Abstract
The degree of polygyny is predicted to influence the strength of direct male–male competition, leading to a high variance in male lifetime reproductive success and to reproduction limited to the prime period of adulthood. Here, we explore the variance in male lifetime reproductive success and reproductive time in an anthropoid primate forming multimale–multifemale groups. Males of this species form dominance hierarchies, which are expected to skew reproduction toward few high-ranking males. At the same time, however, females mate with multiple males (polygynandry), which should limit the degree of polygyny. Using 20 years of genetic and demographic data, we calculated lifetime reproductive success for the free-ranging rhesus macaque (Macaca mulatta) population of Cayo Santiago for subjects that died naturally or reached senescence. Our results show that 1) male lifetime reproductive success was significantly skewed (range: 0–47 offspring; males reproducing below average: 62.8%; nonbreeders: 17.4%), 2) variance in male lifetime reproductive success was 5 times larger than in females, and 3) male lifetime reproductive success was more influenced by variation in fecundity (60%) than longevity (25%), suggesting that some direct male–male competition takes place. However, the opportunity for selection (i.e., standardized variance in male lifetime reproductive success) is low compared with that in other large mammal species characterized by a high degree of polygyny. Moreover, male reproductive life extended much beyond the prime period, showing that physical strength was not required to acquire mates. We conclude that rhesus macaques exhibit a moderate degree of polygyny and, therefore, low levels of direct male–male competition for fertile females, despite the fact that males form linear dominance hierarchies.
Key words: lifetime reproductive success, male–male competition, mammals, opportunity for selection, reproductive skew, reproductive timing, rhesus macaques.
Lay summary
Male rhesus macaques face moderate strength of direct competition for fertile mates, creating opportunity for indirect forms of competition. Using 20 years of genetic and demographic data, we show that variance in lifetime reproductive success is more pronounced in males than in females. Yet, most males reproduce in their lifetime, that surviving as long as possible increases males’ success, and that males reach high fecundity before the full development of secondary sex characteristics.
INTRODUCTION
Sexual selection is proposed to explain the evolution of traits that provide advantages in terms of reproductive rate (or fecundity) rather than in survival (or longevity) (Darwin 1871). This evolutionary process is usually stronger in males than in females. Indeed, because female reproductive rate is limited by gamete production and parental investment, leading to their lifetime reproductive success (LRS) being directly linked to survival and health (Bateman 1948; Trivers 1972). In contrast, male LRS is determined by the number of mating partners that can be acquired per unit of time, leading to a strong influence of fecundity (Bateman 1948; Trivers 1972; Clutton-Brock 1988). This difference between males and females should be particularly pronounced in large placental mammals because 1) female reproductive rate is low and 2) most parental tasks can only be provided by females (e.g., gestation and lactation), leading to male reproductive investment often limited to mating effort, which in turn creates a strong opportunity for sexual selection.
The mating system is expected to influence the extent to which variance in LRS is more pronounced in males than in females and, consequently, the strength and form of sexual selection on male traits (Clutton-Brock 1988, 1989). For example, in a polygynous mating system, a small number of males are able to monopolize fertile females and exclude other males from reproduction, leading to a high variance in male LRS within populations (Wade 1979; Andersson 1994; Arnold and Duvall 1994; e.g., elephant seal, Mirounga angustirostris: Le Boeuf and Reiter 1988; red deer, Cervus elaphus: Pemberton et al. 1992; Marshall et al. 1998; bighorn sheep, Ovis canadensis: Coltman et al. 2002; green swordtail, Xiphophorus helleri: Tatarenkov et al. 2008). This generates a high level of direct male–male competition for fertile females, which selects for male traits that give advantages in contest, such as large male body size and various forms of weaponry (e.g., antlers, long sharp canines; Alexander et al. 1979; Loison et al. 1999). Because reproduction depends on physical strength, only the strongest and largest males of the population are able to reproduce, and male reproduction is expected to peak over few years of high fecundity during the prime period, after which males die or stop reproducing (e.g., Clutton-Brock 1988). As the degree of polygyny decreases and female’s number of mating partners increases, the variance in male LRS is expected to decrease, becoming more similar to that of females (Wade 1979; Andersson 1994; e.g., Soay sheep, Ovis aries: Coltman et al. 1999; European roe deer, Cepreolus capreolus: Vanpé et al. 2008; eastern chipmunks, Tamias striatus: Bergeron et al. 2012). In species where females mate with multiple males, male reproduction relies to a lesser extent on the ability to win fights and, hence, sexual dimorphism in size and weaponry are less pronounced. Moreover, male reproduction is less constrained to the prime period, with longevity directly influencing LRS (e.g., greater horseshoe bats, Rhinolophus ferrumequinum; Rossiter et al. 2006), leading to reproductive timing and life spans that are more similar to those of females than in species facing a high degree of polygyny. Consequently, the extent to which 1) variance in male LRS is pronounced and 2) reproductive timing is limited to a few years of high fecundity should provide some information about the degree of polygyny a given species is facing and, thus, about its mating system.
The standardized variance in LRS (i.e., variance divided by the average in LRS) is often used to compare the degree of polygyny between species, a measure referred to as “opportunity for selection” (Wade and Arnold 1980; Arnold and Wade 1984). This measure is believed to provide an estimation of the upper limit of the strength of the directional sexual selection in a population (Wade 1979; Arnold and Wade 1984; Shuster and Wade 2003; Jones 2009; Klug, Heuschele, et al. 2010). However, this measure is not sufficient to compare the degree of polygyny between species because it can be affected by the sampling method (e.g., number of years, rank, age, and residency status of males included in the calculation) and it only provides an estimation of whether strong selection can take place (e.g., Shuster and Wade 2003; Klug, Heuschele, et al. 2010; Klug, Lindstrom, et al. 2010; Krakauer et al. 2011; Jennions et al. 2012). One way to complement this approach is to explore how variation in fecundity and longevity contributes to the variance in LRS (Arnold and Wade 1984; Brown 1988; e.g., Coltman et al. 1999), and the extent to which reproductive output is concentrated to a few years of high fecundity during prime.
Variance in male LRS has only been assessed in a limited number of large mammals, mainly in ungulate species (cf. Vanpé et al. 2008). The limited work on this topic is likely to be due to the difficulty of obtaining such data. Indeed, assessment of LRS in large mammals is greatly limited by the extended life span. It is particularly difficult to assess LRS for male mammals because males typically disperse multiple times throughout their lives and frequently disappear from the study area (Greenwood 1980; Lawson Handley and Perrin 2007). Moreover, because mating activity is not a reliable estimate of reproductive success and, as opposed to females, males of most species do not maintain close proximity to their litter, assessing reproductive success must be based on genetic paternity analyses. Finally, short-term studies have long been suspected to provide poor estimates of the variance in male LRS. In species facing a high degree of polygyny where male reproduction is limited to a short period of time at their prime, the variance can be overestimated by excluding males that only reproduced before or after the study period (Altmann 1979; Clutton-Brock 1988; Alberts et al. 2006). In contrast, they can underestimate the variation in species facing low degree of polygyny because variation in male longevity contributes to the variance in male LRS (Rossiter et al. 2006; see also Johnston et al. 2013). As such, a complex sampling management maintained over a long period of time is needed to assess male LRS based on genetic paternity assignment in large mammals.
Here, we assess variance in male LRS and reproductive timing for an anthropoid primate, the rhesus macaque (Macaca mulatta) (see Bercovitch 1997; Plavcan 2001). This species forms multimale–multifemale groups (Shultz et al. 2011) in which several immigrant males live year-round with unrelated females. The genetic mating system of this species is unclear. On the one hand, males form linear dominance hierarchies, which suggests a certain degree of polygyny (Altmann 1962). On the other hand, however, the presence of several male competitors facilitates female polyandry (Chapais 1983; Manson 1992), even during the periovulatory phase of the ovarian cycle (Dubuc et al. 2012; see also Chapais 1983; Small 1990). Collectively, evidence accumulated from short-term studies suggests that this species exhibits a low degree of polygyny and, thus, low levels of direct competition for fertile females (Berard 1999; Dubuc et al. 2011). Indeed, there is a relatively low yearly correlation between dominance rank and reproductive success, with the alpha male often not being the most successful sire of the group (Berard et al. 1993, 1994; Widdig et al. 2004; Dubuc et al. 2011). Moreover, males can be highly successful as young adults—that is, when they are not yet fully developed—and can reproduce years beyond their prime (Bercovitch et al. 2003). Finally, males are rarely observed to compete for dominance and rather appear to form dominance hierarchies through queuing (Berard 1999; see also Drickamer and Vessey 1973; Higham et al. 2013), suggesting that the costs of fights are not outweighed by the potential benefits in terms of reproductive output associated with reaching a high-ranking position (van Noordwijk and van Schaik 2004). However, because males of this species have an extended reproductive life (Bercovitch et al. 2003) and residency and tenure lengths can last several years (e.g., Berard 1999), short-term patterns may not provide accurate insights into the degree of polygyny and variance in male LRS in this species.
In order to estimate the degree of polygyny, we compared the variance in LRS and patterns of reproductive timing between males and females of the same population and also compared these characteristics with those of other species. Of particular interest, variance in male LRS has been assessed for one other anthropoid, the mandrill (Setchell et al. 2005a). Although the 2 species are classified as having similar social organization and mating systems, mandrills show a strong yearly correlation between male dominance rank and reproductive success leading to a very pronounced alpha male share in paternity (Setchell et al. 2001; Charpentier et al. 2005), suggesting that the mandrill genetic mating system is characterized by a higher degree of polygyny than that of the rhesus macaque. Supporting this view, mandrills are one of the most sexually dimorphic species of all anthropoids (Setchell et al. 2005b), whereas rhesus macaques rather show moderate levels (Plavcan and van Schaik 1997; Plavcan 2001). Consequently, we predict that the standardized variance in male LRS and the contribution of fecundity will be less pronounced and closer to that of females in rhesus macaques compared with mandrills, with reproduction less limited to the prime period.
Our study was based on 20 years of data taken from the population of Cayo Santiago, using one of the most comprehensive demographic and genetic databases on males for any free-ranging primate population. Indeed, the population lives on an island with several naturally formed groups ranging freely, allowing males to be tracked throughout their lives and their deaths to be confirmed. The sampling method, thus, allows all sexually mature males of the population to be considered when assessing variance in LRS, even those who failed to immigrate into a social group, preventing sampling biases and the pitfalls associated with assessing variance in reproductive success addressed above.
METHODS
Study site and population
The study was conducted on the free-ranging population of rhesus macaques living on Cayo Santiago, a 15-ha island off the coast of Puerto Rico. The colony was established in 1938 when approximately 400 monkeys from various locations in India were transferred to the island (Rawlins and Kessler 1986). Since then, individuals have only been added to the population via natural births. Genetic analyses from pedigree data show no sign of inbreeding (Widdig A, unpublished data). During the study period, the population was composed of 800–1000 individuals divided into 4–14 naturally formed heterosexual troops (average number of groups: 7.5) that ranged freely throughout the island, allowing for intergroup encounters and male dispersal between groups. All research procedures were approved by the Caribbean Primate Research Center (CPRC) and the Institutional Animal Care and Use Committee (IACUC) of the University of Puerto Rico (protocol number 4060105) and the export of DNA samples was approved by the U.S. Fish and Wildlife Service (FWS) (Cites Export permission # 05US101361/9, # 06US112079/9, # 07US133766/9, # 08US163309/9, # 09US200870/9, # 09US230435/9, # 11US28371A/9, Cites Import permission # E-1426/05, # E-1082/06, # E-1207/07, # E-1215/08, # E01146/09, # E-00049/10, # E-00836/11).
Population management
The population is managed by the CPRC. The animals are provisioned with commercial high-protein biscuits distributed once daily in the morning at the 3 food dispensers located in corrals (Rawlins and Kessler 1986). Nevertheless, macaques forage extensively on natural sources, for example, foliage, fruits, insects, and soil, which represent 50% of their feeding time (Marriott 1988; Marriott et al. 1989). Water is offered ad libitum via the collection of rainfall in cisterns and piping of water into drinking basins. There is no medical intervention on the population, aside from a tetanus inoculation given when captured as yearlings and a booster shot at age (Rawlins and Kessler 1982; Kessler et al. 1988, 2006).
Since 1992, all members of the population were sampled for genetic paternity analyses when they were trapped as yearlings for the purpose of individual tattooing and vaccination. Population size and adult sex ratio of ≈1:1.3 has been maintained via a mixture of natural death and culling strategies (see Hernández-Pacheco et al. 2013). The main culling strategy involved random culls of juveniles (46.1%), mainly at 1–2 years of age (1 year old: 13.7%; 2 years old: 23.2%; 3 years old: 9.2%), with a small bias toward males (54%). In addition, the management removed 3 social groups that accounted for 50% of the population in 1984, after which social groups (mainly fissions of well-established groups) were removed in 9 instances over the study period, leading to 15.8±16.5% of the individuals of each cohort culled at some point during their reproductive life (age culled: 8.7±4.3 years old) during the study period. The CPRC provides a detailed demographic database that includes dates of birth and death, birth group, maternal relatedness, dispersal events, and group allocation, and the team of CPRC census takers has monitored the population daily since 1956.
Selection of male and female subjects
We selected as subjects the members of the population that were born between 1987 and 1993, that is, individuals for whom the entire reproductive life could have been covered by the 20-year study period for which comprehensive paternity data were available (birth seasons 1992–2011). As such, our study included males and females that 1) could reach sexual maturity (4 years old; based on Bercovitch and Berard 1993; Bercovitch et al. 2003) not earlier than the first year of our study (1992) and 2) could reach senescence (17 years old; based on Bercovitch 1997) not later than the last year of our study (2011). Hence, the age range of 4–17 years was only used for selection of subjects, with all years of potential reproduction over the entire lifetime being considered in our analysis. We only included individuals who either died of natural causes during the period covered by the study period or who reached the age of the estimated onset of senescence. Therefore, we removed from the analyses all individuals culled by colony management from our analysis as juveniles (N = 539 males and 439 females), regardless of the age at which they were culled, and those for which genetic material was not available (N = 14 males and 20 females). As a result, 211 males and 275 females born between 1987 and 1993 were included as subjects in the study. Most analyses are limited to the subjects that reached sexual maturity (i.e., 4 years old), which represents 86 males and 132 females.
Calculation of LRS
We calculated LRS based on the individuals born between 1992 and 2011 during which yearlings were systematically sampled for genetic paternity analyses. LRS was calculated based on the number of offspring produced that survived their first year of life, that is, offspring that survived the period of life showing the highest mortality (for study population, see Sade et al. 1976; Blomquist 2013; see also Dittus 2004). Of the 4524 infants born between 1992 and 2011, 3311 reached at least 1 year old (73.2%), while 809 died within the first year of their life (17.9%), and 404 were culled along with their mothers during their first months of life (8.9%). Of the remaining 3311 individuals, genetic material was not available or not of sufficient quantity for 126 (3.8%) and 5 (0.15%), respectively; almost all of these cases (116 out of 131) concern infants born in the first 3 years of the study (1992–1994) when sampling efforts were not yet fully implemented. As such, 3180 offspring were considered to calculate variance in LRS.
Paternity assignment
Since 1992, biological samples (mainly blood) were collected from all individuals of the population surviving the first year of life to construct a genetic database using on average 14 markers out of a panel of 21 markers (for sampling details, see Nürnberg et al. 1998; Kulik et al. 2012). Starting in 2008, the CPRC increased the power of analysis of the previous microsatellite marker panel by adding 29 markers, including 1 sex-linked marker (compare Rogers et al. 2006), resulting in a total of 43 markers used in this study. Furthermore, protocols have been optimized over time in order to incorporate a multiple tubes (following Morin et al. 2001) and multiplex approach (Henegariu et al. 1997; Bonhomme et al. 2005).
Excluding the sex-linked marker (DXS2506), our data showed no deviation from Hardy–Weinberg equilibrium or presence of null alleles. The mean number of alleles was 8.10±2.96 per locus, the mean observed heterozygosity across loci was 0.72±0.09, the mean expected heterozygosity was 0.72±0.09, and the mean polymorphism information content over all 43 loci was 0.68±0.10 (all values calculated with Cervus 3.0, Kalinowski et al. 2007). The error rate, calculated as the proportion of mother–offspring dyads that had at least 1 mismatching locus, was 0.02 in the presented data set (N = 3154 mother–offspring dyads).
The database analyzed for this study consists of 20 years of genetic data including 3180 animals sampled (number of markers: mean ± standard deviation: 35.24±5.99, range: 13–43), representing 70.53% of all individuals born between 1992 and 2011 (i.e., those conceived during the 1991–2010 mating seasons). Maternity information from census records was confirmed using genotypic data for 3048 of 3180 total individuals (95.85%) and, if available, this information was subsequently used in paternity analyses. Based on evidence of extragroup paternity (Widdig et al. 2004), all males on the island were considered as potential sires if they fulfilled 2 criteria: males had to be 1) alive at least 1 month before conception of the offspring (i.e., counting back 200 days from the date of birth, following a gestation length estimate of 166.5±7.4; Silk et al. 1993) and 2) older than 1250 days of age (based on youngest possible age at first reproduction; Bercovitch et al. 2003) on the birth date of the offspring.
Paternity was determined for 3107 of 3180 potential offspring sampled (97.70%) using a combination of exclusions and likelihood calculations. In total, 3062 mother–sire–offspring trios had at least 26 overlapping markers, 21 mother–sire–offspring trios had at least 13 overlapping markers (for which new markers could not be used), and 24 sire–offspring duos who had at least 28 overlapping markers. For 3026 of these, the assigned sire had no mismatch with the respective mother–offspring pair, and all other potential sires could be excluded on at least 2 loci. For 12 potential offspring, the assigned sire had no mismatch with the respective mother–offspring pair, but one other potential sire could only be excluded at 1 locus. However, all of these paternity assignments were supported at the 95% confidence level by the maximum likelihood method calculated by CERVUS 3.0. For the remaining 69 potential offspring, only 1 male of the island had a single mismatch; however, we accepted these cases because we typed the mother–father–offspring trio at least on 20 common markers and paternity was confirmed at the 95% confidence level by the maximum likelihood method calculated by CERVUS 3.0. Overall, for 73 offspring, we did not assign paternity either through exclusion or likelihood calculations, with 60 of these born in 1995 or before when sampling efforts were less efficient. However, we felt confident about including these offspring with unknown paternity in our study, as our study males (if present and mature at the time of conception of a given infant) were included in the paternity analysis and consequently excluded as sires by at least 2 mismatches, suggesting that they were not the father of those offspring and that a nonsampled male actually sired the offspring considered. Of the 3107 offspring for which we assigned paternity, a total of 752 offspring were sired by one of the male subjects selected for the study.
Data analyses
Variance in LRS
We used 3 approaches to assess whether LRS was skewed among males and females that reached sexual maturity. Firstly, we calculated the standardized variance in male LRS (I m) in which variance in LRS is divided by the square of the average in LRS (i.e., ) (Wade and Arnold 1980; Arnold and Wade 1984), a method referred to as “opportunity for selection” commonly used in other studies, which allows interspecific comparison (e.g., Madsen and Shine 1994; Coltman et al. 1999; Rossiter et al. 2006; Tatarenkov et al. 2008; Vanpé et al. 2008; Bergeron et al. 2012). Higher I m values indicate that only a fraction of males contribute to the gene pool of the next generation. Based on the range shown for mammalian species (cf. Rossiter et al. 2006; Tatarenkov et al. 2008; Vanpé et al. 2008), we considered values of I m < 1 to show weak opportunity for selection, values between 1 and 3 moderate opportunity, and values >3 high opportunity for selection.
Secondly, we calculated the Nonacs’ B index (Nonacs 2000, 2003) with the program Skew Calculator 2003 (http://www.eeb.ucla.edu/Faculty/Nonacs/PI.html), which tests whether the distribution in LRS is different from expected by chance. Although the actual range depends on the characteristics of the data set, as a general rule, a random distribution generates B values close to 0 or negative, whereas a perfect monopoly generates values close to 1.
Thirdly, we compared the variance in LRS between males and females using a Kolmogorov–Smirnov test (KS test) and divided I m by the standardized variance calculated for females (I m/I f). This ratio allows for estimation of the opportunity for sexual selection by accounting for the variance due to females (Wade and Arnold 1980; Clutton-Brock 1983, 1988; for similar approach, see Vanpé et al. 2008; Bergeron et al. 2012).
Contribution of fecundity and longevity to LRS
We then used 3 approaches to tease apart the contribution of fecundity and longevity to LRS for both males and females. We expected that the higher the opportunity for direct male–male competition, the more pronounced the role of fecundity would be among males.
Firstly, we calculated the relative contribution of longevity (reproductive life span starting at 4 years old) and fecundity (reproductive rate throughout reproductive life) to the total variance in male LRS, an approach that allows interspecific comparison (Arnold and Wade 1984; Brown 1988; for similar approaches, see Madsen and Shine 1994; Coltman et al. 1999; Setchell et al. 2005a; Vanpé et al. 2008). The portion of the total variance due to fecundity is considered to be a good estimator of the potential strength of sexual selection processes, whereas the portion of the variance due to longevity estimates the potential contribution of natural selection processes (Arnold and Wade 1984; Brown 1988). We calculated longevity (L) as the number of mating seasons spent in the population once sexually mature and fecundity (F) as the average yearly reproductive rate during this period (i.e., ≥4 years old). We calculated the contribution of these 2 components to the variance among breeders (i.e., subjects that produced at least 1 offspring) based on the formula [ ], where and are the variance and mean in longevity, respectively, and and are the variance and mean in fecundity (Brown 1988). Q LF was calculated by subtracting the 2 other components of the equation from I. Q LF can be seen as the contribution of the joint variation of longevity and fecundity among breeders, with positive values indicating some degree of positive association between fecundity and longevity (Coltman et al. 1999). F, L, and Q LF are presented as the percentage of contribution to I (e.g., ). Then, in order to assess the contribution of nonbreeders to the variance in LRS, we calculated the contribution of breeders and nonbreeders (i.e., subjects that never reproduced), respectively, as [] and [], where p B is the proportion of breeders, and and are variance and mean of breeders LRS, respectively (Brown 1988; see also Coltman et al. 1999; Vanpé et al. 2008). In a context of high degree of polygyny, one would expect a large portion of the males to never breed, leading to nonbreeders accounting for a large portion of the variance. We then applied this to all males of the population by including the individuals that died before reaching maturity (i.e., immatures) for comparison purposes.
Secondly, we recalculated the skew in LRS, among subjects that reached sexual maturity, this time taking into account male longevity in the calculation of the B index (B Longevity). Indeed, calculation of the B index offers the possibility to take into account differential time access to fertile females, which can influence the skew independently of behavioral tactics (see Nonacs 2000, 2003). In a context of high degree of polygyny, one would expect a skew in male LRS to be detected even if longevity is taken into account. However, in a context of very low degree of polygyny, differential longevity is expected to contribute the variance in LRS, which could lead to a B Longevity being not different from a random distribution, as for females.
Thirdly, we explored how reproductive output and life span are distributed across life for males and females. The higher the degree of polygyny, the more male reproduction is expected to be concentrated during the prime period, and the more males are expected to die at a young age. Based on Bercovitch (1997), males were considered to be at their prime from 9 to 12 years of age, with other age-classes referred to as young (5–8 years old), postprime (13–16 years old), and senescent (17–20 years old). We plotted the average yearly fecundity per age group for males and females in order to visually explore whether male reproduction peaks during their prime or whether it extends over a longer period. In a context of high degree of polygyny, one would expect the difference in fecundity to be limited to the period of prime, with no difference in average yearly output detected between other age categories. We used the Friedman test to compare fecundity between age-classes among males and females (N = 30 males and 74 females that reproduced in all categories). Next, we used a Mann–Whitney U test to compare the maximal fecundity, ages at first and last reproduction, and life span between females and males. Moreover, in order to explore whether all males of different LRS followed the same pattern of reproductive timing, we also compared these 4 dependent variables between 4 male breeder categories based on their LRS using quartiles: top breeders (≥ quartile 3, i.e., top 25%, N = 22), good breeders (between quartiles 2 and 3, N = 22), poor breeders (between quartiles 1 and 2, N = 22), and bottom breeders (between quartiles 0 and 1, i.e., bottom 25%, N = 20) using Kruskal–Wallis test.
Statistical analyses
Statistical analyses were performed in IBM SPSS Statistic 20. Level of significance was set at α = 0.05.
RESULTS
Variance in male LRS
Among males reaching sexual maturity (i.e., ≥4 years old; N = 86), male LRS ranged from 0 to 47 offspring, with an average of 8–9 offspring produced in a males’ lifetime (Table 1). Reproductive life lasted on average 9.78±5.85 years long (range: 1–20) during which males actually produced offspring on average in 4.80±2.97 breeding years (range: 1–12). Male average yearly fecundity was 0.80±0.75 offspring (range: 0–3.1) during their reproductive lives, with 2.01±0.99 offspring (range: 1–6.2) produced during the years they actually bred and 3.54±2.31 offspring (range: 1–12) during their most successful year (maximal yearly fecundity). Top breeders produced on average 21.9±2.3 offspring over their lifetime (range: 13–47), good breeders produced 8.5±0.4 offspring (range: 6–12), poor breeders produced 3.5±0.2 offspring (range: 2–5), and bottom breeders produced 0.25±0.10 offspring (range: 0–1).
Table 1.
Distribution of LRS in both males and females of the population
| All subjects | Sexually mature | Senescent | ||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| N | 211 | 275 | 86 | 132 | 30 | 78 |
| Mean age at death | 9.6 | 12.0 | 12.8 | 15.7 | 19.9 | 19.1 |
| % subjectsa | 100 | 100 | 40.8 | 48.0 | 14.2 | 28.4 |
| % breedersb | 33.6 | 45.8 | 82.6 | 95.5 | 100 | 98.7 |
| % topc | 10.4 | 27.6 | 25.6 | 57.6 | 46.7 | 84.6 |
| Range LRS | 0–47 | 0–16 | 0–47 | 0–16 | 2–47 | 0–16 |
| Average LRS () | 3.6 | 3.7 | 8.7 | 7.7 | 14.3 | 10.0 |
| Variance LRS (σ2) | 58.5 | 22.3 | 98.7 | 15.4 | 131.9 | 7.3 |
We focused the analyses on the individuals that reached the age at sexual maturity (≥4 years old) but also provide figures when considering all the individuals that were born in 1987–1993 (i.e., all subjects) and those that reached senescence (≥17 years old) for comparative purposes.
aPercentage of subjects that reached that age category.
bPercentage of subjects that fit this category that produced at least 1 offspring in their lifetime.
cPercentage of subjects that produced a number of offspring in the top 25% of the range (based on quartile 3). The value for males is 13 offspring and that for females is 8 offspring.
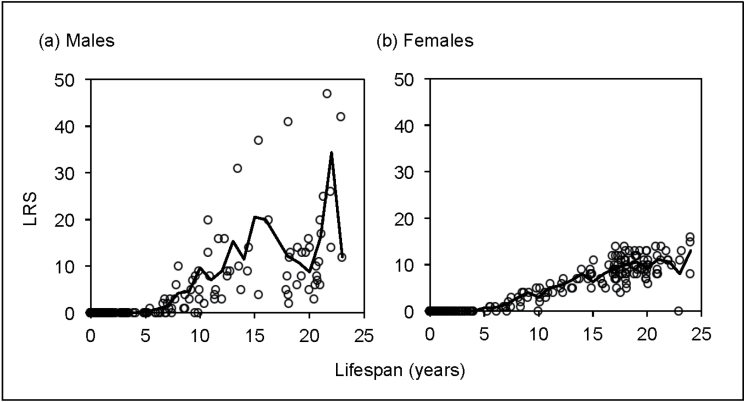
Male LRS was skewed toward certain males in the study population (Figure 1a). A total of 82.6% of all males reaching sexual maturity reproduced, and 37.2% of those produced more offspring than average (Table 1). Hence, 17.4% of mature males never reproduced in their life. Moreover, only 5 males (5.8% of the males) accounted for a quarter of the offspring produced (26.3%), and only 14 males (16.3% of the males) accounted for half of the offspring (49.7%). Variance in male LRS is not highly pronounced, however. Indeed, the opportunity for selection I m = 1.29 is lower than 3 and close to 1, suggesting a moderate to low level (see Methods). Moreover, although the observed distribution of male LRS was significantly different from a random distribution (B = 0.0136, P < 0.001), the B value is far closer to 0 than to 1 and, thus, closer to a random distribution than to a monopoly.
Figure 1.
Distribution of (a) male (N = 211) and (b) female (N = 275) LRS in relation to life span. Each data point illustrates the LRS of a subject, whereas the line illustrates the average LRS of individuals that reached that age. Only males and females who died of natural causes or reached senescence (≥17 years old) were included as subjects in the study; only offspring that reached 1 year of age were considered in the calculation of LRS.
Variance in LRS was more pronounced for males than for females. Indeed, the opportunity for selection for females I f = 0.26 is lower than 1 (suggesting a low level), and the ratio I m/I f is of 4.99, meaning that the opportunity for selection is 5 times higher for males than for females. Accordingly, although LRS was also significantly skewed for females (B = 0.001, P < 0.001), the variance is higher for males than for females (Kolmogorov–Smirnov: Z = 1.482, P-exact = 0.010, N = 86 males, 132 females; Figure 1).
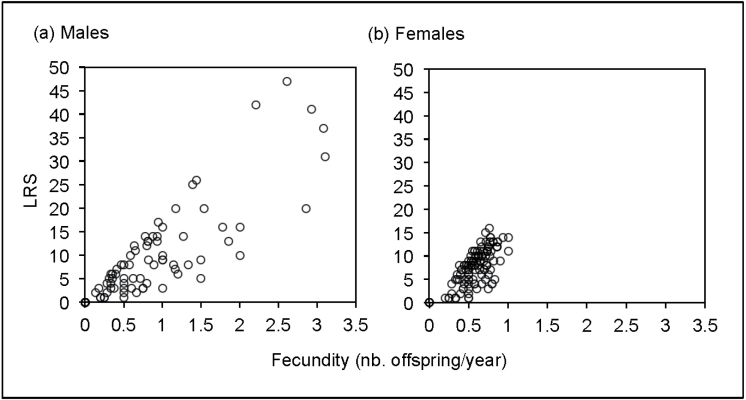
Contribution of fecundity and longevity to male LRS
Fecundity explains 50–60% of the total variance in LRS among breeding males, whereas longevity accounts for only 20–25% (Figures 1a and 2; Table 2). In contrast, fecundity and longevity contribute to 6–8% and 50–60% to the variance in female LRS, respectively (Table 2). Positive Q LF values reveal a positive joint variance of fecundity and longevity for both sexes, although it is over 2 times more pronounced for females (Table 2). Moreover, when nonbreeders are included in the analyses, death before reaching maturity (i.e., immatures) is the main factor explaining the variance in female LRS, whereas variation in fecundity still explains a large part of variance among males (Table 2). Further support of the larger impact of fecundity than longevity in the observed variance is provided by the skewed distribution of male LRS even if life span is accounted for (B Longevity = 0.009, P < 0.001), whereas females show no such effect (B Longevity = −0.0002, P = 0.960).
Figure 2.
Association of LRS with fecundity (i.e., average number of offspring produced per year) for (a) male (N = 86) and (b) female (N = 132) that reached sexual maturity.
Table 2.
Partitioning of total variance in LRS (in %) into its main components for 3 samples: 1) breeders, 2) sexually mature (≥4 years old), and 3) all subjects based on Brown’s (1988) equation
| Components | Males | Females | ||||
|---|---|---|---|---|---|---|
| Breeders | Sexually mature | All subjects | Breeders | Sexually mature | All subjects | |
| Breeders | 100.0 | 83.6 | 57.3 | 100.0 | 81.6 | 27.1 |
| Longevity (L) | 26.0a | 21.7 | 14.9 | 64.9b | 52.9b | 17.6 |
| Fecundity (F) | 62.9b | 52.6b | 36.1a | 7.6 | 6.2 | 2.1 |
| Q LF | 11.0 | 9.2 | 6.3 | 27.5a | 22.4 | 7.5 |
| Nonbreeders | — | 16.4 | 42.7 | — | 18.4 | 72.9 |
| Matures | — | 16.4 | 4.6 | — | 18.4 | 2.9 |
| Immatures | — | — | 38.1a | — | — | 70.0b |
The total variance due to breeders is divided into the contribution of longevity (L), fecundity (F), and their interaction (Q) (see Methods for details). Contribution of nonbreeders (i.e., subjects that did not reproduce during their lifetime) is divided into the contribution of subjects that died before reaching maturity (i.e., “immatures”) and those that reached sexual maturity (i.e., “matures”).
aFactors explaining ≥25% of the variance.
bFactors explaining ≥50% of the variance.
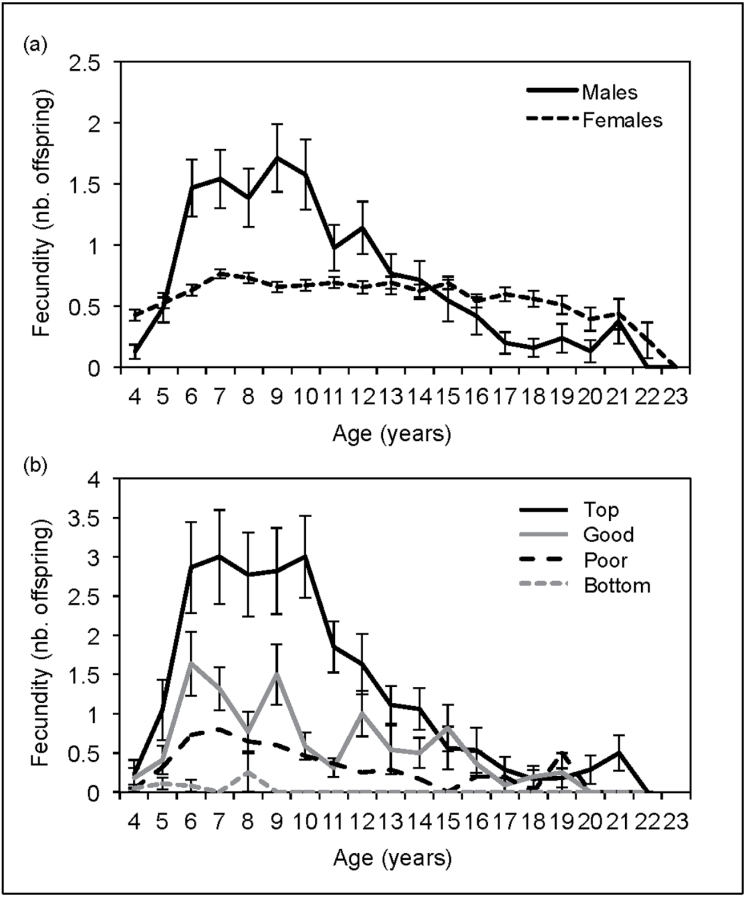
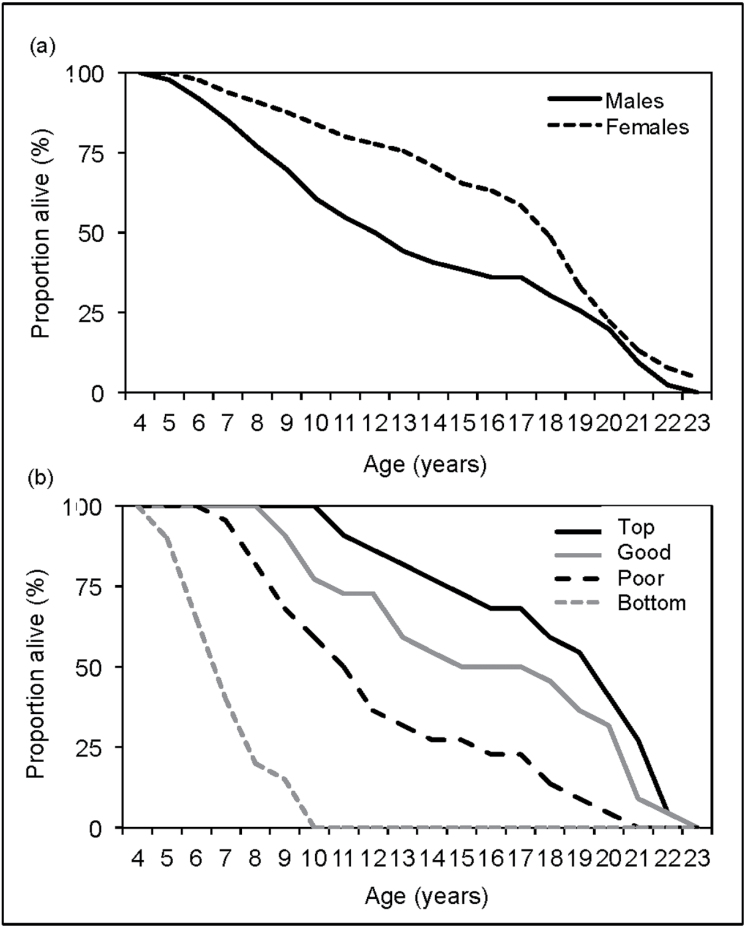
Males’ fecundity varies across lifetime between different age categories (χ2 = 42.383, degrees of freedom [df] = 3, P < 0.001, N = 30). Indeed, in contrast to females, males showed a peak in reproduction at 6–12 years of age, that is, in the young and prime age-classes, after which it constantly decreased (Figure 3a and Table 3). Although female fecundity varies across age categories (χ2 = 9.083, df = 3, P = 0.028, N = 74), this is due to a lower fecundity during the senescence period than in the 3 other periods (Table 3 and Figure 3a). Furthermore, although there is no difference in life span (i.e., age at death) between the sexes (U = 3833.0, Z = −1.671, P = 0.095; N = 71 males and 126 females) (Figure 4a and Table 4), females start reproducing younger (U = 819.0, Z = −9.762, P < 0.001) and stop reproducing later than males (U = 3217.5, Z = −3.277, P = 0.001) (Figure 3a and Table 4). Male reproduction is, therefore, more concentrated over a certain period of time than female reproduction, but less than expected in species facing high degree of polygyny.
Figure 3.
Average ± standard error of the mean (SEM) reproductive output per age (a) for males (N = 86) and females (N = 132) that reached sexual maturity (≥4 years old) and (b) for males classified based on their reproductive success: top (N = 22 males), good (N = 22), poor (N = 22), and bottom (N = 20) breeders. Note that the x axis refers to the age at the time of birth of the offspring (rather than age at conception).
Table 3.
Average ± SEM fecundity (number of offspring/years) for males and females across 4 age categories: young (N = 84 males and 131 females), prime (N = 59 males and 114 females), postprime (N = 38 males and 98 females), and senescent (N = 30 males and 75 females)
| Young (5–8 years old) | Prime (9–12 years old) | Postprime (13–16 years old) | Senescent (17–20 years old) | |
|---|---|---|---|---|
| Males | 1.09±0.13 | 1.31±0.17 | 0.68±0.13 | 0.15±0.05 |
| Females | 0.64±0.02 | 0.65±0.02 | 0.64±0.03 | 0.55±0.04 |
Figure 4.
Proportion of subjects alive per age (a) for males and females and (b) for males classified based on their reproductive success: top (N = 22 males), good (N = 22), poor (N = 22), and bottom (N = 20) breeders.
Table 4.
Average ± SEM age at first reproduction, age at last reproduction, and age at death and fecundity (number of offspring/years) for males (N = 86) and females (N = 132) that reached sexual maturity (≥4 years old), with males classified based on their reproductive success as top (N = 22 males), good (N = 22), poor (N = 22), and bottom (N = 20) breeders
| Fecundity | Age at first | Age at last | Death | |
|---|---|---|---|---|
| Males | 0.79±0.08 | 7.04±0.15 | 12.85±0.52 | 12.78±0.63 |
| Top | 1.65±0.49 | 6.64±0.17 | 15.50±0.52 | 17.45±0.83 |
| Good | 0.85±0.37 | 7.14±0.09 | 13.64±0.28 | 15.27±1.05 |
| Poor | 0.52±0.22 | 7.46±0.05 | 10.82±0.15 | 11.55±0.94 |
| Bottom | 0.07±0.10 | 6.60±0.03 | 6.60±0.10 | 6.25±0.03 |
| Females | 0.60±0.02 | 4.83±0.10 | 14.89±0.42 | 15.07±0.43 |
Although there is no difference in age at first reproduction for males of different breeder categories (Kruskal–Wallis χ2 = 4.843, df = 2, P = 0.088, N = 66), males differ in terms of age at last reproduction (χ2 = 14.590, df = 2, P = 0.001, N = 66) and death (χ2 = 49.446, df = 3, P < 0.001, N = 86). Indeed, in contrast to poor and bottom breeders, top and good breeders typically breed and survive passed their period of prime (Table 3). Therefore, the most successful males of the population reproduced at a higher rate over a longer period of time than least successful males (Figure 3b).
DISCUSSION
Male LRS was significantly skewed in the studied population of rhesus macaques, with only one-third of all males that reached sexual maturity contributing more than average to the genetic pool of the next generation and with approximately one-fifth of sexually mature males not contributing at all over their lifetime. As predicted by sexual selection theory, the standardized variance in LRS was 5 times more pronounced among males than among females, which corresponds to a stronger opportunity for selection on males (Wade 1979; Clutton-Brock 1983, 1988; Andersson 1994; Arnold and Duvall 1994). Moreover, as opposed to females, annual fecundity explained more of the variance in male LRS than longevity (Arnold and Wade 1984; Brown 1988), suggesting that there is an opportunity for sexual selection on male traits in the population.
However, as predicted based on short-term patterns (Berard et al. 1993; Dubuc et al. 2011), the opportunity for selection appears to be low in rhesus macaques when compared with some other large mammalian species (see review in Tatarenkov et al. 2008), suggesting moderate to low degree of polygyny. Indeed, with a I m of 1.29, males of the study population show higher standardized variance than reported for solitary ungulate species that exhibit territoriality (roe deers: I m = 0.75; Vanpé et al. 2008) but lower than those forming harems (Bighorn sheep: I m = 4.52; Coltman et al. 2002) and those living in dominance-based polygynandrous mating systems (Soay sheeps: I m = 3.46; Coltman et al. 1999). In particular, the standardized variance is 4.31 lower in rhesus macaques than in highly sexually dimorphic mandrills (I m = 5.57; calculated based on data from Setchell et al. 2005a), the other anthropoid primate for which data on LRS are available (Setchell et al. 2005a). With values above 5, mandrills show a standardized variance (I) similar to those obtained for other highly sexually dimorphic mammals forming harems (cf. Tatarenkov et al. 2008; Vanpé et al. 2008). Moreover, although the range in male LRS is similar between the 2 species (rhesus macaques: 0–47 vs. mandrills: 0–41 offspring; Setchell et al. 2005a), the percentage of breeders is 2.6 times lower for mandrills (rhesus macaques: 82.6% vs. mandrills: 32.1%; Setchell et al. 2005a). These observations suggest that the degree of polygyny—and thus the strength of direct male–male competition—is much greater in mandrills than in rhesus macaques, despite similarities in their social mating systems.
The difference in degree of polygyny between these 2 species is also supported by the difference in the contribution of fecundity and longevity to the variance in LRS. Although fecundity (F) explained 60% of the variance in male LRS among breeders of the studied rhesus macaque population, longevity (L), a better estimator of natural selection processes, explained 20–25% of the variance alone and 10% in a joint action with fecundity (Q). Those figures echo those obtained in polygynandrous roe deers (F = 56%, L = 33%, Q = 11%; Vanpé et al. 2008) but are in striking contrast to those obtained for highly sexually dimorphic mandrills (F = 7.8%, L = 0.5%, Q = 91.7%; Setchell et al. 2005a). Although fecundity explained 15.6 times more of the variance than longevity in mandrills (as opposed to 2.42 for rhesus macaques), the joint action of fecundity and longevity explained most of the variance in mandrills, a phenomenon that emerges in species characterized by age-dependent mating systems associated with a high degree of polygyny (Coltman et al. 1999). This is due to the fact that mandrills reproduce most at prime (10–14 years old), soon after which male mortality rate is very high (cf. Setchell et al. 2005a), leading to the 2 variables being highly correlated. In rhesus macaques, high male fecundity is maintained from 6 to 12 years old, after which it gradually decreases over a long reproductive life, leading to a weaker association between fecundity and longevity. Examination of reproductive timing in rhesus macaques further suggests a low degree of polygyny in this species. As reported in a previous study (Bercovitch et al. 2003), rhesus males can reach high reproductive success at a young age (i.e., 6 years old), before body size is fully developed (Bercovitch 1997; Bercovitch et al. 2003). Moreover, the most successful breeders of the population both reproduced at a higher fecundity and over a longer period of life than less successful males, showing that extended longevity contributes to male LRS. Together, this suggests that the mating system of rhesus macaques might be less age dependent than that of mandrills, which in turn further supports the view that direct competition through physical fights and intimidation is less important in rhesus macaques.
Together, our results confirm the prediction that the degree of polygyny is lower in rhesus macaques than in mandrills, based on the differences in 1) the strength of short-term association between dominance rank and reproductive success (cf. Charpentier et al. 2005; Dubuc et al. 2011) and 2) degree of sexual dimorphism in size between these species (Plavcan and van Schaik 1997; Plavcan 2001). The reduced degree of polygyny in rhesus macaques is linked to an increased opportunity for female to mate with several mating partners, leading to indirect forms of male–male competition such as sperm competition (Bercovitch and Rodriguez 1993; see also Dubuc et al. 2011). Because anthropoid primate males typically form linear dominance hierarchies, it seems to be often assumed that males in general have the same reproductive strategy: fighting to reach the highest dominance rank possible, which allows access to females through mate guarding (e.g., Paul 2002; Setchell 2008). However, the great interspecific variation in 1) the strength of the correlation between dominance rank and reproductive success over short-term periods (reviewed in Kutsukake and Nunn 2006; Ostner et al. 2008; Gogarten and Koenig 2012), 2) sexual dimorphism (Plavcan 2011), and 3) probability of queuing versus fighting for high-ranking positions (van Noordwijk and van Schaik 2004) does not support this view (Dubuc et al. 2011, 2013). There rather appears to be an important variation in the degree of polygyny and, thus, in the genetic mating system, among multimale–multimale anthropoids, even if these species are considered to have the same social organization and mating system.
Even though very little medical intervention takes place in the study population, the fact that animals are provisioned and face no predation risks and that a proportion of animals are culled to prevent overpopulation might have influenced our results by limiting the variance in longevity and offspring survival. Because our results suggest that longevity contributes to both male and female LRS, one may predict that the variance in LRS might be even more pronounced in wild populations. The main conclusions of our study are robust, however, because they are based on a comparison of males and females of the same population of rhesus macaques and are compared with a population of mandrills also provisioned and lacking predation. Future studies conducted in wild populations of anthropoid primates would be relevant to further confirm the pattern observed here.
In sum, our results suggest that rhesus macaques face moderate degree of polygyny, facilitating polygynandrous mating system. In such a context, the importance of direct male–male competition for fertile females on male trait evolution is reduced, creating opportunities for indirect forms, such as sperm competition, to take place (Harcourt et al. 1995). In addition to its consequence for the dynamics of sexual selection, the extent to which the variance in male LRS is pronounced can also have a significant impact on social evolution. Indeed, whether a large portion or only a fraction of males in the population contributes to the gene pool of a social group is likely to influence the pattern of relatedness among individuals and, thus, the opportunity for kin selection (Altmann 1979; Altmann et al. 1996; Widdig 2013). More studies conducted on species varying in terms of polygyny and male dominance are needed to better understand the effect of the strength of variance in male and female LRS on genetic diversity and relatedness among members of future generations.
FUNDING
The study was conducted within an Emmy-Noether Group funded by the German Research Foundation (grant no. Wi 1808/3-1 awarded to A.W.) and was also supported by a Fonds de Recherche sur la Société et la Culture (FQRSC) postdoctoral fellowship (awarded to C.D.). The population of Cayo Santiago was supported by grant number 8 P40 OD012217-25 from the National Center for Research Resources (NCRR), the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health (NIH), and the Medical Sciences Campus of the University of Puerto Rico (UPR).
Acknowledgments
We appreciate the support Cayo Santiago Field Station staff (especially census takers E. Davila, J. Resto, and G. Caraballo Cruz) for collecting demographic and DNA samples and E. Maldonado for management of demographic data. We thank F. Bercovitch, M. Kessler, J. Berard, M. Krawczak, P. Nürnberg, and J. Schmidtke for starting the genetic database of the Cayo Santiago population; L. Vigilant for providing laboratory access; M. Krawczak and O. Junge for access to the genetic data management program FINDSIRE; S. Bley, L. Muniz, and L. Kulik for improving the genetic paternity database; all field assistants of the Jr Research Group of Primate Kin Selection who assisted in sample collection; and the Max-Planck Institute for Evolutionary Anthropology in Leipzig for their logistic support. We are grateful to D. Pfefferle, L. Kulik, and S. Winters, the editor, and 3 anonymous reviewers for helpful comments on a previous version of the manuscript. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of National Center for Research Resources or Office of Research Infrastructure Programs.
REFERENCES
- Alberts SC, Buchan JC, Altmann J. 2006. Sexual selection in wild baboons: from mating opportunities to paternity success. Anim Behav. 72:1177–1196 [Google Scholar]
- Alexander RD, Hoogland JL, Howard RD, Noonan KM, Sherman PW. 1979. Sexual dimorphisms and breeding systems in pinnipeds, ungulates, primates, and humans. In: Chagnon NA, Irons W, editors. Evolutionary biology and human social behavior: an anthropological perspective. North Scituate (MA): Duxbury Press; p. 402–435 [Google Scholar]
- Altmann J. 1979. Age cohorts as paternal sibships. Behav Ecol Sociobiol. 6:161–164 [Google Scholar]
- Altmann J, Alberts SC, Haines SA, Dubach J, Muruthi P, Coote T, Geffen E, Cheesman DJ, Mututua RS, Saiyalel SN, et al. 1996. Behavior predicts genetic structure in a wild primate group. Proc Natl Acad Sci USA. 93:5797–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann SA. 1962. A field study of the sociobiology of rhesus monkeys, Macaca mulatta . Ann N Y Acad Sci. 102:338–435 [DOI] [PubMed] [Google Scholar]
- Andersson M. 1994. Sexual selection. Princeton (NJ): Princeton University Press [Google Scholar]
- Arnold SJ, Duvall D. 1994. Animal mating systems: a synthesis based on selection theory. Am Nat. 143:317–348 [Google Scholar]
- Arnold SJ, Wade MJ. 1984. On the measurement of natural and sexual selection: applications. Evolution. 38:720–734 [DOI] [PubMed] [Google Scholar]
- Bateman AJ. 1948. Intra-sexual selection in Drosophila . Heredity. 2:349–368 [DOI] [PubMed] [Google Scholar]
- Berard J. 1999. A four-year study of the association between male dominance rank, residency status, and reproductive activity in rhesus macaques (Macaca mulatta). Primates. 40:159–175 [DOI] [PubMed] [Google Scholar]
- Berard JD, Nuernberg P, Epplen JT, Schmidtke J. 1993. Male rank, reproductive behavior, and reproductive success in free-ranging rhesus macaques. Primates. 34:481–489 [Google Scholar]
- Berard JD, Nuernberg P, Epplen JT, Schmidtke J. 1994. Alternative reproductive tactics and reproductive success in male rhesus macaques. Behaviour. 129:177–201 [Google Scholar]
- Bercovitch FB. 1997. Reproductive strategies of rhesus macaques. Primates. 38:247–263 [Google Scholar]
- Bercovitch FB, Berard JD. 1993. Life history costs and consequences of rapid reproductive maturation in female rhesus macaques. Behav Ecol Sociobiol. 32:103–109 [Google Scholar]
- Bercovitch FB, Rodriguez JF. 1993. Testis size, epididymis weight and sperm competition in rhesus macaques. Am J Primatol. 30:163–168 [DOI] [PubMed] [Google Scholar]
- Bercovitch FB, Widdig A, Trefilov A, Kessler MJ, Berard JD, Schmidtke J, Nürnberg P, Krawczak M. 2003. A longitudinal study of age-specific reproductive output and body condition among male rhesus macaques, Macaca mulatta . Naturwissenschaften. 90:309–312 [DOI] [PubMed] [Google Scholar]
- Bergeron P, Montiglio P-O, Réale D, Humphries MM, Garant D. 2012. Bateman gradients in a promiscuous mating system. Behav Ecol Sociobiol. 66:1125–1130 [Google Scholar]
- Blomquist GE. 2013. Maternal effects on offspring mortality in rhesus macaques (Macaca mulatta). Am J Primatol. 75:238–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme M, Blancher A, Crouau-Roy B. 2005. Multiplexed microsatellites for rapid identification and characterization of individuals and populations of Cercopithecidae. Am J Primatol. 67:385–391 [DOI] [PubMed] [Google Scholar]
- Brown D. 1988. Components of lifetime reproductive success. In: Clutton-Brock TH, editor. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago (IL): University of Chicago Press; p. 439–453 [Google Scholar]
- Caballero A. 1994. Developments in the prediction of effective population size. Heredity. 73:657–679 [DOI] [PubMed] [Google Scholar]
- Chapais B. 1983. Reproductive activity in relation to male dominance and the likelihood of ovulation in rhesus monkeys. Behav Ecol Sociobiol. 12:215–228 [Google Scholar]
- Charpentier M, Peignot P, Hossaert-McKey M, Gimenez O, Setchell JM, Wickings EJ. 2005. Constraints on control: factors influencing reproductive success in male mandrills (Mandrillus sphinx). Behav Ecol. 16:614–623 [Google Scholar]
- Clutton-Brock TH. 1983. Selection in relation to sex. In: Bendall DS, editor. Evolution from molecule to man. Cambridge (MA): Cambridge University Press; p. 457–481 [Google Scholar]
- Clutton-Brock TH. 1988. Reproductive success. In: Clutton-Brock TH, editor. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago (IL): University of Chicago Press; p. 472–485 [Google Scholar]
- Clutton-Brock TH. 1989. Mammalian mating systems. Proc R Soc Lond B. 22:339–372 [DOI] [PubMed] [Google Scholar]
- Coltman DW, Festa-Bianchet M, Jorgenson JT, Strobeck C. 2002. Age-dependent sexual selection in bighorn rams. Proc R Soc B. 269:165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltman DW, Smith JA, Bancroft DR, Pilkington J, MacColl AD, Clutton-Brock TH, Pemberton JM. 1999. Density-dependent variation in lifetime breeding success and natural and sexual selection in Soay rams. Am Nat. 154:730–746 [DOI] [PubMed] [Google Scholar]
- Darwin C. 1871. The descent of man, and selection in relation to sex. London: John Murray [Google Scholar]
- Dittus W. 2004. Demography: a window to social evolution. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: a model for the study of social organization. New York: Cambridge University Press; p. 87–112 [Google Scholar]
- Drickamer LC, Vessey SH. 1973. Group changing in free ranging male rhesus monkeys. Primates. 14:359–368 [Google Scholar]
- Dubuc C, Higham J, Engelhardt A. 2013. Towards a theoretical framework for understanding the variation in sexually-selected traits among anthropoid primates. Am J Phys Anthropol. 150(S56):115 [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Engelhardt A, Widdig A. 2011. Testing the priority-of-access model in a seasonally breeding primate species. Behav Ecol Sociobiol. 65:1615–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Widdig A, Engelhardt A. 2012. Do males time their mate-guarding effort with the fertile phase in order to secure fertilisation in Cayo Santiago rhesus macaques? Horm Behav. 61:696–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogarten JF, Koenig A. 2012. Reproductive seasonality is a poor predictor of receptive synchrony and male reproductive skew among nonhuman primates. Behav Ecol Sociobiol. 67:123–134 [Google Scholar]
- Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav. 28:1140–1162 [Google Scholar]
- Harcourt AH, Purvis A, Liles L. 1995. Sperm competition: mating system, not breeding system, affects testes size of primates. Funct Ecol. 9:468–476 [Google Scholar]
- Henegariu O, Heerema NA, Dlouhy SR, Vance GH, Vogt PH. 1997. Multiplex PCR: critical parameters and step-by-step protocol. Biotechniques. 23:504–511 [DOI] [PubMed] [Google Scholar]
- Hernández-Pacheco R, Rawlins RG, Kessler MJ, Williams LE, Ruiz-Maldonado TM, González-Martínez J, Ruiz-Lambides AV, Sabat AM. 2013. Demographic variability and density-dependent dynamics of a free-ranging rhesus macaque population. Am J Primatol. 75:1152–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Heistermann M, Maestripieri D. 2013. The endocrinology of male rhesus macaque social and reproductive status: a test of the challenge and social stress hypotheses. Behav Ecol Sociobiol. 67:19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennions MD, Kokko H, Klug H. 2012. The opportunity to be misled in studies of sexual selection. J Evol Biol. 25:591–598 [DOI] [PubMed] [Google Scholar]
- Johnston SE, Gratten J, Berenos C, Pilkington JG, Clutton-Brock TH, Pemberton JM, Slate J. 2013. Life history trade-offs at a single locus maintain sexually selected genetic variation. Nature. 502:93–95 [DOI] [PubMed] [Google Scholar]
- Jones AG. 2009. On the opportunity for sexual selection, the Bateman gradient and the maximum intensity of sexual selection. Evolution. 63:1673–1684 [DOI] [PubMed] [Google Scholar]
- Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol. 16:1099–1106 [DOI] [PubMed] [Google Scholar]
- Kessler MJ, Berard JD, Rawlins RG. 1988. Effect of tetanus toxoid inoculation on mortality in the Cayo Santiago macaque population. Am J Primatol. 15:93–101 [DOI] [PubMed] [Google Scholar]
- Kessler MJ, Berard JD, Rawlins RG, Bercovitch FB, Gerald MS, Laudenslager ML, Gonzalez-Martinez J. 2006. Tetanus antibody titers and duration of immunity to clinical tetanus infections in free-ranging rhesus monkeys (Macaca mulatta). Am J Primatol. 68:725–731 [DOI] [PubMed] [Google Scholar]
- Klug H, Heuschele J, Jennions MD, Kokko H. 2010. The mismeasurement of sexual selection. J Evol Biol. 23:447–462 [DOI] [PubMed] [Google Scholar]
- Klug H, Lindstrom K, Kokko H. 2010. Who to include in studies of sexual selection is no trivial matter. Ecol Lett. 13:1094–1102 [DOI] [PubMed] [Google Scholar]
- Krakauer AH, Webster MS, Duval EH, Jones AG, Shuster SM. 2011. The opportunity for sexual selection: not mismeasured, just misunderstood. J Evol Biol. 24:2064–2071 [DOI] [PubMed] [Google Scholar]
- Kulik L, Muniz L, Mundry R, Widdig A. 2012. Patterns of interventions and the effect of coalitions and sociality on male fitness. Mol Ecol. 21:699–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake N, Nunn CL. 2006. Comparative tests of reproductive skew in male primates: the roles of demographic factors and incomplete control. Behav Ecol Sociobiol. 60:695–706 [Google Scholar]
- Lawson Handley LJ, Perrin N. 2007. Advances in our understanding of mammalian sex-biased dispersal. Mol Ecol. 16:1559–1578 [DOI] [PubMed] [Google Scholar]
- Le Boeuf BJ, Reiter J. 1988. Lifetime reproductive success in northern elephant seals. In: Clutton-Brock TH, editor. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago (IL): University of Chicago Press; p. 344–362 [Google Scholar]
- Loison A, Gaillard JM, Pélabon C., Yoccoz NG. 1999. What factors shape sexual size dimorphism in ungulates? Evol Ecol Res. 1:611–633 [Google Scholar]
- Madsen T, Shine R. 1994. Components of lifetime reproductive success in adders, Vipera berus . J Anim Ecol. 63:561–568 [Google Scholar]
- Manson JH. 1992. Measuring female mate choice in Cayo Santiago rhesus macaques. Anim Behav. 44:405–416 [Google Scholar]
- Marriott BM. 1988. Time budgets of rhesus monkeys (Macaca mulatta) in a forest habitat in Nepal and on Cayo Santiago. In: Fa JE, Southwick CH, editors. Ecology and behavior of food-enhanced primate groups. New York: Alan R. Liss, Inc; p. 125–149 [Google Scholar]
- Marriott BM, Roemer J, Sultana C. 1989. An overview of the food intake patterns of the Cayo Santiago rhesus monkeys (Macaca mulatta): report of a pilot study. P R Health Sci J. 8:87–94 [PubMed] [Google Scholar]
- Marshall TC, Slate J, Kruuk LE, Pemberton JM. 1998. Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol. 7:639–655 [DOI] [PubMed] [Google Scholar]
- Morin PA, Chambers KE, Boesch C, Vigilant L. 2001. Quantitative PCR analysis of DNA from noninvasive samples for accurate microsatellite genotyping of wild chimpanzees (Pan troglodytes verus). Mol Ecol. 10:1835–1844 [DOI] [PubMed] [Google Scholar]
- Nonacs P. 2000. Measuring and using skew in the study of social behavior and evolution. Am Nat. 156:577–589 [DOI] [PubMed] [Google Scholar]
- Nonacs P. 2003. Measuring the reliability of skew indices: is there one best index? Anim Behav. 65:615–627 [Google Scholar]
- van Noordwijk MA, van Schaik CP. 2004. Sexual selection and the careers of primate males: paternity concentration, dominance acquisition tactics and transfer decisions. In: Kappeler PM, van Schaik CP, editors. Sexual selection in primates: new and comparative. Cambridge (MA): Cambridge University Press; p. 208–229 [Google Scholar]
- Nürnberg P, Sauermann U, Kayser M, Lanfer C, Manns E, Widdig A, Berard J, Bercovitch FB, Kessler M, Schmidtke J, et al. 1998. Paternity assessment in rhesus macaques (Macaca mulatta): multilocus DNA fingerprinting and PCR marker typing. Am J Primatol. 44:1–18 [DOI] [PubMed] [Google Scholar]
- Ostner J, Nunn CL, Schuelke O. 2008. Female reproductive synchrony predicts skewed paternity across primates. Behav Ecol Sociobiol. 19:1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. 2002. Sexual selection and mate choice. Int J Primatol. 23:877–904 [Google Scholar]
- Pemberton JM, Albon SD, Guiness FE, Clutton-Brock TH, Dover DA. 1992. Behavioral estimates of male mating success tested by DNA fingerprinting in a polygynous mammal. Behav Ecol. 3:66–75 [Google Scholar]
- Plavcan JM. 2001. Sexual dimorphism in primate evolution. Yearbk Phys Anthropol. 44:25–53 [DOI] [PubMed] [Google Scholar]
- Plavcan JM. 2011. Understanding dimorphism as a function of changes in male and female traits. Evol Anthropol. 20:143–155 [DOI] [PubMed] [Google Scholar]
- Plavcan JM, van Schaik CP. 1997. Intrasexual competition and body weight dimorphism in anthropoid primates. Am J Phys Anthropol. 103:37–68 [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ. 1982. A five‐year study of tetanus in the Cayo Santiago rhesus monkey colony: behavioral description and epizootiology. Am J Primatol. 3:23–39 [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ. 1986. The history of the Cayo Santiago colony. In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques: history, behavior and biology. Albany (NY): State University of New York Press; p 13–45 [Google Scholar]
- Rogers J, Garcia R, Shelledy W, Kaplan J, Arya A, Johnson Z, Bergstrom M, Novakowski L, Nair P, Vinson A, et al. 2006. An initial genetic linkage map of the rhesus macaque (Macaca mulatta) genome using human microsatellite loci. Genomics. 87:30–38 [DOI] [PubMed] [Google Scholar]
- Rossiter SJ, Ransome RD, Faulkes CG, Dawson DA, Jones G. 2006. Long-term paternity skew and the opportunity for selection in a mammal with reversed sexual size dimorphism. Mol Ecol. 15:3035–3043 [DOI] [PubMed] [Google Scholar]
- Sade DS, Cushing K, Cushing P, Dunaif J, Figueroa A, Kaplan JR, Lauer C, Rhodes D, Scneider J. 1976. Population dynamics in relation to social structure on Cayo Santiago. Yearbk Phys Anthropol. 20:253–262 [Google Scholar]
- Setchell JM. 2008. Alternative reproductive tactics in primates. In: Oliveira RF, Taborsky M, Brockmann HJ, editors. Alternative reproductive tactics: an alternative approach. New York: Cambridge University Press; p. 373–398 [Google Scholar]
- Setchell JM, Charpentier M, Wickings EJ. 2005a. Sexual selection and reproductive careers in mandrills (Mandrillus sphinx). Behav Ecol Sociobiol. 58:474–485 [Google Scholar]
- Setchell JM, Charpentier M, Wickings EJ. 2005b. Mate guarding and paternity in mandrills: factors influencing alpha male monopoly. Anim Behav. 70:1105–1120 [Google Scholar]
- Setchell JM, Lee PC, Wickings EJ, Dixson AF. 2001. Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx). Am J Phys Anthropol. 115:349–360 [DOI] [PubMed] [Google Scholar]
- Shultz S, Opie C, Atkinson QD. 2011. Stepwise evolution of stable sociality in primates. Nature. 479:219–222 [DOI] [PubMed] [Google Scholar]
- Shuster SM, Wade MJ. 2003. Mating systems and strategies. Princeton (NJ): Princeton University Press [Google Scholar]
- Silk J, Short J, Roberts J, Kusnitz J. 1993. Gestation length in rhesus macaques (Macaca mulatta). Int J Primatol 14:95–104 [Google Scholar]
- Small MF. 1990. Consortships and conceptions in captive rhesus macaques (Macaca mulatta). Primates. 31:339–350 [Google Scholar]
- Tatarenkov A, Healey CIM, Grether GF, Avise JC. 2008. Pronounced reproductive skew in a natural population of green swordtails, Xiphophorus helleri . Mol Ecol. 17:4522–4534 [DOI] [PubMed] [Google Scholar]
- Trivers RL. 1972. Parental investment and sexual selection, 1871–1971. In: Campbell B, editor. Sexual selection and the descent of man. London: Heinemann; p. 136–179 [Google Scholar]
- Vanpé C, Kjellander P, Galan M, Cosson J-F, Aulagnier S, Liberg O, Hewison AJM. 2008. Mating system, sexual dimorphism, and the opportunity for sexual selection in a territorial ungulate. Behav Ecol. 19:309–316 [Google Scholar]
- Wade MJ. 1979. Sexual selection and variance in reproductive success. Am Nat. 114:742–747 [DOI] [PubMed] [Google Scholar]
- Wade MJ, Arnold SJ. 1980. The intensity of sexual selection in relation to male sexual behavior, female choice and sperm precedence. Anim Behav. 28:446–461 [Google Scholar]
- Widdig A. 2013. The impact of male reproductive skew on kin structure and sociality in multi-male groups. Evol Anthropol. 22:239–250 [DOI] [PubMed] [Google Scholar]
- Widdig A, Bercovitch FB, Streich WJ, Sauermann U, Nürnberg P, Krawczak M. 2004. A longitudinal analysis of reproductive skew in male rhesus macaques. Proc R Soc B. 271:819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]