Abstract
Background.
The cerebellum plays an important role in mobility and cognition. However, it is unclear which regions of the cerebellum are associated with gait speed and information-processing ability in older adults without overt brain damage.
Methods.
Cross-sectional associations between cerebellar gray matter volumes (GMV), gait speed, and information-processing ability were explored in 231 community-dwelling adults (mean age: 83 years, 48% black, 58% female). We measured gait speed on an automated walkway and information-processing ability on the Digit Symbol Substitution test (DSST). Total and regional cerebellar GMV was measured on 3T-magnetic resonance imaging. Lobar GMV of the cerebellum, obtained by an automated parcellation process, were aggregated based on the cognitive (lobules VI, VII, VIII and crus I, II), sensorimotor (lobules II, IV, V), and vestibular (lobules IX and X) functions ascribed to the cerebellar regions.
Results.
Larger cerebellar GMV correlated with faster gait speed and superior DSST scores (both p < .001) independent of age, gender, atrophy, and small vessel disease. After adjusting for age, gender, and atrophy, larger cognitive cerebellar GMV correlated with both faster gait speed (p = .04) and higher DSST scores (p < .001), larger sensorimotor cerebellar GMV correlated significantly with DSST alone (p = .02), and the vestibular cerebellar GMV with neither. The association between cognitive cerebellar GMV and gait speed was no longer significant after adjusting for DSST score in the linear regression models.
Conclusions.
The relationship between gait speed and cerebellar GMV is influenced by information-processing ability, and this relationship is stronger in subregions ascribed to cognitive than vestibular or sensorimotor functions.
Key Words: Gait, Brain aging, Cognition, Imaging.
Initial reports of the association between mobility impairment and cerebellum were made by Sir Gordon Holmes from observing World War I victims inflicted with gunshot wounds to the cerebellum whose gait he described as irregular, slow, and halting (1). These reports formed the foundation for understanding the role of the cerebellum in gait (reviewed in ref. 2). A combination of truncal instability, disequilibrium, slow gait speed with shortened stride length, increased gait variability, and a wide base of support constitute the hallmark of a cerebellar ataxic gait. Neural causes of age-associated slowing of gait, without other associated gait and balance changes, have primarily focused on cortical changes such as white matter disease and gray matter atrophy with aging. The role of the aging cerebellum in gait has not received similar attention in older adults.
Research on nonmotor functions of the cerebellum is emerging (reviewed by ref. 3). The cerebellum plays an important role in cognitive and emotional processing (4). Cerebellar lesion studies demonstrate that deficits in executive function, attention, and spatial attention can emerge with damage to specific areas of the cerebellum without necessarily causing motor symptoms (5). Studies have explored cognitive functions of the cerebellum by demonstrating activation patterns associated with specific cognitive tasks in healthy populations using functional imaging (reviewed in ref. 6). Specifically, functional imaging studies reported distinct cerebellar regional activation for tasks requiring cognitive and motor functions (7,8). Executive dysfunction attributable to cerebellar pathology is evident on tasks that assess verbal fluency, planning, working memory, divided attention, and set shifting (9–11). Multiple cognitive functions rely on information-processing ability and attention, which are primarily frontal-subcortical functions (12). Information-processing ability is also associated with gait speed in older adults and is likely involved in sensory motor integration (13); both functions are also linked to prefrontal cortex (14–16). Signals transmitted from the prefrontal subcortical regions through the superior cerebellar peduncles to cerebellar regions may influence motor output of the cerebellum. Alternatively, cerebellar regions may have a specialized role for information processing that can influence downstream motor control and gait speed (13). It remains unknown which regions in the cerebellum are associated with information-processing ability and gait speed in older adults. Slow information processing reflects generalized slowing associated with aging that has important implications in mobility decline, cognitive decline, functional ability, and mortality in older adults (13,15). Understanding the role of the cerebellum in information processing and gait speed is therefore important in aging. Controlling for cortical measures could help to shed light on the de novo cerebellar influence on gait speed and information-processing ability.
The aim of this study was to investigate the association between structural measures of cerebellar integrity, gait speed, and information-processing measures in community-dwelling older adults. We hypothesized that greater cerebellar gray matter volume (GMV) would be associated with faster gait and faster information processing, independent of the cortex. As cerebral GMV and small vessel disease are linked to gait speed (16), the above hypothesized associations would remain significant after controlling for cortical atrophy and small vessel disease severity. Furthermore, based on the topographic delineation of the cerebellum (6), the cognitive regions of the cerebellum would be more strongly associated with gait speed than the sensorimotor or vestibular cerebellar regions. Finally, if this association holds, then slower information processing would attenuate the strength of the association between cognitive cerebellum region and gait speed.
Methods
Study Population
This is a cross-sectional evaluation of 231 older adults from the Pittsburgh site of the Health Aging and Body Composition (Health ABC). The Health ABC, a longitudinal study of physical measures in independent community-dwelling older adults aged 70–79 years (17), enrolled a total of 3,075 older adults of whom 1,501 were enrolled in Pittsburgh in years 1996–1998. In 2006–2008, 891 of the 1,501 participants returned for their annual clinic visit at the Pittsburgh Health ABC site and were screened for the Healthy Brain Project. This ancillary study obtained brain magnetic resonance imaging (MRI) in enrolled participants, whereas routine physical and cognitive assessments were conducted under the Health ABC study protocol. Participants were excluded from the Healthy Brain Project if they used assistive devices for walking or if they were ineligible for a 3T-MRI or if no mobility measures were collected concurrent with their Health ABC visit at the time of MRI (resulting in exclusion of 480 of 819 participants). Among the 339 participants that were eligible for the Healthy Brain Project study, 325 received a brain MRI between 2006 and 2008. This report is a secondary analysis of the Healthy Brain Project and Health ABC data. For this analysis, we excluded participants who had a history of Parkinson’s disease or dementia (obtained from self-report, hospital records, and/or medication review), self-reported stroke, modified mini–mental state examination score of 80 or less, and excessive alcohol intake defined here as participants ever reporting having more than five drinks a day. In addition, those who had a 1.5T MRI and those with incomplete regional cerebellar volumetry on 3T-MRI were excluded. This secondary analysis therefore excluded 94 of the 325 participants who completed brain MRI resulting in a sample size of 231. The median age of the study population was 82 years and included 42% African American and 52% women. This study was approved by the Institutional Review Board of both sites (University of Pittsburgh and University of California at San Francisco).
Neuroimaging
A 3T Siemens Tim Trio MR scanner with a Siemens 12-channel head coil was used for obtaining the MRI scans for this study. Axial T1-weighted images (repetition time = 2,300ms; echo time = 3.43ms; inversion time = 900ms; flip angle= 9°; slice thickness = 1 mm; field of view = 256 × 224mm; voxel size = 1 × 1 mm; matrix size = 256 × 224; and number of slices = 176) and fluid-attenuated inversion recovery images (repetition time = 9,160ms; echo time = 89ms; inversion time = 2,500ms; flip angle = 150°; field of view = 256 × 212mm; slice thickness = 3mm; matrix size = 256 × 240; number of slices = 48 slices; and voxel size = 1 × 1 mm) were obtained.
GMV, white matter volume, and cerebrospinal fluid volume were calculated by summing all voxels classified as these tissue types after automated segmentation of the skull-stripped T1-weighted image in native anatomical space (18). To account for brain atrophy, we obtained the atrophy index (1 − total brain volume/total intracranial volume), where the total brain volume was the sum of cerebral and cerebellar GMV, white matter volume, and intraventricular cerebrospinal fluid volume. As a measure of intracranial capacity and head size, we measured intracranial volume as the sum of total brain volume and extraventricular cerebrospinal volume after stripping of the skull and meninges. The atrophy index was used as a covariate in the analysis. Small vessel disease was quantified by assessing volume of white matter hyperintensities (WMH) on T2-weighted MRI by a semiautomated method described previously; WMH volume was normalized to brain volume (19). Cerebellar regions were delineated based on the cerebellum parcellation of Schmahmann and colleagues (20) in the atlas by Tzourio-Mazoyer and colleagues (21).
Volumes of specific cerebellar regions, based on their purported role as cognitive, sensorimotor, and vestibular, were obtained by aggregating regions delineated on the atlas. The cognitive region, which is largely the posterior region of the cerebellum, includes crus I and II, and lobules VI, VII, and VIII. Lobules VI and crus I are involved in working memory; crus II in verbal fluency; lobule VI in spatial tasks; and lobules VI, VII, and VIII and crus I in executive function tasks (6,22,23). These regions were therefore summed to represent the cognitive cerebellum. The sensorimotor cerebellum included lobules III, IV, and V, whereas the vestibular cerebellum included lobules IX and X based on demarcation by Schmahmann and colleagues (20).
Health-Related Characteristics
Demographic data, medical comorbidities (algorithmically assessed from report of physician diagnosis and/or medications taken in the last 2 weeks brought from home to clinic), physical performance, cognitive, psychosocial, and functional measures were collected annually beginning in 1997–1998 as part of the parent Health ABC study (17). Systolic blood pressure was averaged over two readings. The modified mini–mental state examination, a standard test of overall cognitive functions with a score ranging from 0 to 100, was used to exclude participants with an overt cognitive impairment/dementia when self-reported history of dementia or cognitive impairment was lacking. Presence of diabetes mellitus was ascertained from self-report, medical records of physician-diagnosed diabetes, or use of an antidiabetic medication. Peripheral neuropathy was assessed by monofilament test.
Gait Measures
Amongst several gait parameters (spatial—step length, step width; temporal—double support, step time), gait speed was one measure that was related to cerebellar volume (24, 14). Therefore, this analysis focused on gait speed, which was measured on the GaitMatII (EQ Inc., Chalfonte, PA), a computerized gait instrument that is 4 m in length and contains sensors embedded within the mat to enable measurement of various gait parameters. We averaged participants’ gait speed taken over two traverses on the GaitMatII. Prior to data collection, participants performed two practice walks across the walkway. Steady-state gait speed was analyzed by deleting the gait recordings at the two ends of the walkway to account for changes associated with acceleration and deceleration.
Information-Processing Measure
We tested participants’ information-processing ability using the Digit Symbol Substitution test (DSST), a validated measure of information processing commonly used in epidemiological studies (25).In this paper-and-pencil test, participants are provided with a key, showing nine unique symbols corresponding to the numeric digits from 1 to 9. Participants are instructed to use the key and fill in the empty boxes adjacent to the numbers with the corresponding symbols. They are instructed to perform the task as quickly and accurately as possible with a 90-second time limit. Participants have to discriminate the symbols by their spatial organization, process-matching symbols to specific digits, and memorize the symbols as they perform the task in the time allotted.
Statistical Analysis
Age, gender, and atrophy-adjusted Pearson’s correlations were used to analyze the association between cerebellar GMV measures, gait speed, and DSST scores with other variables to identify covariates. Linear regression was used to test the association and quantify the strength of association between cerebellar GMV measures and gait speed, with gait speed and processing speed as the dependent variables. Age, gender, WMH, atrophy, and DSST scores were added incrementally in the regression model. The model was adjusted for DSST when gait speed alone was the dependent variable. Total cerebellar GMV and each regional cerebellar GMV variables were entered into separate stepwise multiple regression models. Variables that were found to be independently associated with gait speed in the univariate models were used as covariates in the multiple regression models. We used a statistical software application (SPSS version 11) to perform the analysis.
Results
Baseline Characteristics
Table 1 represents the baseline characteristics of the 231 participants in this analysis highlighting the demographic characteristics, neuroimaging, performance measures, and comorbidities of this sample at year 10 of the Health ABC study.
Table 1.
Population Characteristics
| Characteristics | Mean (SD) or N (%) |
|---|---|
| Age | 82.9 (2.7) |
| Women | 125 (58.4) |
| White | 144 (62.3) |
| High-school educated | 25 (10.9) |
| BMI | 27.4 (4.4) |
| Total cerebellar gray matter volume (cc) | 66.9 (12.8) |
| Gait speed (m/s) | 1.0 (0.2) |
| Digit symbol substitution test | 38.7 (13) |
| Modified mini–mental state examination | 94.4 (4.7) |
| Diabetes type II | 50 (23.8) |
| Hypertension | 154 (67) |
| Cardiovascular disease | 51 (22.1) |
| Joint pain/arthritis | 9 (4.1%) |
Note: BMI, body mass indesx.
Relationship Between Total Cerebellar GMV, Gait Speed, and Information-Processing Ability
Total cerebellar GMV correlated with gait speed and DSST score (r = .263 and .238, respectively, both p < .001). After controlling for age, gender, cerebral WMH, and cerebral atrophy, the statistical significance was still retained such that larger cerebellar GMV correlated with faster gait speed (r = .140, p = .039) and higher DSST score (r = .220, p = .001). The strength of association between cerebellar GMV and gait speed, with latter as dependent variable in the regression models, showed that larger cerebellar GMV was significantly associated with faster gait speed (β = 0.264, p < .001) even after controlling for atrophy, age, gender, and WMH. After adjusting for DSST, the association between larger cerebellar volume and faster gait speed was no longer statistically significant (Table 2). The association between cerebellar GMV and gait speed remained significant after controlling for diabetes mellitus (β = 0.25, p < .001). Furthermore, addition of peripheral neuropathy and race in the regression models did not substantially alter the significance or strength of the relationship between gait speed and cerebellar volumes.
Table 2.
Association Between Total Cerebellar Gray Matter Volumes and Gait Speed
| Regression Models | Dependent Variable: Gait Speed (standardized beta coefficient, [SE], p value) |
|---|---|
| 1 = Total cerebellar GMV unadjusted | 0.264 (0.013), p < .001 |
| 2 = Model 1 + atrophy | 0.255 (0.013), p < .001 |
| 3 = Model 2 + age | 0.222 (0.013), p = .002 |
| 4 = Model 3 + gender | 0.164 (0.014), p = .024 |
| 5 = Model 4 + WMH | 0.146 (0.013), p = .039 |
| 6 = Model 5 + DSST score | 0.098 (0.014), p = .167 |
Notes: DSST = Digit Symbol Substitution test; GMV = gray matter volume; WMH = white matter hyperintensity.
Unadjusted correlations between cerebral GMV and, gait speed and DSST (r = .229 and .259, respectively, both p < .001) were comparable to that of correlations between cerebellar GMV and, gait speed and DSST (r = .263, p < .001, respectively, both p < .001). We also assessed the strength of associations between individual right and left cerebellar GMV and gait speed and found that these relationships were also statistically significant and comparable to each other (β = 0.25 (right) and 0.26 (left), both p < .001). Adjusting for DSST in the individual regression models for the right and left cerebellar measures association made their respective association with gait speed statistically nonsignificant (β = 0.1, p = .2 for both).
Relationship of Regional Cerebellar Volumes to Gait Speed and Information-Processing Ability
After adjusting for age, gender, and atrophy, larger GMV in the cognitive region of the cerebellum correlated significantly with faster gait speed (r = .139, p = .040) and higher DSST scores (r = .240, p < .001), larger GMV in the sensorimotor region of the cerebellum correlated significantly with DSST (r = .156, p = .021) but marginally with gait speed (r = .128, p = .06), whereas GMV in the vestibular region of the cerebellum did not correlate significantly with gait speed or DSST (p = .178 and .98, respectively).
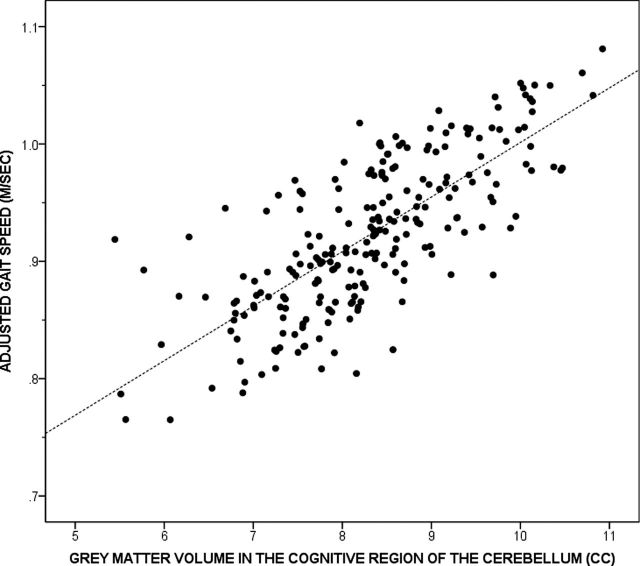
The beta coefficients denoting the strength of association between the two cerebellar regions linked with gait speed in this sample are shown in Table 3. The association between cognitive cerebellar GMV and adjusted gait speed is shown in Figure 1. The strength of association between gait speed and cognitive cerebellar GMV (β = 0.255, p < .001) remained significant after adjusting for atrophy, age, and gender. However, addition of WMH in the model attenuated the strength of association. Addition of DSST in the model attenuated the relationship even further and the association was no longer significant (Table 3). The strength of association between gait speed and sensorimotor cerebellar GMV was significant but weaker than that of the cognitive cerebellar GMV (β = 0.186, p = .006), and the association remained significant after adjusting for atrophy. Addition of age, gender, WMH, and DSST scores in the model attenuated the relationship between gait speed and sensorimotor cerebellar GMV. The association between gait speed and cognitive (β = 0.238, p < .001) and sensorimotor regions (β = 0.186, p = .005) remained significant after adjusting for diabetes mellitus. Addition of peripheral neuropathy and race did not substantially alter the strength of the relationship between gait speed and cerebellar measures.
Table 3.
Association Between Regional Cerebellar GMV and Gait Speed
| Cerebellar Regions | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|
| Cognitive | 0.255 (0.013), p < .001 | 0.245 (0.013), p < .001 | 0.213 (0.013), p = .003 | 0.150 (0.014), p = .039 |
0.128 (0.014), p = .071 |
0.078 (0.014), p = .3 |
| Sensorimotor | 0.186 (0.013), p = .006 | 0.173 (0.013), p = .012 | 0.145 (0.013), p = .034 |
0.127 (0.013), p = .058 |
0.127 (0.012), p = .052 |
0.095 (0.012), p = .2 |
Notes: Beta coefficients, SE, and their corresponding p values are shown for the six models tested.
Model 1: unadjusted.
Model 2: Model 1 further adjusted for atrophy index.
Model 3: Model 2 further adjusted for age.
Model 4: Model 3 further adjusted for gender.
Model 5: Model 4 further adjusted for WMH.
Model 6: Model 5 adjusted for DSST. DSST = Digit Symbol Substitution test; GMV = gray matter volume; WMH = white matter hyperintensity.
Figure 1.
Scatter plot illustrating the association between gray matter volume in the cognitive region of the cerebellum and adjusted gait speed.
To account for cerebral atrophy alone, we also measured the atrophy index without incorporating the cerebellar volumes in the atrophy index. We performed the regressions after accounting for supratentorial effects of atrophy. Removing the cerebellum from the atrophy measure did not substantially alter the strength or statistical significance of the associations between the dependent and independent measures in this analysis.
Discussion
This study found that in our sample of older adults total cerebellar GMV was associated both with gait speed and processing ability independent of age, gender, cerebral atrophy, and small vessel disease; however, adding information-processing measures in the model reduced the strength of the association between cerebellar GMV and gait speed to a statistically nonsignificant relationship. We then parcellated the cerebellum into cognitive, sensorimotor, and vestibular regions based on functional roles ascribed to the cerebellum. The associations between these three regional cerebellar GMV, gait speed, and information-processing ability varied. The cerebellar region with a predominant cognition function was strongly associated with both gait speed and information processing, whereas the region primarily involved in sensorimotor control was significantly associated with information-processing ability, not gait speed; furthermore, cerebellar region involved in vestibular functions was not significantly associated with either gait speed or information-processing ability. Furthermore, similar to the overall cerebellar GMV finding, the strength of the association between the cognitive cerebellar GMV and gait speed diminished with small vessel disease in the model; with DSST in the model, the association between cognitive cerebellar GMV and gait speed was rendered not significant. These findings suggest that specific regions in the cerebellum may play an important role in both mobility and cognition.
Cerebral control of gait includes the premotor cortex, which is involved in learning and storing movement sequences, and the motor cortex (M1), which acts on the signals generated by the premotor cortex in initiating further downstream signaling for voluntary movements of walking. In addition, there is strong evidence in support of the other areas in gait control such as frontal cortex, basal ganglia, and parietal cortex that are instrumental in the control of gait and its adaptation to personal demands and environmental challenges (24,26). Regions involved in gait control are also involved in executive function, such as the dorsolateral prefrontal cortex (Brodmann’s area 46) (27). Executive functions are associated with gait and are important for the control and adaptation of gait under various situations (16,28,29). There are several parallel pathways between cerebellar nuclei and cerebral cortical regions. For instance, the medial cerebellum is connected to primary motor cortex, premotor, and supplementary motor areas (30). The functional connectivity between the lateral regions of the cerebellum, the inferior frontal gyrus and the dorsolateral prefrontal cortex (31) extends further to the premotor and motor areas (32). The feed-forward and feed-back loops that traverse through the superior and middle cerebellar peduncles transmit and receive signals from the projections between the cerebellum and cerebral cortex (33). The cognitive region of the cerebellum, which largely constitutes the lateral part of the cerebellum, appears to be associated with gait speed and speed of processing possibly by the virtue of its reciprocal connections with specific areas of the cerebral cortex. Hence, this region of the cerebellum may be particularly important to the interface between cognition and mobility in older adults.
Is the role of the cerebellum in gait speed partly mediated by information-processing ability? The findings of this study show that while cerebellar GMV is associated with gait speed and information-processing functions, only the cognitive region is significantly associated with gait speed and information-processing ability. Upon controlling for information processing, the relationship between the cognitive region and gait speed is diminished. We did not find strong associations between volume measures of the sensorimotor or vestibular region of the cerebellum and gait speed. Therefore, it appears that the cerebellar role in gait speed control is related to its role in executive function as assessed by speed of mental processing. The attenuation of the relationship between cerebellar volume and gait speed by information-processing speed suggests that the cerebellum may impart executive control of gait that is integral to mobility in older adults in whom multisensory and motor processing, in accordance with varying environmental challenges, are necessary for maintaining one’s gait speed.
We observed that the total cerebellar GMV association with gait speed though attenuated after adjusting for cerebral small vessel disease remained statistically significant suggesting that small vessel disease was not the primary factor involved in overall cerebellar association with gait speed in our sample. These findings are supported by a previous study that showed that the association between gait speed and overall cerebellar volume was independent of WMH (14). However, the relationship between regional cerebellar GMV and gait speed was attenuated by WMH, which can be explained by the fact that WMH in frontosubcortical regions, functionally connected to cognitive regions of the cerebellum, are associated with executive function and mobility in older adults (34–38).
To the best of our knowledge, this is one of the first studies on an older adult population that uses epidemiological data to demonstrate differential associations of regional cerebellar GMV with both gait speed and information processing. Building on evidence from lesion and functional imaging studies that have ascribed functional roles to specific cerebellar regions, the findings of this study demonstrate that the cerebellar regions associated with control of gait speed are also related to information-processing ability in older adults. The cognitive region of the cerebellum appears to have the strongest relationship with gait speed and information-processing ability. These findings extend previous work demonstrating the association between cerebellar GMV, mobility (14,24), and processing speed (13). Slowing of gait and processing ability may be mediated by cerebellar aging process in addition to cerebral cortical changes (13). The association between cognitive and sensorimotor region of the cerebellum with processing speed can be explained by sensory motor integration linking motor abilities to processing abilities in these regions. The strength of association between gait speed and processing speed appears to be stronger in the cognitive region of the cerebellum suggesting that the sensory motor integration in the cerebellum occurs in these regions. The cognitive region of the cerebellum may therefore serve as an important cerebellar interface linking mobility and cognition in older adults.
Processing speed is an important cognitive function that plays an integral role in multiple cognitive processes (39) and also plays an important role in mobility in older adults. It is well known that processing speed and gait speed are closely interrelated (16,24,40,41). It is known that the two functions, processing speed and gait speed, may be mediated through common neural substrates with epicenters in the prefrontal cortices (16). Our findings add to this knowledge and suggest that the relationship between processing speed and gait speed could also hold true at the infratentorial level and specifically may relate to functional specialization of cerebellar regions such that the specific subregions of the cerebellum may be important than others for both information processing and mobility. The prefrontal cortices are linked to cerebellar regions important for executive function control (42,43). It is therefore not surprising that the cerebellar regions that are linked to the prefrontal cortical regions are also related to gait speed and processing speed. However, the association between the cognitive region of the cerebellum and gait speed retained statistical significance differences even after accounting for brain atrophy. This study suggests that an innate cerebellar functional specialization for both processing speed and gait speed may account for the infratentorial relationship between cognition and mobility in older adults.
It is noteworthy that the relationship between gait speed and the sensorimotor portion of the cerebellum was weaker than that of the cognitive region and trended toward not statistically significant after accounting for age, gender, and atrophy. There could be several reasons that could account for this. It is likely that the cerebellar sensorimotor influences on gait are modulated by cortical input to the cerebellum such as from the supplementary area, primary sensorimotor area, thalamus, and the striatum (44,45). Alternately, the relationship between mobility and sensorimotor region may be related to gait parameters other than gait speed such as stride length, which was not explored in this analysis (46). Finally, gender could have a differential effect on the cerebellum (47), but this is not well established (48).
This study has certain limitations that need to be considered. DSST is not purely a test of information processing as it also involves motor dexterity required for writing appropriate symbols on the article and it also taps into other concurrent cognitive processes. However, the DSST remains as a widely used test to measure information-processing ability in cognitive and mobility studies in aging. We excluded those with overt neurodegenerative conditions, stroke, and cognitive impairment but are unable to state that this sample was free of these conditions. Finally, we demarcated the cerebellar regions based on a standard atlas of subdivisions of the cerebellum using an automated parcellation method, which was not used across studies that reported specific motor and nonmotor roles of the cerebellum. As with any automated neuroimaging parcellation procedure, there are certain limitations on achieving optimal accuracy of the subdivisions of the cerebellum.
In summary, this study provides initial evidence, as predicted from lesion and animal studies, that cerebellar volume is linked to information processing and gait speed, with the regions related to executive function processes bearing the strongest relationship with gait speed in this community-dwelling sample of older adults.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, National Institute on Aging grant R01-AG028050, National Institure of Nursing Research (NINR) grant R01-NR012459) and the Pittsburgh Claude D. Pepper Older American’s Independence Center (P30 AG024827).
References
- 1. Holmes G. The Croonian Lectures on the clinical symptoms of cerebellar disease and their interpretation. Lecture II. 1922. Cerebellum. 2007;6(2):148–153; discussion 141. [PubMed] [Google Scholar]
- 2. Sudarsky L. Cerebellar gait and sensory ataxia. In: Bronstein AM, Brandt T, Woollacott MH, Nutt JG, eds. Clinical Disorders of Balance, Gait and Posture. 2nd ed New York, NY: Oxford University Press; 2004:163–173 [Google Scholar]
- 3. Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434 [DOI] [PubMed] [Google Scholar]
- 4. Schmahmann JD, Macmore J, Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162:852–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lagarde J, Hantkie O, Hajjioui A, Yelnik A. Neuropsychological disorders induced by cerebellar damage. Ann Phys Rehabil Med. 2009;52:360–370 [DOI] [PubMed] [Google Scholar]
- 6. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943 [DOI] [PubMed] [Google Scholar]
- 8. Ghilardi M, Ghez C, Dhawan V, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145 [DOI] [PubMed] [Google Scholar]
- 9. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579 [DOI] [PubMed] [Google Scholar]
- 10. Grafman J, Litvan I, Massaquoi S, Stewart M, Sirigu A, Hallett M. Cognitive planning deficit in patients with cerebellar atrophy. Neurology. 1992;42:1493–1496 [DOI] [PubMed] [Google Scholar]
- 11. Gottwald B, Wilde B, Mihajlovic Z, Mehdorn HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. J Neurol Neurosurg Psychiatry. 2004;75:1524–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 12th ed New York, NY: Oxford University Press; 2012 [Google Scholar]
- 13. Eckert MA. Slowing down: age-related neurobiological predictors of processing speed. Front Neurosci. 2011;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1048–1055 [DOI] [PubMed] [Google Scholar]
- 15. Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT, Jr, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simonsick EM, Newman AB, Nevitt MC, et al. Health ABC Study Group. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649 [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57 [DOI] [PubMed] [Google Scholar]
- 19. Nadkarni NK, Studenski SA, Perera S, et al. White matter hyperintensities, exercise, and improvement in gait speed: does type of gait rehabilitation matter? J Am Geriatr Soc. 2013;61:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmahmann JD, Doyon J, McDonald D, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10(3 Pt 1):233–260 [DOI] [PubMed] [Google Scholar]
- 21. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289 [DOI] [PubMed] [Google Scholar]
- 22. Grimaldi G, Manto M. Topography of cerebellar deficits in humans. Cerebellum. 2012;11:336–351 [DOI] [PubMed] [Google Scholar]
- 23. Stoodley CJ. The cerebellum and cognition: evidence from functional imaging Studies. Cerebellum. 2012;11:352–365 [DOI] [PubMed] [Google Scholar]
- 24. Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corp.; 1981 [Google Scholar]
- 26. Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. Neuroimage. 2004;21:568–575 [DOI] [PubMed] [Google Scholar]
- 27. Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010;65:1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Iersel MB, Kessels RP, Bloem BR, Verbeek AL, Olde Rikkert MG. Executive functions are associated with gait and balance in community-living elderly people. J Gerontol A Biol Sci Med Sci. 2008;63:1344–1349 [DOI] [PubMed] [Google Scholar]
- 29. Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548 [DOI] [PubMed] [Google Scholar]
- 30. Scherder E, Eggermont L, Visscher C, Scheltens P, Swaab D. Understanding higher level gait disturbances in mild dementia in order to improve rehabilitation: ‘last in-first out’. Neurosci Biobehav Rev. 2011;35:699–714 [DOI] [PubMed] [Google Scholar]
- 31. Tamada T, Miyauchi S, Imamizu H, Yoshioka T, Kawato M. Cerebro-cerebellar functional connectivity revealed by the laterality index in tool-use learning. Neuroreport. 1999;10:325–331 [DOI] [PubMed] [Google Scholar]
- 32. Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73:167–180 [DOI] [PubMed] [Google Scholar]
- 33. Tamagni C, Mondadori CR, Valko PO, Brugger P, Schuknecht B, Linnebank M. Cerebellum and source memory. Eur Neurol. 2010;63:234–236 [DOI] [PubMed] [Google Scholar]
- 34. Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654 [DOI] [PubMed] [Google Scholar]
- 35. Venkatraman VK, Aizenstein H, Guralnik J, et al. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage. 2010;49:3436–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084 [DOI] [PubMed] [Google Scholar]
- 37. Junqué C, Pujol J, Vendrell P, et al. Leuko-araiosis on magnetic resonance imaging and speed of mental processing. Arch Neurol. 1990;47:151–156 [DOI] [PubMed] [Google Scholar]
- 38. Srikanth V, Phan TG, Chen J, Beare R, Stapleton JM, Reutens DC. The location of white matter lesions and gait–a voxel-based study. Ann Neurol. 2010;67:265–269 [DOI] [PubMed] [Google Scholar]
- 39. Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54 [DOI] [PubMed] [Google Scholar]
- 40. Rosano C, Bennett DA, Newman AB, et al. Patterns of focal gray matter atrophy are associated with bradykinesia and gait disturbances in older adults. J Gerontol A Biol Sci Med Sci. 2012;67:957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin KL, Blizzard L, Wood AG, et al. Cognitive function, gait, and gait variability in older people: a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68:726–732 [DOI] [PubMed] [Google Scholar]
- 42. Schmahmann JD, Pandya DN. Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neurosci Lett. 1995;199:175–178 [DOI] [PubMed] [Google Scholar]
- 43. Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129(Pt 2):290–292 [DOI] [PubMed] [Google Scholar]
- 44. Fukuyama H, Ouchi Y, Matsuzaki S, et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett. 1997;228:183–186 [DOI] [PubMed] [Google Scholar]
- 45. Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–198 [DOI] [PubMed] [Google Scholar]
- 46. Hill KK, Campbell MC, McNeely ME, et al. Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson’s disease. Exp Neurol. 2013;241:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu J, Kobayashi S, Yamaguchi S, Iijima K, Okada K, Yamashita K. Gender effects on age-related changes in brain structure. AJNR Am J Neuroradiol. 2000;21:112–118 [PMC free article] [PubMed] [Google Scholar]
- 48. Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–1087 [DOI] [PubMed] [Google Scholar]