Abstract
This study investigated the overwintering survival and infectivity of free-living gastrointestinal nematode (GIN) stages on pasture. The presence of GIN larvae was assessed on 3 sheep farms in Ontario with a reported history of clinical haemonchosis, by collecting monthly pasture samples over the winter months of 2009/2010. The infectivity of GIN larvae on spring pastures was evaluated using 16 tracer lambs. Air and soil temperature and moisture were recorded hourly. Free-living stages of Trichostrongylus spp. and Nematodirus spp. were isolated from herbage samples. Gastrointestinal nematodes were recovered from all tracer lambs on all farms; Teladorsagia sp. was the predominant species. Very low levels of Haemonchus contortus were recovered from 1 animal on 1 farm. The results suggest that Haemonchus larvae do not survive well on pasture, while Teladorsagia sp., Trichostrongylus spp. and Nematodirus spp. are able to overwinter on pasture in Ontario and are still infective for sheep in the spring.
Résumé
Projet pilote pour faire enquête sur l’hivernage des larves de nématodes gastro-intestinaux libres chez les moutons en Ontario, au Canada. Cette étude a examiné la survie à l’hivernage et le pouvoir infectieux des stades des nématodes gastro-intestinaux (NGI) libres dans les pâturages. La présence de larves de NGI a été évaluée en recueillant des échantillons mensuels dans le pâturage pendant les mois de l’hiver 2009–2010 dans 3 fermes ovines en Ontario avec des antécédents documentés d’hémonchose clinique, tandis que le pouvoir infectieux des larves de NGI sur les pâturages du printemps a été évaluée en utilisant 16 agneaux sentinelles. La température et l’humidité de l’air et du sol ont été notées toutes les heures. Les stades libres de Trichostrongylus spp. et de Nematodirus spp. ont été isolés d’échantillons d’herbage. Les NGI ont été récupérés de tous les agneaux sentinelles dans toutes les fermes et Teladorsagia sp. était l’espèce prédominante. De très faibles taux d’Haemonchus contortus ont été récupérés chez 1 animal dans 1 ferme. Les résultats suggèrent que les larves d’Haemonchus ne survivent pas bien dans le pâturage, tandis que Teladorsagia sp., Trichostrongylus spp. et Nematodirus spp. peuvent survivre l’hiver dans le pâturage de l’Ontario et être toujours infectieux pour les moutons au printemps.
(Traduit par Isabelle Vallières)
Introduction
Gastrointestinal nematodes (GINs) are ubiquitous on sheep farms worldwide, and can cause important economic losses (1). These helminth parasites of sheep have a direct life-cycle, with both free-living and parasitic stages (2). The free-living component of the life cycle involves development of GIN eggs to third-stage infective larvae (L3) in feces, and the subsequent migration onto pasture grasses; larvae are then consumed by definitive hosts (3).
The survival and development of free-living stages is largely influenced by environmental factors, such as air and soil temperature, soil moisture, and air relative humidity (1,4–5). Several studies have reported seasonality in larval numbers on pasture (6–8), and this seasonality has often under-pinned management practices for controlling sheep nematodosis (5). With the emergence of anthelmintic resistance (9), knowledge of the regional factors that influence the dynamics of the free-living stages has assumed an even more important role, as more producers turn to targeted anthelmintic treatment (10) and pasture management strategies to control GIN infections on sheep farms (3). Anthelmintic resistance has recently been reported in sheep flocks in Ontario (11), highlighting the need to improve our knowledge of environmental factors that might influence the presence of GIN on pasture under central Canadian climate conditions.
A recent study on the epidemiology of GIN infections on sheep farms in Ontario and Quebec showed that the most predominant nematode genera during the summer months were Teladorsagia sp., Haemonchus contortus and Trichostrongylus spp. (12). While no recent studies have been conducted to determine the epidemiology of GINs on pasture during the winter months in central Canada, previous studies in the Maritime Provinces (13) and Quebec (14) indicated that both Teladorsagia sp. and Trichostrongylus spp. survived on pasture in the winter, while H. contortus did not. Nonetheless, in recent work conducted in Ontario and Quebec, H. contortus L3s were isolated from herbage samples collected in the spring months, before sheep were put out to graze (15). This finding suggested that H. contortus was able to over-winter on pasture and might have adapted to central Canadian climate conditions. However, the infectivity of these L3s was not determined.
Haemonchus contortus has been described as the single most important parasite affecting sheep, given its high pathogenicity and high biotic potential (7). It is therefore essential to determine whether this parasite has adapted to, and is able to survive, winter weather conditions in central Canada, as this will inform future recommendations on pasture management strategies for the control of GIN. Thus, the objectives of this pilot study were twofold: i) to describe the environmental factors that may affect the over-wintering survival of GINs on 3 commercial sheep farms in south-western Ontario; and ii) to determine if H. contortus larvae are a) able to over-winter on pasture and/or soil under central Canadian winter conditions, and b) capable of establishing a patent infection in naïve tracer lambs the following spring.
Materials and methods
Farm selection
A longitudinal study was conducted between December 2009 and June 2010 in which 3 commercial sheep farms were purposively selected in southwestern Ontario (range between 42°19′N to 44°38′N latitude and 77°98′W to 80°25′W longitude). The sample size was dictated by logistical and financial constraints. Farms were selected based on their willingness to participate in the study, distance from the University of Guelph (within a 200 km radius) due to a requirement for frequent sampling and animal monitoring, and a known history of H. contortus parasitism on the farm. Specifically, both farms A and B reported a veterinary diagnosis of lambs dying of haemonchosis in the summer of 2009, while farm C participated in a study on the epidemiology of GIN parasites in Ontario sheep flocks between 2006 and 2009 (12) and larval cultures performed in 2009 indicated that H. contortus was the predominant parasite species on that farm. Within each farm, a 1-acre representative section of pasture, where GIN-infected ewes and lambs had grazed the previous summer, was selected.
Environmental data
A HOBOware® Pro Data-Logger (Onset Computer Corporation, Bourne, Massachusetts, USA) was set up on the 1-acre section of each of the 3 farms in December, after the sheep were taken off pasture. The data-logger had 4 probes that measured, at hourly intervals, the air temperature (°C) and relative humidity (%) at 1.5 m above ground level, and the soil temperature (°C) and moisture (m3/m3) 5 cm below ground level. These data were downloaded at monthly intervals, from December 2009 to May 2010. It was not possible to collect snow coverage data at the probe level.
Sampling of herbage and soil
Herbage and soil samples were collected monthly during the winter (January to April 2010) from the 1-acre sections on each of the 3 farms. Both herbage and soil samples were collected by walking two “W” routes in the one-acre section and stopping every 20 to 30 paces (16). The herbage was clipped as close as possible to the ground, avoiding fecal and soil contamination. The total amount of herbage collected in each paddock section did not exceed 500 g wet weight. Soil samples were collected using a 30-cm soil auger; core samples were divided into ‘upper 15 cm soil’ (i.e., 0 to 15 cm) and ‘lower 15 cm soil’ (i.e., > 15 to 30 cm) segments (16).
Tracer lambs
In April–May 2010, 16 Rideau-Arcott X Dorset weaned lambs of 3 to 4 months of age, were selected from the Ponsonby Sheep Research Centre at the University of Guelph, weighed, and treated with 10 mg/kg body weight (BW) fenbendazole (Safe-Guard™ Suspension 10%; Intervet Canada, Kirkland, Quebec). The flock at the Ponsonby Sheep Research Centre has been closed for the past 22 y, and from data acquired from repeated monitoring of fecal egg counts (FECs) and necropsy examinations, is considered to be free of infection from all GIN species, except for Nematodirus filicollis. Therefore, the lambs were considered naïve to GIN. Moreover, these animals had never been on pasture, and fecal samples collected from the lambs on day 0 (i.e., when lambs were put on pasture) showed zero GIN FECs.
The tracer lambs were put out to graze for 28 days, starting at the same time the rest of the flock was put on pasture (May 11th on farm A, April 14th on farm B, and May 13th on farm C): 5 tracer animals were placed on each of farms A and B, and 6 animals on farm C. On farms A and C, the same 1-acre sections of paddocks that were sampled in the winter months were fenced off, and the tracer lambs were left to graze on these sections without co-mingling with other sheep. On farm B, the owner wanted the tracer lambs rotationally grazed with the rest of the flock, which was moved every 3 to 4 d onto different fenced-off paddocks, including the paddock with the 1-acre section sampled in the winter. The sheep only grazed the pastures that had been grazed in the previous season but not yet that spring, and the tracer lambs did not return to the same paddock during their 28-day grazing period. Since this was done in April when maximum daily temperatures were still cool, it was improbable that any GIN eggs shed by the rest of the flock would have developed into infective L3s within 3 to 4 d, as this normally takes a minimum of 5 d under optimal environmental conditions (2). After 28 d of grazing, the lambs were weighed and slaughtered at the abattoir of the Food Science Department, University of Guelph. At necropsy, the abomasum, small intestine, and large intestine from each lamb were tied, isolated, and transported in buckets over a period of 1 h to the Ontario Veterinary College (OVC) necropsy room for further processing.
The University of Guelph Animal Care Committee and Research Ethics Board approved all of the animal work (Animal Utilization Protocol Approval No. 09R090) and the method for selection of farmers (Protocol Approval No. 09DC005), respectively.
Laboratory methods
Herbage and soil samples were processed for Baermann testing at the Parasitology Laboratory, Department of Pathobiology, OVC, University of Guelph. Herbage samples were placed in a 23-cm diameter, 2.5 L capacity funnel, fitted with a scientific cleaning wipe (Kimwipes®; Kimberly-Clark, Irving, Texas, USA) that was laid over a removable wire mesh. A small length of hard rubber tubing was attached to the stem of the funnel, and the end of the tubing was fitted with a 50-mL plastic centrifuge tube. The funnel was filled with lukewarm water until the grass sample was submerged, and the sample was left overnight. The next day, the water was removed with a suction apparatus, and the centrifuge tube was detached, centrifuged at 1800 × g for 2 min, and the supernatant discarded. The sediment (approximately 1 mL) was collected; 2 drops of the mixed sediment were transferred to a microscope slide and 1 drop of Lugol’s iodine was added to kill and stain the larvae. A 24 mm × 50 mm cover slip was placed over the mixture and the slide was examined at a magnification of 100 to 400× (as required). This was repeated until all the sediment collected had been examined. All L3s recovered were identified to the genus level and counted. The herbage sample was then transferred onto a tray, dried in an incubator at 37°C until brittle, and its weight recorded. The number of larvae in the herbage samples was expressed as larvae per kg dry matter (DM).
The same Baermann procedure described was used for examination of soil samples, except that samples were submerged in lukewarm water for 48 h. Free-living soil nematodes were differentiated from infective L3s based on morphological features and Lugol-staining properties. Larval recovery was recorded as presence or absence of larvae at a depth of 0 to 15 cm or > 15 to 30 cm below the surface.
The lamb necropsies to determine GIN parasite infection were performed according to methods described by the Ministry of Agriculture, Fisheries and Food (16) and Gasbarre (17). At necropsy, the abomasum, small intestine, and cecum of each lamb were separated, opened, and the entire contents collected in separate buckets. The organs were then each washed in 5 L of lukewarm water, and the contents collected. While mixing vigorously, a 1-L sample was then collected in a pre-labeled plastic bottle and left to stand for 5 to 6 h, after which the top 100 mL was removed and replaced with 100 mL of 40% formaldehyde solution. For each tracer lamb, 200 mL, 100 mL, and 500 mL collected from the abomasum, small intestine, and cecum, respectively, were analyzed. All worms recovered from the washings were counted and identified microscopically to the species level (16).
Statistical analysis
The environmental data were exported into a Microsoft Excel spreadsheet (Microsoft Office Excel, 2007; Microsoft, Redmond, Washington, USA) and then into SAS 9.3 (SAS Institute, Cary, North Carolina, USA) for data cleaning. The data for each month from the 3 farms were merged, then separated into 43 datasets for each of the 4 environmental variables measured (air and soil temperature, soil volumetric water content, and air relative humidity). Only data from December 26th 2009 to May 31st 2010 were kept in the dataset as this corresponded to the relevant study period. Descriptive statistics were carried out to calculate the daily mean, minimum, and maximum measurements on the 3 farms, for each environmental variable.
Herbage, soil, and tracer lamb data were manually entered into a Microsoft Excel spreadsheet, and a Wilcoxon rank-sum non-parametric test was used to compare the total, and genus-specific, number of GINs isolated in the tracer lambs on the 3 farms. An alpha value ≤ 0.05 was considered to be statistically significant.
Results
Farm description
All 3 study farms kept Rideau or Rideau X Polled Dorset breed sheep for meat purposes, practiced out-of-season lambing, and used ultrasound for pregnancy diagnosis. However, the farms had different flock sizes and represented 3 of the 11 Ontario Sheep Marketing Agency districts (18): Farm A was in District 7 and had 2000 breeding ewes; Farm B was in District 6 and had 400 breeding ewes; Farm C was in District 5 and had 130 breeding ewes. Farms B and C also participated in a concurrent study on the peri-parturient egg rise in ewes lambing out-of-season (19), and ewes sampled on the same day the tracer lambs were put on pasture had mean FECs of 393 and 392 epg on farms B and C, respectively.
Environmental data
Air temperature and humidity were similar among the 3 farms. Temperatures remained below freezing point (−1.0°C to −19.0°C) for most of January (the coldest month for the study period December 2009 to May 2010) and February, then increased above freezing point in March, although a dip in the temperature to −5°C was observed between the 26th and 27th of March on all 3 farms. The averages among farms of their average daily minimum and maximum temperatures were −11.0°C in January and 19.9°C in May, respectively.
The overall daily mean air relative humidity was 83.5% in January and 70.3% in May. The differences between the minimum (daytime) and maximum (nighttime) relative humidity reported were most marked between March and May, for 2 reasons: i) this is when precipitation came in the form of rain, compared to snow being the predominant precipitation in December to February; and ii) daily minimum air relative humidity dropped as low as 20% during March to May with the warm dry spring winds, whereas in December to February, it never dropped below 40%.
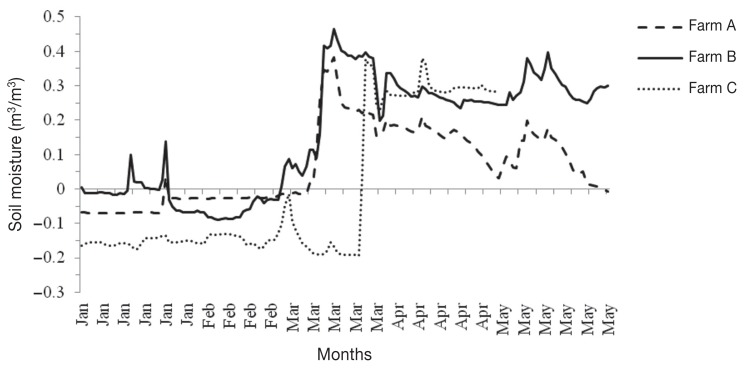
The soil temperature on farms A and B was fairly constant between December and February, while that on farm C fluctuated substantially between December and February, leading to many freeze-thaw cycles (Figure 1), at least in the location of the data-logger. Negligible soil moisture was recorded (negative data) in most of January and February, while positive data were recorded between March and May (Figure 2). However, the soil moisture on farm C was lower (i.e., more negative values) between January and March, with a minimum of −0.20 m3/m3 in early March, and the negative values lasted for 2 more weeks into March, compared to farms A and B.
Figure 1.
Daily maximum, mean, and minimum soil temperatures, recorded by HOBOware® Pro Data-Loggers placed on each of 3 commercial sheep farms in Ontario, Canada, between December 2009 and May 2010.
Figure 2.
Daily mean soil volumetric water content, recorded by HOBOware® Pro Data-Loggers placed on each of three commercial sheep farms in Ontario, Canada, between January and May 2010. Note: Data from May 2010 are not presented for farm C as an error was observed in the recordings.
Reports from the nearest weather stations indicated that, during the period December 2009 to April 2010, snowfall was the lowest recorded amount for the province of Ontario since observations began in 1843 (20).
Herbage and soil samples
Herbage and soil samples were collected from January to March 2010 on farm A, and from January to April 2010 on farms B and C. On farm A, 127.1 L3/kg DM were isolated in the herbage samples collected in March and, of the L3s isolated, 84.7 L3/kg DM (67%) were identified as Trichostrongylus spp., and 42.4 L3/kg DM (33%) were identified as Nematodirus spp. No L3s were isolated in the herbage samples collected in January and February on farm A. Similarly, no L3s were isolated from any of the herbage samples collected on farms B and C. Furthermore, no L3s were isolated in either the “upper 15 cm soil” or “lower 15 cm soil” segments collected from all 3 farms.
Tracer lambs
Gastrointestinal nematodes were recovered from all tracer lambs on all farms. Table 1 presents the mean counts of GINs (adults + immature stages) isolated from the tracer lambs on the 3 farms; Teladorsagia sp. was the predominant species. Very low levels of Haemonchus contortus were recovered from only 1 animal on farm C. There was a significant difference between the mean parasite count for tracer lambs on farm A and farm C (P < 0.0001), and on farm B and farm C (P = 0.0005). The mean count of Teladorsagia circumcincta isolated in tracer lambs on farm C was significantly lower compared with farm A (P = 0.004) and farm B (P = 0.004), while the mean count of Ostertagia trifurcata was significantly higher on farm A compared with farm B (P = 0.016) and farm C (P = 0.002). Lastly, the mean count of Trichostrongylus colubriformis was significantly higher on farm B compared to both farms A (P = 0.016) and C (P = 0.028).
Table 1.
Arithmetic means of gastrointestinal nematode counts (and percentage distribution) for 16 tracer lambs put out to graze, and slaughtered after 28 days, on 3 commercial sheep farms in Ontario, Canada, between April and May 2010
| Farm A N = 5 |
Farm B N = 5 |
Farm C N = 6 |
|
|---|---|---|---|
| Total mean parasite count | 6678a | 6945a | 1023b |
| Range | 5225 to 8050 | 3200 to 10360 | 385 to 1750 |
| Abomasum | |||
| Teladorsagia circumcincta | 5925a (88.7) | 5350a (77.0) | 254b (24.8) |
| Ostertagia trifurcate | 275a (4.1) | 50b (0.7) | 0b (0.0) |
| Haemonchus contortus | 0a (0.0) | 0a (0.0) | 8a (0.8) |
| Small intestine | |||
| Trichostrongylus colubriformis | 106a (1.6) | 690b (9.9) | 142a (13.9) |
| Nematodirus battus | 130a (1.9) | 185a (2.7) | 142a (13.9) |
| Nematodirus spathiger and filicollis | 240a (3.6) | 650a (9.4) | 475a (46.4) |
| Large intestine | |||
| Oesophagostomum columbianum | 2a (0.03) | 20a (0.3) | 2a (0.2) |
N — number of tracer lambs.
Identical superscripts indicate the absence of significant difference, whereas different superscripts following the mean counts indicate significant difference (Wilcoxon rank-sum test; alpha ≤ 0.05).
Discussion
In this study, free-living parasite larvae were recovered from herbage samples collected on farm A in March. The species identified were Trichostrongylus spp. and Nematodirus spp., suggesting that these genera had overwintered on pasture. These parasites have been described as more cold-tolerant than H. contortus, and other studies have similarly shown that they can survive on pasture throughout the winter in temperate areas such as the Maritime Provinces and Quebec, Canada (13–14), Spain (6), and New Zealand (8).
No larvae were isolated from the herbage samples collected in January and February on farm A, or in any of the months on farms B and C. These results are in agreement with previous work conducted on sheep flocks in Ontario and Quebec, where no L3s were recovered from herbage samples collected in January and March (12). In both our study and the study by Mederos et al (12), herbage samples were collected using the standard “W” method, which assumes that infective larvae are distributed evenly on pasture, and that forage availability and use are homogeneous (21). However, larvae distribution and forage availability also depend on other factors, such as stocking density and rate of pasture growth (22). Moreover, parasites are often not randomly distributed on pasture, but concentrated around sheep fecal droppings (21).
In December 2010 and April 2011, herbage samples were again collected from the same 3 farms, but that time the samples were collected purposively within 10 cm of fecal samples. From those samples, free-living larval stages of Trichostrongylus spp., Teladorsagia sp., Haemonchus sp., and Oesophagostomum/Chabertia spp. were isolated from the samples collected in December 2010 on all 3 farms. In April 2011, free-living larval stages of Trichostrongylus spp., Nematodirus sp., Telodorsagia sp. and Oesophagostomum/Chabertia spp. were isolated from pasture samples on all 3 farms (data not presented). Those results suggest that our negative findings in winter 2010 may be due to limitations of the sampling method used, and we therefore recommend future studies take herbage samples within 10 cm of animal feces if the objective is to determine larval over-wintering survival on pasture. Additionally, Waghorn et al (8) estimated the larval extraction efficiencies from herbage and soil samples using the Baermann technique to be 24% and 17%, respectively. These low extraction efficiencies lower the sensitivity of the Baermann technique, and may also have influenced the negative observations in our study.
The use of tracer lambs is a more sensitive test to assess pasture contamination, compared with pasture larval recovery methods, as it represents the GIN infection an animal acquires over a period of time (3,23). In this study, several steps were taken to ensure that any parasites recovered from the tracer lambs were a consequence of ingestion of free-living larvae that had overwintered on pasture from the previous grazing season: i) the pasture used had not been grazed since the previous November; ii) the lambs were GIN-naïve, they originated from a research flock which is known to not have any GIN infection, and the negative status of the lambs was confirmed on FECs the day they were put on pasture; iii) the lambs were treated with a short-acting anthelmintic on the day they were moved to the farms; and iv) on farm B, the lambs were moved every 3 to 4 d to ensure that co-grazing would not lead to cross-infection and false-positive results.
Parasites (adults + immature stages) were isolated from all 16 tracer lambs placed on pasture in April (farm A) and May (farms B and C). However, the mean total parasite count recovered from tracer lambs on farm C was significantly lower (6 times lower) compared with the mean total parasite counts recovered from lambs on farm A or B (Table 1). Moreover, on both farms A and B, Teladorsagia sp. was the predominant parasite recovered, while on farm C, N. spathiger/filicollis was the predominant parasite recovered. This difference in worm counts between farms could be a consequence of different soil temperatures and moisture recorded on the farms and on different levels of pasture contamination. More freeze-thaw cycles were observed on farm C, between December and February, and the volumetric soil water content during the winter months was also lower, compared to the other 2 farms. This could be the result of a difference of level and/or persistence of snow cover on the pastures on the 3 farms and may also explain the lower number of nematodes found in tracer lambs on farm C. During the field visits, the researchers observed that on farm C, the snow coverage was less, and it was often harder to collect soil samples as the ground was deeply frozen, compared to the other 2 farms where the snow coverage was more constant throughout the winter months. O’Connor et al (3) described short-term fluctuations in temperature as being more harmful to GINs, compared with gradual changes in temperature. Moreover, other studies have also suggested that snow can act as a buffer, as it prevents large fluctuations in soil temperature, keeping it at a constant freezing point (23–24). We recognize that the differences observed between parasite counts in tracer lambs on different farms could also be attributed to different parasitism levels in the flocks from the previous year. However, all 3 farms reported clinical signs of gastrointestinal parasitism in their flocks the previous grazing season, suggesting that the level of GIN pasture contamination was high on all 3 farms.
Despite historical evidence of serious clinical haemonchosis problems on the 3 study farms, H. contortus parasites were only isolated in in low numbers from 1 tracer lamb from 1 farm in our study (Table 1). These results are in accordance with recent studies conducted in Spain (6) and Sweden (24), which also found that H. contortus did not overwinter successfully on pasture in temperate winter conditions. In contrast, Teladorsagia sp., Nematodirus spp. and Trichostrongylus spp. were recovered from all the tracer lambs in our study, which suggests that these parasite genera were more tolerant than H. contortus to cold temperatures. In our study, the minimum soil temperature ranged between −20.0°C and −5°C. van Dijk et al (1) reported that −3.0°C was the lowest average temperature at which H. contortus L3 larvae could survive, while both T. circumcincta and, less successfully, Trichostrongylus spp. could survive at −10°C (3). This suggests that, on all 3 farms, the soil temperature in the winter months was not amenable for the survival of H. contortus, at least in the sections of pasture tested.
Paradoxically, H. contortus was only found on farm C, despite farm C having the lowest soil temperatures and more freeze-thaw cycles. A number of reasons could explain this paradox; perhaps the daytime soil temperatures climbing above freezing on farm C allowed H. contortus to survive and remain infective, regardless of the freeze-thaw cycles. Alternatively, the location of the data-logger in the 1-acre pasture was not representative of the pasture environmental conditions, leaving undetected warmer micro-climates away from the data-loggers that were suitable for H. contortus larvae to survive and maintain infectivity.
This descriptive pilot study was conducted on 3 commercial sheep farms over 1 winter season, leading to limited external validity. However, the farms were purposively selected to be representative of the industry and varied geographic distribution of sheep farms with Haemonchus sp. in Ontario, and important differences in both environmental factors and GIN populations were noted among the farms. Enrolment of more farms over a longer sampling period would provide more regional and season-to-season information. Additionally, precise information on snow cover and soil type could be collected to determine whether these factors affect the development, migration, and survival of L3s on pasture. The small probability of H. contortus being able to over-winter on pasture could be exploited to eradicate this parasite from Ontario sheep flocks (25–27); a goal that could be tested in future research. Haemonchus contortus does, however, overwinter as hypobiotic larvae within the host (7), and periparturient ewes have been identified as the primary source of pasture contamination with H. contortus the following spring, when arrested larvae resume development (26). An effective anthelmintic drug could therefore be used to treat ewes before turn-out on pasture, to kill the over-wintering H. contortus nematodes, and prevent pasture contamination (7,27). Unfortunately, recent research in Ontario sheep flocks has shown that resistance to ivermectin and fenbendazole, the 2 most commonly used anthelmintics in Canada, is common, and most of the anthelmintic resistance reported is associated with H. contortus (11). Therefore, more research is required to evaluate selective treatment at lambing with anthelmintics that have been shown to be effective against ivermectin- and fenbendazole-resistant strains of H. contortus, such as the narrow-spectrum anthelmintic closantel (28–29), before eradication of H. contortus could be achieved.
Results from this pilot study on the over-wintering and infectivity of GIN larvae on pasture in the spring suggest that very few Haemonchus larvae were able to over-winter on pasture in the temperate climate of southern Ontario. In contrast, other important parasite genera such as Teladorsagia, Nematodirus, and Trichostrongylus, did survive on pasture during the Ontario winter, and were infective in the spring. These observations need to be taken into consideration when making recommendations on pasture management and timing of anthelmintic treatment for parasite control strategies.
Acknowledgments
The authors are grateful to William Sears for statistical advice, and to Brad De Wolf, Katie Sippel, Kirstie Puskas, Lee Siertsema and Hasani Stewart for laboratory and field assistance. We especially acknowledge the sheep producers who participated in the study. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This research was supported by the Ontario Ministry of Agriculture and Food — New Directions Research Program, with additional financial assistance from the Ontario Sheep Marketing Agency, the Nova Scotia Agricultural College — Organic Science Cluster, and the University of Guelph.
References
- 1.van Dijk J, Sargison ND, Kenyon R, Skuce PJ. Climate change and infectious disease: Helminthological challenges to farmed ruminants in temperate regions. Animal. 2010;4:377–392. doi: 10.1017/S1751731109990991. [DOI] [PubMed] [Google Scholar]
- 2.Taylor MA, Coop RL, Wall RL. Veterinary Parasitology. 3rd ed. Oxford, England: Blackwell Publishing; 2007. pp. 152–162. [Google Scholar]
- 3.O’Connor LJ, Walkden-Brown SW, Kahn LP. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Vet Parasitol. 2006;142:1–15. doi: 10.1016/j.vetpar.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor LJ, Kahn LP, Walkden-Brown SW. Moisture requirements for the free-living development of Haemonchus contortus: Quantitative and temporal effects under conditions of low evaporation. Vet Parasitol. 2007;150:128–138. doi: 10.1016/j.vetpar.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Reynecke DP, Waghorn TS, Oliver AMB, Miller CM, Vlassoff A, Leathwick DM. Dynamics of the free-living stages of sheep intestinal parasites on pasture in the North Island of New Zealand. 2. Weather variables associated with development. NZ Vet J. 2000;59:287–292. doi: 10.1080/00480169.2011.610280. [DOI] [PubMed] [Google Scholar]
- 6.Uriarte J, Llorente MM, Valderrábano J. Seasonal changes of gastrointestinal nematode burden in sheep under an intensive grazing system. Vet Parasitol. 2003;118:79–92. doi: 10.1016/j.vetpar.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Waller PJ, Rudby-Martin L, Ljungström BL, Rydzik A. The epidemiology of abomasal nematodes of sheep in Sweden, with particular reference to over-winter survival strategies. Vet Parasitol. 2004;122:207–220. doi: 10.1016/j.vetpar.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Waghorn TS, Reynecke DP, Oliver AMB, et al. Dynamics of the free-living stages of sheep intestinal parasites on pasture in the North Island of New Zealand. 1. Patterns of seasonal development. NZ Vet J. 2011;59:279–286. doi: 10.1080/00480169.2011.610279. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos E. Anthelmintic resistance in sheep nematodes. Small Rum Res. 2008;76:99–103. [Google Scholar]
- 10.Leathwick DM, Miller CM, Waghorn TS. Development and spatial distribution of the free-living stages of Teladorsagia circumcincta and Trichostrongylus colubriformis on pasture: A pilot study. NZ Vet J. 2011;59:272–278. doi: 10.1080/00480169.2011.610273. [DOI] [PubMed] [Google Scholar]
- 11.Falzon LC, Menzies PI, Shakya KP, et al. Anthelmintic resistance in sheep flocks in Ontario, Canada. Vet Parasitol. 2013;193:150–162. doi: 10.1016/j.vetpar.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Mederos A, Fernández S, VanLeeuwen J, et al. Prevalence and distribution of gastrointestinal nematodes on 32 organic and conventional commercial sheep farms in Ontario and Quebec, Canada (2006–2008) Vet Parasitol. 2010;170:244–252. doi: 10.1016/j.vetpar.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Smith HJ, Archibald RM. The overwinter survival of ovine gastro- intestinal parasites in the Maritime Provinces. Can Vet J. 1965;6:257–266. [PMC free article] [PubMed] [Google Scholar]
- 14.Ayalew L, Gibbs HC. Seasonal fluctuations of nematode populations in breeding ewes and lambs. Can J Comp Med. 1973;37:79–89. [PMC free article] [PubMed] [Google Scholar]
- 15.Mederos A. PhD dissertation. Guelph, Ontario: University of Guelph; 2010. Epidemiological studies of gastrointestinal nematodes in sheep flocks in Ontario and Quebec, Canada. [Google Scholar]
- 16.Ministry of Agriculture, Fisheries and Food. Manual of Veterinary Parasitological Laboratory Techniques. Ministry of Agriculture, Fisheries and Food; London: 1986. pp. 1–65. [Google Scholar]
- 17.Gasbarre LC. Recovery of third stage larvae of Ostertagia ostertagi from the abomasa of experimentally inoculated calves by prolonged saline incubation. Proc Helminthol Soc Wash. 1987;54:160–161. [Google Scholar]
- 18.Ontario Sheep Marketing Agency [homepage on the Internet] [Last accessed May 27, 2014]. Available from: http://www.ontariosheep.org/ABOUTONTARIOSHEEP/Structure.aspx#districts.
- 19.Falzon LC, Menzies PI, Shakya KP, et al. A longitudinal study on the effect of lambing season on the periparturient egg rise in Ontario sheep flocks. Prev Vet Med. 2013;110:467–480. doi: 10.1016/j.prevetmed.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Government of Canada. Climate. [Last accessed May 27, 2014]. [page on the Internet]. Available from: http://climate.weather.gc.ca/advanceSearch/searchHistoricData_e.html.
- 21.Couvillion CE. Estimation of the numbers of trichostrongylid larvae on pastures. Vet Parasitol. 1993;46:197–203. doi: 10.1016/0304-4017(93)90058-u. [DOI] [PubMed] [Google Scholar]
- 22.Familton A, McAnulty R. Sheep nematode larval survival: The epidemiological consequences of findings from recent studies. Proceedings of the Society of Sheep and Beef Cattle Veterinarians of the New Zealand Veterinary Association, Annual Seminar 1974; pp. 185–195. [Google Scholar]
- 23.Stromberg BE. Environmental factors influencing transmission. Vet Parasitol. 1997;72:247–264. doi: 10.1016/s0304-4017(97)00100-3. [DOI] [PubMed] [Google Scholar]
- 24.Troell K, Waller P, Höglund J. The development and overwintering survival of free-living larvae of Haemonchus contortus in Sweden. J Helminth. 2005;79:373–379. doi: 10.1079/joh2005286. [DOI] [PubMed] [Google Scholar]
- 25.Barger IA, Hall E, Dash KM. Local eradication of Haemonchus contortus using closantel. Aust Vet J. 1991;68:347–348. doi: 10.1111/j.1751-0813.1991.tb03100.x. [DOI] [PubMed] [Google Scholar]
- 26.Waller PJ, Rydzik A, Ljungström BL, Törnquist M. Towards the eradication of Haemonchus contortus from sheep flocks in Sweden. Vet Parasitol. 2006;136:367–372. doi: 10.1016/j.vetpar.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Sargison ND, Wilson DJ, Bartley DJ, Penny CD, Jackson F. Haemonchosis and teladorsagiosis in a Scottish sheep flock putatively associated with the overwintering of hypobiotic fourth stage larvae. Vet Parasitol. 2007;147:326–331. doi: 10.1016/j.vetpar.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Uppal RP, Yadav CL, Bhushan C. Efficacy of closantel against fenbendazole and levamisole resistant Haemonchus contortus in small ruminants. Trop Anim Health Prod. 1993;25:30–32. doi: 10.1007/BF02236883. [DOI] [PubMed] [Google Scholar]
- 29.Waruiru RM. Efficacy of closantel, albendazole and levamisole on an ivermectin resistant strain of Haemonchus contortus in sheep. Vet Parasitol. 1997;73:65–71. doi: 10.1016/s0304-4017(97)00065-4. [DOI] [PubMed] [Google Scholar]