Abstract
Objective
Previous research has shown that damage to the left temporal pole (LTP) is associated with impaired retrieval of words for unique entities, including names of famous people and landmarks. However, it is not known whether retrieving names for famous melodies is associated with the LTP. The aim of this study was to investigate the hypothesis that damage to the LTP would be associated with impaired naming of famous musical melodies.
Method
A Melody Naming Test was administered to patients with LTP damage, brain damaged comparison (BDC) patients, and normal comparison participants (NC). The test included various well known melodies (e.g., “Pop Goes the Weasel”). After hearing each melody, participants were asked to rate their familiarity with the melody and identify it by name.
Results
LTP patients named significantly fewer melodies than BDC and NC participants. Recognition of melodies did not differ significantly between groups.
Conclusions
The findings suggest that LTP supports retrieval of names for famous melodies. More broadly, these results extend support for the theoretical notion that LTP is important for retrieving proper names for unique concepts, irrespectively of stimulus modality or category.
Keywords: music, naming, left temporal pole
Famous musical melodies, such as “Twinkle Twinkle Little Star” and “Old MacDonald,” are widely recognized in the western world. While the same musical melody may be represented in multiple ways, the melody itself is a unique, discrete entity. For example, at a birthday party when one person begins singing “Happy Birthday”, others join in, singing in whatever key was spontaneously chosen by the first singer. No matter what key this may be, the melody of “Happy Birthday” is easily and ubiquitously recognized. Similarly, melodies can be played on different instruments and despite the different sounds these make, the melodies tend to be quite recognizable.
Semantically unique items are items that can be characterized by a unique identifier, or name (Gorno-Tempini & Price, 2001). Famous musical melodies are semantically unique items, because they are unique entities that are identified by a proper name, (e.g., “The Star Spangled Banner”). In this way, melodies are similar to other categories of unique entities, such as famous people and landmarks. While musical melodies are similar to famous people and landmarks in this regard, it is uncertain whether the category of famous musical melodies has the same neuroanatomical underpinnings as famous faces and landmarks. Importantly, famous faces and landmarks are both visual stimuli, while melodies are auditory. The neurobiology of this critical difference between these two classes of stimuli is not well understood.
Previous research has frequently investigated the neuroanatomical underpinnings of recognition of famous musical melodies (Hailstone et al., 2009; Hsieh et al., 2011; Peretz et al., 1994; Platel et al., 2003; Steinke et al., 2001). In these studies, subjects have been asked to identify whether a melody is familiar or not, to continue humming the tune of the melody, or to pick the name of the melody from a group of names (in a multiple choice recognition format). Multiple brain regions have been implicated in the recognition of musical melodies, most frequently including regions in the bilateral temporal lobes. However, it is important to note that recognition of famous melodies is different from naming of melodies – the former refers to a set of semantic information that confers “knowing,” while the latter refers to a specific lexical entity that is a proper name. In the current study, we focus on the neuroanatomical basis of naming famous musical melodies, which has been far less studied than recognition.
Only a few studies have investigated the neuroanatomical underpinnings of naming famous musical melodies. Ayotte and colleagues (2000) studied naming of famous musical melodies in patients who had undergone brain surgery for middle cerebral artery aneurysms. The authors found that, compared to normal comparison subjects, patients with left, but not right, hemisphere lesions were impaired at naming famous melodies. However, patients with lesions to the left hemisphere were also found to be impaired at recognizing famous melodies. Therefore, the authors were unable to specify a region that results in isolated deficits in naming famous melodies. Recently, Johnson and colleagues (2011) investigated naming of famous musical melodies, and found that patients with Alzheimer's disease, frontotemporal dementia, and semantic dementia were significantly impaired at naming famous musical melodies compared to normal comparison participants. Additionally, patients with semantic dementia performed significantly poorer than patients with Alzheimer's disease and frontotemporal dementia. However, as in the Ayotte et al. (2000) study, the semantic dementia patients who were impaired at naming famous melodies were also impaired at recognizing these melodies. Using voxel-based morphometry, the authors identified multiple regions in the left temporal lobe that correlated with performance on the famous melodies naming task. Taken together, previous research has provided some preliminary hints that the left temporal lobe is important for naming famous musical melodies. However, neuroanatomical regions responsible solely for naming of famous musical melodies have not been isolated; additionally, a clear mechanism for deficits in naming of famous melodies has not yet been found.
Here, we propose that famous musical melodies are semantically unique items, and therefore, the neuroanatomical substrate underlying naming famous musical melodies is similar to naming other semantically unique items. The left temporal pole (LTP) is a region that is involved in the ability to name semantically unique entities, such as famous faces and landmarks. In a seminal study, Damasio et al. (1996) found that patients with lesions to the left temporal pole were impaired at naming the faces of famous people, but not animals or tools. Functional imaging research has confirmed that the left anterior temporal lobes selectively respond to person-knowledge, as opposed to more general categories of objects (Simmons et al., 2010). Additionally, Tranel (2006) found that patients with LTP damage were impaired at naming famous landmarks (and famous faces), suggesting that the LTP is not just necessary for naming of unique faces, but for multiple categories of semantically unique items. More recently, Drane et al., (2008, 2012) found that patients with left temporal lobectomies were significantly impaired at naming famous faces, while patients with right temporal lobectomies were significantly impaired at recognizing famous faces. These findings are consistent with functional imaging research (Damasio et al., 1996; Grabowski et al., 2001; Griffith et al., 2006; Nielson et al., 2010). Such findings have led to a theoretical framework positing that the LTP is a convergence zone that connects the retrieval of conceptual information about a unique item with the retrieval of the name of that item (Damasio et al., 2004; Tranel et al., 2001; Tranel et al., 2005). Lesions to the left temporal pole disrupt this connection between conceptual information and lexical information, and result in impairments in naming semantically unique items.
Although previous research posits that the LTP is necessary for naming semantically unique entities, it is unclear whether or not musical melodies, which are an auditory stimulus, will be neuroanatomically represented in the same way as other unique entities. Tranel et al. (2005) investigated the naming of concrete (but not unique) entities using both visual and auditory stimuli. In a PET experiment, participants named various tools and animals that were presented either with a picture of the tool/animal or the sound that the tool/animal makes. Naming from both visual and auditory stimuli resulted in similar activations in the inferotemporal region – specifically, the locus of activation differed for the categories (animals v. tools) but not for the sensory modalities (visual v. auditory). While this task did not include naming of semantically unique entities, it provides support for the hypothesis that naming of auditory stimuli may engage similar brain regions to naming of visual stimuli.
The study reported here expands on the previous framework that the left temporal pole is necessary for the naming of unique entities, irrespective of sensory modality or stimulus category, by investigating whether LTP damage is associated with impaired naming of a unique stimulus category: famous musical melodies. Additionally, we expand on the previous literature in music naming and recognition by proposing a specific, focal brain region that, when lesioned, will result in impairment in naming famous musical melodies. We hypothesized that the left temporal pole is associated with retrieving names for specific musical melodies, but not with retrieving semantic knowledge about these melodies. On this basis of this hypothesis, we predicted that: (1) Lesions to the LTP will impair naming of famous musical melodies, and (2) Lesions to the LTP will not impair recognition of famous musical melodies.
Method
Participants
The participants in this study were chosen from the Patient Registry of the University of Iowa Division of Cognitive Neuroscience in the Department of Neurology. There were a total of 20 brain-damaged patients. Participants were grouped on the basis of lesion location into the following groups: patients with damage to the left temporal pole (LTP; n = 10), brain-damaged comparison participants with damage to areas outside of the temporal pole (BDC; n = 10), and normal comparison participants (NC; n = 10). The etiologies of the lesions for the LTP group include: surgical resection for epilepsy treatment (n = 7) and cerebrovascular disease (n = 3); for the BDC group, the etiologies include: surgical resection for epilepsy treatment (n = 2), cerebrovascular disease (n = 6), and surgical resection for treatment of a meningioma (n = 2). The BDC group was included to control for nonspecific effects of having brain damage, and the NC group was included to allow assessment of demographic factors and how those might account for performance on our experimental task. There were no significant differences between these groups in demographic attributes, and of particular importance, the groups were well matched on age and education (Table 1).
Table 1. Demographic Information.
| Group | |||
|---|---|---|---|
| Left TP (n = 10) | BDC (n = 10) | NC (N = 10) | |
| Sex | 4 M, 6 F | 6 M, 4 F | 4 M, 6 F |
| Handedness | 10 R, 0 L | 9 R, 1 L | 9 R, 1 L |
| Age (SD) | 53.0 (10.8) | 60.2 (10.3) | 53.6 (12.3) |
| Education (SD) | 15.2 (3.6) | 14.8 (2.8) | 16.2 (3.0) |
| FSIQ (SD) | 101.0 (16.2) | 110.5 (14.3) | 114.2 (7.9) |
Note: No group differences in age (p = .29, η2 = .08), education (p = .60, η2 =.03), FSIQ (p = .09, η2 =.17).
To be eligible for the study, subjects had to have a strong indication of left-hemisphere language dominance (as determined from neurological, Wada, and neuropsychological testing). Exclusion criteria included hearing impairments (not corrected for by hearing aids) and lesion onset younger than age 18. The participants had been extensively characterized neuropsychologically and neuroanatomically using standard protocols from the Benton Neuropsychology Laboratory and the Laboratory of Brain Imaging and Cognitive Neuroscience (Tranel, 2007). The sample was screened to ensure that none of the participants had general intellectual impairment (as determined by Wechsler Adult Intelligence Scale—Third Edition and Fourth Edition testing). Six subjects in the current sample have scores for the WAIS – III and the remaining subjects have scores for the WAIS – IV; scores on the two tests have been prorated to correspond to one another using the recommendations in the WAIS manual (Wechsler, 2008). Full-scale IQ (FSIQ) in normal comparison subjects was measured using the Wechsler Test of Adult Reading (Wechsler, 2001). There were no significant differences between the LTP and BDC groups on various neuropsychological measures (Table 2). While the LTP group did not score significantly lower than the BDC group on the Boston Naming Test (BNT), the group mean for the LTP subjects was about 8 points lower. This is not surprising, as some degree of visual confrontation naming impairment is common in patients with left temporal lobectomies. Three patients in the LTP group (all who had temporal lobectomies) scored below 48 on the BNT, which may be considered “impaired” according to normative standards (e.g. Kaplan, Goodglass, & Weintraub, 1983). Removing these patients from our dataset did not affect the results of analyses on the experimental task. The remaining seven LTP participants performed within the normal range on the BNT. This suggests that a general naming impairment cannot explain our findings on the experimental melody naming task. All data, including standard neuropsychological measures, neuroanatomical data, and the experimental task, were obtained in the chronic phase of recovery, when participants were at least 3 months post lesion onset. This study was approved by the Institutional Review Board and all participants gave informed consent in accordance with the requirements of the Human Subjects Committee.
Table 2.
Neuropsychological data for lesion groups.
| LTP | BDC | p-value | Effect Size (Cohen's d) | |
|---|---|---|---|---|
| Age at lesion onset | 45.5 (11.7) | 53.1 (13.6) | .199 | .59 |
| Chronicity (time since lesion) | 7.9 (4.1) | 8.6 (8.1) | .811 | .10 |
| BNT(SD) | 48.7 (13.6) | 56.9 (8.1) | .095 | .82 |
| WAIS – VIQ (SD) | 100.9 (16.1) | 108.7 (10.2) | .250 | .58 |
| WAIS – PIQ (SD) | 107.8 (14.4) | 111.7 (9.0) | .511 | .32 |
| WAIS – FSIQ (SD) | 104 (13.2) | 108 (8.1) | .213 | .36 |
| WRAT – Read (SD) | 93.2 (13.4) | 100.3 (11.4) | .234 | .57 |
| AVLT – 30 min recall (SD) | 8.5 (3.6) | 11.0 (3.6) | .153 | .68 |
| CFT – 30 min recall (SD) | 19.0 (6.0) | 21.4 (5.6) | .371 | .41 |
| WCST – Pers. Errors (SD) | 11.8 (9.5) | 19.7 (17.4) | .259 | .56 |
| TMT – B (SD) | 64.9 (25.3) | 55.1 (28.0) | .467 | .36 |
Note: BNT, Boston Naming Test. WAIS, Wechsler Adult Intelligence Scale scores (VIQ, Visual IQ; PIQ, Performance IQ; FSIQ, Full-scale IQ). WRAT, Wide Range Achievement Test scores (Read, Reading Standard Score). AVLT, Auditory-Verbal Learning Test scores (an index of memory function at 30 min). CFT, Complex Figure Test recall scores (an index of memory function at 30 min). WCST, Wisconsin Card Sorting Test Perseverative Errors, an index of reasoning and concept formation (executive functioning). TMT, Trail Making Test Part B scores, an index of divided attention and multi-tasking. There were no significant differences between the groups on any of these neuropsychological tests.
Famous Melodies Naming Task
The Famous Melodies Naming Task consisted of 52 famous melodies with lyrics (e.g., “White Christmas”) or without lyrics (e.g., “The Pink Panther”). Melodies were created using MuseScore, a free music composition and notation software (musescore.org). Each melody consisted of a one-line melody, that is, a melody with no harmonic accompaniment, in a MIDI piano keyboard timbre. Melodies ranged from 8-15 seconds in duration. A set duration was not chosen for all melodies; instead, each melody consisted of two musical phrases. This way, the amount of musical information (that is, the amount of musical phrases) was consistent between all melodies, although the exact amount of time for each melody was not. This prevented songs with slower tempos from having less musical information than songs with faster tempos. This method, of selecting a certain number of musical phases as opposed to a specific length of time, has been used in previous research on melody recognition (e.g., Steinke et al., 2001).
When constructing the stimuli, we kept each melody as canonical as possible, that is, we each melody sounded as much like the “essence” of that particular melody as possible. The melodies were played in various musical keys, with each melody played in its most common key. This was accomplished by searching online for the original sheet music of that particular melody, and using the sheet music to determine key selection.
After hearing each melody, participants rated their familiarity with the melody on a six-point scale ranging from “certain familiarity” (a 6 on the scale) to “certain unfamiliarity” (a 1 on the scale). On this scale, a rating of a 3 or below indicated that the participant was not familiar with the melody, while a rating of a 4 or above indicated that the participant had some degree of familiarity with the melody. This type of scale has been used in previous studies that assessed familiarity of faces in patients with prosopagnosia (Tranel & Damasio, 1988). After listening to the melody, participants were then asked to identify the melody by name. If participants could not identify the melody by name, they were asked to state the lyrics or to continue humming/singing the tune of the melody.
Scoring of Responses
Naming
Each famous melody on the Famous Melodies Naming Test has a unique proper name. If a participant gave the correct name for an item, the response was scored as correct. If the subject produced a close variation (e.g., “Somewhere Over the Rainbow” for “Over the Rainbow”) the response was scored as a “near miss” and was evaluated subsequently (see below). If the subject did not give a name, produced a wrong name (e.g., “Yippi eye ay” for “Zip a dee doo dah”), or gave a vague response (e.g., “a Christmas song”), naming was scored as incorrect. All of the “near miss” responses were individually scored by three raters as either “mostly correct” or “mostly incorrect.” Then, all responses that were rated as “mostly correct” by two or more raters were given a final rating of “correct;” responses that were rated as “mostly incorrect” by two or more raters were given a final rating of “incorrect.”
Recognition
All items that were named correctly were scored as correct recognitions, on the premise that an accurate name can be taken as evidence that the subject recognizes the entity (H. Damasio et al., 2004). If the participant did not give the correct name, the given lyrics were judged. If the participant gave the correct lyrics to the melody, the response was scored as correct recognition. If a participant did not give lyrics, they were asked to continue humming the tune of the melody. If they correctly hummed the tune of the melody, it was scored as correct recognition. If the participant did not do any of the above, they were asked to describe the song in any way that would signify that they recognized the melody. For example, “Pomp and Circumstance” could be (and was for several subjects) described as “the song that is played at graduations” and this was scored as correct recognition but incorrect naming. We went one step further in this process. If none of the above conditions was met (the subject did not produce lyrics, did not hum the tune, and did not give a specific description), the ratings of familiarity (on a six-point familiarity scale) were used to judge recognition. A rating of a 5 or a 6 on the scale was taken to indicate that the participant was moderately familiar to certainly familiar with the melody. Therefore, if a participant rated a song a 5 or a 6 on the familiarity scale, that item was assigned a correct recognition score.
Data Quantification
Neuropsychological data
First, for each participant, we determined which items were correctly recognized, using the guidelines specified above. The naming score was then created from this set of items by taking the number of items correctly named and dividing it by the number of items correctly recognized. Therefore, participants were not penalized for failing to recognize items, as the denominator in the naming score equation was specific to each participant. For example, if a participant only recognized 50% of the items, but correctly named 45% of those items (the ones correctly recognized), the naming score would be 90%. This procedure for calculating naming scores has been used previously (e.g., Damasio et al., 1996, 2004; Tranel et al., 2005; Tranel, 2006), as it helps put the naming scores on a common metric despite the fact that subjects have somewhat differing levels of recognition.
Neuroanatomical data
The neuroanatomical analysis of the lesion patients was based on magnetic resonance imaging data obtained in a 1.5 T scanner. Using Brainvox (Frank et al., 1997) each patient's lesion was reconstructed in three dimensions. The lesion contour for each patient was manually warped into a normal template brain using the MAP-3 method (Fiez, Damasio, & Grabowski, 2000). The overlap of lesions in these volumes, calculated by the sum of n lesions overlapping at any single voxel, is color-coded in Figure 1. As this figure shows, the greatest overlap of LTP patient lesions is in the left temporal pole, defined as Brodmann's area 38. Lesions in the BDC group include areas such as the: right temporal pole, left and right regions in the temporal lobe outside of the temporal pole, and right and left frontal and parietal regions (the lesion overlaps for the BDC group are not shown, as the distribution of lesion locations is varied). No patients in either group had aphasia at the time of the experimental music naming assessment. One patient in the LTP group had damage around Wernicke's area and presented acutely with conduction aphasia (per clinical neuropsychological assessment). The patient's aphasia had recovered by the time of the experimental procedures. In sum, none of the patients in this study had residual aphasia in the chronic epoch.
Figure 1.
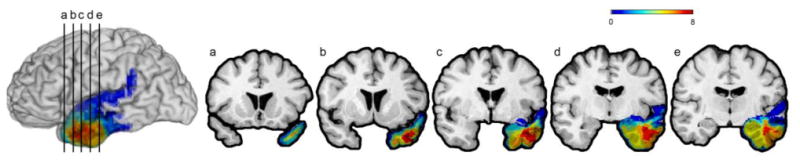
Lesion overlap map of patients with left temporal polar lesions. The left panel depicts a lateral view of the left hemisphere. The five panels to the right depict coronal cuts (a-e) through the left anterior temporal region. Images are shown in radiological convention, with the left hemisphere on the right. The color bar codes maximal lesion overlap, with “hotter” colors (red, yellow) representing higher numbers of lesion overlap.
Data Analysis
The naming and recognition scores of the three groups were compared using one-way analysis of variance (ANOVA) with post-hoc t-tests to compare the groups to each other. Two one-way ANOVAs were conducted, one for recognition score and one for naming score. The use of two one-way ANOVAs is appropriate in this situation because the recognition and naming scores are mostly independent -- due to our calculating the naming score by using the recognition score as the denominator. (The independence of the recognition and naming scores was established further by conducting and examining correlations between these two measures.) Overall, neuroanatomical status (i.e., lesion location) was the independent variable, and naming and recognition performances were the dependent variables.
Results
Across all subjects, scores for musical melody naming and recognition were not highly correlated (r = .100, p = .598; 95% confidence interval (CI): [-.269, .444]). Additionally, naming and recognition scores within the LTP group (r = .236, p = .511; 95% CI: [-.462, .754]), BDC group (r = .279, p = .435; 95% CI: [.-.452, .773]), and NC group (r = - .214, p = .552; 95% CI: [-.743, .480]) were not strongly or significantly correlated (and were in different directions).
The scores for musical melody naming and recognition for each group are depicted in Figure 2. The one-way ANOVA on the recognition score was not significant, F(2,27) = .055, p = .947, η2 = .003. The one-way ANOVA on the naming score was significant, F(2,27) = 10.2, p < .001 η2 = .43. We repeated these contrasts (with ANCOVAs) using FSIQ as a covariate, since the between-group differences in FSIQ were marginally significant (with the LTP group being lower; see Table 1). FSIQ was significantly related to recognition scores, F(1,24) = 11.585, p = .002, η2 = .32. There was no significant effect of group on recognition scores in the ANCOVA, however, F (2,24) = 1.18, p = .323, η2 = .09. FSIQ was significantly related to naming score, F(1,24) = 5.48, p = .028, η2 = .18. The effect of group on the naming scores remained significant after controlling for the effect of FSIQ in the ANCOVA, F(2,24) = 6.15, p = .007, η2 = .34.
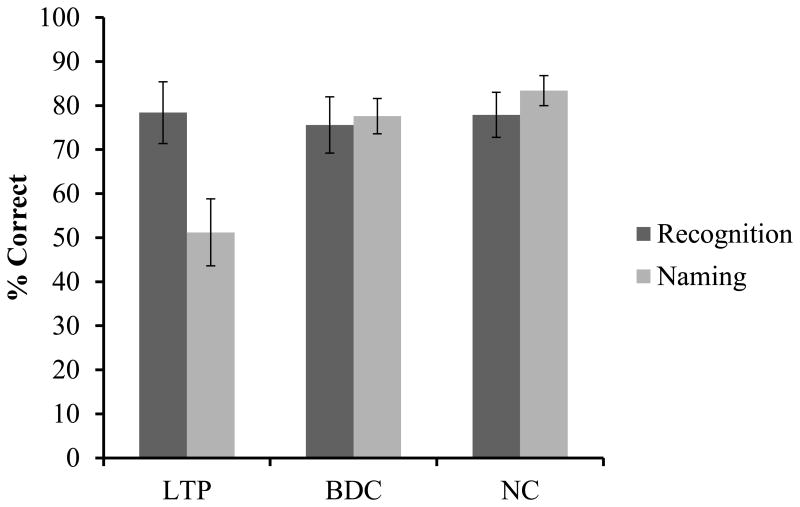
Figure 2.
Performance on recognition and naming for each group. Error bars represent standard error of the mean. The naming score for the LTP group was significantly lower than the naming scores for the BDC and NC groups, per one-way F(7,27) = 10.2, p < .001, η2 =.43. There was no significant group difference for the recognition score F(7,27) = .055, p = .947, η2 = .003.
As the data in Figure 2 show, the LTP group performed below the BDC and NC groups on naming. Post-hoc t-tests were also performed. The naming score for the LTP group was significantly lower than that of the BDC group, t(18) = -3.05, p = .007, and the NC group, t(18) = -3.84, p = .001. The BDC and NC groups did not significantly differ from one another on the naming score t(18) = -1.09, p = .28. Specifically, the mean naming score for the LTP group was 52.1 (SEM = 7.6; 95% CI: [33.9, 68.4]), for the BDC group the mean naming performance was 77.6 (SEM = 4.0; 95% CI: [68.4, 86.7]), and for the NC group the mean naming performance was 83.4 (SEM = 3.4; 95% CI: [75.6, 91.1]). These results show that the 95% confidence interval for the mean of the LTP group does not overlap with those of the other two groups. After controlling for the effect of FSIQ, these group differences remained significant. The LTP group (M = 55.4, SEM = 5.3, 95% CI: [44.4, 66.3]) scored significantly lower than the BDC group (M = 78.2, SEM = 5.6, 95% CI: [66.6, 89.7]; p = .008) and the NC group (M = 80.2, SEM = 5.2, 95% CI: [69.5, 90.9], p = .004). The BDC and NC groups did not significantly differ in naming performance after controlling for FSIQ (p = .789).
Discussion
The results from this study supported both predictions – specifically, that damage to the left temporal pole (LTP) would be associated with impaired naming of musical melodies, but would not affect recognition of these melodies. Overall, these findings support the hypothesis that the LTP is associated with retrieving names for unique identities. Patients with damage to the LTP scored significantly lower than brain-damaged comparison (BDC) and normal comparison (NC) participants on naming famous melodies. The groups did not differ in their recognition of these melodies. Between-group differences in naming performance were not explained by other variables, including age, years of education, IQ, or a general naming defect.
Most of the LTP patients in this study have also been tested with our standard tasks for famous face identification and landmark identification. Specifically, eight LTP patients have completed the Iowa Famous Faces Test and seven of these have also completed the Landmark Test. For both of these other stimulus categories, the LTP patients had impaired naming performances, averaging 1.59 SDs below the normal mean on famous face naming and 2.21 SDs below the normal mean on landmark naming. LTP patients did not have impaired recognition on these tasks. In summary, a direct comparison across different categories of semantically unique items within the same patients suggests that proper name retrieval was impaired across multiple categories. This provides further support for the notion of a heteromodal role of the LTP in mediating the link between semantic knowledge retrieval (recognition) and proper name production (naming).
The theory that the LTP is necessary for naming unique entities was developed from studies that have investigated this effect in various categories of visual stimuli, including famous faces and landmarks (Damasio et al., 1996, 2004; Drane et al., 2008; Tranel, 2006). The current study adds a new dimension to these previous results, showing this same effect in a different stimulus category and different sensory modality, viz., famous musical melodies. While famous musical melodies have some properties similar to those of famous faces and landmarks, such that they are all semantically unique items associated with a specific proper name, musical melodies are different in that they are auditory stimuli. While recent neuroimaging research has investigated neural substrates of one category of auditory stimuli, famous voices (Bethmann et al., 2012), ours is the first study showing that LTP damage is associated with impaired naming (but not recognition) of semantically unique items that are auditory in nature. The current findings are consistent with the idea that famous musical melodies are in fact semantically unique items in a way that parallels famous people and landmarks. This may suggest an expansion of the class of semantically unique items to include famous musical melodies.
A limitation of our study is the relatively modest sample sizes. However, our sample sizes are within the range typically used for lesion studies, and our results display a consistent pattern as well as large effect sizes. Thus, we believe that despite the modest sample sizes, these results are likely to be reliable, valid, and reproducible. Nonetheless, it will be important to replicate the present findings with larger numbers of subjects. We also acknowledge that the etiologies in our patient groups are varied, and it will be important in future work to determine whether different etiologies (e.g., vascular versus surgical) produce different outcomes – we do not expect that this would be the case, given our extensive experience with these different patient populations. We would like to emphasize that a strength of the current study is the Famous Melodies Test, an extensive battery of famous musical melodies. Our Famous Melodies Test, which consists of 52 melodies, is considerably more comprehensive than previous batteries of famous melodies (Hsieh et al., 2011; Johnson et al., 2011; Liegeois-Chauvel et al., 1998; Steinke et al., 2001), likely leading to more reliable outcomes.
Additionally, our study focuses on naming melodies as opposed to recognition of melodies. Previous studies that investigated the naming of famous musical melodies found that patients with impairments in naming also showed impairments in recognition (Ayotte et al., 2000; Johnson et al., 2011). An important feature of our study is that it focuses on impairments in naming famous musical melodies. Our findings thus go beyond previous literature on famous melody recognition, and they also extend support for the theory that the LTP is important for naming unique entities. While our study focuses on a naming deficit in LTP patients, we did not examine the full naming-recognition dissociation; that is, we did not identify patients with recognition deficits but not naming deficits (and we did not target such potential patients in our lesion sampling). Various patterns of dissociations between recognition and naming would be expected, based on previous research. For example, a patient may be impaired at recognition (i.e., only be able to recognize a fraction of the famous melodies), but of those few that were recognized, be unimpaired at naming them—this pattern would demonstrate impaired recognition but intact naming. Such a pattern of dissociation has been found in regard to recognition and naming of famous faces in patients with damage to the right anterior temporal lobe (Drane et al., 2008). One direction for future research would be to use our Melody Naming Task to investigate which regions are associated with isolated deficits in melody recognition, deficits which could be independent of naming defects. Given previous research on recognition in famous faces and melodies, it could be predicted that patients with damage to the right temporal lobe (especially anteriorly) would be impaired at melody recognition but not melody naming.
In the vein of making a distinction between recognition versus naming, it is important to underscore another feature that differs between these two cognitive tasks. In order to name an entity, especially a unique entity, a specific piece of information has to be retrieved, i.e., the name (or for rare stimuli, a few names may be accurate). For example, naming “The Star Spangled Banner” requires coming up with that exact name, and answers like “the national anthem” or “our country's song” are not correct. By contrast, there are many different ways that an entity can be recognized, and that recognition can be signified in a response. In the case of our melodies, for example, one could provide multiple lyrics in the song, a specific description of the song, humming the tune of the song, and so on—all responses that would indicate specific recognition and identification. The unique cognitive (and neural) demands associated with name retrieval are some of the key reasons we have cited in our theoretical formulations in previous papers (e.g., Damasio et al., 2004) as to why naming (and especially proper naming) is exquisitely vulnerable to brain dysfunction, and even more broadly, to factors such as fatigue, alcohol, distraction, and aging. Name retrieval, especially proper name retrieval, is difficult precisely because there is only one right answer. For recognition, by contrast, there are many ways to accomplish successful performance. In short, naming, especially proper naming, is especially vulnerable to brain damage.
Future work could investigate the dissociation between naming unique auditory entities (famous melodies) and naming non-unique auditory stimuli, in a way that would parallel previous work in categories of visual stimuli. For example, it has been shown that lesions to the LTP impair naming of semantically unique visual entities (faces, landmarks), while more posterior temporal lobe lesions impair naming of basic objects such as animals and tools (e.g., Damasio et al., 1996). In the domain of music, future research could investigate differences in naming unique entities (melodies) and basic musical entities (genres, instruments). One could also extend this auditory naming investigation to non-music auditory stimuli, such as whistles, horns, sirens, and the like. Given previous research, we would predict that damage to the LTP would not impair naming of basic auditory or musical stimuli, whereas damage to the posterior left temporal lobe might impair this type of naming.
In summary, we found that patients with damage to the LTP were impaired at naming, but not recognizing, famous musical melodies. The current findings converge with previous research to provide further support for the theory that the LTP region is important for the retrieval of names for unique entities, irrespective of stimulus modality or category.
Acknowledgments
This work was supported by NINDS P01 NS19632. We would like to acknowledge Brett Karlan and Chris Sande for their work testing participants.
References
- Ayotte J, Peretz I, Rousseau I, Bard C, Bojanowski M. Patterns of music agnosia associated with middle cerebral artery infarcts. Brain. 2000;123:1926–1938. doi: 10.1093/brain/123.9.1926. [DOI] [PubMed] [Google Scholar]
- Bethmann A, Scheich H, Brechmann A. The temporal lobes differentiate between the voices of famous and unknown people: An event-related fMRI study on speaker recognition. PloS One. 2012;7(10):e47626. doi: 10.1371/journal.pone.0047626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, Tranel D. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46(5):1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann JG, Phatak V, Loring DW, Gross RE, Hebb AO, Tranel D. Famous face identification in temporal lobe epilepsy: Support for a multimodal integration model of semantic memory. Cortex. 2012:1–20. doi: 10.1016/j.cortex.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Damasio H, Grabowski TJ. Lesion segmentation and manual warping to a reference brain: Intra- and interobserver reliability. Human brain mapping. 2000;9(4):192–211. doi: 10.1002/(SICI)1097-0193(200004)9:4<192::AID-HBM2>3.0.CO;2-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: An interactive, multimodal visualization and analysis system for neuroanatomical imaging. NeuroImage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ. Identification of famous faces and buildings: a functional neuroimaging study of semantically unique items. Brain. 2001;124:2087–2097. doi: 10.1093/brain/124.10.2087. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Human Brain Mapping. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith HR, Richardson E, Pyzalski RW, Bell B, Dow C, Hermann BP, Seidenberg M. Memory for famous faces and the temporal pole: Functional imaging findings in temporal lobe epilepsy. Epilepsy & Behavior. 2006;9(1):173–180. doi: 10.1016/j.yebeh.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Hailstone JC, Omar R, Warren JD. Relatively preserved knowledge of music in semantic dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80(7):808–9. doi: 10.1136/jnnp.2008.153130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S, Hornberger M, Piguet O, Hodges JR. Neural basis of music knowledge: Evidence from the dementias. Brain. 2011;134:2523–2534. doi: 10.1093/brain/awr190. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Chang CC, Brambati SM, Migliaccio R, Gorno-Tempini ML, Miller BL, Janata P. Music recognition in frontotemporal lobar degeneration and Alzheimer disease. Cognitive and Behavioral Neurology. 2011;24(2):74–84. doi: 10.1097/WNN.0b013e31821de326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lee & Febiger; 1983. [Google Scholar]
- Nielson KA, Seidenberg M, Woodard JL, Durgerian S, Zhang Q, Gross WL, Rao SM. Common neural systems associated with the recognition of famous faces and names: An event-related fMRI study. Brain and Cognition. 2010;72(3):491–498. doi: 10.1016/j.bandc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz I, Kolinsky R, Tramo M, Labrecque R, Hublet C, Demeurisse G, Belleville S. Functional dissociations following bilateral lesions of auditory cortex. Brain. 1994;117:1283–301. doi: 10.1093/brain/117.6.1283. [DOI] [PubMed] [Google Scholar]
- Platel H, Baron JC, Desgranges B, Bernard F, Eustache F. Semantic and episodic memory of music are subserved by distinct neural networks. NeuroImage. 2003;20(1):244–256. doi: 10.1016/s1053-8119(03)00287-8. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PSF, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cerebral Cortex. 2010;20(4):813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke W, Cuddy L, Jakobson L. Dissociations among functional systems governing melody recognition after right-hemisphere damage. Cognitive Neuropsychology. 2001;18(5):411–437. doi: 10.1080/02643290125702. [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20(1):1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- Tranel D, Adolphs R, Damasio H, Damasio AR. A neural basis for the retrieval of words for actions. Cognitive Neuropsychology. 2001;18(7):655–674. doi: 10.1080/02643290126377. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR. Non-conscious face recognition in patients with face agnosia. Behavioural Brain Research. 1988;30(3):235–249. doi: 10.1016/0166-4328(88)90166-0. [DOI] [PubMed] [Google Scholar]
- Tranel D, Grabowski TJ, Lyon J, Damasio H. Naming the same entities from visual or from auditory stimulation engages similar regions of left inferotemporal cortices. Journal of Cognitive Neuroscience. 2005;17(8):1293–1305. doi: 10.1162/0898929055002508. [DOI] [PubMed] [Google Scholar]
- Tranel D, Martin C, Damasio H, Grabowski TJ, Hichwa R. Effects of noun-verb homonymy on the neural correlates of naming concrete entities and actions. Brain and Language. 2005;92(3):288–299. doi: 10.1016/j.bandl.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Iltelligence Scale - Fouth Edition. San Antonio, TX: Pearson; 2008. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]