Abstract
Background
Acute kidney injury (AKI) increases the morbidity of critically ill children. Thus, it is necessary to identify better renal biomarkers to follow the outcome of these patients. This prospective case–control study explored the clinical value of a urinary biomarker profile comprised of neutrophil gelatinase lipocalin (uNGAL), fibroblast growth factor-2 (uFGF-2), and epidermal growth factor (uEGF) to follow these patients.
Methods
Urine samples were collected from 21 healthy children, and 39 critically ill children (mean age 7.5 years±6.97 SD) admitted to a pediatric intensive care unit with sepsis or requiring extra corporeal membrane oxygenation (ECMO). uNGAL, uFGF-2, and uEGF levels were measured using ELISA kits during the first 24 h of admission to PICU, at peak of illness, and upon resolution of the critical illness.
Results
On admission, the uNGAL and uFGF-2 levels were increased, and the uEGF levels were decreased, in critically ill children with AKI (n=19) compared to those without AKI (n=20), and healthy controls. A biomarker score using the combined cut-off values of uNGAL, uFGF-2, and uEGF (AUC=0.90) showed the highest specificity to identify children with AKI, relative to each biomarker alone. uNGAL and uFGF-2 on admission showed high sensitivity and specificity to predict mortality (AUC=0.82).
Conclusions
The biomarker profile comprised of uNGAL, uFGF-2, and uEGF increased the specificity to detect AKI in critically ill children, when compared to each biomarker used alone. uNGAL and uFGF-2 may also predict the risk of death. Further validation of these findings in a large sample size is warranted.
Keywords: Acute kidney injury, Neutrophil gelatinase lipocalin (NGAL), Fibroblast growth factor (FGF-2), Epidermal growth factor (EGF), Biomarkers, Sepsis
Introduction
Acute kidney injury (AKI) is considered an independent risk factor for mortality in critically ill children admitted to pediatric intensive care units [1–7]. The current management of these patients is supportive care directed toward reversing the underlying causes of AKI, avoiding nephrotoxic insults, preventing fluid overload, and providing continuous renal replacement therapy (CRRT) [8–11]. However, despite all of these interventions, the mortality and morbidity associated with AKI in critically ill children remains high [4]. In addition, the lack of a standard consensus definition of AKI and the unavailability of reliable biomarkers to detect the early stages of AKI, similar to troponin in acute myocardial infarction [11], also delays the diagnosis and early treatment of these patients [12–15]. A reduction in the glomerular filtration rate (GFR) remains the essential requirement for the clinical diagnosis of AKI. Nonetheless, the assessment of GFR based on the serum creatinine (SCr) levels may be an unreliable early indicator of AKI [16–18]. Therefore, it is necessary to find reliable biomarkers to detect the early stages of AKI and follow the clinical outcome of critically ill children.
Biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), and kidney injury molecule- 1 (KIM-1) have been tested in patients with AKI [19–22]. These biomarkers are considered sensitive to identify the early stages of AKI under different clinical conditions [19, 20, 23–25]. Nevertheless, in patients undergoing systemic infections or multi-organ failure, these biomarkers become less specific in predicting the development of AKI. NGAL is induced in many systemic inflammatory conditions, and is a critical component of the immunity to bacterial infections [23, 26, 27]. In fact, serum NGAL is not a specific predictor of AKI in critically ill children with septic shock [28]. In a similar manner, IL-18 is a pro-inflammatory cytokine that is elevated in all patients with sepsis [16, 21], and KIM-1, which is sensitive to identify patients with acute tubular necrosis [19, 29], shows a weak association with both the need for renal replacement therapy and mortality in patients with AKI. Overall, all of these features limit the ability of NGAL, IL-18, and KIM-1 to identify the early stages of AKI in critically ill children.
Altered renal perfusion and septic shock account for the majority of cases of AKI in critically ill children [1, 7, 8, 30, 31]. Thus, children with sepsis or multisystem organ failure, including those who require extracorporeal membrane oxygenation (ECMO), are at high risk of developing AKI. Recent evidence suggests that renal endothelial injury plays an important role in the pathogenesis of AKI under these clinical circumstances [31–33]. Interestingly, it is unclear whether the candidate biomarkers discussed above can be used to monitor the status of injured renal endothelial cells in critically ill children. Moreover, the release of angiogenic factors in the plasma of critically ill children can be associated with a worse clinical outcome [31]. Furthermore, studies done in experimental animal models of AKI induced by renal ischemia [34, 35] or lipopolysaccharide injections [36, 37], have shown that the administration of angiogenic factors, such as fibroblast growth factor −2 (FGF-2), can affect the outcome of AKI. In addition, previous studies have shown that the urinary levels of epidermal growth factor (EGF) can been used to predict and/or monitor the outcome of AKI, both in children [38–40] and adults [41]. Overall, all these studies suggest that NGAL in combination with FGF-2 and EGF might be a promising candidate biomarker profile to follow the renal outcome of children with AKI-induced by altered renal perfusion or septic shock.
Taking into consideration all of these findings, we performed the current prospective case–control study to test the hypothesis that a urinary biomarker profile comprised of elevated urinary levels of NGAL and FGF-2, and decreased urinary levels of EGF, can enhance our ability to identify the early stages of AKI in critically ill children with sepsis or requiring ECMO therapy. We selected NGAL because it is considered one of the most sensitive biomarkers of AKI in children with septic shock [22, 28] or altered renal perfusion [42]. We selected FGF-2 because it is an angiogenic growth factor released by injured endothelial cells that is involved in the regeneration of renal tubules [43, 44]. Finally, we measured the urinary levels of EGF because it is decreased in the urine of children undergoing acute [38–40] and chronic renal tubular injury [45–48]. In addition, since AKI is considered an independent risk factor for mortality in critically ill children, we conducted another prospective nested case–control study on the ability of these biomarkers to predict mortality.
Materials and methods
Patients
All study subjects were admitted to the pediatric intensive care unit (PICU) or pediatric cardiac intensive care unit (CICU) with septic shock or requiring ECMO. The study protocol was approved by the Institutional Review Board of the Children’s National Medical Center in Washington, DC. Children between the ages of 1 week to 21 years admitted to the PICU and CICU meeting criteria for sepsis or placed on ECMO were included. Seven percent of the children included in the study were less than 30 days old. Control samples were collected from 21 healthy children of similar age range who were seen for minor medical illnesses at the Children’s National Medical Center.
Most of the patients admitted to the ICU with sepsis or to be treated with ECMO had severe respiratory illnesses and respiratory failure, which subsequently lead to bacteremia and septic shock. The cause of sepsis in these children, mostly bacterial and few viral infections, was identified in approximately half of these patients. Some children presented with multi-system organ failure due to septic shock of unclear etiology, and others were estimated to have hospital-acquired infections that lead to a septic picture. The indications for ECMO treatment in these patients were severe respiratory failure refractory to mechanical ventilation or low cardiac output refractory to vasoactive-inotropic support. Other children were admitted to the ICU during the time frame of this study, but were not included in the study. These patients were diagnosed with cardiogenic shock, traumatic or anoxic brain injury, severe burns, acute respiratory failure, hematologic or oncologic emergencies, or needed intensive care for post-operative management of congenital heart diseases, and orthopedic, neurological, or general surgical complications.
Sepsis or sepsis-related syndrome in children (SIRS, sepsis, severe sepsis, septic shock) were defined by the American College of Chest Physician and Society of Critical Care Medicine modified for pediatric populations. Severity of illness scores were calculated by Pediatric Risk and Mortality (PRISM II) scores. Patients were treated with CRRT for fluid overload, oliguria/anuria, and/or renal failure. CCRT was initiated based on the criteria of each individual attending physician in the intensive care unit, on patients who became oliguric, required fluid removal, and/or developed AKI. Patients with known chronic renal diseases or urological malformations, known genetic syndromes with multiple anomalies, angiogenic or solid tumors that are known to increase the serum levels of angiogenic growth factors, and children with tumor lysis syndrome were excluded from the study.
AKI was defined by the pediatric Risk, Injury, Failure, Loss, and End Stage (pRIFLE) criteria created and validated by the Acute Dialysis Quality Initiative Group [12]. Patients were diagnosed with AKI if their estimated creatinine clearance (eCCL) decreased below 50 % of normal values and/or if their urine output was less than 0.5 cc/kg/h for more than 16 h in the absence of pre-existing renal disease. The eCCl was calculated using the Schwartz formula (eCrCl=k* height/serum creatinine) [49]. Serum creatinine values from the current hospitalization were used to determine the baseline eGFR. Clinical data were reviewed in all subjects enrolled in the study. Previous creatinine values and medical records were used to exclude patients with chronic renal failure. In infants under 30 days of age, the diagnosis of AKI was made using criteria previously established for newborns [50, 51].
Sample collection
Urine samples were collected on admission, peak of illness, and at illness resolution before the patients were discharged from the respective intensive care units (ICU). Urine samples collected from critically ill children on admission were used for calculating the sensitivity and specificity of each biomarker. Peak of illness was defined based on the highest requirement for vasoactive-inotropic drugs use, highest ventilation support needed, lowest urine output, and worse multi-systemic organ dysfunction. In eight patients who were clinically unstable, urine samples were collected daily at the estimated peak of illness, and subsequently the most severe day was identified retroactively. In the resolution phase of illness, urine samples were collected from all critically ill children who survived (n=29) prior to their discharge from the ICU. These samples were divided in two groups corresponding to the children who developed and did not develop AKI (n = 12 and 17, respectively). Urine samples collected on admission in children who died (n=10) were also compared to the samples collected from children who survived (n=29). All urine samples were centrifuged at 4,000 × g for 5 min and stored in 1-ml aliquots at −80° C until processed.
Measurement of urinary NGAL, FGF-2, and EGF
Urinary levels of NGAL, FGF-2, and EGF were measured by ELISA using commercially available kits. Briefly, urinary NGAL was measured with the NGAL Rapid ELISA Kit from BioPorto (Gentofte, Denmark), as previously described [22]. The ELISA microtiter plates were coated with a monoclonal antibody against human NGAL. These plates were blocked with buffer containing 1 % bovine serum albumin, coated with 100 μl of urine or standard buffer, and incubated with a biotinylated horseradish-peroxidase (HRP)-conjugated monoclonal antibody. The color-forming substrate TMB (3.3′, 5,5′-tetramethyl benzidine) was added for color development, and read after 30 min at 450 nm with a microplate reader. Inter-and intra-assay coefficient of variation for urinary NGAL was 3–12 %. The assessment of the urinary levels of FGF-2 and EGF was done as previously described [45], using ELISA Quantikine Kits from R&D Systems (Minneapolis, MN). Inter-and intra-assay coefficient of variation for FGF-2 and EGF were 3–9 % and 2–8 %, respectively.
Urinary creatinine levels were determined using the Parameter from R&D Systems, which is based on the Jaffe reaction. Results were expressed in ng (NGAL) or pg (FGF-2 and EGF) as a ratio of the urinary creatinine (UCr) value measured in mg/ml.
All measurements were made in duplicate and the investigators performing the assays were blinded to the sample sources and clinical outcomes.
Statistical analysis
Urinary levels of NGAL, FGF-2, and EGF were expressed as a ratio of urinary creatinine concentration. Data were analyzed using Stata for Windows, Graph Pad Prism 4, and MedCalc software. Urinary NGAL, FGF-2, and EGF levels were expressed as median and inter-quartile range, since the data were nonparametric in nature. The primary outcome variable was development of acute kidney injury per p-RIFLE criteria [5, 12, 13]. Categorical variables were compared using the Chi-square or Fisher’s exact test. Continuous variables were compared using Student’s t test when the data were normally distributed, and the Mann–Whitney U test when the distribution of the data was non-normal. The Kruskal–Wallis one-way analysis of variance test, followed by Dunn’s multiple comparison post hoc, was used to analyze samples from multiple groups that were not normally distributed. Accuracy and validity of each biomarker were assessed by estimating the sensitivity, specificity, positive predictive value, and negative predictive value. Receiver-operating characteristic (ROC) curves were generated for various cut-off points corresponding to the biomarkers under consideration. A perfect marker is expected to have a true-positive fraction = 1 and a false-positive fraction = 0. Multiple logistic regression analysis was performed to determine how the biomarkers selected performed together, as previously described [52]. In addition, we analyzed the results by combining the biomarkers into a score, as described before [53]. For each marker, one point was assigned if the measured value was greater than the corresponding cut-point, and zero points were assigned if it was lower. A cumulative biomarker score was calculated for each patient by adding the points for all three biomarkers combined (0–3).
Results
The clinical characteristics and treatment of all critically ill children included in the study are shown in Table 1. Nineteen out of the 39 critically ill patients included in the study developed AKI. Compared to the critically ill patients who did not develop AKI, those who developed AKI had increased severity of illness score (PRISM II score) and mortality (Table 1). All but three of these AKI patients were in septic shock. The remaining patients developed AKI in association with ECMO treatment and poor renal perfusion. Of the nineteen patients who developed AKI, seven children died prior to ICU discharge. Two of the 19 patients had persistent renal failure for >4 weeks (pRIFLE loss stage), ten patients had anuria/severe oliguria>24 h (pRIFLE renal failure stage), and the remaining ones reached the renal injury stage as defined by the pRIFLE criteria.
Table 1.
Characteristics of patient population
| Critically ill without AKI n=20 | Critically ill with AKI n=19 | p value | |
|---|---|---|---|
| Median age (years with IQR) | 3.5 (0.46; 8) | 14 (0.7; 17) | 0.17 |
| Gender (n, %) | |||
| Male | 8 (40 %) | 8 (43 %) | 1.00 |
| Female | 12 (60 %) | 11 (57 %) | |
| Race (n, %) | |||
| White | 2 (10 %) | 6 (32 %) | 0.12 |
| African American | 10 (50 %) | 10 (53 %) | 1.00 |
| Latin American | 8 (40 %) | 2 (11 %) | 0.06 |
| Asian American | 0 | 1 (5 %) | nd |
| PRISM II score Median (IQR) | 6.5 (4; 11) | 8.5 (7; 13.5) | 0.24 |
| Length of stay in ICU Median (IQR) | 18 (10.75; 25.5) | 16 (10; 25.5) | 0.54 |
| Serum creatinine Mean±SD, peak | 0.58±0.23 | 2.60±1.89 | < 0.001 |
| Non-survivors (n, %) | 3 (15 %) | 7 (37 %) | 0.15 |
| Required CRRT (n, %) | 0 (0 %) | 13 (68 %) | < 0.001 |
| Treated with ECMO (n, %) | 6 (30 %) | 4 (21 %) | 0.71 |
Results were analyzed using either the Mann–Whitney test, Fisher’s exact test, or unpaired t test
IQR inter-quartile range; ICU intensive care unit; CRRT continuous renal replacement therapy; ECMO extracorporeal membrane oxygenation; AKI acute kidney injury
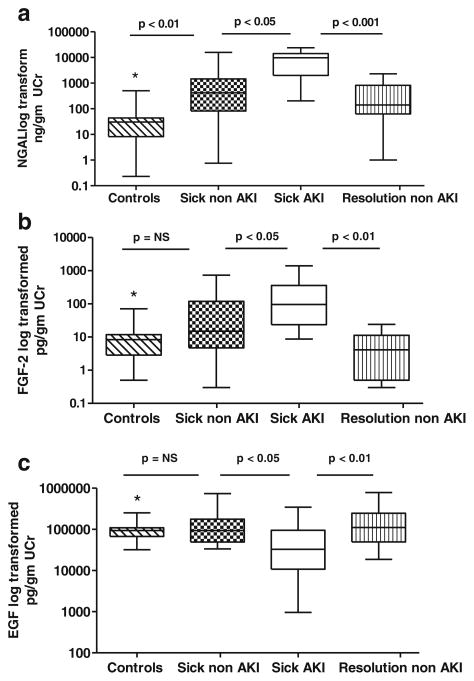
Urinary NGAL, FGF-2, and EGF levels were measured prospectively in 39 children on admission to the ICU. At this time point, the urinary NGAL levels were elevated in all samples collected from critically ill patients, compared to the samples collected from healthy controls (Fig. 1a). The urinary NGAL levels were also moderately increased in critically ill children who did not develop AKI (Fig. 1a), but significantly increased in those who developed AKI (Fig. 1a), when compared to the samples collected at illness resolution in children who did not develop AKI. On admission, the urinary NGAL levels were significantly different between critically ill children who developed AKI compared to those who did not (Fig. 1a). At peak of illness, the urinary NGAL levels were also elevated in samples collected from critically ill children with AKI (median 8,279, IQR 4,150–17,001 ng/mg UCr), when compared to those collected from critically ill children without AKI (median 1,498, IQR 82–9,482 ng/mg UCr; p=< 0.01), and to the urine samples collected at illness resolution in children who did not develop AKI (median=140, IQR 62–814 ng/mg UCr; p<0.001), or healthy controls (median=30, IQR 8–44 ng/mg UCr; p<0.001). The urinary NGAL levels decreased after reaching peak levels in both groups, as the patients recovered from their acute illnesses (Fig. 1a). Figure 2a shows the receiver-operating characteristic curve of NGAL in patients on admission to the ICU as a predictor of AKI. The area under the curve was 0.82 (95 % confidence interval (CI) 0.7040 to 0.9644), with an optimal cut-off value of 1,544 (sensitivity 84 % and specificity 80 %) (95 % CI for sensitivity and specificity=60.42 to 96.62 and 56.34 to 94.27, respectively).
Fig. 1.
Comparison of NGAL (a), FGF-2 (b), and EGF (c) values in urine samples collected from healthy children (controls), critically ill children without acute kidney injury (AKI) (sick non-AKI) on admission to the ICU, critically ill children with AKI (sick AKI) on admission to the ICU, and critically ill children who did not develop AKI at illness resolution prior to their ICU discharge (resolution non-AKI). All values are expressed as a ratio of the urinary creatinine (UCr). NGAL= *controls vs. sick AKI = p<0.001; *controls vs. resolution non-AKI = p=NS. FGF-2=*controls vs. sick AKI = p<0.01; *controls vs. resolution non-AKI = p=NS. EGF=*controls vs. sick AKI = p<0.05; *controls vs. resolution non-AKI = p=NS
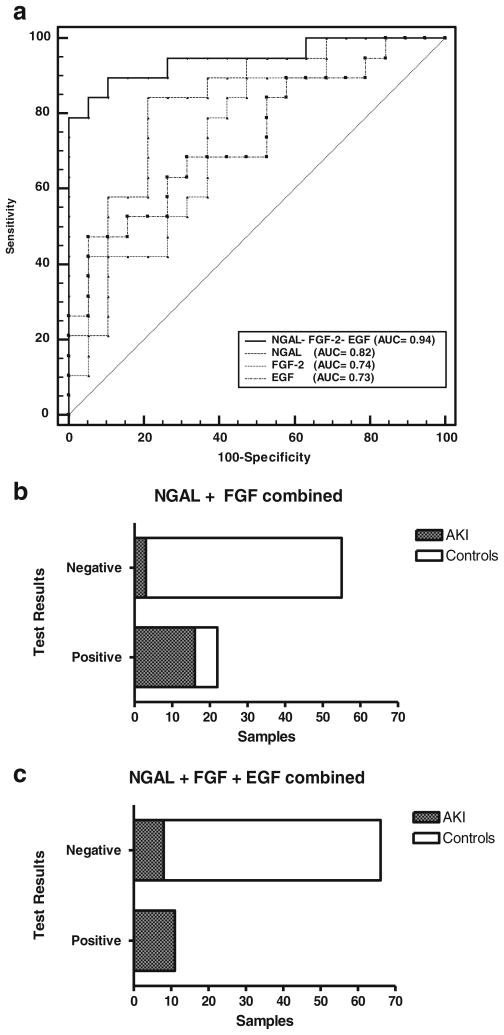
Fig. 2.
a Receiver-operating characteristic (ROC) curve and area under the curve (AUC) for neutrophil gelatinase lipocalin (NGAL), FGF-2, EGF, and all three biomarkers combined, demonstrate the predictive ability of each biomarker profile to detect acute kidney injury (AKI) in critically ill children admitted to the ICU with sepsis or requiring extracorporeal membrane oxygenation (ECMO). b Bar graph showing the results of a contingency table analysis combining urinary cut-off values of (NGAL) (548 ng/mg) and FGF-2 (15.55 pg/mg). A total of 58 control urine samples were collected from healthy controls (n=21), critically ill children without AKI (n=20), and critically ill children without AKI at illness resolution (n=17). A total of 19 urine samples were collected from critically ill children with AKI. c Bar graph showing the results of a contingency table analysis combining the urinary cut-off values of NGAL (548 ng/mg), FGF-2 (15.55 pg/mg), and EGF (31,598 pg/mg). A total of 58 control urine samples and 19 AKI urine samples were collected from the patients described above. All cut-off values are expressed as a ratio of the urinary creatinine (UCr)
In contrast, the FGF-2 levels measured on admission in critically ill children were statistically different only in those patients who developed AKI (Fig. 1b), when compared to healthy controls and the urine samples collected at illness resolution in children who did not develop AKI. The FGF-2 levels in the urine samples collected from healthy controls were not significantly different from those measured in critically ill children without AKI (Fig. 1b). However, the FGF-2 levels in children with AKI were significantly elevated compared to those without AKI (Fig. 1b). Similar results were obtained in samples collected at peak of illness. Briefly, at this time point, the difference between samples collected from critically ill children with and without AKI was statistically significant (sick AKI=median 70, IQR 21–316 vs. sick non-AKI=median 12.7, IQR 7.5–18 pg/mg UCr, respectively; p<0.001). We also found that the urinary FGF-2 values decreased as patients recovered from their critical illnesses (Fig. 1b). Figure 2a shows the receiving operating characteristic curve of FGF-2 in patients on admission to the ICU for the prediction of AKI. The area under the curve was 0.74 (95 % confidence interval 0.58 to 0.90), with an optimal cut-off value of 21.65 (sensitivity 79 %, specificity 63 %) (95 % CI for sensitivity and specificity=54.43 to 93.95 and 38.36 to 83.71, respectively).
On admission, the urinary EGF levels were decreased in critically ill children with AKI (Fig. 1c). In contrast, the urinary EGF levels measured in critically ill children without AKI were not significantly different when compared to healthy controls, or to the urine samples collected at illness resolution in children who did not develop AKI (Fig. 1c). However, the urinary EGF levels in critically ill children with AKI were significantly decreased when compared to all other groups (Fig. 1c). At the peak of illness, the urinary EGF levels were also significantly decreased in samples collected from critically ill children with AKI (sick AKI=-median 18,889, IQR 729–58,889), when compared to those collected from critically ill children without AKI (sick non-AKI=median 56,324, IQR 26,342–142,460 pg/mg, respectively; p<0.02), and to the urine samples collected at illness resolution in children who did not develop AKI (median 111,233, IQR 49,734–246,405; p<0.001). Like FGF-2, the urinary EGF values returned to normal levels as patients recovered from their critical illness (Fig. 1c). Figure 2a shows the receiving operating characteristic curve for the urinary EGF levels in patients on admission to the ICU for the prediction of AKI. The area under the curve was 0.73 (95 % interval 0.58 to 0.90), with an optimal cut-off value of 31,598 (sensitivity 47 %, specificity 94 %) (95 % CI for sensitivity and specificity=24.45 to 71.14 and 73.74. to 99.87, respectively).
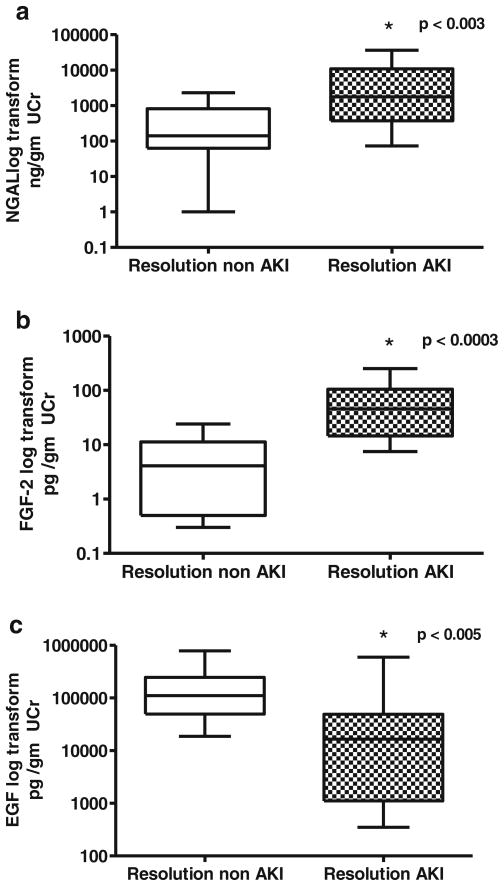
In summary, using the cut-off points for each biomarker described above, NGAL was the most sensitive biomarker to detect AKI in critically ill children, followed by FGF-2. These biomarkers identified approximately 95 and 89 % of the critically ill children with AKI, respectively. However, both biomarkers showed false-positive results in approximately 24 and 32 % of the critically ill patients without AKI, respectively. EGF alone detected approximately 58 % of AKI cases and 7 % of false-positive cases. The combined biomarker profile for urinary NGAL and FGF-2 improved the specificity for detecting AKI in critically ill children, relative to the use of each of these biomarkers alone. Approximately 15 % of the critically ill children tested for the combined biomarker profile showed false-positive results. A contingency table analysis using combined cut-off values of NGAL>584 ng/mg and FGF-2>15.5 pg/mg (Fig. 2b) showed that the sensitivity and specificity for detecting AKI were 84 and 89 %, respectively (95 % CI 0.60 to 0.96 and 0.49 to 0.89). Lastly, by combining all three biomarkers, the specificity for detecting AKI in critically ill children further improved. ROC analysis for all three biomarkers combined (Fig. 2a) shows that the AUC was 0.94 (95 % CI 0.81 to 0.99), 89 % sensitivity, 89 % specificity (95 % CI 66.9 to 98.7 for both), a positive predictive value of 0.85 (95 % CI 0.65 to 0.98), and a negative predictive value of 0.93 % (95 % CI 0.71 to 0.97) (assuming a 40 % prevalence of AKI in our patient population of critically ill children). In a similar manner, ROC analysis using the biomarker scores described in the Materials and methods section (range, 0–3), revealed an AUC=0.90 (95 % CI 0.77 to 0.97). As shown in Fig. 2c, all patients who tested positive for all three biomarkers (score of 3) developed AKI. In contrast, none of the urine samples collected from healthy controls (n=21), critically ill children without AKI (n=20), or critically ill children with normal renal function at illness resolution (n=17), received a score of 3. Finally, when urine samples collected at illness resolution from patients with and without AKI were compared, children with impaired renal function continued to show abnormal biomarkers levels (Fig. 3). These findings suggest that the selected biomarkers may be useful in clinical practice to monitor the renal outcome and recovery of these patients.
Fig. 3.
Neutrophil gelatinase lipocalin (NGAL) (a), FGF-2 (b), and EGF (c) values in urine samples collected from critically ill children at illness resolution immediately prior to their discharge from the ICU. Resolution non-acute kidney injury (AKI) group=urine samples collected at illness resolution in patients with normal serum creatinine (n=17). Resolution AKI group=urine samples collected at illness resolution from patients with abnormal serum creatinine (n=12). UCr=−urinary creatinine. *Non-parametric Mann–Whitney test
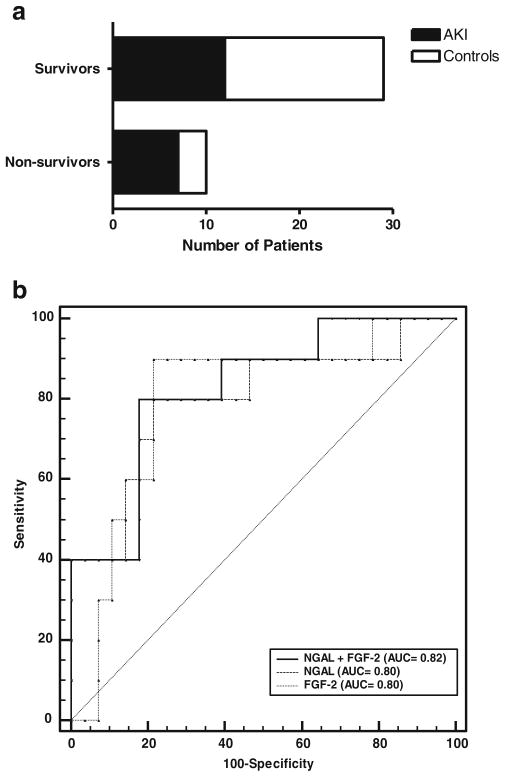
Out of the 39 patients included in this study, ten did not survive. The urinary marker values and characteristics of patients who survived vs. patient who did not survived is listed in Tables 2 and 3. The PRISM scores and mean serum creatinine levels were similar between survivors and non-survivors. Survivors have a longer length of stay in the ICU. CRRTs and ECMO requirement were elevated in non-survivors vs. survivors. The mortality rate in our critically ill patients with AKI was approximately 37 % versus 15 % in those who did not develop AKI (Table 1). We observed that critically ill children who died had increased FGF-2 and NGAL levels (p=0.005 and 0.006, respectively) when compared to patients who survived. ROC analysis to determine the sensitivity and specificity of these biomarkers to predict mortality showed similar AUCs for NGAL, FGF-2, and both biomarkers combined (Fig. 4b). For NGAL, with an optimal cut-off value of 6,808 ng/mg (sensitivity 80 %, specificity 78.6 %), the positive predictive value was 0.48 and the negative predictive value was 0.94 (assuming a mortality prevalence in our patient population of 20 %). For FGF-2, with an optimal cut-off value of 76.2 pg/mg (sensitivity 90 %, specificity 78.6 %), the positive predictive value was 0.52 and the negative predictive value was 0.96. For both biomarkers combined (Fig. 4b), the results were similar (sensitivity 80 %, specificity 82 %), and the positive predictive value was 0.52 and the negative predictive value was 0.94. These results persisted after adjusting for severity of illness scores (PRISM). In contrast, there were no significant differences in the urinary EGF and SCr. levels between patients who survived and those who did not (p=0.39 and 0.30 respectively) (Table 2).
Table 2.
Comparison of serum creatinine (SCr), and urinary neutrophil gelatinase lipocalin (NGAL), FGF-2, and EGF values measured on admission to the intensive care unit in survivors and non-survivors
| Critically ill (survivors) (n=29) | Critically ill (non-survivors) (n=10) | p valuea | |
|---|---|---|---|
| SCr (mg/ml) Mean±SD, peak |
1.53±1.78 | 1.59±1.37 | 0.30 |
| NGAL/uCr (ng/mg) Median (range) |
964 (238; 5,012) | 11,046 (4,014; 40,026) | 0.005 |
| FGF-2/uCr (pg/mg) Median (range) |
19.5 (9.5; 70.5) | 179 (106; 389) | 0.005 |
| EGF/uCr (pg/mg) Median (range) |
58,704 (28,232; 100,250) | 87,250 (26,681; 143,110) | 0.398 |
All results were analyzed using the non-parametric Mann–Whitney test
Table 3.
Characteristics of survivors and non-survivors
| Survivors (n=29) | Non-survivors (n=10) | p value | |
|---|---|---|---|
| Median age (years with IQR) | 2 (0.8; 14) | 8.5 (0.25; 16.8) | 0.85 |
| Gender (n, %) | |||
| Male | 11 (38 %) | 5 (50 %) | 0.70 |
| Female | 18 (62 %) | 5 (50 %) | |
| Race (n, %) | |||
| White | 7 (24 %) | 1 (10 %) | 0.60 |
| African American | 15 (52 %) | 5 (50 %) | 1.0 |
| Latin American | 7 (24 %) | 3 (30 %) | 0.63 |
| Asian American | 0 (0 %) | 1 (10 %) | nd |
| PRISM II score Median (IQR) | 9 (5; 12) | 7 (4.25; 11.25) | 0.46 |
| Length of stay in ICU Median (IQR) | 18.5 (16.5; 26) | 13 (10; 24) | 0.20 |
| Serum creatinine Mean ± SD, peak mg/dl | 1.6±1.4 | 1.5±1.8 | 0.30 |
| AKI (n, %) | 12 (41 %) | 7 (70 %) | 0.15 |
| Required CRRT (n, %) | 8 (27 %) | 4 (40 %) | 0.70 |
| Treated with ECMO (n, %) | 6 (21 %) | 4 (40 %) | 0.70 |
IQR inter-quartile range; ICU intensive care unit; AKI acute kidney injury; CRRT continuous renal replacement therapy; ECMO extracorporeal membrane oxygenation
Fig. 4.
a Mortality in all critically ill children admitted to the ICU with sepsis or requiring extracorporeal membrane oxygenation (ECMO). b Receiver-operating characteristic (ROC) curve and area under the curve (AUC) for neutrophil gelatinase lipocalin (NGAL), FGF-2, and both biomarkers combined, demonstrate the predictive ability of each bio-marker profile to detect mortality in critically ill children admitted to the ICU with sepsis and/or requirement ECMO treatment
Discussion
This pilot study explored the potential clinical value of using a novel urinary biomarker profile to identify AKI in critically ill children. We have shown that elevated urinary levels of NGAL and FGF-2, in combination with decreased urinary levels of EGF, constitutes a sensitive and specific biomarker profile to identify AKI in critically ill children. We also noticed that the changes in the selected urinary biomarkers positively correlates with the outcome of AKI, since as the kidney injury improved, these values were normalized. Finally, we found higher urinary levels of NGAL and FGF-2 in patients who did not survive. These findings suggest that both biomarkers might be useful to predict mortality in critically ill children, although considering the small sample size, a larger study will be required to validate this finding.
In agreement with the results of previous studies in patients with septic shock and other critical illnesses [22, 28], our pilot study suggests that urinary NGAL is a very sensitive but less specific biomarker to identify AKI in critically ill children with sepsis or treated with ECMO, since approximately 24 % of the critically ill children who did not develop AKI tested positive for NGAL. We found that the peak urinary NGAL levels were increased in children AKI, but we did not observe a rise in urinary NGAL levels consistently prior to AKI development in our critically ill patients. Overall, we observed a sixfold increase in median urinary NGAL concentration in critically ill children without AKI compared to controls. It is known that the synthesis and release of NGAL is increased in extrarenal tissues under several inflammatory conditions and that NGAL filtered in the kidney is excreted in the urine [23, 54, 55]. Thus, children with sepsis syndrome or multiorgan failure can show high urinary NGAL levels even in the absence of AKI [26]. NGAL filtered in renal glomeruli is reabsorbed by normal proximal tubular cells, and this process contributes to the increased urinary NGAL levels in children undergoing renal proximal tubular injury. In the kidney, however, NGAL is produced predominately in distal tubular epithelial cells, and the urinary levels of NGAL appear to correlate with the amount of NGAL that is synthesized in these cells [55].
In the current study, the urinary FGF-2 levels were elevated in critically ill children who developed AKI. This is consistent with our previous findings showing elevated urinary levels of FGF-2 in children with classic and atypical forms of HUS [44, 56]. Other studies done in different rodent models of acute kidney injury induced by renal ischemia [34, 35] or lipopolysaccharide injections [36, 37] also support the notion that FGF-2 plays a critical role in the outcome of AKI. In our study, FGF-2 was not as sensitive as urinary NGAL to detect AKI in critically ill children; however, the use of both biomarkers combined improved the specificity of this test to identify AKI, relative to the use of each biomarker alone. In addition, the urinary levels of EGF appear to be very specific, but less sensitive, to detect AKI. Moreover, when combined with FGF-2 and NGAL, EGF improved the specificity of the other two biomarkers combined to diagnose AKI. In clinical practice, the main reason to use biomarkers in children at risk of AKI is to identify and treat patients with AKI before their serum creatinine increases. Thus, to avoid unnecessary treatments in patients at risk, we need highly specific biomarkers for critically ill children. Overall, the combined biomarker profile described above appears to fulfill this goal. Finally, we also observed increased levels of NGAL and FGF-2 in critically ill children who died compared to those who survived. According to our findings, the urinary levels of NGAL and FGF-2 might be useful in assessing the severity of illness and/or to determine the risk of death in children admitted to the ICU with critical illnesses.
Our study has several limitations. First, the sample size was small, and we studied children who developed AKI due to sepsis of different etiologies or altered renal perfusion associated with ECMO. Secondly, we defined AKI by the pRIFLE criteria, which are based on the estimated creatinine clearance and urine output in children older than 1 month of age, however, approximately 7 % of our patients were younger than 1 month. In fact, in previous studies we noted that young children have higher urinary levels of NGAL, FGF-2, and EGF, relative to older children [44, 45, 56]. Nevertheless, even considering the absolute values adjusted to age in our patients, the differences detected between groups persisted, and the cut-off values selected were significantly different from the normal values corresponding to all age groups, including patients younger than 1 month of age. Alternatively, since the formula that we used to estimate the creatinine clearance was validated in children with steady-state renal function, we must recognize that the assessment of the eCCl in patients with AKI must be interpreted with caution. Thus, the possibility of misclassification of AKI using the pRIFLE criteria, or missing patients during the risk stage whenever previous eCrCl values are unavailable, needs to be considered as well. We were also unable to perform subgroup analysis focused on specific causes of AKI or pRIFLE categories due to the limited sample size, or to measure urinary biomarkers in patients who became anuric. The lack of consistency in defining acute kidney injury in the past 15 years also makes comparisons between various AKI studies difficult. Thus, taking into consideration all of these issues, we should acknowledge that further validation of the proposed biomarker profile would require a larger sample size and a multicenter study.
In conclusion, the combined biomarker profile comprised of increased urinary levels of NGAL and FGF-2 and decreased urinary levels of EGF appears to be a very specific test for detecting AKI in critically ill children, relative to the use of each of these biomarkers alone. Further validation of these biomarkers at the onset and upon resolution of AKI in the pediatric ICU can lead to better understanding of the mechanism of AKI, and might help determine the severity and duration of AKI. Finally, both NGAL and FGF-2 appear to be good candidates for predicting the risk of death in these patients. Nonetheless, considering the small sample size, a multicentered cohort study is needed to validate these results.
Acknowledgments
This manuscript was supported by National Institutes of Health Grants R0-1 HL-102497, R0-1HL 55605, R0-1 DK 049419, and U54 HD071601.
Footnotes
Conflict of interest
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Contributor Information
Kitman Wai, Division of Pediatric Critical Care, Children’s National Medical Center, Washington, DC, USA.
Ángel A. Soler-García, Division of Nephrology, Children’s National Medical Center, Washington, DC, USA. Research Center for Molecular Physiology, Children’s National Medical Center, Washington, DC, USA
Sofia Perazzo, Center for Genetic Medicine Research, Children’s National Medical Center, Washington, DC, USA.
Parnell Mattison, Division of Nephrology, Children’s National Medical Center, Washington, DC, USA.
Patricio E. Ray, Email: Pray@cnmc.org, Center for Genetic Medicine Research, Children’s National Medical Center, Washington, DC, USA. Children’s National Medical Center, Room 5543, 5th Floor, 111 Michigan Ave, NW, Washington, DC, USA. Division of Nephrology, Washington, DC, USA. Division of Pediatrics, Washington, DC, USA
References
- 1.Arora P, Kher V, Rai PK, Singhal MK, Gulati S, Gupta A. Prognosis of acute renal failure in children: a multivariate analysis. Pediatr Nephrol. 1997;11:153–155. doi: 10.1007/s004670050247. [DOI] [PubMed] [Google Scholar]
- 2.Williams DM, Sreedhar SS, Mickell JJ, Chan JC. Acute kidney failure: a pediatric experience over 20 years. Arch Pediatr Adolesc Med. 2002;156:893–900. doi: 10.1001/archpedi.156.9.893. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Bailey D, Phan V, Litalien C, Ducruet T, Merouani A, Lacroix J, Gauvin F. Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8:29–35. doi: 10.1097/01.pcc.0000256612.40265.67. [DOI] [PubMed] [Google Scholar]
- 5.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein SL, Devarajan P. Pediatrics: acute kidney injury leads to pediatric patient mortality. Nat Rev Nephrol. 2010;6:393–394. doi: 10.1038/nrneph.2010.67. [DOI] [PubMed] [Google Scholar]
- 7.Basu RK, Devarajan P, Wong H, Wheeler DS. An update and review of acute kidney injury in pediatrics. Pediatr Crit Care Med. 2011;12:339–347. doi: 10.1097/PCC.0b013e3181fe2e0b. [DOI] [PubMed] [Google Scholar]
- 8.Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD. Pediatric acute renal failure: outcome by modality and disease. Pediatr Nephrol. 2001;16:1067–1071. doi: 10.1007/s004670100029. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein SL. Acute kidney injury in children: prevention, treatment and rehabilitation. Contrib Nephrol. 2011;174:163–172. doi: 10.1159/000329394. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein SL, Devarajan P. Acute kidney injury in childhood: should we be worried about progression to CKD? Pediatr Nephrol. 2011;26:509–522. doi: 10.1007/s00467-010-1653-4. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein SL. Acute kidney injury biomarkers: renal angina and the need for a renal troponin I. BMC Med. 2011;9:135. doi: 10.1186/1741-7015-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 13.Askenazi DJ, Bunchman TE. Pediatric acute kidney injury: the use of the RIFLE criteria. Kidney Int. 2007;71:963–964. doi: 10.1038/sj.ki.5002238. [DOI] [PubMed] [Google Scholar]
- 14.Ricci Z, Cruz D, Ronco C. The RIFLE classification for acute kidney injury definition. Am J Surg. 2009;198:152–153. doi: 10.1016/j.amjsurg.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Plotz FB, Bouma AB, van Wijk JA, Kneyber MC, Bokenkamp A. Pediatric acute kidney injury in the ICU: an independent evaluation of pRIFLE criteria. Intensive Care Med. 2008;34:1713–1717. doi: 10.1007/s00134-008-1176-7. [DOI] [PubMed] [Google Scholar]
- 16.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 17.Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27:928–937. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 18.Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, Rogers K, Reid C, Tanner AJ, Easley KA. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 19.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 20.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 21.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 22.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu SY, Pauksen K, Venge P. Serum measurements of human neutrophil lipocalin (HNL) discriminate between acute bacterial and viral infections. Scand J Clin Lab Invest. 1995;55:125–131. doi: 10.3109/00365519509089604. [DOI] [PubMed] [Google Scholar]
- 24.Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36:S159–S165. doi: 10.1097/CCM.0b013e318168c652. [DOI] [PubMed] [Google Scholar]
- 25.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parravicini E, Nemerofsky SL, Michelson KA, Huynh TK, Sise ME, Bateman DA, Lorenz JM, Barasch JM. Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late-onset culture-positive sepsis in very low birth weight infants. Pediatr Res. 2010;67:636–640. doi: 10.1203/PDR.0b013e3181da75c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parravicini E. The clinical utility of urinary neutrophil gelatinase-associated lipocalin in the neonatal ICU. Curr Opin Pediatr. 2010;22:146–150. doi: 10.1097/MOP.0b013e3283369e78. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Washburn KK, Zappitelli M, Arikan AA, Loftis L, Yalavarthy R, Parikh CR, Edelstein CL, Goldstein SL. Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol Dial Transplant. 2008;23:566–572. doi: 10.1093/ndt/gfm638. [DOI] [PubMed] [Google Scholar]
- 30.Otukesh H, Hoseini R, Hooman N, Chalian M, Chalian H, Tabarroki A. Prognosis of acute renal failure in children. Pediatr Nephrol. 2006;21:1873–1878. doi: 10.1007/s00467-006-0240-1. [DOI] [PubMed] [Google Scholar]
- 31.Mankhambo LA, Banda DL, Jeffers G, White SA, Balmer P, Nkhoma S, Phiri H, Molyneux EM, Hart CA, Molyneux ME, Heyderman RS, Carrol ED. The role of angiogenic factors in predicting clinical outcome in severe bacterial infection in Malawian children. Crit Care. 2010;14:R91. doi: 10.1186/cc9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 33.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 34.Villanueva S, Cespedes C, Gonzalez A, Vio CP. bFGF induces an earlier expression of nephrogenic proteins after ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1677–R1687. doi: 10.1152/ajpregu.00023.2006. [DOI] [PubMed] [Google Scholar]
- 35.Villanueva S, Cespedes C, Gonzalez AA, Roessler E, Vio CP. Inhibition of bFGF-receptor type 2 increases kidney damage and suppresses nephrogenic protein expression after ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2008;294:R819–R828. doi: 10.1152/ajpregu.00273.2007. [DOI] [PubMed] [Google Scholar]
- 36.Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, Edwards CK, 3rd, Schuchman EH, Fuks Z, Kolesnick R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med. 1997;186:1831–1841. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattison PC, Soler-Garcia AA, Das JR, Jerebtsova M, Perazzo S, Tang P, Ray PE. Role of circulating fibroblast growth factor-2 in lipopolysaccharide-induced acute kidney injury in mice. Pediatr Nephrol. 2012;27:469–483. doi: 10.1007/s00467-011-2001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Liu W. Effect of asphyxia on urinary epidermal growth factor levels in newborns. J Tongji Med Univ. 1997;17:144–146. doi: 10.1007/BF02888289. [DOI] [PubMed] [Google Scholar]
- 39.Tsau YK, Sheu JN, Chen CH, Teng RJ, Chen HC. Decreased urinary epidermal growth factor in children with acute renal failure: epidermal growth factor/creatinine ratio not a reliable parameter for urinary epidermal growth factor excretion. Pediatr Res. 1996;39:20–24. doi: 10.1203/00006450-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Parwar P, Sonjara S, Ambalavanan N. Urine biomarkers predict acute kidney injury in newborns. J Pediatr. 2012;161(270–275):e271. doi: 10.1016/j.jpeds.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon O, Ahn K, Zhang B, Lockwood T, Dhamija R, Anderson D, Saqib N. Simultaneous monitoring of multiple urinary cytokines may predict renal and patient outcome in ischemic AKI. Ren Fail. 2010;32:699–708. doi: 10.3109/0886022X.2010.486496. [DOI] [PubMed] [Google Scholar]
- 42.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158(1009–1015):e1. doi: 10.1016/j.jpeds.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 43.Ku PT, D’Amore PA. Regulation of basic fibroblast growth factor (bFGF) gene and protein expression following its release from sublethally injured endothelial cells. J Cell Biochem. 1995;58:328–343. doi: 10.1002/jcb.240580307. [DOI] [PubMed] [Google Scholar]
- 44.Ray P, Acheson D, Chitrakar R, Cnaan A, Gibbs K, Hirschman GH, Christen E, Trachtman H. Basic fibroblast growth factor among children with diarrhea-associated hemolytic uremic syndrome. J Am Soc Nephrol. 2002;13:699–707. doi: 10.1681/ASN.V133699. [DOI] [PubMed] [Google Scholar]
- 45.Soler-Garcia AA, Rakhmanina NY, Mattison PC, Ray PE. A urinary biomarker profile for children with HIV-associated renal diseases. Kidney Int. 2009;76:207–214. doi: 10.1038/ki.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiley SC, Chevalier RL. Urinary biomarkers: the future looks promising. Kidney Int. 2009;76:133–134. doi: 10.1038/ki.2009.124. [DOI] [PubMed] [Google Scholar]
- 47.Gupta GK, Milner L, Linshaw MA, McCauley RG, Connors S, Folkman J, Bianchi DW. Urinary basic fibroblast growth factor: a noninvasive marker of progressive cystic renal disease in a child. Am J Med Genet. 2000;93:132–135. doi: 10.1002/1096-8628(20000717)93:2<132::aid-ajmg10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Grandaliano G, Gesualdo L, Bartoli F, Ranieri E, Monno R, Leggio A, Paradies G, Caldarulo E, Infante B, Schena FP. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 2000;58:182–192. doi: 10.1046/j.1523-1755.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 50.Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol. 2009;24:265–274. doi: 10.1007/s00467-008-1060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr. 1984;104:849–854. doi: 10.1016/s0022-3476(84)80479-5. [DOI] [PubMed] [Google Scholar]
- 52.Askenazi DJ, Montesanti A, Hunley H, Koralkar R, Pawar P, Shuaib F, Liwo A, Devarajan P, Ambalavanan N. Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J Pediatr. 2011;159(907–912):e901. doi: 10.1016/j.jpeds.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erdman LK, Dhabangi A, Musoke C, Conroy AL, Hawkes M, Higgins S, Rajwans N, Wolofsky KT, Streiner DL, Liles WC, Cserti-Gazdewich CM, Kain KC. Combinations of host bio-markers predict mortality among Ugandan children with severe malaria: a retrospective case–control study. PLoS One. 2011;6:e17440. doi: 10.1371/journal.pone.0017440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlson M, Raab Y, Seveus L, Xu S, Hallgren R, Venge P. Human neutrophil lipocalin is a unique marker of neutrophil inflammation in ulcerative colitis and proctitis. Gut. 2002;50:501–506. doi: 10.1136/gut.50.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin CS, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17:216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ray PE, Liu XH, Xu L, Rakusan T. Basic fibroblast growth factor in HIV-associated hemolytic uremic syndrome. Pediatr Nephrol. 1999;13:586–593. doi: 10.1007/s004670050749. [DOI] [PubMed] [Google Scholar]