Figure 3.
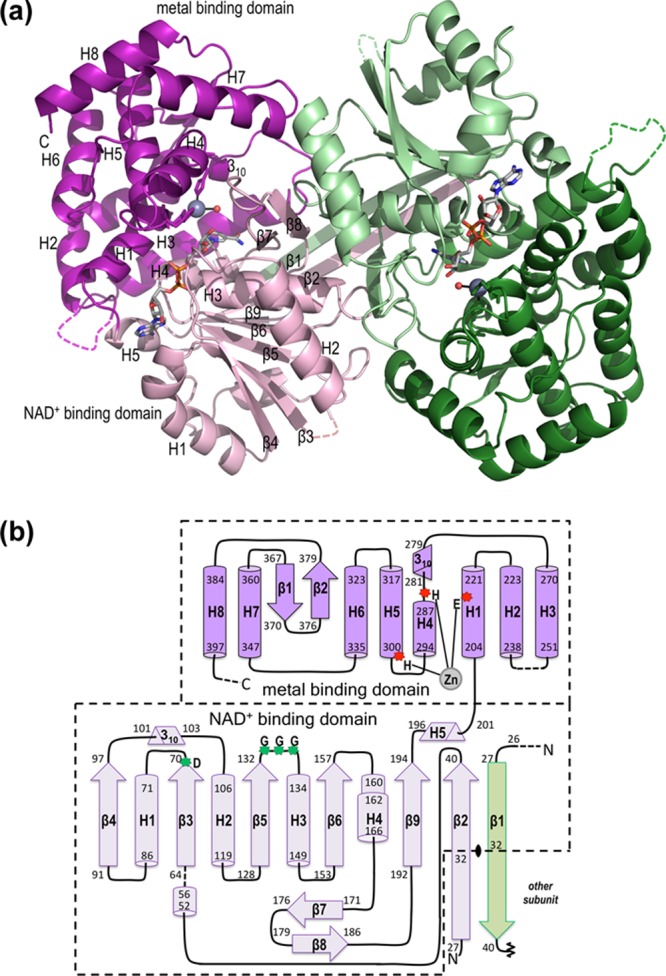
Overall structure and topology of ValA. (a) Ribbon diagrams of the two chains of the ValA dimer are colored in purple and green tones, with the N-terminal NAD+-binding domains in light hues and the C-terminal metal-binding domains in dark hues. The extended β-strands of each subunit involved in the domain-swapped arrangement are visible in the back of the dimer. Dashed lines indicate internal unmodeled backbone segments. The NAD+ and the Zn2+ with its coordinating ligands are shown (colored as in Figure 1). Secondary structural elements in each domain of one monomer are labeled. (b) Topology diagram showing α-helices (cylinders), β-stands (arrows), 310-helices (triangular prisms), and π-helices (wider cylinder) with their respective first and last residues given. The minimal length α- and 310-helices (five and three residues, respectively) are left out of the secondary structure family nomenclature. The domains are colored light and dark purple as indicated, and helices (H) and strands (β) common to the SPCs are named sequentially within each domain. The domain-swapped β-strand containing residues 27–32 from the other subunit of the dimer, but contributing to the purple domain, is colored light green. The crystallographic 2-fold rotation axis (indicated as a black vertical ellipse) relates this β-strand to the residues extending from residue 32 of the purple domain to be part of the β-sheet of the other subunit. We retain the β1 and β2 names for the two parts of the long N-teminal β-strand because they participate in different β-sheets and to maintain in this report a consensus secondary structure nomenclature relevant to the sugar phosphate cyclase superfamily. Dashed lines denote unmodeled backbone segments. The three Zn2+-binding residues (red asterisks) and the glycine-rich turn and acidic residue (green asterisks) that are important for NAD+ binding are indicated.
