Abstract
Objective
We and others previously demonstrated that sirtuin 1 (SIRT-1) regulates apoptosis and cartilage-specific gene expression in human chondrocytes and mouse models. This study was undertaken to determine if SIRT-1 enzymatic activity plays a protective role in cartilage homeostasis in vivo, by investigating mice with SIRT-1 mutations to characterize their cartilage.
Methods
Articular cartilage was harvested from the paws and knees of 5- and 6-month-old wild-type (WT) mice and mice homozygous for SIRT-1tm2.1Mcby (SIRT-1y/y), an allele carrying a point mutation that encodes a SIRT-1 protein with no enzymatic activity (y/y mice). Mice ages 2 days old and 6–7 days old were also examined. Mouse joint cartilage was processed for histologic examination or biochemical analyses of chondrocyte cultures.
Results
We found that articular cartilage tissue sections from y/y mice of up to 6 months of age contained reduced levels of type II collagen, aggrecan, and glycosaminoglycan compared to sections from WT mice. In contrast, protein levels of matrix metalloproteinase 8 (MMP-8), MMP-9, and MMP-13 were elevated in the cartilage of y/y mice. In addition, chondrocyte apoptosis was elevated in SIRT-1 mutant mice as compared to their WT littermates. Consistent with these observations, protein tyrosine phosphatase 1b was elevated in the y/y mice.
Conclusion
Our in vivo findings in this animal model demonstrate that mice with defective SIRT-1 also have defective cartilage, with elevated rates of cartilage degradation with age. Hence, normal cartilage homeostasis requires enzymatically active SIRT-1 protein.
Osteoarthritis (OA) is a multifactorial and complex degenerative disease of the cartilage. Different mechanisms are involved in cartilage degradation, including inflammation, apoptosis, and breakdown of major extracellular matrix (ECM) components, such as type II collagen and aggrecan (1). Aging is one of the most important risk factors linked to OA susceptibility (2,3). More than 50% of adults ages 65 or older reported an arthritis diagnosis, making OA one of the most common diseases in developed countries (4).
Sirtuin 1 (SIRT-1) has been shown to regulate the lifespan and aging in simple eukaryotes (2,5,6). In mammals, SIRT-1 has been reported to play an important role in age-related diseases, such as osteoporosis, diabetes, and cancer (6–8). We have previously shown in vitro that SIRT-1 modulates gene expression in human chondrocytes. SIRT-1 elevates the expression of genes encoding the cartilage ECM in human chondrocytes (9) and enhances the survival of human OA chondrocytes by repressing apoptosis (10,11). Recently, SIRT-1 has been reported to be involved in the pathogenesis of OA by modulating chondrocyte gene expression and hypertrophy (12). Finally, SIRT-1 plays an antiinflammatory role in different tissues by inhibiting the transcription of proinflammatory genes (6).
So far, in vitro studies have suggested a protective role of SIRT-1 in cartilage; however, very few studies have demonstrated these roles in vivo. We previously created SIRT-1–null mice (13,14) and found that they were smaller than wild-type (WT) mice, had craniofacial abnormalities, and their long bones mineralized slower than normal (15). These observations were consistent with the notion that SIRT-1 may play a role in cartilage development and homeostasis. Additional analysis of heterozygous SIRT-1 mice (SIRT-1+/−) showed that they have elevated levels of apoptotic chondrocytes and develop more severe OA with age compared to WT mice (16).
In this study, we investigated the articular cartilage of mice carrying a SIRT-1 protein lacking enzymatic activity (SIRT-1tm2.1Mcby or SIRT-1y/y) and found that these mutant mice were predisposed to develop OA, especially with age. Our results showed an altered cartilage phenotype and an acceleration of the OA process in these SIRT-1 mutant mice due to a higher level of cartilage breakdown and apoptosis (17).
MATERIALS AND METHODS
Animals
Animals carrying a point mutation, SIRT-1tm2.1Mcby (SIRT-1y/y), which ablates SIRT-1 enzymatic activity, and their littermates were used in the experiments. The point mutation encodes a SIRT-1(H355Y) protein with undetectable enzymatic activity, as described by Seifert et al (17). Animals homozygous for this point mutation are referred to as SIRT-1y/y (y/y) mice. All animals were females ages 2 days, 6–7 days, 5 months, or 6 months, and a minimum of 3 animals per strain were used in each experiment. Animals were housed under standard conditions of temperature and light and were fed a standard laboratory diet, with water ad libitum. All procedures were performed in accordance with the National Institutes of Health (NIH) Committees for Animal Use and Care guidelines.
Primary mouse cell culture and cell count
Methods for the isolation of costal cartilage from 6–7-day-old y/y or WT mice and chondrocyte plating were adapted from those described previously (18). Briefly, pieces of costal cartilage were incubated in 3 mg/ml collagenase for 1 hour and 30 minutes at 37°C, carefully isolated from soft tissue in phosphate buffered saline (PBS), and incubated in 0.5 mg/ml collagenase overnight. Chondrocytes were washed, centrifuged, and plated for a 7–10-day period during which they were allowed to propagate. Chondrocytes from passages 0 and 1 were used in the experiments because chondrocytes from these passages maintain their phenotypes. Monolayer cultures were maintained in culture conditions as previously described (18), and growth medium was replaced every 3 days. Prior to analysis, cells were rinsed with PBS, trypsinized with trypsin 0.25%, resuspended in 10 ml of growth medium, and counted with a hemocytometer using a trypan blue exclusion assay.
Protein extraction and immunoblotting
Whole-cell protein extracts from chondrocytes from the hyaline cartilage of 6–7-day-old mice were obtained using the method of Dvir-Ginzberg et al (9). The protein extracts were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10 µg protein/lane) and transferred onto PVDF membranes for immunoblotting. The blots were processed as previously described (9) and probed with antibodies. Blots were developed using an alkaline phosphatase–conjugated secondary antibody and BCIP/nitroblue tetrazolium solution (Invitrogen). Antibodies against protein tyrosine phosphatase 1b (PTP1b), matrix metalloproteinase 13 (MMP-13), MMP-8, and β-actin were purchased from Abcam, SIRT-1 antibody was from Upstate/Millipore, MMP-9 antibody was from R&D Systems, and acetylated NF-κB p65 antibody was from Sigma-Aldrich.
Histologic and immunohistochemical analyses
For immunohistochemical analysis, cartilage samples were fixed in 4% paraformaldehyde for 24–48 hours, dehydrated in a graded series of ethanol baths, embedded in paraffin, and cut into 5-µm sections. Slides were rehydrated and incubated with antibodies specific for mouse anti-aggrecan; mouse anti–type II collagen (Millipore); mouse anti-PTP1b (Abcam); mouse anti–MMP-1, MMP-3, MMP-8, MMP-9, MMP-13, ADAMTS-4, or ADAMTS-5 (all from Abcam); mouse anti–acetylated H4K16 (Cell Signaling Technology); or rabbit anti–acetylated NF-κB p65 (Sigma-Aldrich) and visualized using a broad-spectrum immunohistochemistry kit (diaminobenzidine; Invitrogen).
Sections were stained with Alcian blue to test for the presence of glycosaminoglycans (GAGs) and with Safranin O–light green to examine the subchondral bone and detect OA. Joints of the paws and knees were examined at 5 and 6 months. To identify cells undergoing apoptosis, TUNEL labeling was performed on paraffin-embedded sections, using a TUNEL Apoptosis Detection Kit (for Paraffin-embedded Tissue Sections, Biotin-labeled POD) according to the recommendations of the manufacturer (GenScript). Cell proliferation was examined by bromodeoxyuridine (BrdU) incorporation, carried out with an Amersham cell proliferation labeling reagent, according to the recommendations of the manufacturer (GE Healthcare).
Cartilage defects at 6 months were assessed in accordance with the OA Research Society International (OARSI) histopathology initiative recommendations for histologic assessment of OA in mice (19) and recommendations for grading and scoring of OA cartilage histopathology (20). Immunohistochemical analysis of cartilage breakdown was performed using the Coll2-1 peptide biomarker for collagen breakdown (Artialis), and the results were analyzed according to the method of Ameye et al (21).
Total skeletal staining was performed with basic ethanol–KOH:glycerol alizarin red–Alcian blue, as described by Depew et al (22). Results were analyzed using the Color Atlas of Fetal Skeleton of the Mouse, Rat, and Rabbit (23).
Statistical analysis
Results are presented as the mean ± SD. Statistical analysis was performed using one-way analysis of variance, assuming confidence levels of ≤95% (P ≤ 0.05) to be statistically significant. For immunoblot analysis, 3 different experiments were each repeated twice. For immunohistochemical analysis, the percentage of positively stained cells was determined; stained cells reflected a >10-fold intensity above the background, as determined by scanning densitometry (ImageJ software [NIH; online at http://rsbweb.nih.gov/ij/]). A scoring method adapted from Lee et al (24) was used to evaluate the staining of the tissue matrix. An average of 3 fields in 3 separate sections (1 field per section) from 3 different animals per genotype (y/y or WT) was assessed. Each field was read by 2 different readers in a blinded manner. Cartilage defects were scored in accordance with the recommendations of the OARSI histopathology initiative for scoring OA in mice (19).
RESULTS
Reduced cartilage SIRT-1 enzymatic activity and presence of defects in the cartilage of the skull, spine, rib cages, and joints in y/y mice
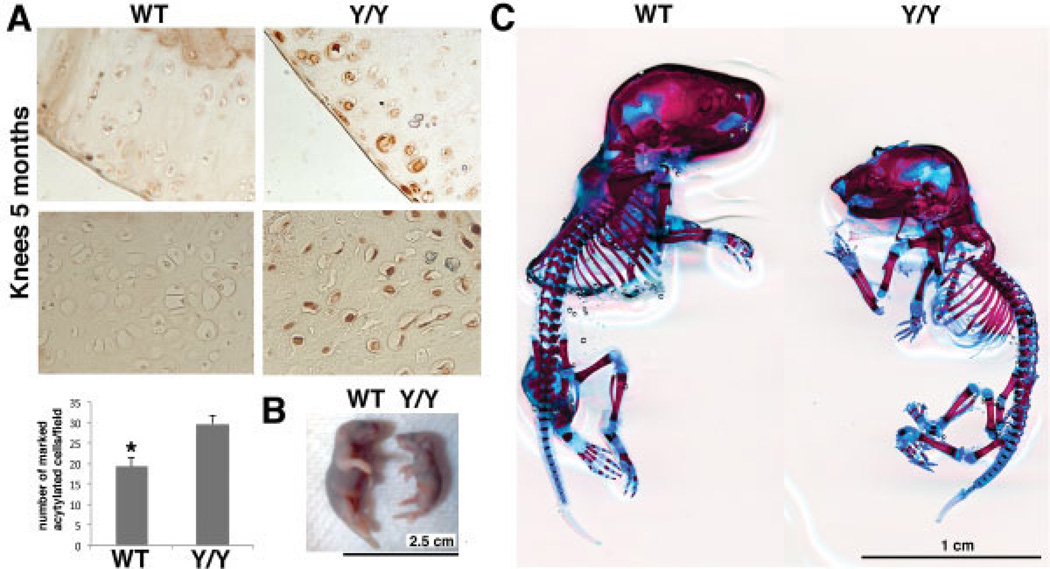
We first confirmed a reduction in SIRT-1 enzymatic activity in the cartilage of the y/y mouse strain by comparing the acetylation of histones in the y/y and WT mice (Figure 1A). Consistent with mouse genotyping, H4K16 staining showed a higher level of acetylation in the cartilage of y/y mice, indicating a lower level of deacetylation of the SIRT-1 histone targets.
Figure 1.
Cartilage phenotype of SIRT-1y/y (y/y) mice. A, Top, Immunohistochemical staining for acetylated H4K16 in knee sections from 5-month-old wild-type (WT) and y/y mice. Top panels show the cartilage surface; bottom panels show the cartilage midzone. Original magnification × 20. Bottom, Number of acetylated H4K16–stained cells per field in WT and y/y mice. An average of 6 fields per section in 3 sections from 3 separate mice per genotype was assessed. The mean number of positively stained cells was increased 1.5 times in the y/y mouse chondrocytes compared to the WT mouse chondrocytes, indicating that the enzymatically inactive SIRT-1 in y/y mice is unable to deacetylate its histone target. Bars show the mean ± SD. * = P = 0.007 versus y/y mice. B, Comparison of the size of a 2-day-old WT mouse and a 2-day-old y/y mouse before skeletal staining. C, Alizarin red–Alcian blue skeletal staining of 2-day-old animals, showing cartilage disparities in the skull, spine, and large joints; disorganization of the rib cages; and major defects of the eyes and all joint cartilage in the y/y mouse.
Skeletal staining of the y/y mice (Figure 1C) confirmed that they were smaller than their normal littermates and had craniofacial and eye defects, as previously reported (13,15). The finding that SIRT-1 enzymatic activity was reduced in the y/y mice (Figure 1A) confirmed that lack of SIRT-1 enzymatic activity plays a role in cartilage-dependent developmental defects (Figure 1B).
We further examined the effect of SIRT-1 enzymatic activity on the skeleton and cartilage (results are available from the corresponding author upon request). The y/y mice had less cartilage in the skull, rib cage, spine, and knee joints, which was attributed to their smaller size (Figure 1C). They exhibited less bone mineralization, suggesting that cartilage hypotrophy results in delayed osteogenesis. Interestingly, the sutures of the calvariae in the y/y mice were not fixed, unlike those in the WT mice. These findings were similar in all of the y/y mice analyzed and in all of the WT mice analyzed (n = 3 mice per group). Interestingly, the y/y mice had more severe matrix disorganization of the rib cage, defects of the eyes, and were smaller compared to WT mice of the same age.
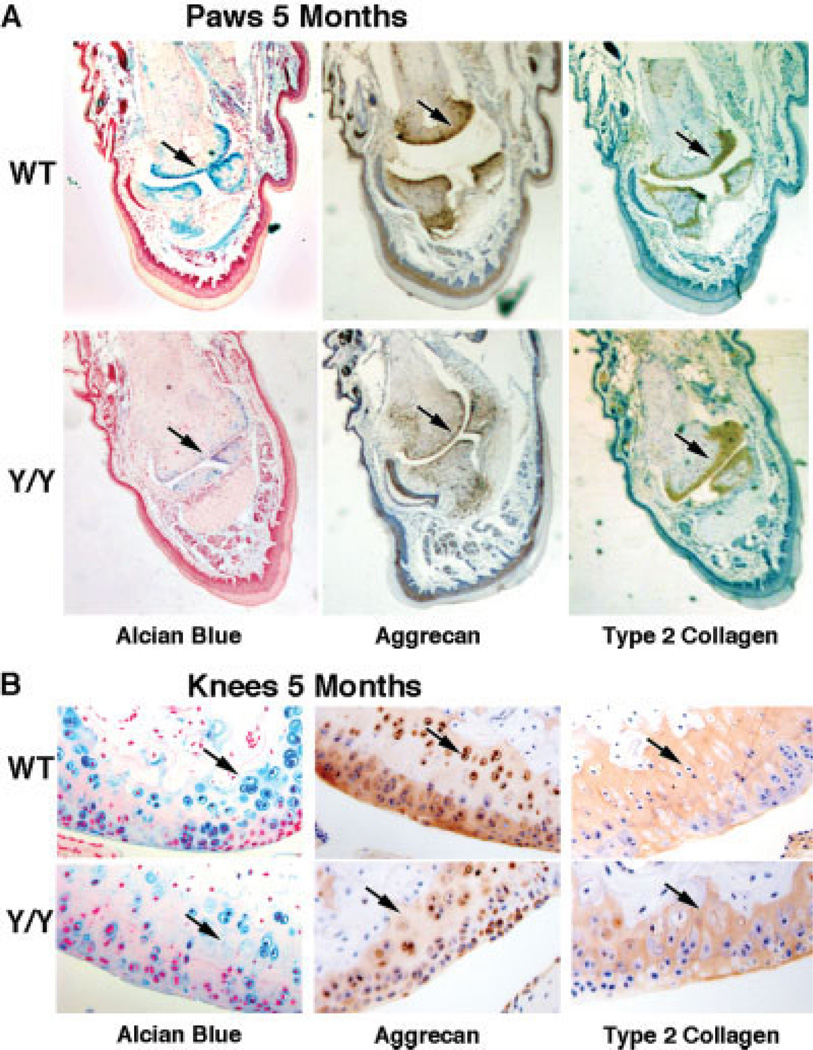
Reduced levels of type II collagen, aggrecan, and GAG in joint articular cartilage tissue from y/y mice compared to WT mice
We investigated the skeletal structure of adult y/y mice (17). Alcian blue staining was carried out to visualize GAG content, which is a hallmark of healthy cartilage. There was less GAG in the paws and knees of y/y mice than in the paws and knees of WT mice at 5 months (Figures 2A and B). The 6-month-old adult y/y animals also had less GAG in the paws and knees than their WT littermates (results not shown). To examine levels of aggrecan and type II collagen, immunohistochemical staining of cartilage was carried out and quantified using densitometry and statistical analysis (Figures 2A and B). Overall, the joints of y/y mice showed less Alcian blue, aggrecan, and type II collagen staining (65%, 50%, and 19% reduction, respectively) than WT mice at 5 months of age.
Figure 2.
Lower glycosaminoglycan (GAG), aggrecan, and type II collagen content in articular cartilage from SIRT-1y/y (y/y) mice than their wild-type (WT) littermates. A, Alcian blue staining (for GAG content), aggrecan staining, and type II collagen staining of paw sections from 5-month-old WT and y/y mice. Alcian blue, aggrecan, and type II collagen staining intensity were reduced by 65%, 50%, and 19%, respectively, in the paws of y/y mice compared to those of WT mice. Arrows indicate the difference in staining intensity for each marker. Original magnification × 10. B, Alcian blue staining (for GAG content), aggrecan staining, and type II collagen staining of knee sections from 5-month-old WT and y/y mice. Staining intensity was reduced in the knees of the y/y mice, similar to the findings in paws. Arrows indicate the difference in staining intensity for each marker. Original magnification × 16.
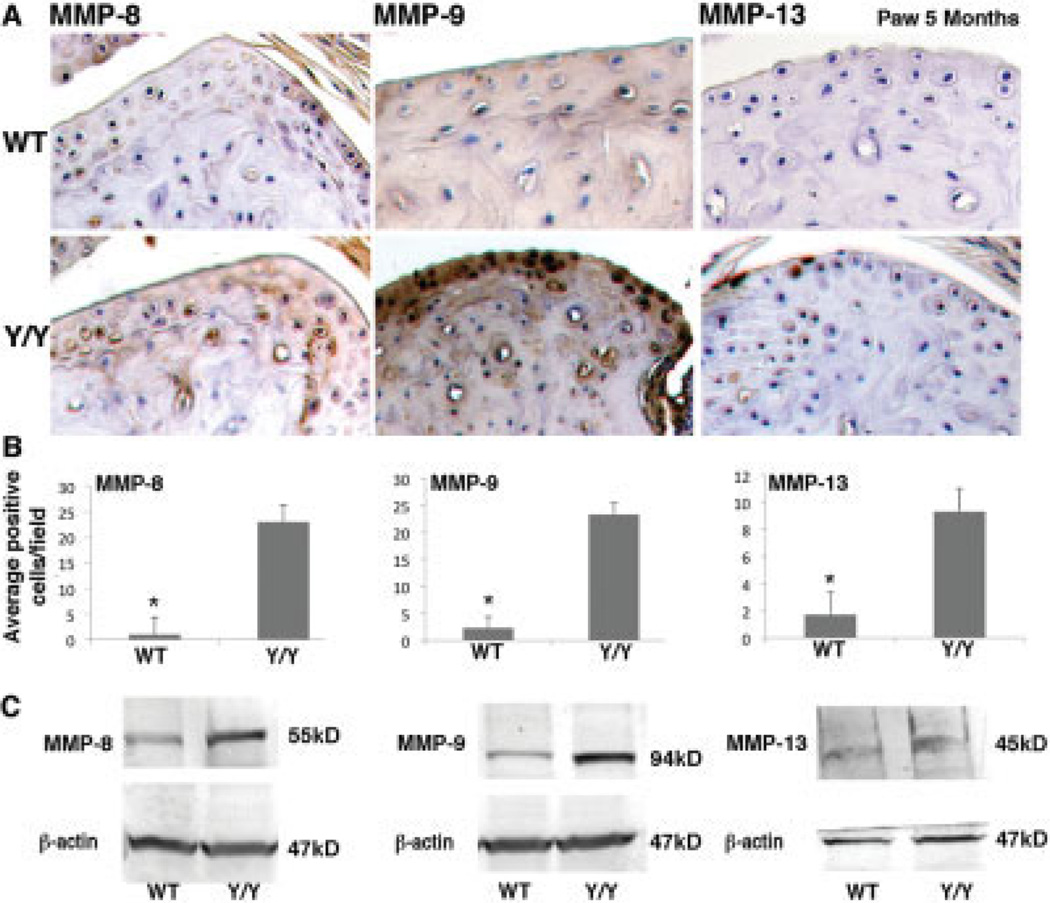
Elevated MMP-8, MMP-9, and MMP-13 protein levels in y/y mice compared to WT mice
To determine whether the catalytic enzymes involved in cartilage degeneration were elevated in the y/y mice, we performed immunohistochemical staining for MMP-1, MMP-3, MMP-8, MMP-9, MMP-13, ADAMTS-4, and ADAMTS-5, all of which have been shown to be elevated in OA (25). Comparisons of the WT and y/y mice at 5 months revealed a statistically significant increase in MMP-8, MMP-9, and MMP-13 protein expression in the cartilage of y/y mice, as determined by immunostaining and Western blot analysis (Figures 3A–C). The y/y mice and WT mice showed similarly low levels of immunostaining for MMP-1, MMP-3, ADAMTS-4, and ADAMTS-5 at 5 months of age (results not shown).
Figure 3.
Elevated levels of matrix metalloproteinase 8 (MMP-8), MMP-9, and MMP-13 protein in SIRT-1y/y (y/y) mouse cartilage. A, Immunohistochemical staining for MMP-8, MMP-9, and MMP-13 in the paws of 5 month-old wild-type (WT) and y/y mice. Hematoxylin counterstained; original magnification × 20. B, Percent of cells stained positively for MMP-8, MMP-9, and MMP-13 in the WT and y/y mice. The percent of positively stained cells per field was determined for an average of 6 fields in 3 sections from 3 separate mice per genotype. Bars show the mean ± SD. * = P = 5.0416 × 10−6 for MMP-8, P = 7.651 × 10−6 for MMP-9, and P = 1.7189 × 10−5 for MMP-13, versus y/y mice. C, Immunoblotting of protein extracts from WT and y/y mice for MMP-8, MMP-9, and MMP-13 protein levels. Results are representative of 3 different experiments and were consistent with the results of the immunohistochemical analysis.
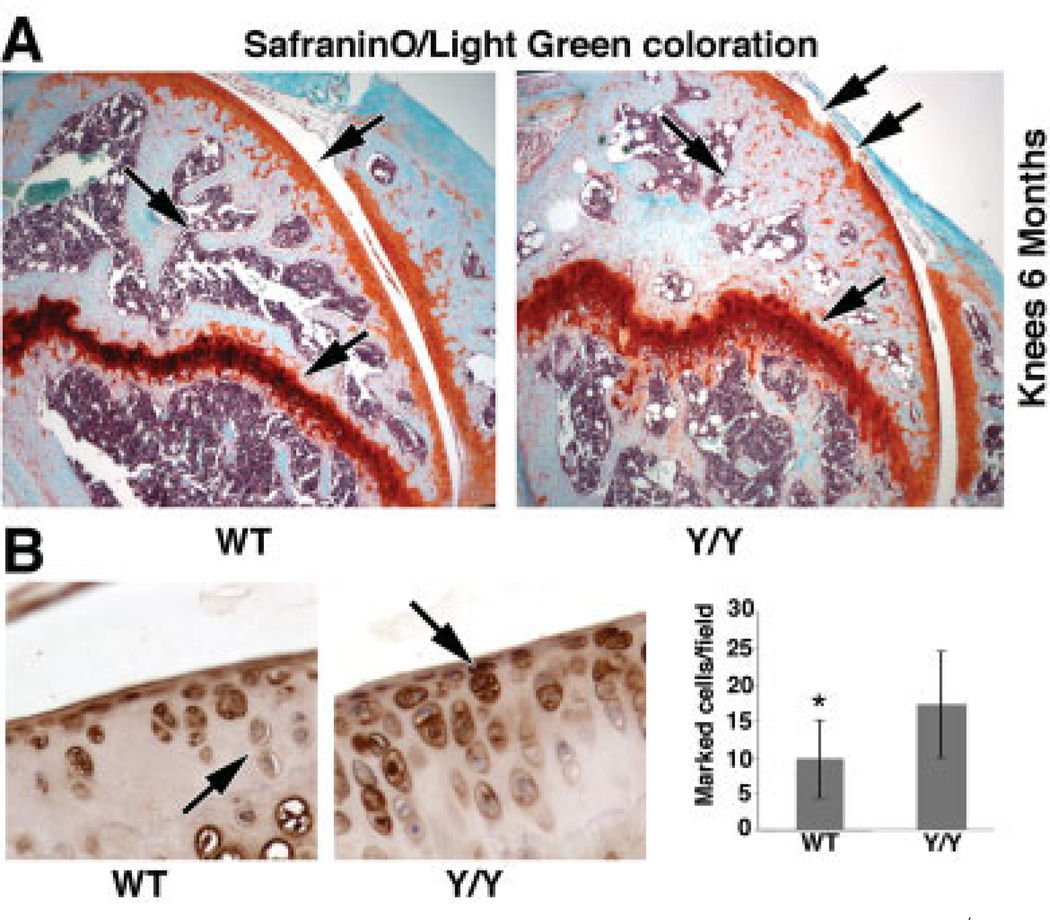
Bone defects and moderate local inflammation of the joint in y/y mice
To examine mouse knee articular cartilage, we used Safranin O–light green staining. As shown in Figure 4A, y/y mice exhibited differences in articular cartilage, growth plate, and subchondral bone morphology compared to WT mice at 6 months. More specifically, the cartilage appeared thinner and eroded in the y/y mice, and the subchondral bone had less trabecular bone volume and thicker trabeculae than in the WT mice. A distinct difference in growth plate thickness was also observed, with a thicker growth plate in the y/y mice (Figure 4A). Immunohistochemical analysis using acetylated NF-κB p65 antibody showed elevated nuclear staining in the y/y mice as compared to their littermates, suggesting that NF-κB may be hyper-activated in the y/y chondrocytes, illustrating a local inflammation due, at least in part, to the absence of SIRT-1 histone deacetylase activity. Moderate local inflammation was seen in 6-month-old WT mice, consistent with an early stage of OA; however, the local inflammation was significantly more severe in the y/y mice (Figure 4B). No systemic inflammation was observed at this age.
Figure 4.
Bone defects and local inflammation in the SIRT-1y/y (y/y) mice. A, Histologic staining of knee sections from 6-month-old wild-type (WT) and y/y mice with Safranin O–light green, showing some erosion and a thinner cartilage layer in the y/y mice. The subchondral bone showed less trabecular bone volume, thicker trabeculae, and thicker growth plate (arrows) in y/y mice compared to WT mice. Original magnification × 16. B, Left, Immunohistochemical staining of knee sections from 6-month-old WT and y/y mice for acetylated NF-κB p65. Arrows indicate the difference in staining intensity. Original magnification × 20. Right, Percent of acetylated p65–stained cells per field in WT and y/y mice. An average of 3 fields in 3 sections from 3 separate mice per genotype was assessed. The mean percent of acetylated p65–stained cells per field was increased 1.7 times in the y/y mice compared to the WT mice, indicating that the enzymatically inactive SIRT-1 in the y/y mice is unable to deacetylate its histone target. Bars show the mean ± SD. * = P = 0.042 versus y/y mice.
Significantly higher level of cartilage breakdown in y/y mice than in WT mice
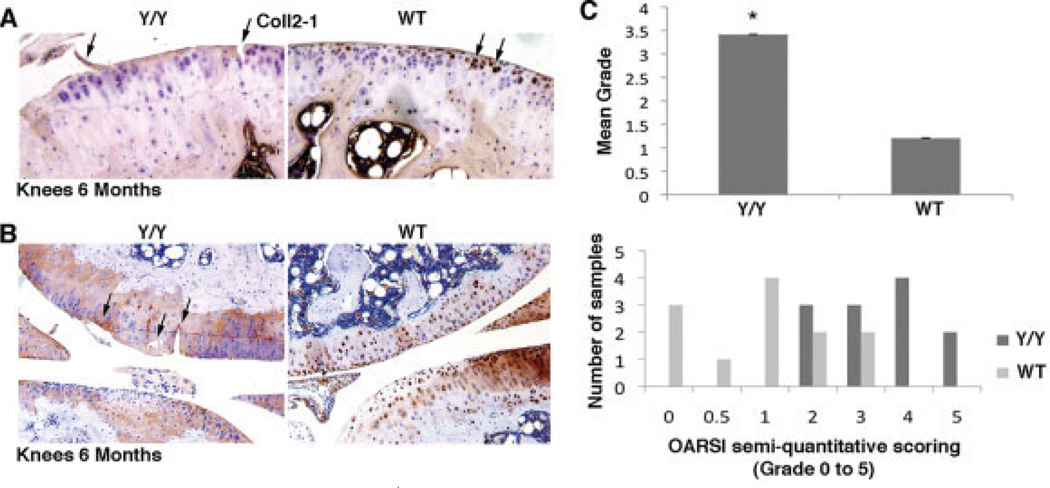
The findings described above suggest that the cartilage degeneration in y/y mice occurred through elevated levels of MMPs and reduced levels of GAGs and type II collagen. We further examined cartilage degeneration using Coll2-1, which is a degradation product of type II collagen and thus serves as a biomarker for cartilage degeneration (20). The WT mouse cartilage showed Coll2-1 cellular localization, but the y/y mouse cartilage showed diffuse matrix staining (Figure 5A). Intracellular staining by Coll2-1 is characteristic of healthy cartilage, whereas diffuse staining of the ECM surrounding the cells is characteristic of unhealthy cartilage (21). Additional analyses were carried out, and cartilage degradation was scored in accordance with the OARSI initiative recommendations for scoring of OA in adult mice (Figures 5B and C). The y/y mice had elevated levels of cartilage degeneration at 6 months, with a mean score of 3.5, corresponding to moderate OA, whereas their WT littermates had a mean score of 1, corresponding to early-stage OA (Figure 5C).
Figure 5.
Elevated rates of cartilage breakdown in SIRT-1y/y (y/y) mice. A, Immunohistochemical staining of knee sections from 6-month-old y/y and wild-type (WT) mice with antibody against the Coll2-1 breakdown peptide. An average of 6 fields in 3 sections from 3 separate mice per genotype was assessed, and representative fields are shown. Erosion of cartilage surface (arrows) and diffuse brown staining around the defects was observed in the y/y mice, whereas the staining was strongly intracellular in the WT mice. Original magnification × 20. B, Anti-aggrecan antibody staining of knee sections from 6-month-old y/y and WT mice, showing significantly higher cartilage degradation in the y/y mice than in the WT mice, with cartilage defects (arrows), such as erosions of the cartilage surface, deeper clefts, and fibrillations. Thirty-six knee sections from each genotype were examined; representative results are shown. Original magnification × 16. C, Cartilage degradation grade, determined in Safranin O–light green–stained sections, for y/y and WT mice. The scoring system was adapted from the Osteoarthritis Research Society International (OARSI) semiquantitative scoring system for OA severity. A score of 0 indicates no degradation; a score of 5 indicates denuded cartilage surface with sclerotic bone. The mean scores were 3.41 for the y/y mice and 1.2 for the WT mice. Twelve samples for each genotype (2 sections of 2 different knees from 3 different mice per genotype) were scored, showing more severe grades for the y/y knees. Bars show the mean ± SD. * = P < 0.05 versus WT mice.
Enhanced apoptosis and elevated PTP1b levels in the cartilage of y/y mice compared to WT mice
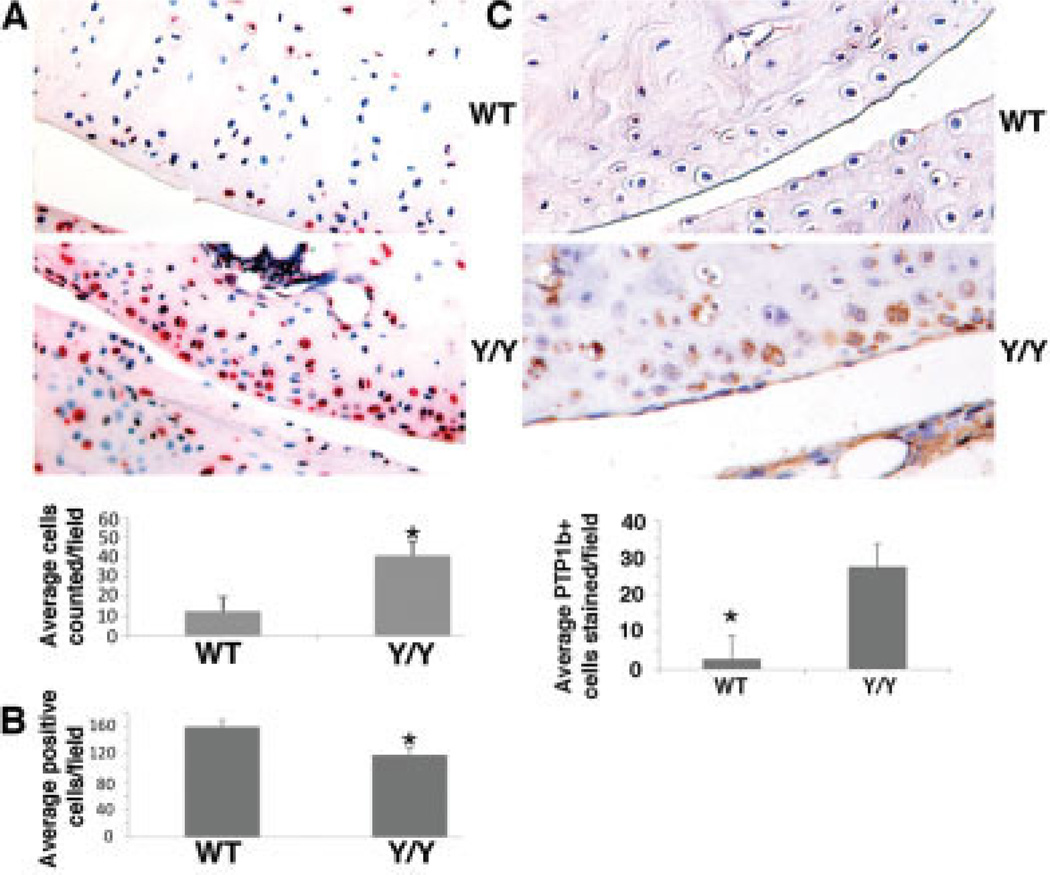
A TUNEL assay of WT and y/y mouse cartilage sections showed significantly more chondrocytes positive for apoptosis in y/y mouse cartilage than in WT mouse cartilage (Figure 6A). Further quantification of chondrocyte numbers within a given cartilage section and in equivalent cartilage regions revealed that the number of chondrocytes per cartilage area was significantly lower in the y/y mice than in the WT mice (Figure 6B). These data support the notion that SIRT-1 contributes to chondrocyte proliferation, and lack of SIRT-1 activity leads to limited growth and repair of cartilage. To confirm these findings, we monitored the rate of chondrocyte division by BrdU incorporation in histologic sections and found a reduced rate of BrdU incorporation in the y/y mouse chondrocyte cell culture as compared to the WT controls (results are available from the corresponding author upon request). As previously reported by Gagarina et al (10), SIRT-1 may prevent chondrocyte apoptosis in a PTP1b–dependent manner. To examine PTP1b expression in vivo, immunohistochemical analysis was carried out. Immunohistochemical analysis of articular cartilage from 5-month-old y/y mice showed PTP1b levels ~10-fold higher than those in WT mouse cartilage (Figure 6C).
Figure 6.
Increased number of apoptotic chondrocytes in SIRT-1y/y (y/y) mice compared to wild-type (WT) mice. A, Top, Sections of knee cartilage from 6-month-old WT and y/y mice. Sections were subjected to a TUNEL assay, and the number of chondrocytes per cartilage region was determined. Original magnification × 10. Bottom, Number of chondrocytes per field in WT and y/y mice. The number of positively stained cells was determined in 3 different sections in 2 different knees of 3 separate mice per genotype. A significantly higher level of apoptosis was observed in the y/y mice than in the WT mice. Bars show the mean × SD. * = P = 0.00238 versus WT mice. B, Number of chondrocytes per cartilage area in the superficial zone of the knee in WT and y/y mice. Cells were counted in 50-µm2 fields in 3 histologic panels for 3 separate mice per genotype. Significantly fewer cells were seen in the y/y mice. Bars show the mean ± SD. * = P = 0.002 versus WT mice. C, Top, Immunohistochemical staining of knee sections from 5-month-old WT and y/y mice for protein tyrosine phosphatase 1b (PTP1b). Original magnification × 10. Bottom, Percent of positively stained cells per field in WT and y/y mice. An average of 6 fields from 3 sections of 3 separate mice per genotype was assessed. Enhanced PTP1b intensity was observed in the y/y mice as compared to the WT mice of the same age (10-fold increase). Bars show the mean ± SD. * = P = 0.0002426 versus y/y mice.
DISCUSSION
A growing body of evidence suggests that the protein deacetylase SIRT-1 plays an important role in cartilage biology (9,10,15,26,27). The present study demonstrates that mice lacking SIRT-1 enzymatic activity possess an altered cartilage phenotype, which is consistent with our previous findings in 9-month-old SIRT-1+/− heterozygous mice (16). Further, we described two different phenomena underlying this phenotype: an increase in chondrocyte apoptosis, and an accelerated rate of cartilage breakdown in these SIRT-1–deficient mice.
The SIRT-1 mutant mice, called y/y mice, were smaller in size compared to WT mice of the same age, which is consistent with the results of previous studies (13,14,28). We hypothesize that the skeletal defects seen in y/y mice at 6 months could be a result of altered cartilage metabolism attributed to variations in skeletal growth and leading to loss of cartilage homeostasis and cellularity with age.
In this study, we used y/y mice, which express a SIRT-1 point mutation that ablates SIRT-1 enzymatic activity (17). Overall, our findings in 6-month-old y/y mice were similar to those in 9-month-old SIRT-1+/− animals. Both had severe OA and elevated chondrocyte cell death with age (16). Here we conclude that much of the age-related cartilage phenotype can be attributed to SIRT-1 enzymatic activity. In order to evaluate a possible cartilage phenotype in the y/y mice, we first analyzed SIRT-1 activity in the cartilage of these mice by monitoring histone acetylation of the SIRT-1 target H4K16 in histology slides of articular cartilage derived from y/y mice and WT mice at 5 months. The results showed a 33% increase in acetylated H4K16 in y/y mice versus WT control (P = 0.0073), consistent with the notion that SIRT-1 enzymatic activity is abrogated in y/y mouse cartilage (Figure 1A). It is now well known that SIRT-1 preferentially deacetylases H4K16, even if H4K16 is not its only target. In this experiment, we confirmed the reduction of enzymatic activity in y/y mouse cartilage. Since the cartilage phenotype is strong in this strain, we conclude that SIRT-1 enzymatic activity is necessary for cartilage protection, and that the lack of SIRT-1 plays a major role in the acceleration of the OA process with age.
We previously showed that SIRT-1 enhances the survival of human OA chondrocytes by repressing PTP1b and activating the insulin-like growth factor receptor pathway (10,15). Previous studies have consistently shown that inhibitors of SIRT-1 and SIRT-2 activity induce cell death through hyperacetylated p53 (29). Di Renzo et al recently proposed a cascade linking skeletal defects and apoptosis to histone hyperacetylation (30). Accordingly, and consistent with our results, altering SIRT-1 activity and levels could lead to enhanced cell death via multiple mechanisms and diminish the protective effect SIRT-1 has on chondrocyte viability in vivo as well as in vitro.
To date, very few studies have demonstrated the role of SIRT-1 in vivo with regard to cartilage biology or age-induced arthritis. Our recent study of WT and haploinsufficient SIRT-1+/− mice compared musculoskeletal features, OA severity scores, and apoptosis in the cartilage in 1-month-old and 9-month-old mice. The results showed a significant decrease in SIRT-1 protein levels in the heterozygous mice compared to their littermates at 1 month. Interestingly, both strains ceased to express full-length SIRT-1 at 9 months with the appearance of the cleaved inactive 75-kd SIRT-1 variant (16,26,27). Also, 9-month-old SIRT-1+/− mice presented enhanced OA severity and chondrocyte apoptosis compared to age-matched WT mice.
An accelerated cartilage degeneration process was observed in y/y mice. Accumulation of MMPs in the cartilage and low levels of type II collagen and aggrecan could predict very weak mechanical properties of cartilage in these mice and susceptibility to cartilage degeneration. Our study showed increased MMP levels, with reduced ECM content in the y/y mice until 6 months of age. However, it is not yet clear if the decreased intensity of aggrecan, type II collagen, and GAG staining observed in the present study was due to the decrease in the cellularity within the cartilage tissue in y/y mice. These data are consistent with the increased severity of OA observed in these mice, especially with age. However, mechanically induced OA, such as the commonly established destabilization of the medial meniscus or anterior cruciate ligament transection models, could possibly show more extreme variations in OA severity in these SIRT-1 mutant mice versus WT controls, rather than natural cartilage occurrences attributed to SIRT-1. Whereas MMP levels were elevated in y/y mice, ADAMTS-4 and ADAMTS-5 aggrecanase levels were slightly increased in both y/y and WT mice and correlated with cartilage breakdown in these mouse strains. It appears that type II collagen breakdown and MMP-13 were most affected and correlated well with OA severity in the SIRT-1–deficient mice.
Recently, the involvement of SIRT-1 in age-related diseases has been described in humans, as well as the role of physical activity in increasing SIRT-1 protein levels (31). SIRT-1 activators have been extensively studied and have demonstrated in vitro attributes (32). However, the relatively high concentrations required to obtain pharmacologic effects have led to a search for more potent SIRT-1 activators. Three SIRT-1–activating molecules have been identified and studied in the treatment of diabetes in mice (33) and may show promise in retarding cartilage destruction in OA.
SIRT-1 plays a role in inflammation (34) and in immune tolerance (35). Therefore, we tested the role of SIRT-1 in secondary inflammation related to OA by measuring the serum levels of the proinflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor α, IL-6, and granulocyte–macrophage colony-stimulating factor. We performed these analyses during the natural age-induced OA observed at 6 months but did not observe any difference in the levels of these cytokines between the y/y mice and WT mice, indicating that there was no systemic inflammation at 6 months. However, using NF-κB p65 pathway checking, we observed significantly higher local inflammation in the y/y mice than in their WT littermates. Further study of inflammation in the OA process in this mouse strain could provide more evidence of the involvement of SIRT-1 in cartilage protection, cartilage degeneration, and inflammation tolerance associated with age.
The findings of the present study highlight the fact that SIRT-1 and its enzymatic activity are necessary for the normal development and homeostasis of cartilage in vivo. In the absence of SIRT-1 enzymatic activity, mice develop characteristics similar to those of OA, one of many late-onset diseases common in aging. This study establishes that SIRT-1 plays a crucial role in normal healthy cartilage homeostasis as well as during skeletal development. The data obtained so far support conducting further studies to develop novel pharmaceutical targets for SIRT-1 activation in cartilage susceptible to damage and degeneration.
ACKNOWLEDGMENTS
The authors wish to acknowledge the following individuals for their valuable assistance: Dr. Yongqing Chen for mouse pictures, Dr. Vittorio Sartorelli for reviewing the manuscript, Evelyne Ralston for providing imaging platforms, and Youngmi Ji for conducting the NF-κB experiment. The authors also acknowledge Dr. David Engel for editing the manuscript and Mr. Richard Booth for assistance in statistical analyses.
Supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Gabay had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Gabay, Dvir-Ginzberg.
Acquisition of data. Gabay, Sanchez, Zaal, Song, He, McBurney.
Analysis and interpretation of data. Gabay, Dvir-Ginzberg, Gagarina.
REFERENCES
- 1.Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40:1–11. doi: 10.3109/03008209909005273. [DOI] [PubMed] [Google Scholar]
- 2.Zhang WG, Bai XJ, Chen XM. SIRT1 variants are associated with aging in a healthy Han Chinese population. Clin Chim Acta. 2010;411:1679–1683. doi: 10.1016/j.cca.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Loeser RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26:371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. for the National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 6.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backesjo CM, Li Y, Lindgren U, Haldosen LA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res. 2006;21:993–1002. doi: 10.1359/jbmr.060415. [DOI] [PubMed] [Google Scholar]
- 8.Zeng L, Chen R, Liang F, Tsuchiya H, Murai H, Nakahashi T, et al. Silent information regulator, sirtuin 1, and age-related diseases. Geriatr Gerontol Int. 2009;9:7–15. doi: 10.1111/j.1447-0594.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 9.Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J Biol Chem. 2008;283:36300–36310. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, et al. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum. 2010;62:1383–1392. doi: 10.1002/art.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 12.Fujita N, Matsushita T, Ishida K, Kubo S, Matsumoto T, Takayama K, et al. Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J Orthop Res. 2011;29:511–515. doi: 10.1002/jor.21284. [DOI] [PubMed] [Google Scholar]
- 13.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, et al. The mammalian SIR2α protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBurney MW, Yang X, Jardine K, Bieman M, Th’ng J, Lemieux M. The absence of SIR2α protein has no effect on global gene silencing in mouse embryonic stem cells. Mol Cancer Res. 2003;1:402–409. [PubMed] [Google Scholar]
- 15.Lemieux ME, Yang X, Jardine K, He X, Jacobsen KX, Staines WA, et al. The Sirt1 deacetylase modulates the insulin-like growth factor signaling pathway in mammals. Mech Ageing Dev. 2005;126:1097–1105. doi: 10.1016/j.mad.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Gabay O, Oppenhiemer H, Meir H, Zaal K, Sanchez C, Dvir- Ginzberg M. Increased apoptotic chondrocytes in articular cartilage from adult heterozygous SirT1 mice. Ann Rheum Dis. 2012;71:613–616. doi: 10.1136/ard.2011.200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seifert EL, Caron AZ, Morin K, Coulombe J, He XH, Jardine K, et al. SirT1 catalytic activity is required for male fertility and metabolic homeostasis in mice. FASEB J. 2012;26:555–566. doi: 10.1096/fj.11-193979. [DOI] [PubMed] [Google Scholar]
- 18.Gabay O, Gosset M, Levy A, Salvat C, Sanchez C, Pigenet A, et al. Stress-induced signaling pathways in hyalin chondrocytes: inhibition by avocado-soybean unsaponifiables (ASU) Osteoarthritis Cartilage. 2008;16:373–384. doi: 10.1016/j.joca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Ameye LG, Deberg M, Oliveira M, Labasse A, Aeschlimann JM, Henrotin Y. The chemical biomarkers C2C, Coll2-1, and Coll2- 1NO2 provide complementary information on type II collagen catabolism in healthy and osteoarthritic mice. Arthritis Rheum. 2007;56:3336–3346. doi: 10.1002/art.22875. [DOI] [PubMed] [Google Scholar]
- 22.Depew MJ. Analysis of skeletal ontogenesis through differential staining of bone and cartilage. Methods Mol Biol. 2008;461:37–45. doi: 10.1007/978-1-60327-483-8_5. [DOI] [PubMed] [Google Scholar]
- 23.Yasuda M, Yuki T, Tanimura T. Color atlas of fetal skeleton of the mouse, rat, and rabbit. Congenit Anom (Kyoto) 1996;36:263–265. [Google Scholar]
- 24.Lee JH, Fitzgerald JB, DiMicco MA, Cheng DM, Flannery CR, Sandy JD, et al. Co-culture of mechanically injured cartilage with joint capsule tissue alters chondrocyte expression patterns and increases ADAMTS5 production. Arch Biochem Biophys. 2009;489:118–126. doi: 10.1016/j.abb.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem. 2011;112:3507–3514. doi: 10.1002/jcb.23298. [DOI] [PubMed] [Google Scholar]
- 26.Dvir-Ginzberg M, Gagarina V, Lee EJ, Booth R, Gabay O, Hall DJ. Tumor necrosis factor α–mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis Rheum. 2011;63:2363–2373. doi: 10.1002/art.30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppenheimer H, Gabay O, Meir H, Haze A, Kandel L, Liebergall M, et al. 75-kd sirtuin 1 blocks tumor necrosis factor α–mediated apoptosis in human osteoarthritic chondrocytes. Arthritis Rheum. 2012;64:718–728. doi: 10.1002/art.33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC, et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 2010;9:844–855. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- 30.Di Renzo F, Broccia ML, Giavini E, Menegola E. VPA-related axial skeletal defects and apoptosis: a proposed event cascade. Reprod Toxicol. 2010;29:106–112. doi: 10.1016/j.reprotox.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Corbi G, Conti V, Scapagnini G, Filippelli A, Ferrara N. Role of sirtuins, calorie restriction and physical activity in aging. Front Biosci (Elite Ed) 2012;4:768–778. doi: 10.2741/417. [DOI] [PubMed] [Google Scholar]
- 32.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 33.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sequeira J, Boily G, Bazinet S, Saliba S, He X, Jardine K, et al. sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314:3069–3074. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]