Abstract
Background
Adults with the apolipoprotein E gene (APOE) alleles e4 and e2 are at high risk of poor neurologic outcome after brain injury. The e4 allele has been associated with cerebral palsy and the e2 allele has been associated with worse neurologic outcome with congenital heart disease. This study was done to test the hypothesis that APOE genotype is associated with outcome among neonates who survive after hypoxic-ischemic encephalopathy (HIE).
Methods
We conducted a cohort study of infants who survived HIE and had 18 – 22 month standardized neurodevelopmental evaluations to assess associations between disability and APOE genotypes e3/e3, e4/-, and e2/-
Results
139 survivors were genotyped. 86 (62%) were e3/e3, 41 (29%) were e4/-, and 14 (10%) were e2/-. 129 infants had genotype and follow-up data; 26% had moderate or severe disabilities. Disability prevalence was 30% and 19% among those with and without e3/e3 genotype, 25% and 26% among those with and without the e2 allele, and 18% and 29% among those with and without the e4 allele. None of the differences were statistically significant. Cerebral palsy prevalence was also similar among genotype groups.
Conclusion
Disability was not associated with APOE genotype in this cohort of HIE survivors.
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) involves complex processes of energy depletion, inflammation, necrosis, apoptosis, and altered development of connectivity after injury (1 – 4). Clinical trials of therapeutic hypothermia have consistently demonstrated reductions in risk of death or impairment; however, the incidence of impairment among surviving cooled infants in clinical trials has consistently been between 40–50% (5). Uncovering associations between genetic variants and risk of adverse outcome after hypoxic-ischemic injury could lead to identification of mechanisms that could be targeted to further improve outcomes.
Apolipoprotein E is the primary apolipoprotein produced in the brain (6). Three allelic variants of the APOE gene, e2, e3, and e4, deriving from two single nucleotide polymorphisms (SNPs), rs429358 (thymine, T or cytosine, C) and rs7412(C or T) result in single amino acid changes in the APOE protein (7 – 9). Presence of the APOE e4 allele has been strongly associated with increased risk for Alzheimer’s disease and poor neurologic outcome after traumatic brain injury and brain hemorrhage among adults (9 – 11). The e4 allele has also been associated with persistence of the cognitive decline seen in adult patients following cardiac bypass surgery (12).
Previous case-control studies in children with cerebral palsy (CP), a morbidity that occasionally follows HIE, have demonstrated associations between the APOE e4 allele and increased severity of CP, microcephaly, and seizures (13, 14). These studies included both term and preterm infants with various forms of CP. In studies assessing APOE genotype and outcome among children with trauma and heart surgery, the e4 allele has been associated with CP, and worse neurodevelopmental outcome (15). The e2 allele has been associated with an increased risk for CP, worse neurodevelopmental outcome and behavior problems among infants with congenital heart disease (16, 17). APOE e3 is the most common allele and e3/e3 homozygous adults are more likely to survive and have better functional outcome following cardiopulmonary resuscitation compared with adults with the less common minor alleles e4 and e2 (18, 19).
No study has reported whether neurologic outcome, including cerebral palsy, among infants with moderate to severe neonatal HIE who survive to discharge is associated with the common variants in the APOE gene associated with neuropathology in adults and CP in younger patients. Because of the strong association between APOE alleles and neurologic outcome after injury in older populations, and the suggestive associations between CP and e2 and e4 alleles in pediatric patients following an array of prior conditions, we conducted a cohort study to assess association between APOE genotypes and neurologic and neurodevelopmental status at 18 – 22 months among neonatal HIE survivors. We hypothesized that among 18 – 22 month survivors of HIE, prevalence of disability would be lowest among those with e3/e3 genotype, and would be higher among infants carrying the APOE e2 or APOE e4 allele. We also included a secondary association analysis of prevalence of cerebral palsy for APOE e3/e3 homozygotes and carriers of e2 and e4 alleles. Because cooling has an impact on outcome after HIE, we also conducted an exploratory analysis to assess whether or not cooling influenced associations between APOE genotype and outcome.
Results
This candidate gene association study enrolled 147 subjects who were participating in the Network’s randomized trial of hypothermia for neonatal HIE or the Network’s study of amplitude integrated electroencephalography (aEEG) in infants with neonatal HIE. (20, 21)The number of infants that were enrolled in the candidate gene study from the two main studies, and number lost to follow-up and the number that died prior to ascertaining the 18 – 22 month outcome, are depicted in Figure 1. Eight (5.4%) infants died before discharge and were not included in the association analyses, six within the first 4 postnatal days, and one at eight days and one at nineteen days. One hundred thirty-nine survivors had DNA samples obtained. Ten (7.2%) infants did not have complete information to define the primary outcome. One hundred twenty-nine infants ultimately had successful DNA extraction, APOE genotyping and survived to have adequate information from follow-up evaluations at 18 – 22 months to determine the primary outcome.
Figure 1. Number of infants enrolled in the APOE candidate gene study from each of the two primary Network studies.
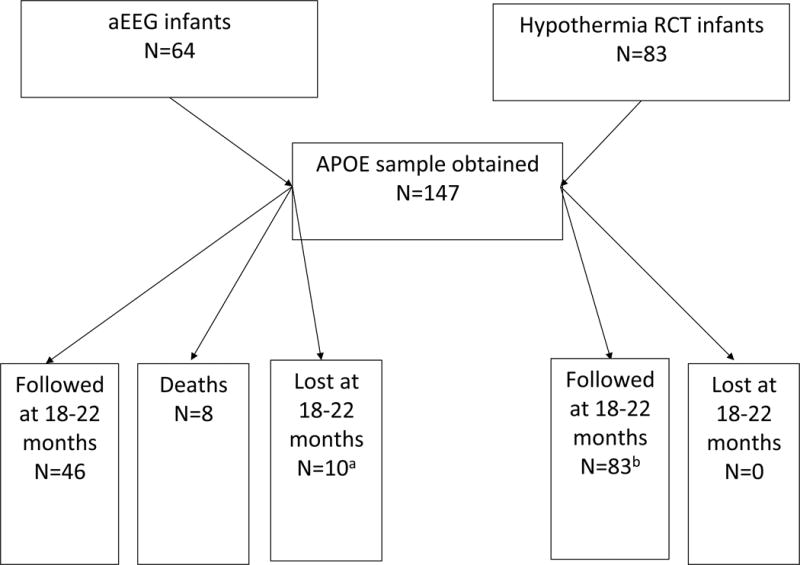
Follow up is defined as non-missing primary outcome (moderate or severe disability) among survivors.
a One infant was lost but was assigned primary outcome through adjudication; data are missing for other follow up outcomes.
b Among aEEG infants, 47 infants were followed but we were only able to assign primary outcome to 46 of them (one had the Bayley exam but was missing CP and hearing data). Ten infants did not have adequate information to allow determination of the primary outcome.
The APOE study cohort appeared to be representative of the infants enrolled in the Network’s Hypothermia and aEEG studies who survived to discharge and follow-up. Survivors in the APOE study who were successfully genotyped, including the 10 who did not have adequate information to define the primary outcome, did not differ in race/ethnicity or intrapartum complications from survivors from the aEEG and Hypothermia studies who were not enrolled in the APOE genotype study. There was good representation of infants who were classified as African-American, Caucasian and Hispanic in the APOE and the non-genotyped Hypothermia and aEEG study populations (Table 1). Infants in the APOE study cohort had higher Apgar scores at 10 minutes and more were cooled compared to infants from the two Network studies who were not enrolled in the APOE study (designated as “other survivors” in Table 1); however, there was no difference in the percentage of infants with moderate (81 vs. 80%) or severe (19 vs. 20%) encephalopathy in the two groups. (Table 1)
Table 1.
Survivors from Hypothermia and aEEG studies with and without genotype samples
| Characteristics | APOE cohort N=139 |
Other survivors N = 80 |
|---|---|---|
| Race/ethnicity | N (%) | N (%) |
| African-American | 45 (32) | 27 (34) |
| Caucasian | 48 (35) | 29 (36) |
| Hispanic | 41 (30) | 22 (28) |
| Other | 5 (4) | 2 (3) |
| Intrapartum complications | ||
| Fetal heart rate decelerations | 97 (70) | 61 (77) |
| Cord prolapse | 24 (17) | 13 (16) |
| Uterine rupture | 16 (12) | 7 (9) |
| Maternal pyrexia | 18 (13) | 6 (8) |
| Shoulder dystocia | 16 (12) | 10 (13) |
| Maternal hemorrhage | 7 (5) | 7 (9) |
| Postpartum characteristics and events | ||
| Apgar < 5 (10 min) * | 75 (66) | 58 (81) |
| Intubation in DR | 121 (87) | 76 (95) |
| CPR >10 min | 126 (91) | 75 (94) |
| No breathing >10 min | 72 (54) | 48 (66) |
| Seizures | 55 (40) | 32 (40) |
| Moderate encephalopathy | 113 (81) | 63 (80) |
| Severe encephalopathy | 26 (19) | 16 (20) |
| Cooling* | 85 (61) | 35 (44) |
p < 0.05
Genotype Frequencies. Among the 139 infants who survived to discharge and had APOE genotypes determined, 10% carried an e2 and 29% an e4 allele. 62% had the APOE e3/e3 genotype. Surviving African-American infants had a higher prevalence of an e4 allele and a lower prevalence of the e3/e3 genotype than either Caucasian or Hispanic infants. (Table 2)
Table 2.
Distribution of apoE2 and E4 allele and 3/3 genotype by race/ethnicity
| APOE | Black N (%) | White N (%) | Hispanic white N (%) | Other N (%) | Total N (%) |
|---|---|---|---|---|---|
| Any e2 allele | 7 (16) | 1 (2) | 2 (5) | 4 (80) | 14 (10) |
| Any e4 allele | 21 (47) | 11 (23) | 9 (22) | 0 (0) | 41 (29) |
| e3/e3 genotype | 19 (42) | 36 (75) | 30 (73) | 1 (20) | 86 (62) |
| total | 47 | 48 | 41 | 5 | 139 |
Prevalence of Outcome for Each Genotype. 26% of the infants in the APOE study who survived to discharge had the primary outcome, moderate or severe disability. Only 2 infants had moderate disability and the rest were severely disabled. The prevalence of moderate or severe disability among infants with the APOE e3/e3 genotype did not differ significantly from those without this hypothesized protective genotype, nor did the prevalence of the primary outcome differ among those with and without the hypothesized risk alleles e4 or e2. (Figure 2) In addition, no differences were found when separate analyses were carried out for infants of different races, with moderate or severe initial encephalopathy, or for infants cooled and not cooled.
Figure 2. Prevalence of moderate/severe disability for infants of different genotypes.
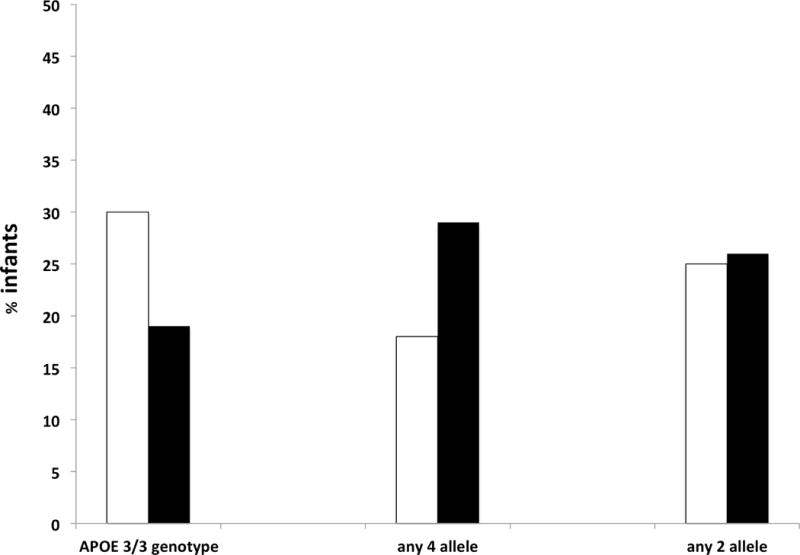
For each genotype, white bars denote the percent of those with the genotype surviving with moderate to severe disability and black bars denote the percent without the genotype surviving with moderate to severe disability. Among 81 infants with APOE e3/e3 genotype and follow-up, prevalence of the primary outcome was 30%, and among the 48 infants not homozygous for e3/e3, prevalence was 19% (p = 0.21). Among 12 infants with the APOE e2 allele the prevalence of primary outcome was 25%, compared with 26% among the 117 infants without the e2 allele (p = 1.00). Among those with the APOE e4 allele (n = 38) the prevalence of the primary outcome was 18%, compared with 29% among the 91 without the e4 allele (p = 0.27).
Similar to what was noted with the primary outcome, the prevalence of Bayley II Mental Developmental Index (MDI) <70 did not differ in the groups with and without the APOE e3/e3 genotype, or with or without an e4 or e2 allele (Figure 3). The prevalence of moderate or severe CP was not significantly different between infants with and without the e3/e3 genotype or between those with or without an e4 or e2 allele (Figure 4).
Figure 3. Prevalence of MDI < 70 among infants of different genotypes.
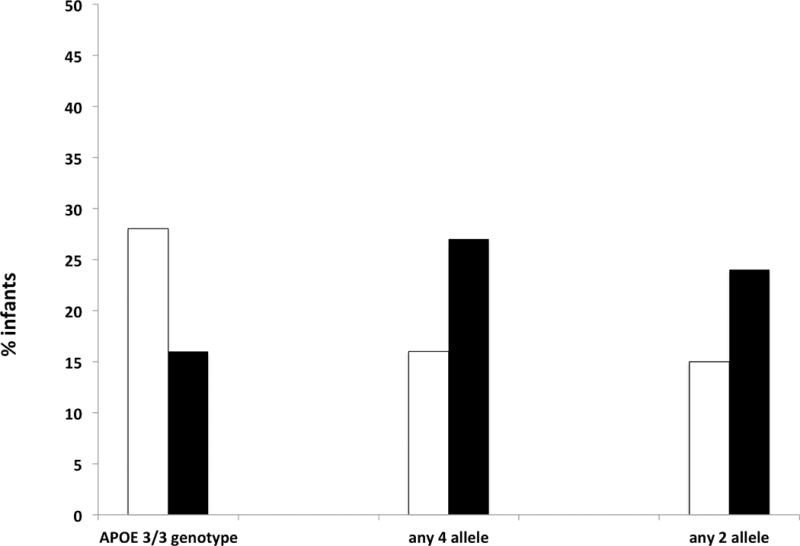
For each genotype, white bars denote the percent of those with the genotype surviving with MDI < 70, and black bars denote the percent without the genotype surviving with MDI < 70. Among 79 infants with APOE e3/e3 genotype and follow-up, prevalence of MDI < 70 was 28%, and among the 49 infants not homozygous for e3, prevalence was 16% (p = 0.20). Among 13 infants with the APOE e2 allele the prevalence of primary outcome was 15%, compared with 24% among the 115 infants without the e2 allele (p = 0.73). Among those with the APOE e4 allele (n = 38) the prevalence of the primary outcome was 16%, compared with 27% among the 90 without the e4 allele (p = 0.25).
Figure 4. Prevalence of moderate to severe cerebral palsy among infants of different genotypes.
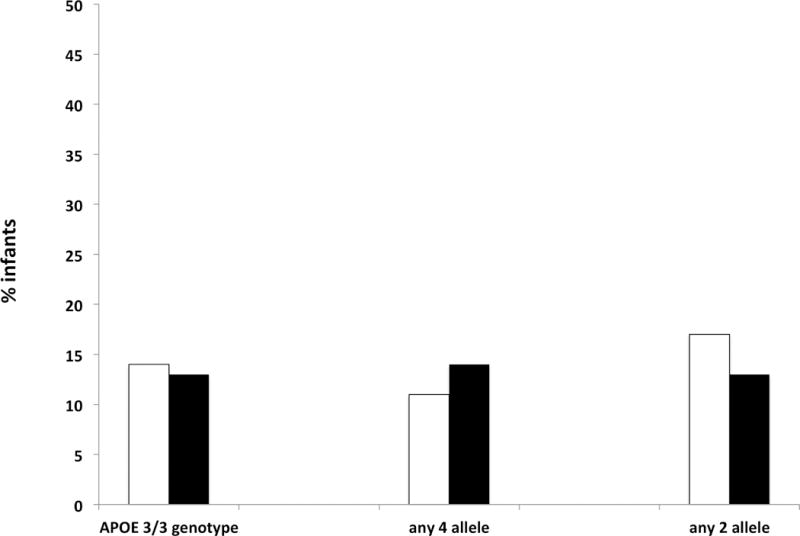
For each genotype, white bars denote the percent of those with the genotype surviving with moderate to severe cerebral palsy, and black bars denote the percent without the genotype surviving with moderate to severe cerebral palsy. Among 80 infants with APOE e3/e3 genotype and follow-up, prevalence of moderate to severe cerebral palsy was 14%, and among the 48 infants not homozygous for APOE e3, prevalence was 13% (p = 1.00). Among 12 infants with the APOE e2 allele the prevalence of moderate to severe cerebral palsy was 17%, compared with 13% among the 116 infants without the e2 allele (p = 0.66). Among those with the APOE e4 allele (n = 38) the prevalence of moderate to severe cerebral palsy was 11%, compared with 14% among the 90 without the e4 allele (p = 0.78).
Discussion
This report is the first to test associations between APOE genotypes and neurologic outcome in a cohort limited to term infants who survived to discharge after neonatal HIE. Previous case – control studies of pediatric populations reporting association between APOE genotypes and CP, a common outcome after HIE, did not target assessment of only infants with neonatal hypoxic-ischemic injury (13, 14). Our results demonstrate no association between APOE genotype and moderate or severe disability, or specifically with CP, in this more restrictive injury phenotype, and no modification from use of hypothermia.
Genetic epidemiology strongly supports associations between the e4 allele and Alzheimer’s disease and poor prognosis after neurologic injury. The association between the e4 allele and Alzheimer’s disease was uncovered using traditional linkage analysis and family studies (22). The association is seen even when unbiased approaches tested the e4 allele among millions of single nucleotide polymorphisms throughout the genome for association with Alzheimer’s disease. (P=2.52 × 10−53) (23).
While the genetic epidemiology supporting links between APOE genotype and Alzheimer’s disease is strong, tests of association between APOE e4 allele and CP among children have not been as consistent. Two recent reports (24, 25) of subjects with CP have not found the strengths of association found in the two earlier reports of association between CP and APOE genotype (13, 14). Wu et al reported results of association studies between candidate gene SNPs and cerebral palsy from a diverse, general population of 334,333 newborn infants from the Kaiser Permanente Health System, inclusive of healthy and sick infants. From this cohort, 138 cases of various types of CP and 165 controls were compared. APOE e4/e3 heterozygotes were associated with CP compared with e3/e3 homozygotes, odds ratio 1.7 (95% confidence interval 1.01–2.9) (24). In the second, more recent Australian case control study of children > 5 years of age with CP, among 55 children with diplegia compared with 281 controls, the odds ratio for CP was 1.87 (95% Confidence Interval 1.03–3.40) for e4/e3 compared with e3/e3; however, among children with quadriplegia (n = 40) compared with controls (n = 249), odds ratio for CP for e2/e3 heterozygotes compared with e3/e3 was 0.23 (0.05–1.00).27 The authors of both studies concluded that due to the multiple comparisons with dozens of candidate SNPs, neither could claim that the associations with APOE genotype were significant; however, both were cautious in eliminating the possibility of genetic links with risk of CP, citing the importance of the potential impact of variation in injury type or risk phenotype as well as variation in the CP phenotype outcomes on their analyses (24, 25). A third study, also among a cohort of children with multiple CP phenotypes with a variety of potential etiologies, has found no associations between APOE genotypes and severity of CP, although prevalence of active epilepsy was higher among children with the e4 allele (26).
Mechanisms for the associations between APOE genotypes and Alzheimer’s disease and poor outcomes after trauma and stroke in older populations are not definitively known. For Alzheimer’s disease, the leading hypothesis is that protein variations caused by the different genotypes lead to differential effects of APOE on amyloid-β accumulation in the brain and its vasculature (9). For the associations with poor outcome after stroke or trauma, there is less certainty (27). Postulated mechanisms associated with the e4 allele in animal studies of various ischemic and traumatic brain injuries include less efficient transport of lipids important for repair and subsequent synaptogenesis, (28)worse protection against oxidative injury (29), and more brain inflammation (30, 31). As oxidative injury, derangements in subsequent connectivity and inflammatory injury are all potential contributors to poor post hypoxic-ischemic injury outcomes (4), the e4 allele’s association with these mechanisms raises suspicions that it would be similarly implicated in worse outcome after HIE. Our results in a small cohort of infants surviving to discharge after neonatal HIE with well-defined outcome phenotypes do not support this hypothesis.
Prevalence of moderate to severe disability in our small study cohort was 18% for those with e4 allele and 29% for those without the e4 allele. This difference was not significant. There is some speculation, however, that the e4 allele may be associated with benefits in younger populations. In a study assessing association between the presence of the e4 allele and severity of cerebral palsy in a population of children with cerebral palsy (61% preterm), there was a trend suggesting infants with one ε4 allele were more likely to be in the low severity group (32). Among children born and raised in poor areas of Mexico and Brazil, the e4 allele has been associated with better 2 year and later outcomes (33, 34). If APOE variants are truly protective early but contribute to risk of Alzheimer’s later in life, the gene may obey the principle of antagonistic pleiotropy, such that despite potentially detrimental effects later in life, certain polymorphisms may be beneficial during critical periods of development when faced with environmental stress. (27, 33) Of note, population prevalence of the e4 allele in our racially mixed study population (29%) is higher than that reported from adult cohorts from multiple regions and ethnicities (2 – 40%) (35, 36). Evidence points to APOE as a critical glial factor in synaptogenesis, which may also subsequently affect long-term synaptic plasticity (37). Wright et al postulate that if e4 promotes myelination, synaptogenesis, or other fatty acid/cholesterol-mediated processes associated with neurodevelopment, then the higher Bayley scores noted among the children in his study cohort may be due to protection against the adverse effects of neurotoxins on these processes by a mechanism related to as yet unknown differences in function in the peptide produced from the APOE gene with the e4 allele (33). An intestinal health related hypothesis has also emerged from a study of Brazilian indigent children with below-median height-for-age z-scores supplemented with retinol and zinc or both, plus glutamine supplementation. In the measures of intestinal barrier function and cognitive outcome, children lacking the e4 allele exhibited negative Pearson correlations between the change in lactulose-to-mannitol ratio over four months and verbal learning and non-verbal intelligence, regardless of receiving any of the 3 interventions. The authors speculate that there is an interaction between APOE genotype and gut trophic factors, which translates into better nutritional, and ultimately, neurocognitive, outcomes (38). Accumulation of larger cohorts of infants with HIE and available genetic samples and accurate neurologic and neurodevelopmental outcome phenotypes are needed to test this finding.
We have utilized our study population to get the most precise estimate possible of associations between APOE genotypes and alleles and outcomes for term infants with HIE. While our study is unique in that it included only infants with HIE, the small study population allowed only a limited number of exploratory tests of association and has limited statistical power to detect significant effects (39, 40). While the estimate may be precise, our findings should be interpreted with caution. A two to three times larger study population with similar proportions of allele frequencies and outcome prevalence among cases and controls would be needed to demonstrate significance with 90% power (41). In addition, the importance of accuracy of injury as well as outcome phenotype to studies of genetic association cannot be underestimated. While we believe the eligibility criteria based on neonatal encephalopathy is a strength of our study, some infants in the study cohort had acute, sentinel events consistent with sudden acute hypoxic-ischemic injury such as umbilical cord prolapse or uterine rupture, while others had encephalopathy at birth without perinatal sentinel events or with multiple intrapartum complications (20). Our study also includes only surviving infants. Our rationale for this is in part a practical one, as we did not ask consent for collecting samples for research DNA analysis in the acute first days and weeks of hospitalization, and, because of our focus on APOE genetic variance playing a role in recovery from moderate to severe injury. Significant differences may have been found in a larger cohort of patients, and if samples were collected at birth so that all infants who died, many of whom had severe encephalopathy, could have been included in the analyses. APOE may have more to do with susceptibility to initial injury rather than recovery from that initial injury and initial recovery.
Although our exploratory study did not reveal significant associations between APOE genotypes and outcomes after HIE, the lower prevalence of CP and MDI < 70 among infants with the e4 allele is of interest, but requires testing in much larger cohorts with both accurate initial neonatal HIE and follow-up neurologic and neurodevelopmental phenotypes.
Conclusion
In this small cohort of surviving infants with neonatal HIE, there was no evidence for an association of the APOE e3/e3 genotype with improved neurodevelopmental outcome at 18 – 22 months, or for an association between presence of either the APOE e2 or e4 allele and increased risk for neurodevelopmental disability. Additional studies are required to confirm these results and explore other potential genetic factors which may influence outcome after neonatal HIE.
Methods
Surviving infants with moderate or severe HIE enrolled in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NICHD) therapeutic hypothermia study (20), and the NICHD study of utility of aEEG for prediction of outcome among infants with HIE were eligible (21). Eligibility criteria for these studies included gestational age of at least 36 weeks and birthweight above 1800 grams. Neonatal HIE was defined as described in the Hypothermia and aEEG studies. Eligibility criteria included a pH of 7.0 or less or a base deficit of 16 mmol/L or more in cord blood or blood gas during the first hour after birth. If, during this interval, a pH was between 7.01 and 7.15, a base deficit was between 10 and 15.9 mmol/L, or a blood gas was not available, additional criteria were required. These included history of an acute perinatal event (e.g., late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, hemorrhage, or cardiorespiratory arrest) and either a 10-minute Apgar score of 5 or less or assisted ventilation initiated at birth and continued for at least 10 minutes. Once these criteria were met all infants underwent a standardized neurologic examination performed by a certified examiner. Infants were candidates for the study when moderate or severe encephalopathy or seizures were present (20, 21). Outcome at 18 – 22 months was assessed by a standard neurologic exam and the Bayley Scales of Infant Development II performed by certified examiners (20, 21). Severe disability in the hypothermia study, and for this study, was defined as any one of the following: MDI<70, Gross Motor Function Classification System (GMFCS) 3–5 (equivalent to moderate/severe CP), hearing impairment requiring aids or bilateral blindness. Moderate disability was defined as Bayley II MDI 70–84 plus any one of the following: GMFCS 2, hearing impaired with no aids or seizure disorder requiring anticonvulsants. The primary outcome for the association analyses was presence of either moderate or severe disability (20, 21).
For DNA collection and analysis, a buccal swab was used to collect DNA prior to hospital discharge or at follow-up. APOE genotypes were determined using restriction fragment length polymorphism measurements. DNA extraction and APOE genotyping was done at Duke. DNA was extracted from the buccal swabs and first amplified using oligonucleotide primers F4 (5′-ACAGAATTCGCCCCGGCCTGGTAcACAC-a3n’d) and F6 (5′-lAAGCITGGCACGGCTGn=cAAGGA-3′). Amplification products of APOE were then subjected to restriction enzyme HhaI digestion, as described by Hixson (42).
Because not every family whose infant was enrolled in the Network’s Hypothermia or aEEG studies was approached for consent for the APOE genotyping study from the time of initiation of the Hypothermia or aEEG study, we compared demographics and perinatal characteristics and events between those enrolled in the genotyping study and those who were not. We used chi-square for categorical and t tests to compare the two groups.
For the primary analyses, we compared prevalence of the primary outcome, moderate or severe disability, among infants with and without the APOE e3/e3 genotype and with or without any e2 or e4 allele by Fisher’s exact test. We also compared prevalence of the components of the primary outcomes, MDI<70 and moderate/severe CP (GMFCS 3 –5), among infants with and without APOE e3/e3 genotype, and with and without the e2 or e4 allele. In separate exploratory analyses, we compared prevalence of outcomes for infants with and without the e3/e3 genotype or alleles (i) among infants of different races, to account for potential allele frequency differences among races, (ii) among infants with different levels of encephalopathy at enrollment and (iii) among infants cooled and those not cooled.
Informed consent was obtained and the study was approved by all sites’ Institutional Review Boards (the list of sites is included in the Supplemental Appendix (online)), including the data coordinating center, RTI International (Research Triangle Park, NC). Because genetic information was generated, a Certificate of Confidentiality was obtained for this study by the data coordinating center.
Acknowledgments
Disclosure: The National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) funded this trial. Principle Investigators and Research Coordinators received salary support as part of the Network grants at each site, and grant numbers are individually listed in the Supplemental Appendix (online).
Footnotes
No authors have financial ties to products in the study or potential/perceived conflicts of interest to disclose.
References
- 1.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30:227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Stone BS, Jiangyang Z, Mack DW, Mori S, Martin LJ, Northington FJ. Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann Neurol. 2008;64:535–546. doi: 10.1002/ana.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller SM, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends in Neurosciences. 2009;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol. 2011;69:743–58. doi: 10.1002/ana.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins RD, Raju T, Edwards AD, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Hypothermia Workshop Speakers and Moderators. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159:851–858. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright RO, Hu H, Silverman EK, et al. Apolipoprotein E genotype predicts 24-month Bayley scales infant development score. Pediatr Res. 2003;54:819–25. doi: 10.1203/01.PDR.0000090927.53818.DE. [DOI] [PubMed] [Google Scholar]
- 7.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–37. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–52. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–23. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 11.Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher KA. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma. 2008;25:279–90. doi: 10.1089/neu.2007.0489. [DOI] [PubMed] [Google Scholar]
- 12.Tardiff BE, Newman MF, Saunders AM, Strittmatter WJ, et al. Preliminary report of a genetic basis for cognitive decline following cardiac operations. Ann of Thorac Surg. 1997;64:715–720. doi: 10.1016/s0003-4975(97)00757-1. [DOI] [PubMed] [Google Scholar]
- 13.Meirelles Kalil Pessoa de Barr E, Rodrigues CJ, de Barros TE, Bevilacqua RG. Presence of apolipoprotein E 4 allele in cerebral palsy. J Pediatr Orthop. 2000;20:786–796. doi: 10.1097/01241398-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Kuroda MM, Weck ME, Sarwark JF, Hamidullah A, Wainwright MS. Association of apolipoprotein E genotype and cerebral palsy in children. Pediatrics. 2007;119:306–13. doi: 10.1542/peds.2006-1083. [DOI] [PubMed] [Google Scholar]
- 15.Lo TY, Jones PA, Chambers IR, et al. Modulating effect of apolipoprotein E polymorphisms on secondary brain insult and outcome after childhood brain trauma. Childs Nerv Sys. 2009;25:47–54. doi: 10.1007/s00381-008-0723-4. [DOI] [PubMed] [Google Scholar]
- 16.Gaynor JW, Gerdes M, Zackai EH, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thoracic Cardiovascular Surgery. 2003;126:1736–45. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 17.Gaynor JW, Nord AS, Wernovsky G, et al. Apolipoprotein E genotype modifies the risk of behavior problems after infant cardiac surgery. Pediatrics. 2009;124:241–50. doi: 10.1542/peds.2008-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiefermeier M, Kollegger H, Madl C, et al. Apolipoprotein E polymorphism: survival and neurological outcome after cardiopulmonary resuscitation. Stroke. 2000;31:2068–73. doi: 10.1161/01.str.31.9.2068. [DOI] [PubMed] [Google Scholar]
- 19.Eisenberg DT, Kuzawa CW, Hayes MG. Worldwide allele frequencies of the human apolipoprotein E gene: climate, local adaptations, and evolutionary history. Am J Phys Anthropol. 2010;143:100–11. doi: 10.1002/ajpa.21298. [DOI] [PubMed] [Google Scholar]
- 20.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 21.Shankaran S, Pappas A, McDonald SA, et al. Predictive value of an early amplitude integrated electroencephalogram and neurologic examination. Pediatrics. 2011;128:e112–120. doi: 10.1542/peds.2010-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 23.Kamboh MI, Demirci FY, Wang X, et al. Genome-wide association study of Alzheimer’s disease. Transl Psychiatry. 2012;2:e117. doi: 10.1038/tp.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu YW, Croen LA, Vanderwerf A, Gelfand AA, Torres AR. Candidate genes and risk for CP: a population-based study. Pediatr Res. 2011;70:642–6. doi: 10.1203/PDR.0b013e31823240dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Callaghan ME, Maclennan AH, Gibson CS, et al. Fetal and maternal candidate single nucleotide polymorphism associations with cerebral palsy: a case-control study. Pediatrics. 2012;129:e414–23. doi: 10.1542/peds.2011-0739. [DOI] [PubMed] [Google Scholar]
- 26.Lien E, Andersen GL, Bao Y, et al. Apolipoprotein E polymorphisms and severity of cerebral palsy: a cross-sectional study in 255 children in Norway. Dev Med Child Neurol. 2013;55:372–7. doi: 10.1111/dmcn.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackman JA, Worley G, Strittmatter WJ. Apolipoprotein E and brain injury: implications for children. Dev Med Child Neurol. 2005;47:64–70. doi: 10.1017/s0012162205000113. [DOI] [PubMed] [Google Scholar]
- 28.Poirier J, Baccichet A, Dea D, Gauthier S. Cholesterol synthesis and lipoprotein reuptake during synaptic remodeling in hippocampus in adult rats. Neuroscience. 1993;55:81–90. doi: 10.1016/0306-4522(93)90456-p. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y, Aono M, Laskowitz DT, Warner DS, Pearlstein RD. Apolipoprotein E protects against oxidative stress in mixed neuronal–glial cell cultures by reducing glutamate. Neurochem Int. 2004;44:107–118. doi: 10.1016/s0197-0186(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 30.Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J Neuroimmunol. 1997;76:70–74. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 31.Laskowitz DT, Fillit H, Yeung M, Toku K, Vitek M. Apolipoprotein E-derived peptides reduce CNS inflammation-implications for therapy of neurological disease. Acta Neurol Scand. 2006;114:15–20. doi: 10.1111/j.1600-0404.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 32.Blackman J, Gurka MJ, Bao Y, Dragulev BP, Chen WM, Romness MJ. Apolipoprotein E and functional motor severity in cerebral palsy. Pediatr Rehabil Med. 2009;2:67–74. doi: 10.3233/PRM-2009-0063. [DOI] [PubMed] [Google Scholar]
- 33.Wright RO, Hu H, Silverman EK, et al. Apolipoprotein E genotype predicts 24-month Bayley scales infant development score. Pediatr Res. 2003;54:819–25. doi: 10.1203/01.PDR.0000090927.53818.DE. [DOI] [PubMed] [Google Scholar]
- 34.Oria RB, Patrick PD, Zhang H, et al. APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr Res. 2005;57:310–6. doi: 10.1203/01.PDR.0000148719.82468.CA. [DOI] [PubMed] [Google Scholar]
- 35.Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann Hum Genet. 1999;63:301–10. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 36.Lewis SJ, Brunner EJ. Methodological problems in genetic association studies of longevity–the apolipoprotein E gene as an example. Int J Epidemiol. 2004;33:962–70. doi: 10.1093/ije/dyh214. [DOI] [PubMed] [Google Scholar]
- 37.Mauch DH, Nagler K, Schumacher S, Goritz, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–7. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 38.Mitter SS, Oriá RB, Kvalsund MP, et al. Apolipoprotein E4 influences growth and cognitive responses to micronutrient supplementation in shantytown children from northeast Brazil. Clinics (Sao Paulo) 2012;67:11–8. doi: 10.6061/clinics/2012(01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ioannidis JN, Ntzani EE, Trikalinos TA, Contopouos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 40.Lohmuller KE, Pearce CL, Pike M, Lander ES, Hirshhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 41.Hattersley AT, McCarthy MI. Genetic Epidemiology 5: What makes a good association study? Lancet. 2005;366:1315–23. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- 42.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]


