradict reports in the literature17,18 that indicated hyperactivity based on increased rCBF using PET in auditory areas of 4 patients. Giraud et al38 found that patients who could modulate tinnitus through gaze had bilateral changes in PET-defined regional cerebral blood flow in temporoparietal association auditory areas but not in the primary auditory cortex.
The current study involved 3-fold more patients, limited confounds by having each patient serve as his or her own control, and effectively excluded potential distortions from excessive head motion that could affect the functional connectivity analysis. We also performed a staged analysis that avoided excessive searches by identifying potential paired seed regions based on significant temporal correlations. Further analysis occurred only for the functional connectivity maps for the brain networks of these seed regions. This involved the TFCE assessment of the t test maps comparing the maneuver and resting states in the same individuals. The lack of a significant result using seeds in auditory areas suggests that the primary auditory cortex did not show hyperactivity in the assessed tinnitus patients.
There are always concerns with acceptance of negative findings. One possibility was that despite a study population 3-fold larger than previously examined, the statistical power might have been insufficient because our patient population was too heterogeneous (ie, wide variation of age, orofacial maneuvers, and THI) to show a small effect of orofacial maneuvers on functional connectivity. A study involving a larger number of patients performing a more stereotypical maneuver would create greater homogeneity, and this might sufficiently reduce variability and thereby reveal even small shifts in functional connectivity caused by somatomotor actions. Obtaining a larger, more homogeneous sample, however, might be a problem. Unlike published reports of a large percentage of tinnitus patients who modified their tinnitus through various head and neck maneuvers,39 we found it quite challenging to recruit sufficient numbers of individuals to complete this study. In addition, the compliance of the participants in performing the orofacial maneuvers cannot be evaluated objectively. Furthermore, we recruited patients whose preferred maneuvers would not cause excessive head motion in the scanner. This selectivity may have resulted in a sampling bias that influenced the results and limited the external validity of this study. We also did not include deep brain or brainstem areas in our analysis, areas that may be involved in tinnitus.
In conclusion, we found no differences in functional connectivity within any cortical neural networks among patients who modulated their tinnitus loudness using various orofacial maneuvers. The novel aspect of this study was the use of fcMRI and a threshold-free permutation analysis to assess possible changes in cortical networks in tinnitus patients able to modulate tinnitus perception with orofacial maneuvers. Future studies may benefit from assessing a larger and more homogeneous group of tinnitus patients able to alter loudness perception with somatomotor maneuvers.
Supplementary Material
Acknowledgments
Sponsorships: None.
Funding source: Partial funding for this project was received through the Washington University Institute of Clinical and Translational Sciences Just-In-Time (JIT) Core Usage Funding Program sponsored, in part, through a grant from the National Institutes of Health–National Center for Research Resources (NCRR) UL1 [RR024992]. Support for Dr Wineland was through the National Institute on Deafness and Other Communication Disorders (NIDCD) National Research Service Award Institutional Research Training Grants [T32–DC000022-26]. Support for Ms Lee was through the Doris Duke Clinical Research Fellowship Program for Medical Students.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Author Contributions
Megan H. Lee, analysis, interpretation, draft, revisions, final version article; Nancy Solowski, conception/design, acquisition, revisions; Andre Wineland, acquisition, interpretation, analysis, draft, revisions, final version article; Oluwafunmilola Okuyemi, conception/design, acquisition, draft; Joyce Nicklaus, design, acquisition, draft; Dorina Kallogjeri, conception/design, analysis, draft; Jay F. Piccirillo, conception/design, interpretation of data, revisions, final version article; Harold Burton, conception/design, acquisition, interpretation, analysis, final version article.
Competing interests: None.
Additional supporting information may be found at http://oto.sagepub.com/content/by/supplemental-data
References
- 1.Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartels H, Staal MJ, Albers FW. Tinnitus and neural plasticity of the brain. Otol Neurotol. 2007;28:178–184. doi: 10.1097/MAO.0b013e31802b3248. [DOI] [PubMed] [Google Scholar]
- 5.Langguth B, Eichhammer P, Kreutzer A, et al. The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus—first results from a PET study. Acta Otolaryngol Suppl. 2006;(556):84–88. doi: 10.1080/03655230600895317. [DOI] [PubMed] [Google Scholar]
- 6.Plewnia C, Reimold M, Najib A, et al. Dose-dependent attenuation of auditory phantom perception (tinnitus) by PET-guided repetitive transcranial magnetic stimulation. Hum Brain Mapp. 2007;28:238–246. doi: 10.1002/hbm.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smits M, Kovacs S, De Ridder D, Peeters RR, van Hecke P, Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus [see comment] Neuroradiology. 2007;49:669–679. doi: 10.1007/s00234-007-0231-3. [DOI] [PubMed] [Google Scholar]
- 8.Lanting CP, De Kleine E, Bartels H, Van Dijk P. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol. 2008;128:415–421. doi: 10.1080/00016480701793743. [DOI] [PubMed] [Google Scholar]
- 9.Rubinstein B, Axelsson A, Carlsson GE. Prevalence of signs and symptoms of craniomandibular disorders in tinnitus patients. J Craniomandib Disord. 1990;4:186–192. [PubMed] [Google Scholar]
- 10.Vielsmeier V, Kleinjung T, Strutz J, Bürgers R, Kreuzer PM, Langguth B. Tinnitus with temporomandibular joint disorders: a specific entity of tinnitus patients? Otolaryngol Head Neck Surg. 2011;145:748–752. doi: 10.1177/0194599811413376. [DOI] [PubMed] [Google Scholar]
- 11.Simmons R, Dambra C, Lobarinas E, Stocking C, Salvi R. Head, neck, and eye movements that modulate tinnitus. Semin Hear. 2008;29:361–370. doi: 10.1055/s-0028-1095895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez TG, Guerra GC, Lorenzi MC, Brandão AL, Bento RF. The influence of voluntary muscle contractions upon the onset and modulation of tinnitus. Audiol Neurootol. 2002;7:370–375. doi: 10.1159/000066155. [DOI] [PubMed] [Google Scholar]
- 13.Pinchoff RJ, Burkard RF, Salvi RJ, Coad ML, Lockwood AH. Modulation of tinnitus by voluntary jaw movements. Am J Otol. 1998;19:785–789. [PubMed] [Google Scholar]
- 14.Phillips JS, Baguley DM, Patel H, Moffat DA. Tinnitus evoked by cutaneous stimulation. Neurology. 2004;63:1756. doi: 10.1212/01.wnl.0000143084.21212.54. [DOI] [PubMed] [Google Scholar]
- 15.Wall M, Rosenberg M, Richardson D. Gaze-evoked tinnitus. Neurology. 1987;37:1034–1036. doi: 10.1212/wnl.37.6.1034. [DOI] [PubMed] [Google Scholar]
- 16.Coad ML, Lockwood A, Salvi R, Burkard R. Characteristics of patients with gaze-evoked tinnitus. Otol Neurotol. 2001;22:650–654. doi: 10.1097/00129492-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Lockwood AH, Wack DS, Burkard RF, et al. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology. 2001;56:472–480. doi: 10.1212/wnl.56.4.472. [DOI] [PubMed] [Google Scholar]
- 18.Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 19.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 20.Cordes D, Haughton VM, Arfanakis K, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems [erratum appears in Proc Natl Acad Sci U S A. 2006;103(36):13560] Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampson M, Tokoglu F, Sun Z, et al. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. Neuroimage. 2006;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanneste S, Plazier M, Ost J, van der Loo E, Van de Heyning P, De Ridder D. Bilateral dorsolateral prefrontal cortex modulation for tinnitus by transcranial direct current stimulation: a preliminary clinical study. Exp Brain Res. 2010;202:779–785. doi: 10.1007/s00221-010-2183-9. [DOI] [PubMed] [Google Scholar]
- 28.Burton H, Wineland A, Bhattacharya M, Nicklaus J, Garcia KS, Piccirillo JF. Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci. 2012;13:3. doi: 10.1186/1471-2202-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierce KJ, Kallogjeri D, Piccirillo JF, Garcia KS, Nicklaus JE, Burton H. Effects of severe bothersome tinnitus on cognitive function measured with standardized tests. J Clin Exp Neuropsychol. 2012;34:126–134. doi: 10.1080/13803395.2011.623120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langguth B, Landgrebe M, Kleinjung T, Sand GP, Hajak G. Tinnitus and depression. World J Biol Psychiatry. 2011;12:489–500. doi: 10.3109/15622975.2011.575178. [DOI] [PubMed] [Google Scholar]
- 31.Schecklmann M, Landgrebe M, Poeppl TB, et al. Neural correlates of tinnitus duration and distress: a positron emission tomography study. Hum Brain Mapp. doi: 10.1002/hbm.21426. published online ahead of print October 22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122:143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 33.Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 34.Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical; 1988. [Google Scholar]
- 35.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 36.Hill J, Dierker D, Neil J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010;30:2268–2276. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I: segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 38.Giraud AL, Chery-Croze S, Fischer G, et al. A selective imaging of tinnitus. NeuroReport. 1999;10:1–5. doi: 10.1097/00001756-199901180-00001. [DOI] [PubMed] [Google Scholar]
- 39.Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153:643–648. doi: 10.1007/s00221-003-1747-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


