Abstract
A distinctive feature of stem cells is their capacity to self-renew to maintain pluripotency. Studies of genetically-engineered mouse models and recent advances in metabolomic analysis, particularly in haematopoietic stem cells, have deepened our understanding of the contribution made by metabolic cues to the regulation of stem cell self-renewal. Many types of stem cells heavily rely on anaerobic glycolysis, and stem cell function is also regulated by bioenergetic signalling, the AKT–mTOR pathway, Gln metabolism and fatty acid metabolism. As maintenance of a stem cell pool requires a finely-tuned balance between self-renewal and differentiation, investigations into the molecular mechanisms and metabolic pathways underlying these decisions hold great therapeutic promise.
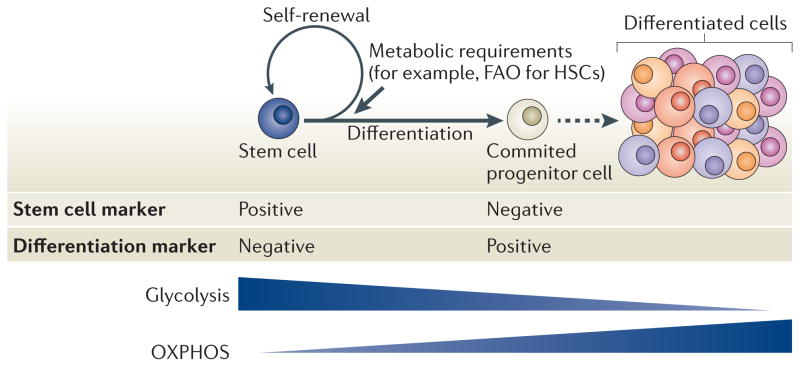
A defining characteristic of stem cells is their dual capacity for self-renewal and multi-potent differentiation1,2. As most mature cells have a limited lifespan, the capacity of stem cells to both replenish ageing mature cells and perpetuate themselves through self-renewal is crucial to the maintenance of tissue homeostasis throughout the lifetime of an organism. The size of stem cell populations depends on the balance between self-renewal and cell differentiation. When the rate of self-renewal (or ‘p’ value) is higher than that of differentiation, the stem cell population expands, whereas when the self-renewal rate is lower than the rate of differentiation, the population declines owing to exhaustion1 (FIG. 1). Cell-intrinsic networks cooperate with signals from the microenvironment to fine-tune the self-renewal capacity of stem cells and to maintain homeostasis3 (FIG. 1). Delineating precisely how stem cell self-renewal is regulated is a key step in our understanding of normal development, ageing and cancer, and will lay the foundation for novel strategies for tissue regeneration and regulation of ageing, as well as new tools to combat degenerative disorders.
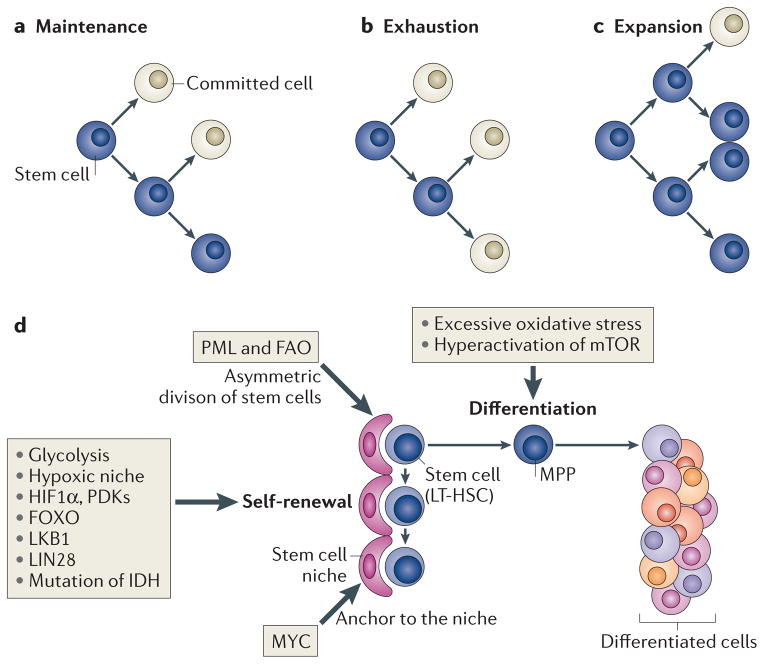
Figure 1. Two specific potentials and cell fates of stem cells.
Stem cells exhibit both self-renewal capacity and pluripotency (parts a,b,c). Asymmetric cell division has been suggested as a regulator of stem cell-fate decisions and is essential for the maintenance of the stem cell compartment (part a). Alterations in the equilibrium of self-renewal and commitment of adult stem cells can affect tissue homeostasis and can lead to stem cell exhaustion (part b) or expansion (part c). Several tissue stem cells (part d) (for example, long-term haematopoietic stem cells (LT-HSCs) in the bone marrow niche) maintain a quiescent state, as this is essential for preserving their self-renewal capacity. Many types of stem cells heavily rely on anaerobic glycolysis to maintain such a quiescent state and are more sensitive to oxidative stress. In hypoxic conditions (such as those found in the stem cell niche), the transcription factor hypoxia-inducible factor 1α (HIF 1α) promotes glycolysis as it induces the expression of pyruvate dehydrogenase kinases (PDKs), which prevent pyruvate from entering the tricarboxylic acid cycle, thus blocking mitochondrial respiration. Forkhead box O (FOXO), liver kinase B1 (LKB1) and LIN28 are crucial to maintain stem cells, and mutation of the gene encoding isocitrate dehydrogenase (IDH) leads to enhanced self-renewal capacity of HSCs. Nutrient-sensitive PI3K–AKT–mTOR pathways, Gln metabolism and fatty acid metabolism also have a crucial role in regulating the balance between quiescence and proliferation of stem cells. The boxes indicate how or which potentials of stem cells are regulated by these factors. FAO, fatty acid oxidation; MPP, multipotent progenitor cell; PML, promyelocytic leukaemia.
Self-renewal is not unique to stem cells. Some types of restricted progenitors and differentiated cells, such as restricted glial progenitors and lymphocytes, can also self-renew4,5, although their differentiation potential is more restricted. During mammalian adult haematopoiesis, asymmetric cell division (FIG. 1) has been suggested to regulate cell-fate decisions and to have a crucial role in ensuring that, during the replenishment of the haematopoietic compartment, a proportion of haematopoietic stem cell (HSC) daughter cells retain stem cell features6–10.
Adult tissue stem cells generally reside within specialized microenvironments, known as stem cell niches, where specific local conditions play a part in maintaining stem cells in a quiescent state, which is essential to preserve their self-renewal capacity. Recent studies have led to an increased understanding of stem cell metabolism and how metabolic pathways may affect homeostasis and quiescence11–22. These studies have been possible because of technical advances, such as the identification of new stem cell markers, which enable the isolation of stem cells with a high degree of purity, and improved metabolomic and transcriptome analyses. These techniques can be combined with established in vivo and in vitro functional assays to assess stem cell activity (including self-renewal and lineage specification)23–26.
In this Review, we first discuss the effect of hypoxia, glycolysis and reduction–oxidation (redox) reactions on stem cell homeostasis, regeneration and ageing. We then describe the roles of the nutrient-sensitive PI3K–AKT–mTOR pathways, Gln metabolism and fatty acid metabolism in regulating the balance between quiescence and stem cell proliferation.
The recent identification of surface markers for both human and mouse HSCs has enabled their purification with high efficiency. This, together with functional assays that have been established for long-term HSCs (LT-HSCs) and the well-characterized properties of the bone marrow (in which HSCs reside), has increased our knowledge of HSC metabolism. Although metabolic pathways affect embryonic stem (ES) cells, as well as stem cells in various adult tissues, we primarily focus on the metabolic networks that regulate stemness in the haematopoietic system and that have clinical implications for haematological diseases.
Glycolysis and the hypoxic niche
Various stem cells, including HSCs, mesenchymal stem cells (MSCs) and neural stem cells (NSCs), reside in a hypoxic niche. Experimental evidence indicates that low oxygen tensions (that is, hypoxia) contribute to the maintenance of an undifferentiated state and influence proliferation and cell-fate commitment23,25,27,28.
Hypoxia promotes HSC maintenance
Quiescent HSCs reside in hypoxic niches in the bone marrow cavity. Despite the relative hypervascularity of this cavity, oxygen concentrations are believed to be lower than in venous blood, owing to high levels of oxygen consumption by proliferating haematopoietic cells.
Indeed, recent direct measurements of oxygen concentration have revealed that oxygen saturation in the bone marrow is low (1.3–4.2%, which corresponds to an oxygen tension (PO2) of 10–32 mm Hg)185. Evidence for the hypoxic conditions in the bone marrow cavity was obtained by integrating two-photon phosphorescence lifetime PO2 measurements with two-photon intravital imaging, which enables the routine imaging of the calvarial bone marrow of living animals. With this microscopy system, it was possible to precisely measure local oxygen tensions in the bone marrow, which further revealed a gradient of declining oxygen levels that begins at the endosteal zone and is lowest in the deep perisinusoidal regions185.
Another group also reported a hypoxic profile in HSCs and in haematopoietic progenitor cells, which is defined by a strong retention of pimonidazole and expression of hypoxia-inducible factor 1α (HIF1α) (see below), using in situ tissue imaging29. However, these data further suggest that low oxidative phosphorylation (OXPHOS) in HSCs and haematopoietic progenitor cells is in part related to cell-specific mechanisms rather than only reflecting differences in environmental oxygen availability. Nevertheless, the emerging view is that HSCs exhibit a hypoxic status with high HIF1α expression (see below). Future challenges include to further determine the dynamics of oxygen availability and to understand the physiological implications of metabolic profiles in stem cells.
Owing to the hypoxic environment in which they reside, HSCs must rely heavily on anaerobic glycolysis, rather than mitochondrial OXPHOS, to support ATP production30,31. Metabolomic analyses of purified HSCs and their progeny have shown that the metabolic profile of HSCs differs from that of committed progenitors. Unlike their progeny, HSCs accumulate high levels of fructose-1,6-bisphosphate and show high pyruvate kinase activity, which indicates active glycolysis. In line with this, HSCs have low levels of phosphoenolpyruvate but increased levels of pyruvate, which are the substrate and product, respectively, of the final ATP-generating step of the glycolytic pathway32. Notably, recent studies have highlighted a key feature of self-renewing HSCs: their need to limit mitochondrial respiration to remain in a quiescent state32–34.
The HIF system in HSCs
A key player in HSC metabolism is HIF1, a transcription factor that is essential for cellular and systemic responses to low oxygen availability35.
HIF1 is a heterodimeric protein that comprises two subunits: HIF1α, the expression of which is stabilized by hypoxia, and HIF-1β, which is constitutively expressed36. When HIF1α associates with HIF1β, they form a transcription factor that binds and activates the promoters of multiple glycolytic genes37.
Recent investigations indicate that the HIF activity within the bone marrow is tightly modulated through intricate regulatory mechanisms and also that HIF1 regulates multiple downstream pathways in parallel. Mathematical models are required to further understand the complexity of this system. However, the scarcity of dynamic experimental data has restricted the capacity to generate such models. The current models relate to steady-state behaviours and do not take into account the dynamics of the hypoxic and HIF response to hypoxia in vitro, and they are also limited to the HIF pathway alone. New mathematical models are required to provide a dynamic and mechanistic understanding of the physical processes that underlie the cellular response to hypoxia38,39. Under normoxic conditions, HIF1 levels decrease through oxygen-mediated degradation of HIF1α. Three prolyl hydroxylases, PHD1, PHD2 and PHD3 (which belong to the family of iron- and 2-oxoglutamate-dependent dioxygenases), are activated by oxygen and they hydroxylate two Pro residues in the O2-dependent degradation (ODD) domain of HIF1α (mainly PHD2 for HIF1α); this primes the protein for degradation by the von Hippel–Lindau (VHL) ubiquitin ligase complex40–42 (FIG. 2). PHDs also catalyse HIF1α Pro hydroxylation in the presence of iron and α-ketoglutarate (αKG; also known as 2-oxoglutarate), which generates succinate (FIG. 2).
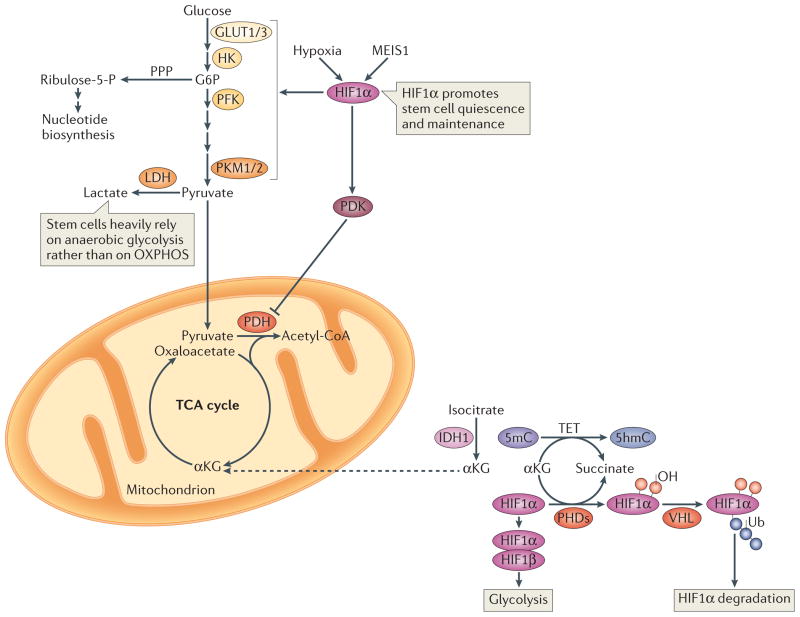
Figure 2. Glycolysis and hypoxia-inducible factor 1α.
Hypoxic conditions and myeloid ecotropic viral integration site 1 (MEIS1) activate hypoxia-inducible factor 1α (HIF1α), which promotes glycolysis and HIF1α-dependent pyruvate dehydrogenase kinase (PDK) activation. PDK in turn prevents pyruvate oxidation by suppressing the pyruvate dehydrogenase (PDH) complex. Such hypoxic conditions are crucial for stem cell maintenance. In normoxic conditions, prolyl hydroxylases (PHDs) catalyse HIF1α hydroxylation in the presence of iron and α-ketoglutarate (αKG), and generate succinate in the process of hydroxylating Pro residues in HIF1α. Hydroxylated HIF1α is targeted for degradation by the von Hippel–Lindau (VHL) ubiquitin ligase complex. A group of αKG-dependent dioxygenases, the ten-eleven translocation (TET) proteins, catalyze the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) in the genome. TET proteins require iron for catalysis and oxidize prime substrates (such as 5mC into 5hmC) with coupled oxidation of αKG (the co-substrate) into succinate and CO2. TET deficiency in mice leads to skewed differentiation and enhanced repopulating capacity of haematopoietic stem cells (HSCs). Isocitrate dehydrogenase 1 (IDH1) catalyzes the oxidative decarboxylation of isocitrate to αKG and CO2. Dashed arrows indicate transport between mitochondria and the cytosol. G6P, glucose-6-phosphate; GLUT, glucose transporter; HK, hexokinase; LDH, lactate dehydrogenase; PFK, phosphofructokinase; PKM, pyruvate kinase muscle isozyme; PPP, pentose phospate pathway; OXPHOS, oxidative phosphorylation; TCA cycle, tricarboxylic acid cycle.
Under hypoxic conditions, hydroxylation is inhibited and HIF1α is stabilized. Decreased oxygen abundance alone is sufficient to inhibit HIF1α hydroxylation, which prevents VHL-dependent HIF1α degradation, which leads to the accumulation of HIF1α–HIF1β complexes on the promoters of glycolytic genes40–43.
When the Hif1a gene (which encodes HIF1α) is depleted in HSCs, the cells exhibit decreased glycolysis and increased mitochondrial metabolism. This leads to HSC exhaustion, which indicates that HIF1α is essential to stem cell function in the haematopoietic system. Mechanistically, HIF1α promotes the expression of pyruvate dehydrogenase kinase 2 (PDK2) and PDK4, which together prevent pyruvate from entering the tricarboxylic acid cycle (TCA cycle), thus blocking mitochondrial respiration (FIG. 2). Furthermore, overexpression of either Pdk2 or Pdk4 rescues the glycolytic and functional defects that have been observed in mouse Hif1a-deficient HSCs, whereas deletion of both Pdk2 and Pdk4 decreases the dependence on anaerobic glycolysis and reduces functional exhaustion, similarly to Hif1a-knockout mice32. As the PDK mimetic, 1-aminoethylphosphonic acid, induces increased levels of intracellular pyruvate and improved LT-HSC maintenance in vitro by promoting cell cycle quiescence, the suppression of glycolytic metabolic influx into mitochondria and the resulting activation of glycolysis through the HIF1α–PDK system could be the primary trigger of HSC quiescence in vivo32. These data suggest that quiescent HSCs in their hypoxic bone marrow niches rely on a HIF1α-driven metabolic programme to prevent engagement of the TCA cycle and maintain anaerobic glycolysis as a source of energy.
HSCs exhibit a range of specific hypoxic responses that are initiated by different members of the HIF family. Whereas HIF1α is the master regulator of O2 homeostasis in most mammalian cells, two HIF1α paralogues exist in HSCs: HIF2α (also known as EPAS1) and HIF3α (REFS 44,45). HIF1α and HIF2α are closely related and share a conserved basic helix–loop–helix (bHLH) domain. Moreover, HIF2α has been implicated in protecting HSCs, haematopoietic progenitor cells and acute myeloid leukaemia cells from endoplasmic reticulum (ER) stress-induced apoptosis46. HIF3α can dimerize with HIF1β to activate transcription, and one of its splice variants functions as a dominant-negative regulator of hypoxia-inducible gene expression47,48. However, clear evidence for a role in haematopoiesis has not yet been found.
Several other potential upstream regulators and downstream targets of HIF1α affect HSC activity. Interestingly, pluripotency-associated transcription factors seem to regulate HSC metabolism. For example, MEIS1 regulates both glycolysis and the oxidative stress response by activation of Hif1a transcription in HSCs30. Moreover, the homeobox protein CBP/p300-interacting transactivator 2 (CITED2) was found to be essential for the maintenance of HSC quiescence, in part by transcriptionally activating Hif1a, thereby increasing HIF activity49–51.
Researchers have also identified extracellular factors that regulate HIF1α activity in HSCs52,53. The soluble protein CRIPTO (also known as TDGF1), and its cell surface receptor 78 kDa glucose-regulated protein (GRP78), have crucial roles in promoting quiescence and in the maintenance of HSCs52. Under hypoxic conditions, HIF1α binds to hypoxia responsive elements (HREs) in the promoter region of Cripto and activates its expression. CRIPTO then binds to GRP78 and stimulates glycolytic metabolism-related proteins. The contribution of secreted cytokines and chemokines to HIF1α expression and HSC metabolism is also under investigation.
Glycolysis and HIF in other adult stem cells
Whereas ES cells are proliferative, most adult stem cells are quiescent — these include LT-HSCs and MSCs in the bone marrow, NSCs in the brain, epidermal stem cells in the hair follicle bulge, and satellite cells in skeletal muscles. These quiescent adult stem cells are generally sensitive to increased intracellular reactive oxygen species (ROS) and avoid cellular damage from ROS to ensure life-long tissue renewal capacity23,25,54.
Like HSCs, MSCs also reside in a hypoxic niche in vivo. Compared to differentiated osteoblasts, human MSCs express higher levels of glycolytic enzymes and lower levels of OXPHOS proteins, which indicates that MSCs rely more on glycolysis for their energy supply than their differentiated progeny (osteoblasts)55. However, MSCs cultured under normoxia are able to use OXPHOS and consume O2 at a high rate, which suggests that glycolysis may be an environmental adaptation for MSCs56. Moreover, the proliferation and colony-forming capacities of MSCs are substantially increased in normoxic conditions57. When MSCs that have expanded under normoxia switch to OXPHOS, their stem cell function is impaired owing to increased senescence. This suggests that hypoxia-induced glycolysis limits MSC proliferation to prevent oxidative stress-induced senescence and to preserve long-term self-renewal potential57. Thus, in at least three types of adult stem cells — LT-HSCs11, MSCs55 and NSCs58 — the induction of ROS in normoxia forces stem cells out of hypoxia-dependent quiescence and into a more proliferative state. This may be a common mechanism for priming stem cell differentiation (as further discussed below).
Glycolysis in pluripotent stem cells
During morula compaction, blastomeres are segregated into the pluripotent inner cell mass (ICM) and trophectoderm, which accelerates metabolic activity and severely increases growth. Furthermore, glycolytic flux is greatly increased with an upregulation of glucose transporter type 1 (GLUT1) and GLUT3 expression, and higher activities of hexokinase (HK) and phosphofructokinase 1 (PFK1) enzymes. As a result, flux into the pentose phosphate pathway (PPP) for nucleotide synthesis also increases (FIG. 2).
Interestingly, mitochondria in the ICM have a lower membrane potential59, which is indicative of reduced mitochondrial function, and pluripotent ES cells derived from in vitro cultured ICMs show high rates of glycolysis60,61. A switch from OXPHOS to glycolysis is also seen during the conversion of somatic differentiated cells into induced pluripotent stem (iPS) cells and reflects metabolic reprogramming61,62. This activation of glycolysis accompanied by high PPP activity is thus the preferred metabolic state of rapidly proliferating cells63,64.
The reprogramming factor LIN28A (also known as LIN28), which is highly expressed in both mouse and human ES cells, binds to a large number of mRNAs encoding mitochondrial and glycolytic enzymes in human ES cells65–67. LIN28A has been used to increase the efficiency of human fibroblast reprogramming to iPS cells66. Interestingly, LIN28A and its paralogue LIN28B are also important in tissue development, organismal growth, metabolism and somatic reprogramming. LIN28A and LIN28B have emerged as factors that define stemness in several tissue lineages, including fetal HSCs, skeletal myoblasts during muscle regeneration and spermatogonia67–72. In mammalian ES cells, LIN28A was shown to block the production of mature let-7 microRNAs (miRNAs) through binding to let-7 pre-miRNA, thereby influencing mRNA translation and regulating self-renewal. Importantly, LIN28A- and LIN28B-mediated let-7 repression regulates glucose metabolism by modulating the insulin–PI3K–mTOR signalling pathway73. Further experimental evidence will clarify the function of LIN28-dependent metabolic programming in the maintenance of stem cell homeostasis.
Linked and parallel pathways to glycolysis
In cultured ES cells, levels of OXPHOS are low. ATP synthesis seems to be decoupled from O2 consumption by the mitochondrial electron transport chain (ETC), and is more dependent on glycolysis74. It has been suggested that mitochondrial oxidation operates both to recycle NAD+ and to keep the TCA cycle ‘turning’ to generate fatty acids and amino acids75, and that high glycolytic ATP levels force ETC reactions to run in reverse76. However, the precise mechanisms of these processes remain unclear.
Notably, mouse ES cells are characterized by a unique method of amino acid catabolism. A crucial amino acid for mouse ES cell growth and for maintaining them in a pluripotent state is Thr77. Mouse ES cells and iPS cells have extremely high levels of the Thr-catabolizing enzyme L-Thr dehydratase (TDH; also known as SDH) relative to differentiated cells61,77. TDH converts Thr to Gly and acetyl-coenzyme A (CoA). Gly is then used by the mitochondrial enzyme Gly decarboxylase (GLDC) to generate the folate one-carbon pool to promote nucleotide synthesis and rapid proliferation of mouse ES cells1. Furthermore, TDH increases the synthesis of S-adenosylmethionine (SAM), leading to a high ratio of SAM/S-adenosylhomocysteine (SAH) and high levels of trimethylation of histone H3 at Lys4 (H3K4me3), which is also thought to contribute to the maintenance of pluripotency and the proliferation of ES cells61.
The PPP produces ribose-5-phosphate and NADPH for the synthesis of DNA, RNA and fatty acids (FIG. 2). In a manner similar to the Warburg effect in cancer cells, ES cells show increased activity in the PPP, which enables rapid nucleotide synthesis for cell proliferation63,64,78. The major functions of the PPP are to protect cells against oxidative stress by producing NADPH, which is necessary for the regeneration of glutathione and to act as an alternative pathway to glycolysis. Interestingly, recent studies have identified a HIF1α-induced reciprocal metabolic switch between the PPP and glycolysis. Expression of PPP enzymes and glucose flux throughout both the PPP and the TCA cycle are reduced under hypoxia, whereas glycolysis enzymes are upregulated79,80. Thus, the anabolic PPP is active in proliferating cells but is suppressed under acute hypoxic stress, which indicates that cells switch to direct glycolysis for increased protection against hypoxic damage. The contributions of this metabolic switch to the function of quiescent stem cells in the hypoxic niche have not yet been explored.
Switch to oxidative metabolism
Mitochondria are central organelles in carbohydrate, lipid and amino acid metabolisms of most cells, and their role in stem cells is complex (FIG. 3). There seems to be only a small number of rounded and non-fused mitochondria (with underdeveloped cristae) in human and mouse ES cells81–84. Moreover, mitochondria in HSCs are relatively inactive85, whereas ROS levels (linked to mitochondrial activity) are much higher in lineage-committed progenitors than in HSCs30,86. This suggests that when HSCs undergo differentiation a burst of robust mitochondrial metabolism supplies the energy for this rapid transition.
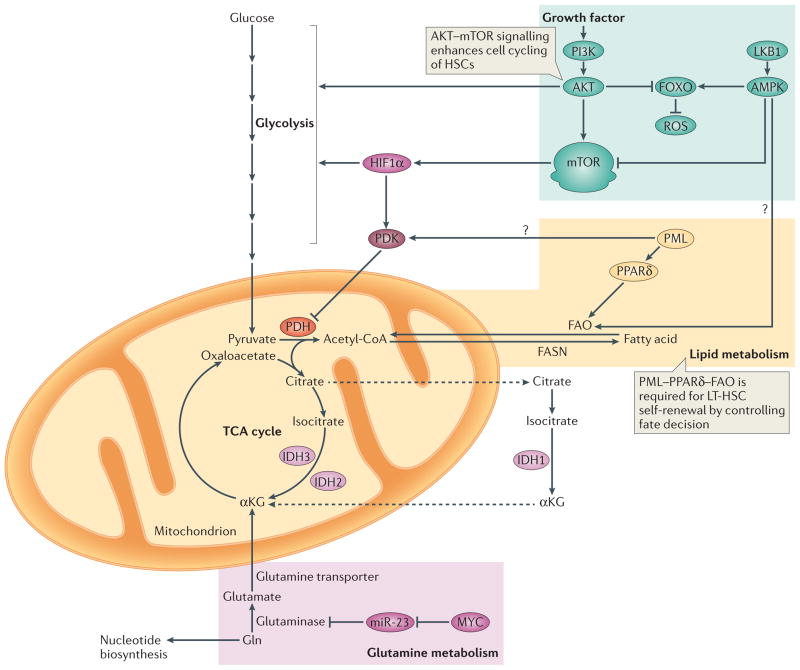
Figure 3. Overview of metabolic requirements in stem cells.
PI3K–AKT signalling promotes the production of reactive oxygen species (ROS) by repressing the forkhead box O (FOXO)-mediated stress response. This is accompanied by increased glycolysis and activation of mTOR, which promotes cell cycling of haematopoietic stem cells (HSCs) (indicated by green background). MYC controls the balance between HSC self-renewal and differentiation183,184 (indicated by pink background). Gln is converted to glutamate by glutaminase, which is under the control of MYC, in part through microRNA-23 (miR-23), and its role in stem cell homeostasis is currently being elucidated. The promyelocytic leukaemia protein (PML)–peroxisome proliferator activator receptor-δ (PPARδ) pathway for fatty acid oxidation (FAO) is required for long-term haematopoietic stem cell (LT-HSC) self-renewal and quiescence (indicated by orange background). Isocitrate dehydrogenases (IDHs) are a family of enzymes that are responsible for catalyzing the oxidative decarboxylation of isocitrate into α-chetoglutarate (αKG). IDH1 functions in the cytosol and peroxisomes, whereas IDH2 and IDH3 are both localized in the mitochondria. Dashed arrows indicate transport between mitochondria and the cytosol. Question marks indicate interesting links between metabolic programmes, although these connections have not been formally tested in stem cells. Acetyl-CoA, acetyl-coenzyme A; FASN, fatty acid synthase; LKB1, liver kinase B1; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; TCA cycle, tricarboxylic acid cycle.
The regulation and coordination of mitochondrial metabolism in mouse HSCs has recently been studied by blocking mitochondrial respiration through conditional inactivation of Ptpmt1 (protein tyrosine phosphatase, mitochondrial 1), which encodes a PTEN-like mitochondrial phosphatase34. Ptpmt1-deficient HSCs were unable to initiate differentiation-associated divisions, which blocked HSC differentiation and resulted in rapid haematopoietic failure. Mechanistically, the substrates of PTPMT1, phosphatidylinositol phosphates (PIPs), increased the activation of mitochondrial uncoupling protein 2 (UCP2), which in turn inhibited glucose-derived pyruvate oxidation in mitochondria. Hence, Ptpmt1-deficient HSCs and progenitor cells responded to decreased mitochondrial respiration by increasing their levels of anaerobic glycolysis34.
These data suggest that HSC metabolism can be reprogrammed on the basis of functional demands. Self-renewing HSCs, for example, heavily rely on anaerobic glycolysis to maintain their quiescent state and need to rapidly switch to mitochondrial OXPHOS to meet the robust energy demands associated with differentiation.
Oxidative stress and the ROS rheostat
ROS are toxic by-products of oxidative metabolism. Although important for physiological processes, such as intracellular signal transduction, and for combating pathogens, excessive levels of ROS can impair cellular function by damaging lipids, proteins, RNA and DNA. To remain functional throughout the life of an organism, stem cells must minimize ROS damage — it has been suggested that a ‘ROS rheostat’ monitors stem cell fate decisions with regard to self-renewal or commitment11– 14,87–90 (FIG. 4).
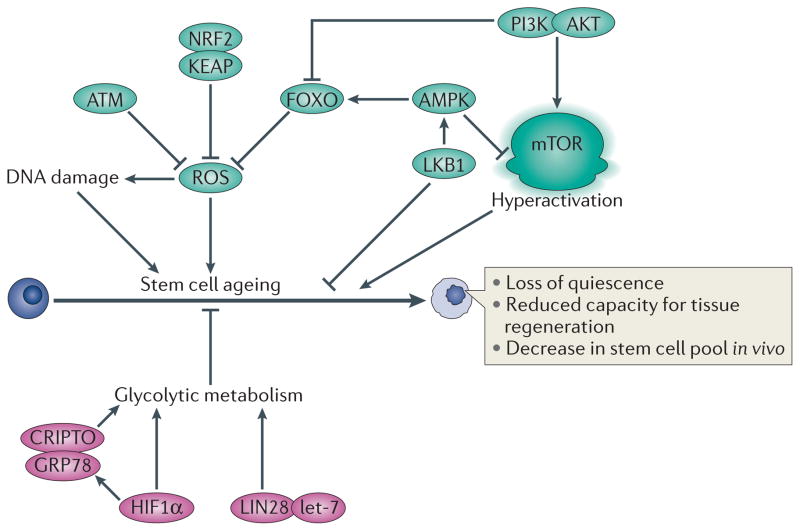
Figure 4. A ROS rheostat tightly regulates cellular ROS to maintain stemness and inhibit stem cell ageing.
Increased levels of reactive oxygen species (ROS) activate the p38 MAPK–p16 pathway, which results in defective self-renewal activity and increased stem cell senescence. Therefore, the cellular reduction–oxidation (redox) status is crucial to stem cell function and low oxidation levels are required to maintain the quiescent state of long-term haematopoietic stem cells (LT-HSCs) and to prevent their exhaustion. The ‘ROS rheostat’ has been proposed to function by inhibiting excess ROS, maintaining important stem cell characteristics (that is, quiescence and self-renewal potential) and preventing stem cell ageing. This rheostat would include an immense cellular antioxidant defence system (for example, forkhead box O (FOXO), ataxia telangiectasia mutated (ATM)–BH3-interacting domain death agonist (BID) pathway) and a relatively inactive PI3K–AKT–mTOR pathway, all operating under hypoxic niche conditions. GRP78, 78 kDa glucose-regulated protein; HIF1α, hypoxia-inducible factor 1α; KEAP, Kelch-like ECH-associated protein 1; LKB1, liver kinase B1; NRF2, nuclear factor erythroid 2-related factor 2.
The protective FOXO pathway
The forkhead box protein O (FOXO) family of transcription factors could be components of such a rheostat. These tumour suppressors protect stem cells and other cells from oxidative damage, thus reducing mutagenesis and extending cellular lifespan91. FOXO proteins activate the expression of genes that encode superoxide dismutase and catalase enzymes (required for the detoxification of ROS) (FIG. 3), and of genes that promote quiescence11,14,90. FOXO activity is in turn positively regulated by AMP kinase (AMPK) and negatively regulated by PI3K pathway signalling, as AKT (activated by PI3K) phosphorylates FOXO proteins to cause its translocation out of the nucleus (FIGS 3,4). PI3K-mediated AKT activation and FOXO exclusion from the nucleus are associated with the loss of HSC quiescence and terminal differentiation in vitro92.
Deletion of three members of the FOXO family, Foxo1, Foxo3 and Foxo4, leads to increased levels of ROS in mice, as well as accelerated HSC proliferation and depletion11,90. Disruption of Foxo3 alone increased ROS levels in HSCs14 and led to their depletion, although to a lesser extent than was observed in triple-knockout mice, which lacked Foxo1, Foxo3 and Foxo4 (REF. 90). These defects can be partially rescued by treatment with the antioxidant N-acetyl-L-Cys.
FOXO proteins also have a crucial role in NSC fate decisions58,93. In the nervous system, NSCs generate new neurons that are necessary for cognitive function. Adult Foxo3−/− mice have fewer NSCs than their wild-type counterparts, and cultured Foxo3−/− NSCs exhibit decreased self-renewal and an impaired ability to generate different neural lineages. Gene expression profiling suggests that FOXO3 regulates the NSC pool by inducing a gene expression programme that preserves quiescence, prevents premature differentiation and controls oxygen metabolism. The oxidative stress response that is mediated by FOXO3 is rapidly deactivated following NSC differentiation, whereas administration of an antioxidant rescues aberrant proliferation or loss of self-renewal capacity of Foxo3−/− NSCs. These results suggest that FOXO-dependent cellular metabolism is necessary to prevent excessive ROS generation in NSCs.
Enzymes involved in ROS breakdown, including superoxide dismutase and catalase, had reduced activity in Foxo-deficient HSCs, suggesting a potential mechanism for ROS accumulation. It is worth noting that FOXO3 also regulates mitochondrial ROS accummulation and inhibits apoptotic cell death in neuronal cells, and neuroblastoma tumour cells. Moreover, cellular ROS levels are affected by the FOXO3 targets BIM (BCL-2-interacting mediator of cell death), BCL-XL, and survivin94. BIM activation by FOXO3 causes mitochondrial depolarization, which results in ROS production, whereas FOXO3 inhibits ROS generation through inactivation of survivin. Additionally, BCL-XL inhibition by FOXO3 also restricts ROS generation by inactivating BIM. The downstream effectors of FOXO3 that regulate ROS in HSCs remain to be determined.
An indirect effect of cellular metabolism on stem cell activity is achieved through autophagy. Autophagy has been shown to be essential to the development of the blood system, as shown by dysregulated fetal and postnatal haematopoiesis that has been observed in mice lacking the autophagy gene Atg7 (REF. 95). Importantly, FOXO3A maintains a pro-autophagy gene expression programme to protect HSCs through autophagic responses during metabolic stress when nutrients are limited96. It is an open question whether this protective autophagic response occurs in the stem cells of other tissues.
The ATM pathway
Ataxia telangiectasia mutated (ATM) protein, a kinase that is activated in response to DNA damage, is required for normal DNA repair and the avoidance of oxidative stress97. Atm-deficient HSCs accumulate ROS, which activates p38 MAPK and induces p16 (also known as INK4A; encoded by CDKN2A)12,13 (FIG. 4). NSCs also require Atm to avoid genomic instability, abnormal proliferation and depletion98. These defects have been partially rescued in mice by treatment with N-acetyl-L-Cys or a p38 MAPK inhibitor. Although it remains unclear why ROS accumulate in the absence of ATM, these data demonstrate a link between DNA repair, ROS levels and stem cell maintenance.
Importantly, investigators have identified BH3-interacting domain death agonist (BID), a BCL-2 homology 3 (BH3)-only BCL-2 family member, as one of the important ATM effectors in stem cells; it has been demonstrated that ATM-mediated BID phosphorylation plays a part in maintaining the quiescence of HSCs87,88. These data imply that selected BCL-2 proteins might coordinate mitochondrial and nuclear activities through ROS to regulate cell fate decisions; how precisely BID modulates mitochondrial ROS to regulate stem cell function, however, remains unknown.
Low levels of ROS in stem cell maintenance
Although ROS levels must be kept low to prevent tissue damage, it is becoming clear that very low levels of intracellular ROS in HSCs are essential to maintain HSC quiescence99 (FIG. 4).
ROS contribute to HSC ageing and senescence, and excessive ROS generation induces apoptotic cell death in HSCs11–14,88,90,100. These fail-safe mechanisms prevent the spread of HSC damage (such as DNA damage) to the rest of the haematopoietic system, and their function is very similar to that of oncogene-induced senescence (OIS). Indeed, similar pathways are used by both cellular processes. In OIS, the pyruvate dehydrogenase (PDH) complex is activated to promote the use of glucose-derived pyruvate in the TCA cycle, which in turn leads to an increase in cellular respiration — an element of the OIS programme that is as essential as the induction of p16 and p15 (also known as INK4B; encoded by CDKN2B)102.
Recent studies in zebrafish have reported that HSC proliferation may occur when ROS levels are maintained at low levels. It was shown that HIF1α regulates blood formation, and HIF1α is known to mediate dose-dependent effects of glucose uptake and metabolism on the timing and magnitude of HSC production through mitochondria-derived ROS102. Intriguingly, germ stem cells require ROS for self-renewal through NADPH oxidase (NOX1)103.
This proliferative behaviour of HSCs may be linked to the cellular events that promote the proliferation of committed progenitor cells in the bone marrow compartment. Consistent with this hypothesis, haematopoietic progenitor cells exhibit higher intracellular ROS levels, and inhibition of mitochondrial metabolism blocks the differentiation of HSCs and increases the HSC pool87,88. Importantly, ROS were shown to be required for recovery after bone marrow injury, which involves ROS-dependent proliferation of HSCs and progenitor cells104. This further supports the hypothesis of a proliferative response of HSCs to intracellular ROS levels.
Thus, after bone marrow damage or in other situations in different tissues, robust mitochondrial metabolism supplies the energy for the rapid onset of HSC proliferation, which is accompanied by low levels of ROS to minimize adverse effects on stem cell function.
Glucose and amino acid metabolism
When stem cells proliferate, the PI3K–AKT–mTOR pathway is activated by growth factors and nutrients such as glucose and amino acids105 (FIG. 3). Increased PI3K activity leads to increases in cellular phosphatidylinositol-3,4-bisphosphate (PtdIns(4,5)P2) and PtdIns-3,4,5-trisphosphate (PtdIns(3,4,5)P3) levels. The binding of 3-phosphoinositides induces a conformational change in AKT, which enables the co-recruited 3-phosphoinositide-dependent protein kinase 1 (PDPK1) to access the activation loop.
Nutrient sensing by PI3K–AKT–mTOR signalling
Constitutively active AKT signalling causes accelerated proliferation and depletion of HSCs106,107. Moreover, deletion of Pten, a PIP3 phosphatase that induces the constitutive activation of PI3K–AKT signalling, results in an accelerated cell cycle, which eventually leads to HSC exhaustion108,109. The haematopoietic reconstitution capacity of Pten−/− HSCs is, however, restored by deletion of Tp53 or Cdkn2a (REF. 110). Conditional knockout of an inhibitor of mTOR, tuberous sclerosis 1 (Tsc1), likewise led to overactivation of the mTOR pathway, which results in the loss of quiescence and repopulation capacity in HSCs111,112.
Intracellular ROS levels and the number of mitochondria are increased in Tsc1-deleted HSCs. These data indicate that mTOR is a crucial modulator of stem cell maintenance and that two tumour suppressors, Pten and Tsc1 act as important negative regulators of PI3K–mTOR signalling in HSCs.
The PI3K–mTOR pathway has essential roles in other tissue stem cells. For example, depletion of Pten aberrantly increases the proliferation of NSCs113, and Tsc1 and Tsc2 genes are essential for neural differentiation and migration of NSCs and neuronal progenitor cells114. Similarly, mTOR regulates intestinal stem cell (ISC) function. Calorie restriction reduces signalling by mTOR complex 1 (mTORC1) in Paneth cells, which constitute the intestinal stem cell niche, resulting in an increase in ISC number and function115.
Using nutrient-sensing pathways, stem cells maintain energy production by collectively inhibiting key processes (for example, OXPHOS) and increasing others (for example, glycolysis), and this interplay is key to the maintenance of stem cells in various tissues.
The LKB1–AMPK pathway in stem cells
The tumour suppressor liver kinase B1 (LKB1; also known as STK11) is a Ser/Thr kinase that acts upstream of the AMPK and mTOR pathways (FIG. 3). LKB1 is inactivated in the Peutz–Jeghers familial cancer syndrome and is currently viewed as a master regulator of cellular metabolism that restricts cell growth under energetically unfavourable conditions116. Increased cellular AMP/ATP ratios result in increased AMPK activation through its phosphorylation by LKB1, leading to inhibition of mTORC1, which in turn reduces cell growth and proliferation117. LKB1 also modulates metabolism through other AMPK targets, such as the FOXO transcription factors in mammalian cells.
LKB1 has crucial roles in regulating HSC function16–18. Conditional deletion of Lkb1 in the mouse haematopoietic system leads to a loss of quiescence in HSCs and to a transient increase in the number of haematopoietic progenitors, which is followed by a decline in the number of haematopoietic cells in the bone marrow and progressive pancytopenia. The fact that increased cell cycling was not observed in mature blood cells suggests that LKB1 is necessary to maintain quiescence specifically in HSCs. Lkb1 depletion in HSCs led to a cell-autonomous defect in bone marrow regeneration capacity, as mutant HSCs failed to rescue lethally irradiated recipient mice and as they were out-competed by wild-type HSCs in competitive reconstitution assays16–18. Surprisingly, however, these effects occurred independently of AMPK–mTOR and FOXO signalling, as treatment with an AMPK activator, the mTORC1 inhibitor rapamycin or administration of antioxidants did not rescue the effects of Lkb1 deficiency.
Intriguing suggestions have recently been made for potential downstream effectors of LKB1 signalling. For example, global metabolic profiles using a combination of either liquid or gas chromatography and mass spectrometry platforms (LC/MS or GC/MS) have demonstrated that LKB1 mutants show alterations in lipid metabolism17; and transcriptome analysis of Lkb1-deleted HSCs revealed changes in the expression of genes involved in the metabolic pathway of peroxisome proliferator-activating receptor (PPAR), such as downregulation of PPARγ co-activators (PGCs)16. This suggests that LKB1 may affect components of the PGC–PPAR pathway for lipid metabolism (also discussed below), which results in an impaired HSC function. Further studies are needed to determine how a metabolic sensor such as LKB1 can balance proliferation and quiescence in self-renewing HSCs.
Gln metabolism in stem cell homeostasis
Glutaminolysis, a key energy source for proliferative cells (including cancer cells), is a mitochondrial pathway that is initiated by the deamination of Gln by glutaminase, yielding glutamate and ammonia. Glutamate is then converted through a second deamination step into a TCA cycle intermediate, αKG. The conversion of Gln to αKG is catalysed by either glutamate dehydrogenase (producing a second molecule of ammonia) or one of several transaminases, which convert αKeto acids into their corresponding amino acids (FIG. 3). Glutaminolysis also supports the production of molecules, such as glutathione and NADPH, which protect cells from oxidative stress118,119.
Glutaminolysis is regulated by MYC, a potent oncogene well-known for its ability to drive cell cycle progression and boost glycolytic metabolism120 (FIG. 3). In contrast to the clear contributions of Gln metabolism to cancer development and progression, little is known regarding its role in stem cell homeostasis. Further investigation will be required to elucidate the role of MYC-induced glutaminolysis, and its relationship with glucose metabolism, in stem cell self-renewal.
Fatty acid metabolism and stem cell self-renewal
As for glucose and Gln, fatty acid metabolism supports both the biosynthetic and bioenergetic requirements of cell proliferation and survival. Lipids are essential components of plasma and organelle membranes, and fatty acids from either de novo synthesis or stored fat can function as secondary messengers for specific signal transduction pathways. For instance, fatty acid consumption through β-oxidation can provide a key alternative pathway that supports cancer cell survival when glucose metabolism becomes limiting121–123 (FIG. 3).
Stem cell research in mice has demonstrated that fatty acid oxidation (FAO) supports mouse blastocyst ICM growth124,125. In addition, the disruption of carnitine O-palmitoyltransferase (CPT), the rate-limiting enzyme for FAO, leads to ATP depletion and decreased resistance to nutrient deprivation, which indicates a key role for fatty acids in the generation of energy in mouse ES cells125.
Although this remains a relatively unexplored field, recent studies have provided direct evidence for fatty acid metabolism and promyelocytic leukaemia protein (PML) having a crucial role in the self-renewal of HSCs by controlling cell fate decisions126. The PML tumour-suppressor gene, which was originally cloned at the break point of the t(15;17) chromosomal translocation of acute promyelocytic leukaemia, has a key role in stem cell biology126. Deletion of Pml leads to loss of quiescence in HSCs, which results in their transient amplification and subsequent exhaustion. In models of chronic myeloid leukaemia (CML), PML was required for the maintenance of leukaemia-initiating cells (LICs), which suggested a new therapeutic approach based on targeting quiescent LICs through the pharmacological inhibition of PML126. Importantly, a PML-dependent metabolic reprogramming of tumour cells was identified that increases breast cancer cell survival by acting as a promoter of PPAR through deacetylation of PGC1α121 (FIG. 3).
Current knowledge of PML in breast cancer was used to study HSC biology. PML activates PPARδ, a nuclear receptor that plays a key part in stem cell maintenance22. The fact that PPARδ levels and signalling output are reduced during the differentiation of HSCs inspired further investigations into the role of fatty acid metabolism in HSC maintenance, which led to the finding that the PML–PPARδ pathway and FAO function together to control HSC asymmetric division and thus self-renewal22. These findings strongly suggest that metabolic changes accompany the tight regulation of asymmetric division of HSCs, which suggests that metabolic reprogramming may more generally underlie stem cell differentiation.
The link between the regulation of fatty acid metabolism by PML and the control of HSC division was explored by determining the symmetry of these divisions through a binary immunophenotypical assay that takes advantage of two surface markers, TIE2 (also known as TEK) and CD48 (REFS 22,127,128). Depletion of Ppard or Pml, together with mitochondrial FAO inhibition, resulted in a symmetric commitment of HSCs (divisions giving rise to two committed HSC daughter cells) in vitro and in vivo. Conversely, pharmacological PPARδ activation increased asymmetric division, thereby supporting the maintenance of the HSC population22,129 (FIG. 5). These findings suggest a link between nuclear organization, transcriptional control and lipid metabolism in decisions underlying asymmetric cell division, but they also have major implications for the therapeutic manipulation of HSCs.
Figure 5. Coordinated regulation of stem cell function by metabolism.
Stem cell equilibrium is bioenergetically and biosynthetically balanced through proper regulation of the flux of pathways that metabolize glucose, Gln and/or fatty acids. Mitochondria in stem cells are relatively inactive and stem cells heavily rely on anaerobic glycolysis30, whereas oxidative phosphorylation (OXPHOS) is associated with differentiation, as well as impaired stem cell function. Fatty acid metabolism has recently been identified as a critical factor in the self-renewal of haematopoietic stem cells (HSCs) through the control it exerts over stem cell fate decisions22. FAO, fatty acid oxidation.
First, the apparent benefits of PPAR and FAO to stem cell maintenance have brought attention to PPARδ activators as therapeutic targets, especially in conditions in which HSC function must be maximized (as in bone marrow transplantation). Activation of PPAR signalling is expected to enable long-term engraftment with a lower number of donor HSCs. This should markedly reduce the discomfort of bone marrow aspiration by minimizing the number of needle insertions required, which could in turn lead to increased numbers of registered bone marrow donors. Furthermore, higher levels of FAO should enable successful engraftment with a minimal amount of transplanted HSCs, even from donated peripheral blood.
It is worthy of note that modulators of these pathways have already been developed, and selective PPARδ activators have undergone clinical trials for the treatment of metabolic disorders such as dyslipidemia130, which exemplifies the therapeutic potentials of targeting energy metabolism131,132. Moreover, the strategy of targeting HSC lipid metabolism for therapy could possibly be applied to other stem cell types of different tissues with high rates of FAO, such as liver and heart muscle.
Second, as altering the balance of stem cell division causes haematological disorders, it is tempting to propose that the use of pharmacological inhibitors of FAO to promote LIC exhaustion may be an alternative therapeutic strategy to targeting leukaemic cells133. Bioinformatic analyses suggest that PPARD is highly expressed in some incurable haematological malignancies, such as chronic lymphoid leukaemia (see the Oncomine database). Further investigation is needed to determine the conditions under which this approach might be beneficial to potentially develop a therapy for leukaemia and possibly for other cancers.
Increased levels of ROS can lead to HSC exhaustion12,13,134. FAO may contribute to ROS management as it is an important source of NADPH122. FAO generates one molecule of acetyl-CoA in each oxidation cycle and two in the last cycle122. Acetyl-CoA then enters the TCA cycle and together with oxaloacetate gives rise to citrate, which after export to the cytoplasm can enter two metabolic chain reactions to produce cytosolic NADPH122. This FAO-derived cytosolic NADPH is key to counteracting oxidative stress135,136, and FAO also acts to sustain ATP levels and NADPH production during metabolic stress123,125,136. In addition, it has been suggested that FAO-dependent NADPH production could be relevant to the survival of leukaemic cells16–18,112,133,137. As FAO-regulatory pathways are relevant to HSC maintenance, it is tempting to speculate that FAO might likewise provide HSCs with the NADPH required to minimize intracellular ROS production and prevent stem cell exhaustion.
Moreover, a recent report has demonstrated that NSCs and neuronal progenitor cells require fatty acid synthase (FASN)-dependent lipogenesis for their proliferation and the subsequent maintenance of neurogenesis in mice138. Specifically, thyroid hormone-inducible hepatic protein (THRSP; also known as SPOT14) acts as a molecular brake on FASN-dependent lipogenesis to ensure quiescence of adult NSCs and progenitor cells. The detailed cellular mechanisms remain to be elucidated, but these data indicate that a potential transition exists from FAO in quiescent stem cells to FASN in proliferating progenitors.
Importantly, although both pyruvate oxidation and FAO are central providers of acetyl-CoA, the precise mechanisms that determine the different fates of acetyl-CoA derived from either pyruvate oxidation or FAO are still unclear. As FAO-derived citrate would be oxidized into NADH in the TCA cycle, one theoretical possibility is that low levels of OXPHOS forces mitochondrial citrate to be released into the cytosol. The resulting cytosolic citrate could be used for fatty acid synthesis but may contribute to the remodelling of the HSC lipidome by creating a particularly useful fatty acid that promotes HSC self-renewal, while depleting less useful fatty acids absorbed from the serum. To confirm this hypothesis, however, it should be first confirmed that malic enzymes and/or isocitrate dehydrogenase (IDH) have a higher activity in LT-HSCs compared to committed progenitors. Several other possibilities should also be considered: first, other mechanisms downstream of PPARδ are also relevant and, in addition to its use as energy source, the FAO process itself could control specific pathways; second, the contribution of further PPARδ-dependent and FAO-independent pathways cannot be entirely ruled out, which may function in HSC maintenance; third, FAO may be a major provider of acetyl-CoA in HSCs, which leads to a shortage of oxaloacetate to feed the TCA cycle. Oxaloacetate can be produced from pyruvate through pyruvate carboxylase, which raises the possibility of glycolysis-derived pyruvate being used for oxaloacetate synthesis rather than oxidation to generate acetyl-CoA; fourth, acetyl-CoA molecules generated by FAO could also be used as a substrate for altering the histone acetylation patterns139 that are responsible for epigenetic control of stem cell fate.
During cell division, whereas every daughter cell receives an equal complement of chromosomes, not all organelles, including mitochondria, are symmetrically transmitted to the progeny in many cell types140. This was thought to be important for proper cell function and fate, especially during stem cell asymmetric division. Therefore, further study is needed to determine how PML–PPAR–FAO pathways affect the asymmetrical segregation of cellular organelles and cell-fate determinants.
Key open questions
The identification of key metabolic pathways in the control of HSC activity raises several questions. Nucleotide synthesis in HSCs is not as well-characterized as in cancer cells and ES cells. Cancer cells are thought to divert glycolytic intermediates into amino acid and nucleotide synthesis for cell proliferation64. Accordingly, proliferative mouse ES cells have shown increased activity in PPP, which enables rapid nucleotide synthesis63,78,141 (FIG. 2). However, the precise regulation of nucleotide synthesis in quiescent HSCs and its role in cell fate decisions remain to be determined. The involvement of pyruvate kinase M2 (PKM2; also known as PKM) in HSC maintenance also remains to be explored. Increased conversion of glucose to lactate is a crucial feature of many cancer cells, in which PKM2 is preferentially expressed and tightly regulates anabolic metabolism142,143 (FIG. 2). It is known that modulation of the catalytic activity of PKM2 regulates the synthesis of DNA and lipids that are required for cell proliferation, and the need for pyruvate kinase is apparent in non-proliferating tumour cells143. Given its pleiotropic effects on cancer biology, PKM2 presents an attractive target for therapy. Notably, important contributions of PKM2 to carcinogenesis, through a negative feedback loop that controls cellular redox homeostasis, have recently been identified. In cancer cells, inhibition of PKM2 by ROS contributes to cellular antioxidant responses144. It is clear that the functional analysis of PKM2 in stem cells has only just begun.
Another unexplored issue is the link between stem cell metabolism and epigenetics. Research has highlighted the important roles of epigenetic regulation in many cellular pathways that are involved in both carcinogenesis and stem cell self-renewal145,146, as well as associations between epigenetic machineries and metabolism-related genes. Acetyl-CoA metabolism, for example, is now regarded as a major regulator of histone acetylation, which might regulate stem cell activity25,139. αKG is likewise believed to function as a cofactor for dioxygenase enzymes, including ten–eleven translocation (TET) family DNA hydroxylases and possibly for the PHD enzymes that control HIF1α stability (FIG. 2). The mutant forms of IDH1 or IDH2 that are found in myeloid malignancies reduce the availability of αKG while producing 2-hydroxyglutarate (2-HG)15,19, which restricts HIF1α hydroxylation, and activating glycolysis23. Additionally, there is increasing evidence for metabolic roles of non-coding RNA and for other crucial links between miRNA and epigenetics in stem cell biology68,73,147–149. Specifically, it has been demonstrated that RNA-binding proteins LIN28A and/or LIN28B regulate glycolytic metabolism by modulating insulin–PI3K–mTOR signalling through the tumour suppressor miRNA let-7 (REFS 73,150) (FIG. 4). How metabolism integrates epigenetic and genetic programmes to regulate HSC function while coordinating cell fate decisions, however, remains an important open question. Perhaps most intriguingly of all, it remains to be determined whether the metabolic rate of HSCs, or its carbon and energy source, determines self-renewal capacity. Distinctions between metabolic regulation of HSCs and committed progenitors suggest that metabolic reprogramming may contribute to the reprogramming of mature cells to the pluripotent state. Further studies of stem cell metabolism should also reveal how stem cells switch from a quiescent to an actively proliferating state.
Conclusions and perspectives
Several metabolic pathways are emerging as regulatory elements of stem cell maintenance, as balanced energetic and biosynthetic demands cooperatively maintain the self-renewal capacity and quiescent status of HSCs (FIGS 1,5). Moreover, many of these features are shared with other tissue self-renewing stem cells and, surprisingly, with highly proliferative cancer cells.
Ongoing investigations, through both genetic and pharmacological approaches, will undoubtedly illuminate the metabolic requirements associated with stem cell fate decisions (BOX 1). Increasing evidence for the existence of self-renewing cancer stem cells (also known as cancer-initiating cells (CICs)) in solid tumours has led to the emergence of an important new area of cancer research151–155, and new insights into how metabolism may affect CIC activity are emerging from studies of the metabolism of highly proliferating bulk cancer cells156 (BOX 2). These studies have the potential to be very influential in the clinic, as we believe that targeted metabolic reprogramming will eventually increase and extend patient health and well-being through the fine-tuning of stem cell function.
Box 1. Technical challenges to study the metabolism of stem cells.
Metabolomic and bioinformatic analyses have driven recent discoveries in the field of stem cell metabolism. However, further technological innovation will be needed to elucidate the detailed metabolic fluxes inside the purified stem cell compartment. Current methods of isotope-tracing in gas or liquid chromatography mass spectrometry (GC-MS or LC-MS) have high sensitivity but are not able to track real-time changes in metabolic fluxes in vivo. New techniques of real-time in vivo metabolic analysis will be required to unravel the dynamic and rapid kinetics of metabolic reaction in stem cells.
The limited number of stem cells in a niche compartment has also proved a challenge to researchers. New technologies, including multi-isotope imaging mass spectrometry (MIMS)158 and/or Gln imaging159, now enable us to measure stable isotope incorporation with submicrometre resolution. Further innovations in metabolomic profiling at the single-cell level should facilitate many advances in the characterization and quantification of metabolites, even given the small cell populations available.
Recently identified stem cell markers have enabled researchers to sort stem cells with especially high levels of purity, and the functional robustness of haematopoietic stem cells (HSCs) has been validated by in vivo transplantation assays with small numbers of HSCs or even single cells22,127,160. Moreover, technological advances in transcriptome analysis have enabled us to carry out single-cell gene expression assays160, which have been key to identifying the regulatory mechanisms of stem cell self-renewal. The combination of single-cell metabolomic profiling and advanced transcriptome analysis of the purified stem cell compartment will undoubtedly lead to a deeper understanding of the novel cell fate determinants which maintain stemness.
Furthermore, the ability to compare metabolites between stem cells and committed progenitors, or genetically manipulated stem cells, will help us to more clearly define the critical metabolic events and/or metabolic pathways that may be dysregulated in various diseases and disorders. And perhaps most importantly, single-cell metabolomic assays will lead to the comparison of metabolic profiles between one daughter cell and another one from the same parent cell, enabling us to determine whether cell-to-cell heterogeneity in gene transcription has a role in cell fate.
Box 2. Cancer cell metabolism.
Many features of self-renewing stem cells are often shared with tumour cells, so how metabolism affects tumour cell activity may be informative with respect to stem cell activity.
The TCA cycle and tumorigenesis
Current research has uncovered links between cellular events, such as the dysregulation of haematopoietic stem cell (HSC) function, leukaemia and metabolic programming. Mutations in these genes have been frequently identified in myeloid malignancies, such as myelodysplastic syndrome (MDS) and acute myeloid leukaemia161,162. Recent characterizations of gene mutations in isocitrate dehydrogenase 1 (IDH1) and IDH2 have revealed leukaemic or stem cell-specific roles15,19–21. Both IDH1 and IDH2 convert isocitrate to α-ketoglutarate (αKG; which enters the tricarboxylic acid (TCA) cycle) and reside in the cytoplasm and mitochondria, respectively. Leukaemia-associated IDH1- or IDH2-mutant enzymes convert αKG to 2-hydroxyglutarate (2-HG), an inhibitory metabolite of ten-eleven translocation 2 (TET2)15,163–165 (FIG. 2). Expression of mutated IDH1 or IDH2, or Tet2 depletion, impairs haematopoietic differentiation and is accompanied by increased HSC self-renewal, which results in pro-leukaemogenic effects15,166 (FIG. 1).
IDH genes are also mutated in more than 75% of low-grade gliomas and secondary glioblastomas167–169, and are likely to be involved in the early stage of gliomagenesis. Mutation of IDH genes that cause excess accumulation of 2-HG increases the risk for malignant gliomas through alteration of DNA and histone methylation, and increased hypoxia-inducible factor 1α (HIF1α) activity21,170,171. IDH mutations have been further identified in other tumours at a lower frequency, including thyroid carcinomas172, cartilaginous tumours173,174 and intrahepatic cholangiocarcinomas175,176.
Gln metabolism in tumorigenesis
Increased levels of glucose metabolism that is driven by MYC promotes glucose-dependent cancer cell growth and survival177,178. The dependence of MYC-expressing cells on oxidative metabolism may be due to increased Gln metabolism179,180. Indeed, some cancer cells are unable to survive in the absence of exogenous Gln181. Further connections between MYC and glutaminolysis have recently been revealed; MYC directly regulates the transcription of Gln transporters181 and also increases expression of glutaminase by repressing microRNA-23 (miR-23)182 (FIG. 3). Intriguingly, MYC also controls the balance between HSC self-renewal and differentiation183,184. Myc-deficient HSCs can still proliferate normally, but most of their progenitors cease proliferation entirely. In addition, the differentiation of these HSCs into committed progenitors is inhibited, as their upregulated adhesion molecules anchor the stem cells in the bone marrow niche.
For instance, attention has been directed towards identifying small-molecule inhibitors of PHDs, which might lead to a clinical strategy for protecting the myocardium against ischemia and reperfusion injury (through a mechanism termed hypoxic preconditioning) 157. However, we should be cautious about inhibiting HIF proteins and glycolysis as this may have potentially broad biological effect. Organ-specific targeting should also be important to minimize potential systemic effects. Predicting clinical outcomes will depend on a deeper understanding of the regulatory networks that link glycolysis to HSC activity and mitochondrial biogenesis in the hypoxic niche of the bone marrow.
Specific activators and inhibitors of metabolic pathways have already been developed, and some have been tested in humans for the treatment of metabolic disorders. The tumour suppressor PML was recently shown to have a key role in HSC maintenance, and is thus emerging as an excellent example of a potential therapeutic target in stem cells126. Indeed, the effectiveness of an arsenic-mediated drug that targets PML is now being assessed in a clinical trial. It should be noted that the regulation of PPAR–FAO by PML seems to be an essential rheostat that fine-tunes asymmetric cell divisions, which are important for HSC maintenance22. Further investigations will define exactly how metabolic reprogramming is involved in controlling the delicate equilibrium of stem cells and will yield findings that are relevant to the development of new therapies.
Acknowledgments
The authors thank C. Lin, A. Sasaki and A. Carracedo Pérez for their comments and discussion on metabolic pathways in stem cells. T.S. is supported by the Seventh Framework Programme of the European Union under grant agreement number 306240 (SystemAge) and a Grant-in-Aid from the Japan Society for the Promotion of Science. K.I. is supported by grants from the US National Institutes of Health (NIH) (R00CA139009 and R01DK98263). The authors apologize to those whose work could not be discussed owing to space limitations.
Glossary
- Self-renewal
The capacity to propagate stem cells with a differentiation potential (potency) that is similar to that of the mother stem cell
- Asymmetric cell division
One of the proposed models to explain the regulation of cell-fate decisions, which play a crucial part in stem cell self-renewal. During asymmetric division, on daughter cell remains a stem cell, while the other one becomes committed
- Hypoxia
A state of reduced oxygen pressure below a certain threshold, which restricts the function of organs, tissues or cells. It has a role in regulating stem cell behaviour. A partial oxygen pressure (PO2) of <40 mm Hg in arterial blood constitutes hypoxia
- Glycolysis
A metabolic pathway that generates ATP and converts one molecule of glucose into two molecules of pyruvate or lactate. Glycolysis occurs in the presence (aerobic glycolysis) or absence (anaerobic glycolysis) of oxygen
- Long-term HSCs
(LT-HSCs). Haematopoietic stem cells (HSCs) that are defined functionally by their ability to mediate the long-term repopulation of all haematopoietic lineages (known as long-term repopulating activity) after transfer to lethally irradiated recipients
- Pimonidazole
An effective and non-toxic exogenous 2-nitroimidazole marker for hypoxia. It forms adducts with thiol groups in proteins, peptides and amino acids specifically in hypoxic cells
- Oxidative phosphorylation
(OXPHOS). The mitochondrial reactions that generate and harness energy released from the oxidation of nutrients such as pyruvate, through a proton gradient, to synthesize ATP
- Normoxic conditions
Normal partial oxygen pressure (PO2) levels range from 150 mm Hg in the upper airway to ~5 mm Hg in the retina
- Tricarboxylic acid cycle
(TCA cycle). Also known as the citric acid cycle and Krebs cycle. Cyclic series of enzyme-catalysed chemical reactions that form a key part of aerobic respiration in cells. Each complete turn of the cycle generates one GTP, two CO2, one FADH2 and three NADH molecules
- Reactive oxygen species
(ROS). Reactive molecules, such as hydrogen peroxide and superoxide anion. ROS form as by-products of cellular respiration and ionizing radiation, and are potentially damaging to cell structures and other molecules causing oxidative stress
- Membrane potential
An important parameter of mitochondrial function that is crucial for maintaining the physiological function of the respiratory chain to generate ATP. A great loss of the membrane potential renders cells depleted of energy, followed by death
- Metabolic reprogramming
Stem cell-specific programmes that may drive the dependency of stem cells on specific nutrients, such as glucose, fatty acids and Gln, as well as impose changes to the wiring of metabolic pathways to maintain stemness
- Catabolism
A set of metabolic pathways that breaks down molecules (for example, nucleic acids, lipids and proteins) into smaller units (for example, nucleotides, fatty acids and amino acids) to release energy
- Cristae
Folds in the inner membrane of mitochondria, which are studded with proteins, such as ATP synthase, and which increase the surface area for chemical reactions to occur (for example, cellular respiration)
- p38 MAPK
A member of the MAPK family that is activated by various environmental stresses and inflammatory cytokines. It is involved in processes such as cell differentiation, apoptosis and autophagy
- p16
(also known as INK4A; encoded by CDKN2A). A tumour suppressor protein, which inhibits the activities of CDK4 and CDK6. This leads to cell cycle arrest and has key roles in important cellular physiological processes such as senescence and apoptosis
- Oncogene-induced senescence
(OIS). A tumour suppressor mechanism that prevents the expansion of cells bearing activated oncogenes, which is caused by aberrant activation of oncoproteins and tumour suppressors
- Pancytopenia
An abnormal reduction in the number of erythrocytes, leukocytes and platelets in the blood
- Glutaminolysis
Biochemical reactions in which Gln is broken down to Glu, Asp, CO2, pyruvate, lactate, Ala and citrate
- Fatty acid oxidation
(FAO). The dehydrogenation, hydration, oxidation and thiolysis reactions that cleave off the two-carbon acetyl-coenzyme A (CoA) from fatty acids, which then feeds into the tricarboxylic acid cycle (TCA) cycle. It also includes the generation of NADH and FADH2, which are used by the electron transport chain
Footnotes
Competing interests statement
The authors declare no competing interests.
Contributor Information
Keisuke Ito, Email: keisuke.ito@einstein.yu.edu.
Toshio Suda, Email: sudato@z3.keio.jp.
References
- 1.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 2.Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–313. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- 4.He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- 5.van der Lugt NM, et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 6.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 7.Lansdorp PM. Intrinsic control of stem cell fate. Stem Cells. 1997;15(Suppl 1):223–225. doi: 10.1002/stem.5530150830. discussion 225–227. [DOI] [PubMed] [Google Scholar]
- 8.Suda T, Suda J, Ogawa M. Single-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc Natl Acad Sci USA. 1983;80:6689–6693. doi: 10.1073/pnas.80.21.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suda T, Suda J, Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci USA. 1984;81:2520–2524. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalf D. Lineage commitment in the progeny of murine hematopoietic preprogenitor cells: influence of thrombopoietin and interleukin 5. Proc Natl Acad Sci USA. 1998;95:6408–6412. doi: 10.1073/pnas.95.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa ME, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan B, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurumurthy S, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. Together with references 16 and 17, this study demonstrates that the protein LKB1, which lies at the crossroad of energy metabolism and cell growth, seems to regulate HSC dynamics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tefferi A, et al. IDH1 and IDH2 mutation studies in 1473 patients with chronic-, fibrotic- or blast-phase essential thrombocythemia, polycythemia vera or myelofibrosis. Leukemia. 2010;24:1302–1309. doi: 10.1038/leu.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward PS, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito K, et al. A PML–PPAR-δe pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nature Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. This study shows that PML acts as a critical rheostat responsible for fine-tuning tissue homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. A comprehensive review on oxygen homeostasis, the hypoxic niche and energy metabolism for maintenance of HSC function and long-term self-renewal. [DOI] [PubMed] [Google Scholar]
- 24.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. An important review of recent studies of the balance among glycolysis, mitochondrial OXPHOS and oxidative stress in various forms of stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, et al. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 2013;18:325–332. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nature Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nombela-Arrieta C, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simsek T, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suda T. Hematopoiesis and bone remodeling. Blood. 2011;117:5556–5557. doi: 10.1182/blood-2011-03-344127. [DOI] [PubMed] [Google Scholar]
- 32.Takubo K, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12:49–61. doi: 10.1016/j.stem.2012.10.011. This study demonstrates that glycolytic metabolic status governed by PDK acts as a cell cycle checkpoint that modulates HSC quiescence and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warr MR, Passegue E. Metabolic makeover for HSCs. Cell Stem Cell. 2013;12:1–3. doi: 10.1016/j.stem.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Yu WM, et al. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 2013;12:62–74. doi: 10.1016/j.stem.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 36.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 37.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavadas MA, Nguyen LK, Cheong A. Hypoxia-inducible factor (HIF) network: insights from mathematical models. Cell Commun Signal. 2013;11:42. doi: 10.1186/1478-811X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen LK, et al. A dynamic model of the hypoxia-inducible factor 1α (HIF-1α) network. J Cell Sci. 2013;126:1454–1463. doi: 10.1242/jcs.119974. [DOI] [PubMed] [Google Scholar]
- 40.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 41.Epstein AC, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 42.Ivan M, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci USA. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barger JF, Plas DR. Balancing biosynthesis and bioenergetics: metabolic programs in oncogenesis. Endocr Relat Cancer. 2010;17:R287–304. doi: 10.1677/ERC-10-0106. [DOI] [PubMed] [Google Scholar]
- 44.Semenza GL. Targeting HIF-1 for cancer therapy. Nature Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 45.Simon MC, Liu L, Barnhart BC, Young RM. Hypoxia-induced signaling in the cardiovascular system. Annu Rev Physiol. 2008;70:51–71. doi: 10.1146/annurev.physiol.70.113006.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rouault-Pierre K, et al. HIF-2α protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell Stem Cell. 2013;13:549–563. doi: 10.1016/j.stem.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3α. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- 48.Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J Biol Chem. 2002;277:32405–32408. doi: 10.1074/jbc.C200328200. [DOI] [PubMed] [Google Scholar]
- 49.Kranc KR, et al. Cited2 is an essential regulator of adult hematopoietic stem cells. Cell Stem Cell. 2009;5:659–665. doi: 10.1016/j.stem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du J, et al. HIF-1α deletion partially rescues defects of hematopoietic stem cell quiescence caused by Cited2 deficiency. Blood. 2012;119:2789–2798. doi: 10.1182/blood-2011-10-387902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du J, et al. Cited2 is required for the maintenance of glycolytic metabolism in adult hematopoietic stem cells. Stem Cells Dev. 2014;23:83–94. doi: 10.1089/scd.2013.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miharada K, et al. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell. 2011;9:330–344. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Miharada K, et al. Hematopoietic stem cells are regulated by Cripto, as an intermediary of HIF-1α in the hypoxic bone marrow niche. Ann NY Acad Sci. 2012;1266:55–62. doi: 10.1111/j.1749-6632.2012.06564.x. [DOI] [PubMed] [Google Scholar]
- 54.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 55.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 56.Pattappa G, Heywood HK, de Bruijn JD, Lee DA. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J Cell Physiol. 2011;226:2562–2570. doi: 10.1002/jcp.22605. [DOI] [PubMed] [Google Scholar]
- 57.Pattappa G, et al. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods. 2013;19:68–79. doi: 10.1089/ten.TEC.2011.0734. [DOI] [PubMed] [Google Scholar]
- 58.Renault VM, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Blerkom J. Mitochondria in early mammalian development. Semin Cell Dev Biol. 2009;20:354–364. doi: 10.1016/j.semcdb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Kondoh H, et al. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- 61.Shyh-Chang N, et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339:222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Folmes CD, et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varum S, et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng S, et al. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells. 2011;29:496–504. doi: 10.1002/stem.591. [DOI] [PubMed] [Google Scholar]
- 66.Balzer E, Moss EG. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 2007;4:16–25. doi: 10.4161/rna.4.1.4364. [DOI] [PubMed] [Google Scholar]
- 67.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12:395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 70.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng K, Wu X, Kaestner KH, Wang PJ. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev Biol. 2009;9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shyh-Chang N, et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155:778–792. doi: 10.1016/j.cell.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu H, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30:4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shyh-Chang N, Zheng Y, Locasale JW, Cantley LC. Human pluripotent stem cells decouple respiration from energy production. EMBO J. 2011;30:4851–4852. doi: 10.1038/emboj.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forman NG, Wilson DF. Energetics and stoichiometry of oxidative phosphorylation from NADH to cytochrome c in isolated rat liver mitochondria. J Biol Chem. 1982;257:12908–12915. [PubMed] [Google Scholar]
- 77.Wang J, et al. Dependence of mouse embryonic stem cells on threonine catabolism. Science. 2009;325:435–439. doi: 10.1126/science.1173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manganelli G, et al. Modulation of the pentose phosphate pathway induces endodermal differentiation in embryonic stem cells. PLoS ONE. 2012;7:e29321. doi: 10.1371/journal.pone.0029321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Kathagen A, et al. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol. 2013;126:763–80. doi: 10.1007/s00401-013-1173-y. [DOI] [PubMed] [Google Scholar]
- 80.Zhao F, et al. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1α-induced metabolic reprograming. Oncogene. 2010;29:2962–2972. doi: 10.1038/onc.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho YM, et al. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348:1472–1478. doi: 10.1016/j.bbrc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 82.Chung S, Arrell DK, Faustino RS, Terzic A, Dzeja PP. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J Mol Cell Cardiol. 2010;48:725–734. doi: 10.1016/j.yjmcc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 84.St John JC, et al. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Clon Stem Cells. 2005;7:141–153. doi: 10.1089/clo.2005.7.141. [DOI] [PubMed] [Google Scholar]
- 85.Norddahl GL, et al. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell. 2011;8:499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Inoue S, et al. Mitochondrial respiration defects modulate differentiation but not proliferation of hematopoietic stem and progenitor cells. FEBS Lett. 2010;584:3402–3409. doi: 10.1016/j.febslet.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 87.Maryanovich M, et al. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nature Cell Biol. 2012;14:535–541. doi: 10.1038/ncb2468. [DOI] [PubMed] [Google Scholar]
- 88.Maryanovich M, Gross AA. ROS rheostat for cell fate regulation. Trends Cell Biol. 2013;23:129–134. doi: 10.1016/j.tcb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 89.Weiss CN, Ito K. DNA damage response, redox status and hematopoiesis. Blood Cells Mol Dis. 2013;52:12–18. doi: 10.1016/j.bcmd.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. Together with references 11 and 14, this study demonstrates an important role of FOXO proteins in the maintenance and integrity of stem cells. [DOI] [PubMed] [Google Scholar]
- 91.Paik JH, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamazaki S, et al. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 2006;25:3515–3523. doi: 10.1038/sj.emboj.7601236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paik JH, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hagenbuchner J, Ausserlechner MJ. Mitochondria and FOXO3: breath or die. Front Physiol. 2013;4:147. doi: 10.3389/fphys.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mortensen M, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011;208:455–467. doi: 10.1084/jem.20101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Warr MR, et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–327. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair. 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 98.Allen DM, et al. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev. 2001;15:554–566. doi: 10.1101/gad.869001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nitta E, et al. Telomerase reverse transcriptase protects ATM-deficient hematopoietic stem cells from ROS-induced apoptosis through a telomere-independent mechanism. Blood. 2011;117:4169–4180. doi: 10.1182/blood-2010-08-297390. [DOI] [PubMed] [Google Scholar]
- 101.Kaplon J, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 102.Harris JM, et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood. 2013;121:2483–2493. doi: 10.1182/blood-2012-12-471201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morimoto H, et al. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 2013;12:774–786. doi: 10.1016/j.stem.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 104.Lewandowski D, et al. In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood. 2010;115:443–452. doi: 10.1182/blood-2009-05-222711. [DOI] [PubMed] [Google Scholar]
- 105.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kharas MG, Gritsman K. Akt: a double-edged sword for hematopoietic stem cells. Cell Cycle. 2010;9:1223–1224. doi: 10.4161/cc.9.7.11362. [DOI] [PubMed] [Google Scholar]
- 107.Kharas MG, et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–1415. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yilmaz OH, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 109.Zhang J, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 110.Lee JY, et al. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell. 2010;7:593–605. doi: 10.1016/j.stem.2010.09.015. In this study, mTOR activation depletes HSCs through a tumour suppressor response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen C, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gan B, et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci USA. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Groszer M, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 114.Zhou J, et al. Tsc1 mutant neural stem/progenitor cells exhibit migration deficits and give rise to subependymal lesions in the lateral ventricle. Genes Dev. 2011;25:1595–1600. doi: 10.1101/gad.16750211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yilmaz OH, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 118.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Son J, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dang CV, et al. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 121.Carracedo A, et al. A metabolic prosurvival role for PML in breast cancer. J Clin Invest. 2012;122:3088–3100. doi: 10.1172/JCI62129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nature Rev Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schafer ZT, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dunning KR, et al. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83:909–918. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- 125.Zaugg K, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–1051. doi: 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ito K, et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 128.Arai F, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 129.Ito K, Ito K. Newly Identified Roles of PML in Stem Cell Biology. Front Oncol. 2013;3:50. doi: 10.3389/fonc.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Riserus U, et al. Activation of peroxisome proliferator-activated receptor (PPAR) δ promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 131.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 132.Wagner KD, Wagner N. Peroxisome proliferator-activated receptor beta/delta (PPARβ/δ) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol Ther. 2010;125:423–435. doi: 10.1016/j.pharmthera.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 133.Samudio I, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010;120:142–156. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hosokawa K, et al. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem Biophys Res Commun. 2007;363:578–583. doi: 10.1016/j.bbrc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 135.Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807:726–734. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 136.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661– 665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Caro P, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Knobloch M, et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Galdieri L, Vancura A. Acetyl-CoA carboxylase regulates global histone acetylation. J Biol Chem. 2012;287:23865–23876. doi: 10.1074/jbc.M112.380519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lerit DA, Smyth JT, Rusan NM. Organelle asymmetry for proper fitness, function, and fate. Chromosome Res. 2013;21:271–286. doi: 10.1007/s10577-013-9350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Filosa S, et al. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem J. 2003;370:935–943. doi: 10.1042/BJ20021614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 143.Israelsen WJ, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Anastasiou D, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nature Rev Cancer. 2012;12:599–612. doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
- 146.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nature Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 147.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 2010;140:445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 148.Song SJ, et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via tet-family-dependent chromatin remodeling. Cell. 2013;154:311–324. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song SJ, et al. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell. 2013;13:87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu H, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nature Genet. 2010;42:626–630. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 152.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 153.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 154.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nature Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 155.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 156.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 157.Sen Banerjee S, et al. HIF-prolyl hydroxylases and cardiovascular diseases. Toxicol Mech Methods. 2012;22:347–358. doi: 10.3109/15376516.2012.673088. [DOI] [PubMed] [Google Scholar]
- 158.Steinhauser ML, et al. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature. 2012;481:516–519. doi: 10.1038/nature10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Qu W, et al. Synthesis of optically pure 4-fluoroglutamines as potential metabolic imaging agents for tumors. J Am Chem Soc. 2011;133:1122–1133. doi: 10.1021/ja109203d. [DOI] [PubMed] [Google Scholar]
- 160.Hosokawa K, et al. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell. 2010;6:194–198. doi: 10.1016/j.stem.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 161.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mardis ER, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wise DR, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sasaki M, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488:656–659. doi: 10.1038/nature11323. Important report that demonstrates mechanistic links between an Idh1 mutation, human acute myeloid leukaemia and the induction of a leukaemic DNA methylation signature in a mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kats LM, et al. Proto-oncogenic role of mutant idh2 in leukemia initiation and maintenance. Cell Stem Cell. 2014 doi: 10.1016/j.stem.2013.12.016. http://dx.doi.org/10.1016/j.stem.2013.12.016. [DOI] [PMC free article] [PubMed]
- 166.Cimmino L, Abdel-Wahab O, Levine RL, Aifantis I. TET family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell. 2011;9:193–204. doi: 10.1016/j.stem.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yan H, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 2010;16:387–397. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 169.Yang H, Ye D, Guan KL, Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res. 2012;18:5562–5571. doi: 10.1158/1078-0432.CCR-12-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhao S, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1α. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Christensen BC, et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst. 2011;103:143–153. doi: 10.1093/jnci/djq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Murugan AK, Bojdani E, Xing M. Identification and functional characterization of isocitrate dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys Res Commun. 2010;393:555–559. doi: 10.1016/j.bbrc.2010.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Amary MF, et al. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nature Genet. 2011;43:1262–1265. doi: 10.1038/ng.994. [DOI] [PubMed] [Google Scholar]
- 174.Pansuriya TC, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nature Genet. 2011;43:1256–1261. doi: 10.1038/ng.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Borger DR, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang P, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32:3091–3100. doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gottlob K, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 179.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brunelle JK, et al. c-Myc sensitization to oxygen deprivation-induced cell death is dependent on Bax/Bak, but is independent of p53 and hypoxia-inducible factor-1. J Biol Chem. 2004;279:4305–4312. doi: 10.1074/jbc.M312241200. [DOI] [PubMed] [Google Scholar]
- 181.Wise DR, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gao P, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wilson A, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. Demonstrates that MYC controls the balance between HSC self-renewal and differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Laurenti E, Wilson A, Trumpp A. Myc’s other life: stem cells and beyond. Curr Opin Cell Biol. 2009;21:844–854. doi: 10.1016/j.ceb.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 185.Spencer JA, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014 doi: 10.1038/nature13034. http://dx.doi.org/10.1038/nature13034. [DOI] [PMC free article] [PubMed]