Abstract
Purpose
Osteoporosis is a severe complication of spinal cord injury (SCI). Many exercise modalities are used to slow bone loss, yet their efficacy is equivocal. This study examined the effect of activity-based therapy (ABT) targeting the lower extremities on bone health in individuals with SCI.
Methods
Thirteen men and women with SCI (age and injury duration = 29.7 ± 7.8 and 1.9 ± 2.7 years) underwent 6 months of ABT. At baseline and after 3 and 6 months of training, blood samples were obtained to assess bone formation (serum procollagen type 1 N propeptide (PINP) and bone resorption (serum C-terminal telopeptide of type I collagen (CTX), and participants underwent dual-energy X-ray absorptiometry scans to obtain total body and regional estimates of bone mineral density (BMD).
Results
Results demonstrated significant increases (p < 0.05) in spine BMD (+4.8 %; 1.27 ± 0.22–1.33 ± 0.24 g/cm2) and decreases (p < 0.01) in total hip BMD (−6.1 %; 0.98 ± 0.18–0.91 ± 0.16 g/cm2) from 0 to 6 months of training. BMD at the bilateral distal femur (−7.5 to −11.0 %) and proximal tibia (− 8.0 to −11.2 %) declined but was not different (p > 0.05) versus baseline. Neither PINP nor CTX was altered (p> 0.05) with training.
Conclusions
Chronic activity-based therapy did not reverse bone loss typically observed soon after injury, yet reductions in BMD were less than the expected magnitude of decline in lower extremity BMD in persons with recent SCI.
Keywords: Osteoporosis, Paralysis, Exercise training, Bone turnover, Bone mineral density, Spinal cord injury
Introduction
A serious complication of spinal cord injury (SCI) is marked reductions in bone mineral density (BMD) in the initial 6 months post-injury (Garland et al. 2001) which is greatest in weight-bearing sites such as the tibia and femur (Dauty et al. 2000). Bone loss enhances fracture onset, causing severe clinical, psychological, and financial complications. Therefore, early intervention to slow bone loss, reduce fracture risk, and improve health status in individuals with SCI is essential.
Many exercise modes have been used to slow bone loss, yet their efficacy is equivocal. For example, previous studies (Belanger et al. 2000; Chen et al. 2005) conducted in persons with chronic SCI varying in injury severity revealed increased BMD at the knee after 6 months of functional electrical stimulation exercise (FES) performed 2.5–5.0 h/week. Similar enhancements in BMD were also revealed in individuals completing FES (Mohr et al. 1997; Frotzler et al. 2008) as well as body weight supported-treadmill training (Coupaud et al. 2009). In contrast, other investigations using these modalities of exercise demonstrated a maintenance (Eser et al. 2003; Giangregorio et al. 2006) or reduced BMD (Forrest et al. 2008) in response to chronic training. However, Clark et al. (2007) reported that single mode exercise by itself is unable to slow bone loss in persons with SCI, which merits investigation into potential benefits of multiple exercise modalities on bone status in this population.
Although low BMD is one of the strongest risk factors for fracture (Chen et al. 2005), Bauman et al. (2005) cited limitations of DXA to assess fracture risk and changes in bone status in response to intervention such as exercise training. To better understand changes in bone status, various biomarkers can be used to assess changes in bone formation and bone resorption. In a recent review, Vasikaran et al. (2011) identified human serum procollagen type 1 N propeptide (PINP) and serum C-terminal telopeptide of type I collagen (CTX) as the preferred reference standards for assessing bone formation and bone resorption due to widespread assay availability, their ability to detect intervention-induced changes in bone status prior to BMD changes, and knowledge of these markers’ variability in human samples. Although their application is relatively widespread in able-bodied individuals such as women with osteoporosis, only a few studies have addressed changes in bone turnover in individuals with SCI. Bauman et al. (2005 reported similar PINP values in persons with chronic SCI versus able-bodied subjects, and moreover, PINP did not change with long-term ingestion of a vitamin D analog. With the exception of one study (Gordon et al. 2013) showing no effects of gait training on PINP and CTX in chronic SCI, changes in bone turnover in individuals with SCI undergoing exercise training are not well understood.
To date, no study has examined the effects of multimodal exercise on bone health in persons with SCI. Frost (1987) stated that bone requires a sufficient threshold amount of strain to promote bone growth. Furthermore, it has been reported that 6–12 months of high volume (4–5 h/week) FES training (Chen et al. 2005; Frotzler et al. 2008) significantly increases knee BMD in this population. Whether completion of multiple training modes focusing on loading of the lower extremities, as utilized in activity-based therapy (ABT) (Sadowsky and McDonald 2009), elicits a positive effect upon bone is unknown. Consequently, this novel study examined the effect of 6 months of ABT on BMD and bone turnover in men and women with SCI. It was hypothesized that 6 months of ABT would increase bone formation markers versus baseline, and that spine BMD would be increased with no change demonstrated in hip or knee BMD. Data from this study may potentially alter rehabilitation practices and modify the design of future studies using exercise as a strategy to slow osteoporosis in persons with SCI.
Methods
Participants
Adult men and women with acute (<3 year post-injury, n = 11) and chronic (>3 year post-injury, n = 2) SCI were recruited to participate in this investigation. This classification was used as bone seems to reach a steady-state approximately 3-year post-injury (Eser et al. 2004). Their physical characteristics are demonstrated in Table 1. Five individuals were identified as Caucasian, three as Hispanic, three as Middle Eastern, and two as African-American. To be eligible, subjects met the following inclusion criteria: completion of no formal ABT in the previous year, complete or incomplete SCI, injury level at or lower than C2, non-ventilator dependent, and physician’s permission to engage in an intense exercise program. Prospective participants were excluded if they had completed formal rehabilitation in the preceding year, lacked the physical function to complete training or had excess pain, were taking medications that alter bone health other than calcium or vitamin D supplements, had medical conditions besides paralysis that alter bone metabolism, such as diabetes or hyperthyroidism, were peri- or post-menopausal, suffered an acute infection or illness, or experienced previous upper or lower-body extremity fractures. After providing their health-history via a brief survey, they provided informed consent to participate in the study, which was approved by the University Institutional Review Board.
Table 1.
Participant baseline characteristics
| Subject | Age (year) | Mass (kg) | Injury duration (year) | Injury level | Severity | Completeness | PA (h/week) |
|---|---|---|---|---|---|---|---|
| Ma | 19.0 | 53.9 | 0.6 | C5–C6 | T | C | 4.6 |
| Mb | 29.0 | 73.4 | 2.9 | T10 | P | C | 8.0 |
| M | 40.0 | 101.0 | 1.0 | C4 | T | C | 9.0 |
| M | 36.0 | 80.1 | 0.5 | T12 | P | C | 7.3 |
| F | 23.0 | 90.9 | 0.7 | T12 | P | C | 13.0 |
| M | 23.0 | 79.1 | 0.3 | C5–C6 | T | I | 6.0 |
| M | 41.0 | 79.2 | 0.8 | T3–T4 | P | C | 4.7 |
| M | 30.0 | 61.4 | 9.3 | C4 | T | I | 6.0 |
| M | 29.0 | 78.0 | 4.5 | T12 | P | I | 10.0 |
| F | 23.0 | 55.5 | 0.6 | T3–T4 | P | I | 8.0 |
| Mc | 30.0 | 89.4 | 0.9 | C5–C6 | T | I | 12.0 |
| M | 42.0 | 67.0 | 0.2 | L1 | P | C | 6.0 |
| M | 29.0 | 62.7 | 1.2 | T8 | P | I | 8.0 |
| Mean ± SD | 29.4 ± 7.8 | 75.7 ± 14.4 | 1.9 ± 2.7 | NA | NA | NA | 7.8 ± 2.7 |
M male, F female, T tetraplegic, P paraplegic, C complete, I incomplete, PA hours of activity-based therapy per week
Withdrew from the study at 5 months to attend college out of state
Withdrew from the study at 1.5 months due to unrelated leg injury
Withdrew from the study at 1 month due to unrelated injury
Design
Participants with SCI initiated 6 months of intense training at a local activity-based therapy rehabilitation center. During a single session at baseline and at 3 and 6 months, they underwent dual-energy X-ray absorptiometry (DXA) scans to determine bone mineral density at various sites. In addition, blood samples were obtained to measure changes in bone turnover and a 4-day food log was completed. Time of day was standardized within subjects across all trials. All training was supervised by experienced personnel and targeted regions below the level of injury. Compliance to training was monitored by staff at the facility on a daily basis.
Activity-based training
Participants performed 2–3 h/day of activity-based therapy (ABT) targeting regions below the level of injury (80 % for those with quadriplegia and 100 % for paraplegia) a minimum of 2 day/week for 6 months at the facility. We (Harness and Astorino 2011) recently showed that acute completion of this regimen elicits intensities ranging from 1.9 to 3.2 SCI METs which is similar to that reported for circuit training and resistance exercise (Collins et al. 2010) yet lower than evoked from arm ergometry or wheelchair ambulation (Perret et al. 2010). Activity-based therapy promotes activation of the neurological levels located both above and below the injury level using rehabilitation therapies (Sadowsky and McDonald 2009) and was previously shown (Harness et al. 2008) to enhance motor gains in persons with chronic SCI. This high volume of training has been previously shown to alter bone mass in persons with acute and chronic SCI (de Bruin et al. 1999; Frotzler et al. 2008). Training was individualized for each client based on their baseline function, and progression was instituted daily based on participant tolerance to training and acquisition of gains. Over the 6 month study, time performing active assistive exercises and passive gait training generally decreased, while time performing resistance training and active gait training, which present greater skeletal loading, increased. Load bearing progressed from more supportive exercises (i.e., elbows and knees or using a standing frame) to less supportive (i.e., high kneeling or using parallel bars to stand).
During the study, ABT consisted of the following modalities: active assistive exercise (Yang and Gorassini 2006) was completed up to 1.5 h/week either supine or prone depending on the exercises performed. It consisted of helping the subject through different ranges of motion and providing a resistance less than gravity. Subjects were asked to attempt to actively assist or resist the movement performed. Upper/lower body and core resistance training was performed 1.5–2.0 h/week with the specific exercise and resistance dependent on the client’s functional ability. A concentric or eccentric contraction was performed against gravity or a resistance greater than gravity. During load bearing which was performed 1.5 h/week, the hands/elbows and/or feet/knees remained in contact with the ground and body weight (or a percentage) was supported through the extremities (Dietz et al. 2002). During cycle ergometry, clients completed 15–30 min of continuous exercise on a hand cycle (SCIFIT, Tulsa, OK, USA) or stationary bicycle (StarTrac V-Bike, Irvine, CA, USA) at various intensities (Dietz et al. 2002). Gait training included several modes of assisted and unassisted walking (Maegele et al. 2002) as well as body weight-supported mechanized elliptical training as recently employed (Harness et al. 2008). Once a subject was able to initiate stepping, they were transitioned to body weight supported-treadmill training and overground walking. At this time, subjects were assisted by up to four specialists based on their ability to control their upper body as well as their lower extremities. Passive gait training occurred for 15–30 min/week in five individuals and active gait training occurred 15–60 min/week in eight individuals. Vibration training was completed 10–30 min/ week during which any part of the body contacted the vibration platform (Power Plate NA, Inc., Northbrook, IL, USA) (van Nes et al. 2004). This exercise mode stimulates neuromuscular activity of the engaged limb. Lastly, functional electrical stimulation of the quadriceps, gluteals, and hamstrings was completed approximately 30 min/week on an FES bike (RT300, Restorative Therapies Inc., Baltimore, MD, USA). Amplitude, pulse width, and frequency were set on an individual basis based on each subject’s response to the stimulation.
Dual-energy X-ray absorptiometry (DXA)
Total-body bone mineral content (BMC) and bone mineral density (BMD) as well as regional determinations at the lumbar spine (L1–L4), proximal femur, and knee were obtained using DXA (software version 13.5, Lunar Prodigy Advance, GE Healthcare, Madison, WI, USA). Regions of interest from the proximal femur scan included total hip, femoral neck, and greater trochanter. Regions of interest from the knee scan included proximal tibia and distal femur. Analyses were performed by the same technician who followed standard quality control procedures developed by the manufacturer. Coefficients of variation (CV) for the lumbar spine, proximal femur, and knee BMD in our lab are less than 1.0 %, and for whole-body BMD, 1.2 %, respectively.
Initially, the subject was placed on the bone scanner for a few minutes to minimize onset of muscle spasm. They were instructed to remain still, while total-body BMD, bilateral proximal femur, and lumbar spine measurements were obtained according to the manufacturer’s specifications. Lastly, bilateral knee BMD was assessed following an existing protocol developed by Shields et al. (2005) for individuals with SCI. Leg length was measured from the distal border of the greater trochanter of the hip to the distal end of the femur. Using lumbar spine scan mode, the laser was positioned at the mid-tibia at a distance = 30 % of the leg length, below the knee joint. Procedures for analyzing proximal tibia and distal femur BMD followed an established protocol (Shields et al. 2005). Total body mass (in kg) was assessed as the sum of fat mass, lean mass, and BMC from the whole-body scan. Bone status at all sites was expressed in absolute units (g/cm2 for BMD and g for BMC) as well as using a mean percent change (% across both limbs) score from 0 to 6 months of training.
Bone turnover markers
A fasting blood sample (10 mL) was obtained from the antecubital vein (21 G × 1.25 in BD Eclipse™ Vacutainer® Holder, Becton–Dickinson and Company, Franklin Lakes, NJ, USA) to assess markers of bone formation (PINP) and bone resorption (CTX). These samples were placed into a collection tube that was inverted five times to promote clotting. Tubes were then centrifuged at 1,200g for 10 min (Fisher HealthCare Model 614B, Hanover Park, IL, USA). Serum aliquots were placed into 2 mL Eppendorf tubes and frozen at −80 °C for later analysis by ELISA using commercially available kits (Cusabio, Hubei Province, China for PINP and IDS PLC, Scottsdale, AZ for CTX). All samples were run in duplicate using ≤2 kits from the same batch, and the mean concentration was reported. Intraassay and interassay coefficient of variations were equal to ≤8 and 10 % for PINP and ≤6 and 10 % for CTX. The detection range for these assays was 0.2–1.2 ng/mL for PINP and 0–2.5 ng/mL for CTX. Changes in these biomarkers were expressed in ng/mL for CTX and PINP. Complete data were recorded for eight participants, as three individuals dropped out of the study (see “Results”) and blood could not be drawn in two individuals at one time-point of the study.
Assessment of dietary intake
Participants were required to complete a 4-day food log (including 2 weekend days) at baseline and at 3 and 6 months. They were encouraged to actively denote all food and drink ingested (including supplements) during this period with specific instructions to describe method of preparation, portion sizes, and brands where applicable. This information was confirmed during each visit and used to determine total caloric as well as macronutrient (fat, carbohydrate, and protein in g) and calcium (in mg) intake using a commercially-available website (http://ndb.nal.usda.gov/ndb/foods/list).
Data analysis
Data are reported as mean ± standard deviation (SD) and were analyzed using SPSS Version 20.0 (Chicago, IL, USA). Initially, normality of all variables was examined. One-way analysis of variance with repeated measures was used to examine changes in all variables in response to training. The Greenhouse-Geisser correction was used to account for the sphericity assumption. If a significant F ratio was obtained, Tukey’s post hoc test was used to identify differences between means. Partial eta-squared (η2) was used as an estimate of effect size. Statistical significance was established as p < 0.05.
Results
Due to onset of injury unrelated to training (n = 2) and choice to leave the region and initiate college out of state (n = 1), only ten men and women with SCI completed the training protocol. Data from subjects who dropped out of the study remained in all subsequent analyses where applicable. Compliance to training (minimum of at least 2 h/days for 2 days/week, mean volume = 7.9 ± 2.6 h/week of ABT) was equal to 100 % during the study. As two male participants had chronic SCI (4.5 and 9.3 years, respectively), their changes in BMD and bone turnover were much different than remaining subjects who were more in the acute phase of SCI during which bone loss may occur for up to 3-year post-injury (Eser et al. 2004). As participants were comprised of both tetraplegics and paraplegics with complete and incomplete injury, ANOVA was also used to examine if injury severity or level altered resultant responses to chronic ABT. For all analyses, these results were similar to those displayed for the entire population, and no training X group interactions or between-group differences in any variable were revealed.
Changes in total-body and regional BMD and BMC
Total-body BMD declined by 2.5 % (p = 0.003, η2 = 0.47, range −4.9 to 1.8 %) from 0 to 6 months which was accompanied by significant reductions (−6.1 %) in total hip BMD (p = 0.001, η2 = 0.59) at both 3 and 6 months. BMD at the right femoral neck (−5.2 %, p = 0.001 for right and p = 0.04 for left, η2 = 0.46) consistently declined from 0 to 6 months; whereas, left femoral neck BMD declined from 0 to 3 months after which it was maintained. Right trochanter BMD at 6 months was significantly lower (p < 0.05) than values at 0 and 3 months, although there was no difference from 0 to 3 months. In contrast, BMD at the left trochanter declined from 0 to 3 and then at 6 months (−7.4 %, p = 0.01 and 0.001 for right and left, η2 = 0.44). Total-body BMC decreased by 2.2 % during the study (p = 0.07). Spine BMD increased by 4.8 % (p = 0.006, η 2 = 0.44, range 0.9–15.8 %) at 6 months of training compared to baseline. These data are demonstrated in Table 2.
Table 2.
Changes in bone mineral density and bone mineral content in response to training
| Parameter | Baseline | 3 months | 6 months |
|---|---|---|---|
| Total-body BMD (g/cm2) | 1.244 ± 0.18 | 1.224 ± 0.17* | 1.213 ± 0.16* |
| Right total hip (g/cm2) | 0.96 ± 0.20 | 0.93 ± 0.18* | 0.90 ± 0.17*,# |
| Left total hip (g/cm2) | 0.99 ± 0.17 | 0.95 ± 0.17* | 0.92 ± 0.15*,# |
| Right femoral neck (g/cm2) | 0.98 ± 0.16 | 0.96 ± 0.15* | 0.93 ± 0.14*,# |
| Left femoral neck (g/cm2) | 0.99 ± 0.17 | 0.96 ± 0.17* | 0.95 ± 0.14* |
| Right trochanter (g/cm2) | 0.75 ± 0.21 | 0.73 ± 0.19 | 0.70 ± 0.16*,# |
| Left trochanter (g/cm2) | 0.79 ± 0.18 | 0.75 ± 0.16* | 0.72 ± 0.15* |
| Spine BMD (g/cm2) | 1.27 ± 0.22 | 1.31 ± 0.21* | 1.33 ± 0.24* |
| BMC (g) | 3 317.9 ± 884.8 | 3 253.0 ± 775.9 | 3 243.7 ± 759.5 |
| Right distal femur BMD (g/cm2) | 0.92 ± 0.28 | 0.87 ± 0.34 | 0.82 ± 0.31 |
| Left distal femur BMD (g/cm2) | 0.92 ± 0.28 | 0.88 ± 0.29 | 0.85 ± 0.27 |
| Right proximal tibia BMD (g/cm2) | 0.88 ± 0.35 | 0.85 ± 0.39 | 0.81 ± 0.37 |
| Left proximal tibia BMD (g/cm2) | 0.89 ± 0.40 | 0.85 ± 0.33 | 0.79 ± 0.30 |
BMD bone mineral density, BMC bone mineral content
p < 0.05 from baseline
p < 0.05 from 3 months
Due to complications with the scan mode early in the study, knee scans were not usable in a few participants, so complete knee BMD data were obtained from only seven individuals. BMD at the distal femur and proximal tibia declined, but these differences were not significant (p = 0.13 and 0.29) (Table 2).
Individual responses
A 1.0 % change in BMD (greater than our DXA CV) is considered a clinically significant change and has implications for fracture risk (Black et al. 1992). Frequency of participants exhibiting clinically significant reductions in BMD was equal to 70 % for the total hip and 80 % for the femoral neck. Nine of 10 participants showed meaningful increases in spine BMD in response to training. The two participants with chronic SCI showed increased total body (1.0 and 1.8 %), total femur (0.5 and 1.3 %), and trochanter BMD (2.6 and 6.8 %) in response to training. Only one participant with acute SCI, a 23-year-old woman with complete SCI at T12, revealed minimal change in BMD at the total hip (−0.6 %) with training, yet other individuals revealed mean percent change equal to −8.4 %. These data are demonstrated in Figs. 1 and 2.
Fig. 1.
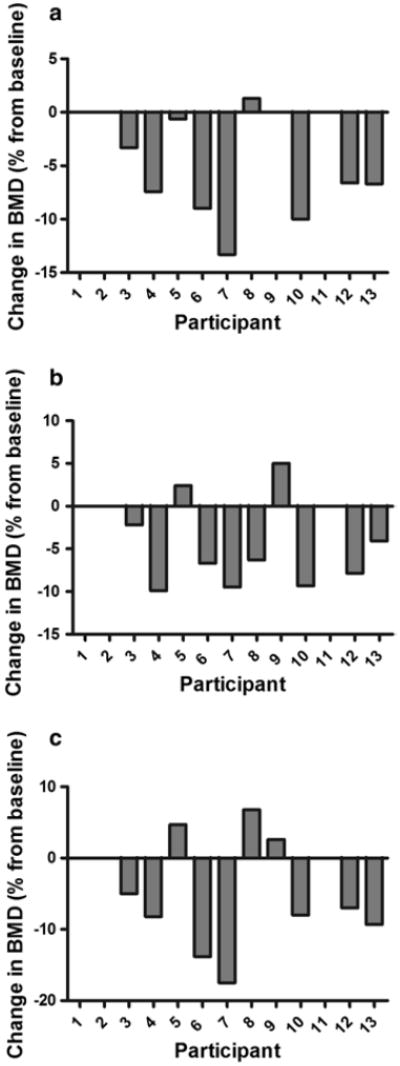
Individual changes in BMD at the a total hip, b femoral neck, and c greater trochanter in response to 6 months of activity-based therapy. Note that participants 8 and 9 have chronic SCI, and subjects 1, 2, and 11 did not complete the study
Fig. 2.
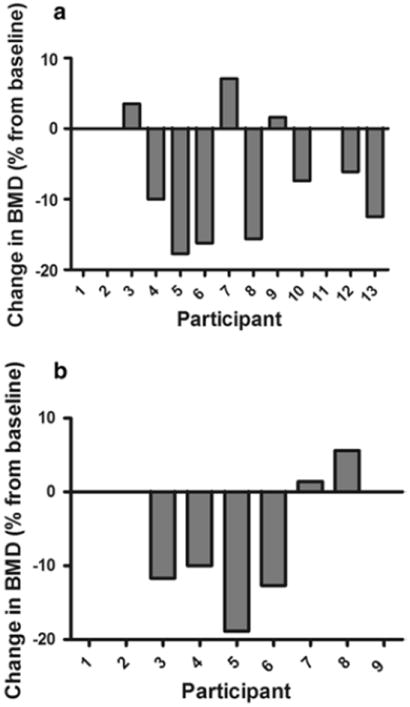
Individual changes in BMD at the a distal femur and b proximal tibia in response to 6 months of activity-based therapy. Note that participants 8 and 9 have chronic SCI, and subjects 1, 2, and 11 did not complete the study. For the proximal tibia, no data were obtained for subjects 10, 12, and 13
Changes in bone turnover
PINP did not change (p = 0.57) from baseline (18.2 ± 10.8 ng/mL) to 3 (17.8 ± 9.9 ng/mL) and 6 mo (18.7 ± 11.8 ng/mL) of the study and ranged from 8.5 to 48.7 ng/mL across participants. These values are slightly lower to those reported by Eastell et al. (2011) ranging from 16.0 to 75.8 μg/L for young women. Similarly, training had no effect (p = 0.29) on serum CTX which was equal to 0.55 ± 0.19, 0.62 ± 0.21, and 0.65 ± 0.15 ng/mL at 0, 3, and 6 months, respectively.
Dietary intake
There was no change (p = 0.27) in total energy intake during the study (1,766.5 ± 399.5, 1,740.6 ± 367.8, and 1,835.7 ± 366.8 kcal at 0, 3, and 6 months, respectively). With the exception of carbohydrate intake which was greater (p = 0.04, η2 = 0.30) at 6 months (244.7 ± 55.8 g) versus 0 (223.3 ± 70.4 g) and 3 mo (216.5 ± 54.1 g) of the study, there was no change (p > 0.05) in fat (31.6 ± 6.9, 31.9 ± 4.7, and 29.3 ± 5.9 %) or protein intake (18.1 ± 3.2, 19.4 ± 2.2, and 17.1 ± 4.6 %). Calcium intake was unchanged (p = 0.10) at 3 (765.6 ± 264.7 mg) and 6 months (890.2 ± 383.7 mg) of the study versus baseline (726.0 ± 265.0 mg).
Discussion
The primary aim of the present study was to determine the effect of intense chronic activity-based therapy targeting regions below the level of injury on BMD and bone turnover in persons with SCI. Existing studies varying in exercise modality, training duration and/or intensity, and participant level and duration of injury reveal no change (Giangregorio et al. 2005), increased (Belanger et al. 2000), or diminished BMD (Clark et al. 2007) at various sites in response to exercise training. Nevertheless, the majority of these studies did not measure bone turnover which provides a more sensitive measure of bone health than BMD alone. Completion of 6 months of ABT was unable to ameliorate bone loss of the total body and hip, but reduced the expected magnitude of bone loss seen soon after SCI by about 50 % compared to previous studies (Biering-Sorenson et al. 1990; Alekna et al. 2008). Spine BMD was increased, and markers of bone formation and resorption were unchanged with training. Overall, this modality of training seems to augment lumbar spine BMD and potentially slow bone loss in men and women with SCI, although alternative strategies are needed to reverse osteoporosis of the lower extremities in this population.
Previously-documented changes in DEXA-derived BMD in response to exercise training in persons with SCI are widely equivocal due to differences in subject population, training modality, specific device used to assess BMD, as well as specific characteristics of training including intensity, duration, and frequency employed across studies. In one study, men with chronic SCI performed 150 min/week of functional electrical stimulation (FES) for 6 months, and data revealed marked increases (10–12 %) in knee BMD, although femoral neck BMD was reduced by almost 3 % (Chen et al. 2005). In men with acute SCI completing 3 day/week of FES cycling exercise for 3 month, loss of bone at the distal femur was less than that in a non-exercising control group (Lai et al. 2010). In individuals with acute (n = 3) and chronic SCI (n = 11), 6 months of FES training (5 days/week for up to 1 h/day) increased knee BMD versus control subjects (Belanger et al. 2000), an outcome attributed to training at an intensity surpassing the mechanostat threshold developed by Frost (1987). In response to 12 months of FES cycling performed 30 min/day, 3 day/week by men and women with chronic SCI, proximal tibia BMD was increased (p < 0.05) by 10 % although there was no change in femoral neck BMD (Mohr et al. 1997). However, completion of 5 day/week of FES for 6 months (Clark et al. 2007) exhibited similar losses in BMD at the femur (−8.4 to 10.8 %) and total body (−1.9 to −3.0 %) in clients with acute SCI versus patients who did not exercise. However, compliance to training was low (61 % of sessions) and training was at low intensities. In the current study, participants completed an average of 30 min/week of FES as one component of training, although five participants did not regularly complete this modality. Overall, previous findings indicate that bone is typically responsive to high-volume FES training in persons with chronic SCI, although as shown in the current study, training was ineffective in preserving BMD at the hip or knee in individuals with acute SCI.
Locomotor training is a common exercise modality employed in SCI, and consists of both passive and active gait training as used in the present study. However, its ability to alter bone mass in the SCI is relatively minimal. In persons with acute SCI, 48 sessions of body weight supported-treadmill training did not slow bone loss at the hip or knee (Giangregorio et al. 2005). Data from a 12 months follow-up study by these authors (Giangregorio et al. 2006) revealed no significant changes in BMD although responses were quite variable across subjects, as participants with chronic SCI exhibited maintenance or small increases in knee BMD; whereas, those with acute SCI typically revealed reductions in BMD. In a male with complete acute SCI, total body (−1.5 %) and leg BMD (−6.7 %) was lower than pre-training values in response to 9 months of this mode of training (Forrest et al. 2008). In response to up to 20 weeks of training on an ambulation system (Needham-Shropshire et al. 1997), no change in femur BMD was revealed in men and women with complete SCI. Ultimately, locomotor training by itself may be most useful in promoting ambulation in persons with SCI yet its ability to improve BMD in both acute and chronic SCI seems limited.
The BMD changes observed in the present study are lower than those observed in response to alendronate therapy in men and women with recent SCI (Gilchrist et al. 2007). In this study, patients with acute SCI were randomized to 12 months of placebo or alendronate therapy, and data revealed a preservation in total-body and femoral BMD that did not occur with the placebo treatment. When parathyroid hormone administration was combined with gait training in persons with chronic SCI over a 1-year period, no change in BMD was revealed, although markers of bone turnover were augmented (Gordon et al. 2013). Overall, exercise training such as activity-based therapy may promote smaller changes in BMD than medications, although the physiological and psychological benefits of regular physical activity in the SCI cannot be overlooked. The potential synergistic effects of exercise combined with anti-resorptive medication on BMD in individuals with acute SCI remain to be determined and is an important topic to be investigated, as medications may further slow bone loss induced by exercise training.
Data revealing variable alterations in BMD with training across individuals in the present study corroborate results from a previous study in chronic SCI (Frotzler et al. 2008), although these authors cited that neither age, frequency of training, nor injury duration was associated with bone adaptation to training. For example, a 36-year-old male (baseline total hip and distal femur BMD = 1.15 and 1.30 g/cm2, respectively) with acute complete SCI at T12 exhibited 7 and 10 % losses in total hip and distal femur BMD at 6 months, similar to decreases seen in men with acute SCI (baseline distal femur BMD = 1.09 g/cm2) undergoing 6–8 months of body weight supported-treadmill training (Giangregorio et al. 2005). In contrast, a 23-year-old female with acute complete SCI at T12 revealed minimal attenuation in hip BMD from 0 to 6 months (0.77–0.76 g/cm2) despite significant losses at the knee (−15.1 %). Similarly, one male with chronic SCI experienced little change in total hip BMD yet had relatively low femoral and knee BMD at baseline (0.79 and 0.57 g/cm2, respectively). Previous data show that 6 months of training increased distal femur BMD by 11 % in men with chronic SCI who had low baseline values (0.72 g/cm2) (Chen et al. 2005). However, these scores are still below a fracture threshold for the knee developed by Garland and Adkins (2001) equal to 0.87 g/cm2. Overall, the mean change in total hip and knee BMD observed in the current study is about 50 % of that reported soon after SCI in the absence of weight-bearing (Biering-Sorenson et al. 1990; Alekna et al. 2008), so it appears that our intervention was able to slow but not reverse bone loss in persons with SCI, as shown with chronic standing (Alekna et al. 2008). In fact, declines in total-body (−2.5 %) and hip BMD (−6.9 %) reported in the current study (Table 2) are quite similar to values demonstrated by Alekna et al. (2008) (−2.5 and −6.6 %) in men and women with complete SCI performing standing during the second year post-SCI, and are much lower than individuals in their study who did not stand (−2.9 and −12.9 %). Taken together, these results suggest that the skeletal loading induced via prolonged standing and ABT may be sufficient to slow bone loss in acute SCI as compared to no weight-bearing. Further study is needed to verify this claim and investigate inter-subject differences in bone changes to chronic exercise training in persons with SCI.
Inability of ABT as used in the present study to reverse bone loss in SCI may also be attributed to the specific composition of said training. Closer examination of each participant’s training diary revealed that active assistive exercise (25.0 %, range 14.5–37.2 %) and load bearing (21.3 %, range 16.7–27.0 %) constituted almost half the total training volume; whereas, upper/lower-body resistance training (18.1 %), passive/active gait training (11.0 %), core strength training (9.3 %), and FES (5.5 %) represented lower portions of ABT. Of these modalities, only FES has been shown to increase BMD in this population (Mohr et al. 1997; Chen et al. 2005) and directly targets the muscles of the lower limbs, so the relatively minimal use of FES may explain the lack of positive changes in BMD. However, this ABT program is of similar volume and duration to FES regimens shown to have beneficial effects on BMD in SCI (Frotzler et al. 2008), yet it may not exert sufficient strain on bone to promote increases in bone mineral density of the lower extremity. Nevertheless, load bearing does require the client to support some percentage of his/her body weight either standing (100 %), on their hand and knees (up to 50 %), via kneeling (100 %—lower leg weight), or on the Total Gym (45–60 %). During active assistive exercise, the rehabilitation specialist manually manipulates the limbs by putting pressure on the foot/hand etc., which would be loading the bones/joints both longitudinally and horizontally depending upon the actions being performed.
Based on the information presented above, what is the best exercise regimen for clinicians to employ seeking to slow bone loss in persons with SCI? Astorino and Witzke (2012) demonstrated that greater exercise volume (4.9 h/week vs. 2.3 h/week), duration (11.0 vs. 6.7 months), and compliance (>80 % of sessions) were characteristics of studies showing increased or maintenance in BMD as compared to those revealing significant declines. In regards to modality, body weight support treadmill training (Giangregorio et al. 2005; Forrest et al. 2008) and FES (Eser et al. 2003; Clark et al. 2007) did not alter lower extremity BMD in acute SCI, as it continued to decline. In another study (Lai et al. 2010), FES slowed bone loss at the distal femur yet did not alter femoral neck BMD compared to a control group. In response to extremely high compressive loads, 3 years of FES led to smaller declines in tibial BMD versus the untrained limb in persons with acute SCI (Shields et al. 2006). Compared to non-standing, daily standing (>1 h/day) for 2 years diminished BMD decline in the legs (−24.9 vs. −33.8 %), lumbar spine (−14.3 vs. −16.8 %), and whole body (−11.2 vs. −14.5 %) in men and women with recent SCI (Alekna et al. 2008). Overall, exercise training does not typically reverse lower extremity BMD in persons with recent injury, and only those regimens emphasizing loading equal to (Alekna et al. 2008) or greater than body weight (e.g., Shields et al. 2006) may have the potential to slow bone loss attendant with acute SCI.
The large individual variability in BMD changes to exercise training merits the use of bone turnover markers to better clarify changes in bone status. In the current study, CTX was used as a marker of bone resorption due to its low within-subject variability and responsiveness to intervention (Vasikaran et al. 2011). In men and women (age = 50.0 ± 12.0 year) with chronic SCI, CTX was equal to 0.26 ng/mL and ranged from 0.05 to 0.70 ng/mL (Hummel et al. 2012). Our values are higher than these data yet similar to those observed in women with osteoporosis (Rogers et al. 2009). Gordon et al. (2013) demonstrated no change in CTX in response to 6 months of gait training combined with parathyroid administration in persons with chronic SCI, yet training volume (<2 h/week) and compliance to training were low (70 %). Previous studies (Bloomfield et al. 1996; Mohr et al. 1997) showed no effect of exercise training on bone resorption in persons with SCI, yet hydroxyproline and deoxyproline were measured instead of PINP and CTX, which are considered more sensitive markers of bone turnover. In a cross-sectional study (Chain et al. 2012), no difference in urinary CTX was revealed between sedentary and active men with chronic SCI. In the current study, CTX was unchanged (p > 0.05) with training, and CTX widely varied as subjects showed reduced (n = 2), increased (n = 3), and little change in CTX (n = 3) in response to training. This variability may be attributed to seasonal changes in bone turnover inherent in longitudinal studies as well as failure to adhere to pretest guidelines such as fasting and abstaining from exercise, as recently reported (Vasikaran et al. 2011).
Our data reveal that bone formation represented by changes in PINP did not change with 6 months of ABT, which is supported by previous findings (Dauty et al. 2000; Gordon et al. 2013). A previous study (Bauman et al. 2005) in patients with acute SCI (22–65 day post-injury; baseline PINP levels = 13–35 ng/mL) also revealed no change in this biomarker in response to 12 months of bisphosphonate therapy. However, the experimental group was only composed of six participants and there was large variability in the PINP results within subjects. Yet in women with osteoporosis (Delmas et al. 2009), PINP decreased by 56 % in response to 36 months of zoledronic acid ingestion. However, previous studies demonstrated unchanged (Giangregorio et al. 2006) or increased (Bloomfield et al. 1996; Carvalho et al. 2006) bone formation as represented by osteocalcin levels in response to prolonged exercise training in this population, although participants had chronic SCI. In individuals with acute SCI, training did not alter bone formation as measured by osteocalcin (Giangregorio et al. 2005), which is supported by results showing no stimulation of bone formation in persons with acute and chronic SCI (Reiter et al. 2007). It may be that intense activity-based therapy as completed in the current study cannot sufficiently impact bone turnover to stimulate bone formation in acute SCI. Furthermore, PINP was not associated with fracture risk in post-menopausal women with osteoporosis (Delmas et al. 2009), so its utility to identify risk in persons with SCI remains to be determined. Nevertheless, a local effect on bone that was undetected by these biomarkers cannot be dismissed, as findings from animals (Kodama et al. 2000) and humans (Vainionpää et al. 2009) suggest that mechanical loading and exercise may promote structural changes in bone without attendant changes in these systemic biomarkers. Despite the increased expense and time needed to assay multiple biomarkers, clinicians may consider assessing multiple indices of bone formation (PINP, osteocalcin, and total alkaline phosphatase) and resorption (CTX, hydroxyproline, and tartrate-resistant acid phosphatase 5b) in exercise training studies to yield a more robust understanding of changes in bone status.
Chain et al. (2012) reported a synergistic effect of calcium intake and exercise training on changes in BMD in the SCI. Nevertheless, mean calcium intake determined in our population was approximately 75 % of the recommended dose of 1,000 mg/day needed to optimize bone health (IOM 2010). In addition, only 3 of 10 participants typically met the recommended dose. Many individuals were unaware of the role of calcium as well as vitamin D in modifying bone status. Furthermore, it has been reported (Bauman et al. 1995) that persons with SCI tend to avoid calcium-containing foods that are typically fortified with vitamin D, which may reduce bone health. However, in a recent cross-sectional study (Hummel et al. 2012), almost 40 % of men and women with chronic SCI had a vitamin D deficiency despite supplementing both calcium and vitamin D, so further research is merited to identify optimal doses of these constituents to slow bone loss and reduce fracture risk.
This study faces a few limitations. First, individuals were undergoing different volumes of specialized activity-based therapy that was individualized for each participant based on his/her function and tolerance to training on a daily and weekly basis. However, there was no association between frequency of training and change in BMD (p > 0.05, data not reported). Dissimilar changes in BMD and bone turnover may occur in response to more traditional single-mode rehabilitation such as FES or gait training. Only 6 months of training was instituted, and a better understanding of changes in bone health would occur if training persisted for a longer duration and, moreover, if compressive load on the skeleton was determined. With the exception of one participant who initiated training 3 months post-injury, training was instituted ≥6 months post-SCI when bone resorption slows versus the weeks immediately after injury (Edwards et al. 2013), making it plausible that losses in BMD could have been further minimized with earlier intervention (de Bruin et al. 1999; Dauty et al. 2000). Data were obtained from a small heterogeneous sample of young men and women with SCI, so this sample does not allow examination of individual associations of gender, level and completeness of injury, or ethnicity on bone health. Nevertheless, age and injury duration (Frotzler et al. 2008) or injury level (Eser et al. 2003) do not seem to mediate bone’s adaptation to training and did not alter observed responses to ABT in the current study. DXA only provides a two-dimensional image of bone, and use of peripheral quantitative computed tomography allows measurement of changes in cortical and trabecular bone which decline in acute SCI (Roberts et al. 1998; Eser et al. 2004). In addition, a control group was not recruited so we are unsure if observed changes in BMD would be different than found in a nonintervention group. However, individuals with acute SCI not partaking in exercise demonstrated greater loss in tibial (de Bruin et al. 1999) and lower extremity BMD (Alekna et al. 2008) than those completing weight-bearing exercise.
In conclusion, bone loss at the hip and knee was evident despite completion of 6 months of intense activity-based therapy, although spine BMD was enhanced. Results showed no change in bone turnover in response to ABT. Our data corroborate previous results showing that intense exercise training may attenuate the dramatic decline in hip and knee BMD observed in the early stages of SCI, although inclusion of a non-exercising control group would verify this result. Nevertheless, the exercise regimen as completed in the present study was relatively time-consuming and labor-intensive, so a minimum amount of exercise should be identified in persons with SCI to elicit similar beneficial effects on bone health.
Acknowledgments
This project was funded by a grant from the National Institutes of Health SC3GM095416-02. The authors also thank the subjects for their dedication to this project as well as Brian J. Martin, M.S., L.V.N., John Lyon R.N., and Katya Geronimo L.V.N. for performing the blood draws.
Abbreviations
- ABT
Activity based therapy
- BMC
Bone mineral content
- BMD
Bone mineral density
- C
Cervical
- CTX
Serum C-terminal telopeptide of type I collagen
- DXA
Dual-energy X-ray absorptiometry
- FES
Functional electrical stimulation
- L
Lumbar
- PINP
Procollagen type 1 N propeptide
- SCI
Spinal cord injury
Footnotes
Conflict of interest None.
Contributor Information
Todd Anthony Astorino, Email: astorino@csusm.edu, Department of Kinesiology, California State University, San Marcos, 333. S. Twin Oaks Valley Road, UNIV 320, San Marcos, CA 92096-0001, USA.
Eric T. Harness, Project Walk® Spinal Cord Injury Recovery Center, Carlsbad, CA, USA
Kara A. Witzke, Department of Kinesiology, California State University, San Marcos, 333. S. Twin Oaks Valley Road, UNIV 320, San Marcos, CA 92096-0001, USA Department of Sport and Exercise Science, Oregon State University – Cascades, Bend, OR, USA.
References
- Alekna V, Tamulaitiene M, Sinevicius T, Juocevicius A. Effect of weight-bearing activities on bone mineral density in spinal cord injured patients during the period of the first two years. Spinal Cord. 2008;46:727–732. doi: 10.1038/sc.2008.36. [DOI] [PubMed] [Google Scholar]
- Astorino TA, Witzke KA. Does exercise training slow bone loss in the spinal cord injured? Efficacy, technical considerations, and questions that remain to be answered. Nova Science Publishers Inc.; Hauppauge NY: 2012. [Google Scholar]
- Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44(12):1612–1616. doi: 10.1016/0026-0495(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Morrison N, Zhang RL, Schwartz E. Effect of a vitamin D analog on leg bone mineral density in patients with chronic spinal cord injury. J Rehabil Res Devel. 2005;42(5):625–634. doi: 10.1682/jrrd.2004.11.0145. [DOI] [PubMed] [Google Scholar]
- Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000;81:1090–1098. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- Biering-Sorenson F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, forearm, and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Black DM, Cummings SR, Genant HK, Nevitt MC, Palermo L, Browner W. Axial and appendicular bone density predict fractures in older women. J Bone Min Res. 1992;7(6):633–638. doi: 10.1002/jbmr.5650070607. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone. 1996;19(1):61–68. doi: 10.1016/8756-3282(96)00109-3. [DOI] [PubMed] [Google Scholar]
- Carvalho DCL, Garlipp CR, Bottini PV, Afaz SH, Moda MA, Cliquet A. Effect of treadmill gait on bone markers and bone mineral density of quadriplegic subjects. Brazilian J Med Biol Res. 2006;39:1357–1363. doi: 10.1590/s0100-879x2006001000012. [DOI] [PubMed] [Google Scholar]
- Chain A, Koury JC, Bezerra FF. Physical activity benefits bone density and bone-related hormones in adult men with cervical spinal cord injury. Eur J Appl Physiol. 2012;112:3179–3186. doi: 10.1007/s00421-011-2303-7. [DOI] [PubMed] [Google Scholar]
- Chen SC, Lai CH, Chan WP, Hunag MH, Tsai HW, Chen JJ. Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil. 2005;27(22):1337–1341. doi: 10.1080/09638280500164032. [DOI] [PubMed] [Google Scholar]
- Clark JM, Jelbart M, Rischbieth H, Strayer J, Chatterton B, Schultz C, Marshall R. Physiological effects of lower extremity functional electrical stimulation in early spinal cord injury: lack of efficacy to prevent bone loss. Spinal Cord. 2007;45:78–85. doi: 10.1038/sj.sc.3101929. [DOI] [PubMed] [Google Scholar]
- Collins EG, Gater D, Kiratli J, Butler J, Hanson K, Langbein WE. Energy cost of physical activities in persons with spinal cord injury. Med Sci Sports Exerc. 2010;42(4):691–700. doi: 10.1249/MSS.0b013e3181bb902f. [DOI] [PubMed] [Google Scholar]
- Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. National Academy Press; Washington, DC: 2010. [Google Scholar]
- Coupaud S, Jack LP, Hunt KJ. Muscle and bone adaptations after treadmill training in incomplete spinal cord injury: a case study using peripheral quantitative computed tomography. J Musculoskelet Neuronal Interact. 2009;9(4):288–297. [PubMed] [Google Scholar]
- Dauty M, Perroun Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–309. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Frey-Rindova P, Herzog RE, Dietz V, Dambacher MA, Stussi E. Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch Phys Med Rehabil. 1999;80:214–220. doi: 10.1016/s0003-9993(99)90124-7. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Munoz F, Black DM, Cosman F, Boonen S, Watts NB, Kendler N, Eriksen EF, Mesenbrink PG, Eastell R. Effects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. J Bone Min Res. 2009;24(9):1544–1551. doi: 10.1359/jbmr.090310. [DOI] [PubMed] [Google Scholar]
- Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125(12):2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- Eastell R, Rogers A, Ni X, Krege JH. Effects of raloxifene and alendronate on bone turnover as assessed by procollagen type I N-terminal propeptide. Osteoporos Int. 2011;22:1927–1934. doi: 10.1007/s00198-010-1380-5. [DOI] [PubMed] [Google Scholar]
- Edwards WB, Schnitzer TJ, Troy KL. Bone mineral loss at the proximal femur in acute spinal cord injury. Osteoporos Int. 2013;24:2323–2328. doi: 10.1007/s00198-013-2323-8. [DOI] [PubMed] [Google Scholar]
- Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H, Stussi E. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest. 2003;33:412–419. doi: 10.1046/j.1365-2362.2003.01156.x. [DOI] [PubMed] [Google Scholar]
- Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Forrest GF, Sisto SA, Barbeau H, Kirshblum SC, Wilen J, Bond Q, Bentson S, Asselin P, Cirnigliaro CM, Harkema S. Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AIS-B spinal cord injury. J Spinal Cord Med. 2008;31(5):509–521. doi: 10.1080/10790268.2008.11753646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost HM. Bone ‘mass’ and the ‘mechanostat’: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, de Donaldson N, Eser P. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43:169–176. doi: 10.1016/j.bone.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Garland DE, Adkins RH. Bone loss at the knee in spinal cord injury. Top Spinal Cord Inj Rehabil. 2001;6:37–46. [Google Scholar]
- Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D. Regional osteoporosis in women who have a complete spinal cord injury. J Bone Joint Surg Am. 2001;83(A8):1195–1200. doi: 10.2106/00004623-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, McCartney N. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005;43:649–657. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- Giangregorio LM, Webber CE, Hicks AL, Phillips SM, Craven BC, Bugaresti JM, McCartney N. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab. 2006;31(3):283–291. doi: 10.1139/h05-036. [DOI] [PubMed] [Google Scholar]
- Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, Heard A, Reilly P, Marshall K. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endo Metab. 2007;92(4):1385–1390. doi: 10.1210/jc.2006-2013. [DOI] [PubMed] [Google Scholar]
- Gordon KE, Wald M, Schnitzer TJ. Effect of parathyroid hormone combined with gait training on bone density and bone architecture in people with chronic spinal cord injury. PM R. 2013;5(8):663–671. doi: 10.1016/j.pmrj.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Harness ET, Astorino TA. Acute energy cost of multi-modal activity based therapy in persons with spinal cord injury. J Spinal Cord Med. 2011;34(5):495–500. doi: 10.1179/2045772311Y.0000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harness ET, Yozbatiran N, Cramer SC. Effects of intense exercise in chronic spinal cord injury. Spinal Cord. 2008;46(11):733–737. doi: 10.1038/sc.2008.56. [DOI] [PubMed] [Google Scholar]
- Hummel K, Craven BC, Giangregorio L. Serum 25(OH)D, PTH, and correlates of suboptimal 5(OH)D in persons with chronic spinal cord injury. Spinal Cord. 2012;50:812–816. doi: 10.1038/sc.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y, Umemura Y, Nagasawa S, Beamer WG, Donahue LR, Rosen CR, Baylink DJ, Farley JR. Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice. Calcif Tissue Int. 2000;66(4):298–306. doi: 10.1007/s002230010060. [DOI] [PubMed] [Google Scholar]
- Lai CH, Chang WH, Chan WP, Peng CW, Shen LK, Chen JJ, Chen SC. Effects of functional electrical stimulation cycling exercise on bone mineral density loss in the early stages of spinal cord injury. J Rehabil Med. 2010;42(2):150–154. doi: 10.2340/16501977-0499. [DOI] [PubMed] [Google Scholar]
- Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotrauma. 2002;19(10):1217–1229. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]
- Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G, Kjaer M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int. 1997;61:22–25. doi: 10.1007/s002239900286. [DOI] [PubMed] [Google Scholar]
- Needham-Shropshire BM, Broton JG, Klose KJ, Lebwohl N, Guest RS, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 3. Lack of effect on bone mineral density. Arch Phys Med Rehabil. 1997;78:799–803. doi: 10.1016/s0003-9993(97)90190-8. [DOI] [PubMed] [Google Scholar]
- Perret C, Berry H, Hunt KJ, Donaldson N, Kakebeeke TH. Feasibility of functional electrical stimulated cycling in subjects with spinal cord injury: an energetics assessment. J Rehabil Med. 2010;42:873–875. doi: 10.2340/16501977-0611. [DOI] [PubMed] [Google Scholar]
- Reiter AL, Volk A, Vollmar J, Fromm B, Gerner HJ. Changes of basic bone turnover parameters in short-term and long-term patients with spinal cord injury. Eur Spine J. 2007;16:771–776. doi: 10.1007/s00586-006-0163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endo Metab. 1998;83:415–422. doi: 10.1210/jcem.83.2.4581. [DOI] [PubMed] [Google Scholar]
- Rogers A, Glover SJ, Eastell R. A randomized, double-blinded, placebo-controlled trial to determine the individual response in bone turnover markers to lasofoxifene therapy. Bone. 2009;45:1044–1052. doi: 10.1016/j.bone.2009.07.089. [DOI] [PubMed] [Google Scholar]
- Sadowsky CL, McDonald JW. Activity-based restorative therapies: concepts and applications in spinal cord injury-related neurorehabilitation. Dev Disabil Res Rev. 2009;15(2):112–116. doi: 10.1002/ddrr.61. [DOI] [PubMed] [Google Scholar]
- Shields RK, Schlechte J, Dudley-Javoroski S, Zwart BD, Clark SD, Grant SA, Mattiace VM. Bone mineral density after spinal cord injury: a reliable method for knee assessment. Arch Phys Med Rehabil. 2005;86(10):1969–1973. doi: 10.1016/j.apmr.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RK, Dudley-Javoroski W, Frey Law LA. Electrically induced muscle contractions influence bone density decline after spinal cord injury. Spine. 2006;31(3):548–553. doi: 10.1097/01.brs.0000201303.49308.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainionpää A, Korpelainen R, Vihriälä E, Rinta-Paavola A, Leppäluoto J, Jämsä T. Effect of impact exercise on bone metabolism. Osteoporos Int. 2009;20(10):1725–1733. doi: 10.1007/s00198-009-0881-6. [DOI] [PubMed] [Google Scholar]
- van Nes IJ, Guerts AC, Hendricks HT, Duysen J. Short-term effects of whole-body vibration on postural control in unilateral chronic stroke patients: preliminary evidence. Am J Phys Med Rehabil. 2004;83(11):867–873. doi: 10.1097/01.phm.0000140801.23135.09. [DOI] [PubMed] [Google Scholar]
- Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- Yang JF, Gorassini M. Spinal and brain control of human walking: implications for retraining of walking. Neuroscientist. 2006;12(5):379–389. doi: 10.1177/1073858406292151. [DOI] [PubMed] [Google Scholar]


