Abstract
Non-inflammatory fibrosis of the subsynovial connective tissue (SSCT) is a hallmark of carpal tunnel syndrome (CTS). The etiology of this finding and its relationship to the development of CTS remain poorly understood. Recent studies have found that transforming growth factor-β (TGF-β) plays a central role in fibrosis. The purpose of this study was to investigate the expression of TGF-β and connective tissue growth factor (CTGF), a downstream mediator of TGF-β, in the pathogenesis of CTS. We compared SSCT specimens from 26 idiopathic CTS patients with specimens from 10 human cadaver controls with no previous diagnosis of CTS. Immunohistochemistry was performed to determine levels TGF-β1, CTGF, collagen 1(Col1) and collagen 3 (Col3) expression. TGF-β1(P<0.01), CTGF (P<0.01), and Col3 (P<0.01) were increased in SSCT of CTS patients compared with control tissue. In addition, a strong positive correlation was found between TGF-β1 and CTGF, (R2=0.80, p<0.01) and a moderate positive correlation between Col3 and TGF-β1 (R2=0.49, p<0.01). These finding suggest that there is an increased expression of TGF-β and CTGF, a TGF-β regulated protein, and that this TGF-β activation may be responsible for SSCT fibrosis in CTS patients.
Keywords: Carpal Tunnel Syndrome, Subsynovial Connective Tissue, TGF-β, CTGF
INTRODUCTION
Increased fibrosis is a hallmark of the development and pathology of carpal tunnel syndrome (CTS). Furthermore, this fibrosis is unique as it is primarily described as non-inflammatory fibrosis of subsynovial connective tissue (SSCT).1,2 There are some cytokines and receptors that are differentially regulated in the SSCT of CTS patients,1,3–5 but whether they play a pathogenic role in the SSCT fibrosis is still unknown.
Previous studies of other pathological disorders have found that transforming growth factor - β (TGF-β) plays a central role in fibrosis,6,7 especially in non-inflammatory progressive fibrosis, which is typically present in CTS patients.8 TGF-β’s induction of fibrosis ranges from inhibition of extracellular matrix (ECM) degradation by decreasing matrix metalloprotease activity or stimulating production of tissue inhibitors of metalloproteases.9 In addition, TGF-β induces synthesis of ECM through multiple signal transduction pathways.7,10,11 Therefore, TGF-β is an important target for antifibrotic therapy.12,13
While there are three structural isoforms of TGF-β (TGF-β1, 2, 3), TGF-β1 is primarily implicated in fibrosis.6,14 TGF-β1 gene expression is up-regulated in response to tissue injury, and excessive or sustained production of TGF-β1 is a hallmark of fibrosis in many tissues.6
Connective tissue growth factor (CTGF) is a putative regulator of progressive fibrosis and is known to be itself regulated by TGF-β.15–18 CTGF is a heparin-binding 38-kDa cysteine-rich peptide that promotes cell proliferation, collagen synthesis, and chemotaxis in mesenchymal cells. The regulation of CTGF appears to be controlled primarily at the level of gene expression, and even transitory exposure of fibroblasts to TGF-β is sufficient to induce sustained CTGF expression.18 However, the regulatory relationship between TGF-β and CTGF and resulting increase of ECM production has not been explored in the CTS context. Therefore, the aim of this project was to compare the expression of two fibrotic cytokines (TGF-β1 and CTGF) and two ECM markers (Collagen 1 and 3) in the SSCT of CTS patients and control specimens.
METHODS
A total of 64 CTS patients who had had SSCT biopsy specimens sent to our Department of Laboratory Medicine and Pathology during carpal tunnel release between 1991 and 2009 were evaluated. All patients had diagnostic neurophysiological testing, including electromyography and nerve conduction studies performed according to American Association of Electrodiagnostic Medicine standards, 19 with the diagnosis of carpal tunnel syndrome confirmed in each case. The Mayo Clinic severity scale was used to assess the neurophysiologic severity of each patient’s CTS.20 In this scale, CTS is divided into groups on the basis of neurophysiologic findings. These groups are:20 “mild CTS, prolonged (relative or absolute) sensory or mixed nerve action potential (NAP) distal latency (orthodromic, antidromic, or palmar) ± sensory nerve action potential (SNAP) amplitude below the lower limit of normal; moderate, mild findings plus and (relative or absolute) prolongation of median motor distal latency; severe; prolonged median motor and sensory distal latencies, with either an absent SNAP or mixed NAP, or low amplitude or absent thenar compound muscle action potential amplitude (CMAP), with needle examination often revealing fibrillations, reduced recruitment, and motor unit potential changes.” Sensory nerve conduction was considered abnormal if the amplitude of the nerve action potential was less than 50 μV, the distal latency was more than 2.3 ms, or the median-to-ulnar palmar latency difference exceeded 0.3 ms when the palmar latency was 2.2 ms or less. Motor nerve conduction was considered abnormal if the amplitude of the thenar compound muscle action potential was less than 4 mV or the distal latency was greater than 4.5 ms (at a distance of 7 cm). The medical charts were reviewed to exclude patients with a history of diabetes, glucose intolerance, thyroid disease, rheumatoid arthritis, osteoarthrosis, degenerative joint disease, flexor tendinitis, gout, hemodialysis, sarcoidosis, amyloidosis, peripheral nerve disease, or traumatic injuries to the ipsilateral arm.
Ultimately, 26 idiopathic CTS patients (11 female and 15 male) with mean age of 55 years old (range from 37 – 74) were included in this study. Regarding the Mayo Clinic severity score, there were 8 severe, 11 moderate and 7 mild cases. All specimens had initially been given a pathological diagnosis of non-inflammatory fibrous tissue. The control group consisted of 3 female and 7 male fresh cadavers (mean: 76 years, range: 56- 95) from which subsynovial connective tissue of the ulnar bursa of the carpal tunnel was obtained. We used cadaver tissue for the control because we thought that the risks of research subject harm from opening a normal carpal tunnel to obtain tissue outweighed the research benefits. In addition, opportunities to obtain such tissue incidentally in the course of other surgery in the region had substantial limitations as well, since the indicated local procedure, typically for fracture, tumor, or arthritis treatment, were likely to have an effect on the SSCT. Thus, we considered fresh cadaver tissue the best compromise, when considering all the risks and benefits.
A chart review was performed on each member in the control group; to be sure the individuals met the same exclusion criteria and in addition did not have an antemortem diagnosis of carpal tunnel syndrome. Demographic data, including age and gender, were obtained and recorded for both patient and cadaver control.
Immunohistochemistry
Both CTS patient and control specimens were formalin-fixed and paraffin embedded, after which 4μm-thick sections were made. For immunohistochemistry, the sections were deparaffinized in xylene, rehydrated with graded ethanol, and washed with 0.1 M phosphate-buffered saline (PBS, pH 7.4). To inactivate the endogenous peroxidase, the sections were treated with 0.1% H2O2 in 0.1 M PBS for 30 minutes at room temperature (RT). After washing for 10 minutes with in 0.1 M PBS containing 0.3% Triton X-100 (PBST), the sections were treated with 2% bovine serum albumin (BSA) for blocking at RT. After washing for 10 minutes with in 0.1 M PBST, the sections were incubated overnight with a primary antibody against TGFβ1 (Abcam, Cambridge, MA), CTGF (Santa Cruz Biotechnology, Santa Cruz, CA), Collagen 1(Col1) (Abcam, Cambridge, MA) and Collagen 3(Col3) (Acris Antibodies, San Diego, CA) diluted 1:200 for TGFβ1 and CTGF, 1:500 for Col1 and Col3 in PBST at 4C°. Negative control staining for IHC were completed by two methods: 1) primary antibody was removed; and 2) primary antibody was replaced by normal species matched sera to the primary antibody. After washing for 30 minutes with in 0.1 M PBST, the sections were incubated for 2 hours with second antibody (Vector Laboratories, Burlingame, CA) diluted 1:500 in PBST at RT. They were washed for 30 minutes with PBST and placed for 2 hours in avidin-biotin-peroxidase complex (ABC kit, Vector Laboratories, Burlingame, CA) diluted 1:1000 in PBST at RT. The immunoreaction were rendered visible by reaction with 0.05 M Tris-HCl buffer (pH 7.6) containing 0.01% 3,3′-diaminobenzidine (DAB) (Sigma-Aldrich, St Louis, MO), and 0.0003% H2O2 for 30 minutes at RT. Finally, slides were counterstained with hematoxylin QS (Vector Laboratories), dehydrated and mounted on glass slides.
Eight different areas were randomly chosen from each slide under a light microscope with the 400x objective. Digital images were made of each area. To analyze the TGF-β1 and CTGF expression, the percentage of positive cells was measured in each image. For the analysis of Col1 and Col3, cellSens Image Analysis Software (Olympus, Tokyo, Japan) was used to quantify the positive staining areas. Human tendon and a 21 day post-operative canine skin wound were used as positive controls for Col1 and Col3, respectively. Positive control slides were stained and 8 digital images were randomly taken by the same procedures. Observers identified the threshold of the positive area from the digital images of the positive controls on the image software. By using the identified threshold from positive controls, the positive staining areas in both CTS patients and controls were digitally identified. Intraclass correlation coefficients (ICCs) were assessed for the intra and inter-observer reliability of this analysis. Two observers performed the same procedures and measured the positive areas from 30 images which were selected from CTS patients and controls. The measurement of ICCs was performed twice in varied order and separated by at least one week.
Statistical Methods
Results were expressed as a mean ± standard deviation (SD). To assess normality, the Shapiro-Wilk test was used. The Mann-Whitney test was compared the percentage of TGF-β1 and CTGF positive cells, and the positive area of Col1 and Col3 of CTS patients. Spearman’s rank correlation coefficient was used for analysis of correlation between TGF-β1, CTGF, Col1, Col3 and severity of the CTS. Values of p<0.05 were considered to be statistically significant for all the statistical analyses, which were performed with SPSS 17.0 software (SPSS Inc., Chicago, IL). An effect size was calculated by a pilot study which was performed to detect differences in TGF-β and CTGF between CTS patients and controls. The effect size for TGF-β1was 1.44, and CTGF was 1.58. From the effect size, we estimated that a sample size of 9 for TGF-β1, and of 8 for CTGF in each group would have 80% power at a significance level of p<0.05.
RESULTS
The Shapiro-Wilk result was statistically significant (p<0.05), showing that the data were not normally distributed.
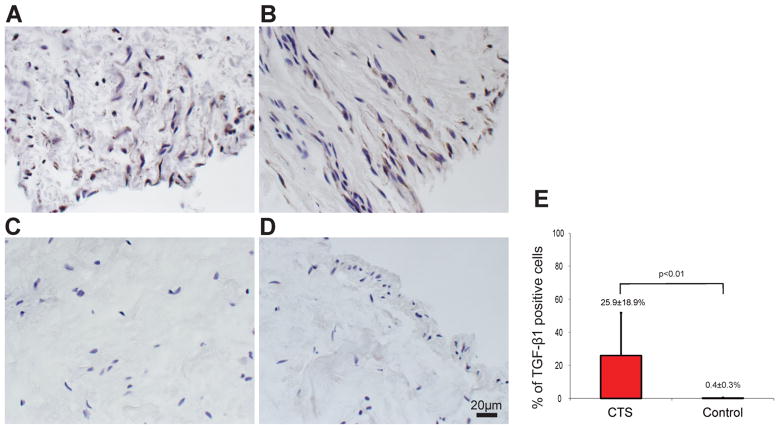
Figure 1 shows the characteristic staining of CTS (Figs 1A and B) and control (Figs 1C and D) specimens. The percentage of TGF-β1 positive cells was 25.9±18.9% in CTS patients and 0.4±0.3% in control (p<0.01) (Fig. 1E).
Figure 1.
TGF-β1 positive cells were identified in SSCT (A and B) in the CTS patients. In the controls, there were few TGF-β1 positive cells in SSCT(C and D). There were significantly more TGF-β1 positive cells in the CTS patients than in the controls (E).
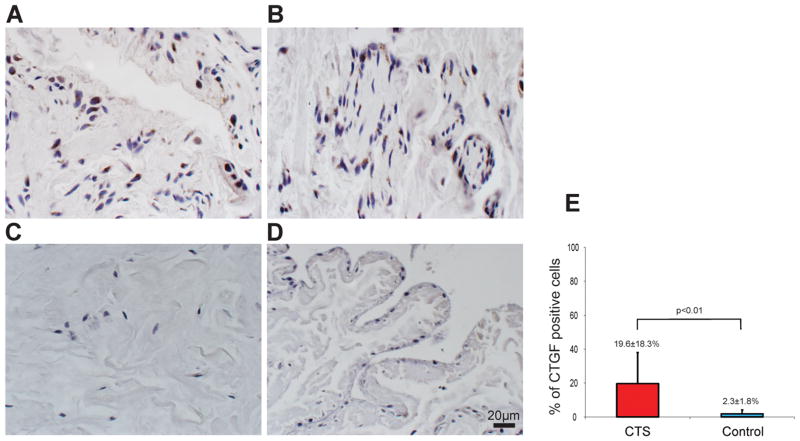
CTGF positive cells were also identified in the SSCT in both CTS patient and control groups (Fig. 2A–D). However, there were few CTGF positive cells in the SSCT (Fig. 2C and D) in the control group compared to the CTS patient group. The percentage of CTGF positive cells was 19.6±18.3% in CTS patients and 2.3±1.8% in control (p<0.01) (Fig. 2E).
Figure 2.
CTGF positive cells were identified in SSCT (A and B). There were few CTGF positive cells in the SSCT (C and D) in the control group. There were significantly more CTGF positive cells in the CTS patient than in the controls (E).
The Col1 positive area was 14563.5±4708.0μm2 [0][0]i[0]n CTS patients and 14585.9±5073.6μm2 in control specimens. The Col3 positive area was 14738.0±7972.9μm2 in CTS patients and 3824.5±3412.0μm2 in control. There was no difference in the mean Col1 positive area between CTS patient and control (p=0.95) (Fig. 3A). In contrast the mean Col3 positive area in the CTS patients SSCT was significantly larger than that in control specimens (p<0.01) (Fig. 3B).
Figure 3.
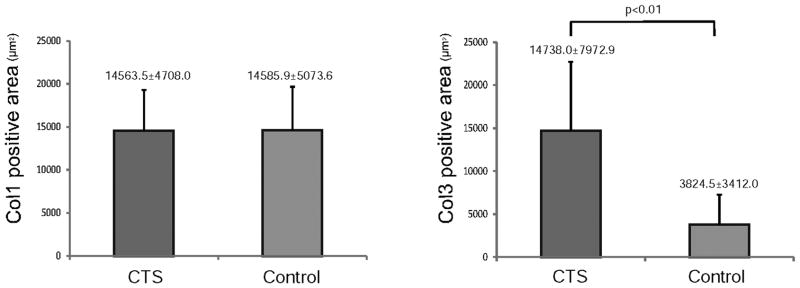
Comparison of the Col1 (A) and Col3 (B) positive area between CTS patients and controls. There was no difference in Col1 positive area comparing CTS patient and controls (A), but the Col3 positive area in the CTS patients was significantly larger than that in the controls (B).
The intra-observer reliability for TGF-β1, CTGF, Col1 and Col3 was 0.987(95% confidence interval (CI), 0.973 to 0.994), 0.992(95%CI, 0.958 to 0.991), 0.915(95%CI, 0.834 to 0.957), and 0.962 (95%CI, 0.924 to 0.981), respectively. The inter-observer reliability for TGF-β1, CTGF, Col1 and Col3 was 0.940 (95%CI, 0.871 to 0.972), 0.981(95%CI, 0.958 to 0.991), 0.935(95%CI, 0.845 to 0.970), and 0.990(95%CI, 0.291 to 0.998), respectively.
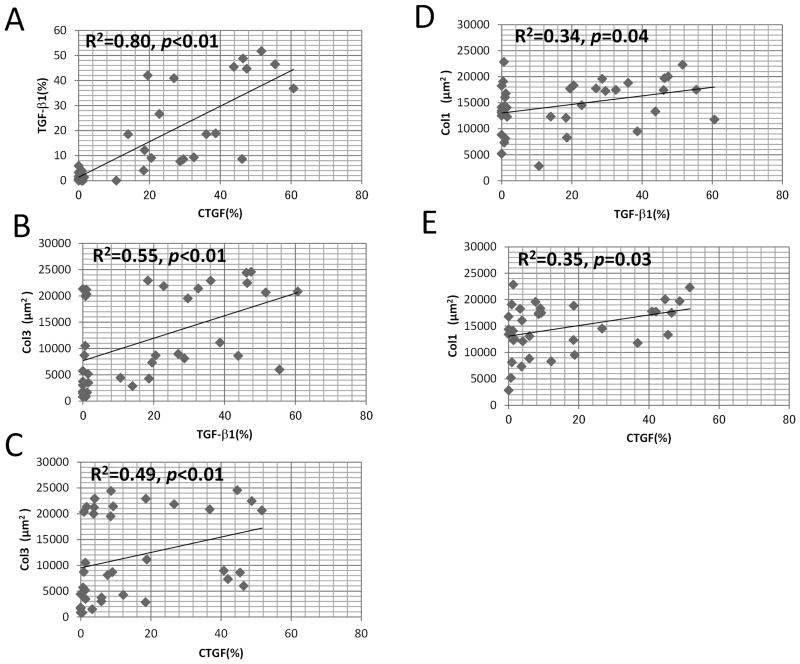
A strong positive correlation was found between TGF-β1 and CTGF (R2=0.80, p<0.01) (Fig. 4A), a moderate positive correlation was present between Col3 and TGF-β1 (R2=0.55, p<0.01) (Fig. 4B) and Col3 and CTGF (R2=0.49, p<0.01) (Fig. 4C), and a low positive correlation was present between Col1 and TGF-β1 (R2=0.34, p=0.04) (Fig. 4D) and Col1 and CTGF (R2=0.35, p=0.03) (Fig. 4E). There was no correlation between the severity of the carpal tunnel syndrome, and TGF-β1(p=0.74), CTGF(p=0.12), Col1(p=0.13) or Col3(p=0.16) expression.
Figure 4.
Spearman rank order correlation coefficient between TGF-β1, CTGF, Col1 and Col3. A strong positive correlation were found between TGF-β1 and CTGF(A), moderate positive correlation between Col3 and TGF-β1 (B), Col3 and CTGF (C), low positive correlation between Col1 and TGF-β (D) and Col1, and CTGF (E).
DISCUSSION
Here we have demonstrated that TGF-β, CTGF and Col3 are overexpressed in the SSCT of CTS patients. As discussed below, these findings suggest that the fibrosis typically found in patients with CTS may be TGF-β mediated.
The TGF-β family of multifunctional growth factors is involved in the regulation and dysregulation of cell proliferation, adhesion, migration, differentiation, epithelial to mesenchymal transition and extracellular matrix (ECM) production. TGF-β also controls the expression of various components of the ECM, including type I collagen, type III collagen and fibronectin.21–23 Border et al.6 noted that “in both animals and humans, acute, limited injury is accompanied by only a transient increase in TGF-β1, and fibrosis does not occur”. They also stated that “With repeated injury, the increase in TGF-β1 production is sustained, leading to the progressive deposition of extracellular matrix and tissue fibrosis.”6 Indeed, overexpression of TGF-β1 by adenoviral vector-mediated gene transfer of active TGF-β1 to rat lungs results in chronic and fibrosis including pathological deposition of ECM such as dysregulated collagen, fibronectin, and elastin deposition.8,24,25 TGF-β signaling is mediated through TGF-β receptors. The signaling through second messengers including the canonical Smad activation and non-canonical kinase cascades. Upon phosphorylation, receptor Smad2/3 bind to co-Smad4 and translocates to the nucleus, and regulates gene expression.26,27 Verrecchia et al.28 have found collagen gene promoters in human dermal fibroblasts were induced by TGF-β1 and required Smad3 activation. In addition, TGF-β decreases proteinase activity, including metalloproteinases, which then inhibit ECM degradation, resulting in increased fibrosis.22
Rodeo et al.29 reported overexpression of TGF-β and its receptor, with deposition of collagen III in the idiopathic capsular fibrosis of the shoulder joint. Ettema et al.4 also reported an increased Col3 and TGF-β receptor Type I (TGF-βRI) in CTS patients and showed an association between Col3 and TGF-βRI.
Further, we demonstrated that CTGF expression was increased in the SSCT in CTS patients as compared to normal human control tissue. CTGF expression also had a strong correlation with TGF-β1. Many human fibrotic diseases and experimental models, such skin, kidney, lung, and liver fibrosis, are typified by increased CTGF expression.30–32 Indeed the correlation of the combined effects of TGF-β and CTGF expression on pathological fibrosis has been revealed in a mouse model of skin fibrosis. Separate and transitory exposure to either TGF-β or CTGF alone resulted in transient effects, where as combined exposure results in a chronic fibrotic response.33,34 These study suggested that CTGF and TGF-β act synergistically to promote chronic fibrosis.
The synthesis of CTGF is stimulated by specific growth factors, particularly TGF-β and endothelin-1, as well as environmental changes such as hypoxia.35 TGF-β1 mediated induction of CTGF mRNA in fibroblast occurs within 30 min of TGF-β1 treatment, without involving de novo protein synthesis.18 A functional Smad element resides within the CTGF promoter; Smad3 and 4 potently activates, whereas Smad 7 suppresses.36 Additional elements, including Ets-1 and a Sp1 site have been shown to play a role in basal activity of the CTGF promoter.36,37 In addition, a study by Qi et al.38 reports the requirement for CTGF profibrotic expression in renal fibroblasts must occur in the presence of TGF-β signaling as the pro-fibrotic effect of CTGF was blocked by pan-specific TGF-β and TGF-β type II receptor neutralizing antibodies. Active pro-fibrobitic signaling requires active TGF-β signaling to promote fibrotic ECM production.
Col3 is widely distributed in Col1 containing tissues.39 This homotrimeric molecule that consists of three α1(III) chains is also abundant in elastic tissues, however absent in bone.39 In normal skin, Col1 and Col3 exist in a ratio of approximately 4:1. In hypertrophic scars, the percentage of Col3 may be as high as 33%, which has a correlation with expression of TGF-β1 mRNA.40,41 In the current study, we demonstrated high expression of Col3 in CTS patients, with a positive correlation with TGF-β1. Furthermore, the ratio of Col1 to Col3 is 4:1 in normal tissue and nearly 1:1 in CTS patient tissue. These combined findings suggest that TGF-β signaling might be important in the development of SSCT fibrosis in CTS.
While the inciting pathological mechanism of CTS remains elusive, one hypothesis is that CTS results from a cycle of progressive injury, damage and fibrosis in the SSCT during repetitive finger and wrist movement.42–44 With repeated injury, the increase in TGF-β1 production may be sustained, leading to progressive fibrosis of the SSCT via increased expression of fibrotic pathways, as reflected in the increased expression of CTGF and Col3 that we observed. In this study, all patient tissues were diagnosed as showing non-inflammatory fibrosis. A number of previous studies have already shown that the SSCT in CTS patients is non-inflammatory.1,2,45 Although it is well known that TGF-β plays an important role during acute inflammatory fibrosis and remodeling processes, implying a need for an inflammation response, recent evidence reports that CTS fibrosis may occur primarily through oxidative stress, which is also TGF-β mediated.46 Regardless, if CTS has a low level of initial inflammation or an oxidative stress response, it is clear that TGF-β is highly expressed in CTS patients, and CTGF, which is regulated, at least in part, by TGF-β, is also highly expressed.
Potential limitations of this study include sample size and distribution. We used a sample of convenience, specimens which had already been collected at our institution for clinical diagnosis. Thus, these cases were not randomly selected. In addition, since CTS primarily affects patients in their 40’s and 50’s, it is difficult to match the case and control age. As noted above, we considered the use of cadaver controls to be the best option given that SSCT tissue harvest is not a benign procedure, and therefore normal specimens could not, in our opinion, be obtained ethically from normal living subjects. Another potential limitation of this study is the staining process that we used for TGF-β1 and CTGF. TGF-β1 and CTGF are expressed in normal fibroblasts.47,48 Overexpression of these growth factors may be a sign of excessive connective tissue fibrosis, as shown in other studies.6,35 However, while we found overexpression of TGF-β1 and CTGF in the SSCT of CTS patients compared to normal controls, we lacked an internal positive control for TGF-β1 and CTGF, aside from our normal specimens themselves. However, given the robust significance of both TGF-β1 and CTGF expression in CTS patient tissue we feel strongly that our results are valid. In summary, we have demonstrated an increased expression of TGF-β1, CTGF and Col3 in CTS patients, suggesting that TGF-β signaling might be responsible for the SSCT fibrosis commonly seen in CTS patients.
Footnotes
This work was supported by gNIH/NIAMS R01 AR049823, T32 AR056950, and F32 AR063596
References
- 1.Donato G, Galasso O, Valentino P, et al. Pathological findings in subsynovial connective tissue in idiopathic carpal tunnel syndrome. Clin Neuropathol. 2009;28:129–135. doi: 10.5414/npp28129. [DOI] [PubMed] [Google Scholar]
- 2.Schuind F, Ventura M, Pasteels JL. Idiopathic carpal tunnel syndrome: histologic study of flexor tendon synovium. J Hand Surg Am. 1990;15:497–503. doi: 10.1016/0363-5023(90)90070-8. [DOI] [PubMed] [Google Scholar]
- 3.Hirata H, Nagakura T, Tsujii M, et al. The relationship of VEGF and PGE2 expression to extracellular matrix remodelling of the tenosynovium in the carpal tunnel syndrome. J Pathol. 2004;204:605–612. doi: 10.1002/path.1673. [DOI] [PubMed] [Google Scholar]
- 4.Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86-A:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Kim JK, Koh YD, Kim JS, Hann HJ, Kim MJ. Oxidative stress in subsynovial connective tissue of idiopathic carpal tunnel syndrome. J Orthop Res. 2010;28:1463–1468. doi: 10.1002/jor.21163. [DOI] [PubMed] [Google Scholar]
- 6.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 7.Ihn H. The role of TGF-beta signaling in the pathogenesis of fibrosis in scleroderma. Arch Immunol Ther Exp (Warsz) 2002;50:325–331. [PubMed] [Google Scholar]
- 8.Ask K, Bonniaud P, Maass K, et al. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int J Biochem Cell Biol. 2008;40:484–495. doi: 10.1016/j.biocel.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35:661–664. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- 11.Pohlers D, Brenmoehl J, Loffler I, et al. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Daniels CE, Wilkes MC, Edens M, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3:1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 14.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 15.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 17.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 18.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- 19.Medicine AAoE. Guidelines in electrodiagnostic medicine. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1992;15:229–253. doi: 10.1002/mus.880150218. [DOI] [PubMed] [Google Scholar]
- 20.Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1997;20:1477–1486. doi: 10.1002/(sici)1097-4598(199712)20:12<1477::aid-mus1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Ihn H, Yamane K, Kubo M, Tamaki K. Blockade of endogenous transforming growth factor beta signaling prevents up-regulated collagen synthesis in scleroderma fibroblasts: association with increased expression of transforming growth factor beta receptors. Arthritis Rheum. 2001;44:474–480. doi: 10.1002/1529-0131(200102)44:2<474::AID-ANR67>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 23.Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987;105:1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolb M, Margetts PJ, Sime PJ, Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1327–1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 25.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 27.Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 28.Verrecchia F, Vindevoghel L, Lechleider RJ, et al. Smad3/AP-1 interactions control transcriptional responses to TGF-beta in a promoter-specific manner. Oncogene. 2001;20:3332–3340. doi: 10.1038/sj.onc.1204448. [DOI] [PubMed] [Google Scholar]
- 29.Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickiewicz TL. Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder. J Orthop Res. 1997;15:427–436. doi: 10.1002/jor.1100150316. [DOI] [PubMed] [Google Scholar]
- 30.Abou-Shady M, Friess H, Zimmermann A, et al. Connective tissue growth factor in human liver cirrhosis. Liver. 2000;20:296–304. doi: 10.1034/j.1600-0676.2000.020004296.x. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Clarkson MR, Duggan J, Brady HR. Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 2000;58:1389–1399. doi: 10.1046/j.1523-1755.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- 32.Paradis V, Dargere D, Vidaud M, et al. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology. 1999;30:968–976. doi: 10.1002/hep.510300425. [DOI] [PubMed] [Google Scholar]
- 33.Mori T, Kawara S, Shinozaki M, et al. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Chujo S, Shirasaki F, Kawara S, et al. Connective tissue growth factor causes persistent proalpha2(I) collagen gene expression induced by transforming growth factor-beta in a mouse fibrosis model. J Cell Physiol. 2005;203:447–456. doi: 10.1002/jcp.20251. [DOI] [PubMed] [Google Scholar]
- 35.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 36.Holmes A, Abraham DJ, Sa S, et al. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- 37.Holmes A, Abraham DJ, Chen Y, et al. Constitutive connective tissue growth factor expression in scleroderma fibroblasts is dependent on Sp1. J Biol Chem. 2003;278:41728–41733. doi: 10.1074/jbc.M305019200. [DOI] [PubMed] [Google Scholar]
- 38.Qi W, Twigg S, Chen X, et al. Integrated actions of transforming growth factor-beta1 and connective tissue growth factor in renal fibrosis. Am J Physiol Renal Physiol. 2005;288:F800–809. doi: 10.1152/ajprenal.00179.2004. [DOI] [PubMed] [Google Scholar]
- 39.Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhang K, Garner W, Cohen L, Rodriguez J, Phan S. Increased types I and III collagen and transforming growth factor-beta 1 mRNA and protein in hypertrophic burn scar. J Invest Dermatol. 1995;104:750–754. doi: 10.1111/1523-1747.ep12606979. [DOI] [PubMed] [Google Scholar]
- 41.Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176:26S–38S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 42.Ugbolue UC, Hsu WH, Goitz RJ, Li ZM. Tendon and nerve displacement at the wrist during finger movements. Clin Biomech (Bristol, Avon) 2005;20:50–56. doi: 10.1016/j.clinbiomech.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 43.van Tulder M, Malmivaara A, Koes B. Repetitive strain injury. Lancet. 2007;369:1815–1822. doi: 10.1016/S0140-6736(07)60820-4. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C, Ettema AM, Osamura N, et al. Gliding characteristics between flexor tendons and surrounding tissues in the carpal tunnel: a biomechanical cadaver study. J Orthop Res. 2007;25:185–190. doi: 10.1002/jor.20321. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs PC, Nathan PA, Myers LD. Synovial histology in carpal tunnel syndrome. J Hand Surg Am. 1991;16:753–758. doi: 10.1016/0363-5023(91)90208-s. [DOI] [PubMed] [Google Scholar]
- 46.Demirkol A, Uludag M, Soran N, et al. Total oxidative stress and antioxidant status in patients with carpal tunnel syndrome. Redox Rep. doi: 10.1179/1351000212Y.0000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183:225–237. doi: 10.1007/s00408-004-2534-z. [DOI] [PubMed] [Google Scholar]
- 48.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002;118:402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]