Abstract
Background
We evaluated whether aliskiren, valsartan, or the combination was protective following myocardial infarction (MI) through effects on matrix metalloproteinase (MMP)-9.
Methods and Results
C57BL/6J wild type (WT, n=94) and MMP-9 null (null, n=85) mice were divided into 4 groups at 3 h post-MI: saline (S), aliskiren (A; 50 mg/kg/d), valsartan (V; 40 mg/kg/d), or A+V and compared to no MI controls at 28 d post-MI. All groups had similar infarct areas, and survival rates were higher in the null mice. The treatments influenced systolic function and hypertrophy index, as well as extracellular matrix (ECM) and inflammatory genes in the remote region, indicating that primary effects were on the viable myocardium. Saline treated WT mice showed increased end systolic and diastolic volumes and hypertrophy index, along with reduced ejection fraction. MMP-9 deletion improved LV function post-MI. Aliskiren attenuated the increase in end systolic volume and hypertrophy index, while valsartan improved end diastolic volumes and aliskiren + valsartan improved the hypertrophy index only when MMP-9 was absent. Extracellular matrix and inflammatory gene expression showed distinct patterns among the treatment groups, indicating a divergence in mechanisms of remodeling.
Conclusions
This study shows that MMP-9 regulates aliskiren and valsartan effects in mice. These results in mice provide mechanistic insight to help translate these findings to post-MI patients.
Keywords: myocardial infarction, matrix metalloproteinase, proteomics, remodeling, inflammation, extracellular matrix, MMP-9, aliskiren, valsartan
Introduction
Adverse remodeling of the left ventricle (LV) following myocardial infarction (MI) is a significant cause of congestive heart failure. While short-term post-MI survival has dramatically improved over the past decades, there remains a need to develop therapeutic strategies to limit or prevent the progression to heart failure. The outcome of LV remodeling post-MI determines the extent of LV dysfunction that will develop, and the extent of LV dysfunction predicts the development of heart failure and mortality [1]. Currently, there is a need for more effective treatments to improve LV remodeling outcomes post-MI. In order to develop optimal therapeutic strategies, a better understanding of the mechanisms that drive LV remodeling is needed.
The extracellular matrix (ECM) is a key regulator of LV remodeling, and the matrix metalloproteinases (MMPs) are a key family of proteases that modulate the ECM response. A significant MMP involved in ECM degradation and LV remodeling is MMP-9. MMP-9 processes a very broad repertoire of both ECM and non-ECM substrates that have implications for post-MI remodeling. For example, MMP-9 proteolyzes collagen, elastin, fibronectin, galectin-3, laminin, pro-MMP-2, pro-MMP-13, and vitronectin [2]. Non-ECM substrates of MMP-9 that have roles in post-MI remodeling include interleukin (IL)-1β, IL-8, platelet factor 4, endothelin I, and α2-macroglobulin [2]. A detailed understanding of substrate functions and the affinity of MMP-9 for this wide range of substrates is needed for a better understanding of MMP-9 roles as a mediator of post-MI remodeling.
ECM degradation, mediated by MMP-9 and amplified by inflammation, is a major pathway that stimulates post-MI remodeling. MMP-9 increases early post-MI [3], MMP inhibition decreases MMP-9 levels and improves outcomes [4, 5], and MMP-9 gene deletion reduces LV remodeling [6]. In humans, plasma MMP-9 levels predict cardiovascular mortality, as coronary artery disease patients with the highest MMP-9 levels at baseline show the greatest cardiovascular mortality rates at the 4 year follow-up [7]. Plasma MMP-9 and TIMP-1, but not NTproBNP, correlate with increased end diastolic volumes, implicating MMP-9 as a clinical biomarker of LV remodeling and adverse prognosis [8]. Further, we have previously identified a role of MMP-9 in the pro-inflammatory response, suggesting a possible regulating mechanism [9].
Aliskiren is a direct renin inhibitor and is the only member of a new class of anti-hypertensive agents. Valsartan is an angiotensin II receptor blocker. Both have been shown to exert mechanisms beyond blood pressure reduction, and these mechanisms also involve MMP-9 [10, 11]. In multiple animal disease models, there is a robust induction of renin, angiotensin-converting enzyme (ACE), and angiotensin type I receptor (AT1R) during the inflammatory response. Aliskiren reduces the levels of multiple genes in the renin-angiotensin-aldosterone system pathway [12]. ACE inhibitors repress adverse LV remodeling in post-MI patients and improve LV function [13]. Similar to aliskiren and valsartan, ACE inhibitors target the renin-angiotensin-aldosterone pathway and have anti-inflammatory effects. Fujiwara and colleagues showed that valsartan inhibits both macrophage infiltration and MMP-9 levels in an abdominal aortic aneurysm model [14].
Both compounds have anti-inflammatory roles, but whether they affect LV remodeling post-MI and if MMP-9 regulates this response have not been explored. We hypothesized that the two agents may have effects on post-MI remodeling by modulating inflammation and fibrosis through direct and indirect effects on MMP-9. Accordingly, we examined the ability of aliskiren and valsartan, alone or in combination and through MMP-9, to influence post-MI remodeling in a mouse model of permanent artery occlusion.
Methods
A more complete description of the methods is included in the supplementary methods file.
Mice
All animal procedures were conducted according to the “Guide for the Care and Use of Laboratory Animals” (Eighth edition, revised 2011) and were approved by the institutional animal care and use committee at the University of Texas Health Science Center at San Antonio. Young C57BL/6J WT (4.5±1.5 months, n=94) and MMP-9 null (4.5±1.5 months, n=85) male and female mice were used in this study. The MMP-9 null mice were generated in the laboratory of Dr. Zena Werb and were backcrossed into the C57BL/6J background in the laboratory of Dr. Lynn Matrisian [15, 16].
WT and MMP-9 null mice were randomly divided into five groups, and each group had a minimum of n=6 (n=3 male and n=3 female) surviving mice. The five groups were: day (d) 0 naïve no MI controls, MI with saline, MI with aliskiren (A, 50 mg/kg/d), MI with valsartan (V, 40 mg/kg/d), and MI with aliskiren and valsartan (A+V). MI was induced by permanently ligating the left anterior descending coronary artery as previously described [17]. At 3 h post-MI, the drugs were administered by osmotic pump (Alzet; 2004; Cupertino, CA). Animals were all sacrificed at 28 d post-MI.
Blood Pressure and Echocardiography
Blood pressure was noninvasively acquired with the MC4000 Blood Pressure Analysis System (Hatteras Instruments, Cary, NC). Transthoracic echocardiography was performed using the Visual Sonics Vevo 770 system (VisualSonics) with a 30-MHz image transducer. Mice were anesthetized with 0.5–2% isoflurane in a 100% oxygen mix.
Tissue and Plasma Collection
Mice were sacrificed at 28 d post-MI under 2% isoflurane in a 100% oxygen mix [9]. Following heparin injection (4 units/ g BW), blood was collected from the carotid artery and plasma was isolated by centrifugation [18]. The heart was arrested in diastole by apical injection of cardioplegic solution, and the heart was divided into LV and right ventricle [19]. The heart was stained with 1% 2,3,5 triphenyltetrazolium chloride, and the infarct area was determined using Photoshop (Adobe).
Immunohistochemistry
The mid-section of the LV was fixed in zinc-formalin and paraffin embedded. Sections (5 μm) were cut, and the sections were stained for collagen by picrosirius red staining or for macrophages by immunohistochemistry.
Real Time PCR
The apex and base sections of the LV were separated into infarcted (LVI) and non-infarcted remote (LVC) regions and snap frozen in liquid nitrogen for gene array analysis. Inflammatory, ECM, and adhesion molecule genes were evaluated for mRNA levels using the Inflammatory Cytokines and Receptors and the Extracellular Matrix and Adhesion Molecules arrays (Qiagen PAMM-011A and PAMM-013A, Valencia, CA). Total RNA was extracted by homogenization in TRIzol reagent (Invitrogen, 15596-026, Grand Island, NY) according to the manufacturer protocol, and cDNA was synthesized with RT2 First Strand Kit (Qiagen 330401). Results were normalized to 5 housekeeping genes (actinb, gapdh, hprt1, gusb, and Hsp90ab1) and the results were reported as 2−ΔCt values x1000 for inflammatory genes and x100 for ECM genes.
Plasma Proteomic Profiling
Plasma samples (100 μl) were analyzed for rodent multi-analyte proteomic profiling by Myriad RBM (Austin, TX).
Statistical and Data Analyses
All results were reported as mean±SEM. Statistical analyses were performed using statistical software JMP (Cary, NC), SAS (Cary, NC), or Graphpad’s Instat (La Jolla, CA). For the biclustering analysis, expression levels of ECM and inflammatory genes from the three drug treatment groups were normalized to the average expression levels of each gene from the saline treated MI group and expressed as a fold change. A Sparse Singular Value Decomposition (SSVD) biclustering algorithm was applied to microarray data collected from ECM and inflammatory panels [20]. Biclustering categorized genes and treatments with respect to similarity scores on fold change of each gene. Groups of genes were assigned based on their patterns of change. All other statistical comparisons were analyzed by ANOVA with Student-Newman Keuls post-test. A p<0.05 was considered significant.
Results
MMP-9 null had improved survival post-MI, compared to WT mice
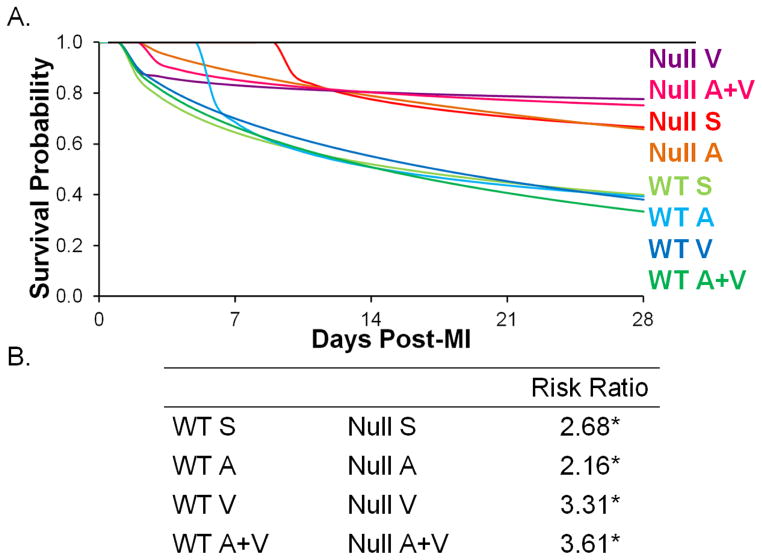
There was a significant difference in survival probabilities between the two genotypes. Null mice had higher survival rates than WT mice, for all groups (Figure 1). The WT A+V mice had the lowest survival rate, while the null V mice had the highest survival rates.
Figure 1.
(A) Survival probability of each treatment group indicated higher survival rates in all null groups compared to WT. (B) Risk ratio (group 1/group 2 risks of death) showed a higher risk for death in all WT groups. * p<0.05 null compared to respective WT counterpart.
Blood Pressure did not change with treatment groups
Blood pressure was not lowered by any of the treatments, indicating that the effects seen were not directly due to a blood pressure-lowering mechanism (Table 1). This result was expected, as the doses given were below those needed to decrease blood pressure [10, 21].
Table 1.
Blood Pressure and Necropsy Data
| WT D0 | WT Saline | WT A | WT V | WT A+V | Null D0 | Null Saline | Null A | Null V | Null A+V | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size (n) | 6 | 9 | 9 | 11 | 10 | 7 | 10 | 14 | 16 | 17 |
| Blood Pressure | ||||||||||
| Heart Rate (bpm) | N/A | 670±15 | 622±21 | 575±34 | 559±82 | N/A | 675±21 | 655±23 | 651±23 | 596±24 |
| Blood Pressure; systolic (mmHg) | N/A | 97±4 | 112±8 | 107±3 | 101±3 | N/A | 96±3 | 104±3 | 100±3 | 100±4 |
| Blood Pressure; diastolic (mmHg) | N/A | 82±4 | 82±7 | 85±5 | 81±5 | N/A | 75±5 | 80±3 | 77±3 | 80±4 |
| Necropsy | ||||||||||
| Infarct Area (%) | N/A | 32±2 | 33±2 | 35±2 | 36±3 | N/A | 30±2 | 35±2 | 37±2 | 35±2 |
Data are reported as AVG±SEM. Blood Pressure data was acquired without anesthesia, and none of the groups were statistically significantly different. N/A- not applicable; A- aliskiren, V-valsartan
Echocardiography reveals MMP-9 deletion or aliskiren treatment improved systolic function and hypertrophy index
No significant changes were found in the infarct areas among the groups (p=0.24). This indicates that all mice were given consistent initial ischemic injuries at baseline and is consistent with starting therapy at 3h post-MI.
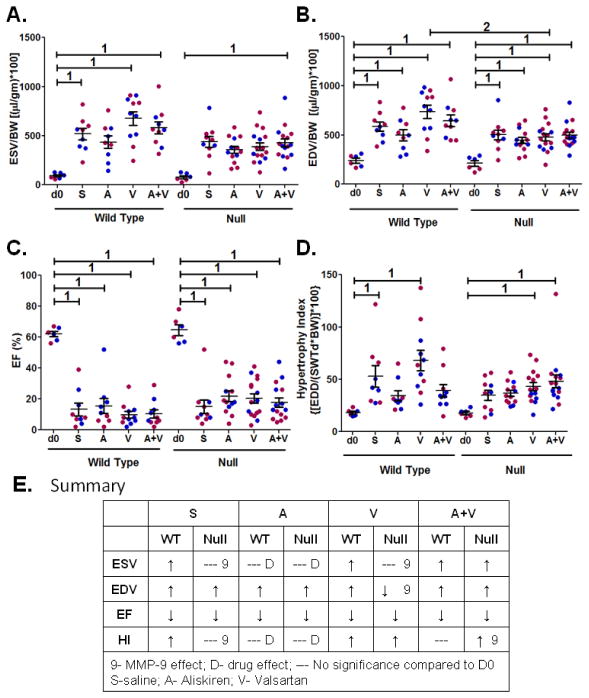
By echocardiography, end systolic volume (ESV) increased in WT saline, V, and A+V groups compared to day 0 controls, and this effect was attenuated in the WT A group (Figure 2A). MMP-9 deletion improved ESV for all groups except A+V. Aliskiren treatment attenuated the dilation at the end systolic stage in both WT and MMP-9 null mice, indicating this effect was MMP-9 independent. Overall, the prominent MMP-9 and drug effects were to improve systolic function.
Figure 2.
Dilation and hypertrophy responses, but not EF, were altered by drug or MMP-9 deletion. (A) End systolic volume (ESV), normalized to body weight. (B) End diastolic volume (EDV), normalized to body weight. (C) Ejection fraction (EF). (D) Hypertrophy index (HI). Volume data were normalized to body weight and multiplied by 100, and data are mean±SEM. Individual data points are shown in blue for males and red for females. n=6–16/group; 1 p<0.05 compared to d0 no MI control, 2 p<0.05 null compared to respective WT. (E) Summary table illustrates effects on ESV, EDV, EF, and HI is due to MMP-9 or treatments.
For end diastolic volume (EDV), all groups showed increased volumes compared to the day 0 controls, regardless of the drug treatment or MMP-9 deletion (Figure 2B). The MMP-9 null V group was the only one with attenuated EDV compared to WT V, indicating that a valsartan effect on EDV was MMP-9 dependent.
EF was reduced equally among the drug treatments and genotype; the improvement in ESV seen with A treatment or MMP-9 deletion was not sufficient to improve EF (Figure 2C). The hypertrophy index revealed differences among genotypes and drug treatments (Figure 2D). This index was elevated in the WT saline group, and attenuated by A and A+V treatments, but not V. Aliskiren attenuated the hypertrophy index in both wild type and MMP-9 null mice, independent of MMP-9. Treatment with valsartan did not show any effect on hypertrophy index whether in wild type or with MMP-9 deletion. A+V, in the setting of MMP-9 deletion, increased the hypertrophy index. This demonstrates that the hypertrophy index response to A+V treatment, but not aliskiren or valsartan, was MMP-9 dependent.
Overall, the echocardiography results revealed that the dilation and hypertrophy responses, but not EF, were altered by MMP-9 deletion or treatment (Figure 2E). The majority of effects were seen in systolic function, rather than diastolic function. This indicates that long-term effects were likely through action on the remote region rather than the infarct region.
Macrophage numbers and collagen levels were similar among d 28 post-MI groups
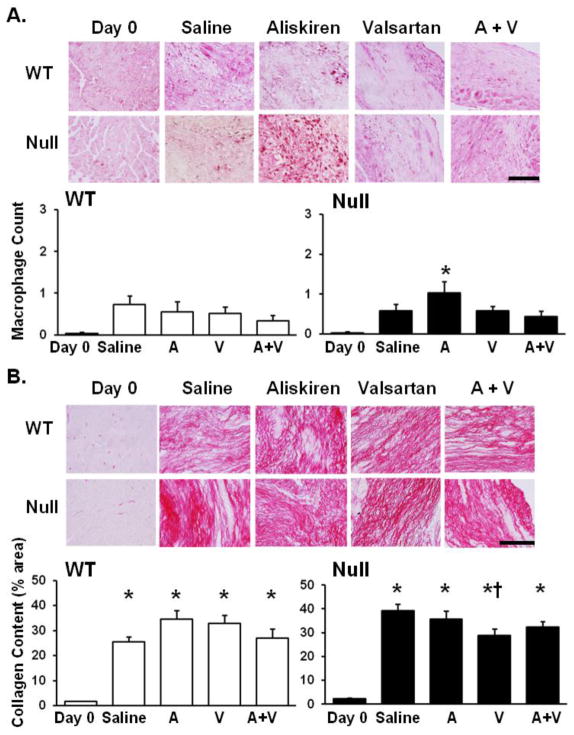
Mac 3 staining was performed on all groups to determine the macrophage numbers at d 28 post-MI, a time when macrophage infiltration has normally returned towards baseline. Wild type A, V, and A/V, as well as null V and A/V groups in comparison to respective saline groups showed no significant difference in macrophage numbers. The null A group showed significantly elevated macrophage numbers compared to d0 controls, indicating a prolonged inflammatory response in comparison to other null groups (p<0.05; Figure 3A).
Figure 3.
(A) At day 28 post-MI, macrophage numbers were not significantly elevated in any of the WT groups, indicating a return of macrophage numbers to pre-MI levels. Macrophage infiltration in the null aliskiren treated LV was significantly increased compared to null d0, suggesting a prolonged inflammatory response compared to the other null groups. Macrophage count is represented by the percentage area stained with Mac-3. Images were taken at 60x magnification and the scale bar is 100 μm. (B) By PSR staining, collagen deposition in the infarct region was increased in all MI groups compared to respective d0 controls, and collagen levels were lower in the null valsartan-treated group compared to saline treated post-MI LV, indicating differences in collagen organization. n=6–14/group; * p<0.05 compared to d0 no MI control, † p<0.05 compared to the saline MI control. Images were taken at 60x magnification and the scale bar is 100 μm.
Picrosirius red staining demonstrated that collagen deposition of all post-MI groups was elevated compared to d0 controls (Figure 3B). The null V group showed a lower amount of total collagen deposition compared to the null saline group (p<0.05).
Extracellular Matrix Gene Array reveals MMP-9 and treatment dependent effects occur only in the remote region (Figure 4, Tables 2 and 3 and Supplementary Tables 1, 2, and 5)
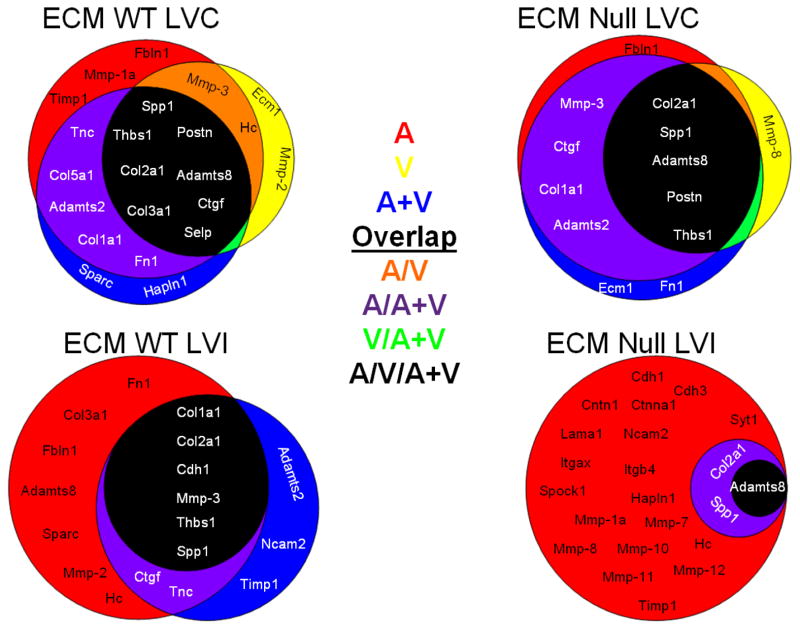
Figure 4.
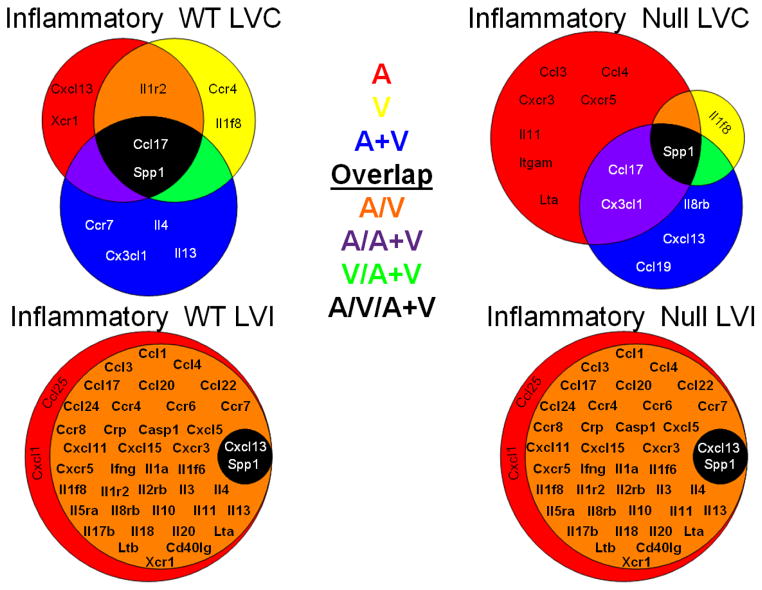
Extracellular matrix (ECM) genes are influenced by the individual drug treatments in both the remote and infarct regions. Venn diagrams of the dominant ECM genes determined by biclustering analysis (with all drug treatment groups compared as the fold change over the saline treated MI group). The groups are shown by red (for aliskiren, A), yellow (for valsartan, V), or blue (for the combination A+V). Genes in common between two groups are represented by orange (A group and V group, red + yellow), purple (A group and A/V group, orange + yellow), or green (V group and A+V group, yellow + blue). The genes in common to all three treatment groups are shown in the black circle. The Venn diagrams illustrate the effects of the three drug treatments compared to the untreated MI, both in the presence and absence of MMP-9. The diagrams illustrate that each drug treatment differentially affects the ECM response. n=6–8/group
Table 2.
List of ECM genes altered in the remote and infarct region of wild type mice, grouped by patterns of change. Note that all genes changes were in the remote region.
| LVC | ||||
|---|---|---|---|---|
| Gene | WT Saline | WT Aliskiren | WT Valsartan | WT A+V |
| Adamts2, Adamts5, Cd44, Col1a1, Col4a1, Col4a2, Col4a3, Col5a1, Ecm1, Emilin1, Fbln1, Fn1, Mmp14, Mmp3, Ncam1, Postn, Selp, Sgce, Sparc, Tgfbi, Thbs1, Thbs3, Timp1, Timp2, Tnc, Vcan | ⬆* | ⬆* | ⬆* | ⬆* |
| Cdh2, Cdh4, Lamb3, Mmp9, Mmp15 | ⬇* | ⬇* | ⬇* | ⬇* |
| Col6a1, Lamb2 | ⬆* | ns | ns | ns |
| Col3a1, Itgax | ⬆* | ns | ⬆* | ⬆* |
| Itga5 | ns | ⬆* | ns | ns |
| Hapln1 | ns | ⬆*† | ⬇§ | ⬇§ |
| Itgal | ns | ⬇* | ns | ⬇* |
| Entpd1, Itgb3 | ns | ⬆* | ⬆* | ⬆* |
| Ctgf | ns | ns | ⬆* | ns |
| Icam1, Spp1 | ns | ns | ⬆* | ⬆* |
| Cdh1, Col2a1 | ns | ns | ns | ⬆* |
| LVI | ||||
| Gene | WT Saline | WT Aliskiren | WT Valsartan | WT A+V |
| No changes | ||||
Arrows depict direction of change; black boxes show MI effects and white boxes show drug effects. n=6/group. A= aliskiren, V= valsartan.
p<0.05 vs. day 0;
p<0.05 vs. saline;
p<0.05 vs. Aliskiren
Table 3.
List of ECM genes altered in the remote and infarct region of MMP-9 null mice, grouped by patterns of change. Note that all changes were in the remote region.
| LVC | ||||
|---|---|---|---|---|
| Gene | Null Saline | Null Aliskiren | Null Valsartan | Null A+V |
| Adamts2, Col4a3, Col5a1, Ecm1, Fn1, Itgb3, Mmp2, Mmp3, Postn, Sparc, Thbs3, Timp3, Tnc, Vcan | ⬆* | ⬆* | ⬆* | ⬆* |
| Cdh2, Cdh4, Mmp15 | ⬇* | ⬇* | ⬇* | ⬇* |
| Ctgf | ⬆* | ns | ns | ns |
| Fbln1, Itgb4, Timp2 | ns | ⬆* | ns | ns |
| Cd44 | ⬆* | ⬆* | ns | ns |
| Tgfbi | ⬆* | ⬆* | ns | ⬆* |
| Mmp13 | ⬇* | ⬇* | ns | ⬇* |
| Itgav | ns | ⬆* | ns | ns |
| Itgam, Mmp14, Ncam1 | ns | ⬆* | ⬆* | ns |
| Col1a1 | ns | ⬆* | ⬆* | ns |
| Itga5, Timp1 | ns | ns | ⬆* | ns |
| Col4a2 | ns | ns | ns | ⬆* |
| LVI | ||||
| Gene | WT Saline | WT Aliskiren | WT Valsartan | WT A+V |
| No changes | ||||
Arrows depict direction of change; black boxes show MI effects and white boxes show drug effects. n=6/group. A= aliskiren, V= valsartan.
p<0.05 vs. day 0
Figure 4 summarizes the biclustering analysis for the ECM genes, which was based on fold-change similarity patterns. For ECM genes in WT LVC (Figure 4, upper left panel), 18 genes contributed 80% of the overall ECM response in A (red background), 12 in V (yellow background), and 15 in A+V (blue background). The intersection of the three groups (black background) contained 8 genes (Adamts8, Col2a1, Col3a1, Ctgf, Postn, Selp, Spp1, and Thbs1) in common across treatments. Fbln1, MMP-1a, and Timp1 were affected by A only; Ecm1 and Mmp-2 by V only; and Sparc and Hapln1 by A+V only. Two genes in the orange background (Mmp-3 and Hc) were affected by both A and V as individual treatments, while 5 genes were altered by both A and A+V.
For ECM genes in WT LVI (Figure 4, lower left panel), 15 genes contributed 80% of the overall ECM response in A (red background), 8 in V (yellow background), and 11 in A+V (blue background). Interestingly, 8 genes affected by V were also affected by A treatment, and 6 out of these 8 genes (Col1a1, Col2a1, Cdh1, Mmp-3, Thbs1, and Spp1) were in common to all 3 treatments (black background). Three genes (Adamts2, Ncam2, and Timp1) were affected by A+V treatment only. Our analysis suggests that drug effects in WT were more active in the LVC remote region than the LVI infarct region at day 28 post-MI.
For ECM genes in MMP-9 null LVC (Figure 4, upper right panel), 10 genes contributed 80% of the overall ECM response in A (red background), 6 in V (yellow background), and 11 in A+V (blue background). The intersection of the three groups (black background) contained 5 genes (Adamts8, Col2a1, Postn, Spp1, and Thbs1) in common across treatments. Fbln1 was affected only in A, which was similar to WT LVC and indicates this effect is MMP-9 independent. Mmp-8 was affected by V only, while Ecm1 and Fn1 were affected by A+ V only. There were 4 genes (purple background; Adamts2, Ctgf, Col1a1, and Mmp-3) affected by A and A+V.
For ECM genes in MMP-9 null LVI (Figure 4, lower right panel), 22 genes explained 80% of the A response (red background). Out of the 22 genes affected by A, 3 genes (Adamts8, Col1a1, and Spp1) were also affected by A+V. The intersection of the three groups (black background) contained 1 gene (Adamts8) in common across treatments. In WT LVI, only A affected Adamsts8 expression, indicating that the effect of A treatment on Adamts8 was MMP-9 regulated, but was MMP-9 independent in the V or A+V treatment groups.
Tables 2 and 3 list only the up- and down-regulated ECM genes in the WT. Among the 44 genes altered, 26 genes were up-regulated and 5 genes down-regulated in all WT groups at day 28 (first 2 lines). These indicate gene changes that are MI-dependent and not treatment influenced. Our results demonstrate that the individual treatments followed different downstream mechanisms to regulate specific genes in the remote region of WT mice. For example, Cola1 and Lamb2 increased post-MI in the saline group, and these increases were attenuated by all 3 treatments. Aliskiren treatment increased Hapln1 expression significantly compared to saline, V, and A+V groups. Overall, all 3 treatment groups stimulated ECM production in the remote region. MMP-9 deletion increased ECM production in the A group and decreased ECM in the V and A+V groups; and these effects were seen in the remote region. Overall, this data suggests strong MMP-9 dependence.
Inflammatory Gene Array reveals that changes are predominantly in the remote region (Figure 5, Tables 4 and 5 and Supplementary Tables 3, 4, and 6)
Figure 5.
Inflammatory genes are influenced by drug treatments in both the remote and infarct regions. Venn diagrams of the dominant inflammatory genes determined by biclustering analysis (with all drug treatment groups compared as the fold change over the saline treated MI group). The groups are shown by red (for aliskiren, A), yellow (for valsartan, V), or blue (for the combination A+V). Genes in common between two groups are represented by orange (A group and V group, red + yellow), purple (A group and A/V group, orange + yellow), or green (V group and A+V group, yellow + blue). The genes in common to all three treatment groups are shown in the black circle. The Venn diagrams illustrate the effects of the three groups compared to the untreated MI, both in the presence and absence of MMP-9. n=6–8/group
Table 4.
List of inflammatory genes altered in the remote and infarct region of WT mice, grouped by patterns of change. Note that the majority of changes were in the remote region.
| LVC | ||||
|---|---|---|---|---|
| Gene | WT Saline | WT Aliskiren | WT Valsartan | WT A+V |
| Ccl11 | ⬇* | ⬇* | ⬇* | ⬇* |
| Il15 | ⬇* | ns | ⬇* | ns |
| Ccl4 | ⬆* | ⬇† | ns | ⬆*§ |
| Ccr5 | ⬆* | ns | ⬆* | ⬆*†§‡ |
| Il11 | ⬆* | ns | ns | ⬆*†§‡ |
| Ccl7 | ns | ⬇* | ns | ns |
| Cxcl9 | ns | ⬇* | ⬇* | ns |
| Abcf1, Spp1 | ns | ⬆* | ⬆* | ⬆* |
| Cxcl5 | ns | ns | ⬇* | ns |
| Ccr3, Itgam | ns | ns | ns | ⬆*‡ |
| Casp1, Cx3cl1, Il1r1 | ns | ns | ns | ⬆* |
| LVI | ||||
| Gene | WT Saline | WT Aliskiren | WT Valsartan | WT A+V |
| Ccl25, Ccr10 | ns | ns | ns | ⬆†§‡ |
Arrows depict direction of change; black boxes show MI effects and white boxes show drug effects. n=6/group. A= aliskiren, V= valsartan.
p<0.05 vs. day 0;
p<0.05 vs. saline;
p<0.05 vs. aliskiren,
p<0.05 vs. valsartan
Table 5.
List of inflammatory genes altered in the remote and infarct region of MMP-9 null mice, grouped by patterns of change. Note that all changes were in the remote region.
| LVC | ||||
|---|---|---|---|---|
| Gene | Null Saline | Null Aliskiren | Null Valsartan | Null A+V |
| Ccl9, Ccl19 | ⬆* | ⬆* | ⬆* | ⬆* |
| Ccl7, Ccl11, Cxcl9, Il15, Pf4 | ⬇* | ⬇* | ⬇* | ⬇* |
| Mif | ⬆* | ns | ns | ns |
| Cx3cl1 | ⬆* | ⬆* | ns | ns |
| Tnfrsf1b | ⬆* | ⬆* | ⬆* | ns |
| Casp1 | ⬆* | ns | ⬆* | ns |
| Ccl8 | ⬆* | ns | ns | ⬆* |
| LVI | ||||
| Gene | WT Saline | WT Aliskiren | WT Valsartan | WT A+V |
| No changes | ||||
Arrows depict direction of change; black boxes show MI effect and white boxes show drug effect. n=6/group. A= aliskiren, V= valsartan.
p<0.05 vs. day 0
Figure 5 summarizes the biclustering analysis for the inflammatory genes, which was based on fold-change similarity patterns. For inflammatory genes in WT LVC (Figure 5, upper left panel), 5 genes contributed 80% of the overall inflammatory response in A (red background), 5 in V (yellow background), and 6 in A+V (blue background). The intersection of the three groups (black background) contained 2 genes (Ccl17 and Spp1) in common across treatments. Two genes (Cxcl13 and Xcr1) were affected only by A, two genes (Ccr4 and Il1f8) were affected only by V, and 4 genes (Ccr7, Cx3cl1, Il4, and Il13) were affected only by A+V. Only 1 gene (orange background; Il1r2) was affected by both A or V individually but not by A+V.
For inflammatory genes in WT LVI (Figure 5, lower left panel), 42 genes contributed 80% of the overall inflammatory response in A (red background) and 40 in V. The intersection of the three groups (black background) contained two genes (Cxcl13 and Spp1) in common across treatments. Because this was the same effect observed in the MMP-9 null LVI (Figure 5, lower right panel), this indicates that all of the effects on inflammation in the LVI were MMP-9 independent and that by day 28 post-MI the infarct is more stable than the remote region.
For inflammatory genes in null LVC (Figure 5, upper right panel), 10 genes contributed 80% of overall inflammatory response in A (red background), 2 in V (yellow background), and 6 in A+V (blue background). The intersection of the three groups (black background) contained only 1 gene (Spp1) in common across treatments. Similar to WT LVC, null LVC Il1f8 was regulated by V treatment in an MMP-9 independent manner. Aliskiren treatment regulated 7 genes (Ccl3, Ccl4, Cxcr3, Cxcr5, Il11, Itgam, and Lta) in null LVC that were different from those regulated by Aliskiren in WT (Cxcl13 and Xcr1), suggesting that Aliskiren has different effects depending on the presence or absence of MMP-9. This is consistent with A+V combination treatment, which also regulates a separate set of genes in null LVC (Ccl19, Cxcl13, and Il8rb) compared to WT LVC (Ccr7, Cx3cl1, Il4, and Il13).
Tables 4 and 5 lists only the up- and down-regulated inflammatory genes in the WT. Ccl11 was the only gene that was MI-dependent (it decreased post-MI) and not treatment influenced. Further, the mechanisms of action of the treatments were different depending upon whether the drug was given alone or in combination. For example, in the WT the decrease in Il15 was attenuated by A or A+V; the increase in Ccl4 was attenuated by A; and the increase in Ccr5 was attenuated in A but exacerbated in A+V. In general, A and V reduced inflammation, albeit through effect on different genes, while A+V amplified the inflammatory response. Overall, the inflammatory response to drug treatments showed significant diversity at day 28 post-MI for both WT and null groups, indicating inflammatory responses were MMP-9 dependent and drug specific.
Plasma proteomic profiling revealed TIMP-1 differences (Supplementary Table 7)
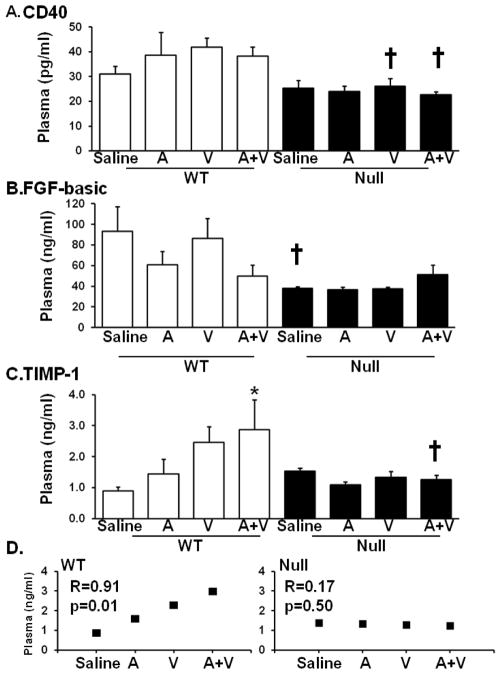
At d 28 post-MI, 3 plasma analytes were different among the groups (Figure 6). CD40 and FGFβ levels were both lower in the null saline MI mice compared to the WT saline MI mice. CD40 levels were decreased in all null groups compared to their respective WT groups and WT S group. TIMP-1 levels showed a step-wise increase in the WT MI mice, but not the null groups. The A+V WT group showed significantly increased levels compared to the saline MI WT group. A regression analysis showed a linear correlation between treatment groups and TIMP-1 levels (R=0.91, p=0.01); this correlation was not seen in the null mice.
Figure 6.
Plasma proteomic profiling performed at day 28 revealed 3 genes (CD40, FGF-β and TIMP-1) out of 59 that were significantly altered by drug treatment. (A) CD40 levels were lower in V and A+V null mice post-MI, compared to their respective WT MI. (B) FGF-β was significantly lower in the null saline group compared to the WT saline group. (C and D) TIMP-1 showed a positive linear correlation between drug treatments in the WT mice, but not the null mice. n=6–17/group, * p<0.05 compared to the saline control, † p<0.05 compared to the respective WT counterpart.
Discussion
The objective of this study was to evaluate whether aliskiren or valsartan, alone or in combination, had effects on LV remodeling through MMP-9 dependent mechanisms when provided 3 hours post-MI. The significant findings of this study were: (1) MMP-9 deletion improved survival post-MI; (2) Dilation and hypertrophy responses, but not EF, were altered by MMP-9 deletion or treatment, and all 3 treatments showed some beneficial responses; and (3) effects of treatments on extracellular matrix and inflammatory responses were primarily in the LV remote regions, and MMP-9 deletion altered the patterns of response.
MMP-9 deletion, but not drug treatments, increased post-MI survival. This effect on survival was not due to blood pressure differences, as systolic and diastolic blood pressures were not different among any of the groups. A number of large outcome trials, including the Valsartan in Acute Myocardial Infarction (VALIANT) Trial, showed that treatment with valsartan reduces all-cause mortality and morbidity in patients with MI [22, 23]. Part of this protection occurred through lowering the incidence of atrial fibrillation and reducing circulating levels of endothelial dysfunction markers [23]. The dose used in the VALIANT trial (80 mg/kg/day) was twice the dose used in the current study (40 mg/kg/day). Studies performed in rodents have shown varying effects of valsartan in post-MI remodeling, depending on the time when the treatment began post-MI, the dosage of the drug given, and duration of the treatment. Gervais and colleagues showed that treatment with valsartan at 50 mg/kg/day, given from day 7 post-MI for 6 weeks, limited the development of cardiac hypertrophy in Wistar rats [24]. Higashikuni et al showed that, in mice, valsartan at 25 mg/kg/day, given from 3–28 days post MI, ameliorated ventricular remodeling [25]. The previous studies and our own data suggest that the dosage and the timing of treatment have significant and differential effect on LV remodeling. These studies and a review by Cho and colleagues underscore that the appropriate dose for valsartan remains to be determined [26]. Our survival results showed improvement with MMP-9 deletion but not with the drug treatments. The echocardiography results, however, differentiate the groups. One note to make is that it is possible there is a survivor effect in this study; since more WT mice died post-MI than the nulls, the echo changes we see at day 28 may not be as dramatic as what we might see at day 3 or day 7 post-MI. Whether the change in remodeling seen through the differences in extracellular matrix and inflammatory gene responses translates to a different template for future events was not examined.
Post-MI, the fibroblast is the primary cell type that secretes collagen and other ECM components to form the infarct scar. Activated myofibroblasts are first seen at d 3 and are abundant by 1 wk post-MI in mice and humans [27–31]. Fibroblasts regulate remodeling by secreting ECM to influence myocardial scar structural properties. The Lijnen laboratory has shown that angiotensin II infusion increases cardiac fibroblast mediated collagen I and collagen III mRNA synthesis, and this increase was prevented by treatment with the angiotensin type I receptor inhibitor losartan [32]. Similar to this past report, Col1a1 and Col3a1 were upregulated in the WT saline group compared to day 0, and these increases were attenuated by MMP-9 deletion. The lower collagen deposition observed in the scar in the valsartan treated null group indicates that temporal and spatial collagen responses varied by genotype and treatment.
In the WT aliskiren treated remote region, Itga5 was higher and Itgax was lower compared to the saline MI group. These genes have previously been shown to be altered by AT1R and ACE inhibitors, which may help to explain the change in cardiac fibroblast ECM response [12, 33, 34]. These genes (Itga5 and Itgax) were not changed in the null mice treated with aliskiren compared to the saline MI control. This suggests that downstream regulation of ECM genes varies across drug treatments to produce quantitatively and qualitatively different infarct scars, which translates to different LV remodeling phenotypes.
Osteopontin (Spp1), connective tissue growth factor (Ctgf), and intercellular adhesion molecule 1 (Icam1) all increased in WT mice treated with valsartan. None of these increases were seen in the MMP-9 null mice treated with valsartan. These three proteins, interacting with MMP-9, could be the reason for worsened LV dysfunction in valsartan treated wild type group compared to valsartan treated null group. Spp1 (osteopontin) is a known MMP-9 substrate, although the consequence of osteopontin cleavage has not been explored [35].
Ctgf is upstream of transforming growth factor β signaling and could explain the increase in collagen III seen with valsartan treatment. We have previously shown that the expression of pro-fibrotic CTGF increases with age, and this increase was also diminished by MMP-9 deletion [36]. The increase in Icam1 suggests that valsartan may alter macrophage phenotype [14]. Meanwhile, the null valsartan group also showed the least accumulation of collagen, which may suggest differences in collagen organization or cell-matrix adhesion properties.
The inflammatory array results reveal both genotype and drug shifts during the remodeling landscape. Out of 84 inflammatory genes analyzed, none were significantly different in the WT infarct region. This is in contrast to the 5 genes (3 increased and 2 decreased) in the remote region, which suggests that by day 28 post-MI, the infarct region is becoming quiescent while the remote region is becoming active. Treatment effects may primarily be through influencing still viable myocytes in the remote region. This is consistent with the echocardiography data, where systolic function and hypertrophy were affected by treatment. Of note, single drug treatment strategies were more effective in further downregulating genes compared to the combination therapy strategy.
The macrophage is the primary cell type that regulates the chronic inflammatory phase post-MI [37]. Macrophages facilitate wound healing by engulfing necrotic myocytes and apoptotic neutrophils and secreting angiogenic molecules and growth factors to stimulate endothelial cells and activate fibroblasts. Chemoattractants locally produce, recruit, and confine macrophages to the injury site [38]. Activated macrophages, in turn, produce cytokines, chemokines, and proteases- including MMP-9 [39, 40]. Macrophage migration inhibitory factor (Mif) was not significantly changed in wild type compared to day 0 and its expression was upregulated in the saline and aliskiren treated null groups. This suggests that Mif is regulated both by MMP-9 as well as aliskiren treatment. The increased levels of Mif may cause delayed clearance of macrophages at day 28 post-MI which is consistent with higher expression of macrophages observed in aliskiren treated null group.
Our results highlight the role of MMP-9 in mediating drug effects on LV function, inflammation, and fibrosis. Aliskiren treatment yielded positive effects on cardiac function of both wild type and MMP-9 null mice post-MI and valsartan treatment had beneficial effects on cardiac function of MMP-9 null mice post-MI. Since aliskiren has been withdrawn from clinic use, renin intervention to treat MI may not be therapeutically useful. However, our results in mice provide mechanistic insight that the therapeutic effect of renin-angiotensin system inhibitors aliskiren and valsartan may be dependent, at least in part, on MMP-9.
Supplementary Material
Highlights.
We examined Aliskiren and Valsartan effects on MMP-9 dependent remodeling post-MI.
Drug effects depend on responses that are MMP-9 dependent.
Infarct and remote region responses persist through day 28 post-MI.
The main effects of the drug treatments were on the remote region.
Acknowledgments
Acknowledgements and Funding Sources
We acknowledge excellent technical support from Qiuxia Dai and funding support from the National Institutes of Health EB009496 and 1SC2 HL101430 to Y-FJ; and from Novartis, NIH/NHLBI HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center, HL051971 and R01 HL075360, the Max and Minnie Tomerlin Voelcker Fund, and the Veteran’s Administration (Merit) to MLL.
Footnotes
Disclosures
Novartis provided unrestricted funds for this study through a pre-clinical animal research agreement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaudron P, Kugler I, Hu K, Bauer W, Eilles C, Ertl G. Time course of cardiac structural, functional and electrical changes in asymptomatic patients after myocardial infarction: their inter-relation and prognostic impact. J Am Coll Cardiol. 2001;38:33–40. doi: 10.1016/s0735-1097(01)01319-5. [DOI] [PubMed] [Google Scholar]
- 2.Rugmani Padmanabhan Iyer NLP, Fields Gregg B, Lindsey Merry L. The history of matrix metalloproteinases: milestones, myths, and misperceptions. American Journal of Physiology - Heart and Circulatory Physiology. 2012;303:H919–H30. doi: 10.1152/ajpheart.00577.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, et al. Matrix-Dependent Mechanism of Neutrophil-Mediated Release and Activation of Matrix Metalloproteinase 9 in Myocardial Ischemia/Reperfusion. Circulation. 2001;103:2181–87. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- 4.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, et al. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;15:3063–70. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 5.Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J. Early Short-Term Treatment With Doxycycline Modulates Postinfarction Left Ventricular Remodeling. Circulation. 2003;108:1487–92. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 6.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H232–39. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 7.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, et al. Plasma Concentrations and Genetic Variation of Matrix Metalloproteinase 9 and Prognosis of Patients With Cardiovascular Disease. Circulation. 2003;107:1579–85. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 8.Kelly D, Khan SQ, Thompson M, Cockerill G, Ng LL, Samani N, et al. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008;29:2116–24. doi: 10.1093/eurheartj/ehn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamilpa RKR, Cigarroa J, IV, Dai Q, Escobar GP, Martinez H, Jimenez F, et al. CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. American Journal of Physiology Heart and Circulatory Physiology. 2011;300:H1418–26. doi: 10.1152/ajpheart.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller DN, Derer W, Dechend R. Aliskiren--mode of action and preclinical data. J Mol Med. 2008;86:659–62. doi: 10.1007/s00109-008-0330-6. [DOI] [PubMed] [Google Scholar]
- 11.Muller DN, Luft FC. Direct renin inhibition with aliskiren in hypertension and target organ damage. Clin J Am Soc Nephrol. 2006;1:221–8. doi: 10.2215/CJN.01201005. [DOI] [PubMed] [Google Scholar]
- 12.Stegbauer JLD, Seubert S, Ellrichmann G, Manzel A, Kvakan H, Muller DN, et al. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proceedings of the National Academy of Science. 2009;106:14942–47. doi: 10.1073/pnas.0903602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon SD, Skali H, Bourgoun M, Fang J, Ghali JK, Martelet M, et al. Effect of angiotensin-converting enzyme or vasopeptidase inhibition on ventricular size and function in patients with heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) echocardiographic study. American heart journal. 2005;150:257–62. doi: 10.1016/j.ahj.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara Y, Shiraya S, Miyake T, Yamakawa S, Aoki M, Makino H, et al. Inhibition of experimental abdominal aortic aneurysm in a rat model by the angiotensin receptor blocker valsartan. Int J Mol Med. 2008;22:703–8. [PubMed] [Google Scholar]
- 15.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–22. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin MD, Carter KJ, Jean-Philippe SR, Chang M, Mobashery S, Thiolloy S, et al. Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res. 2008;68:6251–9. doi: 10.1158/0008-5472.CAN-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindsey ML, Dobrucki LW, Bouges S, Mingoia JT, McClister DM, Jr, Su H, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocaridal infarction. American Journal of Physiology Heart and Circulatory Physiology. 2006;290:H232–39. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 18.Chiao YADQ, Zhang J, Lin J, Lopez EF, Ahuja SS, Chou YM, et al. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Ciculation: Cardiovascular Genetics. 2011;4:455–63. doi: 10.1161/CIRCGENETICS.111.959981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michael LH, Ballantyne CM, Zachariah JP, Gould KE, Pocius JS, Taffet GE, et al. Myocardial infarction and remodeling in mice: effect of reperfusion. Am J Physiol. 1999;277:H660–8. doi: 10.1152/ajpheart.1999.277.2.H660. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y, Church GM. Biclustering of expression data. Proc Int Conf Intell Syst Mol Biol. 2000;8:93–103. [PubMed] [Google Scholar]
- 21.Zheng F, Zeng YJ, Plati AR, Elliot SJ, Berho M, Potier M, et al. Combined AGE inhibition and ACEi decreases the progression of established diabetic nephropathy in B6 db/db mice. Kidney Int. 2006;70:507–14. doi: 10.1038/sj.ki.5001578. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer MA, McMurray JJV, Velazquez EJ, Rouleau J-L, Køber L, Maggioni AP, et al. Valsartan, Captopril, or Both in Myocardial Infarction Complicated by Heart Failure, Left Ventricular Dysfunction, or Both. New England Journal of Medicine. 2003;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 23.Black HR, Bailey J, Zappe D, Samuel R. Valsartan: more than a decade of experience. Drugs. 2009;69:2393–414. doi: 10.2165/11319460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Gervais M, Richer C, Fornes P, De Gasparo M, Giudicelli JF. Valsartan and coronary haemodynamics in early post-myocardial infarction in rats. Fundam Clin Pharmacol. 1999;13:635–45. doi: 10.1111/j.1472-8206.1999.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 25.Higashikuni Y, Takaoka M, Iwata H, Tanaka K, Hirata Y, Nagai R, et al. Aliskiren in combination with valsartan exerts synergistic protective effects against ventricular remodeling after myocardial infarction in mice. Hypertension research : official journal of the Japanese Society of Hypertension. 2012;35:62–9. doi: 10.1038/hr.2011.136. [DOI] [PubMed] [Google Scholar]
- 26.Cho YR, Kim YD, Park TH, Park K, Park JS, Baek H, et al. The impact of dose of the angiotensin-receptor blocker valsartan on the post-myocardial infarction ventricular remodeling: study protocol for a randomized controlled trial. Trials. 2011;12:247. doi: 10.1186/1745-6215-12-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovascular Research. 2000;46:250–56. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 28.Lijnen PJ, Petrov VV, Fagard RH. Induction of Cardiac Fibrosis by Transforming Growth Factor-[beta]1. Molecular Genetics and Metabolism. 2000;71:418–35. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 29.Willems I, Havenith M, De Mey J, Daemen M. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–75. [PMC free article] [PubMed] [Google Scholar]
- 30.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, et al. Altered fibroblast function following myocardial infarction. Journal of Molecular and Cellular Cardiology. 2005;39:699–707. doi: 10.1016/j.yjmcc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Lijnen PPV. Antagonism of the renin-angiotensin-aldosterone system and collagen metabolism in cardiac fibroblasts. Methods and Findings in Experimental & Clinical Pharmacology. 1999;21:215–27. doi: 10.1358/mf.1999.21.3.534832. [DOI] [PubMed] [Google Scholar]
- 33.Mervaala EMMD, Park JK, Schmidt F, Lohn M, Breu V, Dragun D, et al. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension. 1999;33:389–95. doi: 10.1161/01.hyp.33.1.389. [DOI] [PubMed] [Google Scholar]
- 34.Clarke NEFM, Porter KE, Lambert DW, Turner AJ. Angiotensin converting enzyme (ACE) and ACE2 bind integrins and ACE2 regulates integrin signalling. Public Library of Science (PLoS) One. 2012;7:e34747. doi: 10.1371/journal.pone.0034747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takafuji VFM, Unsworth E, Goldsmith P, Wang XW. An Osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene. 2007;26:6361–71. doi: 10.1038/sj.onc.1210463. [DOI] [PubMed] [Google Scholar]
- 36.Giles JT, Fert-Bober J, Park JK, Bingham CO, 3rd, Andrade F, Fox-Talbot K, et al. Myocardial citrullination in rheumatoid arthritis: a correlative histopathologic study. Arthritis Res Ther. 2012;14:R39. doi: 10.1186/ar3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–58. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin Shedding of Syndecan-1 Regulates Chemokine Mobilization and Transepithelial Efflux of Neutrophils in Acute Lung Injury. Cell. 2002;111:635–46. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Hashimoto S, Toyoda N, Nagai S, Yamazaki N, Dong HY, et al. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96:2584–91. [PubMed] [Google Scholar]
- 40.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, et al. An HMG-CoA Reductase Inhibitor, Cerivastatin, Suppresses Growth of Macrophages Expressing Matrix Metalloproteinases and Tissue Factor In Vivo and In Vitro. Circulation. 2001;103:276–83. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.