Abstract
In response to the high rates of colonization and infection by multidrug-resistant gram-negative bacilli (MDR GNB), many military treatment facilities (MTFs) have implemented additional infection control practices, such as active surveillance cultures for asymptomatic colonization. Results of surveillance cultures (June 2009 – May 2012) collected from patients at Landstuhl Regional Medical Center (Landstuhl RMC), Germany, and three U.S. MTFs were analyzed to evaluate trends in MDR GNB colonization over time and across facilities. At Landstuhl RMC, 6.6 percent of patients were colonized on admission with MDR GNB compared to 12.4 percent of patients admitted to the participating U.S. MTFs. Escherichia coli was the predominant organism, representing 82.4 percent of MDR isolates at Landstuhl RMC and 67.1 to 83.3 percent at U.S. MTFs. Other common MDR GNB included Acinetobacter calcoaceticus-baumannii complex and Klebsiella pneumoniae. Although Pseudomonas aeruginosa was often isolated from the surveillance cultures, it was never multidrug-resistant. Annual rates of MDR GNB colonization were not significantly different over the three-year period. Ongoing research includes assessment of predictive factors for MDR GNB colonization and the relationship between colonization and infection.
BACKGROUND
Numerous reports have described infection and colonization with multidrug-resistant gram-negative bacilli (MDR GNB) among wounded and hospitalized military service members during the conflicts in Afghanistan and Iraq.1–7 The spread of some resistant organisms, such as Acinetobacter calcoaceticus-baumannii (ABC) complex, is thought to be primarily healthcare-associated. Studies of healthy service members in the United States have not demonstrated significant colonization with MDR GNB prior to deployment,8–10 Additionally, MDR GNB have not been reported to be common in the soil in deployed locations,11 and war wounds have not been found to be colonized with MDR GNB at the time of injury.12 Potential sources for MDR GNB introduction include acquired resistance of endogenous flora due to antibiotic pressure or acquisition of resistant strains from the healthcare system.
The increase in infections associated with MDR GNB at U.S. military treatment facilities (MTFs) led to the implementation of several infection control practices, including active surveillance cultures to detect asymptomatic colonization and preemptive contact isolation of patients while awaiting surveillance culture results. In 2003, three U.S. MTFs (Brooke Army Medical Center [Brooke AMC], National Naval Medical Center, and Walter Reed Army Medical Center [Walter Reed AMC]) began performing surveillance cultures for ACB at the time of hospital admission; however, data were not routinely collected from all care areas and colonization with other MDR GNB was not evaluated. In 2005, Landstuhl Regional Medical Center (Landstuhl RMC) in Germany initiated screening in order to gather ACB colonization data from patients arriving directly from combat zones. Analysis of surveillance culture data collected from 2005 through 2009 demonstrated a decrease in ACB colonization from seven percent to one percent at Landstuhl RMC and from 21 percent to 4 percent at the U.S. MTFs.13
In an effort to further understand MDR GNB colonization, a prospective longitudinal study at Walter Reed AMC conducted in 2008 entailed collection of surveillance cultures at the time of admission and during hospitalization from six different anatomic sites of both injured service members and non-deployed subjects.6 Evaluation of the cultures determined that the groin was the most sensitive anatomic site for the detection of MDR GNB colonization. The prevalence of MDR GNB colonization at admission was 23 percent and eight percent among deployed and non-deployed beneficiaries, respectively, and included not only ACB, but also high rates of multidrug-resistant (MDR) Escherichia coli and Klebsiella pneumoniae. Based on this study and others, Walter Reed AMC, National Naval Medical Center, Brooke AMC, and Landstuhl RMC aligned their surveillance culture programs in 2009 and began routinely swabbing the groins of new admissions to evaluate for colonization with any MDR GNB.6
This report presents surveillance data on MDR GNB colonization from June 2009 through May 2012 on injured service members admitted to Landstuhl RMC and the three U.S. MTFs after their surveillance culture programs were aligned.
METHODS
For this report, groin surveillance cultures performed on service members injured in Iraq or Afghanistan within three days of admission to Landstuhl RMC or the U.S. MTFs were analyzed.13 The analysis included only the first swab collected from each patient at admission. Results from the surveillance cultures were captured at all four MTFs as part of a prospective study on infectious complications following deployment-related injuries (The Department of Defense – Department of Veterans Affairs, Trauma Infectious Disease Outcomes Study [TIDOS]).14 Clinical information and microbiology results from deployed subjects admitted to Landstuhl RMC, Walter Reed AMC, National Naval Medical Center (Walter Reed National Military Medical Center after September 2011), or Brooke AMC (San Antonio Military Medical Center after September 2011) were collected through TIDOS as part of an infectious disease module of the Department of Defense Trauma Registry.
Gram-negative bacilli were defined as multidrug-resistant if they were determined to be resistant to three or more of the following antibiotic classes, aminoglycosides, β-lactams, carbapenems, and fluoroquinolones, or if they produced extended spectrum β-lactamases, or if they produced K. pneumoniae carbapenemases.15 Antimicrobial susceptibility tests were performed for all bacterial strains using automated systems (BD Phoenix [BD Biosciences, Sparks, MD] or Vitek 2 [bioMérieux Inc., Hazelwood, MO]), in addition to testing with disc diffusion and E-test methods as recommended by the Clinical and Laboratory Standards Institute and per routine at each hospital.16
McNemar’s test, which evaluates paired proportions, was used to compare the proportion of subjects colonized between Landstuhl RMC and the U.S. MTFs. Trend comparisons used the GENMOD procedure with binomial distribution in SAS® version 9.3. P-values less than 0.05 were considered statistically significant.
RESULTS
Study population
The study population consisted predominantly of male (98%) service members from the Army (63%) and the Marine Corps (29%) with a median age of 24 years. The majority of patients were medically evacuated to Landstuhl RMC due to injuries sustained through explosions (65%) or gunshots (20%) (data not shown).
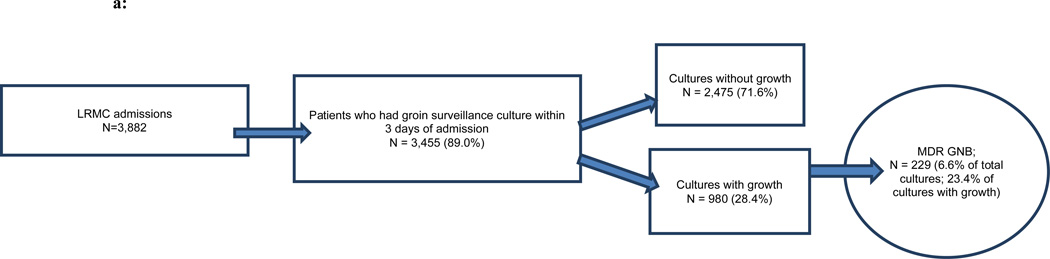
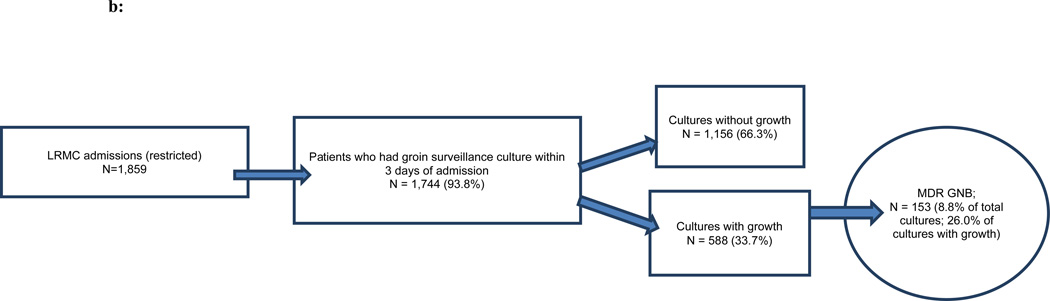
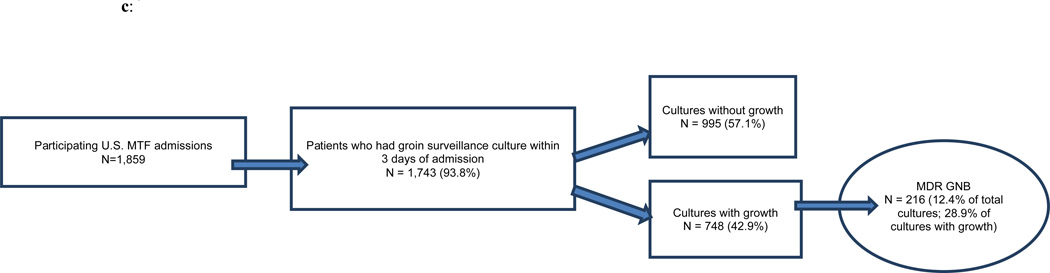
Of the 3,882 military personnel admitted to Landstuhl RMC, 48 percent (n=1,859) were transferred to one of the participating U.S. MTFs (Figures 1a,b,c). Examination of monthly admissions combined for the three years indicated a seasonal pattern of admissions at Landstuhl RMC and the subsequent U.S. MTFs with the highest number of subjects admitted during the summer and early autumn months and the lowest number during the winter (Figures 2a,b).
FIGURE 1.
a: Landstuhl Regional Medical Center (LRMC) admissions and active surveillance cultures for asymptomatic colonization, June 2009–May 2012
MDR GNB – multidrug-resistant gram-negative bacilli
b: Landstuhl Regional Medical Center (LRMC) admissions and active surveillance cultures for asymptomatic colonization, restricted to the population of patients who transferred to the U.S. Military Treatment Facility (MTF), June 2009–May 2012
MDR GNB – multidrug-resistant gram-negative bacilli
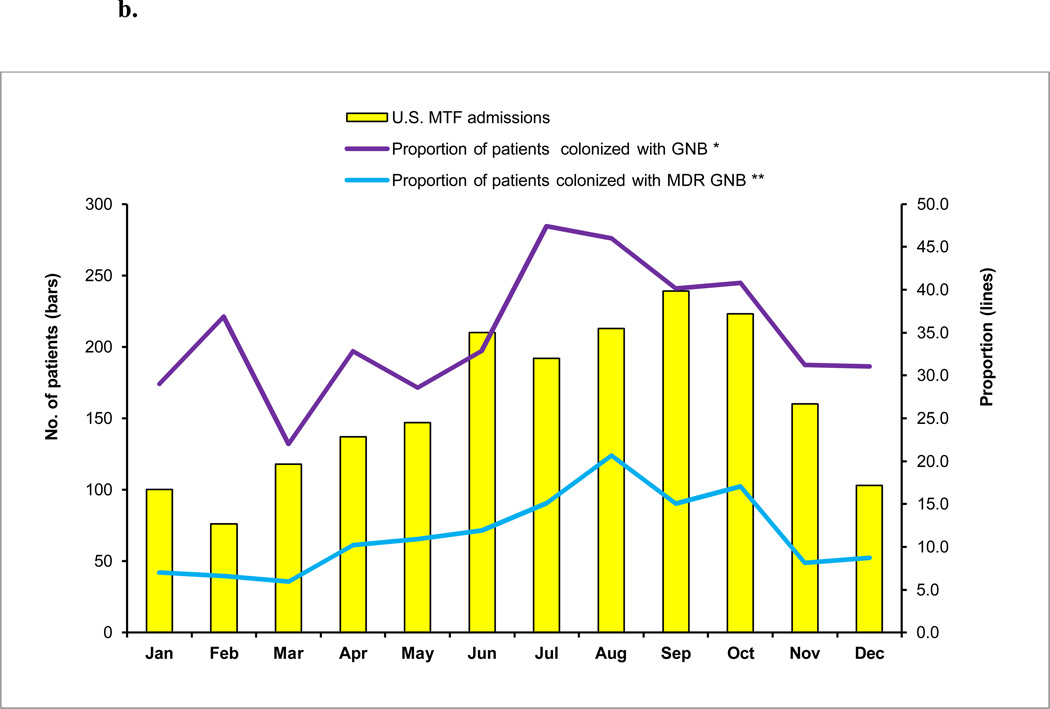
c: U.S. Military Treatment Facility (MTF) admissions and active surveillance cultures for asymptomatic colonization, June 2009–May 2012
MDR GNB – multidrug-resistant gram-negative bacilli
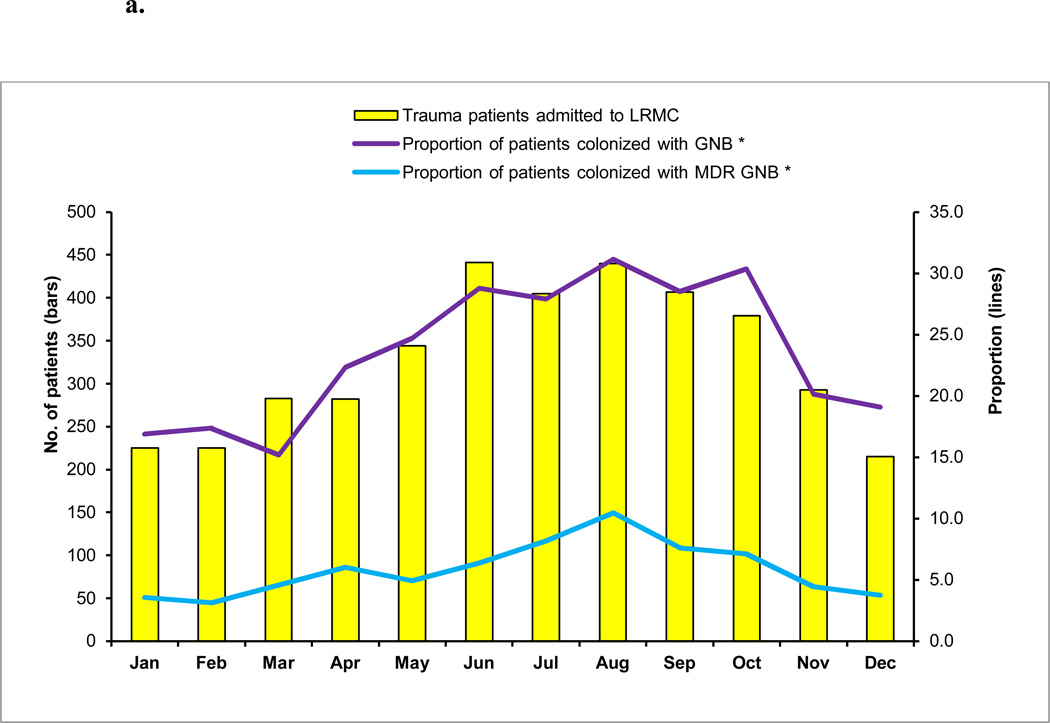
FIGURE 2.
a. Multidrug-resistant (MDR) gram-negative bacilli (GNB) colonization at Landstuhl Regional Medical Center (LRMC) by month of admission, June 2009–May 2012
* p<0.0001 for test of trend over time
b. Multidrug-resistant (MDR) gram-negative bacilli (GNB) colonization at U.S. Military Treatment Facilities (MTFs) by month of admission, June 2009–May 2012
* p=0.0042 for test of trend over time; ** p<0.0001 for test of trend over time
Proportion with MDR GNB
Within three days of admission, 89.0 percent of patients at Landstuhl RMC and 93.8 percent of patients at the U.S. MTFs admitted from a deployment location had surveillance cultures performed (Figures 1a,b,c). Only approximately one-fourth of the surveillance cultures from Landstuhl RMC grew bacteria (Figure 1a). Of the total patients cultured (n=3,455), 6.6 percent grew MDR GNB at Landstuhl RMC. Limiting the analysis of Landstuhl RMC patients with surveillance cultures to those who ultimately transferred to one of the participating U.S. MTFs (n=1,744), 8.8 percent were colonized at Landstuhl RMC with MDR GNB (Figure 1b). After admission to an U.S. MTF (n=1,743), the percentage of surveillance cultures that grew MDR GNB significantly increased to 12.4 percent (p<0.0001) when compared to the same population at Landstuhl RMC (Figure 1c).
Similar to the pattern of admissions, the highest proportion of patients colonized with MDR GNB at Landstuhl RMC occurred in August (10%), while the lowest proportion colonized was in February (3%) (Figure 2a). The proportion of patients at the U.S. MTFs colonized with MDR GNB followed the same pattern with the highest proportion of colonization occurring in August (21%) and the lowest in March (6%) (Figure 2b).
Restricting the study population to a subset of military personnel injured in combat demonstrated an increased percentage of medical evacuees at Landstuhl RMC who received surveillance cultures upon admission (95%), of which nine percent grew MDR GNB. At U.S. MTFs, 89 percent of those injured during combat had surveillance cultures performed and 16 percent of those subjects were colonized with MDR GNB. At both Landstuhl RMC and the U.S. MTFs, the percentage of MDR GNB colonization among combat wounded was statistically greater compared to the non-combat group (p<0.0001) (data not shown).
The most prevalent colonizing organisms identified at Landstuhl RMC and the U.S. MTFs were E. coli, K. pneumoniae, and Enterobacter aerogenes (Table 1). E. coli was not only the most common colonizing organism, but also the most frequent MDR GNB recovered (total of 387 isolates) accounting for 82.4 percent of MDR GNB at Landstuhl RMC and 67.1 to 83.3 percent at the U.S. MTFs. The next most frequent MDR GNB cultured included: ACB, K. pneumoniae, and Enterobacter cloacae. Pathogen distribution was similar among the U.S. MTFs, although at Brooke AMC the percentage of MDR E. coli was lower and the percentages of MDR ACB and K. pneumoniae were higher compared to the other sites (data not shown).
TABLE 1.
Most common colonizing bacteria isolated from gram-negative bacilli active surveillance cultures, 1 June 2009–31 May, 2012
| Organism | Overall (% of total) |
LRMC (% of total) |
NNMCa (% of total) |
WRAMCb (% of total) |
BAMC (% of total) |
WRNMMCc (% of total) |
|---|---|---|---|---|---|---|
| Escherichia coli | 1039 (50.3) | 606 (51.3) | 144 (49.7) | 147 (52.1) | 128 (49.8) | 14 (25.9) |
| Klebsiella pneumoniae | 179 (8.7) | 88 (7.4) | 26 (9.0) | 30 (10.6) | 30 (11.7) | 5 (9.3) |
| Enterobacter aerogenes | 153 (7.4) | 96 (8.1) | 18 (6.2) | 18 (6.4) | 17 (6.6) | 4 (7.4) |
| Acinetobacter calcoaceticus baumannii | 143 (6.9) | 84 (7.1) | 23 (7.9) | 20 (7.1) | 16 (6.2) | 0 (0.0) |
| Pseudomonas aeruginosa | 130 (6.3) | 45 (3.8) | 37 (12.8) | 19 (6.7) | 18 (7.0) | 11 (20.4) |
| Enterobacter cloacae | 116 (5.6) | 80 (6.8) | 10 (3.4) | 9 (3.2) | 13 (5.1) | 4 (7.4) |
| Citrobacter spp. | 83 (4.0) | 57 (4.8) | 6 (2.1) | 9 (3.2) | 8 (3.1) | 3 (5.6) |
| Pseudomonas spp. (non-aeruginosa) | 47 (2.3) | 33 (2.8) | 2 (0.7) | 6 (2.1) | 4 (1.6) | 2 (3.7) |
| Proteus mirabilis | 41 (2.0) | 20 (1.7) | 5 (1.7) | 6 (2.1) | 7 (2.7) | 3 (5.6) |
| Serratia marcescens | 35 (1.7) | 15 (1.3) | 7 (2.4) | 8 (2.8) | 3 (1.2) | 2 (3.7) |
| Totald | 2065 | 1182 | 290 | 282 | 257 | 54 |
LRMC=Landstuhl Regional Medical Center; NNMC=National Naval Medical Center; BAMC=Brooke Army Medical Center; WRAMC=Walter Reed Army Medical Center; WRNMMC=Walter Reed National Military Medical Center
NNMC was combined with WRAMC to form WRNMMC in September 2011; therefore, NNMC is limited to data from June 2009 through August 2011
WRAMC was combined with NNMC to form WRNMMC in September 2011; therefore, WRAMC is limited to data from June 2009 through August 2011
WRNMMC was established September 2011; therefore, WRNMMC is limited to data from September 2011 through May 2012
Total for each column includes bacterial isolates not incorporated as part of the top ten overall colonizing bacteria, therefore, the sum of the isolates listed for each column equals less than the total
Antibiotic Resistance of MDR GNB
E. coli and K. pneumoniae isolates were primarily MDR due to extended spectrum β-lactamase production, whereas ACB isolates much more frequently demonstrated resistance to multiple antimicrobial classes (Table 2). Of the extended spectrum β-lactamase-producing E. coli, 47 percent were also resistant to fluoroquinolones and 11 percent were resistant to aminoglycosides (data not shown). Of the extended spectrum β-lactamase-producing K. pneumoniae, approximately one-third were resistant to fluoroquinolones and aminoglycosides (data not shown). Most Pseudomonas aeruginosa and E. aerogenes isolates were susceptible to all antibiotic classes.
TABLE 2.
Antibiotic resistance among common colonizing gram-negative bacilli, active surveillance cultures, 1 June 2009–31 May, 2012
| Organism | Total Number of Isolatesa |
MDR Isolates (% of total) |
ESBL producers (% of MDR Isolates) |
Aminoglycosides (% resistantb) |
β-lactams (% resistantb) |
Carbapenems (% resistantb) |
Fluoroquinolones (% resistantb) |
|---|---|---|---|---|---|---|---|
| Escherichia coli | 1039 | 387 (37.2) | 385 (99.5) | 69 (6.6) | 391 (37.6) | 3 (0.3) | 348 (33.5) |
| Klebsiella pneumoniae | 179 | 40 (22.3) | 40 (100) | 13 (7.3) | 40 (22.3) | 0 (0.0) | 23 (12.8) |
| Enterobacter aerogenes | 153 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.3) | 0 (0.0) | 0 (0.0) |
| Acinetobacter calcoaceticus baumannii | 143 | 64 (44.8) | 0 (0.0) | 48 (33.6) | 52 (36.4) | 63 (44.1) | 70 (49.0) |
| Pseudomonas aeruginosa | 130 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (2.3) | 1 (0.8) | 11 (8.5) |
| Enterobacter cloacae | 116 | 4 (3.4) | 4 (100) | 2 (1.7) | 12 (10.3) | 1 (0.9) | 8 (6.9) |
| Citrobacter spp. | 83 | 1 (1.2) | 1 (100) | 2 (2.4) | 2 (2.4) | 0 (0.0) | 8 (9.6) |
| Pseudomonas spp. (non-aeruginosa) | 47 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 0 (0.0) | 2 (4.3) |
| Proteus mirabilis | 41 | 2 (4.9) | 2 (100) | 0 (0.0) | 3 (7.3) | 0 (0.0) | 3 (7.3) |
| Serratia marcescens | 35 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) |
ESBL=extended spectrum β-lactamase; MDR=multidrug-resistant
Total number of isolates refers to both resistant isolates (shown here) and non-resistant isolates. In addition, an isolate may be resistant to more than one antimicrobial agent and will be counted under each applicable column.
The percentage reflects the proportion of organisms resistant to that antibiotic class divided by the total number of isolates of that organism
Annual Trends
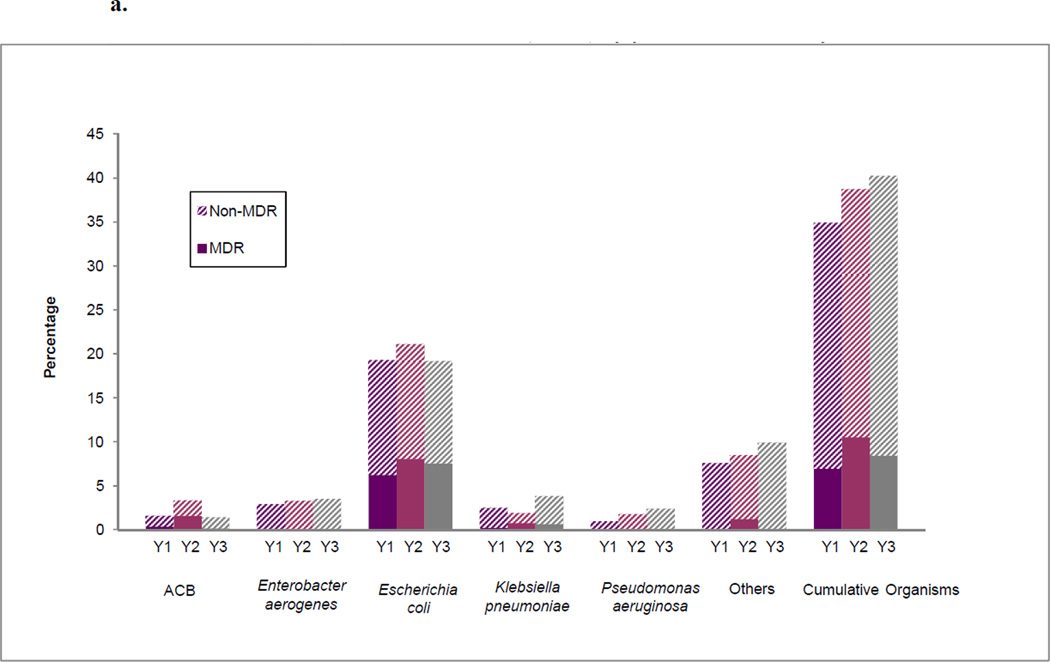
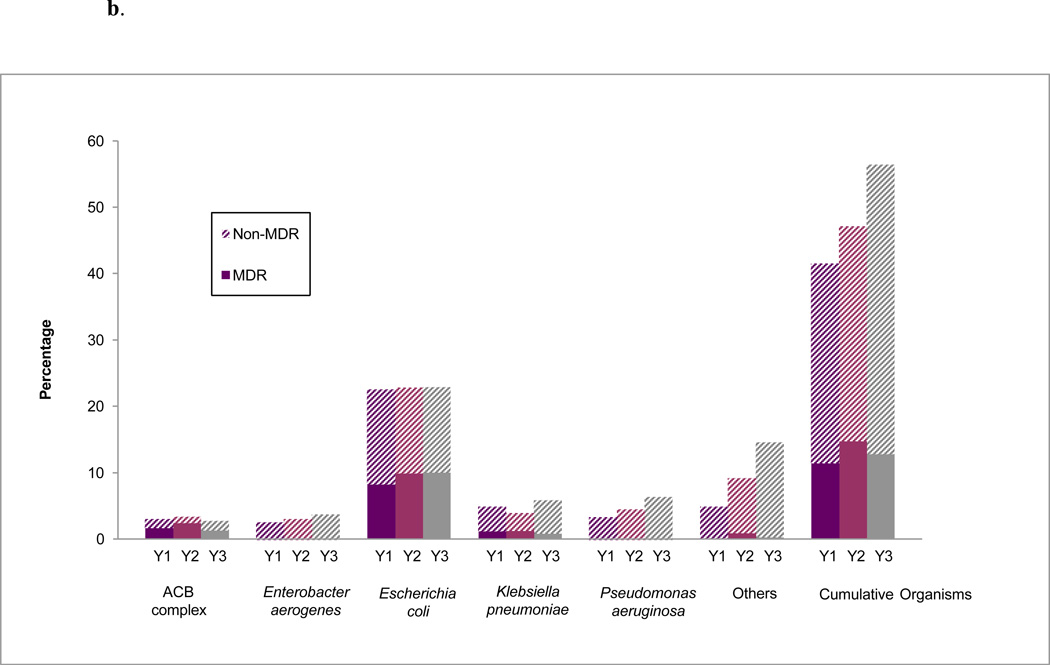
Overall, the percentage of patients colonized with MDR GNB at Landstuhl RMC among the population who received surveillance cultures and transferred to the U.S. MTFs (n=1,744) was 7.0 percent, 10.6 percent, and 8.5 percent during the first (June 2009 – May 2010), second (June 2010 – May 2011), and third (June 2011 – May 2012) years, respectively (Figure 3a). The percentages at the U.S. MTFs were 11.5 percent, 14.9 percent, and 12.9 percent, respectively (Figure 3b). Although the rates of MDR GNB colonization were generally highest in the second year of the study and declined during the third, the differences were not statistically significant at either Landstuhl RMC or the U.S. MTFs.
FIGURE 3.
a. Percentage of surveillance cultures with colonizing isolates at Landstuhl Regional Medical Center (LRMC) among patients transferred to U.S. military treatment facilities (MTFs)a by year, June 2009–May 2012
ACB=Acinetobacter calcoaceticus baumannii; MDR=Multidrug resistant
a The proportion is the number of LRMC patients with a particular organism among the total patients admitted to LRMC in that year that were transferred to a U.S. MTF
Y1=June 2009–May 2010; Y2-June 2010–May 2011; Y3=June 2011–May 2012
b. Percentage of surveillance cultures with colonizing isolates at U.S. military treatment facilities (MTFs)a,b by year, June 2009–May 2012
ACB=Acinetobacter calcoaceticus baumannii; MDR=multidrug resistant
a U.S. MTF data were combined for yearly analysis;
b The proportion is the number of U.S. MTF patients having a particular organism among the total patients admitted to the U.S. MTFs in that year
Y1=June 2009–May 2010; Y2-June 2010–May 2011; Y3=June 2011–May 2012
EDITORIAL COMMENT
This report summarizes three-years (June 2009 – May 2012) of results from active surveillance culturing for MDR GNB of deployed U.S. service members who were hospitalized at Landstuhl RMC and several U.S. MTFs. A higher overall percentage of MDR GNB was observed at U.S. MTFs compared to Landstuhl RMC. E. coli was the most frequently cultured MDR GNB recovered at both Landstuhl RMC and U.S. MTFs, followed by ACB, K. pneumoniae, and E. cloacae. In addition, P. aeruginosa and E. aerogenes commonly grew from active surveillance cultures; however, the isolates were almost always susceptible to multiple classes of antibiotics.
Colonization with MDR GNB has been reported in prior studies in relation to deployed personnel. An evaluation of colonization among service members admitted to Walter Reed AMC from Landstuhl RMC in 2008 found that 23 percent were colonized with MDR organisms at Walter Reed AMC admission.6 In another analysis, 21 percent of subjects admitted to Walter Reed AMC, National Naval Medical Center, or Brooke AMC in 2005 were colonized with MDR ACB; however, the colonization rate decreased to four percent in 2009. A comparable decrease was reported at Landstuhl RMC with seven percent of subjects colonized with MDR ACB at admission in 2005 and one percent in 2009. The overall MDR organism colonization rate in 2009 was three percent at admission to Landstuhl RMC and 13 percent at the U.S. MTFs. In addition, extended spectrum β-lactamase-producing E. coli represented the most prevalent colonizing organism.13
Similar to the previous analyses, the report herein demonstrates that rates of MDR GNB colonization are higher at the U.S. MTFs (12.4%) than at Landstuhl RMC (8.8% of subjects that transfer to a participating U.S. MTF). The reasons for this remain unclear, but may be related to nosocomial acquisition through the evacuation chain, longer time available for bacterial growth, different culturing techniques, or greater administration of antibiotics. Another similarity to the prior studies is that the percentage of patients colonized with MDR ACB has decreased since the early 2000s. Moreover, the three-year data reported here indicate that the mechanism responsible for the majority of drug resistance seen in colonizing isolates is extended spectrum β-lactamase production. Future studies should investigate risk factors for extended spectrum β-lactamase production in this patient population.
A key finding of this study is that service members who sustained combat-related injuries had significantly higher rates of MDR GNB colonization (9% and 16% at Landstuhl RMC and U.S. MTFs, respectively) than the overall population admitted to Landstuhl RMC and, subsequently, to one of the three U.S. MTFs. Although MDR GNB colonization in those with combat-related trauma is higher than in the overall population, deployed subjects without combat injuries who transit through the same evacuation chain continue to have significant rates of MDR GNB colonization that warrant similar infection control practices.
The findings of this study should be interpreted with consideration that the surveillance culture data were collected from U.S. MTFs involved in the TIDOS project. The patients at these participating U.S. MTFs (approximately 50% of subjects transferred from Landstuhl RMC) tended to be more severely injured than those transferred to other U.S. MTFs; therefore, it is uncertain if the surveillance culture results are generalizable to all injured service members transferred from Landstuhl RMC.
Prevention and control of MDR organism transmission has become a national priority. The emergence of MDR GNB limits the use of specific antimicrobial agents, lengthens the time of convalescence, and may lead to increases in morbidity and mortality. The Healthcare Infection Control Practices Advisory Committee (HICPAC) has published guidelines on how to manage these organisms in healthcare settings.17 The HICPAC guidelines recommend using a combination of techniques to control MDR GNB including surveillance cultures to detect asymptomatic colonization and preemptive contact precautions until culture results return. This report demonstrates sustained levels of MDR GNB colonization at Landstuhl RMC and select U.S. MTFs over the past three years, which warrants the continuation of surveillance cultures and contact precautions. Future studies should focus on the relationship between MDR GNB colonization and infection, the effectiveness of surveillance cultures plus contact isolation for decreasing nosocomial transmission of MDR GNB, and risk factors for the propagation of extended spectrum β-lactamases in the deployed population.
Acknowledgments
The authors are indebted to the Infectious Disease Clinical Research Program TIDOS study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
Author Disclosure Statement: Support for this work (IDCRP-024) was provided by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health, under Inter-Agency Agreement Y1-AI-5072, and the Department of the Navy under the Wounded, Ill, and Injured Program.
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the National Institute of Health or the Department of Health and Human Services, the Department of Defense or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government.
References
- 1.Murray CK. Epidemiology of infections associated with combat-related injuries in Iraq and Afghanistan. J Trauma. 2008;64(Suppl):S232–S238. doi: 10.1097/TA.0b013e318163c3f5. [DOI] [PubMed] [Google Scholar]
- 2.Murray CK, Wilkins K, Molter NC, et al. Infections complicating the care of combat casualties during Operations Iraqi Freedom and Enduring Freedom. J Trauma. 2011;71(Suppl 1):S62–S73. doi: 10.1097/TA.0b013e3182218c99. [DOI] [PubMed] [Google Scholar]
- 3.O’Shea MK. Acinetobacter in modern warfare. Int J Antimicrob Agents. 2012;39:363–375. doi: 10.1016/j.ijantimicag.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb Mortal Wkly Rep. 2004;53:1063–1066. [PubMed] [Google Scholar]
- 5.Scott P, Deye G, Srinivasan A, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 6.Weintrob AC, Roediger MP, Barber M, et al. Natural history of colonization with gram-negative multidrug-resistant organisms among hospitalized patients. Infect Control Hosp Epidemiol. 2010;31(4):330–337. doi: 10.1086/651304. [DOI] [PubMed] [Google Scholar]
- 7.Paolino K, Erwin D, Padharia V, et al. In vitro activity of colistin against multidrug-resistant gram-negative bacteria isolated at a major Army hospital during the military campaigns in Iraq and Afghanistan. Clin Infect Dis. 45(1):140–141. doi: 10.1086/518710. [DOI] [PubMed] [Google Scholar]
- 8.Griffith ME, Ellis MW, Murray CK. Acinetobacter nares colonization of health US soldiers. Infect Control Hosp Epidemiol. 2006;27(7):787–788. doi: 10.1086/505923. [DOI] [PubMed] [Google Scholar]
- 9.Griffith ME, Ceremuga JM, Ellis MW, et al. Acinetobacter skin colonization of US Army soldiers. Infect Control Hosp Epidemiol. 2006;27(7):659–661. doi: 10.1086/506596. [DOI] [PubMed] [Google Scholar]
- 10.Griffith ME, Lazarus DR, Mann PB, et al. Acinetobacter skin carriage among US Army soldiers deployed in Iraq. Infect Control Hosp Epidemiol. 2007;28(6):720–722. doi: 10.1086/518966. [DOI] [PubMed] [Google Scholar]
- 11.Keen EF, III, Mende K, Yun HC, et al. Evaluation of potential environmental contamination sources for the presence of multidrug-resistant bacteria linked to wound infections in combat casualties. Infect Control Hosp Epidemiol. 2012;33(9):905–911. doi: 10.1086/667382. [DOI] [PubMed] [Google Scholar]
- 12.Murray CK, Roop SA, Hospenthal DR, et al. Bacteriology of war wounds at the time of injury. Mil Med. 2006;171(9):826–829. doi: 10.7205/milmed.171.9.826. [DOI] [PubMed] [Google Scholar]
- 13.Hospenthal DR, Crouch HK, English JF, et al. Multidrug-resistant bacterial colonization of combat-injured personnel at admission to medical centers after evacuation from Afghanistan and Iraq. J Trauma. 2011;71(1 Suppl):S52–S57. doi: 10.1097/TA.0b013e31822118fb. [DOI] [PubMed] [Google Scholar]
- 14.Tribble DR, Conger NG, Fraser S, et al. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma. 2011;71(Suppl 1):S33–S42. doi: 10.1097/TA.0b013e318221162e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Division of Healthcare Quality Promotion, Detection and Control of Infectious Diseases, Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) Manual (Patient Safety Component)-Protocol: Multidrug-resistant Organism (MDRO) and Clostridium difficile-Associated Disease (CDAD) Module. Atlanta, Georgia: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 16.Clinical and Laboratory Standards Institute (CLSI) Wayne, Pennsylvania: CLSI; 2009. Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline – Third edition. CLSI document M39-A3. [Google Scholar]
- 17.Siegel JD, Rhinehart E, Jackson M, et al. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35(10) Suppl 2:S165–S193. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]