Abstract
Background
Customization of the tracheoesophageal (TE) voice prosthesis (VP) is often preferred over surgical closure to prevent aspiration around the VP in laryngectomized patients with an enlarged tracheoesophageal puncture (TEP), but has not been thoroughly evaluated.
Study Design
Single-institution prospective trial.
Methods
A prospective trial was conducted to evaluate the effectiveness of a customized VP with the addition of an enlarged tracheal and/or esophageal collar in patients with leakage around an enlarged TEP. Absence of leakage around the VP after placement defined immediate effectiveness. Long-term success was defined by the prevention of adverse events related to leakage during the study period. Events that defined failure included: permanent gastrostomy dependence, aspiration pneumonia, and/or surgical TEP closure.
Results
Twenty-one patients with enlarged TEP were enrolled (2003-2006). Insertion of a customized VP was unsuccessful in 1 patient; 145 customizations were performed in the remaining 20 patients (median: 3.5 customizations) during the trial period. Seventy-seven percent (112/145) of customizations prevented leakage immediately after VP insertion. The most common adverse event was dislodgement of the prosthesis (11%) or the collar alone (7%) in 18% (26/145) of customized VP placements. Six patients who died of disease were not evaluable for long-term outcomes. Long-term success was achieved in 80% (12/ 15) of evaluable patients who avoided permanent gastrostomy, aspiration pneumonia, and surgical TEP closure.
Conclusions
Prosthetic customization offers an effective method to prevent leakage around the VP in many patients with an enlarged TEP, thereby preserving TE voice while avoiding surgical closure in this high risk population.
Keywords: laryngectomy, speech, alaryngeal, tracheoesophageal puncture, voice prosthesis
INTRODUCTION
Tracheoesophageal (TE) speech is the most highly regarded form of alaryngeal voice rehabilitation because it allows restoration of near-normal voice after total laryngectomy (TL).1 TE speech production depends on a puncture that is created between the posterior tracheal and anterior esophageal wall through which a unidirectional voice prosthesis (VP) is inserted to allow shunting of pulmonary airflow into the esophagus for sound production while avoiding aspiration during swallowing. The method requires the occlusion of the tracheostoma using a digit or automatic speaking valve to redirect airflow through the prosthesis for phonation. Although a variety of complications have been reported after tracheoesophageal puncture (TEP),1-3 circumferential enlargement of the TEP ultimately results in leakage around the TE voice prosthesis and remains one of the most challenging problems to manage. Aspiration pneumonia remains a significant and potentially life-threatening complication of an enlarged TEP.4
Enlargement of the TEP has been postulated to occur from a variety of sources including tumor recurrence, fibrosis of irradiated tissues, malnourishment, uncontrolled diabetes, and smoking.3,5 A recent multivariate analysis performed at our institution found advanced nodal disease, postoperative stricture, and locoregional recurrence/distant metastasis to be the most significant risk factors for an enlarged puncture. Additionally, preoperative nutritional status and extended resection were also found to be associated with greater risk of enlargement of the TEP.5
Several treatment alternatives have been proposed to manage the enlarged TEP with varying success. Surgical options include a submucosal purse-string suture around the enlarged TEP and complete closure of the TEP. Conservative methods such as temporary removal of the VP to facilitate stenosis of the TE tract and TEP site injections have often been preferred over surgery. Customization of the VP offers another conservative alternative to prevent leakage around a VP as a result of an enlarged TEP that is non-invasive and frequently preferred over surgical alternatives.6 Most commonly, a tracheal or esophageal silicone collar that is bigger in diameter than the TEP, has been added to the VP to prevent leakage and aspiration around the prosthesis. Previous studies7,8 have provided preliminary evidence but, to date, the optimal customization (tracheal or esophageal) and long-term benefit of customization have not been thoroughly analyzed or described. Therefore, the purpose of this study was to prospectively identify the immediate and long-term effectiveness of a customized VP using tracheal and/or esophageal collars to prevent aspiration around the VP in patients with an enlarged TEP. Thus, the intent of this initial trial was to evaluate success of individualized customizations based on presenting TEP problems; this paper does not present an algorithm for management.
MATERIALS AND METHODS
A prospective clinical trial was conducted to investigate the effectiveness of a customized VP to prevent aspiration around the VP in patients with an enlarged TEP. An enlarged TEP was defined as one that resulted in leakage around the VP unresponsive to standard prosthetic management.4 The study participants included those who had enlarged TEP with leakage around the VP who failed to respond to other measures such as resizing and replacing a standard VP, temporary removal of the VP, and placement of a small diameter catheter to facilitate stenosis of the TEP. All eligible patients were recruited within the Section of Speech Pathology and Audiology at The University of Texas MD Anderson Cancer Center between December, 2003 and June, 2006. Informed consent was obtained prior to placement of a custom VP. Outcomes were prospectively collected for a minimum of 2 years post-enrollment or until death. The trial was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Customization was achieved by adding a tracheal and/or esophageal silicone collar (0.3 to 0.8 mm thickness) to an appropriately sized, commercially available VP. The diameter of the esophageal and/or tracheal collars was increased to ensure surface coverage of the enlarged TEP. Based on visual inspection, the thickness and shape of the collar were modified to conform to, and cover any irregularities at the TEP site. Tracheal collars (diameter range: 13 - 25 millimeters) were secured under the strap of the VP. All esophageal collars (diameter range: 18 -35 millimeters) were adhered to the distal flange of the prosthesis using a medical-grade silicone glue (NuSil Silicone Technology, Carpenteria, CA) and allowed to dry before insertion.
The approach to customization was individualized for each patient, and did not follow a pre-determined algorithm. The type of customization (i.e., enlarged esophageal and/or tracheal collar) was selected based on visual inspection of the enlarged TEP and the clinical presentation of each participant. Our initial approach is always to use a commercially-available standard VP with an enlarged flange. When the standard device does not provide adequate surface coverage to prevent aspiration, customization is undertaken. Enlarged esophageal collars must be adhered to the esophageal flange and allowed to cure prior to insertion. Despite the need for advanced preparation, placement of a VP with an enlarged esophageal collar is often our first choice to optimize coverage of the enlarged puncture and potentially lower the risk of unintentional dislodgement into the airway. In the event of persistent aspiration around the VP, an enlarged tracheal collar may be added after the custom VP is inserted. Conversely, enlarged tracheal collars alone are often preferred in the setting of esophageal stricture, or placed temporarily while a VP is fabricated with an enlarged esophageal flange.
Customized VPs were inserted using a 16 or 20 FR gel-cap (InHealth Technologies, Carpenteria, CA); a tapered gel cap (InHealth Technologies, Carpenteria, CA) was used for customizations that could not be folded into either the 16 or 20 FR gel cap. In some cases, the customized prosthesis was inserted into the TEP using a modified Provox® loading device, particularly when the esophageal collar was 25 millimeters or larger in diameter (Atos Medical, Hörby, Sweden). Figure 1 shows examples of customized collars and prostheses.
Data were prospectively collected at each clinic visit. The type of customization including the diameter and location of the collars, complications, and patient complaints were documented at each follow up visit. Leakage was assessed immediately post-fitting by visual examination of the TEP during multiple liquid swallows and then recorded. Disease characteristics, medical comorbidities, and treatment variables were also collected from the electronic medical record.
The immediate and long-term effectiveness of the VP customization were recorded. Immediate success was defined by the absence of leakage around the VP after the customized VP was placed. Long-term effectiveness was assessed at final follow-up and was defined as the prevention of adverse events related to leakage around the VP over the study period. Permanent feeding tube dependence, aspiration pneumonia, and/or surgical closure of the TEP defined adverse events. End-of-life cases who died of disease within 6-months of study participation were excluded from evaluation of long-term effectiveness because of multiple confounding medical variables that prevented accurate assessment of long-term success.
Descriptive statistics were calculated to summarize sample characteristics, and rates of immediate and long-term effectiveness. Rates of effectiveness were compared by type of customization, extent of surgery (total laryngectomy versus total laryngopharyngectomy), and radiation exposure. Statistical associations between groups for categorical variables were analyzed using Fisher's exact test. Statistical significance was considered α-level 0.05. Statistical analyses were performed using the STATA data analysis statistical software, version 10.0 (StataCorp LP, College Station, TX).
RESULTS
Sample Characteristics
Twenty-one patients met inclusion criteria for this study. The median age was 60 years (range: 51-87) and 86% were male. Thirteen patients (62%) underwent total laryngectomy for T3 or T4 disease and 5 patients (24%) for recurrent disease. Fifteen patients (71%) underwent total laryngectomy and 6 patients (29%) underwent total laryngopharyngectomy. Nineteen patients (90%) received radiation therapy. Median time from surgery to study enrollment for leakage around the prosthesis was 16 months (range: 3-100 months). During the study period, 10 patients (48%) had locoregional or distant disease, one of whom was cured of disease. Therefore, at last follow-up (median 17 months), 12 patients (57%) were living disease-free, 3 (14%) were living with disease, and 6 (29%) had died of disease. Table I summarizes sample characteristics.
I.
Table Patient Demographics (N=21)
| n | % | |
|---|---|---|
| Sex | ||
| Male | 18 | 85.71% |
| Female | 3 | 14.29% |
| Age at consent, yr | ||
| Median | 60.17 | |
| Range | 51.17 -87.42 | |
| T- Classification | ||
| 1/2 | 0 | 0% |
| 3 | 3 | 14.29% |
| 4 | 10 | 47.62% |
| Recurrent at time of TL | 5 | 23.81% |
| Unknown or N/A* | 3 | 14.29% |
| N- Classification | ||
| N0 | 3 | 14.29% |
| N+ | 10 | 47.62% |
| Recurrent at time of TL | 5 | 23.81% |
| Unknown or N/A* | 3 | 14.29% |
| Surgical Procedure | ||
| Total laryngectomy | 15 | 71.43% |
| Total laryngopharyngectomy | 6 | 28.57% |
| Reconstruction | ||
| None | 15 | 71.43% |
| ALT | 5 | 23.81% |
| Jejunum | 1 | 4.76% |
| Radiation | ||
| No | 2 | 9.52% |
| Preoperative treatment | 6 | 28.57% |
| Postoperative treatment | 13 | 61.90% |
| Chemotherapy | ||
| No | 12 | 57.14% |
| Yes | 9 | 42.86% |
| Timing of TEP | ||
| Primary | 5 | 23.81% |
| Secondary† | 16 | 76.19% |
| Recurrent disease during study period | ||
| No | 11 | 52.38% |
| Yes | 10 | 47.62% |
| Smoking at Diagnosis | ||
| Never | 2 | 9.52% |
| Current | 13 | 61.90% |
| Former | 6 | 28.57% |
| History of stricture | ||
| No | 16 | 76.19% |
| Yes | 5 | 23.81% |
| Reflux‡ | ||
| No | 12 | 57.14% |
| Yes | 9 | 42.86% |
Includes single case of Chondrosarcoma.
Includes two patients who received primary and secondary punctures; enlargement occurred after secondary tracheoesophageal puncture (TEP).
As noted by medication history.
TL = total laryngectomy; N/A = not available; ALT = anterolateralthigh.
Outcomes were assessed in 20 of 21 patients who received customizations. One patient could not be fit with a customized prosthesis because of a deeply recessed TEP.
Types of Prosthetic Customizations
Of the 20 patients who received customizations, seven patients (35%) received an enlarged tracheal collar only as the initial type of customization, one (5%) an esophageal collar only, and 12 (60%) had both tracheal and esophageal collars initially placed. Sixteen patients (80%) required more than one custom prosthesis during the study period. A total of 145 voice prostheses were customized in 20 patients (median number of customizations per patient: 3.5, range: 1-47). Table II provides details regarding prosthetic customizations. Overall, a custom esophageal collar was added to 39% (56/145) of VPs, and 8% (11/145) included only tracheal collar customizations. The majority of modifications 54% (78/145) included both tracheal and esophageal collar customizations. Among the 89 customizations using tracheal collars, 74 (83%) were not adhered.
Table II.
Description and Outcomes of Customized Prostheses
| Customization N=145 n (%) | Successful* n (%) | |
|---|---|---|
| Type | ||
| Tracheal Collar Only | 11 (7.59%) | 7 (63.64%) |
| Esophageal Collar Only | 56 (38.62%) | 43 (76.79%) |
| Both (Tracheal and Esophageal) Collars | 78 (53.79%) | 62 (79.49%) |
| Increased Resistance† | ||
| No | 37 (25.52%) | |
| Yes | 108 (74.48%) |
Prevention of leakage immediately after insertion
Glue was placed on a standard prosthesis or a commercially-available prosthesis with increased resistance.
Of the customizations, 75% had a modification to increase airflow resistance through the VP because leakage was also occurring through the device. We added silicone glue to the valve of a standard VP in 21% (30/145), and 54% (78/145) of customizations were made to a commercially available VP with increased resistance.
Immediate Effect of Custom VP
The customized collars prevented leakage immediately after fitting in 77% (112/145) of customizations. Persistent leakage around the custom VP occurred immediately after 23% (33/145) of customizations requiring further intervention. Figure 2 illustrates the immediate effect of customization to prevent leakage around the VP. Rates of immediate success were higher after customizations using esophageal collars (with or without tracheal collars) compared with tracheal collars alone (78% vs. 64%, P = .272). Although rates of immediate success were higher in patients who had total laryngectomy compared with total laryngopharyngectomy (79% vs. 71%, P = .361), and in non-irradiated patients compared with previously irradiated patients (100% vs. 75%, P = .070), these differences were not statistically significant. Eighteen percent of customizations (11% custom prostheses, 7% unadhered tracheal collars only) were unintentionally dislodged from the TEP; however, only 2% (3/145; two custom prostheses, one unadhered tracheal collar) were aspirated but were easily retrieved without further complication.
The need to replace a VP can occur for a variety of indications other than leakage around the VP. Our data revealed two clinical scenarios requiring replacement of the customized VP that were important to calculations regarding device life of the customized VP. The first group comprised 45% (65/145) of all customizations and included devices that required replacement for other indications besides leakage around the VP. The second group included 55% (80/145) of customizations that required replacement specifically because of failure of the customized VP to consistently prevent leakage around the VP over time. The mean duration of effectiveness was 56 days (range, 1-362 days) before customized prostheses required replacement for recurrent leakage around the device.
Long-term Success of Custom VP
Long-term outcomes were available in 71% of patients (15/21). Six patients (29%) died of their disease within 6 months of study participation, and were excluded from the analysis of long-term outcomes because of confounding medical complications. Long-term success was achieved in 80% (12/15) of evaluable patients who avoided permanent gastrostomy, aspiration pneumonia, and surgical TEP closure at a median follow-up of 23 months. Fifty-eight percent (7/12) of patients were able to resume the use of a standard, non-customized VP and 42% (5/12) were successfully managed at last follow-up using customized VPs. The 3 unsuccessful patients included 1 who required surgical closure of the TEP and 2 patients who had recurrent pneumonia despite customizations (one died of his comorbidities, and one continues to speak using his TEP despite recurring pneumonias). Neither extent of surgery (total laryngectomy versus total laryngopharyngectomy, P = 1.000) nor radiation history (nonirradiated versus irradiated, P = 1.000) were significantly associated with long-term success of prosthetic customization. Long-term outcomes are illustrated in Figure 3.
DISCUSSION
Despite the relatively simple and straightforward method of placing a TE voice prosthesis to allow alaryngeal voice restoration after total laryngectomy, leakage of food and saliva around the VP continues to be a frustrating and potentially life threatening problem that is difficult to manage. Enlargement of the TEP has been estimated to occur in up to 20% of patients with TEP. The adverse effects of this complication can be significant.3,4,6 We have previously reported a 39% risk of pneumonia and a 14% rate of chronic feeding tube dependence in patients with intractable leakage resulting from an enlarged TEP that is inadequately managed.4 These rates are notable in a population for whom the risk of aspiration and pneumonia should be relatively minimal because of the separation of the airway from the digestive tract.
The results of our study showed that leakage around a TE voice prosthesis can be immediately prevented most of the time (77%) by modifying the prosthesis to include an enlarged collar around the device that effectively shields the puncture from food, liquid, and saliva seeping around it. Furthermore, we found that 80% of patients with enlarged TEPs who were evaluable long-term were successfully managed with the customized VP and avoided permanent gastrostomy, aspiration pneumonia, and surgical TEP closure at their last follow up, regardless of the extent of surgery or their radiation history. Our data also support that of other investigators who found that a small silicone collar placed on the tracheal end of the VP is often successful in preventing leakage around the VP.8 However, our clinical experience has shown that simply adding a tracheal collar in patients with leakage around the VP is not always successful in preventing long-term leakage in all patients. Therefore, our study evaluated the effectiveness of the use of the tracheal collar alone compared with the use of a VP with a customized esophageal collar, and further analyzed customizations that required both tracheal and esophageal modifications. We found that only 8% of customizations successfully prevented leakage using a tracheal collar alone while 39% required a prosthesis with a customized esophageal flange alone. However, the majority of customizations (54%) required the use of a prosthesis with both customized esophageal and tracheal collars to prevent long-term leakage around the VP. We surmise the greater effectiveness using an esophageal customized collar may be a function of the ability to achieve better surface coverage of the esophageal mucosa versus the peri-TEP tracheal mucosa that is often irregular and difficult to consistently cover especially as the prosthesis pistons and moves within the TE tract during swallowing, speech production, and even quiet breathing.
We found the time to customize the esophageal flange slightly longer than placement of a tracheal collar, and the insertion of the VP with a customized esophageal flange was not as straightforward as simply slipping an enlarged collar on the tracheal side of the VP. However, neither the time nor the effort spent customizing the VP was found to hinder insertion or effectiveness. It has been our experience that conservative alternatives such as the modification of a VP, even those that require slightly more clinician effort, are much preferred to surgical alternatives particularly in this high risk population.
In addition, 75% of the customizations we performed for leakage around the VP were made to prostheses that also had modifications, ours or the manufacturer's, that provided increased resistance to airflow and valve opening. Our clinical experience has shown that leakage around a VP is often accompanied by early leakage through it. Although the reason for this is not clear, we suspect that changes in intraluminal pressure, particularly during swallowing, may contribute to prosthetic leakage both around and through the device. It is likely that modifications to the esophageal collar along with modifications to valve resistance may improve the overall duration of effectiveness using a customized VP in some patients. Further comparisons are needed.
We also believe that it is advantageous to customize the VP to the specifications of the defect. That is, we visually inspected the enlarged TEP sites so that we could customize the tracheal or esophageal flange to optimally approximate the size and shape of the enlargement. We have found that a round collar of the same diameter added to the tracheal end of the prosthesis does not provide optimal coverage in all cases. In addition, an unsecured tracheal collar represents an elevated risk for aspiration. Our data demonstrated that the dislodgement rate of 18% was elevated as a result of using unadhered tracheal flanges in half of our customizations as we defined this complication based on dislodgement of either the collar itself or the entire prosthesis. Our findings suggest that the risk of dislodgement may be lowered by as much as 7%, from 18% to 11%, by securing the tracheal collar. Our practice now is to firmly attach tracheal collars to the voice prosthesis with glue or other means to avoid dislodgement and prevent the adverse event of aspiration of the collar.
CONCLUSION
Customization of the VP using a tracheal and/or esophageal collar offers an effective method to control leakage around the VP in select patients with an enlarged TEP. We believe that the most effective customizations are those tailored to the defect, in shape, size, and thickness of the customized collar. In select patients, the use of a customized esophageal collar may provide longer prevention of leakage around the VP compared with the use of a tracheal collar alone. However, it is likely that most patients will benefit from both. The immediate effectiveness of prosthetic customization is attractive in a clinical setting, but our long-term success rates are even more promising as this alternative may avoid the need for subsequent surgical closure of the TEP.
Fig. 1.
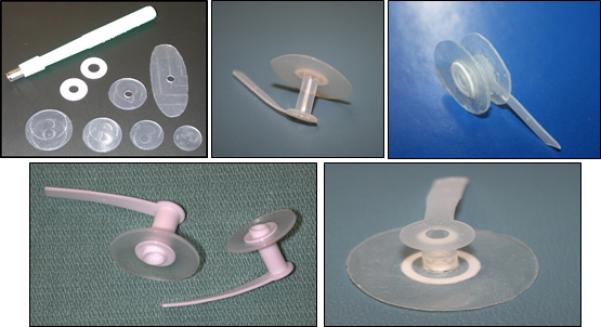
Examples of customized collars and tracheoesophageal voice prostheses.
Fig. 2.
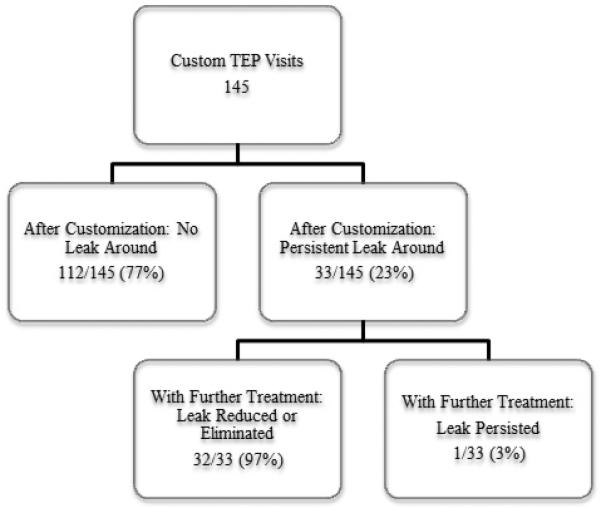
Immediate effectiveness of customized prostheses on leakage around the tracheoesophageal voice prosthesis. TEP = tracheoesophageal puncture.
Fig. 3.
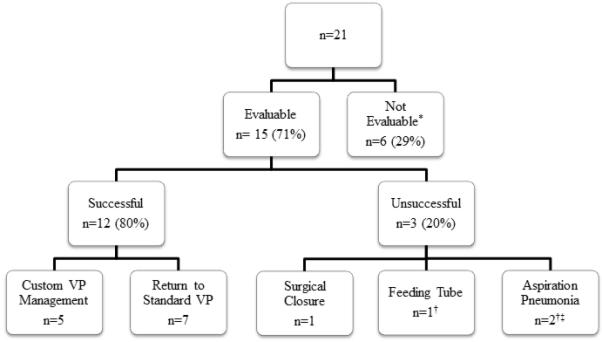
Long-term effectiveness of customized tracheoesophageal voice prostheses (VP). *Died of disease within 6 months of study participation. †One patient experienced both aspiration pneumonia and required a feeding tube. ‡One patient continues to speak despite recurring episodes of aspiration pneumonia.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Mr. Wei-Han Kan, Ms. Martha Portwood, and Mrs. Janet Hampton for their help with data analysis and manuscript submission. Their support was invaluable.
Footnotes
Presented at the Triological Society Combined Sections Meeting, Miami Beach, Florida, 01/29/2011
Financial Disclosures and Conflicts of Interest: None
BIBLIOGRAPHY
- 1.Lewin JS, Bishop-Leone JK, Forman AD, Diaz EM., Jr. Head Neck. 2001;Further experience with Botox injection for tracheoesophageal speech failure.23:456–460. doi: 10.1002/hed.1059. [DOI] [PubMed] [Google Scholar]
- 2.Zafereo ME, Weber RS, Hutcheson KA, et al. Academy of Head and Neck Surgery Annual Meeting. Phoenix, AZ: 2009. The influence of timing and previous radiation on complications and speech outcomes with tracheoesophageal punctures. [Google Scholar]
- 3.Op de Coul BM, Hilgers FJ, Balm AJ, et al. A decade of postlaryngectomy vocal rehabilitation in 318 patients: a single Institution's experience with consistent application of provox indwelling voice prostheses. Arch Otolarngol Head Neck Surg. 2000;126:1320–1328. doi: 10.1001/archotol.126.11.1320. [DOI] [PubMed] [Google Scholar]
- 4.Hutcheson KA, Lewin JS, Sturgis EM, Risser J. Outcomes and adverse events of enlarged tracheoesophageal puncture after total laryngectomy. Laryngoscope. 2011;121:1455–1461. doi: 10.1002/lary.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutcheson KA, Lewin JS, Risser J, Sturgis EM. Multivariable analysis of risk factors for enlarged tracheoesophageal puncture after total laryngectomy. Head Neck. 2012;34:557–567. doi: 10.1002/hed.21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutcheson KA, Lewin JS, Sturgis EM, Kapadia A, Risser J. Enlarged tracheoesophageal puncture after total laryngectomy: a systematic review and meta-analysis. Head Neck. 2011;33:20–30. doi: 10.1002/hed.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kress P, Schafer P, Schwerdtfeger FP, Kress P, Schafer P, Schwerdtfeger FP. The custom-fit voice prosthesis, for treatment of periprothetic leakage after tracheoesophageal voice restoration [in German]. Laryngorhinootologie. 2006;85:496–500. doi: 10.1055/s-2006-925081. [DOI] [PubMed] [Google Scholar]
- 8.Hilgers FJ, Soolsma J, Ackerstaff AH, Balm FJ, Tan IB, van den Brekel MW. A thin tracheal silicone washer to solve periprosthetic leakage in laryngectomies: direct results and long-term clinical effects. Laryngoscope. 2008;118:640–645. doi: 10.1097/MLG.0b013e31816067d5. [DOI] [PubMed] [Google Scholar]


