Abstract
Blk was identified two decades ago as a B cell-specific member of the Src family of tyrosine kinases. Recent studies, however, have discovered that Blk is expressed in many cell types outside of the B lineage, including early thymic precursors, IL-17-producing γδ T cells and pancreatic β-cells. In light of these recent discoveries, we performed a more comprehensive analysis of Blk expression patterns in hematopoietic cells and found that Blk is differentially expressed in mature B cell subsets, with marginal zone (MZ) B cells expressing high levels, B1 B cells expressing intermediate to high levels, and follicular (FO) B cells expressing low levels of Blk. To determine whether these differences in Blk expression levels reflected differential requirements for Blk in MZ, B1 and FO B cell development, we analyzed the effects of reducing and eliminating Blk expression on B cell development. We report that both Blk-haploinsufficiency and Blk-deficiency impaired the generation of MZ B cells. Moreover, although there were fewer MZ B cells in Blk+/− and Blk−/− mice compared to Blk+/+ mice, Blk mutant MZ B cells were hyper-responsive to B cell receptor stimulation, both in vitro and in vivo. Thus, this study has revealed a previously unappreciated role for Blk in the development and activation of MZ B cells.
Keywords: B cells, B cell receptor, Development, Kinases, Signal Transduction
INTRODUCTION
Members of the Src family of tyrosine kinases (SFKs) are activated in response to many different stimuli and, consequently, are involved in a multitude of signaling pathways.1,2 SFKs are expressed in both hematopoietic and non-hematopoietic cells; however, some family members exhibit a restricted pattern of expression in hematopoietic cells.1,2 Lck and Blk are examples of SFKs whose expression has been reported to be restricted to specific hematopoietic lineages; Lck is expressed in T lineage cells while Blk is expressed in B lineage cells.1–3 However, in contrast to Lck, which has an essential, non-redundant role in T cell development,4 Blk was shown to be dispensable for B cell development.5 Based on these findings, it was concluded that Lyn and Fyn, two broadly expressed SFKs that are found in B lineage cells, could compensate for the loss of Blk during B cell development.5
Although Blk was initially thought to be a B cell-specific SFK, recent studies have shown that it is expressed in a variety of cell types outside of the B cell lineage.6,7 One group found Blk to be expressed in both human and mouse pancreatic β-cells, where, in response to glucose, it enhances insulin synthesis and secretion.6 Notably, mutations in BLK, which act to reduce Blk activity and/or expression, have been identified in patients with maturity-onset diabetes of the young, a form of diabetes characterized by an autosomal pattern of inheritance and an early age at onset.6 In addition, we discovered that Blk is expressed in progenitor populations from the bone marrow (BM) and thymus, in immature thymocytes, in γδ thymocytes, and in a small subset of mature γδ T cells that have the potential to produce the proinflammatory cytokine IL-17.7 Importantly, Blk is required for the development of T cells and IL-17-producing γδ T cells, as Blk-deficient mice display reduced thymus cellularity during ontogeny and generate significantly fewer IL-17-producing γδ T cells than wild-type (Blk-sufficient) mice.7
In light of the discovery that the expression pattern of Blk is much broader than initially reported, we sought to perform a more comprehensive analysis of Blk expression levels in hematopoietic cells using our recently developed intracellular (i.c.) flow cytometric assay. During this analysis, as a consequence of using B cell subsets as positive staining controls for Blk, we noted that Blk is differentially expressed in mature B cell subsets. Specifically, Blk was found to be expressed at high levels in marginal zone (MZ) B cells, at intermediate to high levels in B1 B cells, and at low levels in follicular (FO) B cells. To determine whether these differences in Blk expression levels reflected differential requirements for Blk in MZ, B1 and FO B cell development, we analyzed the effects of reducing Blk levels on B cell development in mice expressing one functional allele of Blk (Blk+/−). In addition, we analyzed Blk−/− mice, as the effects of Blk-deficiency on MZ B cell development have not been previously examined.5 Here, we report that both reducing and eliminating Blk expression impaired the development of MZ B cells. Paradoxically, although there were fewer MZ B cells in Blk+/− and Blk−/− mice compared to Blk+/+ mice, Blk mutant MZ B cells were hyper-responsive to BCR stimulation, both in vitro and in vivo. Lastly, we found that, with age, Blk+/− and Blk−/− mice exhibit a breach in B cell tolerance, as evidenced by the detection of serum anti-nuclear Abs (ANA). Thus, Blk joins the list of signaling molecules that are required for the development and activation of MZ B cells.
RESULTS
Blk is differentially expressed in mature B cell subsets
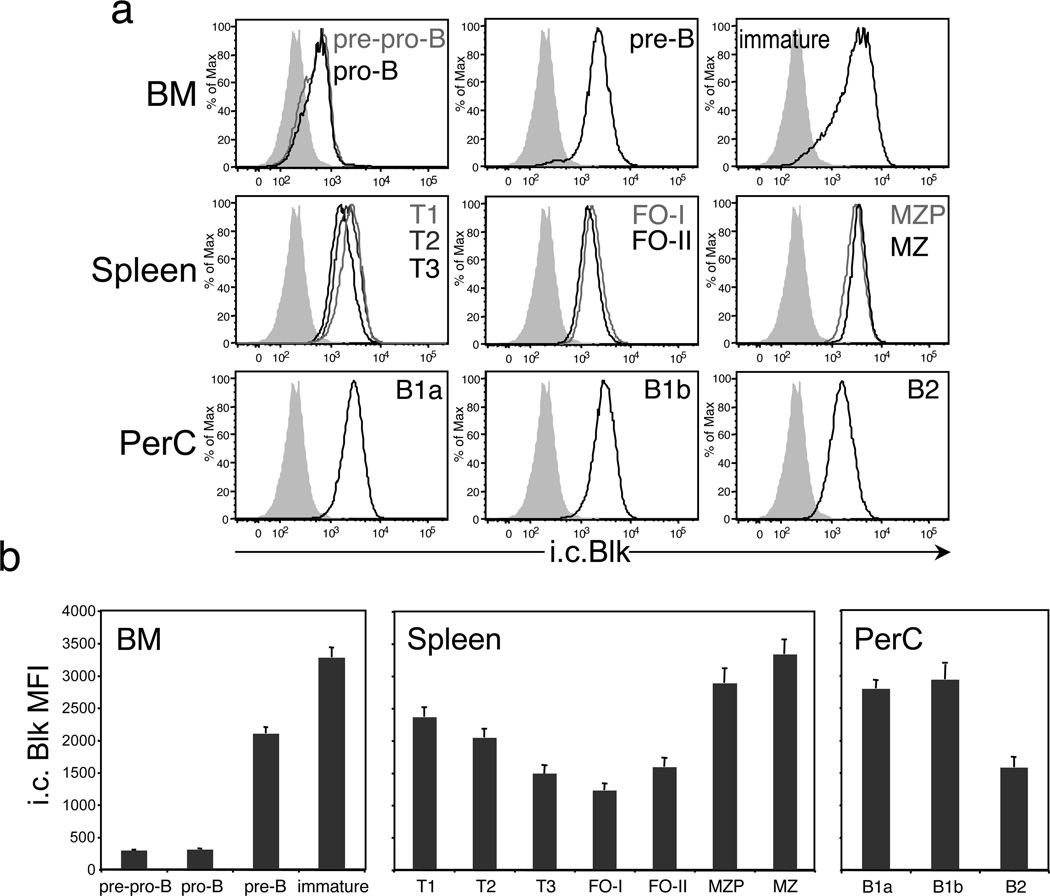
We recently developed an i.c. flow cytometric assay to measure Blk protein expression at the single cell level.7 Using this assay, we found that Blk is detected in pre-pro-B cells, the earliest identifiable B cell precursors in the BM, and that its expression continues to increase as B lineage cells mature through the pro-B and pre-B stages to reach the immature B cell stage (Figures 1a and b). In contrast to the BM, where Blk expression levels were upregulated during differentiation, Blk expression levels in splenic B cell subsets were, for the most part, downregulated in comparison. Immature B cells that are generated in the BM migrate to the spleen, where they mature and become transitional (T) B cells. T B cells in turn give rise either to mature MZ B cells, through intermediates known as MZ precursors (MZP), or to mature FO-I B cells. A second type of FO B cell, known as the FO-II B cell, has recently been described.8 FO-II B cells share functional abilities with FO-I B cells (i.e., T-dependent humoral responses) but differ from FO-I B cells in their developmental requirements and in their ability to serve as precursors to both MZ B cells and FO-I B cells.8 The splenic B cell subsets that expressed Blk at levels lower than those in immature B cells were the T, FO-II, and FO-I B cell subsets (Figures 1a and b). In contrast, MZP and MZ B cells expressed Blk at levels comparable to or higher than those in immature B cells (Figures 1a and b). Since MZ B cells are the progeny of T and/or FO-II B cells,8–11 the relatively high Blk expression levels in MZP and MZ B cells compared to T and FO-II B cells suggest that Blk expression is upregulated when cells adopt the MZ B cell fate. Notably, the difference in the regulation of Blk expression in mature splenic B cell subsets resulted in MZ B cells expressing twice as much Blk as FO-I B cells (Figure 1b).
Figure 1.
Blk is differentially expressed in immature and mature B cell subsets. (a) Histograms show representative staining of the i.c. levels of Blk in gated populations of immature and mature B cell subsets in the BM, spleen and PerC of C57BL/6 mice. In the BM, these subsets include pre-pro-B (IgM− B220+ CD43+ c-Kitlo), pro-B (IgM− B220+ CD43+ c-Kit−), pre-B (IgM− B220+ CD43−), and immature (IgM+ B220lo) B cells. In the spleen, the subsets include T1 (B220+ CD93+ IgMhi CD23−), T2 (B220+ CD93+ IgMhi CD23+), T3 (B220+ CD93+ IgMlo CD23+), FO-I (CD19+ IgDhi IgMlo CD21+), FO-II (CD19+ IgDhi IgMhi CD21+), MZP (CD19+ IgDhi IgMhi CD21hi), and MZ (CD19+ IgDlo IgMhi CD21hi) B cells. In the PerC, the subsets include B1a (IgM+ CD5lo CD11b+), B1b (IgM+ CD5− CD11b+) and B2 (IgM+ CD5− CD11b−) B cells. Cells from all tissues were stained and acquired on the same day. Data are representative of two independent experiments, with 3 mice per experiment. Solid gray histograms represent Blk staining levels in mature CD4+ T cells, which express negligible levels of Blk by real-time RT-PCR analysis.7 (b) Summary of the data presented in a. Comparison of i.c. Blk levels, presented as mean fluorescence intensity values, in gated immature and mature B cell subsets.
In addition, we measured Blk expression levels in B1 B cells, a second B cell lineage that resides primarily in the peritoneal and pleural cavities. Interestingly, both B1a and B1b B cell subsets in the peritoneal cavity (PerC) expressed Blk at relatively high levels, whereas the resident B2 B cells expressed Blk at levels comparable to those observed in splenic FO-I B cells (Figures 1a and b). These data demonstrate that Blk is differentially expressed in mature B cell subsets, with MZ B cells expressing the highest levels of Blk, followed by B1 B cells, and then by FO B cells.
Impaired MZ B cell development in Blk+/− and Blk−/− mice
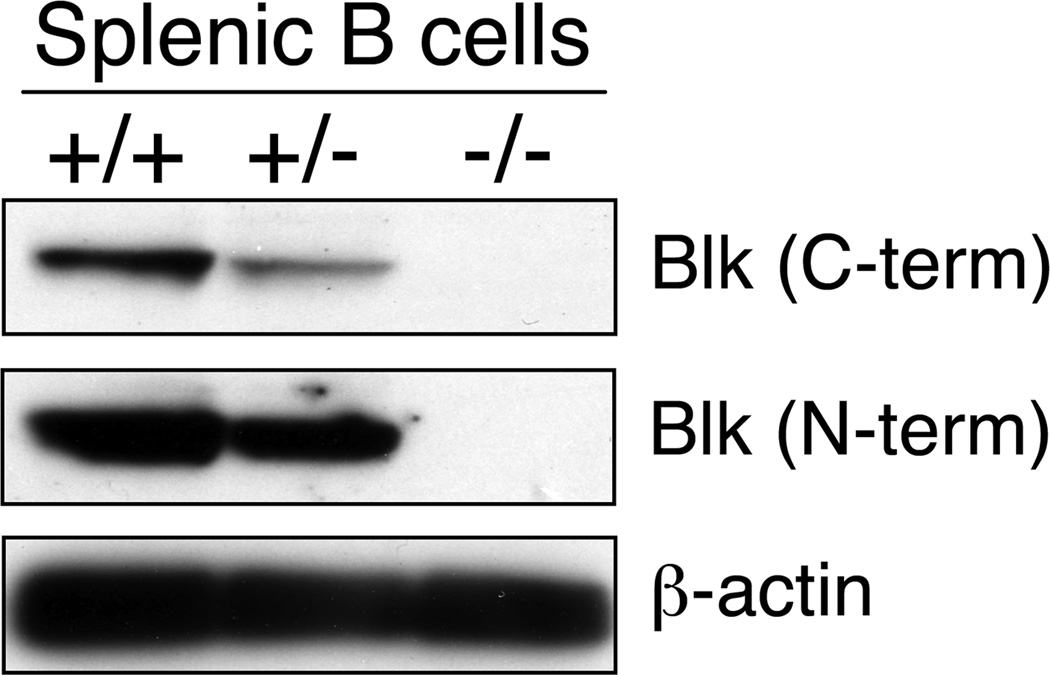
The difference in Blk expression levels between MZ and FO B cells suggested a difference in the requirement for Blk in MZ and FO B cell development. To test this, we analyzed splenic B cell development in not only Blk−/− mice but also Blk+/− mice, as their B cells have reduced levels of Blk [55% of wild-type B cells (Figure 2)]. Using various gating strategies,8,12,13 we noted significant differences in the relative percentages and absolute numbers of several splenic B cell subsets in Blk+/+, Blk+/− and Blk−/− mice (Figures 3a, b, and Table 1). First, we found that the percentages and numbers of both MZP and MZ B cells were dramatically decreased in Blk+/− and Blk−/− mice compared to Blk+/+ mice. Remarkably, the percentage and number of FO-I B cells were similar among all three genotypes, indicating that reducing or eliminating Blk expression impairs the development of MZ but not FO-I B cells. Interestingly, we also found that the percentages and numbers of T1 and FO-II B cells were significantly increased in Blk+/− and Blk−/− mice. As these B cell subsets are precursors to both MZ and FO-I B cells,8–11 the increase in their numbers in Blk+/− and Blk−/− mice suggest that reducing or eliminating Blk blocks MZ B cell development at an early stage in the FO versus MZ B cell fate decision.
Figure 2.
Comparison of Blk Expression Levels in B cells from Blk+/+, Blk+/− and Blk−/− mice. Western blot analysis of B cell lysates from Blk+/+, Blk+/− and Blk−/− mice (50 µg of protein/lane). Blots were probed with two different anti-Blk antibodies. Densitometric analysis of the bands shows a ~45% decrease in Blk levels in Blk+/− B cells compared to Blk+/+ B cells. Data are representative of three independent experiments.
Figure 3.
Effects of Blk-haploinsufficiency and -deficiency on B cell development in the spleen and peritoneal cavity. (a) Phenotypic analysis of splenocytes from age-matched Blk+/+, Blk+/− and Blk−/− mice. Left panel, dot plots show representative CD93 versus B220 staining profiles on total splenocytes. The percentages of immature transitional B cells (CD93+ B220+) and mature B cells (CD93− B220+) are shown. Middle panels, dot plots show representative IgM versus CD23 staining profiles on gated immature transitional and mature B cell subsets. The percentages of T1, T2, T3 and FO-I B cells are shown. Right panel, dot plots show representative CD21 versus CD1d staining profiles on gated CD23− IgMhi B cells. The percentage of MZ B cells (CD21hi CD1d+) is shown. (b) Dot plots show representative IgD versus IgM staining profiles on total splenocytes. The percentage of IgDhi IgMhi B cells is shown. Adjacent histograms show CD21 surface levels on gated IgDhi IgMhi B cells. The percentages of FO-II B cells (CD21+ IgDhi IgMhi) and MZP cells (CD21hi IgDhi IgMhi) are shown. Data are representative of 4 to 6 mice per genotype. (c) Dot plots show representative CD11b versus CD5 staining on IgM+ PerC cells within the lymphoid gate. The percentages of B1a (CD11b+ CD5+ IgM+) and B1b (CD11b+ CD5− IgM+) cells are shown. Data are representative of 3 to 5 mice per genotype.
Table 1.
Absolute numbers of splenic B cell subsets in wild-type and Blk mutant mice1
| Genotype | T1 | T2 | FO-II | FO-I | MZP | MZ |
|---|---|---|---|---|---|---|
| Blk+/+ | 2.5 ± 0.1 | 1.5 ± 0.1 | 4.7 ± 0.3 | 24.9 ± 1.1 | 1.5 ± 0.04 | 2.8 ± 0.4 |
| Blk+/− | 4.0 ± 0.32 | 1.6 ± 0.1 | 6.5 ± 0.42 | 27.3 ± 2.6 | 0.8 ± 0.13 | 1.2 ± 0.12 |
| Blk−/− | 3.9 ± 0.23 | 2.6 ± 0.12 | 8.3 ± 0.43 | 29.1 ± 2.0 | 0.5 ± 0.073 | 0.5 ± 0.13 |
Mean×106 ± SEM. n ≥ 4 mice per genotype.
p ≤ 0.01 compared with Blk+/+.
p ≤ 0.001 compared with Blk+/+.
Peritoneal B1 B cell numbers are increased in Blk−/− but not Blk+/− mice
Our expression study also demonstrated that B1 B cells express higher levels of Blk than FO-I B cells (Figure 1b). To determine whether B1 B cells require relatively high levels of Blk for their development, we performed flow cytometric analysis on PerC cells from both Blk+/− and Blk−/− mice. We found that reducing Blk levels did not affect B1 B cell development, as the percentages and numbers of B1a and B1b B cell subsets were comparable in Blk+/+ and Blk+/− mice (Figure 3c and Table 2). Blk-deficiency, on the other hand, did affect B1 B cell development. There were marked increases in not only the relative percentages of B1a and B1b B cells but also the absolute numbers of these B1 subsets, as a result of the significant increase in the overall cellularity of the PerC in Blk−/− mice compared to Blk+/+ mice (Figure 3c and Table 2). Therefore, even though both MZ and B1 B cells express relatively high levels of Blk compared to FO-I B cells, Blk-deficiency has opposite effects on their development, with Blk−/− mice generating fewer MZ B cells but more B1 B cells than Blk+/+ mice. Blk-haploinsufficiency, in contrast, only affected the generation of MZ B cells. Together, these data underscore differences in the developmental requirements of MZ and B1 B cells.
Table 2.
Absolute numbers of B1 B cell subsets in the peritoneal cavity of wild-type and Blk mutant mice1
| Genotype | Total | B1a | B1b |
|---|---|---|---|
| Blk+/+ | 143.3 ± 12.4 | 2.8 ± 0.3 | 1.4 ± 0.2 |
| Blk+/− | 200.7 ± 42.6 | 3.3 ± 0.3 | 1.0 ± 0.1 |
| Blk−/− | 270.1 ± 30.32 | 7.5 ± 1.13 | 1.9 ± 0.042 |
Mean×104 ± SEM. n ≥ 3 mice per genotype.
p ≤ 0.05 compared with Blk+/+.
p ≤ 0.01 compared with Blk+/+.
Humoral responses to a T-independent antigen are enhanced in Blk+/− and Blk−/− mice
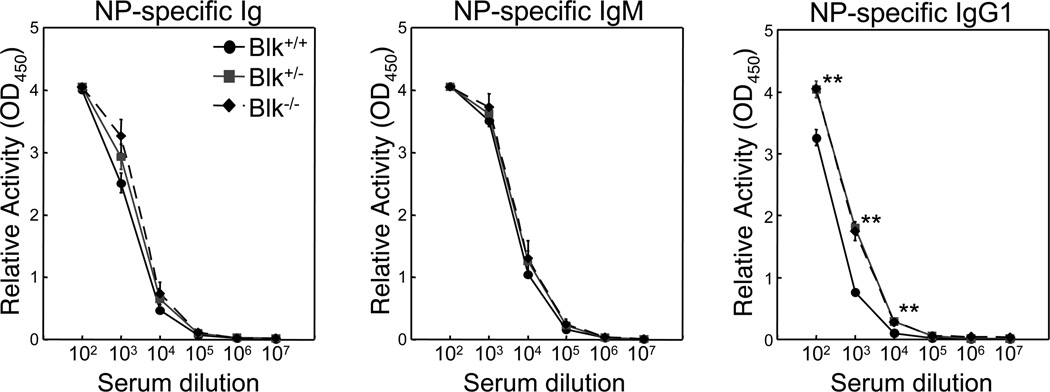
Several mutant mouse strains with reduced numbers of MZ B cells exhibit impaired responses to T-independent antigens.14–19 Because MZ B cell numbers are reduced in Blk+/− and Blk−/− mice, it was of interest to determine whether their functional response to a T-independent antigen was also impaired. Because the MZ B cell compartment is specifically targeted when T-independent antigens are injected intravenously,20 we immunized Blk+/+, Blk+/− and Blk−/− mice with NP-Ficoll, a type 2 T-independent antigen, by this route. Surprisingly, we found that the serum levels of NP-specific Ig were increased in both Blk+/− and Blk−/− mice compared to Blk+/+ mice, albeit not significantly (Figure 4). Furthermore, even though serum levels of NP-specific IgM were equivalent among the three genotypes, serum levels of NP-specific IgG1 were significantly higher in Blk+/− and Blk−/− mice than in Blk+/+ mice (Figure 4). These findings demonstrate that MZ B cells in Blk+/− and Blk−/− mice exhibit an enhanced humoral response to a T-independent antigen.
Figure 4.
Effects of Blk-haploinsufficiency and -deficiency on the humoral responses to a T-independent antigen. Age-matched Blk+/+, Blk+/− and Blk−/− mice were immunized intravenously with NP-Ficoll, a type 2 T-independent antigen. 7 days later, sera were collected and analyzed. Relative amounts of NP-specific serum Ig, IgM and IgG1 in immunized Blk+/+, Blk+/− and Blk−/− mice. **p ≤ 0.01. Data represent 3 to 4 mice per genotype.
We also evaluated the ability of Blk+/− and Blk−/− mice to produce antibodies in response to a T-dependent antigen (i.e., NP-OVA). Blk+/+, Blk+/− and Blk−/− mice were immunized with alum-precipitated NP-OVA and, 14 days later, their sera were collected and analyzed. No significant differences were observed among Blk+/+, Blk+/− and Blk−/− mice in their serum levels of NP-specific Ig, IgM or IgG1 (data not shown). Thus, these results demonstrate that Blk-haploinsufficiency and Blk-deficiency have no appreciable effects on the humoral response to a T-dependent antigen.
MZ B cells from Blk+/− and Blk−/− mice are hyper-responsive to BCR stimulation
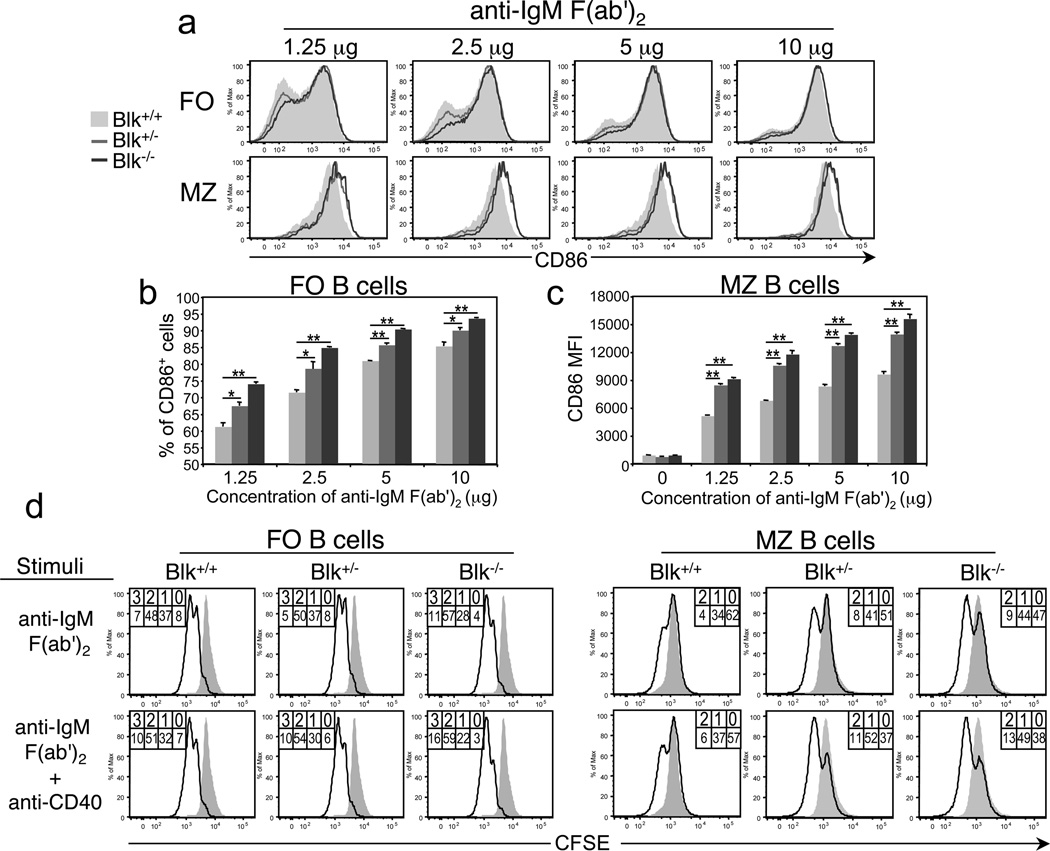
To understand how Blk+/− and Blk−/− mice could mount a robust humoral response to NP-Ficoll with significantly fewer MZ B cells than Blk+/+ mice, we evaluated the effects of Blk-haploinsufficiency and Blk-deficiency on B cell activation in vitro. We first compared the ability of MZ and FO B cells from Blk+/+, Blk+/− and Blk−/− mice to upregulate CD86 expression following antibody-mediated cross-linking of IgM. Notably, we observed striking differences in the CD86 expression levels on activated MZ B cells from wild-type and Blk mutant mice, with Blk+/− and Blk−/− MZ B cells expressing, on average, 1.7-fold higher levels of CD86 than Blk+/+ MZ B cells following activation with anti-IgM (Figure 5a and c). In contrast, no differences were detected among FO B cells from the three genotypes in their surface levels of CD86 following BCR stimulation (Figure 5a). Instead, we found that the percentages of Blk+/− and Blk−/− FO B cells with increased CD86 expression were consistently higher than that of wild-type FO B cells across the concentration range of anti-IgM used to simulate antigen-BCR ligation (Figure 5a and b). The enhanced ability of B cells from Blk+/− and Blk−/− mice to respond to the low doses of anti-IgM indicate that both Blk-haploinsufficiency and Blk-deficiency lower the threshold of B cell activation.
Figure 5.
Effects of Blk-haploinsufficiency and -deficiency on B cell activation in vitro. (a) Upregulation of CD86 expression following BCR stimulation. Splenocytes from Blk+/+, Blk+/− and Blk−/− mice were plated and then stimulated with varying doses (1.25, 2.5, 5, and 10 µg/ml) of goat anti-mouse F(ab')2 anti-IgM for 24 hours. Histograms show CD86 expression on gated MZ (CD21hi CD1d+ IgDlo) and FO (CD23+ IgDhi) B cells from Blk+/+ (shaded histogram), Blk+/− (gray histogram) and Blk−/− (dark gray histogram) mice. Data are representative of three independent experiments. (b) Summary of FO B cell data presented in a. Comparison of the percentages of CD86+ FO B cells among Blk+/+ (light gray bars), Blk+/− (gray bars) and Blk−/− (dark gray bars) mice as a function of antigen dose. *p ≤ 0.05, **p ≤ 0.01. (c) Summary of MZ B cell data presented in a. Comparison of the mean fluorescence intensity of CD86 on MZ B cells from Blk+/+ (light gray bars), Blk+/− (gray bars) and Blk−/− (dark gray bars) mice as a function of antigen dose. **p ≤ 0.01. (d) Comparison of proliferative responses following BCR stimulation. Purified splenic B cells were labeled with CFSE and then stimulated with soluble anti-IgM (Fab’)2 (10 µg/ml) or with soluble anti-IgM (Fab’)2 (10 µg/ml) and anti-CD40 monoclonal antibody (1 µg/ml). After 72 hours, cells were harvested, stained with monoclonal antibodies against various surface antigens to identify and gate FO and MZ B cells subsets, and then proliferation was assessed by flow cytometric analysis. The percentage of cells within each CFSE peak is given. The overlay histograms show representative CFSE levels in unstimulated B cells. Data are representative of two independent experiments, with 4 mice per genotype.
Next, we compared the proliferative responses of MZ and FO B cells from Blk+/+, Blk+/− and Blk−/− mice to BCR stimulation by measuring the extent of CFSE dilution. Blk+/− MZ B cells, but not Blk+/− FO B cells, underwent more rounds of cell division than their wild-type counterparts following BCR stimulation (Figure 5d). In contrast, both Blk−/− MZ and FO B cells exhibited increased proliferation compared to Blk+/+ MZ and FO B cells under similar stimulating conditions (Figure 5d). Proliferative responses by FO and MZ B cells to stimulation with LPS, IL-4, CpG, or Pam3Cys (a TLR1/2 agonist), however, were comparable among Blk+/+, Blk+/− and Blk+/− mice (data not shown). These in vitro data, combined with the in vivo data, show that Blk-haploinsufficiency and Blk-deficiency enhance the expansion and functional responses of MZ B cells.
B cells from Blk−/− mice exhibit enhanced ERK activation following BCR cross-linking
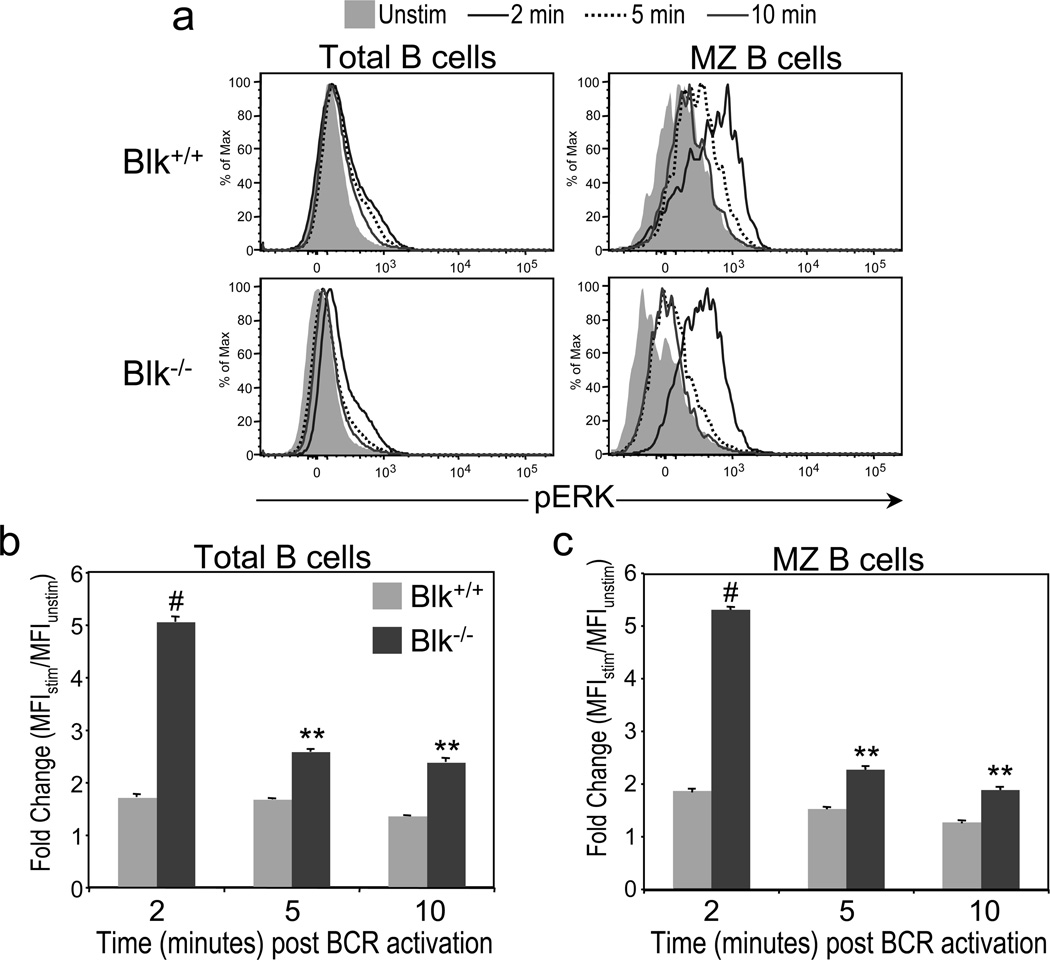
To gain insight into how Blk regulates signal transduction by the BCR, we compared the ability of B cells from Blk+/+ and Blk−/− mice to activate Syk, a proximal signaling event, and ERK, a distal signaling event, after BCR ligation. Using phospho-specific flow cytometry, we detected no significant difference between Blk+/+ and Blk−/− B cells in either the magnitude or the kinetics of Syk activation after BCR stimulation (data not shown). However, we did note a dramatic difference in the magnitude of ERK activation not only between total Blk+/+ and Blk−/− B cells (Figure 6a and b) but also between Blk+/+ and Blk−/− MZ B cells (Figure 6a and c), following BCR cross-linking. Given that B cells from Blk−/− mice exhibit enhanced BCR-mediated ERK activation, we conclude that Blk is negative regulator of BCR signaling.
Figure 6.
Effects of Blk-deficiency on BCR signal transduction. Splenocytes from Blk+/+ and Blk−/− mice were stimulated for 0, 2, 5 and 10 minutes with 10 µg/ml of goat anti-mouse F(ab')2 anti-IgM, stained with monoclonal antibodies against various surface antigens in addition to anti-pERK, and then analyzed by flow cytometric analysis. (a) Sample histograms for intracellular pERK in unstimulated (shaded histogram) and stimulated total B cells (left panel) and MZ B cells (right panel) from Blk+/+ (top panel) and Blk−/− (bottom panel) mice. Comparison of the kinetics and magnitude of ERK activation in B cells (b) and MZ B cells (c) from Blk+/+ and Blk−/− mice. Data are presented as fold change, which was calculated by dividing the geometric mean fluorescence intensity (MFI) of the stimulated sample (MFIstim) by the MFI of the unstimulated sample (MFIunstim). **p ≤ 0.01, #p ≤ 0.001. Data are representative of three independent experiments.
Blk+/− and Blk−/− mice develop autoimmunity with age
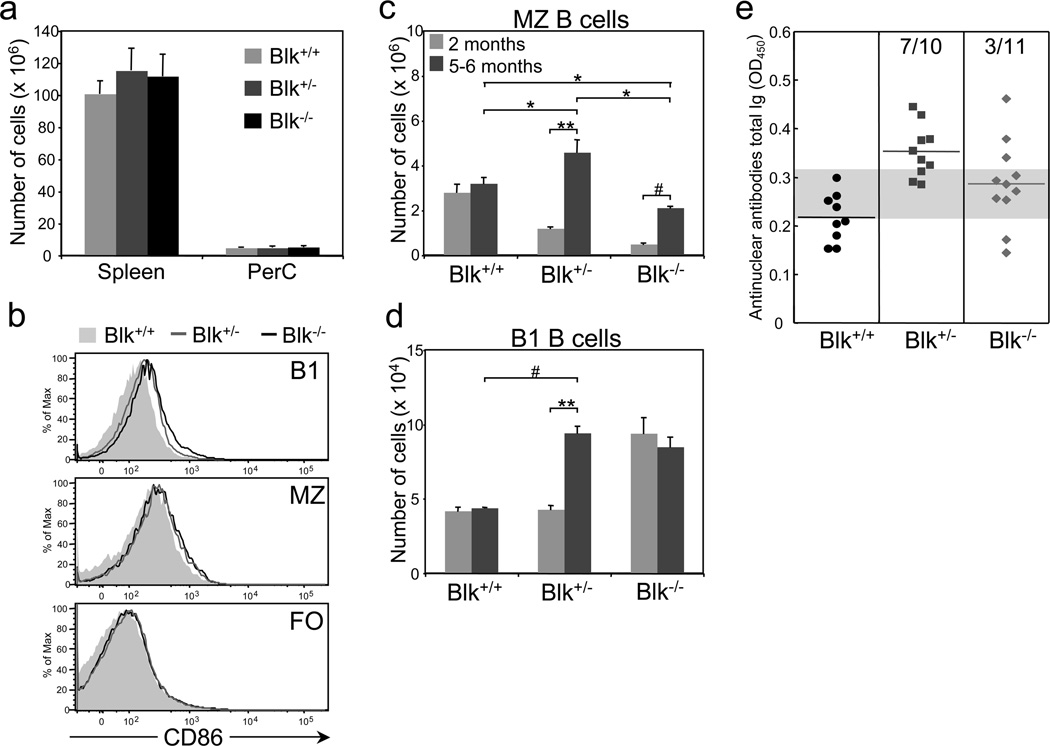
Since there is a correlation in many mouse models between hyper-responsive B cells and autoimmunity (21–25), we sought to determine whether Blk+/− and Blk−/− mice develop autoimmunity with age. Several phenotypes, such as splenomegaly, emergence of B cells with an activated phenotype, and increased numbers of MZ and B1 B cells, have been reported in autoimmune-prone mice (reviewed in 26,27). In 5 to 6-month-old Blk+/− and Blk−/− mice, we did not detect any significant differences in the cellularity of either the spleen or PerC compared to age-matched Blk+/+ mice (Figure 7a). However, we did note differences in their B cell surface phenotype, with MZ and B1 B cells from aged Blk+/− and Blk−/− mice expressing higher levels of CD86 than their age-matched wild-type counterparts (Figure 7b). Interestingly, CD86 surface levels on FO B cells were equivalent among all three genotypes. Consistent with MZ and B1 B cells from aged Blk+/− and Blk−/− mice displaying an activated phenotype, we observed dramatic changes in their numbers in aged Blk+/− and Blk−/− mice (Figure 7c and d). MZ B cell numbers were significantly higher in aged Blk+/− and Blk−/− mice than in young (2-month-old) Blk+/− and Blk−/− mice (Figure 7c). In fact, the number of MZ B cells in aged Blk+/− mice was even higher than that in aged Blk+/+ mice, while that in aged Blk−/− mice was still lower than that in aged Blk+/+ mice (Figure 7c). In aged Blk+/− mice, we also noted a significant increase in B1 B cell numbers relative to aged Blk+/+ mice as well as to young Blk+/− mice (Figure 7d). The number of B1 B cells in aged Blk−/− mice, on the other hand, was unchanged compared to young Blk−/− mice. These data demonstrate that there are age-related changes in the phenotype and number of the MZ and B1 B cell populations in Blk+/− and Blk−/− mice.
Figure 7.
Aged Blk+/− and Blk−/− mice display autoimmune phenotypes. (a) Comparison of the absolute numbers of splenocytes and PerC cells in 5 to 6-month old Blk+/+, Blk+/− and Blk−/− mice. Data represent 3 to 6 mice per genotype. (b) Comparison of the surface levels of CD86 on B1, MZ and FO B cells from aged Blk+/+, Blk+/− and Blk−/− mice. Data are representative of 3 to 6 mice per genotype. Comparison of the number of MZ (c) and B1 (d) B cells in young (2 months of age) and aged (5 to 6 months of age) Blk+/+, Blk+/− and Blk−/− mice. *p ≤ 0.05, **p ≤ 0.01, #p ≤ 0.001. Data are representative of 3 to 6 mice per genotype. (e) Sera were collected from 6-month-old Blk+/+, Blk+/− and Blk+/− mice and analyzed for the presence of ANA by ELISA. Data are presented as OD450 readings for a 1:100 dilution of serum. Symbols represent individual mice. Gray horizontal bar marks the upper limit (Blk+/+ mean plus 2 standard deviations) of the normal range. Numbers of Blk+/− and Blk−/− mice that exhibit serum ANA levels above the upper limit of the normal range are shown.
Because MZ and B1 B cells are known to be a source of autoantibodies (28–32), we assayed the sera of aged Blk+/− and Blk−/− mice for the presence of autoantibodies. We found that the serum levels of ANA were significantly higher in 6-month-old Blk+/− mice than in age-matched Blk+/+ mice (p ≤ 0.001; Figure 5d). Although the ANA serum levels were also increased in 6-month-old Blk−/− mice relative to age-matched Blk+/+ mice (p ≤ 0.05; Figure 7e), there was variability in the serum ANA levels among the individual aged Blk−/− mice. Accordingly, using the wild-type mean OD450-value plus 2 standard deviations as the upper limit of the normal range, we were able to classify 70% of Blk+/− mice and 27% of Blk−/− mice as autoantibody producers. Therefore, when Blk expression is reduced and, to a lesser extent, absent, there is a breach in B cell tolerance.
DISCUSSION
Two decades ago, Blk emerged on the scene as an SFK expressed primarily in B cells (3). Blk, like Lyn and Fyn (the other two major SFKs expressed in B lineage cells), was shown to be physically associated with the BCR complex and to be activated following BCR engagement (33–35). Notably, out of all three SFKs, Blk displayed the strongest activation index following BCR cross-linking, suggesting that it was the predominant SFK in the BCR-coupled signaling pathway (33,36). Further studies showed that, following BCR stimulation, all three SFKs phosphorylate distinct substrates (37) and differ in their ability to bind to signaling intermediates in the BCR signaling cascade (36–38). It was, therefore, a surprise when no apparent defects in B cell development or activation were observed in Blk−/− mice (5). As a result, very few groups continued to study Blk, and its functions and mechanisms of action remain poorly defined.
There has been a renewed interest in Blk in light of recent studies showing that Blk is not only expressed in non-B lineage cells but is also indispensable for the development and/or function of these cells (6,7). Accordingly, we performed a more comprehensive analysis of Blk expression patterns in hematopoietic cells with the goal of identifying other hematopoietic lineages that express Blk. During this analysis, while using B cell subsets as positive staining controls, we found that Blk is differentially expressed among MZ, FO and B1 B cells, with MZ and B1 B cells expressing approximately twice as much Blk as FO B cells. The dynamics of Blk expression during splenic B cell development show that the differential expression of Blk between MZ and FO B cells is due to its upregulation in MZP and MZ B cells. The upregulation of Blk is crucial for the development and activation of the MZ B cell lineage, as mice with reduced levels, or a loss, of Blk generate MZ B cells that are fewer in number but exhibit enhanced in vitro and in vivo responses to BCR stimulation compared to wild-type mice.
Previous studies have compared Blk transcript and Blk protein levels between MZ and FO B cells but not among MZ, FO and B1 B cells. The studies that compared Blk expression between MZ and FO B cells did not report a difference in its expression between these two subsets (39,40). An explanation for this discrepancy is that, in the study that compared gene expression in MZ and FO B cells following microarray analysis, differential expression between the two subsets was restricted to a mean fold change of >2 (39). The results of our flow assay demonstrated a two-fold difference in Blk expression between MZ and FO B cells, which falls below the >two-fold limit set up in the microarray study. To explain the results from the biochemical study that reported no difference in Blk protein expression between MZ and FO B cells by Western blot analysis (40), we propose that lysis in the detergent Nonidet P-40 did not solubilize all the cellular compartments where Blk is associated in MZ B cells. Our flow assay, on the other hand, detects Blk in any cellular compartment, as long as the epitope is available for binding.
Our re-examination of the role of Blk in B cell development and activation demonstrated that a loss of Blk expression affected MZ and B1 B cell development as well as humoral responses to a T-independent antigen. These findings are in stark contrast to those of the initial study that reported that Blk−/− mice exhibit no defects in B cell development and function (5). An explanation for the contradictory results is that the initial study was published a decade ago, prior to the development of the complex gating strategies that are used to identify MZP and MZ B cell subsets (8,13,41). In point of fact, there was no quantification of MZP and MZ B cells in the original report (5). Another explanation, which is not mutually exclusive, is that the different genetic backgrounds of the Blk−/− mice used in the two studies (i.e., 129/Sv in the first study and C57BL/6 in our study) differentially modify the effects of Blk-deficiency. Consistent with this explanation are the reports that Fyn-deficiency has different effects on T cell activation and function depending on the genetic background of the Fyn−/− mouse (42–45).
SFKs can be positive or negative regulators of cell signaling (reviewed in 1,2,46). The finding that B cells from Blk+/− and Blk−/− mice are hyper-responsive to BCR stimulation, as evidenced by their enhanced ERK activation and cellular proliferation, indicate that Blk negatively regulates the BCR signaling response. Based on these findings, we propose that the activation threshold of a B cell is proportionate to its cellular level of Blk. This idea is supported by the comparison of the proliferative responses of wild-type MZ and FO B cells, in which Blklo FO B cells outproliferate Blkhi MZ B cells following BCR stimulation (Figure 4d; 10).
Assigning Blk the role as a negative regulator of BCR signal transduction has important implications for B cell subset fate decisions. Evidence is accumulating to support a role for BCR signal strength in the generation of B1, FO and MZ B cells, although it is not clear whether BCR signal strength determines cell fate, maintains the cell fate decision or both (reviewed in 9,10,26,47). According to the current model, a strong signal is required for the generation of B1 B cells, an intermediate to strong signal is required for the generation of FO B cells, and a weak signal is required for the generation of MZ B cells. Our data support this model, as Blk-deficiency, which augments the BCR signaling response, resulted in an increased number of B1 cells and a decreased number of MZ B cells. Interestingly, FO-I B cell numbers were not significantly different between Blk+/+ and Blk−/− mice, suggesting that the effects that Blk-deficiency has on the BCR signaling response are still within the range allowed for FO B cell generation and/or survival.
Blk as a negative regulator of BCR signal transduction also has important implications in the control of B cell tolerance. Both B1 B cells and MZ B cells express relatively high levels of this negative regulator compared to FO B cells. Since MZ and B1 B cells are positively selected by self-antigens (reviewed in 9,10,26,47), their relatively high expression of Blk may be a mechanism by which a high BCR signaling threshold is set in these potentially self-reactive cells. This proposed mechanism may explain why, when signaling thresholds are lowered as a result of reduced Blk levels, there is a breach in tolerance, as evidenced by the detection of ANA in aged Blk+/− mice. We posit that the expanded MZ and B1 B cell populations, both of which are known to be a source of autoantibodies (28–32), contribute to the elevated serum levels of ANA in aged Blk+/− mice. Along these lines, the finding that fewer aged Blk−/− mice than Blk+/− mice produce ANA may be explained by the fact that their MZ B cell numbers, although increased compared to young Blk−/− mice, are approximately 50% of those in Blk+/− mice. Thus, we predict that the percentage of Blk−/− mice with elevated serum levels of ANA will increase with age, providing that their MZ B cell numbers continue to increase.
An allele in the promoter region of BLK, which results in its reduced transcription, is one of the novel genetic alleles that were recently shown to associate with susceptibility to systemic lupus erythematosus (SLE) (48). Our study of Blk-haploinsufficient mice, in which Blk expression levels are reduced to levels comparable to those in individuals homozygous for the risk allele (48 and data not shown), should provide insight into how this genetic variant promotes development of SLE. However, it is important to note that this risk allele, since it is located in a promoter region, also affects the transcription of an adjacent gene, C8orf13, which is ubiquitously expressed and currently has no known function (48). Interestingly, the risk allele has the inverse effect on C8orf13 transcription, with B cells from individuals homozygous for the risk allele displaying twice as much C8orf13 message as B cells from individuals lacking the risk allele (48). Although it is not known whether changes in the expression of both gene products are required to confer susceptibility to SLE, it is clear that reducing Blk levels alone can result in a breach in tolerance and autoimmunity.
In summary, we have discovered that Blk is required not only for the development and activation of MZ B cells but also for the control of B cell tolerance.
METHODS
Mice
C57BL/6J (Blk+/+) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA), whereas B6-Blktm1 (Blk−/−) mice5 were provided by A. Tarakhovsky (Rockefeller University, New York, NY, USA). All mice used in this study were bred and maintained in the Department of Laboratory Animal Resources at SUNY Upstate Medical University in accordance with the specifications of the Association for Assessment and Accreditation of Laboratory Animal Care. Mouse protocols were approved by the SUNY Upstate Medical University Committee on the Humane Use of Animals. All mice were used at 6 to 10 weeks of age unless otherwise noted.
Antibodies and reagents
Monoclonal antibodies used for flow cytometric analysis and magnetic bead separation included anti-CD1d (1B1), anti-CD4 (RM4-5), anti-CD5 (53-7.3), anti-CD8a (53-6.7), anti-CD11b (M1/70), anti-CD19 (6D5), anti-CD21 (7E9), anti-CD23 (B3B4), anti-CD43 (S7), anti-B220 (RA3-6B2), anti-CD86 (GL-1), anti-CD93 (AA4.1), anti-CD117 (2B8), anti-I-Ab (AF6-120.1), and anti-IgM (RMM-1), anti-IgD (11-26c.2a), anti-TCRβ (H57-597), anti-TCRγδ (UC7-13D5), anti-Gr-1 (RB6-8C5), anti-NK1.1 (PK136), and anti-TER-119 (TER-119), which were purchased from BioLegend (San Diego, CA, USA), eBioscience (San Diego, CA, USA) and BD Pharmingen (San Jose, CA, USA). Antibodies used in intracellular flow cytometric assays were anti-Blk (Cell Signaling Technology, Danvers, MA, USA), FITC-conjugated donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA) and anti-phosphoERK1/2 (BD Pharmingen).
Purification of splenic B cells
Splenic B cells were purified by negative selection using magnetic bead separation. Briefly, spleen cells from Blk+/+, Blk+/− and Blk−/− mice were stained for 10 min with FITC-conjugated anti-CD4, anti-CD8, anti-TCRβ, anti-TCRγδ, anti-NK1.1, anti-CD11b, anti-Gr-1, and anti-TER-119 monoclonal antibodies, washed, and then incubated with anti-FITC beads (Miltenyi, Auburn, CA, USA) for 15 min, with all steps at 4°C. The purity of the resulting cell population was typically ≥95% CD19+, as assessed by flow cytometric analysis.
B cell activation assays
Splenic B cells were purified by negative selection and then labeled with CFSE (eBioscience) as per the manufacturer’s instructions. 3 × 106 B cells were plated and then stimulated with 10 µg/ml goat anti-mouse F(ab')2 anti-IgM (Jackson ImmunoResearch, West Grove, PA, USA) and/or 1 µg/ml anti-CD40 monoclonal antibody (1C10; BioLegend). 72 hours later, B cells were harvested, stained with monoclonal antibodies against various surface antigens, and then analyzed by flow cytometric analysis.
To assess activation marker upregulation after BCR stimulation, 3 × 106 splenocytes were plated and then stimulated with varying doses (1.25, 2.5, 5, and 10 µg/ml) of goat anti-mouse F(ab')2 anti-IgM (Jackson ImmunoResearch). 24 hours later, cells were harvested, stained with monoclonal antibodies against various surface antigens, including anti-CD86, and then analyzed by flow cytometric analysis.
To assess the kinetics of ERK activation after BCR stimulation, 2 × 106 splenocytes were stimulated for different times with 10 µg/ml of goat anti-mouse F(ab')2 anti-IgM (Jackson ImmunoResearch), stained with monoclonal antibodies against various surface antigens in addition to anti-pERK, and then analyzed by flow cytometric analysis.
Flow cytometric analysis
Flow cytometric analysis for both surface and intracellular antigens was performed as previously described.7,49,50
Western Blot analysis
B cells were lysed in a buffer containing 20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, protease inhibitors (Roche Laboratories, Indianapolis, IN, USA), and 1% NP-40 Alternative (EMD Biosciences, La Jolla, CA, USA). Proteins within the cleared B cell lysates were resolved by SDS-PAGE, transferred to PVDF membranes, and blotted with antibodies against the N-terminus of Blk (Santa Cruz Biotechnology, Santa Cruz, CA, USA), the C-terminus of Blk (Cell Signaling Technology) and β-actin (Sigma Aldrich, St. Louis, MO, USA).
Immunizations and NP-specific ELISAs
To measure humoral responses to a type 2 T-independent antigen, mice were immunized intravenously with 10 µg NP-Ficoll (Biosearch Technologies, Novato, CA, USA). Sera were collected 7 days post immunization.
Serially diluted serum samples were plated in duplicate onto ELISA plates that had been coated with NP26-BSA (Biosearch Technologies). NP-specific Igs were identified with HRP-conjugated isotype-specific antibodies (SouthernBiotech, Birmingham, AL, USA). Data are presented as OD450 readings for all serum dilutions.
Autoantibody ELISA
Serum levels of anti-nuclear autoantibodies (ANA) were measured by ELISA using a kit from Alpha Diagnostics International (San Antonio, TX, USA), following the manufacturer’s instructions.
ACKNOWLEDGMENTS
We thank Dr. Alexander Tarakhovsky for the Blk−/− mice. We also thank Drs. Paul Love and Michael Princiotta for helpful advice and critical review of the manuscript. This work was supported by the Hendricks Fund for Medical Research and NIH AI081068 to S.M.H and NIH CA102667 to R.R.
References
- 1.Lowell CA. Src-family kinases: Rheostats of immune cell signaling. Mol. Immunol. 2004;41:631–643. doi: 10.1016/j.molimm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Palacios EH, Weiss A. Function of the Src-family kinases, lck and fyn, in T cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 3.Dymecki SM, Niederhuber JE, Desiderio SV. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990;247:332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- 4.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 5.Texido G, Su IH, Mecklenbräuker I, Saijo K, Malek SN, Desiderio S, Rajewsky K, Tarakhovsky A. The B-cell-specific Src-family kinase Blk is dispensable for B-cell development and activation. Mol. Cell. Biol. 2000;20:1227–1233. doi: 10.1128/mcb.20.4.1227-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borowiec M, Liew CW, Thompson R, Boonyasrisawat W, Hu J, Mlynarski WM, El Khattabi I, Kim SH, Marselli L, Rich SS, et al. Mutations at the BLK locus linked to maturity onset diabetes of the young and β-cell dysfunction. Proc. Natl. Acad. Sci. U.S.A. 2009;106:14460–14465. doi: 10.1073/pnas.0906474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laird RM, Laky K, Hayes SM. Unexpected role for the B cell-specific Src family kinase B lymphoid kinase in the development of IL-17-producing γδ T cells. J. Immunol. 2010;185:6518–6527. doi: 10.4049/jimmunol.1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cariappa A, Boboila C, Moran ST, Liu H, Shi HN, Pillai S. The recirculating B cell pool contains two functionally distinct, long-lived, posttransitional, follicular B cell populations. J. Immunol. 2007;179:2270–2281. doi: 10.4049/jimmunol.179.4.2270. [DOI] [PubMed] [Google Scholar]
- 9.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 10.Martin F, Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 11.Allman D, Srivastava B, Lindsey RC. Alternative routes to maturity: branch points and pathways for generating follicular and marginal zone B cells. Immunol. Rev. 2004;197:147–160. doi: 10.1111/j.0105-2896.2004.0108.x. [DOI] [PubMed] [Google Scholar]
- 12.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 13.Cariappa A, Liou H-C, Horwitz BH, Pillai S. Nuclear Factor κb is required for the development of marginal zone B lymphocytes. J. Exp. Med. 2000;192:1175–1182. doi: 10.1084/jem.192.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guinamard R, Okigaki M, Schlessinger J, Ravetch J. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 15.Girkontaite I, Missy K, Sakk V, Harenberg A, Tedford K, Pötzel T, Pfeffer K, Fischer K-D. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat. Immunol. 2001;2:855–862. doi: 10.1038/ni0901-855. [DOI] [PubMed] [Google Scholar]
- 16.Samardzic T, Marinkovic D, Danzer CP, Gerlach J, Nitschke L, Wirth T. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 2002;32:561–567. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Yu M, Podd A, Wen R, Chrzanowska-Wodnicka M, White GC, Wang D. A critical role of Rap1b in B-cell trafficking and marginal zone B-cell development. Blood. 2008;111:4627–4636. doi: 10.1182/blood-2007-12-128140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheikl T, Reis B, Pfeffer K, Holzmann B, Beer S. Reduced notch activity is associated with an impaired marginal zone B cell development and function in Sly1 mutant mice. Mol. Immunol. 2009;46:969–977. doi: 10.1016/j.molimm.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Al-Shami A, Wilkins C, Crisostomo J, Seshasayee D, Martin F, Xu N, Suwanichkul A, Anderson SJ, Oravecz T. The adaptor protein Sh2d3c is critical for marginal zone B cell development and function. J. Immunol. 2010;185:327–334. doi: 10.4049/jimmunol.1000096. [DOI] [PubMed] [Google Scholar]
- 20.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 21.Cyster JG, Goodnow CC. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 22.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 23.Inaoki M, Sato S, Weintraub BC, Goodnow CC, Tedder TF. CD19-regulated signaling thresholds control peripheral tolerance and autoantibody production in B lymphocytes. J. Exp. Med. 1997;186:1923–1931. doi: 10.1084/jem.186.11.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JH, Avitahl N, Cariappa A, Friedrich C, Ikeda T, Renold A, Andrikopoulos K, Liang L, Pillai S, Morgan BA, Georgopoulos K. Aiolos regulates B cell activation and maturation to effector state. Immunity. 1998;9:543–553. doi: 10.1016/s1074-7613(00)80637-8. [DOI] [PubMed] [Google Scholar]
- 25.O'Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cellspecific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J. Exp. Med. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol. Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 27.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv. Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int. Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- 29.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J. Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 30.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J. Immunol. 2004;172:625–635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 31.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun. Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher CA, Groom JR, Woehl B, Leung H, Mackay C, Mackay F. Development of autoimmune nephritis in genetically asplenic and splenectomized BAFF transgenic mice. J. Autoimmun. 2011;36:125–134. doi: 10.1016/j.jaut.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Burkhardt AL, Brunswick M, Bolen JB, Mond JJ. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark MR, Campbell KS, Kazlauskas A, Johnson SA, Hertz M, Potter TA, Pleiman CM, Cambier JC. The B cell antigen receptor complex: association of Ig-α and Ig-β with distinct cytoplasmic effectors. Science. 1992;258:123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- 35.Lin J, Justement LB. The MB-1/B29 heterodimer couples the B cell antigen receptor to multiple src family protein tyrosine kinases. J. Immunol. 1992;149:1548–1555. [PubMed] [Google Scholar]
- 36.Aoki Y, Isselbacher KJ, Cherayil BJ, Pillai S. Tyrosine phosphorylation of Blk and Fyn Src homology 2 domain-binding proteins occur in response to antigen-receptor liagtion in B cells and constitutively in pre-B cells. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4204–4208. doi: 10.1073/pnas.91.10.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malek SN, Desiderio S. SH2 domains of the protein-tyrosine kinases Blk, Lyn, and Fyn(T) bind distinct sets of phosphoproteins from B lymphocytes. J. Biol. Chem. 1993;268:22557–22565. [PubMed] [Google Scholar]
- 38.Pleiman CM, Clark MR, Timson Gauen LK, Winitz S, Coggeshall KM, Johnson GL, Shaw A, Cambier JC. Mapping of sites on the Src family protein tyrosine kinases p55blk, p59fyn, and p56lyn which interact with the effector molecular phospolipase C- γ2, microtubule-associated protein kinases, GTPase-activating protein, and phosphatidylinositol 3-kinase. Mol. Cell. Biol. 1993;13:5877–5887. doi: 10.1128/mcb.13.9.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kin NW, Crawford DM, Liu J, Behrens TW, Kearney JF. DNA microarray gene expression profile of marginal zone versus follicular B cells and idiotype positive marginal zone B cells before and after immunization with Streptococcus pneumoniae. J. Immunol. 2008;180:6663–6674. doi: 10.4049/jimmunol.180.10.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Martin F, Oliver AM, Kearney JF, Carter RH. Antigen receptor proximal signaling in splenic B-2 cell subsets. J. Immunol. 2001;166:3122–3129. doi: 10.4049/jimmunol.166.5.3122. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava B, Quinn WJ, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J. Exp. Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasuda K, Nagafuku M, Shima T, Okada M, Yagi T, Yamada T, Minaki Y, Kato A, Tani-Ichi S, Hamaoka T, Kosugi A. Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipidenriched microdomains in lipid rafts in resting T cells. J. Immunol. 2002;169:2813–2817. doi: 10.4049/jimmunol.169.6.2813. [DOI] [PubMed] [Google Scholar]
- 43.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates TH2 differentiation and PKC-θ-mediated activation of NF-κB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Davidson D, Shi X, Zhang S, Wang H, Nemer M, Ono N, Ohno S, Yanagi Y, Veillette A. Genetic evidence linking, SAP, the X-linked lymphoproliferative gene product, to Src-related kinase FynT in TH2 cytokine regulation. Immunity. 2004;21:707–717. doi: 10.1016/j.immuni.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Mamchak AA, Sullivan BM, Hou B, Lee LM, Gilden JK, Krummel MF, Locksley RM, DeFranco AL. Normal development and activation but altered cytokine production of Fyn-deficient CD4+ T cells. J. Immunol. 2008;181:5374–5385. doi: 10.4049/jimmunol.181.8.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 47.Hardy RR. B-1 B cell development. J. Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 48.Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, Lee AT, Chung SA, Ferreira RC, Pant PV, et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N. Engl. J. Med. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- 49.Laird RM, Hayes SM. Roles of the Src tyrosine kinases Lck and Fyn in regulating γδTCR signal strength. PLoS ONE. 2010;5:e8899. doi: 10.1371/journal.pone.0008899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayes SM, Li LQ, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]