Abstract
Adeno-associated virus (AAV) vectors delivered through the systemic circulation successfully transduce various target tissues in animal models. However, similar attempts in humans have been hampered by the high prevalence of neutralizing antibodies to AAV, which completely block vector transduction. We show in both mouse and nonhuman primate models that addition of empty capsid to the final vector formulation can, in a dose-dependent manner, adsorb these antibodies, even at high titers, thus overcoming their inhibitory effect. To further enhance the safety of the approach, we mutated the receptor binding site of AAV2 to generate an empty capsid mutant that can adsorb antibodies but cannot enter a target cell. Our work suggests that optimizing the ratio of full/empty capsids in the final formulation of vector, based on a patient's anti-AAV titers, will maximize the efficacy of gene transfer after systemic vector delivery.
Introduction
Adeno-associated viral (AAV) vector–mediated gene transfer has shown potential as a therapeutic platform for inherited and metabolic diseases (1). Systemic delivery of AAV vectors through the bloodstream is a safe, noninvasive, and potentially effective strategy to target a variety of organs, including liver (1–3) and muscle (4). However, with a prevalence of 30 to 60% in humans (5, 6), neutralizing antibodies (NAbs) to AAV constitute a major obstacle, and other studies have shown that these NAbs, even at relatively low titers, block gene transfer when vector is delivered through the vasculature (2, 7, 8). Moreover, cross-reactivity of anti-AAV antibodies results in neutralization of a wide range of AAV serotypes (5), eliminating the obvious solution of switching AAV serotype.
Thus far, two clinical studies in which an AAV vector was delivered through the systemic circulation have been conducted; both studies targeted the liver to express coagulation factor IX (F.IX) to treat hemophilia B. In one study, a single-stranded AAV2 vector expressing the F.IX transgene was delivered through the hepatic artery to severe hemophilia B subjects at doses of 8 × 1010, 4 × 1011, and 2 × 1012 vector genomes (vg)/kg (2). Efficacy was observed in only one subject, who received the highest vector dose, 2 × 1012 vg/kg, and who exhibited peak F.IX (transgene product) plasma levels of ∼10% of normal. A second subject infused with the same vector dose, with pretreatment anti-AAV NAb titer of 1:17, failed to achieve detectable levels of transgene expression. The subjects infused with lower doses had no detectable NAbs and did not show any evidence of transgene expression (2).
In a second study, a self-complementary AAV8 vector expressing the F.IX transgene was administered through peripheral vein infusion to severe hemophilia B subjects at doses similar to those administered in the AAV2 study: 2 × 1011, 6 × 1011, and 2 × 1012 vg/kg (1). All subjects enrolled in the AAV8 trial had evidence of transgene expression above baseline levels, even though some of the subjects had low but detectable levels of anti-AAV8 NAbs (1). Peak F.IX plasma levels at the high vector dose were 8 to 12% of normal, similar to the high dose of the AAV2 trial, suggesting that the vectors used in the two studies had comparable potency. The vectors used in the two studies differed in empty capsid content because the AAV2 vector preparation was essentially empty capsid–free (9) and the AAV8 vector contained a 5-fold (5X) to 10-fold (10X) excess of empty capsids (10).
One common aspect of both studies is that, at the higher vector doses tested, activation of capsid-specific CD8+ T cells was associated with an increase in serum liver enzymes and loss of F.IX transgene expression (1, 2, 11), likely caused by immune-mediated clearance of transduced hepatocytes. Therefore, although administration of higher vector doses increases the efficiency of AAV transduction, the activation of capsid-specific T cell immunity, as a function of capsid load (1, 12), may eventually limit the efficacy of gene transfer.
The current study was undertaken to explore the role of empty capsids as a factor in the difference in outcome in the low-dose cohorts of the two trials. Our underlying hypothesis was that the presence of an excess of empty capsids effectively absorbs low-level neutralizing antibodies (NAbs) and non-NAbs, permitting transduction even in their presence. Our work demonstrates that the inhibitory effect of anti-AAV antibodies can be overcome by adding empty capsids to the final formulation of AAV vector, and that the higher the antibody titer, the higher the dose of empty capsids required to overcome the inhibitory antibodies. Because the empty capsid is not immunologically inert, however (13, 14), we performed additional experiments using a noninfectious AAV mutant derived from AAV2 (15), showing that the mutant capsid has markedly lower immunogenicity compared to native empty capsids and is nonetheless equally effective at adsorbing antibodies. Application of these findings to the development of personalized final formulations of vector product for intravascular delivery will facilitate safe, effective AAV-mediated gene transfer in settings in which vectors are delivered through the systemic circulation.
Results
AAV empty capsids allow for vector delivery in the presence of NAbs
Using a cell-based assay to measure anti-AAV8 NAbs (16), we demonstrated that empty capsids greatly reduce neutralizing activity of intravenous immunoglobulin (IVIg) and human serum in vitro (fig. S1). To test the effect of empty capsids on AAV vector transduction in vivo, we used a mouse model of anti-AAV antibody responses (8) in which mice were passively immunized with human IVIg injected intraperitoneally 24 hours before vector administration (Fig. 1A). We injected intravenously an AAV8 vector expressing human F.IX (AAV8-hF.IX) into naïve mice or mice that were passively immunized with a low dose of IVIg (0.5 mg per mouse), sufficient to result in an anti-AAV8 NAb titer ranging from 1:1 to 1:3 (Fig. 1, A and B). Vector doses of 1 × 109 and 5 × 109 vg per mouse, or the same vector doses formulated in 10X excess AAV8 empty capsids, gave rise to similar levels of hF.IX in plasma in naïve animals. IVIg immunization effectively blocked most liver transduction by vectors formulated in phosphate-buffered saline (PBS), whereas formulation of vectors in 10X empty capsids rescued transgene expression (Fig. 1B). Vector gene copy number measured in livers collected from animals receiving 5 × 109 vg of AAV8-hF.IX confirmed these findings (Fig. 1C) and showed similar vector biodistribution in mice receiving vector only or vector formulated in empty capsids (vide infra).
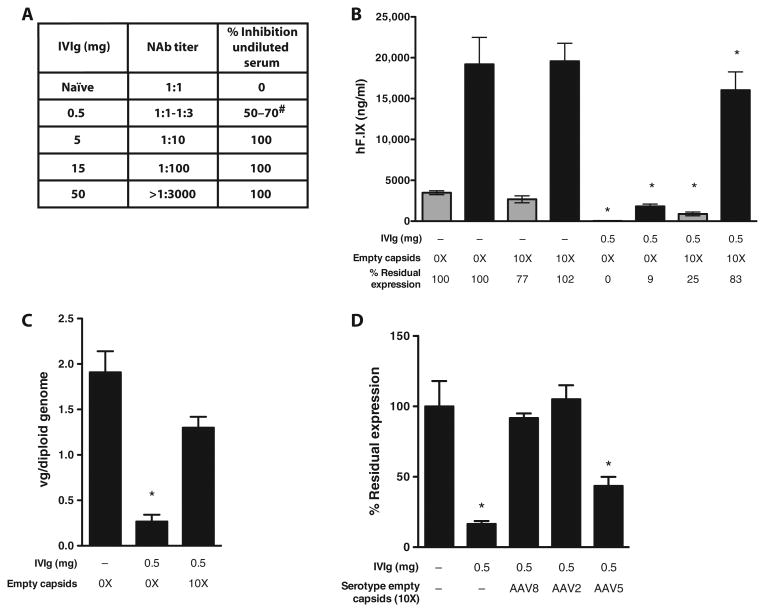
Fig. 1. AAV empty capsids prevent vector neutralization by anti-AAV NAbs in vivo in mice.
(A) Anti-AAV8 antibody analysis in naïve mice injected intraperitoneally with PBS only or mice passively immunized intraperitoneally with 0.5, 5, 15, or 50 mg of IVIg (n = 5 per group). The analysis was performed 24 hours after immunization. “NAb titer” represents the reciprocal serum dilution at which <50% inhibition of the reporter vector signal was measured in the NAb assay. “% Inhibition undiluted serum” represents the inhibition of the reporter gene signal observed when undiluted test serum was mixed with an equal volume of a solution containing the reporter vector. #, range. (B) Male C57BL/6 mice (n = 5 per group) were injected intraperitoneally with 0.5 mg of IVIg (resulting in a NAb titer of 1:1 to 1:3) or injected with PBS (−). After 24 hours, animals received either 1 × 109 vg (gray bars) or 5 × 109 vg (black bars) of an AAV8-F.IX vector alone (0X) or formulated with a 10X excess of AAV8 empty capsids [1 × 1010 capsid particles (gray bars) or 5 × 1010 capsid particles (black bars)]. hF.IX transgene levels in plasma at week 8 after vector delivery are shown as averages; error bars, SEM. *P < 0.05 versus naïve mice (two-tailed, unpaired t test). (C) Vector gene copy number in mouse livers collected at week 12 after AAV8-F.IX gene transfer at a vector dose of 5 × 109 vg per animal. Results are shown as average copy number of five livers. Error bars, SEM. Experimental groups are the same as shown in (B). *P < 0.05 versus naïve mice (two-tailed, unpaired t test). (D) Empty capsids from alternate AAV serotypes protect AAV8-F.IX vector from NAb neutralization. Male C57BL/6 mice (n = 5 per group) were passively immunized with 0.5 mg of IVIg or injected with PBS (−) intraperitoneally. Twenty-four hours after, animals received 5 × 109 vg of an AAV8-F.IX vector alone (−) or formulated with a 10X excess of AAV8, AAV2, or AAV5 empty capsids. The percent residual expression is calculated relative to the F.IX transgene plasma levels in naïve animals receiving the AAV-F.IX vector only. *P < 0.05 versus naïve mice (two-tailed, unpaired t test).
AAV8-F.IX vector transduction in the presence of anti-AAV8 NAbs was equally rescued by AAV8 or AAV2 empty capsids, whereas AAV5 empty capsids were not as effective (Fig. 1D), a result in agreement with the lower degree of conservation of the capsid amino acid sequence of this serotype.
These results indicate that empty AAV capsids can increase the efficiency of transduction of AAV vectors delivered systemically in the presence of anti-AAV antibodies.
Empty capsid formulation overcomes high-titer Nabs
To test whether it is possible to define the optimal amount of empty capsid content in AAV preparations based on the baseline anti-AAV NAb titer, mice passively immunized with various amounts of IVIg (Fig. 1A) received 5 × 109 vg per mouse of vector alone or formulated in increasing amounts of empty AAV8 capsids (Fig. 2, A to D). In the presence of low-titer anti-AAV8 NAb titers (1:1 to 1:3, Fig. 2A), formulation of vector in a 10X excess of empty capsids completely rescued AAV vector transduction, and empty capsid excess of up to 100X the AAV8-F.IX dose did not inhibit vector transduction. In this experiment, a 1000X excess of AAV8 empty particles resulted in a ∼60% loss of transgene expression, likely due to interference of empty capsids with AAV8-F.IX vector receptor binding on hepatocytes (Fig. 2A). Conversely, the same excess of AAV2 empty capsid did not alter the efficiency of liver transduction of the AAV8-F. IX vector (fig. S2).
Fig. 2. Addition of empty capsid in defined amounts based on pretreatment NAb titers can overcome transduction barrier posed by anti-AAV antibodies.
(A to D) Percent residual F.IX transgene expression in mice immunized with 0.5 mg (A), 5.0 mg (B), 15 mg (C), or 50 mg (D) of IVIg and injected 24 hours later with 5 × 109 vg per mouse of AAV8-F.IX vector alone or vector formulated in excess empty capsids as indicated in the x axes. The percent residual expression is calculated relative to the F.IX transgene product plasma levels in naïve animals (No IVIg) receiving the AAV-F.IX vector alone. Results are shown as average residual expression (n = 5 animals per group); error bars represent the SEM. *P < 0.05 versus naïve mice (two-tailed, unpaired t test).
In the presence of NAb titers of 1:10 and 1:100, full rescue of liver transduction was obtained with an excess of 50X and 100X empty capsids, respectively (Fig. 2, B and C). Decreased expression levels of F.IX were observed at 100X excess empty capsids in mice with a NAb titer of 1:10; this was likely due to experimental variability. When mice were immunized with high doses of IVIg, resulting in very high NAb titers (>1:3000), we were not able to rescue liver transduction even by adding a 1000X excess of empty capsids (Fig. 2D). Although anti-AAV8 NAb titers higher than 1:1000 are rarely found in humans naïve to AAV vectors (table S1), they are common in subjects dosed with AAV vectors, suggesting that formulation of vector in empty capsids may need to be coupled with other strategies [such as plasmapheresis (17) or pharmacological methods (16)] to allow for vector readministration.
These results support the hypothesis that AAV empty capsids enhance liver transduction after systemic vector delivery in the presence of anti-AAV NAbs in a dose-dependent fashion, with higher NAb titers requiring more excess empty capsid to overcome vector neutralization.
AAV empty capsids act as decoys for anti-AAV antibodies
We hypothesized that empty capsids act as decoys for anti-AAV antibodies, thus protecting AAV vectors from neutralization. To test this hypothesis, mice passively immunized to a NAb titer 1:10 received an AAV8-F.IX vector alone or formulated in empty capsids (Fig. 3A). Plasma was collected 1 day after vector delivery and assayed for immunoglobulin G (IgG)–capsid complexes and for anti-AAV8 NAb titer. For titration of immune complexes in plasma, we constructed a standard curve with known quantities of AAV8 capsid incubated with IVIg (Fig. 3B). After vector administration, no immune complexes were detectable in the plasma of naïve mice (Fig. 3, A and C, group 1), whereas IgG-capsid complexes were detectable in IVIg-injected animals receiving vector only or vector and empty capsids (Fig. 3, A and C, groups 2 to 6), with the highest amounts of complexes detectable in animals from the 50X and 100X empty capsid groups (Fig. 3, A and C, groups 5 and 6). Absorption of anti-capsid antibodies by empty capsids resulted in a dose-dependent drop in anti-AAV8 NAb titer in mice 1 day after vector delivery (Fig. 3A) and, ultimately, in the detection of increasing levels of hF.IX transgene expression (Fig. 3D). These data support the hypothesis that empty AAV capsids act as a decoy for anti-AAV NAbs, thus protecting AAV vectors from antibody-mediated neutralization.
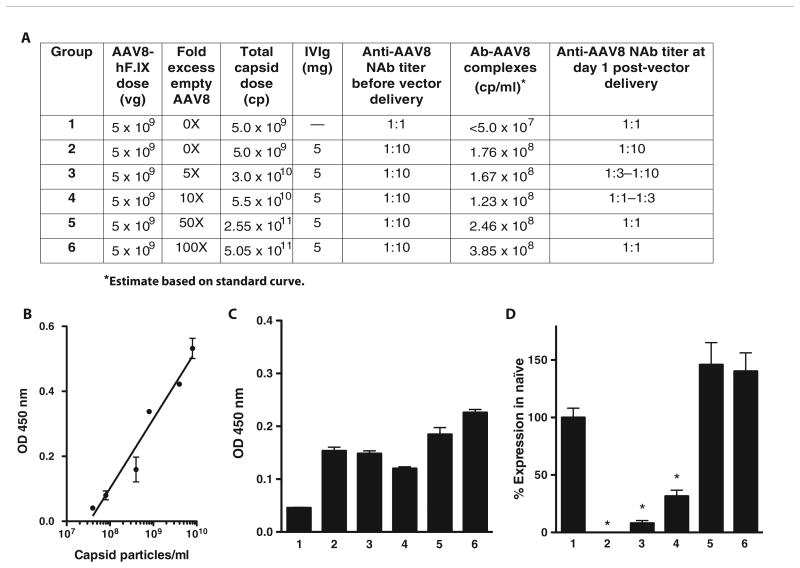
Fig. 3. Detection of antibody-capsid immune complexes after vector AAV delivery.
(A) Outline of experimental groups and summary of results. Mice (n = 5 per group) received 5 × 109 vg of AAV8-F.IX 24 hours after the administration of 5 mg of IVIg (groups 2 to 6) or saline (group 1, naïve control) intraperitoneally. Vector was formulated in increasing amounts of AAV8 empty capsids, from 0X to 100X. Twenty-four hours after vector administration, plasma was collected and assayed for immune complexes and anti-AAV8 NAb titers. (B) Standard curve used for the quantification of immune complexes prepared with known amounts of AAV8 capsid particles incubated with IVIg for 1 hour at 37°C. Each sample was tested in duplicate. (C) Detection of immune complexes after AAV vector delivery. An increasing amount of immune complexes is detectable at increasing capsid doses. Each sample was tested in triplicate. (D) F.IX transgene expression levels 4 weeks after vector administration. At the same AAV8-F.IX vector dose, increasing amounts of F.IX expression are measured at higher empty capsid doses and when circulating antibody-capsid immune complexes are detected. F.IX levels are expressed as percent of F.IX levels in naïve mice. *P < 0.05 versus group 1 (two-tailed, unpaired t test). vg, vector genomes; cp, capsid particles; OD, optical density. Error bars represent the SEM.
Empty capsid decoys are safe and effective in nonhuman primates
On the basis of the molecular weight of one AAV particle, ∼3750 kD (18), 1 × 1014 virions correspond to ∼0.6 mg of protein, less than the standard doses of recombinant proteins administered repeatedly, for example, in the clinical management of hemophilia (19). This may account for the lack of toxicity observed in humans receiving high doses of AAV vectors intravenously (1, 2).
We used rhesus macaques, which are natural hosts for AAV8 (20), to further assess the safety and efficacy of our approach. Similar to humans, despite the low NAb titer, baseline undiluted sera of the selected animals had a neutralizing activity in vitro ranging from 0 to 80% (Table 1). At a dose of 1 × 1012 vg/kg of AAV8-hF.IX, of the two animals that received vector alone, one (1001) expressed the hF.IX transgene only transiently because of the development of an anti-hF.IX antibody, a finding previously reported in rhesus macaques (21–23); the other animal, 1002, reached plateau hF.IX plasma levels of 75 ng/ml. In contrast, the animal injected with vector formulated in 9X empty AAV8 capsids, 2001, reached plateau levels of transgene expression of ∼380 ng/ml, ∼5X higher (Fig. 4A). Animals 1002 and 2001 had a similar anti-AAV8–neutralizing activity at baseline (Table 1).
Table 1. Summary of studies in nonhuman primates.
vg, vector genomes; cp, capsid particles.
| Animal ID | Vector dose (vg/kg) | Empty capsids (cp/kg) | Baseline NAbs | |
|---|---|---|---|---|
|
| ||||
| Anti-AAV8 NAb titer | % Inhibition undiluted serum | |||
| 1001 | 1 × 1012 | — | 1:1 | 50 |
| 1002 | 1 × 1012 | — | 1:1 | 34 |
| 2001 | 1 × 1012 | 9 × 1012 | 1:1 | 32 |
| 3001 | 2 × 1012 | — | 1:1–1:3 | 57 |
| 3002 | 2 × 1012 | — | 1:1 | 10 |
| 5001 | 2 × 1012 | — | 1:1 | 0 |
| 4001 | 2 × 1012 | 1.8 × 1013 | 1:1–1:3 | 80 |
| 4002 | 2 × 1012 | 1.8 × 1013 | 1:1–1:3 | 55 |
| 6001 | 2 × 1012 | 0.8 × 1013 | 1:1 | 43 |
Fig. 4. Safety and efficacy of coadministration of AAV8-F.IX vectors and AAV8 empty capsids in rhesus macaques.
(A) Plasma hF.IX levels in male rhesus macaques receiving an AAV8-hF.IX vector at doses of 1 × 1012 or 2 × 1012 vg/kg. Animals received the AAV8-hF.IX vector in PBS (1001, 1002, 3001, 3002, and 5001) or formulated in excess of empty AAV8 capsids (2001, 4001, 4002, and 6001). Results are shown as average hF.IX levels in plasma at weeks 4 to 12 (weekly measurements). Error bars represent the SEM. Statistical analysis of hF.IX expression levels was performed with two-tailed, unpaired t test. (B) Antibody-capsid circulating immune complexes measured after vector administration. Serum samples were collected at baseline and at days 1, 2, 14, 21, and 28 after vector delivery and assayed for the presence of immune complexes. Results are shown as optical densities (OD). Error bars represent the SD of the average of triplicate testing. When detectable, immune complexes are present only briefly after vector delivery.
Dose escalation to 2 × 1012 vg/kg led to similar results. One of the three animals dosed with vector only, 3001, did not achieve detectable levels of circulating hF.IX transgene product. The animal did not develop anti-hF.IX antibodies, and the low AAV vector genome copy number in liver (table S2) suggests that even low-titer NAbs (Table 1) completely blocked vector transduction. Animals 3002 and 5001, both with very low baseline antibody levels (Table 1), expressed hF.IX at ∼120 ng/ml, and 5001 of 250 ng/ml. 5001 developed an anti-hF.IX antibody 9 weeks after vector delivery.
The three animals dosed with vector formulated in empty capsids—4001, 4002, and 6001 (all with detectable baseline NAbs; Table 1)—expressed hF.IX at higher levels than the vector only group, between ∼300 and 450 ng/ml (Fig. 4A and fig. S3). AAV vector biodistribution was performed in a subset of animals, showing no differences among experimental groups (table S2). Similarly, a survey of histopathological findings in liver, kidney, lung, or spleen by an independent pathologist showed no alterations, consistent with results from serum clinical chemistry analysis (table S3). Monitoring of IgG-AAV8 immune complexes in plasma collected from all animals at baseline and up to 4 weeks after vector delivery showed rapid clearance, within 2 days after vector delivery, of these complexes from the circulation (Fig. 4B). Finally, anti–capsid B and T cell responses were equivalent in all experimental groups (fig. S4, A and B), with no evidence of enhanced immunogenicity triggered by the administration of vector formulated in empty capsids in the presence of antibodies.
These results confirm, in a large animal model, findings in vitro and in vivo in mice on the enhancing effect of empty capsids on AAV vector transduction efficiency; they also support the safety of the approach.
Mutant empty capsids show efficacy and reduced immunogenicity
Entry of empty capsids into a target cell would likely increase major histocompatibility complex (MHC) class I presentation of capsid epitopes (12–14, 24). Thus, although increasing the total capsid dose (empty particles and DNA-bearing vector particles) helps to evade anti-AAV humoral responses, it may increase the likelihood of triggering potentially harmful T cell responses against the AAV capsid (1, 2, 11). To reduce the ability of empty capsids to enter a cell, we introduced two mutations within the AAV2 capsid known to disrupt the virus receptor binding (15). The resulting vector, AAV585/8, was poorly infective in vitro (fig. S5A) and failed to transduce the liver in vivo (fig. S5, B and C), whereas it effectively protected an AAV8 vector from antibody neutralization in vitro (fig. S5D). In vivo, empty AAV585/8 capsids protected AAV8-F.IX vectors from NAbs (Fig. 5A); because this mutant capsid was derived from AAV2, which does not seem to compete with AAV8 for receptor binding (fig. S2), it could be added to AAV8-F.IX in 1000X excess without resulting in inhibition of liver transduction (Fig. 5A and fig. S6). Furthermore, the AAV585/8 mutant effectively protected from anti-AAV neutralization several AAV capsids, which were otherwise effectively neutralized by anti-AAV antibodies (Fig. 5B). Only partial rescue of expression was observed with AAVdj, likely due to the presence of unique B cell epitopes in this serotype, which are not represented in the AAV585/8 mutant decoys (Fig. 5B). The presence of anti-AAV antibodies at the time of vector delivery did not affect vector genome biodistribution, except in the case of liver where it was reduced (fig. S6), and administration of AAV585/8 capsids 1 hour before AAV8 vector delivery, as opposed to coadministering them with the vector, did not result in a significant increase in F.IX transgene expression levels (fig. S7).
Fig. 5. The AAV585/8 capsid mutant effectively protects AAV vector neutralization by NAbs and does not infect cells.
(A) Male C57BL/6 mice (n = 5 per group) were passively immunized with 0.5 mg of IVIg (+) or injected with PBS (−). Twenty-four hours later, animals received 5 × 109 vg of an AAV8-F.IX vector alone (0X) or formulated with a 10X or 1000X excess of AAV585/8 empty capsids (10X AAV585/8 and 1000X AAV585/8, respectively) or 1000X excess of AAV8 empty capsids (1000X AAV8). Results are shown as percent of expression of F.IX in naïve mice. Error bars represent the SEM. *P < 0.05 versus vector alone in naïve animals (two-tailed, unpaired t test). (B) Naïve (−) or passively immunized mice (+) were given a 5 × 109 vg (AAVdj) or 5 × 1010 vg (AAV2, AAV5, and AAV6) of vectors expressing F.IX. Vector was given alone (black bars) or formulated in a 10X excess of AAV585/8 empty capsids (red bars). Results are shown as percent of expression of F.IX in naïve mice. Error bars represent the SEM. *P < 0.05 versus vector alone in naïve animals (two-tailed, unpaired t test). (C to H) AAV2 (C to E) and AAV585/8 (F to H) vector internalization in HHL5 human hepatocytes. Cells were treated for 4 hours at a multiplicity of infection (MOI) of 1 × 105 with the AAV vectors and subsequently intracellularly stained with the anti-AAV2 monoclonal antibody A20, which binds equally both AAV2 and AAV585/8 (fig. S5E). The gates in (C) and (F) indicate the percent of AAV signal localized intracellularly; the gates in (D) and (G) indicate the percent of the AAV signal localized in early endosomes; the gates in (E) and (H) indicate the percent of AAV signal localized in the nuclei. (I) CTL assay with HHL5 human hepatocytes. Target cells were transduced overnight at increasing MOIs of AAV2 (black line) or AAV585/8 (red line) vectors and incubated with human leukocyte antigen–matched AAV-specific effector cells at an effector/target ratio of 10:1. Percent cytotoxicity is calculated after background subtraction relative to the maximum cell lysis obtained by treating targets with Triton X-100. Error bars, SEM of triplicate readings.
Flow imaging of human hepatocytes transduced in vitro with AAV2 or AAV585/8 vectors showed that AAV585/8 was unable to enter the cells (Fig. 5, C to H); this was also associated with the inability of AAV585/8 capsid to trigger killing of target human hepatocytes in vitro in a cytotoxic T lymphocyte (CTL) assay (Fig. 5I) (13, 14).
These results suggest that noninfective mutant AAV capsids enhance vector transduction in the presence of anti-AAV NAbs for a variety of AAV serotypes. Furthermore, they suggest that decreased cellular uptake of mutant AAV capsids potentially reduces the risk of flagging transduced hepatocytes for immune-mediated destruction.
Discussion
Intravascular delivery of AAV vectors is a safe, simple, and minimally invasive gene delivery approach that can be used to target multiple tissues (1, 2, 4). However, studies to date have shown that the human immune response generates at least two roadblocks to successful intravascular delivery of AAV vectors, specifically NAbs that efficiently block transduction when vector is delivered through the circulation, and AAV capsid-specific CD8+ T cells, which can eventually destroy the transduced cells, resulting in loss of the donated gene. The difficulty for the gene therapist is that the obvious solution to the first obstacle, using higher doses of vector, tends to encroach on successful avoidance of the second.
Subjects enrolled in the AAV2-F.IX liver trial for hemophilia B (2) and the more recent AAV8-F.IX liver study (1) had low levels of anti-AAV antibodies and received similar vector doses. However, although in the AAV2 study none of the subjects in the low- and mid-dose cohorts exhibited detectable levels of transgene expression (2), subjects in the AAV8 trial had detectable levels of clotting factor (>1%) at comparable vector doses (1). Possible explanations for the different outcome of these studies could be the higher tropism of AAV8 vectors for hepatocytes compared with AAV2 vectors (20) or the fact that the AAV8 vector carried a self-complementary genome, postulated to drive higher transgene expression levels (22, 25). The fact that published studies in nonhuman primates showed roughly equivalent levels of F.IX transgene expression after the delivery of AAV8 or AAV2 vectors carrying the same transgene expression cassette (7, 21) and the fact that, in both the AAV2 and AAV8 trials, subjects injected at the highest vector dose (2 × 1012 vg/kg) had similar peak F.IX transgene product plasma levels (1, 2) do not support these hypotheses. We thus focused on the differences in AAV vector preparations, because the vector used in the AAV2 study was devoid of empty AAV capsids (9), whereas the vector used in the AAV8 study contained up to 10X empty capsids (10).
Here, we show that inclusion of empty capsids in AAV vector preparations greatly reduces neutralizing activity of human serum over a wide range of titers. This is in agreement with previous work showing that AAV vector preparations obtained by column chromatography, thus containing variable amounts of empty capsids, are less prone to neutralization (8). Our data indicate that low-titer NAbs are sufficient to block liver transduction of vector preparations devoid of empty capsids delivered through the circulation. Addition of excess empty capsids restores vector transduction in a dose-dependent manner, even at high anti-AAV NAb titers, without resulting in a significant inhibition of vector transduction, except when added in large excess (1000X). Experiments in nonhuman primates, which have a similar anti-AAV antibody profile to humans (26), support the validity of our hypothesis.
These results suggest that it is feasible to personalize the formulation of AAV vector preparations, by measuring the baseline anti-AAV antibody titer of each patient and adding defined amounts of empty capsid to the final formulation based on this titer, to achieve efficient and consistent gene transfer. They also highlight the importance of coupling therapeutics (AAV vectors) with effective, predictive diagnostic assays (AAV antibody assays). Finally, it is important to recognize that this modification to the final formulation of the infusate, whereas useful when vector is delivered through the intravascular space, is unnecessary in other settings (27–29).
Empty AAV capsids, however, do enter target cells (24); thus, one limitation of our strategy is that increasing the capsid dose is likely to add to the total amount of capsid antigen being presented on MHC class I (13, 14) and contribute to the recognition of transduced cells by capsid-specific CD8+ T cells (1, 2, 11).
To address this issue, we mutagenized the AAV2 capsid to render it unable to enter a cell and access the MHC class I presentation pathway within the target cell. This mutant capsid effectively protected AAV vectors from neutralization in vitro and in vivo as efficiently as untreated, native empty AAV capsids, with the added advantage that it could be added in up to 1000X excess without competing with AAV8 vectors for receptor binding.
One potential limitation of this strategy derives from the fact that professional antigen-presenting cells can engulf antigens by pinocytosis (30), in addition to receptor-mediated uptake, thus making noninfectious mutant capsids potentially immunogenic, at least to some extent. One additional limitation is that this approach was evidenced by our studies in passively immunized mice, showing that large excess of empty capsids was not able to overcome titer >1:3000, suggesting that a combination of strategies may be needed to overcome very high NAb titers, which may be found in human after AAV vector administration (29). Finally, results with the mutant capsid AAVdj indicate that the success of our approach depends on the presence of the same B cell epitopes on both the therapeutic vector and the capsid decoys. One implication of this finding is that escape from antibody-mediated neutralization could be obtained using individual capsid proteins, compared to whole capsid particles, although in designing the approach one should take into account B cell epitopes, which are nonlinear and may not be represented in linear sequences of the capsid.
Anti-AAV antibodies are highly prevalent in humans, cross-react with several AAV serotypes (5), and may interfere with the efficiency of gene transfer after systemic AAV vector delivery by either neutralizing the vector, increasing its clearance, or both. Data in mice suggest that the hurdle of NAbs to AAV could be overcome by reducing antibody titers transiently for about 4 hours, thus allowing the vector to transduce the target hepatocytes (31). Strategies to reduce these titers through plasmapheresis or by pharmacologic means have shown only limited efficacy (17, 32). Similarly, use of balloon catheters combined with saline flush has shown some efficacy but only for liver gene transfer in the presence of low to moderate NAb titers (33).
Additional strategies tested include the conjugation of AAV vectors with polyethylene glycol (34, 35), which was only partially beneficial at protecting the virus from neutralization and led to reduced infectivity. Recent work from several laboratories explored the use of alternate AAV serotypes (6, 36) or AAV capsid mutants that escape anti-AAV antibodies (37). However, these strategies suffer from several limitations, mainly the fact that antibodies to AAV cross-react with several AAV serotypes [Fig. 5B and (5)] and the fact that it would be difficult from a regulatory standpoint to personalize the gene transfer vector serotype based on a subject's NAbs. Further, engineered escape mutant capsids are likely to have altered tissue tropism, and also are likely to only partially evade AAV, as shown here with AAVdj, a capsid developed with shuffling techniques and selected for its resistance to neutralization by IVIg (38), which is still susceptible to antibody-mediated neutralization (Fig. 5B).
The approach presented here has several advantages: (i) it does not involve chemical modification of the AAV vector or AAV empty capsids; (ii) the AAV585/8 mutant capsids can be produced at high titers with the same current good manufacturing practice process as AAV vectors; (iii) the AAV585/8 capsids do not compete with vector for receptor binding; (iv) the empty capsid content can be adjusted according to a subject's NAb titer; and (iv) it is a strategy effective with a wide range of AAV serotypes. Finally, one implication of our results is that the total capsid dose determines the ability of a vector to escape antibody neutralization; thus, for lower vector doses, a higher excess of empty capsids is needed to reach the critical amount of capsids needed to adsorb circulating antibodies.
In summary, this work provides a strategy to enhance the efficiency of intravascular gene transfer for the individual patient and to increase the proportion of the population that can undergo successful intravascular gene delivery. The use of empty AAV capsid in carefully titrated amounts, based on the titer of baseline anti-AAV antibodies, represents a strategy to overcome the widespread prevalence of humoral immunity to AAV in humans. In effect, the approach allows one to increase the dose in the circulation to overcome humoral immunity without increasing the intracellular burden of capsid, which triggers cellular immunity. The development of this approach of personalized final formulations of vector for delivery through the systemic circulation will greatly expand the cohort of patients who can undergo successful gene delivery to vital organs.
Materials and Methods
AAV vectors and empty capsids
AAV vectors were prepared as previously described (39). Genome-containing vectors and empty AAV capsid particles were purified by cesium chloride gradient centrifugation (40). The AAV vectors used in the in vivo experiments expressed hF.IX under the control of a liver-specific promoter (2).
The AAV585/8 mutant of AAV2 was obtained by introducing two lysine-to-alanine mutations at positions 585 and 588, thus disrupting the receptor binding domain (15). Mutations were introduced with the QuikChange Site-Directed Mutagenesis Kit (Stratagene Agilent Technologies). AAVdj (38) was provided by M. Kay (Stanford University).
Human serum and cell samples
All human samples used in the study were collected under protocols approved by the Children's Hospital of Philadelphia and the University of Pittsburgh Institutional Review Boards.
Supplementary Material
www.sciencetranslationalmedicine.org/cgi/content/full/5/194/194ra92/DC1
Materials and Methods
Fig. S1. AAV empty capsids prevent vector neutralization by anti-AAV NAbs in vitro.
Fig. S2. Large excess of AAV2 empty capsids does not inhibit AAV8-F.IX–mediated live transduction.
Fig. S3. Analysis of hF.IX expression levels and anti-hF.IX IgG levels in nonhuman primates.
Fig. S4. Analysis of capsid-specific humoral and cellular immune responses in nonhuman primates.
Fig. S5. Characterization of the AAV585/8 mutant capsid.
Fig. S6. Vector genome biodistribution in mice receiving an AAV8-F.IX vector formulated in AAV8 or AAV585/8 empty capsids.
Fig. S7. Timing of AAV585/8 empty capsid administration does not have a major influence on hF.IX transgene expression.
Table S1. NAb titers to AAV88 in a cohort of hemophilia B subjects.
Table S2. AAV8-F.IX vector biodistribution in nonhuman primates.
Table S3. Clinical chemistry data over time in nonhuman primates dosed with AAV8-F.IX vectors alone formulated in a 9X excess of AAV8 empty capsids.
Acknowledgments
We thank L. Couto for helping with the manuscript preparation and M. Kay for supplying the AAVdj capsid plasmid. We also thank S. Tichenor, A. Brice, and M. Vinlove for assistance with the primate studies. Finally, we thank M. Ragni for coordinating the collection of samples from the hemophilia B subjects.
Funding: Supported by the Howard Hughes Medical Institute, the Center for Cellular and Molecular Therapeutics at the Children's Hospital of Philadelphia, and the NIH (grant P01 HL078810).
Footnotes
Author contributions: X.M.A., G.P., Y.C., R.J.D., D.J.H., M.Y., L.E., C.J.H., A.F., C.H., and E.B.-T. ran the experiments. A.T. and S.Z. prepared the vectors used in the study. G.M.P. assisted with human sample collection. F.M., X.M.A., J.F.W., and K.A.H. designed the experiments and wrote the manuscript.
Competing interests: F.M., X.M.A., G.M.P., J.F.W., and K.A.H. are inventors in a pending patent on the formulation of AAV vectors for highly efficient transgene delivery (U.S. Provisional Patent Application Serial No. 61/682,019). J.F.W. and K.A.H. have consulted for companies developing AAV-based gene therapeutics. All other authors declare no competing interests.
Contributor Information
Federico Mingozzi, Email: fmingozzi@genethon.fr.
Katherine A. High, Email: high@email.chop.edu.
References and Notes
- 1.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O'Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS, Malani N, Anguela XM, Sharma R, Ivanciu L, Murphy SL, Finn JD, Khazi FR, Zhou S, Paschon DE, Rebar EJ, Bushman FD, Gregory PD, Holmes MC, High KA. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011;475:217–221. doi: 10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arruda VR, Stedman HH, Haurigot V, Buchlis G, Baila S, Favaro P, Chen Y, Franck HG, Zhou S, Wright JF, Couto LB, Jiang H, Pierce GF, Bellinger DA, Mingozzi F, Nichols TC, High KA. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115:4678–4688. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 6.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, Zhou S, Scallan CD, Sommer J, Vijay S, Mingozzi F, High KA, Pierce GF. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, Couto LB, Pierce GF. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 9.Wright JF. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 2008;15:840–848. doi: 10.1038/gt.2008.65. [DOI] [PubMed] [Google Scholar]
- 10.Allay JA, Sleep S, Long S, Tillman DM, Clark R, Carney G, Fagone P, McIntosh JH, Nienhuis AW, Davidoff AM, Nathwani AC, Gray JT. Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum Gene Ther. 2011;22:595–604. doi: 10.1089/hum.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, Ragni MV, Manno CS, Sommer J, Jiang H, Pierce GF, Ertl HC, High KA. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 12.Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2011;11:321–330. doi: 10.2174/156652311796150354. [DOI] [PubMed] [Google Scholar]
- 13.Finn JD, Hui D, Downey HD, Dunn D, Pien GC, Mingozzi F, Zhou S, High KA. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther. 2010;18:135–142. doi: 10.1038/mt.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, Hasbrouck NC, Zhou S, Murphy SL, Maus MV, Mingozzi F, Orange JS, High KA. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119:1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opie SR, Warrington KH, Jr, Agbandje-McKenna M, Zolotukhin S, Muzyczka N. Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding. J Virol. 2003;77:6995–7006. doi: 10.1128/JVI.77.12.6995-7006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mingozzi F, Chen Y, Edmonson SC, Zhou S, Thurlings RM, Tak PP, High KA, Vervoordeldonk MJ. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013;20:417–424. doi: 10.1038/gt.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF, Moullier P, Benveniste O, Masurier C. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19:2084–2091. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman MS. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci USA. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benson G, Auerswald G, Elezović I, Lambert T, Ljung R, Morfini M, Remor E, Salek SZ. Immune tolerance induction in patients with severe hemophilia with inhibitors: Expert panel views and recommendations for clinical practice. Eur J Haematol. 2012;88:371–379. doi: 10.1111/j.1600-0609.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- 20.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, Zhou S, Wright JF, Jiang H, Pierce GF, Arruda VR, High KA. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, Tuddenham EG, Kemball-Cook G, McIntosh J, Boon-Spijker M, Mertens K, Davidoff AM. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D, Nawathe S, Waddington SN, Bronson R, Jackson S, Donahue RE, High KA, Mingozzi F, Ng CY, Zhou J, Spence Y, McCarville MB, Valentine M, Allay J, Coleman J, Sleep S, Gray JT, Nienhuis AW, Davidoff AM. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson JS, Samulski RJ. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 26.Hurlbut GD, Ziegler RJ, Nietupski JB, Foley JW, Woodworth LA, Meyers E, Bercury SD, Pande NN, Souza DW, Bree MP, Lukason MJ, Marshall J, Cheng SH, Scheule RK. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol Ther. 2010;18:1983–1994. doi: 10.1038/mt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: An open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 28.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, Leonard DG, Johnson FA, McClelland A, Scallan C, Skarsgard E, Flake AW, Kay MA, High KA, Glader B. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 30.Norbury CC. Drinking a lot is good for dendritic cells. Immunology. 2006;117:443–451. doi: 10.1111/j.1365-2567.2006.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy SL, Li H, Zhou S, Schlachterman A, High KA. Prolonged susceptibility to antibody-mediated neutralization for adeno-associated vectors targeted to the liver. Mol Ther. 2008;16:138–145. doi: 10.1038/sj.mt.6300334. [DOI] [PubMed] [Google Scholar]
- 32.Mingozzi F, Chen Y, Murphy SL, Edmonson SC, Tai A, Price SD, Metzger ME, Zhou S, Wright JF, Donahue RE, Dunbar CE, High KA. Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol Ther. 2012;20:1410–1416. doi: 10.1038/mt.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mimuro J, Mizukami H, Hishikawa S, Ikemoto T, Ishiwata A, Sakata A, Ohmori T, Madoiwa S, Ono F, Ozawa K, Sakata Y. Minimizing the inhibitory effect of neutralizing antibody for efficient gene expression in the liver with adeno-associated virus 8 vectors. Mol Ther. 2013;21:318–323. doi: 10.1038/mt.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le HT, Yu QC, Wilson JM, Croyle MA. Utility of PEGylated recombinant adeno-associated viruses for gene transfer. J Control Release. 2005;108:161–177. doi: 10.1016/j.jconrel.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Lee GK, Maheshri N, Kaspar B, Schaffer DV. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol Bioeng. 2005;92:24–34. doi: 10.1002/bit.20562. [DOI] [PubMed] [Google Scholar]
- 36.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartel M, Schaffer D, Büning H. Enhancing the clinical potential of AAV vectors by capsid engineering to evade pre-existing immunity. Front Microbiol. 2011;2:204. doi: 10.3389/fmicb.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y, Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 40.Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V, Edmonson SA, Africa L, Zhou S, High KA, Bosch F, Wright JF. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17:503–510. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- 41.Murphy SL, Bhagwat A, Edmonson S, Zhou S, High KA. High-throughput screening and biophysical interrogation of hepatotropic AAV. Mol Ther. 2008;16:1960–1967. doi: 10.1038/mt.2008.210. [DOI] [PubMed] [Google Scholar]
- 42.Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, Cochrane M, Gray E, Tuddenham EG, Davidoff AM. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
www.sciencetranslationalmedicine.org/cgi/content/full/5/194/194ra92/DC1
Materials and Methods
Fig. S1. AAV empty capsids prevent vector neutralization by anti-AAV NAbs in vitro.
Fig. S2. Large excess of AAV2 empty capsids does not inhibit AAV8-F.IX–mediated live transduction.
Fig. S3. Analysis of hF.IX expression levels and anti-hF.IX IgG levels in nonhuman primates.
Fig. S4. Analysis of capsid-specific humoral and cellular immune responses in nonhuman primates.
Fig. S5. Characterization of the AAV585/8 mutant capsid.
Fig. S6. Vector genome biodistribution in mice receiving an AAV8-F.IX vector formulated in AAV8 or AAV585/8 empty capsids.
Fig. S7. Timing of AAV585/8 empty capsid administration does not have a major influence on hF.IX transgene expression.
Table S1. NAb titers to AAV88 in a cohort of hemophilia B subjects.
Table S2. AAV8-F.IX vector biodistribution in nonhuman primates.
Table S3. Clinical chemistry data over time in nonhuman primates dosed with AAV8-F.IX vectors alone formulated in a 9X excess of AAV8 empty capsids.