Abstract
Head and neck squamous cell carcinoma (HNSCC) is an immunosuppressive malignancy. Interest in developing novel immunotherapies in HNSCC has been reawakened by the success of cetuximab, a therapeutic monoclonal antibody (mAb) against the epidermal growth factor receptor which likely relies on immune as well as anti-signaling mechanisms. We focus on novel therapeutic mAb in current clinical development against established mechanisms of immune evasion in HNSCC, targeting: tumor antigens (TA), with resultant potential to induce antibody-dependent cell-mediated cytotoxicity and T cell activation; immunosuppressive cytokines; co-stimulatory Tumor Necrosis Factor (TNF)-family receptors; and co-inhibitory immune checkpoint receptors. Clinical trials of immunotherapeutic mAb as monotherapy, in combination with cytolytic standard therapies exposing TA or in combination with other immunomodulatory mAb, are urgently needed in HNSCC.
Introduction
Head and neck squamous cell carcinoma (HNSCC), the sixth leading incident cancer worldwide1, has been recognized as an immunosuppressive disease. HNSCC induces a tumor-permissive cytokine profile2, 3, qualitative and quantitative lymphocyte deficiencies4–6, anergy in major immune effector cells7–10, and poor antigen-presentation.11–13 An increasing proportion of HNSCC in North America and Europe is caused by oral infection with human papillomavirus (HPV)14–16, rather than the classic exposures of tobacco and alcohol. Whether caused by environmental carcinogenesis or transformation by HPV oncogenes, HNSCC thwarts immune surveillance, recognition and destruction, which must be reversed to maximize therapeutic efficacy.
Early clinical trials in HNSCC exploited available immunostimulatory cytokines, which faltered clinically due to disinterest in local delivery or prohibitive systemic toxicity.17–19 Three parallel advancements have reawakened enthusiasm for the development of novel immunotherapies in HNSCC: 1) realization of the contribution of immune mechanisms to the clinical activity of cetuximab20, 21, the monoclonal antibody (mAb) against the epidermal growth factor receptor (EGFR) approved in HNSCC by the U.S. Food and Drug Administration (FDA) in 2006; 2) progressive preclinical insights into specific, targetable immune escape mechanisms important to the survival of HNSCC tumors; 3) previously unimagined clinical responses in non-small cell lung cancer (NSCLC), a non-immunogenic solid tumor similar to HNSCC, to phase I therapy with an immune checkpoint mAb.22, 23 In the interest of prioritizing rational clinical investigations, this review will examine the immunotherapeutic mAb currently in human trials for cancer patients, in the specific context of immune escape mechanisms in HNSCC. Immunotherapeutic mAbs will be conceptually divided into those which target tumor antigens (TA), immunosuppressive cytokines, TNF receptor (TNFR) costimulatory molecules, or immune checkpoint receptors (Table 1).
Table 1.
Therapeutic Monoclonal Antibodies to Overcome Immune Escape in HNSCC
| Drug (Company) | Target | IgG subclass | Phase of development, human cancer | HNSCC development |
|---|---|---|---|---|
| Tumor Antigen Targeted Monoclonal Antibodies | ||||
| Cetuximab (Bristol-Myers Squibb, Eli Lilly) | EGFR antagonist | IgG1 | III/IV (FDA-approved HNSCC, NSCLC, colon cancer) | Phase III/IV |
| Panitumumab (Amgen) | EGFR antagonist | IgG2 | III/IV (FDA-approved colon cancer) | Phase II/III |
| Onartuzumab (Genentech) | cMet antagonist (single-arm Fab) | IgG1 | II/III | --- |
| Pertuzumab (Genentech) | HER2 antagonist | IgG1 | III | --- |
| AV-203 (Aveo) | HER3 antagonist | IgG1 | I | Phase I (monotherapy; cetuximab combination) |
| MM-121 (Merck) | HER3 antagonist | IgG2 | I/II | --- |
| RO5479599 (Roche) | HER3 antagonist | Glyco-engineered | I/II | --- |
| Cixutumumab (Eli Lilly) | IGFR antagonist | IgG1 | II | Phase 0-II (neoadjuvant monotherapy; cetuximab combination) |
| Cytokine Targeted Monoclonal Antibodies | ||||
| Bevacizumab (Genentech) | VEGF neutralizer | IgG1 | III/IV (FDA approved in NSCLC, colon) | Phase III (platinum chemotherapy +/−) |
| Ficlatuzumab (Aveo) | HGF neutralizer | IgG1 | I/II | Phase I (cetuximab combination; cisplatin-radiation combination) |
| AMG 102 (Amgen) | HGF neutralizer | IgG2 | I/II | --- |
| Fresolimumab (Genzyme) | TGF-β neutralizer | IgG4 | --- | |
| Siltuximab (Janssen Biotech) | IL-6 neutralizer | IgG1 | I/II | --- |
| TNF Receptor Targeted Monoclonal Antibodies | ||||
| CP-870,893 (Pfizer) | CD40 agonist | IgG2 | I | --- |
| OX40 mAb (AgonOx, Providence Health) | OX40 agonist | IgG2 | I | --- |
| Urelumab (Bristol-Myers Squibb) | CD137 agonist | IgG4 | I | --- |
| PF-05082566 (Pfizer) | CD137 agonist | IgG2 | I | --- |
| Immune Checkpoint Targeted Monoclonal Antibodies | ||||
| Ipilimumab (Bristol-Myers Squibb) | CTLA4 | IgG1 | III/IV (FDA approved in melanoma | Phase I (cetuximab-radiation combination) |
| Tremelimumab (Pfizer) | CTLA4 | IgG2 | III | --- |
| Nivolumab (Bristol-Myers Squibb) | PD-1 | IgG4 | I/II/III | --- |
| Lambrolizumab (Merck) | PD-1 | IgG4 | I/II | --- |
| BMS-936559 (Bristol-Myers Squibb) | PD-L1 | IgG4 | I | --- |
TA-Targeted mAbs
Although cytotoxic T lymphocytes (CTL) specific for p53, EGFR, or the HPV E7 oncoprotein have been detected in HNSCC patients,24–26 the nascent adaptive immune response is ineffective. Due to selective loss of human leukocyte antigen (HLA) I and functional deficiency in antigen processing machinery, HNSCC tumor cells avoid recognition and destruction by extant TA-specific CTLs.11, 27 Recent evidence confirms that cetuximab, a chimeric, IgG1-isotype mAb which blocks the extracellular domain of EGFR, potentiates both innate and adaptive immune responses against endogenous TAs20 – indicating that TA-targeted mAb have broader immunogenic potential than is currently being exploited.
In HNSCC, cetuximab development was compelled by the frequent finding of over-expression of EGFR correlating with advanced stage, radiation resistance, and poor survival.28, 29 Indeed, cetuximab increased response rate and overall survival combined with radiation in locally advanced HNSCC, or with platinum-based chemotherapy in recurrent HNSCC, ultimately gaining FDA approval for both indications.30, 31 Cetuximab is frequently described as the first molecularly targeted agent in HNSCC, a deserved appellation; yet certain conundrums prompted the search for additional, immunologic mechanisms of action. First, despite over-expression of EGFR in more than 90% of HNSCC32, cetuximab monotherapy demonstrates a response rate of only 10–15% in recurrent disease.33 Moreover, over-expression or amplification of EGFR does not predict clinical benefit from cetuximab, and activating EGFR mutations are not found in HNSCC, underscoring the absence of a predictive molecular marker.34, 35 Second, non-immunogenic small molecule inhibitors of the intracellular tyrosine kinase domain of EGFR have not demonstrated clinical benefit in randomized trials in HNSCC.36, 37 Third, despite rapid abrogation of EGFR phosphorylation and tumor proliferation upon mAb exposure in preclinical models38, in vitro tumor cell apoptosis or lysis requires the presence of lymphocytes.11
Dissection of the immunologic actions of cetuximab provides guidance for further development of TA-targeted mAb in HNSCC. Specifically, cetuximab-coated HNSCC tumor cells activate the antibody-binding receptor (FcγR IIIa) on natural killer (NK) cells, the major effector cell of innate immunity. Binding between the constant Fc component of the IgG mAb and FcγR IIIa instigates antibody-dependent cellular cytotoxicity (ADCC).21, 39, 40 Moreover, activation of FcγR IIIa upregulates NK cell secretion of IFN-γ, an immunostimulatory cytokine which promotes maturation of dendritic cells (DC). NK-DC cross-priming enhances antigen processing and presentation by DCs, ultimately promoting activation of TA-specific CTL. Of interest, the repertoire of TA-specific CTLs induced by cetuximab is not restricted to EGFR. Preclinical modeling also demonstrates cross-priming of CTLs specific for MAGE-3, a second TA.20 These findings suggest three broad and interacting strategies to augment the therapeutic potential of cetuximab and other TA-targeted mAb: 1) potentiating ADCC; 2) promoting DC maturation; 3) releasing suppression of CTLs.
Fundamentally, ADCC requires binding between FcγR IIIa on NK cells and the IgG Fc region on the mAb-coated tumor cell, an interaction which may be influenced by an intrinsic patient factor, FcγR polymorphisms, or a drug factor, the IgG isotype subclass. Differential patient response to mAb including rituximab in lymphoma (anti-CD20), trastuzumab in breast cancer (anti-HER2), and cetuximab in colorectal cancer has been correlated with FcγR IIIa polymorphisms, thought to reflect Fc binding affinities.41–43 While these findings raise the possibility of patient selection for mAb therapy by ADCC capacity, a similar preclinical correlation in HNSCC was not validated in patients. The VV FcγR IIIa genotype favorably influenced NK cytolysis of HNSCC tumor cells in ADCC assays44, but was not associated with cetuximab outcome in HNSCC patients where it was found in only 5% of cases.20 Thus, enhancing mAb activity by patient selection for favorable polymorphisms is not currently suitable for the clinic.
The FcγR binding partner, the IgG Fc subclass of the mAb itself, appears increasingly important to the efficacy of TA-targeted mAb. In HNSCC, the case in point is panitumumab. Panitumumab is a fully humanized IgG2 mAb specific for the same EGFR epitope as cetuximab. In contrast to IgG1, the IgG2 isotype has a low binding affinity for FcγR IIIa, and is ineffective at mediating ADCC by NK cells.20, 21, 45 IgG2 does bind FcγR IIa on myeloid-lineage lytic cells, triggering ADCC, however the clinical importance of this mechanism is unclear.45 Of note, panitumumab does not induce the NK-DC cross-priming underlying the adaptive immune response.20 Unlike cetuximab, panitumumab did not improve survival when combined with platinum-based chemotherapy in recurrent HNSCC, although a secondary analysis noted improvement in patients with p-16 negative tumors, a surrogate marker for HPV within the oropharynx.46 In a phase II trial in the locally advanced setting, panitumumab-radiotherapy was inferior to cisplatin-radiotherapy for the primary endpoint of 2-year locoregional control.47 The failure of panitumumab to make therapeutic inroads in HNSCC, despite identical anti-signaling properties to cetuximab, has further elevated the hypothesis that immune mechanisms are critical to cetuximab’s activity. This insight should inform development of subsequent TA-targeted mAb in HNSCC, with prioritization of IgG1 subclass drugs.
Innate or acquired resistance to cetuximab is frequently associated with compensatory signaling by alternate growth factor receptors, e.g. HER2, HER3, cMet, and insulin growth factor receptor (IGFR).48 In this light, TA-specific mAb targeting parallel growth factor receptors are rational candidates for investigation in HNSCC. Representative IgG1-isotype mAb are in clinical development. Trastuzumab and pertuzumab target HER2-overexpressing breast cancer; co-targeting HER2 with both drugs in a HER2-expressing xenograft model augmented ADCC.49 Thus, combinatorial mAb strategies are desirable, and investigation of immune correlates will be critical to guiding development. For example, AV-203, an anti-HER3mAb, is under phase I evaluation in combination with cetuximab (NCT01603979). This trial includes a cetuximab-resistant HNSCC cohort, a setting where both signaling and immune resistance mechanisms may occur. While cixutumumab, an anti-IGFR mAb failed to improve PFS alone or in combination with cetuximab as compared to cetuximab monotherapy50, it is now under investigation in a window-of-opportunity trial in patients with operable HNSCC (NCT00957853). In this model, both signaling and immune mechanisms could be investigated in paired tumor specimens.
While FcγR-Fc binding is required for ADCC, the NK cell’s capacity for cytolysis is significantly amplified by the immunostimulatory cytokines IL-12 and TNFα, secreted by activated DCs following TA uptake and presentation. In the development of IgG1-isotype mAb in HNSCC, judicious co-investigation of two ADCC adjuncts would appear rational: exogenous cytokines, such as IL-12, or toll-like receptor (TLR) agonists. Intravenous IL-12 has been studied in phase I combination with paclitaxel and trastuzumab in HER2-expressing cancers, exploiting the ADCC mechanism.51 In operable HNSCC, neoadjuvant tumoral injections of IL-12 resulted in migration of NK cells to the primary tumor and draining lymph nodes, and increased IFN-γ secretion52; these promising immunomodulatory findings underpin an ongoing phase II study of cetuximab and subcutaneous IL-12 in recurrent HNSCC (NCT01468896). VTX-2337, a TLR8 agonist, activates innate immunity, amplifying phagocytosis, immunostimulatory cytokine secretion, and antigen presentation by DCs.53–56, 57 Randomized trials evaluating cetuximab with/without VTX-2337 (NCT01334177) or platinum-based chemotherapy and cetuximab, with/without VTX-2337 (NCT01836029), are recruiting.
Cytokine-Targeted mAbs
The immunosuppressive cytokine profile of the HNSCC microenvironment is driven by aberrant regulation of the signal transducer and activator of transcription (STAT) family. HNSCC tumors and immune cells demonstrate deficient immunostimulatory STAT1 signaling, and excess immunosuppressive STAT3 signaling.3, 58, 59 The STAT1/STAT3 activation imbalance results in dominant production of TGF-β1, IL-6, IL-10 and vascular endothelial growth factor (VEGF) by HNSCC tumors and tumor-associated macrophages.60 These cytokines inhibit NK cell cytolysis, DC maturation, and CTL activation while inducing regulatory T cells (Treg).7, 58, 61–63 Tumor-associated fibroblasts reinforce immunosuppression through secretion of hepatocyte growth factor (HGF), a paracrine cytokine which promotes HNSCC proliferation and metastasis through cMet signaling while inhibiting DC maturation.64, 65 As immunosuppressive cytokines in sera of HNSCC patients correlate longitudinally with poor response and relapse2, cytokine-neutralizing mAb may be particularly relevant therapeutic adjuncts. Specifically, mAb targeting VEGF and HGF are in clinical development in HNSCC, whereas mAb neutralizing TGF-β1 and IL-6 are under study in other malignancies.
Bevacizumab is an anti-VEGF IgG1 subclass mAb which increased survival when combined with carboplatin-paclitaxel chemotherapy in advanced NSCLC,66 and VEGF and microvascular density are negative prognostic indicators in HNSCC.67, 68 While the phase II combination of bevacizumab-cetuximab had disappointing efficacy in recurrent HNSCC69, a randomized phase III ECOG trial testing frontline platinum-based chemotherapy with/without bevacizumab is ongoing (NCT00588770).
Ficlatuzumab (IgG1) and rilotumumab (IgG2) are humanized mAb which neutralize HGF, preventing ligation of the oncogenic cMet receptor. As HGF/cMet signaling is implicated in resistance to standard HNSCC therapies70–72, trials combining these agents with cetuximab, cisplatin and/or radiation are in development. Bearing in mind that HGF inhibits DC maturation, companion immune biomarkers should be incorporated into early phase design.
Fresolimumab is an IgG4 subclass mAb which neutralizes all TGF-β isoforms, under phase I study in myeloproliferative disorders, kidney cancer and melanoma (NCT01291784; NCT00356460). In a HNSCC xenograft model, adding a preclinical TGF-β mAb to cetuximab prevented resistance to NK cell cytolysis mediated by TGF-β1.73 Siltuximab is an IgG1 chimeric mAb against IL-6, under phase II evaluation in kidney cancer74, prostate cancer and multiple myeloma (NCT00385827; NCT01484275). As TGF-β1 and IL-6 are key immunosuppressive STAT3 cytokines, and no direct STAT3 inhibitors are available, both agents are of significant interest in HNSCC.
TNFR-Family Targeted mAbs
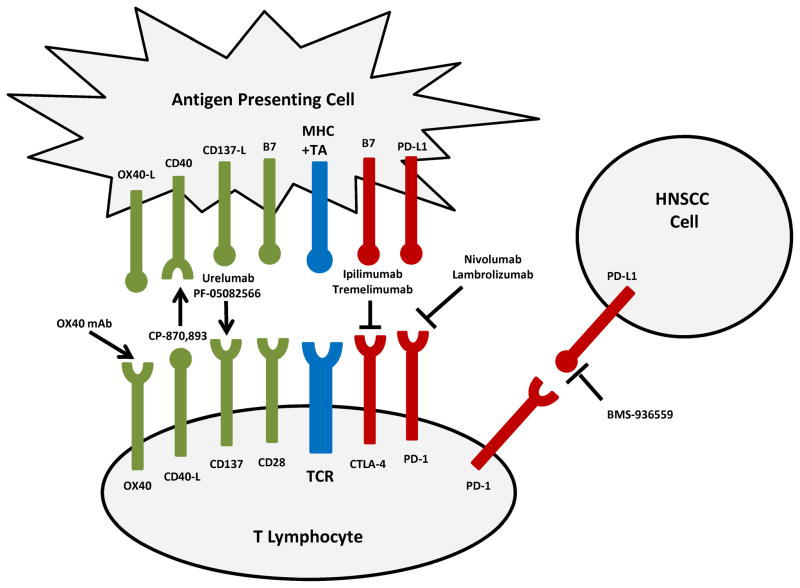
Ultimately, effective immune eradication of tumor requires priming of the TA-specific, HLA-I restricted, CD8(+) CTL by APCs. Binding of the T cell receptor (TCR) by its cognate TA-HLA I complex is insufficient for differentiation of the naïve CTL. TA recognition can be followed by anergy vs. activation, depending upon the balance of co-inhibitory vs. co-stimulatory intercellular signaling across the lymphocyte-APC “immune synapse” (Figure 1). Priming, cytolytic capacity, and memory cell differentiation require predominance of co-stimulatory signaling by the TNFR superfamily of accessory surface receptors. Agonist mAb to TNFR co-stimulatory members including CD40, OX40, and CD137 are in early clinical development.75 In most cases, these mAb demonstrate synergy with other immunomodulatory therapies including the cytolytic modalities of chemotherapy and radiation; TA-targeted mAb; or T cell checkpoint inhibitors.
Figure 1.
Signaling by CD40, a TNFR expressed by APCs, dramatically magnifies APC priming capacity. Binding of CD40 on DCs by its ligand CD40L, present solely on activated CD4(+) T helper cells, triggers immunostimulatory cytokine secretion, upregulation of antigen processing machinery, and CTL priming. In HNSCC, CD40/CD40L expression on APCs/lymphocytes decreases with advancing stage. Moreover, monocytic CD40 expression increases post-operatively, suggesting this pathway is actively suppressed during HNSCC immune evasion.76 Agonist CD40 mAb have been developed to substitute for the critical role of the T helper cell in licensing DC.77 A point of theoretical controversy is that HNSCC cell lines and human tumors express CD40, where ligation promotes proliferation and angiogenesis in preclinical models.78, 79 Nonetheless, in the first clinical trial evaluating CD40 agonism with recombinant human CD40L, a patient with refractory HNSCC sustained a long-term complete response.80 CP-870,893, a humanized IgG2 mAb with CD40 agonist activity, demonstrated efficacy in melanoma during phase I evaluation.81 CP-870,893 may be more active when added to treatments which release TA, such as cytotoxic chemotherapy or radiation. Phase I combinations with carboplatin-paclitaxel or gemcitabine showed immunologic and clinical responses.82, 83
Tumor-infiltrating lymphocytes (TILs) in HNSCC display an anergic phenotype associated with low expression of the co-stimulatory TNFRs, OX40 and CD137.10 OX40 signaling supports survival, clonal expansion, inflammatory cytokine production, and memory function in T helper cells; conversely, suppression by T regulatory cells (Tregs) is relieved.84, 85 Although not explicitly evaluated in HNSCC preclinical models, OX40 agonism has arrested both immunogenic and non-immunogenic solid tumors86; however as with CD40, more robust responses were seen upon combination with cytolytic treatments.87 An OX40 agonist mAb is under phase I study in combination with radiation in breast and prostate cancers (NCT01642290; NCT01303705). CD137 signaling enhances proliferation, inflammatory cytokine production, cytolytic capacity and survival of CTLs88; like OX40, ligation of CD137 on Tregs can paradoxically release immunosuppression.89 CD137 on NK cells also co-stimulates ADCC. A significant proportion of HNSCC patients receiving cetuximab demonstrate upregulation of CD137 on NK cells, which correlating with the VV and VF FcγR IIIa polymorphisms.90 In HNSCC preclinical models, cetuximab induces CD137 upregulation on NK cells; sequential treatment with a CD137 mAb enhances cytolytic activity.90 Urelumab is a humanized IgG4 agonist mAb against CD137 which has been evaluated in dose-finding trials in melanoma, NSCLC and lymphoma. As serious hepatotoxicity limited dose-escalation, lower-dose phase I studies have resumed, including a combination trial with rituximab (NCT01471210; NCT01775631). Similarly, PF-05082566 is an IgG2 CD137 agonist mAb under evaluation in combination with rituximab (NCT01307267). In HNSCC, combinatorial trials with cetuximab, chemotherapy or radiation are warranted.
Immune Checkpoint-Targeted mAbs
The therapeutic complement to agonism of co-stimulatory TNFRs is blockade of co-inhibitory receptors. HNSCC TILs are characterized by high expression of the co-inhibitory receptors cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and programmed-death 1 (PD-1), so-called “immune checkpoints.”10, 91 CTLA-4 is a surface glycoprotein progressively expressed by CTLs following TCR ligation. CTLA-4 down-modulates immune response to chronic antigen stimulation, preventing autoimmunity; however in the case of cancer, CTLA-4 promotes inappropriate tolerance and immune escape. Tregs in the HNSCC microenvironment constitutively express CTLA-4.92 CTLA-4 and the major co-stimulatory receptor CD28 compete for B7 ligands, CD80 and CD86 (Figure 1). Thus, mAb blockade of CTLA-4 frees CD28 to bind B7 and propagate the necessary TCR co-stimulatory signal. Inhibition of Treg CTLA-4 signaling also releases CTL suppression, potentiating TA-targeted cytolysis.93 This novel therapeutic paradigm culminated in the first FDA approval of an immune checkpoint inhibitor for melanoma, an IgG4 anti-CTLA-4 mAb, ipilimumab.94 Ipilimumab has intriguing potential to reverse immunosuppression in HNSCC, alone or in combination with other immunogenic therapies. Of particular interest to trial design, CTLA-4 inhibition potentiates the abscopal effect when combined with fractionated radiotherapy: in breast and colon cancer radiation experiments, a preclinical CTLA-4 mAb inhibited out-of-field secondary tumor growth.95 A phase I trial of cetuximab, ipilimumab and intensity modulated radiotherapy in the management of locally advanced HNSCC is now accruing (NCT01860430).
Like CTLA-4, PD-1 is an inhibitory member of the B7-CD28 family of co-receptors. Following TCR activation, PD-1 is expressed by multiple immune cells including CTLs, NK cells, B lymphocytes, monocytes and DCs. The PD-1 ligand, PD-L1, is expressed broadly on non-hematopoietic tissue in response to IFN-γ; thus PD-1 ligation is thought to broadly protect against autoimmunity and immune destruction of normal tissue during infection.96 PD-L1 is also expressed in the majority of HNSCC, and is explicitly linked to the immune-privileged, invasive front of HPV-transformed HNSCC.97–99 Infiltration by PD-1 expressing T cells is associated with favorable prognosis in HPV-associated disease; this paradox highlights the importance of prior immune response and the therapeutic potential of restoring it by PD-1 blockade.91 Therapeutic targeting of PD-1 or PD-L1 block the co-inhibitory signal at the immune synapse. Nivolumab, a humanized IgG4 anti-PD-1 mAb, was investigated in advanced solid tumors.23 Objective response rates in this heavily treated population were notable in renal cell carcinoma (27%), melanoma (28%), and NSCLC (18%). An important preliminary observation correlated objective response with PD-L1+ tumors. BMS-936559, a humanized IgG4 mAb which inhibits binding of PD-L1 to both PD-1 and CD80, also resulted in objective responses in a phase I study for patients with pretreated, advanced solid tumors – including NSCLC (10%). Anti-PD-1 and PD-L1 mAb are of pressing interest for therapeutic development in HPV-positive and HPV-negative HNSCC.
Conclusion
Successful development of novel immunotherapeutic mAb in HNSCC can be guided by recent insights into the immune mechanisms of cetuximab, the first FDA-approved immunotherapy in HNSCC, as well as dissection of immune evasion by HNSCC. In addition to blocking EGFR signaling, cetuximab induces ADCC, DC maturation, and cross-priming of EGFR-specific CTLs – immune mechanisms which could be augmented by co-treatment with other TA-targeted mAb, exogenous IL-12 or TLR stimulation, mAb to neutralize immunosuppressive cytokines, mAb to enhance antigen presentation, or mAb favorably influencing co-stimulatory vs. co-inhibitory receptor signaling. However, the strategic vision for novel therapeutic mAb should not be limited to co-administration with cetuximab. HNSCC exploits numerous redundant and mutually reinforcing immune escape mechanisms, including an imbalanced STAT1/STAT3 cytokine profile, downregulation of antigen processing and presentation, underexpression of co-stimulatory receptors and overexpression of co-inhibitory receptors in TILs. Targeting these nodes of immunosuppression may be of independent therapeutic value, and holds the promise of synergy with standard cytotoxic modalities exposing TA – including chemotherapy and radiation. Clinical trials evaluating monotherapeutic and combinatorial mAb strategies, including combinations with cytolytic therapy, are of urgent priority.
Supplementary Material
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. Journal of Clinical Oncology. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Allen C, Duffy S, Teknos T, et al. Nuclear factor-kappaB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res. 2007;13:3182–3190. doi: 10.1158/1078-0432.CCR-06-3047. [DOI] [PubMed] [Google Scholar]
- 3.Leibowitz MS, Andrade Filho PA, Ferrone S, Ferris RL. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother. 2011;60:525–535. doi: 10.1007/s00262-010-0961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:3755–3762. doi: 10.1158/1078-0432.CCR-04-0054. [DOI] [PubMed] [Google Scholar]
- 5.Whiteside TL. Immunobiology of head and neck cancer. Cancer Metastasis Rev. 2005;24:95–105. doi: 10.1007/s10555-005-5050-6. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 7.Dasgupta S, Bhattacharya-Chatterjee M, O’Malley BW, Jr, Chatterjee SK. Inhibition of NK cell activity through TGF-beta 1 by down-regulation of NKG2D in a murine model of head and neck cancer. J Immunol. 2005;175:5541–5550. doi: 10.4049/jimmunol.175.8.5541. [DOI] [PubMed] [Google Scholar]
- 8.Bauernhofer T, Kuss I, Henderson B, Baum AS, Whiteside TL. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. Eur J Immunol. 2003;33:119–124. doi: 10.1002/immu.200390014. [DOI] [PubMed] [Google Scholar]
- 9.Young MR, Wright MA, Lozano Y, Matthews JP, Benefield J, Prechel MM. Mechanisms of immune suppression in patients with head and neck cancer: influence on the immune infiltrate of the cancer. Int J Cancer. 1996;67:333–338. doi: 10.1002/(SICI)1097-0215(19960729)67:3<333::AID-IJC5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Baruah P, Lee M, Odutoye T, et al. Decreased levels of alternative co-stimulatory receptors OX40 and 4–1BB characterise T cells from head and neck cancer patients. Immunobiology. 2012;217:669–675. doi: 10.1016/j.imbio.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Albaitero A, Nayak JV, Ogino T, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–3409. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 12.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–3895. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 13.Ferris R, Whiteside TL, Ferrone S. Clinical significance of downregulated antigen processing machinery in head and neck cancer. Clinical Cancer Research. 2006 doi: 10.1158/1078-0432.CCR-05-2750. in press. [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer-systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 17.Hadden J, Verastegui E, Barrera JL, et al. A trial of IRX-2 in patients with squamous cell carcinomas of the head and neck. International Immunopharmacology. 2003;3:1073–1081. doi: 10.1016/S1567-5769(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 18.De Stefani A, Forni G, Ragona R, et al. Improved survival with perilymphatic interleukin 2 in patients with resectable squamous cell carcinoma of the oral cavity and oropharynx. Cancer. 2002;95:90–97. doi: 10.1002/cncr.10654. [DOI] [PubMed] [Google Scholar]
- 19.Urba SG, Forastiere AA, Wolf GT, Amrein PC. Intensive recombinant interleukin-2 and alpha-interferon therapy in patients with advanced head and neck squamous carcinoma. Cancer. 1993;71:2326–2331. doi: 10.1002/1097-0142(19930401)71:7<2326::aid-cncr2820710725>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava RM, Lee SC, Andrade Filho PA, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albers AE, Ferris RL, Kim GG, Chikamatsu K, DeLeo AB, Whiteside TL. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at tumor sites. Cancer Immunol Immunother. 2005;54:1072–1081. doi: 10.1007/s00262-005-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuler PJ, Boeckers P, Engers R, et al. EGFR-specific T cell frequencies correlate with EGFR expression in head and neck squamous cell carcinoma. J Transl Med. 2011;9:168. doi: 10.1186/1479-5876-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albers A, Abe K, Hunt J, et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:11146–11155. doi: 10.1158/0008-5472.CAN-05-0772. [DOI] [PubMed] [Google Scholar]
- 27.Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical significance. Immunol Res. 2005;33:113–133. doi: 10.1385/IR:33:2:113. [DOI] [PubMed] [Google Scholar]
- 28.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 29.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 30.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 31.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 32.Grandis JR, Tweardy DJ. TGF-alpha and EGFR in head and neck cancer. J Cell Biochem Suppl. 1993;17F:188–191. doi: 10.1002/jcb.240531027. [DOI] [PubMed] [Google Scholar]
- 33.Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112:2710–2719. doi: 10.1002/cncr.23442. [DOI] [PubMed] [Google Scholar]
- 34.Licitra L, Storkel S, Kerr KM, et al. Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: Analysis of data from the EXTREME and CRYSTAL studies. Eur J Cancer. 2013;49:1161–1168. doi: 10.1016/j.ejca.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Licitra L, Mesia R, Rivera F, et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann Oncol. 2011;22:1078–1087. doi: 10.1093/annonc/mdq588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martins RG, Parvathaneni U, Bauman JE, et al. Cisplatin and Radiotherapy With or Without Erlotinib in Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomized Phase II Trial. J Clin Oncol. 2013;31:1415–1421. doi: 10.1200/JCO.2012.46.3299. [DOI] [PubMed] [Google Scholar]
- 37.Argiris A, Ghebremichael M, Gilbert J, et al. Phase III Randomized, Placebo-Controlled Trial of Docetaxel With or Without Gefitinib in Recurrent or Metastatic Head and Neck Cancer: An Eastern Cooperative Oncology Group Trial. J Clin Oncol. 2013;31:1405–1414. doi: 10.1200/JCO.2012.45.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lango MN, Shin DM, Grandis JR. Targeting growth factor receptors: integration of novel therapeutics in the management of head and neck cancer. Curr Opin Oncol. 2001;13:168–175. doi: 10.1097/00001622-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011;50:248–254. doi: 10.1007/s12026-011-8231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kondadasula SV, Roda JM, Parihar R, et al. Colocalization of the IL-12 receptor and FcgammaRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-gamma. Blood. 2008;111:4173–4183. doi: 10.1182/blood-2007-01-068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 42.Kim DH, Jung HD, Kim JG, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood. 2006;108:2720–2725. doi: 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 43.Varchetta S, Gibelli N, Oliviero B, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67:11991–11999. doi: 10.1158/0008-5472.CAN-07-2068. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Albaitero A, Lee SC, Morgan S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58:1853–1864. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol. 2010;184:512–520. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- 46.Vermorken JB, Stohlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 47.Giralt J, Trigo J, Nuyts S, et al. Phase 2, randomized trial (CONCERT02) of panitumumab plus radiotherapy compared with chemoradiotherapy in patients with unresected, locally advanced squamous cell carcinoma of the head and neck. Annals of Oncology. 2012;23(Supplement 9):ix334–ix347. doi: 10.1016/S1470-2045(14)71200-8. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 50.Glisson BS, Tseng JE, Marur S, et al. Randomized Phase II Trial of Cixutumumab Alone or with Cetuximab for Refractory Recurrent/Metastatic Squamous Cancer of Head and Neck. J Clin Oncol. 2013:29. [Google Scholar]
- 51.Bekaii-Saab TS, Roda JM, Guenterberg KD, et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther. 2009;8:2983–2991. doi: 10.1158/1535-7163.MCT-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Herpen CM, Looman M, Zonneveld M, et al. Intratumoral administration of recombinant human interleukin 12 in head and neck squamous cell carcinoma patients elicits a T-helper 1 profile in the locoregional lymph nodes. Clin Cancer Res. 2004;10:2626–2635. doi: 10.1158/1078-0432.ccr-03-0304. [DOI] [PubMed] [Google Scholar]
- 53.Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 54.Schnurr M, Chen Q, Shin A, et al. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105:2465–2472. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- 55.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 56.Lu H, Dietsch GN, Matthews MA, et al. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin Cancer Res. 2012;18:499–509. doi: 10.1158/1078-0432.CCR-11-1625. [DOI] [PubMed] [Google Scholar]
- 57.Stephenson RM, Lim M, Matthews M, Dietsch G, Hershberg R, Ferris RL. TLR8 stimulation enhances cetuximab-mediated natural killer cell lysis of ead andn eck cancer cells and dendritic cell cross-priming of EGFR-specific CD8+ T cells. Cancer Immunol Immunother. 2013 doi: 10.1007/s00262-013-1437-3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 59.Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97–102. doi: 10.1016/j.imlet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 62.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 63.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 64.Leef G, Thomas SM. Molecular communication between tumor-associated fibroblasts and head and neck squamous cell carcinoma. Oral Oncol. 2013;49:381–386. doi: 10.1016/j.oraloncology.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singhal E, Sen P. Hepatocyte growth factor-induced c-Src-phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway inhibits dendritic cell activation by blocking IkappaB kinase activity. Int J Biochem Cell Biol. 2011;43:1134–1146. doi: 10.1016/j.biocel.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 67.Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:624–630. doi: 10.1007/s00432-005-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lionello M, Staffieri A, Marioni G. Potential prognostic and therapeutic role for angiogenesis markers in laryngeal carcinoma. Acta Otolaryngol. 2012;132:574–582. doi: 10.3109/00016489.2011.652308. [DOI] [PubMed] [Google Scholar]
- 69.Argiris A, Kotsakis AP, Hoang T, et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2012 doi: 10.1093/annonc/mds245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun S, Wang Z. Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129:2337–2348. doi: 10.1002/ijc.25927. [DOI] [PubMed] [Google Scholar]
- 71.Knowles LM, Stabile LP, Egloff AM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15:3740–3750. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Bacco F, Luraghi P, Medico E, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst. 2011;103:645–661. doi: 10.1093/jnci/djr093. [DOI] [PubMed] [Google Scholar]
- 73.Bedi A, Chang X, Noonan K, et al. Inhibition of TGF-beta enhances the in vivo antitumor efficacy of EGF receptor-targeted therapy. Mol Cancer Ther. 2012;11:2429–2439. doi: 10.1158/1535-7163.MCT-12-0101-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossi JF, Negrier S, James ND, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–1053. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sathawane D, Kharat RS, Halder S, et al. Monocyte CD40 expression in head and neck squamous cell carcinoma (HNSCC) Hum Immunol. 2013;74:1–5. doi: 10.1016/j.humimm.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Posner MR, Cavacini LA, Upton MP, Tillman KC, Gornstein ER, Norris CM., Jr Surface membrane-expressed CD40 is present on tumor cells from squamous cell cancer of the head and neck in vitro and in vivo and regulates cell growth in tumor cell lines. Clin Cancer Res. 1999;5:2261–2270. [PubMed] [Google Scholar]
- 79.Cao W, Cavacini LA, Tillman KC, Posner MR. CD40 function in squamous cell cancer of the head and neck. Oral Oncol. 2005;41:462–469. doi: 10.1016/j.oraloncology.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 80.Vonderheide RH, Dutcher JP, Anderson JE, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 81.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 82.Vonderheide RH, Burg JM, Mick R, et al. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology. 2013;2:e23033. doi: 10.4161/onci.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 85.Kitamura N, Murata S, Ueki T, et al. OX40 costimulation can abrogate Foxp3+ regulatory T cell-mediated suppression of antitumor immunity. Int J Cancer. 2009;125:630–638. doi: 10.1002/ijc.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 87.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pollok KE, Kim YJ, Zhou Z, et al. Inducible T cell antigen 4–1BB. Analysis of expression and function. J Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 89.So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4–1BB. Cytokine Growth Factor Rev. 2008;19:253–262. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kohrt HE, Houot R, Goldstein M, et al. Targeting CD137 to enhance the antitumor efficacy of cetuximab by stimulation of innate and adaptive immunity. J Clin Oncol. 2013:31. [Google Scholar]
- 91.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 92.Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 93.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quezada SA, Peggs KS. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br J Cancer. 2013;108:1560–1565. doi: 10.1038/bjc.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47:1148–1153. doi: 10.1016/j.oraloncology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 98.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a Role of the PD-1:PD-L1 Pathway in Immune Resistance of HPV-Associated Head and Neck Squamous Cell Carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.