Abstract
Insect cells are often glycoengineered using DNA constructs encoding foreign glyocoenzymes under the transcriptional control of the baculovirus immediate early promoter, ie1. However, we recently found that the delayed early baculovirus promoter, 39K, provides inducible and higher levels of transgene expression than ie1 after baculovirus infection (Lin and Jarvis, 2013). Thus, the purpose of this study was to assess the utility of the 39K promoter for insect cell glycoengineering. We produced two polyclonal transgenic insect cell populations in parallel using DNA constructs encoding foreign glycoenzymes under either ie1 (Sfie1SWT) or 39K (Sf39KSWT) promoter control. The surface of Sfie1SWT cells was constitutively sialylated, whereas the Sf39KSWT cell surface was only strongly sialylated after baculovirus infection, indicating Sf39KSWT cells were inducibly-glycoengineered. All nine glycogene-related transcript levels were induced by baculovirus infection of Sf39KSWT cells and most reached higher levels in Sf39KSWT than in Sfie1SWT cells at early times after infection. Similarly, galactosyltransferase activity, sialyltransferase activity, and sialic acid levels were induced and reached higher levels in baculovirus-infected Sf39KSWT cells. Finally, two different recombinant glycoproteins produced by baculovirus-infected Sf39KSWT cells had lower proportions of paucimannose-type and higher proportions of sialylated, complex-type N-glycans than those produced by baculovirus-infected Sfie1SWT cells. Thus, the 39K promoter provides baculovirus-inducible expression of foreign glycogenes, higher glycoenzyme activity levels, and higher human-type N-glycan processing efficiencies than the ie1 promoter, indicating that this delayed early baculovirus promoter has great utility for insect cell glycoengineering.
Keywords: baculovirus expression vector system, glycoengineering, baculovirus promoters, insect cell glycosylation
1. Introduction
The baculovirus-expression vector system (BEVS; reviewed in Jarvis, 2009) is a protein production platform comprised of a recombinant baculovirus and its lepidopteran insect cell host. One advantage of the BEVS over mammalian platforms is the lower risk of contamination with adventitious agents infectious for humans. One advantage of the BEVS over prokaryotic expression systems is its ability to provide eukaryotic protein modifications, including N-glycosylation. However, due to low or undetectable levels of terminal glycosyltransferase activities (Altmann et al., 1993; Butters et al., 1981; Hooker et al., 1999; Stollar et al., 1976), the apparent absence of CMP-sialic acids (Hooker et al., 1999; Tomiya et al., 2001), and the presence of a processing beta-N-acetylglucosaminidase (Altmann et al., 1995; Geisler et al., 2008), insect cells produce much simpler N-glycans than mammalian cells. The major processed N-glycans produced by insect cells are paucimannose-type structures, not terminally sialylated, complex-type structures like those produced by mammalian cells. This difference between insect and mammalian N-glycosylation effectively precludes any opportunity to use the BEVS to produce therapeutic glycoproteins because the circulatory half lives and, therefore, biological activities of these products are known to be significantly lower in the absence of terminal sialic acids (Byrne et al., 2007; Egrie and Browne, 2001; Ngantung et al., 2006; Solá and Griebenow, 2010).
We have addressed this problem in previous studies by engineering the BEVS to produce complex N-glycans. Our approaches involved incorporating foreign genes encoding glycosyltransferases, enzymes involved in sialic acid and CMP-sialic acid biosynthesis, and a CMP-sialic acid transporter into either baculovirus expression vectors (Hill et al., 2006; Jarvis and Finn, 1996; Jarvis et al., 2001; Seo et al., 2001; Tomiya et al., 2003) or insect cell lines (Aumiller et al., 2003; Aumiller et al., 2012; Breitbach and Jarvis, 2001; Geisler and Jarvis, 2012a; Hollister et al., 2002; Hollister and Jarvis, 2001; Hollister et al., 1998; Mabashi-Asazuma et al., 2013). The latter efforts yielded new, glycoengineered insect cell lines capable of producing biantennary, terminally sialylated N-glycans. However, the sialylation efficiencies ranged from 0.1% (Mabashi-Asazuma et al., 2013) to 40% (Toth, unpublished), depending on the cell line and recombinant glycoprotein being produced. Thus, we recently re-focused our efforts on the ultimate goal of producing glycoengineered BEVS that can provide 100% efficient human-type N-glycan processing leading to uniform sialylation.
In all previous insect cell glycoengineering efforts, we used the baculovirus immediate early 1 (ie1) promoter for foreign glycogene expression. The baculovirus ie1 gene encodes a major transcriptional activator and is expressed immediately after viral infection. The ie1 and other baculovirus immediate early genes are transcribed by the host RNA polymerase II, with no requirement for de novo synthesis of any other viral gene products. Therefore, the ie1 promoter is constitutively active in uninfected insect cells and is useful for insect cell glycoengineering (Guarino and Summers, 1986; Jarvis et al., 1990). However, baculoviruses also have three other temporally distinct classes of genes, delayed early, late, and very late, which are not expressed in uninfected insect cells because they require de novo synthesis of other gene products for transcription (Guarino and Summers, 1986; Lu and Miller, 1997). We recently compared the utility of baculovirus promoters from each temporal class for foreign gene expression in transformed insect cells (Lin and Jarvis, 2013). We found that the delayed early 39K promoter, derived from one of the most abundantly expressed early genes (Smith et al., 1982), provided baculovirus-inducible expression of the reporter protein, secreted alkaline phosphatase (SEAP). We also found that the 39K promoter induced higher levels of SEAP activity than any other promoter examined, including ie1. This finding prompted us to undertake the current study, which was designed to examine the utility of the 39K promoter for glycoengineering insect cells with higher efficiencies of human-type N-glycan processing.
To accomplish this goal, we isolated two new, polyclonal glycoengineered insect cell populations by parallel genetic transformation of Sf9 with a set of nine glycogenes placed under the transcriptional control of either the ie1 or 39K promoter. We then compared their growth properties, foreign glycogene expression levels, selected foreign glycosyltransferase activity levels, sialic acid production levels, and N-glycan processing efficiencies using two different recombinant glycoproteins as models. Our data demonstrated that the 39K promoter generally supported higher levels of foreign glycogene expression at early times after infection, which led to higher levels of glycosytransferase activities, sialic acid production, and N-glycan processing, as compared to the ie1 promoter. Therefore, our results demonstrated that utilization of the 39K, rather than the ie1 promoter for foreign glycogene expression is one approach that can be used to increase the efficiency of human-type N-glycan processing in the BEVS.
2. Materials and methods
2.1 Plasmid constructions
The set of plasmids encoding nine different glycoenzymes under the control of the ie1 promoter, which was used to produce Sfie1SWT cells, has been described previously (Table 1). A new set of plasmids encoding the same nine glycoenzymes under the control of the 39K promoter, which was used to produce Sf39KSWT cells, was constructed as detailed in Table 1. In general terms, construction of this new set of plasmids involved replacing the DNA sequence encoding SEAP in p39K-hr5-SEAP (Lin and Jarvis, 2013) with the DNA sequences encoding the relevant glycoenzymes in each ie1 plasmid. Thus, the resulting 39K plasmids were identical to the ie1 plasmids except for the promoter. pIE1Neo, which was used as a selectable marker for the isolation of Sfie1SWT and Sf39KSWT cells, has been described previously (Jarvis et al., 1990).
Table 1.
Glycoenzyme constructs used in this study.
| Glycoenzyme* | ie1 plasmid (reference) | 39K plasmid | Method used for 39K plasmid construction |
|---|---|---|---|
| MGAT1 | pIE1GlcNAcTI (Hollister et al., 2002) | p39KHRGlcNAcTI | 2.7 kb NotI-HindIII fragment from pIE1GlcNAcTI subcloned into NotI-HindII sites of p39K-SEAP |
| MGAT2 | pIE1GlcNAcTII (Hollister et al., 2002) | p39KHRGlcNAcTII | 2.6 kb BglII-HindIII fragment from pIE1GlcNAcTII subcloned into BglII-HindIII sites of p39K- SEAP |
| B4GALT1 | pIE1HR3HyGalT(F282) (Geisler, in prep) | p39KHR3HyGalT(F282) | 1.0 kb BglII-NruI fragment from pIE1HR3HyGalT(F282) subcloned into BglII-PmeI sites of p39K- SEAP |
| GNPE | pIE1HREcGNPE (Geisler and Jarvis, 2012a) | p39KHREcGNPE | 2.0 kb BglII-HindIII fragment from pIE1EcGNPE subcloned into BglII-HindIII sites of p39K-SEAP |
| SAS | pIE1HRMmSAS (Geisler and Jarvis, 2012a) | p39KHRMmSAS | 2.4 kb NotI-HindIII fragment from pIE1HRMmSAS subcloned into NotI-HindIII sites of p39K-SEAP |
| CMAS | pIE1HRMmCSAS (Geisler and Jarvis, 2012a) | p39KHRMmCSAS | 2.1 kb BamHI-XbaI fragment from pIE1MmCSAS subcloned into BglII-XbaI sites of p39K-SEAP |
| CSAT | pIE1-hCSAT (Mabashi-Asazuma et al., 2013) | p39K-hCSAT | 2.3 kb BglII-HindIII fragment from pIE1-hCSAT subcloned into BglII-HindII sites of p39K-SEAP |
| ST3GAL4 | pIE1-hST3GAL4b (Mabashi, in prep) | p39K-hST3GAL4b | 1.8 kb BamHI-XbaI fragment from pIE1-hST3GAL4b subcloned into BglII-XbaI sites of p39K-SEAP |
| ST6GAL1 | pIE1HRHyST6Δcys (Geisler, in prep) | p39KHRHyST6Δcys | 1.9 kb SacII(3′ overhangs removed with T4 DNA Polymerase)-XbaI fragment from pIE1HRHyST6Δcys subcloned into NotI(5′ overhangs filled with T4 DNA Polymerase)- XbaI sites of p39K-SEAP |
HUGO nomenclature, except CSAT, whose HUGO symbol is SLC35A1
2.2. Cells and viruses
Sf9, Sfie1SWT, and Sf39KSWT cells were routinely maintained as shake-flask cultures in ESF 921 medium (Expression Systems, Woodland, CA) at 28°C and 125 rpm. Sfie1SWT and Sf39KSWT cells are new, polyclonal insect cell populations isolated for this study by transforming Sf9 cells using a previously described modified calcium phosphate transfection protocol (Harrison and Jarvis, 2007a, b). The DNA mixtures used to make Sfie1SWT cells included 2.1 μg each of pIE1GlcNAcTI, pIE1GlcNAcTII, pIE1HRHyGalT(F282), pIE1HREcGNPE, pIE1HRMmSAS, pIE1HRMmCSAS, pIE1-hCSAT, pIE1HRST3Gal4b, pIE1HRHyST6Gal1Δcys and 1 μg of pIE1Neo (Table 1). The DNA mixtures used to make Sf39KSWT cells included 2.1 μg each of p39KGlcNAcTI, p39KGlcNAcTII, p39KHRHyGalT(F282), p39KHREcGNPE, p39KHRMmSAS, p39KHRMmCSAS, p39K-hCSAT, p39KHRST3Gal4b, pIE139KHyST6Gal1Δcys and 1 μg of pIE1Neo (Table 1). The cells were selected in TNM-FH supplemented with 10% fetal bovine serum (Fisher Scientific) and 0.1% (w/v) pluronic F68, which is designated “complete” TNM-FH, plus 1 mg/ml geneticin (Calbiochem). After amplification in complete TNM-FH without geneticin, the cells were transferred directly into ESF 921 medium. The Sfie1SWT and Sf39KSWT cells used for the experiments described in this manuscript ranged from passages 5 to 11 and 3 to 28, respectively.
To determine their growth curves, Sf9, Sfie1SWT, or Sf39KSWT maintained in ESF 921 medium were seeded into 125 ml shake flask cultures containing 50 ml of fresh ESF 921 medium at a density of 1.5 × 106 cells/ml. Triplicate samples were removed every 24 h for 5 days and viable cell densities were determined using a Countess® automated cell counter (Invitrogen, Carlsbad, CA).
AchEPO-His, a recombinant baculovirus encoding a C-terminally 6X histidine-tagged version of human erythropoietin (hEPO-His) under the transcriptional control of the polyhedrin promoter, has been described previously (Mabashi-Asazuma et al., 2013). Ac-E1ecto, a recombinant baculovirus encoding an N-terminally 8X histidine-tagged version of the Western equine encephalitis virus E1 glycoprotein (E1-ecto) under the transcriptional control of the polyhedrin promoter, also has been described previously (Toth et al., 2011).
2.3. Virus infections
Sf9, Sfie1SWT, or Sf39KSWT cells were seeded into 500 ml DeLong flasks containing 200 ml of ESF 921 medium, grown to log phase (2 × 106 cells/ml), and infected with AchEPO-His at a multiplicity of infection of 3 plaque-forming units per cell. After a 1 h adsorption period, the inocula were removed and the cells were washed with ESF 921 media, resuspended in ESF 921 at a density of 2×106 cells/ml, and returned to the shake flasks. Samples were then removed at several times after infection and used for the assays described in sections 2.5–2.8.
2.4. Cell surface lectin staining
Sf9, Sfie1SWT, or Sf39KSWT cells were seeded into 6-well plates at a density of 2×106 cells/well, allowed to adhere, and mock-infected or infected for 48 h with AchEPO-His, as described above. At that time point, the cells were stained with Sambucus nigra agglutinin (SNA; Vector Laboratories, Burlingame, CA) to probe for cell surface sialylation, as described previously (Mabashi-Asazuma et al., 2013).
2.5. RT-PCR assays
At various times after infection with AchEPO-His, total RNA was extracted from 5×106 Sf9, Sfie1SWT, or Sf39KSWT cells using the RNA-Solv® (Omega Biotech) reagent according to the manufacturer’s protocol. The RNA preparations were treated with RNAse-free DNAse I (New England Biolabs), and then 400 ng of each were reverse transcribed at 50°C for 30 min with Thermoscript Reverse Transcriptase (Life Technologies) and oligo(dT)31-VN (5′-TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN-3′) as the primer. The resulting cDNA preparations were treated with RNAse H and used for PCR reactions with Taq Polymerase and Crimson Taq Buffer (New England Biolabs). The PCR conditions included an initial denaturation step of 95°C for 30 seconds, followed by 30 (MGAT1, MGAT2, B4GALT1, SAS, ST3GAL4b, SfRPL3) or 33 (GNPE, CMAS, CSAT, ST6GAL1) cycles of denaturation at 95°C for 15 seconds, annealing at 50°C for 20 seconds, and extension at 68°C for 30 seconds. The sequences of the primers used for RT-PCR assays are given in Table 2.
Table 2.
Primers used in this study.
| Transcript* | Primer | Sequence (5′-3′) |
|---|---|---|
| MGAT1 | hGNTI-Fw | ctgctcctcttcttctgg |
| hGNTI-Rv | cgatcttgtagtagccctg | |
| MGAT2 | hMGAT2-Fw | ggtgcatcaatgctgagt |
| hMGAT2-Rv | tgcataccacagtctcca | |
| B4GALT1 | bB4GalT1-Fw | tgagtttaacatacctgtggac |
| bB4GalT1-Rv | ccccagtagttattaggaaatc | |
| GNPE | EcGNPE-Fw2 | tggcattagcggcagaac |
| EcGNPE-Rv2 | Ttcatcgctgtgttgtacc | |
| SAS | mSAS-Fw | ggatgcagtcaatggaca |
| mSAS-Rv | gctaggttgaagatgtcttc | |
| CMAS | mCMAS-Fw | cgacaagactgggatgga |
| mCMAS-Rv | gacacttcattgccgaga | |
| CSAT | hSLC35A1-Fw | atggctgccccgagagacaat |
| hSLC35A1-Rv | tcacacaccaataactctctcctttgaag | |
| ST3GAL4b | hST3Gal4b-Fw | taagctctttggcaactactc |
| hST3Gal4b-Rv | tgtctgggttgttttctactt | |
| ST6GAL1 | HyST6Gal1-Fw | acaaggactccacatactca |
| rST6GAL1-Rv | ccattaaacctcagaactgc | |
| RPL3 | SfRPL3-Fw | acatcgaaactcctcatggtct |
| SfRPL3-Rv | tcttgataaccttgccatcctt |
HUGO nomenclature, except CSAT, whose HUGO symbol is SLC35A1
2.6. Enzyme activity assays
At various times after infection with AchEPO-His, aliquots of each suspension culture containing 5×106 (B4GALT1 and ST6GAL1 assays) or 2×107 (sialic acid assays) Sf9, Sfie1SWT, or Sf39KSWT cells were pelleted, washed once with cold 1X PBS, and then lysed in B4GALT1 (10 mM Hepes, pH 7.4; 140 mM NaCl; 20 mM MnCl2; 0.5% NP-40), ST6GAL1 (100 mM sodium cacodylate, pH 6.4; 10 mM MgCl2; 2 mM CaCl2;1.5% CF-54, Roche protease inhibitor cocktail), or thiobarbituric acid (TBA) assay buffer (1% SDS). The cell extracts were frozen and thawed, samples were clarified by centrifugation (15000×g for 5 min), and then protein concentrations were measured using a commercial bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). B4GALT1 and ST6GAL1/ST3GAL3 activities were measured using samples containing 100 μg of total protein, as described previously (Aumiller et al., 2012), except the reactions were quenched by adding an equal volume of 10% trichloroacetic acid rather than cold buffer, and glass fiber filters were washed immediately after the addition of samples, without drying. Sialic acid determinations were performed using TBA assays, as described previously (Geisler and Jarvis, 2012a), except we added 5 mg of sample to each assay.
2.7. Expression and purification of hEPO-His and E1-ecto
Sf9, Sfie1SWT, or Sf39KSWT cell cultures were infected with either AchEPO-His or Ac-E1ecto as described in section 2.3 and the secreted products were purified from cell- and virus-free culture supernatants at 48 h post-infection (hpi), essentially as described previously (Mabashi-Asazuma et al., 2013). Each purified protein preparation was quantified by BCA assays and purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie brilliant blue staining.
2.8. Western and lectin blotting
The hEPO-His isolated from each baculovirus-infected cell line was treated with PNGase F (New England Biolabs, Ipswich, MA), neuraminidase (New England Biolabs), or their respective buffers alone, according to the manufacturer’s instructions. The proteins were then resolved by SDS-PAGE and transferred to Immobilon-P membranes (Millipore). Western blots were performed using a rabbit polyclonal antibody against hEPO (U-Cy Tech, The Netherlands) and lectin blots were performed with SNA, both as described previously (Mabashi-Asazuma et al., 2013). Lectin blots with Maackia amurensis lectin (MAL) were performed by blocking the membranes with Tris-buffered saline (TBS) containing 1% Tween-20 for 2 h at room temperature, and then probing with biotinylated MAL-I (Vector Laboratories) at a final concentration of 3 mg/ml in MAL buffer (10 mM Tris pH 7.5, 150 mM NaCl, 0.2% Tween-20, 0.08% sodium azide) overnight at 4°C. The membranes were washed 6 times for 5 min with TBS, and then probed for 1 h with 1 μg/mL of streptavidin-alkaline phosphatase (Vector Laboratories) in TBS containing 0.5% Tween. The signals were developed using a standard chromogenic assay for alkaline phosphatase activity (Blake et al., 1984).
2.9. N-glycan profiling
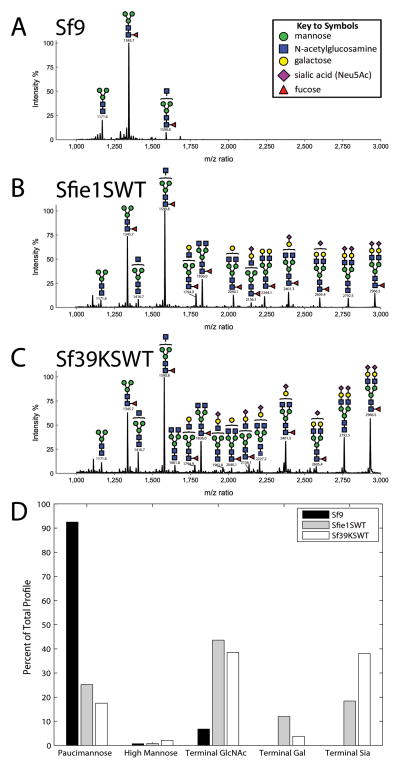
Samples of the hEPO-His (15 μg) or E1-ecto (5 μg) were purified from each cell line as described in section 2.7, diluted to a volume of 0.8 ml with 0.1M ammonium bicarbonate buffer, pH 8.5 (AmBic buffer), supplemented with 0.1 ml of 0.1 M DTT in AmBic buffer, and incubated for 1 h at 37°C. This was followed by the addition of 0.1 ml of 0.5 M iodoacetamide in AmBic buffer and another 1 h incubation at room temperature in the dark. The reduced and alkylated proteins were then treated with trypsin (30 ug/ml) overnight at 37°C. Residual trypsin activity was destroyed by boiling the samples for 5 min, and then the proteins were buffer exchanged into 60% acetonitrile using a SampliQ C18 column (Agilent Technologies). The samples were evaporated by lyophilization, re-dissolved in 0.1 ml of AmBic Buffer, and exhaustively digested with PNGase F (New England Biolabs). The spent reactions were applied to pre-conditioned C18 SepPak cartridges (Waters Corp., Milford, MA) and the flow-through plus one 5% (v/v) aqueous acetic acid wash were pooled, evaporated, and permethylated, as described previously (Dell et al., 1994). The permethylated N-glycan derivatives were extracted into chloroform, pooled with several aqueous washes, re-evaporated, and resuspended in acetonitrile. After being mixed 1:1 with 2,5-dihydroxybenzoic acid matrix (10 mg/ml in 50% acetonitrile in water), samples were spotted onto a matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) target plate. Data acquisition was performed manually on a Model 4700 Proteomics Analyzer equipped with an Nd:YAG laser (Applied Biosystems, Framingham, MA) and 1,000 shots were accumulated in the reflectron positive ion mode. The MALDI-TOF MS profiles were analyzed and major peaks were manually assigned to specific N-glycan structures and quantified using Glycoworkbench 2.0 software (Ceroni et al., 2008). These peaks were labeled in the profiles and their relative prevalence calculated and presented in the bar graphs shown in Figs. 7 and 8.
Fig. 7.
N-glycan profiling of hEPO-His by MALDI-TOF MS. Sf9, Sfie1SWT, and Sf39KSWT cells were infected with AchEPO-His and the secreted hEPO-His product was harvested and affinity-purified at 48 hpi, as described in section 2. After enzymatic release and permethylation, the N-glycans from each hEPO-His preparation were profiled by MALDI-TOF MS, as described in section 2. The figure shows the N-glycan profiles from the hEPO-His produced by (A) Sf9, (B) Sfie1SWT, or (C) Sf39SWT cells. The annotated m/z values refer to the monoisotopic peaks. The bar graph (D) shows a quantitative analysis of the relative percentages of paucimannose-type structures and hybrid- or complex-type structures with terminal N-acetylglucosamine, galactose, and/or sialic acid residues detected on the purified hEPO-His produced by Sf9, Sfie1SWT, or Sf39KSWT cells.
Fig. 8.
N-glycan profiling of E1-ecto by MALDI-TOF MS. N-glycans were isolated from affinity-purified E1-ecto produced by Sf9 (A), Sfie1SWT (B), and Sf39KSWT (C) cells and analyzed by MALDI-TOF-MS, as described in the legend to Figure 7. The annotated m/z values refer to the monoisotopic peaks. The peaks marked with asterisks had m/z values that did not correspond to any glycan structures in the databases and are most likely contaminants. The bar graph (D) shows a quantitative analysis of the relative percentages of paucimannose-type structures and hybrid- or complex-type structures with terminal N-acetylglucosamine, galactose, and/or sialic acid residues detected on the E1-ecto produced by Sf9, Sfie1SWT, or Sf39KSWT cells.
3. Results
3.1. Isolation of transgenic Sf9 cells glycoengineered with genes under ie1 or 39K control
To examine the impact of the 39K promoter on glycogene expression and the efficiency of human-type N-glycan processing in lepidopteran insect cells, we isolated two polyclonal transgenic Sf9 cell populations, designated Sfie1SWT and Sf39KSWT, in parallel. These cells were transformed with two sets of nine glycogene constructs that were identical except for their transcriptional promoters, which were derived from either baculovirus ie1 or 39K genes, respectively (Table 1). Five of the glycogenes (MGAT1, MGAT2, B4GALT1, ST3GAL4, ST6GAL1) encoded glycosyltransferases involved in N-glycan elongation, three (SAS, CMAS, GNPE) encoded enzymes involved in sialic acid and CMP-sialic acid production, and one (CSAT; Mabashi-Asazuma et al., 2013) encoded a CMP-sialic acid transporter.
The baculovirus ie1 promoter, which is constitutively active in uninfected insect cells, is most commonly used for insect cell transformation and was the promoter used for all of our previous insect cell glycoengineering efforts (Aumiller et al., 2003; Geisler and Jarvis, 2012a; Hollister et al., 2002; Hollister and Jarvis, 2001; Mabashi-Asazuma et al., 2013). In contrast, the baculovirus 39K promoter, a delayed early promoter, is not active in uninfected cells because it requires one or more virus-encoded transcription factors (Passarelli and Guarino, 2007). The results of a recent study in our laboratory suggested that the 39K promoter might provide inducible and, perhaps, higher levels of glycogene expression than the ie1 promoter (Lin and Jarvis, 2013). If this hypothesis was correct, the 39K promoter would have great utility for insect cell glycoengineering because it would eliminate the potential negative selection pressure imposed by constitutive transgene expression and potentially increase the efficiency of human-type N-glycan processing obtained after baculovirus infection of the glycoengineered cells.
After isolating Sfie1SWT and Sf39KSWT cells, we compared their growth curves to those of the parental Sf9 cells in suspension cultures. All three cell types had similar exponential growth curves for 48 h before reaching a plateau at 72 h (Fig. 1). However, Sfie1SWT and Sf39KSWT achieved lower final densities than Sf9 cells, as observed previously with some other glycoengineered insect cell lines (Aumiller et al., 2003; Geisler and Jarvis, 2012a). Importantly, Sfie1SWT and Sf39KSWT cells had virtually identical growth curves, indicating that the observed difference in growth relative to the parental cell line was not due to constitutive expression of the nine glycogenes by Sfie1SWT cells.
Fig. 1.
Sfie1SWT and Sf39KSWT cell growth curves. Sf9, Sfie1SWT, and Sf39KSWT cells were seeded into fresh ESF 921 medium at 1.5×106 cells/ml. Triplicate samples were taken at various times after seeding and viable cell numbers were measured with an automated cell counter, as described in section 2. The average viable cell densities were plotted as the number (×106) of cells/mL against incubation time (h).
3.2. Cell surface sialylation
We subsequently examined the glycoengineered phenotypes of uninfected and baculovirus-infected Sfie1SWT and Sf39KSWT cells by using SNA to probe for cell surface sialylation, with the parental Sf9 cells as a negative control. As expected, Sf9 cells were not stained with SNA before infection or at 48 h after infection, indicating they had no detectable cell surface sialylation (Fig. 2A). In contrast, Sfie1SWT cells were clearly stained with SNA both before infection and at 48 h after infection, indicating they had constitutive cell surface sialylation, which is representative of the glycoengineered phenotype (Fig. 2A). This was expected because, as mentioned above, the ie1 promoter can provide transgene expression in uninfected and infected insect cells and, in glycoengineered insect cells, this leads to the production of sialylated cell surface glycoconjugates. Uninfected Sf39KSWT cells exhibited SNA staining levels that were barely above background, but significantly higher levels at 48 h after infection (Figs. 2A and B). These results indicated that both Sfie1SWT and Sf39KSWT cells had been effectively glycoengineered to produce human-type, terminally sialylated N-glycans and that Sf39KSWT cells were glycoengineered in a baculovirus-inducible manner.
Fig. 2.
Cell surface sialylation. Sf9, Sfie1SWT, and Sf39KSWT cells were seeded into 6-well plates, allowed to adhere, and then mock-infected or infected with AchEPO-His for 48 h. At that time point, the cells were stained with SNA as described in section 2.
3.3. Transgene expression
Semi-quantitative reverse transcriptase–polymerase chain reaction (RT–PCR) assays were performed to examine the expression of each transgene in baculovirus-infected Sfie1SWT and Sf39KSWT cells. Total RNA from the parental Sf9 cells was used as a negative control, and a primer pair specific for SfRPL3, an endogenous ribosomal gene, was used as a positive control. In addition, assays were performed with and without the addition of RT to assess the possibility of DNA contamination. No amplification products were detected when the RT-PCR assays were performed without RT, indicating there was no detectable DNA contamination (Fig. 3, RT−). In contrast, RT-PCR assays with RT, the SfRPL3 primer pair, and total RNA isolated from each cell type at each time point all yielded an amplification product of the correct size (Fig. 3, SfRPL3, RT+). RT-PCR assays with each transgene-specific primer pair and total RNA from Sf9 cells produced no amplification products at any time point (Fig. 3, lanes 1). RT-PCR assays with each transgene-specific primer pair and total RNA from Sfie1SWT cells yielded clear amplification products of the expected sizes at 0 hpi (Fig. 3, 0 hpi, lanes 2). In contrast, none of the RT-PCR assays with transgene-specific primer pairs and total RNA from Sf39KSWT cells yielded any amplification products of the expected sizes at 0 hpi, except for assays with the GNPE primer pair, which yielded a very weak product that was barely above background (Fig. 3, 0 hpi, lanes 3). In fact, this latter amplification product was so weak that it did not show up in the bar graph produced after quantification and normalization of the RT-PCR products (Fig. 4). At 4 hpi and beyond, all RT-PCR assays with transgene-specific primer pairs and total RNA isolated from either Sfie1SWT or Sf39KSWT cells yielded amplification products of the expected sizes. In addition, the relative intensities of the RT-PCR products obtained with both cell types increased after 0 hpi and, in most cases, slightly higher intensities were obtained with total RNA from baculovirus-infected Sf39KSWT cells, as compared to Sfie1SWT cells, especially at early times (4–12 h) after infection (Fig. 4). Together, these results demonstrated that both Sfie1SWT and Sf39KSWT cells expressed each of the nine foreign glycogenes. Sfie1SWT cells expressed these genes constitutively and expression levels increased upon baculovirus infection, as expected from previous results (Hollister et al., 2002; Hollister and Jarvis, 2001; Jarvis, 1993). In contrast, Sf39KSWT cells expressed the transgenes in a baculovirus-inducible manner and the levels of expression obtained after baculovirus infection were generally slightly higher at early time points after infection, as compared to those observed with Sfie1SWT.
Fig. 3.
Transgene expression. Sf9, Sfie1SWT, and Sf39KSWT cells were infected with AchEPO-His and total RNA was isolated from each culture at various times after infection. Samples of the RNA were then used for RT-PCR assays, as described in section 2. Each reaction was performed in the presence (+) or absence (−) of reverse transcriptase to assess DNA contamination of the RNA preparations. The primer pairs used for these assays (Table 2) were specific for each individual transgene or SfRPL3, an endogenous ribosomal protein gene, which was used as a normalization control, as indicated by the labels on the left. Lanes 1, Sf9; lanes 2, Sfie1SWT; lanes 3, Sf39KSWT.
Fig. 4.
Semi-quantitative analysis of transgene expression. The relative intensities of the amplification products shown in Fig. 3 were quantitated using BioRad Quantity One software. The plots show the band intensities for the indicated transgene and cell line at each time point normalized to band intensities for SfRPL3 for the corresponding cell line and time point.
3.4. B4GALT1 and ST6GAL1 activities and sialic acid levels
The next set of experiments was designed to determine whether the generally higher glycogene transcript levels observed in Sf39KSWT produced higher glycoenzyme activities, as compared to Sfie1SWT cells. Galactosyltransferase assays were performed to measure B4GALT1 activity, sialyltransferase assays were performed to measure ST6GAL1 and/or ST3GAL4 activities, and TBA assays were performed to measure sialic acid levels. The latter reflects the activity of two transgenes; GNPE, which converts N-acetylglucosamine-6-phosphate to mannosamine-6-phosphate, and SAS, which converts mannosamine-6-phosphate to N-acetylneuraminic acid-9-phosphate. An endogenous phosphatase subsequently converts N-acetylneuraminic acid-9-phosphate to N-acetylneuraminic acid, the most abundant sialic acid.
Protein samples were prepared from baculovirus-infected Sfie1SWT and Sf39KSWT cells at the same times after infection as the RNA samples and assayed for the relevant enzyme activities, as described in section 2. The average results were plotted as the levels of activity after subtracting the background observed in baculovirus-infected Sf9 cells at the corresponding time after infection. In agreement with the RT-PCR results, B4GALT1 (Fig. 5A) and sialyltransferase (Fig. 5B) activities were detected in Sfie1SWT, but not Sf39KSWT immediately after infection. Starting at 4 hpi, B4GALT1 and sialyltransferase activities were detected in both cell types, and each activity increased with time of infection. Notably, both B4GALT1 and sialyltransferase activities reached higher levels in Sf39KSWT than in Sfie1SWT cells and remained higher throughout the course of infection. In both cell types, the levels of B4GALT1 and sialyltransferase activities decreased at late times after infection. The reason for this decrease is unclear, but this same result was observed previously with other glycoengineered insect cell lines (Hollister et al., 2002; Hollister and Jarvis, 2001).
Fig. 5.
Glycoenzyme activities. Sf9, Sfie1SWT, and Sf39KSWT were infected with AchEPO-His and samples were taken for analysis of glycoenzyme activities at various times after infection, as described in section 2. Samples were assayed in duplicate (B4GALT1 and ST6GAL1) or triplicate (TBA) and values at each time point are presented as background-subtracted average −/+ standard deviation, where background is the average value for Sf9 cells at each time point. (A) B4GALT1 assay, (B) ST6GAL1 assay, (C) TBA assay for sialic acid. One-way ANOVA analysis showed that there was a statistically significant difference between values for Sfie1SWT and Sf39KSWT cells in all assays. For the B4GALT1 and TBA assays, the value for Sfie1SWT was significantly (P<0.05) greater than the value for Sf39KSWT at 0 hpi. For all assays, the values for Sf39KSWT were significantly (P< 0.05) greater than for Sfie1SWT at 12, 18, 24, and 36 hpi.
The results of the sialic acid assays (Fig. 5C) also indicated that baculovirus-infected Sf39KSWT cells had higher levels of relevant glycoenzyme activities than Sfie1SWT cells. Starting at about 12 hpi, Sf39KSWT cells had more sialic acid, with a steady increase throughout the course of infection, which ultimately reached about seven-fold higher levels than those observed in Sfie1SWT cells.
3.5. Impact of 39K-mediated glycogene expression on human-type N-glycan processing
Next, we used a recombinant form of human erythropoietin as a model to examine the impact of the promoter used for glycogene expression on the efficiency of human-type N-glycan processing in the BEVS. Sf9, Sfie1SWT, and Sf39KSWT cells were infected with AchEPO-His and the secreted hEPO-His product was affinity-purified from the cell culture supernatants at 48 hpi. The yield and purityof each purified protein was assessed by BCA assays and SDS-PAGE with Coomassie brilliant blue staining, respectively (data not shown).
We initially examined the nature of the N-glycans on the hEPO-His preparations from each cell type by Western and lectin blotting coupled with endoglycosidase treatments, as described in section 2. The western blotting results showed that the hEPO-His produced by Sf9 cells migrated as one discrete band, whereas the hEPO-His produced by Sfie1SWT and Sf39KSWT cells migrated as a smear extending downward from a position of higher electrophoretic mobility (Figs. 6A and B, α-hEPO). This difference in the migration of the hEPO-His preparations isolated from the parental and glycoengineered cells reflected differences in N-glycosylation and sialylation, as evidenced by the fact that they were eliminated by PNGase F treatment and the slowest migrating species in the smear were eliminated by neuraminidase treatment. SNA and MAL blotting confirmed that the hEPO-His produced by both glycoengineered cell types contained α2,6- and α2,3-linked sialic acids (Figs. 6A and B, SNA and MAL). The lectin blotting results also suggested that the hEPO-His produced by Sf39KSWT contained more sialic acid than the hEPO-HIS produced by SfieSWT cells, as the former had slightly more SNA and significantly more MAL-I reactivity. This interpretation was indirectly supported by the observation that the hEPO-His produced by Sf39KSWT cells had relatively less RCA reactivity (Figs. 6A and B, RCA), because a higher level of sialylation would reduce the numbers of terminal galactose residues available for RCA binding.
Fig. 6.
Western and lectin blotting analysis of purified hEPO-His. Sf9, Sfie1SWT, and Sf39KSWT cells were infected with AchEPO-His and the secreted hEPO-His product was harvested and affinity-purified at 48 hpi, as described in section 2. (A) Analysis of purified hEPO-His by western blotting with α-hEPO and lectin blotting with RCA, SNA, or MAL after treatment with PNGase F buffer alone (−) or PNGase F (+). (B) Analysis of purified hEPO-His by western blotting with α-hEPO and lectin blotting with RCA, SNA, or MAL after treatment with neuraminidase buffer alone (−) or neuraminidase (+). Lanes 1, Sf9; lanes 2, Sfie1SWT; lanes 3, Sf39KSWT. * marks the PNGase F protein, which cross-reacted with the anti-hEPO antibody and all lectins.
We also used MALDI-TOF MS to more directly and comprehensively profile the N-glycosylation patterns of hEPO-His preparations from baculovirus-infected Sf9, Sfie1SWT, and Sf39KSWT cells. Briefly, the N-glycans from samples of each purified hEPO-His preparation were enzymatically released, permethylated, and their structures analyzed by MALDI-TOF MS, as described in section 2. The results showed that the vast majority of the N-glycans on the hEPO-His produced by Sf9 cells had mostly paucimannose-type structures, with small proportions of high mannose structures and hybrid-type structures containing a single terminal N-acetylglucosamine residue (Figs. 7A and D). In contrast, the N-glycans on the hEPO-His produced by Sfie1SWT and Sf39KSWT cells had a mixture of paucimannose-type and humanized hybrid- and complex-type N-glycans with terminal N-acetylglucosamine, galactose, and/or sialic acid residues (Figs. 7B–D). Most importantly, the hEPO-His produced by Sf39KSWT cells appeared to have a higher proportion of human-type N-glycans, particularly a higher proportion of terminally sialylated structures. Quantitative analysis revealed that the proportions of paucimannose-type structures on the hEPO-His produced by Sf9, Sfie1SWT, and Sf39KSWT were 93%, 25%, and 18%, respectively (Fig. 7D). These differences reflected the impact of glycoengineering, in general, and revealed that transgenes containing the 39K promoter provided a slightly higher efficiency than the ie1 promoter. More strikingly, the quantitative analysis also revealed that the proportion of sialylated N-glycans was over two-fold higher (39% vs. 18%) and the proportion of galactosylated N-glycans was lower (4% vs. 12%) when hEPO-His was produced in Sf39KSWT, as compared to Sfie1SWT cells (Fig. 7D). Together, the results of the lectin blotting and MALDI-TOF MS analyses indicated that 39K-mediated glycogene expression provides a higher efficiency of human-type N-glycan processing, at least for hEPO-His, in glycoengineered insect cells.
To determine if the higher human-type N-glycan processing efficiency observed with hEPO-His would be observed with another recombinant glycoprotein, we analyzed the N-glycosylation profiles of E1-ecto purified from baculovirus-infected Sf9, Sfie1SWT, and Sf39KSWT cells. N-glycans were enzymatically released from the purified E1-ecto preparations, permethylated, and their structures analyzed by MALDI-TOF MS, as described in section 2. The results showed that the majority of the N-glycans on E1-ecto produced by Sf9 cells had paucimannose-type structures (Figs. 7A and D). In contrast, the N-glycans on E1-ecto produced by Sfie1SWT and Sf39KSWT cells had lower proportions of paucimannose-type and higher proportions of hybrid-type and humanized, complex-type N-glycans (Figs. 8B–D). Like hEPO-His, the E1-ecto produced by Sf39KSWT cells was more efficiently processed than E1-ecto produced by Sfie1SWT cells, as it had a greater than twofold higher proportion of sialylated N-glycans (40% vs 13%) and a lower proportion of galactosylated N-glycans (4% vs 8%; Fig. 8D). These results show that the higher human-type N-glycan processing efficiency provided by insect cells transformed with glycogenes controlled by the 39K promoter is not restricted to hEPO-His.
4. Discussion
The BEVS has great potential for the production of therapeutic glycoproteins for clinical use in human patients, but this potential is restricted by the inability to produce glycoproteins with authentic, human-type N-glycans. Glycoengineering has been used to address this problem, but additional work is needed to create BEVS with the highest possible efficiencies of human-type N-glycan processing. Based on the results of a recent study in our laboratory (Lin and Jarvis, 2013), we hypothesized that human-type N-glycan processing efficiencies might be increased by glycoengineering insect cells with foreign glycogenes containing the 39K, rather than the ie1 promoter. In this study, we tested this hypothesis by generating a matched pair of polyclonal glycoengineered Sf9 cell populations transformed with a set of nine glycogenes placed under the transcriptional control of either a baculovirus ie1 or 39K promoter.
Sfie1SWT and Sf39KSWT cells both had glycoengineered phenotypes, which were expressed constitutively by the former and induced by baculovirus infection in the latter. Sfie1SWT and Sf39KSWT cells had similar growth curves and reached similar final densities, which were lower than the final density reached by Sf9 cells. It is possible that this reflects a metabolic load imposed by low-level transgene expression. In fact, we detected GNPE transcripts in uninfected Sf39KSWT cells, but the levels were only barely above background. Thus, we speculate that the lower maximal cell densities observed relative to Sf9 cells more likely reflects the polyclonal nature of the transgenic insect cell lines. This is supported by the results of a previous study, in which we observed no loss of phenotype in a glycoengineered insect cell line expressing a subset of six of these nine genes over the course of >300 passages in culture (Aumiller et al., 2012). These results indicated that constitutive expression of up to nine different transgenes did not exert a strong negative selection pressure, as it did not lead to loss of the glycoengineered phenotype during long-term continuous passage of glycoengineered insect cell lines. However, we have observed phenotypic instability in a related glycoengineering project involving the addition of a constitutively expressable bacterial gene designed to eliminate glycoprotein fucosylation in insect cells (Mabashi-Asazuma et al., 2014). Thus, we further speculate that the relative inactivity of the 39K promoter in uninfected insect cells and its baculovirus-inducibility might be advantageous for this type of glycoengineering effort in the future.
Transgene expression was baculovirus-inducible and transcript levels were generally higher in baculovirus-infected Sf39KSWT than in baculovirus-infected Sfie1SWT cells. In addition, our analysis of a subset of glycoenzyme activities demonstrated that the general increase in RNA levels led to increased enzyme activity levels. As these results were consistent with those obtained in our previous study (Lin and Jarvis, 2013), we examined the relative impacts of ie1- and 39K-mediated glycogene expression on the efficiency of human-type N-glycan processing obtained after baculovirus infection. Initially, we examined glycosylation of hEPO-His, a model glycoprotein produced during baculovirus infection of the parental or glycoengineered insect cells. Western and lectin blotting analyses indicated that the hEPO-His produced by Sf39KSWT cells was more highly sialylated than the hEPO-His produced by Sfie1SWT cells. This conclusion was confirmed and extended by MALDI-TOF MS profiling of the enzymatically released N-glycans from the purified hEPO-His preparations, as the results showed that the Sf39KSWT cells provided a higher efficiency of human-type N-glycan processing than Sfie1SWT cells, with a total of 39% terminally sialylated N-glycans. The remainder consisted mainly of paucimannose-type (18%) and hybrid- and complex-type (39%) structures with one or two terminal N-acetylglucosamine residues. We obtained analogous results using another recombinant glycoprotein, E1-ecto, indicating that the impact of the 39K promoter on human-type N-glycan processing is not restricted to hEPO-His. Thus, while it did not drive the efficiency to 100%, using the 39K promoter for expression constructs increases glycogene transcript levels, glycoenzyme activities, andhuman-type N-glycan processing efficiencies in glycoengineered insect cells.
The remaining pool of paucimannose-type structures might reflect the presence of a processing beta-N-acetylglucosaminidase in Sf9 cells (Altmann et al., 1995; Geisler et al., 2008), which competes with MGAT1 and MGAT2 to drive the production of paucimannose-type structures (Geisler and Jarvis, 2012b; Wagner et al., 1996a). An oft-stated goal of insect cell glycoengineering has been to reduce or eliminate this competing activity, as this might be another way to increase the efficiency of human-type N-glycan processing. Another approach is to increase MGAT1 activity to enable this enzyme to out-compete the processing beta-N-acetylglucosaminidase, as described previously (Wagner et al., 1996b). Increasing MGAT1, MGAT2, and/or B4GALT1 activities also might eliminate the remaining pool of hybrid structures with terminal N-acetylglucosamine residues. In addition to using a promoter, such as 39K, that can increase these enzyme activities, it might be possible to obtain higher human-type N-glycan processing efficiencies by sequence optimization, protein engineering, and/or glycoengineered insect cell cloning.
Finally, it is possible that endogenous pools of UDP-galactose and UDP-N-acetylglucosamine, which are the donor substrates for B4GALT1 and MGAT1/MGAT2, respectively, are not sufficient to support optimal levels of the respective enzyme activities. Import of these donor substrates into the Golgi apparatus also could be a bottleneck. Thus, introduction of glycogenes encoding the enzymes required for the production and import of these nucleotide sugars might be yet another way to try to increase human-type N-glycan processing efficiencies in an effort to drive the process to homogeneity in glycoengineered insect cells. We are currently pursuing efforts to address each of these possibilities and to create glycoengineered BEVS that can provide 100% efficiency of human-type N-glycan processing.
Highlights.
39K promoter provides baculovirus-inducible glycogene expression.
39K promoter provides higher glycogene expression than ie1 early in infection.
39K promoter induces higher glycoenzyme activities than ie1.
39K promoter use improves N-glycan processing efficiency.
Acknowledgments
This work was supported by Award Numbers GM49734 and GM102982 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or National Institutes of Health. The MALDI-MS data were acquired at the Core Facilities for Protein Structural Analysis at Academia Sinica, supported under the Taiwan National Core Facility Program for Biotechnology (NSC102-2319-B-001-003).
Abbreviations
- 39K
baculovirus delayed early gene encoding phosphoprotein of 39 kDa in apparent molecular weight
- AmBic
ammonium bicarbonate buffer
- BCA
bicinchoninic acid
- B4GALT1
β1,4-galactosyltransferase I
- BEVS
baculovirus expression vector system
- CMAS
CMP-sialic acid synthetase
- CSAT
CMP-sialic acid transporter
- E1-ecto
8X-histidine tagged form of the ectodomain of Western equine encephalitis virus E1 glycoprotein
- GNPE
N-acetyl-D-glucosamine-6-phosphate 2′-epimerase
- hEPO
human erythropoietin
- hEPO-His
6X-histidine tagged form of human erythropoietin
- hpi
hours post-infection
- ie1
immediate early gene 1
- MAL
Maackia amurensis lectin
- MALDI-TOF MS
matrix-assisted laser desorption/ionization-time of flight mass spectrometry
- MGAT1
β1,2-glucosaminyltransferase I
- MGAT2
β1,2-glucosaminyltransferase II
- RCA
Ricinus communis agglutinin
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SAS
sialic acid-9-phosphate synthase
- SEAP
secreted alkaline phosphatase
- SNA
Sambucus nigra agglutinin
- ST3GAL3
α2,3-sialyltransferase III
- ST6GAL1
α2,6-sialyltransferase I
- PNGase F
peptide-N-glycosidase F
- TBA
thiobarbituric acid
- TBS
Tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann F, Kornfeld G, Dalik T, Staudacher E, Glössl J. Processing of asparagine-linked oligosaccharides in insect cells. N-acetylglucosaminyltransferase I and II activities in cultured lepidopteran cells. Glycobiology. 1993;3:619–625. doi: 10.1093/glycob/3.6.619. [DOI] [PubMed] [Google Scholar]
- Altmann F, Schwihla H, Staudacher E, Glössl J, März L. Insect cells contain an unusual, membrane-bound beta-N-acetylglucosaminidase probably involved in the processing of protein N-glycans. J Biol Chem. 1995;270:17344–17349. doi: 10.1074/jbc.270.29.17344. [DOI] [PubMed] [Google Scholar]
- Aumiller JJ, Hollister JR, Jarvis DL. A transgenic insect cell line engineered to produce CMP-sialic acid and sialylated glycoproteins. Glycobiology. 2003;13:497–507. doi: 10.1093/glycob/cwg051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller JJ, Mabashi-Asazuma H, Hillar A, Shi X, Jarvis DL. A new glycoengineered insect cell line with an inducibly mammalianized protein N-glycosylation pathway. Glycobiology. 2012;22:417–428. doi: 10.1093/glycob/cwr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Analyt Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Breitbach K, Jarvis DL. Improved glycosylation of a foreign protein by Tn-5B1-4 cells engineered to express mammalian glycosyltransferases. Biotechnol Bioengr. 2001;74:230–239. doi: 10.1002/bit.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters TD, Hughes RC, Vischer P. Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunicamycin. Biochim Biophys Acta. 1981;640:672–686. doi: 10.1016/0005-2736(81)90097-3. [DOI] [PubMed] [Google Scholar]
- Byrne B, Donohoe GG, O’Kennedy R. Sialic acids: carbohydrate moieties that influence the biological and physical properties of biopharmaceutical proteins and living cells. Drug Discovery Today. 2007;12:319–326. doi: 10.1016/j.drudis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res. 2008;7:1650–1659. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- Dell A, Reason AJ, Khoo KH, Panico M, McDowell RA, Morris HR. Mass spectrometry of carbohydrate-containing biopolymers. Meth Enzymol. 1994;230:108–132. doi: 10.1016/0076-6879(94)30010-0. [DOI] [PubMed] [Google Scholar]
- Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP) Nephrol Dial Transpl. 2001;16(Suppl 3):3–13. [PubMed] [Google Scholar]
- Geisler C, Aumiller J, Jarvis D. A fused lobes gene encodes the processing beta-N-acetylglucosaminidase in Sf9 cells. J Biol Chem. 2008;283:11330–11339. doi: 10.1074/jbc.M710279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, Jarvis DL. Innovative use of a bacterial enzyme involved in sialic acid degradation to initiate sialic acid biosynthesis in glycoengineered insect cells. Metab Engr. 2012a;14:642–652. doi: 10.1016/j.ymben.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, Jarvis DL. Substrate specificities and intracellular distributions of three N-glycan processing enzymes functioning at a key branch point in the insect N-glycosylation pathway. J Biol Chem. 2012b;287:7084–7097. doi: 10.1074/jbc.M111.296814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino LA, Summers MD. Functional mapping of a trans-activating gene required for expression of a baculovirus delayed-early gene. J Virol. 1986;57:563–571. doi: 10.1128/jvi.57.2.563-571.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RL, Jarvis DL. Transforming lepidopteran insect cells for continuous recombinant protein expression. Meth Mol Biol. 2007a;388:299–316. doi: 10.1007/978-1-59745-457-5_15. [DOI] [PubMed] [Google Scholar]
- Harrison RL, Jarvis DL. Transforming lepidopteran insect cells for improved protein processing. Meth Mol Biol. 2007b;388:341–356. doi: 10.1007/978-1-59745-457-5_17. [DOI] [PubMed] [Google Scholar]
- Hill DR, Aumiller JJ, Shi X, Jarvis DL. Isolation and analysis of a baculovirus vector that supports recombinant glycoprotein sialylation by SfSWT-1 cells cultured in serum-free medium. Biotechnol Bioengr. 2006;95:37–47. doi: 10.1002/bit.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister J, Grabenhorst E, Nimtz M, Conradt H, Jarvis DL. Engineering the protein N-glycosylation pathway in insect cells for production of biantennary, complex N-glycans. Biochemistry. 2002;41:15093–15104. doi: 10.1021/bi026455d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister JR, Jarvis DL. Engineering lepidopteran insect cells for sialoglycoprotein production by genetic transformation with mammalian beta 1,4-galactosyltransferase and alpha 2,6-sialyltransferase genes. Glycobiology. 2001;11:1–9. doi: 10.1093/glycob/11.1.1. [DOI] [PubMed] [Google Scholar]
- Hollister JR, Shaper JH, Jarvis DL. Stable expression of mammalian beta 1,4-galactosyltransferase extends the N-glycosylation pathway in insect cells. Glycobiology. 1998;8:473–480. doi: 10.1093/glycob/8.5.473. [DOI] [PubMed] [Google Scholar]
- Hooker AD, Green NH, Baines AJ, Bull AT, Jenkins N, Strange PG, James DC. Constraints on the transport and glycosylation of recombinant IFN-gamma in Chinese hamster ovary and insect cells. Biotechnol Bioengr. 1999;63:559–572. doi: 10.1002/(sici)1097-0290(19990605)63:5<559::aid-bit6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Jarvis DL. Effects of baculovirus infection on IE1-mediated foreign gene expression in stably transformed insect cells. J Virol. 1993;67:2583–2591. doi: 10.1128/jvi.67.5.2583-2591.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis DL. Baculovirus-insect cell expression systems. Meth Enzymol. 2009;463:191–222. doi: 10.1016/S0076-6879(09)63014-7. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Finn EE. Modifying the insect cell N-glycosylation pathway with immediate early baculovirus expression vectors. Nat Biotechnol. 1996;14:1288–1292. doi: 10.1038/nbt1096-1288. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Fleming JA, Kovacs GR, Summers MD, Guarino LA. Use of early baculovirus promoters for continuous expression and efficient processing of foreign gene products in stably transformed lepidopteran cells. Nat Biotechnol. 1990;8:950–955. doi: 10.1038/nbt1090-950. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Howe D, Aumiller JJ. Novel baculovirus expression vectors that provide sialylation of recombinant glycoproteins in lepidopteran insect cells. J Virol. 2001;75:6223–6227. doi: 10.1128/JVI.75.13.6223-6227.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Jarvis DL. Utility of temporally distinct baculovirus promoters for constitutive and baculovirus-inducible transgene expression in transformed insect cells. J Biotechnol. 2013;165:11–17. doi: 10.1016/j.jbiotec.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Miller LK. Regulation of Baculovirus Late and Very Late Gene Expression. In: Miller LK, Wagner RR, editors. The Baculoviruses. Plenum Press; New York, NY: 1997. pp. 193–216. [Google Scholar]
- Mabashi-Asazuma H, Kuo CW, Khoo KH, Jarvis DL. A novel baculovirus vector for the production of nonfucosylated recombinant glycoproteins in insect cells. Glycobiology. 2014;24:325–340. doi: 10.1093/glycob/cwt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabashi-Asazuma H, Shi X, Geisler C, Kuo CW, Khoo KH, Jarvis DL. Impact of a human CMP-sialic acid transporter on recombinant glycoprotein sialylation in glycoengineered insect cells. Glycobiology. 2013;23:199–210. doi: 10.1093/glycob/cws143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngantung FA, Miller PG, Brushett FR, Tang GL, Wang DI. RNA interference of sialidase improves glycoprotein sialic acid content consistency. Biotechnol Bioengr. 2006;95:106–119. doi: 10.1002/bit.20997. [DOI] [PubMed] [Google Scholar]
- Passarelli AL, Guarino LA. Baculovirus late and very late gene regulation. Curr Drug Targets. 2007;8:1103–1115. doi: 10.2174/138945007782151324. [DOI] [PubMed] [Google Scholar]
- Seo NS, Hollister JR, Jarvis DL. Mammalian glycosyltransferase expression allows sialoglycoprotein production by baculovirus-infected insect cells. Prot Expr Purif. 2001;22:234–241. doi: 10.1006/prep.2001.1432. [DOI] [PubMed] [Google Scholar]
- Smith GE, Vlak JM, Summers MD. In vitro translation of Autographa californica nuclear polyhedrosis virus early and late mRNAs. J Virol. 1982;44:199–208. doi: 10.1128/jvi.44.1.199-208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solá RJ, Griebenow K. Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy. BioDrugs. 2010;24:9–21. doi: 10.2165/11530550-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V, Stollar BD, Koo R, Harrap KA, Schlesinger RW. Sialic acid contents of sindbis virus from vertebrate and mosquito cells. Equivalence of biological and immunological viral properties. Virology. 1976;69:104–115. doi: 10.1016/0042-6822(76)90198-7. [DOI] [PubMed] [Google Scholar]
- Tomiya N, Ailor E, Lawrence SM, Betenbaugh MJ, Lee YC. Determination of nucleotides and sugar nucleotides involved in protein glycosylation by high-performance anion-exchange chromatography: sugar nucleotide contents in cultured insect cells and mammalian cells. Analyt Biochem. 2001;293:129–137. doi: 10.1006/abio.2001.5091. [DOI] [PubMed] [Google Scholar]
- Tomiya N, Howe D, Aumiller JJ, Pathak M, Park J, Palter KB, Jarvis DL, Betenbaugh MJ, Lee YC. Complex-type biantennary N-glycans of recombinant human transferrin from Trichoplusia ni insect cells expressing mammalian beta-1,4-galactosyltransferase and beta-1,2-N-acetylglucosaminyltransferase II. Glycobiology. 2003;13:23–34. doi: 10.1093/glycob/cwg012. [DOI] [PubMed] [Google Scholar]
- Toth AM, Geisler C, Aumiller JJ, Jarvis DL. Factors affecting recombinant Western equine encephalitis virus glycoprotein production in the baculovirus system. Prot Expr Purif. 2011;80:274–282. doi: 10.1016/j.pep.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Geyer H, Geyer R, Klenk HD. N-acetyl-beta-glucosaminidase accounts for differences in glycosylation of influenza virus hemagglutinin expressed in insect cells from a baculovirus vector. J Virol. 1996a;70:4103–4109. doi: 10.1128/jvi.70.6.4103-4109.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Liedtke S, Kretzschmar E, Geyer H, Geyer R, Klenk HD. Elongation of the N-glycans of fowl plague virus hemagglutinin expressed in Spodoptera frugiperda (Sf9) cells by coexpression of human beta 1,2-N-acetylglucosaminyltransferase I. Glycobiology. 1996b;6:165–175. doi: 10.1093/glycob/6.2.165. [DOI] [PubMed] [Google Scholar]