Abstract
Background
Research to understand variability at the highest end of the cognitive performance distribution has been scarce. Our aim was to define a cognitive endophenotype based on exceptional episodic memory performance (EM) and to investigate familial aggregation of EM in families from the Long Life Family Study.
Methods
Using a sample of 1911 non-demented offspring of long-lived probands, we created a quantitative phenotype, EM (memory z-score >= 1.5), and classified LLFS families as EM and Non-EM families based on the number of EM offspring. We then assessed differences in memory performance between LLFS relatives in the parental generation of EM families and those in Non-EM families using multivariate analysis adjusted for APOE genotype.
Results
LLFS relatives in the proband generation from EM families showed better episodic memory performance than those from Non-EM families (β=0.74, SE=0.19, p =1.4 × 10-4).
Conclusions
We demonstrated that there is a familial correlation of the EM endophenotype, suggesting that genetic variants might influence memory performance in long-lived families.
Introduction
Human cognitive performance is highly variable. Family, twin, and adoption studies have documented strong evidence that genetic factors contribute to variation in the normal range of cognitive performance (Butcher, et al., 2008, Deary, et al., 2006). In contrast, little is known about the genetic factors that contribute to exceptionally high levels of cognitive performance. Quantitative genetic research in the normal range of cognitive variation has shown that virtually all cognitive tasks show appreciable heritability (Plomin and DeFries, 1998). For episodic memory for example, we and others (Johansson, et al., 1999, Wilson, et al., 2011) have consistently reported heritability estimates between 30% and 60%, indicating that much of the observed variability in this cognitive domain is genetically influenced. Subsequent efforts have been aimed at identifying the specific genes responsible for the heritability of specific cognitive abilities. Most of the research has focused on conditions associated with cognitive disabilities, because these conditions can provide important clues to the potential effect of genes on cognition. As a result, hundreds of single-gene defects have been described as impairing cognitive development (Freund and Reiss, 1991, Reiss, et al., 1995). However, variability in normal cognitive function is most likely the result of many different genes interacting with each other and with non-genetic factors as well. In fact, genome-wide association studies (GWAS) of general cognitive ability have demonstrated that many genes of small effect make up 60% of the heritable variation in the trait (Butcher, et al., 2008, Davis, et al., 2010). Similarly, the majority of the research on exceptional cognitive abilities has explored the contribution of both genetics and environmental factors to the differences observed among individuals with exceptional abilities. As with estimates found for normal variation, findings from the small number of studies which have assessed cognitive exceptionality in twins reported high heritability estimates for high cognitive function (Haworth, et al., 2009, Petrill, et al., 2009, Plomin and Haworth, 2009, Saudino, et al., 1994).
The biological pathways that influence longevity are still unknown. We and others (Barral, et al., 2012, Fried, et al., 1998, Korten, et al., 1999, Schupf, et al., 2003) have documented a significant association between preserved cognitive function and successful aging. It is likely that cognitive traits, such as exceptional memory, might represent one of the several endophenotypes contributing to exceptional survival.
In this study, we take into account the complex quantitative nature of cognitive performance and define a phenotype based on the exceptional performance of episodic memory exhibited by offspring in the Long Life Family Study cohort. We aim to evaluate whether there is a familial clustering of the exceptional memory phenotype (EM) within LLFS families that would suggest its potential value for further genetic studies.
Material and Methods
LLFS cohort
Characteristics of the LLFS cohort have been described elsewhere (Barral, et al., 2012).
Cognitive assessment: Episodic memory domain
Using the non-demented offspring of the LLFS probands as a normative sample (N=1911), we computed demographically adjusted z-scores for two memory tests, immediate and delayed recall of Story A from the Wechsler Memory Scale Revised (Wechsler, 1987) using linear regression models adjusting for sex, age and education. These demographically adjusted scores were then averaged to obtain the episodic memory score.
EM quantitative trait
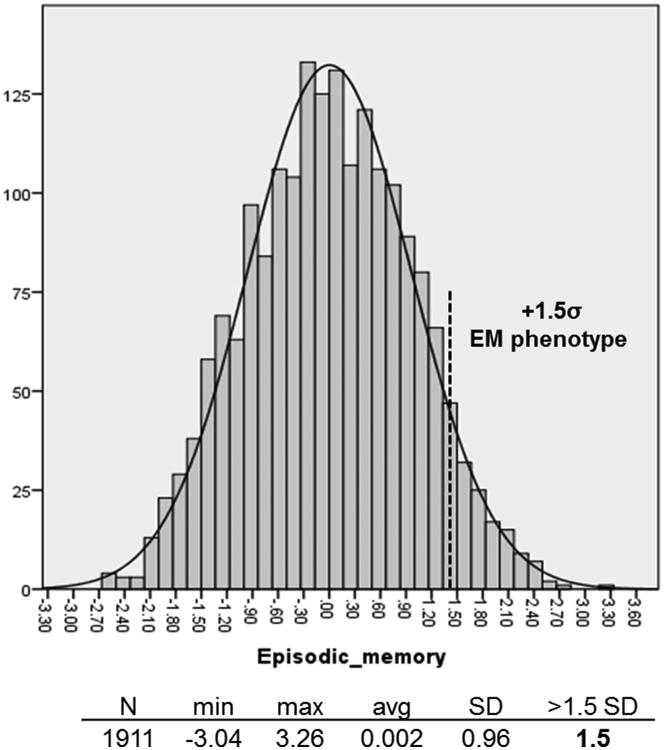
In the normative sample, the episodic memory distribution had a mean value of 0.002 (SD=0.96), ranging from a minimum z-score of -3.04 to a maximum z-score of 3.26 (Figure 1). We used a threshold of 1.5 standard deviations above the mean score to declare that an individual has exceptional memory (episodic memory z-score ≥ 1.5). We used this threshold to identify EM subjects in the entire offspring generation of the LLFS cohort (non-demented offspring and their unrelated non-demented spouses, N= 2547). Based on the number of offspring with EM, we categorized the LLFS families as families with and without exceptional memory, EM families (families with at least two EM offspring) and Non-EM families (families with one or none EM offspring).
Figure 1.
Episodic memory distribution in the normative sample of non-demented offspring of LLFS probands.
Comparison groups
We investigated differences in memory performance of probands and their siblings in EM families and probands and their relatives in Non-EM families. Secondary analysis assessed differences in performance for three comparison groups: i) spouses of the LLFS' offspring in the EM families versus spouses of the LLFS's offspring in the Non-EM families, ii) offspring of the Non-EM families versus the entire group of spouses in the offspring generation and iii) offspring of the EM families versus the entire group of spouses.
Statistical analysis
To assess differences in memory performance between the different comparison groups, we used General Linear Models and Generalized Estimating Equations (GEE). For those group comparisons involving relatives of the LLFS's probands, GEE allows adjustments for differences in family size and accommodates the dependence among related individuals without assuming the joint distribution of the whole family. All multivariate analyses were adjusted for APOE genotype (genotypes at the APOE locus were recoded into two categories after excluding heterozygous individuals' ε2ε4: 1) having no APOE ε4 allele and 2) having at least one copy of the APOE ε4 allele). Analyses were not demographically adjusted as the dependent variable was already adjusted for age, sex, and education.
Results
The LLFS dataset used in this study was restricted to families without missing values for the cognitive tasks and demographic variables considered, consisting of 2971 subjects from 557 two-generation LLFS families. When our selection criterion was applied to the entire offspring generation of the LLFS cohort (N=2547), we identified 18 EM families (N=405 subjects) and 539 Non-EM families (N=2566 individuals). Table 1 summarizes the results from the different linear models considered. Results from our primary comparison analysis showed that probands and their siblings in EM families achieved significantly higher scores on episodic memory compared with probands and their siblings in Non-EM families (estimated average episodic memory of 0.56 (SE=0.19) versus -0.18 (SE=0.05), p = 1.4 × 10-4).Secondary analysis showed that spouses of the LLFS's offspring in EM families demonstrated no significant difference in performance compared with spouses from Non-EM families (estimated average episodic memory of 0.20, (SE=0.08) versus 0.02 (SE=0.04), p=0.069). The Non-EM offspring have significantly worse memory performance than the entire group of spouses (estimated average episodic memory of -0.12, (SE=0.03) versus 0.06 (SE=0.04), p = 6 × 10-5). As expected, GEE models showed that EM offspring have exceptional memory scores when compared with the entire group of unrelated spouses (data not shown). Inclusion of APOE did not change the effect of the estimates in any of the models considered (data not shown).
Table 1. Demographics and linear regression equations results for the LLFS comparison groups.
| LLFS comparison groups | N | age avg (SD) | edu avg (SD) | % females | APOE %e4 | β (SE) | p | episodic memory avg (SD) |
|---|---|---|---|---|---|---|---|---|
| parental generation EM families | 21 | 85 (4.8) | 7 (4.3) | 71 | 2 | 0.74 (0.19) | 1.4 × 10-4 | 0.56 (0.19) |
| parental generation Non-EM families | 297 | 86 (5.9) | 11 (2.9) | 57 | 13 | -0.18 (0.05) | ||
|
| ||||||||
| offspring Non-EM families | 1612 | 61 (8.0) | 13 (2.6) | 60 | 19 | -0.18 (0.04) | 6 × 10-5 | -0.12 (0.03) |
| spouses | 636 | 61 (8.3) | 12 (3.4) | 49 | 24 | 0.06 (0.04) | ||
Discussion
We found evidence for significant familial clustering of our quantitative trait, exceptional memory performance (EM), within families ascertained on the basis of their exceptional survival. The parental generation of the LLFS's offspring with exceptional memory showed significantly better performance for this cognitive domain when compared to the parental generation of LLFS's offspring without exceptional memory.
The offspring without exceptional memory demonstrated significantly worse memory performance when compared with their spouses (β=-0.18, SE=0.04, p= 6 × 10-5), and offspring with exceptional memory demonstrated a significantly better memory performance when compared with their spouses (β=1.82, SE=0.07, p <0.001). These findings are consistent with the fact that the selection criterion applied to the LLFS offspring biased the groups toward exceptional versus non-exceptional cognition whereas the spousal control groups included individuals at all levels of cognition.
Previous studies on exceptional cognitive skills focused primarily on general cognitive ability (g) and explored the heritability of g using twin studies, usually involving a small number of samples (Petrill, et al., 1998, Plomin, et al., 1993, Saudino, et al., 1994). In a well powered analysis using data from 11,000 twin pairs, Haworth and colleagues (Haworth, et al., 2009) investigated genetic and non-genetic influences on g for the top 15% of the distribution and found substantial contribution of genetic variation while influence of shared environmental effects for high cognitive abilities was only moderate. Their results substantially overlap with previous findings for the normal distribution, suggesting that the etiology of exceptional cognitive abilities differs quantitatively and not qualitatively from that of the normal distribution of cognitive abilities. We will be able to address this hypothesis in future studies by estimating the heritability of the EM phenotype in the LLFS cohort.
Our findings suggest that EM may be one of the several biological pathways contributing to the exceptional survival of the LLFS families. Demonstration of familial clustering warrants further genetic studies to determine the specific genetic contributions to the EM phenotype. Identifying specific genes that contribute to the EM phenotype will help to identify people and pathways likely to have exceptional longevity.
The limitations of our study include: 1) We defined the EM phenotype using a fairly liberal definition of EM (1.5 SD above the mean), reasoning that this cutoff will capture those individuals with high memory without significantly penalizing sample size and statistical power and 2) There might be other unexamined reasons explaining the familial clustering of the EM phenotype such as shared genes for an endophenotype that influences cognitive performance and/or shared non-genetic factors among family members.
We investigated the familial aggregation of exceptional memory in a family cohort selected based on the exceptional survival of their probands. We have confirmed that exceptional episodic memory performance aggregates strongly within LLFS families. Future genetic studies are needed to identify genetic and non-genetic factors contributing to exceptional cognition.
Acknowledgments
Sponsored by the National Institute on Aging (NIA cooperative agreements U01-AG023712, U01-AG23744, U01-AG023746, U01-AG023749 and U01-AG023755) Danish 1905-cohort is funded by NIH/NIA, P01 AG08761. The Danish Aging Research Center is funded by the VELUX Foundation Special thanks to Heidi Dubrouillet, Project Manager at the Division of Statistical Genomics of Washington University School of Medicine
References
- Barral S, Cosentino S, Costa R, Matteini A, Christensen K, Andersen SL, Glynn NW, Newman AB, Mayeux R. Cognitive function in families with exceptional survival. Neurobiol Aging. 2012;33(3):619 e1–7. doi: 10.1016/j.neurobiolaging.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher LM, Davis OS, Craig IW, Plomin R. Genome-wide quantitative trait locus association scan of general cognitive ability using pooled DNA and 500K single nucleotide polymorphism microarrays. Genes Brain Behav. 2008;7(4):435–46. doi: 10.1111/j.1601-183X.2007.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis OS, Butcher LM, Docherty SJ, Meaburn EL, Curtis CJ, Simpson MA, Schalkwyk LC, Plomin R. A three-stage genome-wide association study of general cognitive ability: hunting the small effects. Behav Genet. 2010;40(6):759–67. doi: 10.1007/s10519-010-9350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Spinath FM, Bates TC. Genetics of intelligence. Eur J Hum Genet. 2006;14(6):690–700. doi: 10.1038/sj.ejhg.5201588. [DOI] [PubMed] [Google Scholar]
- Freund LS, Reiss AL. Cognitive profiles associated with the fra(X) syndrome in males and females. Am J Med Genet. 1991;38(4):542–7. doi: 10.1002/ajmg.1320380409. [DOI] [PubMed] [Google Scholar]
- Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. Jama. 1998;279(8):585–92. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Martin NW, Martin NG, Boomsma DI, Bartels M, Posthuma D, Davis OS, Brant AM, Corley RP, Hewitt JK, Iacono WG, McGue M, Thompson LA, Hart SA, Petrill SA, Lubinski D, Plomin R. A twin study of the genetics of high cognitive ability selected from 11,000 twin pairs in six studies from four countries. Behav Genet. 2009;39(4):359–70. doi: 10.1007/s10519-009-9262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Whitfield K, Pedersen NL, Hofer SM, Ahern F, McClearn GE. Origins of individual differences in episodic memory in the oldest-old: a population-based study of identical and same-sex fraternal twins aged 80 and older. J Gerontol B Psychol Sci Soc Sci. 1999;54(3):173–9. doi: 10.1093/geronb/54b.3.p173. [DOI] [PubMed] [Google Scholar]
- Korten AE, Jorm AF, Jiao Z, Letenneur L, Jacomb PA, Henderson AS, Christensen H, Rodgers B. Health, cognitive, and psychosocial factors as predictors of mortality in an elderly community sample. J Epidemiol Community Health. 1999;53(2):83–8. doi: 10.1136/jech.53.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrill SA, Kovas Y, Hart SA, Thompson LA, Plomin R. The genetic and environmental etiology of high math performance in 10-year-old twins. Behav Genet. 2009;39(4):371–9. doi: 10.1007/s10519-009-9258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrill SA, Saudino K, Cherny SS, Emde RN, Fulker DW, Hewitt JK, Plomin R. Exploring the genetic and environmental etiology of high general cognitive ability in fourteen- to thirty-six-month-old twins. Child Dev. 1998;69(1):68–74. [PubMed] [Google Scholar]
- Plomin R, DeFries JC. The genetics of cognitive abilities and disabilities. Sci Am. 1998;278(5):62–9. doi: 10.1038/scientificamerican0598-62. [DOI] [PubMed] [Google Scholar]
- Plomin R, Emde RN, Braungart JM, Campos J, Corley R, Fulker DW, Kagan J, Reznick JS, Robinson J, Zahn-Waxler C, et al. Genetic change and continuity from fourteen to twenty months: the MacArthur Longitudinal Twin Study. Child Dev. 1993;64(5):1354–76. [PubMed] [Google Scholar]
- Plomin R, Haworth CM. Genetics of high cognitive abilities. Behav Genet. 2009;39(4):347–9. doi: 10.1007/s10519-009-9277-9. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Freund LS, Baumgardner TL, Abrams MT, Denckla MB. Contribution of the FMR1 gene mutation to human intellectual dysfunction. Nat Genet. 1995;11(3):331–4. doi: 10.1038/ng1195-331. [DOI] [PubMed] [Google Scholar]
- Saudino KJ, Plomin R, Pedersen NL, McClearn GE. The etiology of high and low cognitive ability during the second half of the life span. Intelligence. 1994;19:353–71. [Google Scholar]
- Schupf N, Costa R, Tang MX, Andrews H, Tycko B, Mayeux R. Preservation of cognitive and functional ability as markers of family longevity. Neurobiol Aging. 2003 doi: 10.1016/j.neurobiolaging.2003.11.010. DOI. 10.1016. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Wilson RS, Barral S, Lee JH, Leurgans SE, Foroud TM, Sweet RA, Graff-Radford N, Bird TD, Mayeux R, Bennett DA. Heritability of different forms of memory in the Late Onset Alzheimer's Disease Family Study. J Alzheimers Dis. 2011;23(2):249–55. doi: 10.3233/JAD-2010-101515. [DOI] [PMC free article] [PubMed] [Google Scholar]